Psychiatric Aspects of Child and Adolescent Obesity
Abstract
Objective: To review the past 10 years of published research on psychiatric aspects of child and adolescent obesity and highlight information mental health professionals need for preventing obesity in youths and diagnosing and treating it. Method: Researchers performed computerized and manual searches of the literature and summarized the most relevant articles. Results: The growing epidemic of child and adolescent obesity deserves attention for its immediate mental health and long-term medical complications. Mental health professionals working with obese youths should be aware of recent advances in neuroscience, genetics, and etiologies associated with obesity. Those who assess and treat obese youth should view obesity as a chronic disease. Currently, no approved pharmacological or surgical approaches exist to treat childhood obesity. Conclusions: Health care providers should focus on modest weight-loss goals that correlate with significant health benefits. The most effective treatments include substantial parental involvement. Mental health professionals should help obese children build self-esteem to help them lead full lives regardless of weight.
It is currently not known why children and adolescents become obese, or what environmental, genetic, and psychiatric pathways lead to obesity. Child and adolescent psychiatrists have an ambiguous role in treating obesity in individuals who do not suffer from psychiatric disorders. This review critiques empirical studies published in the past 10 years concerning the psychiatric assessment, course, causes, sequelae, comorbidity, and treatment of childhood obesity.
A major challenge for this 10-year review and synthesis was the exponential growth of research and review articles on all aspects of this epidemic. Because of the enormity of the pediatric literature on obesity, this review focuses on the psychiatric aspects of childhood obesity. Therefore, it is beyond the scope of this 10-year review to critically synthesize many nonpsychiatric aspects of childhood obesity in general.
Obesity is immensely costly at a national level, as well as mentally and physically costly to those who suffer from it. A safe and successful treatment of obesity remains elusive, particularly during maintenance.
Definition of obesity
Body mass index (BMI) is the simplest and most common assessment tool for categorizing childhood obesity and is calculated by dividing weight (kg) by height (m2). BMI charts for boys and girls can be found at www.cdc.gov/growthcharts. BMI accurately reflects the proportion of excess body fat and correlates with markers of secondary complications of obesity and long-term mortality (Barlow and Dietz, 1998). An expert panel on obesity evaluation and treatment recommends that a BMI greater than the 95th percentile for age and sex should be used as a cutoff for medical evaluation of obesity (Barlow and Dietz, 1998). In older adolescents, a BMI in the 95th percentile is associated with elevated blood pressure and lipid profiles, increasing risks of obesity-related disease and mortality (Barlow and Dietz, 1998). A patient with a BMI above the 85th percentile or a large increase in BMI should be observed closely for secondary complications. To maintain consistency with the existing literature, this review will use the term “obesity” to describe a BMI greater than or equal to the 95th percentile and “overweight” for BMI greater than or equal to the 85th percentile.
Prevalence
Rates of childhood and adolescent obesity have increased dramatically in the past decade. According to a recent article in the Journal of the American Medical Association based on results from the 1999–2000 National Health and Nutrition Examination Survey (NHANES), more than 15% of youths ages 6 through 19 were obese, and more than 10% of children ages 2 through 5 were obese (Ogden et al., 2002). The percentages of obese children and adolescents were relatively stable over NHANES I (1971–1974) and II (1976–1980) but doubled to 11% during NHANES III (1988–1994) and then increased again by 4% during NHANES IV (1999–2000) (National Center for Health Statistics [NCHS], 1999).
This recent increase is particularly evident among non-Hispanic black and Mexican-American adolescents. For youths in these groups, the prevalence of obesity increased more than 10% between 1988 to 1994 and 1999 to 2000. As a result, more than 23% of non-Hispanic black and Mexican-American adolescents were obese in 1999 to 2000 (Ogden et al., 2002). African-American girls ages 6 to 19 have the highest rates of obesity, slightly higher than Native Americans and Hispanics (NCHS, 1999). These figures merit further research into cultural and racial influences on the prevalence of childhood obesity.
Medical and psychosocial sequelae
Although many of the effects of childhood obesity do not manifest themselves until adulthood, the obese child may suffer immediate consequences from his or her condition. The medical sequelae that threaten the young obese child include cardiovascular disease, endocrine and pulmonary problems, and orthopedic, gastroenterological, and neurological difficulties (Strauss, 1999).
A 40-year follow-up study revealed a twofold increase in the rate of cardiovascular disease and hypertension and a threefold increase of diabetes in obese children compared with normal-weight children (Mossberg, 1989). A 50-year follow-up to the Harvard Growth Study of 1922 to 1935 showed that obese adolescent boys ages 13 to 18 experienced twice the mortality rate from cardiovascular disease in adulthood. Being obese as an adolescent was a better predictor of overall mortality than being obese as an adult (Strauss, 1999).
Endocrine malfunction caused by obesity results in insulin resistance, associated with the onset of type 2 diabetes mellitus. The number of children with type 2 diabetes mellitus increased 10-fold between 1982 and 1992, and more than 90% of these patients had a BMI above the 90th percentile (Pinhas-Hamiel et al., 1996). It is noteworthy that most of these patients exhibited no symptoms or unrelated symptoms of type 2 diabetes mellitus. Data from the Bogalusa Heart Study showed the development of type 2 diabetes mellitus in 2.4% of the obese adolescents by age 30 compared to none in the lean population (Srinivasan et al., 1996).
Recent research on asthma and child obesity has demonstrated mixed results, and most findings suggest no correlation between the two disorders (Brenner et al., 2001; Chinn and Rona, 2001; Von Kries et al., 2001; Von Mutius et al., 2001). Multiple studies of obese children have shown that as many as 94% suffer from a sleep abnormality, most often sleep apnea (Mallory et al., 1989; Marcus et al., 1996; Silvesti et al., 1993). Obese children with obstructive sleep apnea demonstrate clinically significant decrements in learning and memory function compared with obese children without obstructive sleep apnea (Rhodes et al., 1995).
Orthopedic problems, such as slipped capital epiphyses and Blount’s disease, occur in obese children. Approximately 50% to 70% of patients with slipped capital epiphyses are obese. Hip pain, knee pain, or altered gait in an obese child could result from this event (Kelsey et al., 1972; Strauss, 1999). Blount’s disease involves bowing of the legs and tibial torsion from bearing unequal or early excess weight, and one study reported that up to 80% of children with Blount’s disease were obese (Dietz et al., 1982). Obesity accounts for 8% to 33% of cases of gallstones observed in children, and it has been estimated that the risk of gallstone formation is approximately 4.2 times greater in 14- to 20-year-old obese girls than in their peers of normal weight (Friesen and Roberts, 1989; Halcomb et al., 1980; Honore, 1980). Studies have also shown a positive correlation between obesity and pseudotumor cerebri (Corbett et al., 1982; Durcan et al., 1988; Scott et al., 1997; Sugerman et al., 1997).
No data suggest that childhood obesity contributes directly to an increased risk of cancer in adulthood. However, a high-fat childhood diet may increase long-term cancer risks, because excess fat intake in adults correlates with an increased risk of breast, uterine, cervical, colon, prostate, and pancreatic cancer (Deslypere, 1995; Weisburger, 1997).
During young adulthood, the psychosocial consequences of obesity can include fewer years of education, lower family income, higher poverty rates, and lower marriage rates (Dietz, 1997; Maffeis and Tato, 2001). The challenge of preventing such sequelae is similar to that associated with youth smoking. In both cases, health care professionals must consider how to persuade youths to change their behavior even though the negative physical consequences are so distant.
Natural course
Obese children are at great risk for adulthood obesity. Leading predictors are parental weight status and the child’s adiposity (amount of fatty tissue) after age 10. Both obese and nonobese children have twice the risk of adulthood obesity when at least one parent is obese (NCHS, 1999; Whitaker et al., 1997). For children ages 3 to 9 years, both their BMIs and their parents’ BMIs are predictors of later obesity. Furthermore, 80% of obese adolescents ages 10 to 14 with at least one obese parent remain obese (Whitaker et al., 1997). Although a high percentage of obese children become obese adults, this does not mean that most obese adults were obese as children.
As children mature, their own adiposity and BMI become more accurate predictors of future obesity than parental weight. Klish (1998) reports that 25% of obese 6-year-olds become obese adults and 75% of obese 12-year-olds remain obese. Other studies supporting these data, such as Whitaker et al. (1997) and Styne (2001), estimate that one third to one half of obese 6-year-olds become obese adults.
Pediatric predictors of childhood obesity and subsequent adult obesity
Gestational events and the adiposity rebound period (see below) may influence childhood-onset obesity leading to adult obesity. Studies have observed that famine during gestation can either increase or decrease later obesity prevalence, and “infants with higher neonatal amniotic fluid concentrations of insulin show significantly increased obesity at age 6 years” (Strauss, 1999, p. 8). Infants whose mothers had gestational diabetes have a 50% greater risk of obesity at age 10 (French et al., 1995; Styne, 2001). Although some studies indicate that breast-feeding protects against obesity, others indicate either no influence or an increased risk of becoming obese (Ebbeling et al., 2002; Parsons et al., 1999; Strauss, 1999). Parsons et al. (1999) found a strong correlation between low socioeconomic status during early life and adult obesity.
Many studies on childhood obesity causes have focused on adiposity rebound, a normal childhood developmental event. Rebound is the increase of BMI that occurs after the normal decrease in adiposity between infancy and approximately age 5 to 7. Some studies identify early adiposity rebound in childhood (before age 5) as an obesity predictor (French et al., 1995; Styne, 2001). Parsons et al. (1999) and Strauss (1999) discuss early maturation, noted in several studies, as an increased obesity risk. However, no clear consensus exists concerning how early adiposity rebound or early maturation influences obesity.
Researchers cannot reliably predict which obese children will become obese adults. Intervention studies are needed in high-risk early childhood populations, and pinpointing responsible factors should be a national priority.
National health care cost of child obesity
Obesity-associated annual hospital costs, the only U.S. cost data available that isolate childhood obesity, have more than tripled in the past 20 years, from $35 million in 1979 to 1981 to $127 million in 1997 to 1999 (Wang and Dietz, 2002). These data reflect an increase in the percentage of discharges with obesity-related diseases, particularly obesity (197% increase), gallbladder disease (228% increase), and sleep apnea (436% increase). The increased incidence of hospital stays related to obesity-associated diseases serves as further evidence of the increasing prevalence of obesity in children and adolescents.
There are no published reports on the national costs of childhood obesity. However, in 1995, the total cost of health care attributed to adult obesity was $99.2 billion, $51.64 billion of which involved direct medical costs for obesity-related diseases (Wolf and Colditz, 1998).
The median reimbursement rate for pediatric obesity treatment is estimated to be 11%. This rate varies greatly among different types of insurance policies, although there are no significant variations based on a child’s sex, race, or degree of obesity (Tershakovec et al., 1999).
Social issues and pressures: stigma and self-esteem
Obesity stigmatizes young children even before adolescence, placing them outside the social norms. When shown drawings of children of different sizes, children rank obese classmates as the least desirable playmates.
Studies on self-esteem in obese children report inconsistent results. Many indicate that obese children and adolescents have moderately lower self-esteem than nonobese peers (French et al., 1995; Manus and Killeen, 1995; Pesa et al., 2000; Stradmeijer et al., 2000; Strauss, 2000). However, other studies reveal no differences in self-esteem between population-based groups of obese children and adolescents and nonobese controls (Gortmaker, 1993; Renman et al., 1999; Rumpel and Harris, 1994). Furthermore, studies finding lower self-esteem report that self-esteem is not significantly lower in obese populations once body image is controlled for (French et al., 1995; Pesa et al., 2000). The most consistently replicated finding is that obese children and adolescents have a more negative body image than their peers (Buddeburg-Fisher et al., 1999; French et al., 1995; Israel and Ivanova, 2002; Manus and Killeen, 1995; Pesa et al., 2000; Renman et al., 1999; Rumpel and Harris, 1994). Overweight children as young as age 5 can develop low self-esteem and negative body image (Ebbeling et al., 2002). A study completed by Grilo et al. (1994) demonstrated that “the greater the frequency of being teased about weight and shape while growing up, the more negative one’s appearance is regarded, and the greater the degree of body dissatisfaction in adulthood” (p. 448).
Self-esteem in obese children varies with gender and age. Females are at greater risk for self-esteem problems because body image is an important component of their self-esteem (Manus and Killeeen, 1995; Pesa et al., 2000). Some obese females develop lower self-esteem as they go through puberty into adolescence (Israel and Ivanova, 2002; Stradmeijer et al., 2000). Some studies report that severely obese females express lower self-esteem than moderately obese females, which suggests that self-esteem relates to the severity of obesity (Israel and Ivanova, 2002). In female adolescents and children, dieting is associated with lower self-esteem (Pesa et al., 2000). The frustrating dieting cycle of losing and regaining weight may itself contribute to lower self-esteem. In addition, dieting correlates with perceived, not actual, body weight. Therefore, although some studies show that an adolescent female’s severity of obesity is correlative with lower self-esteem, dieting behavior, regardless of the severity of obesity, is an indicator of lower self-esteem.
Few studies have examined relationships between self-esteem and race and culture in obese individuals. One study did report that whereas obese white and Latin American females had significantly lower levels of self-esteem than age- and race-matched controls, obese and nonobese African-American girls did not differ significantly on this construct (Strauss, 2000).
Another factor influencing self-esteem is parental concern for the child’s well-being. For 5-year-old obese girls, the mothers’ restriction of food and the fathers’ opinion of the child’s obesity were significantly associated with the child’s negative self-perception (Davison and Birch, 2001). Stradmeijer et al. (2000) found that parental concern in obese 10- to 16-year-olds more significantly correlated with self-esteem problems than did BMI. Parental acceptance or lack of concern may be a protective factor for self-esteem.
Clinical populations show a clear relationship between lower self-esteem and obesity (Rumpel and Harris, 1994). Obesity in children seeking clinical treatment is often more severe than in children in the general population and may correlate with lower self-esteem. However, no data are available to support this relationship in community samples because subjects with obesity as severe as that seen in clinically referred patients are rare. Therefore, associations between the severity of obesity and low self-esteem may be more difficult to assess in a population-based study. One hypothesis is that clinically referred children represent a subgroup of obesity associated with especially low self-esteem. In addition, parents who seek clinical treatment for their child’s obesity may exhibit more concern, an indicator for negative self-esteem in children as described above.
Manus and Killeen (1995) and Israel and Ivanova (2002) indicate that some obese children and adolescents with normal self-esteem use compensatory methods to protect themselves from lower self-esteem. Manus and Killeen suggest that obese children use discounting (diminishing the importance of domains in which they are less competent) as a coping mechanism. For instance, such children may view their physical appearance as unimportant and place more importance on other domains in which they excel. They also use distortion (enhancing their perception of competence) by underestimating their actual body size or weight. Similarly, Israel and Ivanova suggest that both obese girls and boys place decreased emphasis on their physical self-esteem and increased emphasis on other self-esteem dimensions to maintain general self-esteem. Further research is needed on why some obese children are more susceptible than others to low self-esteem.
Neuroscience of feeding and weight maintenance
For a complete review of the neuroscience and neuroendocrinology involved in eating, see Schwartz (2001) and Lustig (2001).
The hypothalamus regulates energy balance and food intake. Leptin, an adipocyte (fat cell) hormone, and insulin, both present in proportion to fat stores in the body, have a high density of receptors in the hypothalamus. The presence of leptin and insulin activates the anorectic branch of the hypothalamus (which decreases food intake) and inhibits the orexigenic branch (which stimulates food intake) (Fig. 1).
Conversely, the absence of these hormones activates the orexigenic branch and inhibits the anorectic branch, increasing food intake. In addition, peripherally generated signals such as low plasma glucose, cortisol, and the recently discovered hormone ghrelin also increase food intake. A detailed description of these processes is beyond the scope of this review.
Irregular hypothalamus development or damage to hypothalamic neurons may cause some rare cases of obesity, generally before age 10. Certain disorders causing abnormal hypothalamus development, such as Prader-Willi, Bardet-Biedl, Cohen, or Carpenter syndrome, may predispose children to obesity (Lustig, 2001). Tumors may also damage the hypothalamus. Diencephalic tumors, more common in children than adults, are associated with early-onset childhood obesity (Lustig, 2001).
Other brain regions influence hypothalamic control and food intake through projections to the ventral medial hypothalamus (VMH). Dopaminergic brain areas affect eating behavior and motivation. In the 1960s doctors treated weight problems with stimulants such as dextroamphetamine, which have subsequently been shown to alter intrasynaptic dopamine levels (Towell et al., 1988). Further implicating dopamine in obesity, Wang et al. (2001), in a positron emission study, found that adults with fewer dopamine (DA2) brain receptors had greater BMIs. Identifying abnormalities in brain receptors or function in obese children and adolescents could lead to earlier, more precise identification of susceptible individuals. Surprisingly, there are few brain imaging studies on idiopathic obese adults and none on children.
Other important neurotransmitters controlling eating and weight include serotonin (5-HT) and norepinephrine (NE). Serotonergic neurons, which project to areas of the VMH, originate in the raphe nuclei. Studies on 5-HT reuptake inhibitors reveal that serotonin induces satiety and decreases food intake. The role of NE in food regulation is not completely understood. However, studies report that noradrenergic alpha-2 receptor agonists induce food intake, while alpha-1 receptor agonists reduce food intake (Lustig, 2001). Understanding how neurotransmitters and the hypothalamus regulate food intake will permit research into more specialized obesity treatments.
Genetics
Farooqi and O’Rahilly (2000) provide a complete review of the molecular genetics of childhood obesity.
Twin studies estimate that genetic factors account for 50% to 90% of the variance in BMI. Estimates from numerous adoption, parent–offspring, and sibling studies indicate that genetic factors account for 20% to 80% of the variance in factors associated with obesity development. Such factors include energy intake and expenditure (Klish, 1998; Maes et al., 1997). Maes et al. (1997) examined more than 25,000 twin pairs and 50,000 biological and adoptive relatives and found the strongest BMI correlations among children range from 0.74 for monozygotic twins and 0.32 for dizygotic twins to 0.25 for siblings. The wide range (20%–80%) of estimated influence of genetic factors is due to the large number of studies and very divergent methodologies used.
Several family studies have provided evidence for the role of genetics in obesity, suggesting that 30% to 50% of obesity phenotypes are inherited (Pérusse and Bouchard, 1999a). In mouse and human models, researchers have identified over 200 genes that affect metabolism, food intake, energy expenditure, and other weight-related functions. Mouse models of obesity resulting from environmental factors also exist (Yager, 2000).
Although genes may set biological limits for metabolism and other weight-related mechanisms, behavior and environment influence variations within these limits.
Genes may affect behavior, such as calorie expenditure, by influencing the amount someone moves or fidgets (Levine et al., 1999). Specific genes and mechanisms underlying such behaviors have not been identified, but genetic and familial patterns have been examined. Pérusse and Bouchard (1999a) cited one family study showing that children of active parents tended to be more active, while children of less active parents were less active. This study did not parse out genetic and environmental effects. Another study that Pérusse and Bouchard (1999a) cited found that parents who tended to eat carbohydrates and fatty foods had children who desired similar diets.
Gene–environment interactions are an important aspect of obesity genetics. Intra-twin studies show that similar genotypes respond with little variance to the environment, while inter-twin studies show that dissimilar genotypes vary greatly in environmental response (Pérusse and Bouchard, 1999b). For example, infant biological twins have more similar overfeeding responses than comparisons across twin pairs, who display a large variation in response (Strauss, 1999).
The most common genetic cause of human obesity is mutations in the melanocortin 4 receptor (MC4R) coding region. Whereas most genetic forms of obesity are rare recessive disorders, mutations in the MC4R gene cause dominant and recessive obesity (Lubrano-Berthelier et al., 2003). In some studies, mutations in this gene have accounted for up to 6% of all cases of severe obesity.
Medical and psychosocial causes of childhood obesity
Although rare in the population at large, systemic medical conditions can result in obesity. Table 1 lists most of the medical causes. However, fewer than 10% of the child obesity cases seen have endogenous causes; more than 90% are idiopathic (Moran, 1999). This large idiopathic percentage is responsible for the child obesity epidemic of the past 30 years (Strauss, 1999).
Decreased physical activity
The average child watches 3 to 5 hours of television daily—a worrisome fact, considering that adult men who watch more than 3 hours of television daily are twice as likely to be obese than those who watch less than an hour daily (Tucker and Friedman, 1989). A large randomized control trial by Robinson (1999b) compared two populations of children at similar public schools. One population received a 6-month classroom curriculum to reduce television, videotape, and video-game use, while the other population did not. Robinson found that compared to the control population, children in the intervention group had statistically significant decreases in BMI and other adiposity measurements.
The Muscatine Heart Study showed that pubertal and postpubertal children spend only 8 to 10 minutes per day in aerobic activity (Janz et al., 1992). In a recent longitudinal study by Kim et al. (2002), activity declined 100% for African-American girls and 64% for non-Hispanic white girls by year 10 of the study. In addition, 56% of the African-American girls and 31% of the non-Hispanic white girls reported no habitual leisure-time activity. These results represent significant declines in the physical activity of adolescent girls and no doubt contribute substantially to the growing obesity epidemic.
Increased fat intake
Nutritional surveys show that obesity levels relate to the amount of fat consumed. Studies of dietary preference have shown that normal-weight people crave high-carbohydrate foods, while obese people crave high-fat foods (Drewnowski et al., 1991). Americans eat approximately one third of meals outside the home, often at fast-food restaurants, where fat constitutes 45% to 55% of most food selections’ caloric content. The physiological response to high-fat meals suggests that fat intake should suppress appetite (i.e., delayed gastric emptying). However, the reverse appears to be true. A 1994 study showed that young adults eat similar amounts of food regardless of the fat content (Stubbs et al., 1994). As a result, individuals consuming high-fat foods are more likely to gain weight. Parents influence their children’s eating habits by their example (Ebbeling et al., 2002). These habits tend to remain with the individual throughout adulthood. Children as young as age 3 exhibit increased preferences for high-fat foods if their parents are obese.
Social factors
Social factors associated with obesity include neglect, abuse, and generally nonsupportive home environments (Strauss, 1999). Neglected children are nine times more likely than others to become obese (Lissau and Sorensen, 1994). Adults seeking treatment for obesity demonstrate a fourfold increase in the prevalence of childhood sexual abuse, as well as a twofold increase in nonsexual abuse compared with a control population (Fellitti, 1993). One psychosomatic theory of obesity is that food provides comfort and therefore that eating serves as a compensatory mechanism for children who have survived traumatic experiences or who live in difficult environments (Parsons et al., 1999; Strauss, 1999). Thus, obese children may overeat as a consequence of environmental deprivation or as a result of depression, somatization, or familial abuse (Christoffel and Forsyth, 1989; Fellitti, 1993).
Obesity: a psychiatric or behavior disorder?
Overeating, the chronic inability to control how much is eaten, results in obesity and causes suffering, stigmatization, and social cost, but it is not classified as a behavioral or psychiatric disorder. The International Classification of Diseases (ICD) categorizes obesity as a general medical condition. DSM-IV does not characterize it as a psychiatric disorder “because it has not been established that obesity is consistently associated with a psychological or behavioral syndrome” (American Psychiatric Association, 1994, p. 539). However, binge-eating disorder, seen in high numbers of obese individuals, is a separate proposed DSM-IV disorder (Yanovski et al., 1993).
For obese children and adolescents, who are commonly teased and bullied, obesity is often associated with psychological and behavioral symptoms (see below). Whether pediatric obesity should be labeled a psychiatric disorder is debatable and requires empirical/epidemiological studies. “Compulsive eating” as a symptom has received very little attention in published studies and also merits further research.
Prevalence of psychiatric disorder in obesity
Psychiatric disorders: cause or consequence?
Many population-based studies have found high rates of psychological disorders in obese children and adolescents, especially in females. Buddeburg-Fisher et al. (1999) found higher rates of such disorders as somatoform, mood, pain, and anxiety in overweight Swiss high school girls. They also reported a correlation between poorer body image and increased psychiatric comorbidity. However, in a study of 3,197 adolescent females, Pesa et al. (2000) found that after controlling for body-image dissatisfaction, the difference in psychopathology between nonobese and obese females ages 15 to 17 was insignificant. Erickson et al. (2000) found similar results in a study of third graders. In another population-based study of 10,000 adolescents, Gortmaker (1993) found no correlation initially or at 7-year follow-up between obesity and psychological comorbidity.
In population-based studies, a significant difference in behavior (not disorders) is evident between obese and nonobese children. Stradmeijer et al. (2000) used parent and teacher reports on two groups of children, prepubertal (ages 10–13) and adolescent (ages 13–16). They found that obese children and adolescents had significantly more behavior problems, as reported by their mothers and teachers, than nonobese peers. Behavior was worse in the prepubertal age group.
Some research indicates a significant difference in psychiatric comorbidity between obese children who are clinically referred and obese children in the general population. Those seeking clinical treatment have increased levels of depression, anxiety, somatoform, and eating disorders (Britz et al., 2000; Epstein et al., 1994a; Sheslow et al., 1993; Wallace et al., 1993). Britz et al. (2000) reported that the rate of eating disorders was six times higher in the obese patient group than the population-based control group. The disorders included bulimia nervosa, eating disorders not otherwise specified (EDNOS), and anorexia nervosa. Sixty percent of the females and 35.3% of the males reported binge-eating episodes in which the quantity of food intake exceeded that of most people under similar circumstances. Wallace et al. (1993) found that 32% of obese children had depression, and Sheslow et al. (1993) reported a rate of 50%. These data parallel findings that clinically referred obese adults have higher psychiatric comorbidity than population-based obese adults (Britz et al., 2000; Pesa et al., 2000). Spitzer et al. (1993) performed a multisite study of binge-eating disorder (BED) involving 1,785 adult subjects and found that 29% of the subjects in weight control programs met criteria for BED. A study by Yanovski et al. (1993) found that 43% of the 128 obese adult subjects met criteria for BED.
The incidence of psychological disorders in clinically referred obese patients may diminish when alternative factors are controlled for. Two studies by Epstein et al. (1994a, 1996) compared psychopathology in obese patients and controls covarying mother’s psychopathology and child’s BMI. Results indicated significant between-group differences in psychological problems, showing “58% of boys and 44% of girls met criteria on at least one Child Behavior Checklist/4–18 (CBCL) behavioral problem scale” (Achenbach, 1991; Epstein et al., 1996, p. 65). However, after controlling for maternal psychopathology, no significant difference remained in the psychological comorbidity and socioeconomic status of the obese pediatric population.
In summary, the evidence cited above shows behavioral problems in subgroups of obese children, but there is no clear indication of higher rates of psychiatric comorbidity in the general population of obese children. Friedman and Brownell (1995) suggest research to identify protective factors and vulnerabilities influencing the development of psychopathology in obese children.
Obesity in general appears not to result from currently classified psychiatric disorders. Weight gain sometimes accompanies such psychiatric disorders as depression, and some Axis I disorders are associated with overeating, but most obese subjects do not have a diagnosis. This provides strong evidence that psychiatric disorders do not cause obesity. Psychiatric investigators often fail to examine the temporal relationship between the onset of classic psychiatric disorder and weight gain. One exception is recent work by Goodman and Whitaker (2002), who found that depressed mood at baseline independently predicted obesity at follow-up after controlling for variables, including race and socioeconomic status. They also found that depressed mood at baseline predicted obesity among those who were not obese at baseline. Pine et al. (1997), in a longitudinal study of 644 adolescents, found a significant relationship between adolescent symptoms of conduct disorder and subsequent increased BMI in young adulthood. They also found significant gender differences in psychological comorbidity in adolescents who later became obese young adults. The results showed a positive correlation between obesity and depression in females, but no correlation for males. A lack of BMI data during the subjects’ adolescence limits interpretation of the results.
Long-term, epidemiological studies similar to those by Pine et al. (1997) and Goodman and Whitaker (2002) provide the methodology to examine temporal relationships between the onset of obesity and the onset of psychiatric disorders. With regard to substance abuse, studies by Neumark-Sztainer et al. (1997), Strauss and Mir (2001), and Wichstrom (1995) all concluded that there was no correlation between adolescent weight and the use of substances, which included tobacco, marijuana, and alcohol. Anecdotally, tobacco and stimulants have been used for appetite suppression in youths. Epidemiological data about the incidence and prevalence of substance abuse in the obese adolescent population are not yet available.
Medical and psychiatric evaluation
Obese patients should be thoroughly evaluated to identify any medical or psychiatric conditions that may affect the course of treatment.
Medical assessment
A complete medical assessment is prudent, especially in children with short stature, low IQ, or both. These factors are warning signs that childhood-onset obesity may stem from medical causes.
Underlying Syndromes of Obesity.
Underlying syndromes of obesity may be genetic (e.g., Prader-Willi, Cohen syndromes) or endocrinological (e.g., hypothyroidism, Cushing’s syndrome) (Barlow and Dietz, 1998). While these exogenous causes of obesity are rare, they should be ruled out before weight-management treatment is prescribed (Table 1).
Psychiatric assessment
No studies exist that compare different methods for psychiatric assessment of obese children. Therefore, our recommendation is to use well-validated instruments previously used in normal and psychiatric populations. As noted below, assessment tools do exist for conditions most commonly associated with eating disorders.
Depression.
Results from several studies (but not all) (e.g., Csabi et al., 2000; Wallace et al., 1993) suggest a higher rate of depression among obese children than among children of normal weight. The most commonly used screening device for pediatric depression is the Children’s Depression Inventory (CDI), a 27-item, symptom-oriented scale (Kovacs, 1985). Normative data from several independent groups of healthy children and adolescents (Knight et al., 1988; Smucker et al., 1986) suggest that the CDI is a valid and highly reliable measure. In addition, the CDI has been effectively used in several studies involving obese children (e.g., Sheslow et al., 1993; Wallace et al., 1993). However, one noteworthy disadvantage of the CDI is that it is exclusively a self-report measure. Therefore, if a physician suspects depression, it may be useful to supplement the CDI with a parent-report measure such as the Child Behavior Checklist (Achenbach and Ruffle, 2000). Depressed children should not participate in a weight-control program unless they do so with concurrence of a mental health expert. If the child’s depression remains untreated, the weight-control program may be futile or even harmful (Barlow and Dietz, 1998).
Other Psychiatric and Psychosocial Symptoms.
In addition to depression, low self-esteem and anxiety have also been found to relate to obesity in children and adolescents in some studies (Morgan et al., 2002; Sheslow et al., 1993). The Perceived Competence Scale for Children (PCSC) (Harter, 1988) is a validated measure that has reliably assessed both general and dimensional (cognitive, social, and physical) self-esteem in multiple samples of obese children and adolescents (French et al., 1995; Israel and Ivanova, 2002). The self-esteem scale developed by Resnick et al. (1997) for Add Health analysis has excellent reliability (Cronbach’s α=.85). Higher scores indicate lower self-esteem. The State-Trait Anxiety Inventory (STAIC) (Spielberger, 1973) is a validated and reliable measure of current anxiety as well as of propensity for anxiety in both children and adolescents (Muris et al., 2002; Papay and Hedl, 1978).
Eating Disorders.
The Stunkard and Messick Three-Factor Eating Questionnaire (TFEQ) is designed to evaluate three aspects of eating behavior: restraint, disinhibition, and hunger (Stunkard and Messick, 1985). Boschi et al. (2001) used the TFEQ to evaluate adults seeking participation in a diet and therapy program and determined that the test measures a wide range of eating behavior. However, it was not sufficiently sensitive or specific to identify the presence or absence of a pathological behavior. Also, it has not been used in adolescent subjects. Eating disorders such as anorexia and bulimia have been found to be associated with obesity (Neumark-Sztainer et al., 1999).
The Eating Disorder Examination is a standardized, semistructured interview that is considered the gold standard for the assessment of the psychopathology of eating disorders (Fairburn and Cooper, 1993). This measure has been found to be reliable in a variety of independent adult samples (Cooper et al., 1989; Rizvi et al., 2000), and a pediatric version is available that has been successfully administered to obese children and adolescents (Burrows and Cooper, 2002). Primary-care physicians should also inquire about disordered eating behaviors (e.g., use of diet pills, laxatives, diuretics, or tobacco), which occur with a higher frequency in obese adolescents (Irving and Neumark-Sztainer, 2002). Like children suffering from depression, children with eating disorders require psychological treatment and may not benefit from a weight-control program unless they are under the supervision of an experienced therapist.
Assessment of readiness to change
For a weight-management program to succeed, the obese patient must be ready to change his or her lifestyle. An unsuccessful weight-management program may not only diminish the child’s self-esteem but may also impair future weight-loss efforts. Two reliable measures that have been successfully used to assess weight-management program readiness in obese children and adolescents are the Children’s Eating Behavior Inventory (Archer et al., 1991) and the Children’s Eating Attitude Test (Braet and Van Strien, 1997; Maloney et al., 1988).
If the patient is a young child, the readiness of the parent overseeing the weight-management program should also be assessed. Signs that the child or family is not ready for change include lack of concern about the child’s obesity, belief that the obesity is inevitable, and belief that the child is incapable of losing weight (Barlow and Dietz, 1998). Obese children and their family members who are not ready to undergo treatment may benefit from motivational counseling (Berg-Smith et al., 1999). The physician should also be sure that the patient is not regularly using drugs or alcohol, as substance abuse is likely to hinder program adherence.
Treatment for child and adolescent obesity
Review articles
A detailed review of more than 70 randomized controlled studies of child and adolescent obesity treatment programs is beyond the scope of this review of psychiatric aspects of child and adolescent obesity. Two excellent pediatric-based reviews of the literature by Epstein et al. (1998) and Jelalian and Saelens (1999) are highly recommended. It is important for child and adolescent psychiatrists to be familiar with the fundamental findings of that literature.
Despite discouraging results in adult long-term weight-loss trials, a pediatric 10-year randomized treatment study (Epstein et al., 1994b) examining behavioral family-based treatment showed that 34% of participants (who entered the study at ages 6–12) decreased percentage overweight by 20% or more, and 30% were not obese 10 years later. This long-term result demonstrates the importance of family support for modifying eating and activity behaviors and suggests major differences between early family-based approaches and late adult trials. The study stands alone and needs replication and refinement. Epstein et al. (1990) also found that when parents were targeted for weight loss as well, children showed a 15% decrease in weight over 10 years. Other research has shown that when both parents are active, their children are approximately six times more likely to be active than when neither parent is active (Moore et al., 1991). For best results, treatment strategies must employ diet and exercise intervention.
Behavior goals
The recommendations of the Expert Committee on Obesity Evaluation and Treatment provide the best and most concise guidelines for practitioners treating child and adolescent obesity. The committee agrees that the primary goal should be developing healthy eating and activity habits, not achieving ideal body weight (Barlow and Dietz, 1998). Eating and physical activity patterns are partly learned behaviors and can be changed. The ideal program should modify the environment that shapes behavior. Family influence is the most important factor in child obesity treatment. Multiple studies have shown that when the family was included in the treatment program, short-term and long-term success rates were much higher. The committee has outlined four skills for families (Barlow and Dietz, 1998, p. 35):
| 1. | Becoming aware of current eating habits, activity, and parenting behavior | ||||
| 2. | Identifying problem eating and activity behavior | ||||
| 3. | Modifying current behavior in small, permanent steps and making additional changes only when previous changes are firmly in place | ||||
| 4. | Watching behavior and recognizing problems that arise as the child becomes more independent, family schedules change, or other changes occur that alter the treatment plan | ||||
Medical and weight goals
Improvement or resolution of secondary medical complications of obesity should motivate the patient and family (Barlow and Dietz, 1998). The first step in weight control for all obese children beyond age 2 should be to hold weight constant (i.e., not gain any weight). The child can achieve this goal through modest changes in diet and activity. Therefore, a sufficient goal for many children is prolonged weight maintenance, allowing their BMI to decrease as they grow in height and develop. A first step should be the demonstration that the child’s weight can be held constant. Only then should clinicians encourage weight loss of a pound per month. A BMI below the 85th percentile is an appropriate goal. The primary goal, however, is healthy eating and increased activity.
Therapy to achieve above goals
The committee recommendations offer clear guidelines for a general approach to therapy and parenting skills, highlighting techniques used in the most successful outcomes (Barlow and Dietz, 1998). A review by Robinson (1999a, p. S52) highlights the most effective behavioral treatments for child obesity. These include:
| • | A group format with individualized behavioral counseling | ||||
| • | Parent participation | ||||
| • | Frequent sessions | ||||
| • | A long treatment duration | ||||
| • | A simple and explicit diet that produces a caloric deficit | ||||
| • | A physical activity program emphasizing choice and reinforcing reduced sedentary behaviors | ||||
| • | Making changes in the home and family environments to help reduce cues and opportunities associated with calorie intake and inactivity | ||||
| • | Increased cues and opportunities for physical activity | ||||
| • | Self-monitoring | ||||
| • | Goal setting and contracting | ||||
| • | Parenting skills training | ||||
| • | Skills for managing high-risk situations | ||||
| • | Skills for maintenance and relapse prevention | ||||
Adult studies have shown that more frequent sessions and a longer treatment duration produce more weight loss and long-term maintenance (Robinson, 1999a). The same approach would likely be beneficial for children and adolescents.
Diet and exercise
Increase in activity and reduction in caloric intake
For weight loss, obese children must expend more energy than they consume, by increasing activity and decreasing caloric intake. This includes limiting time in front of the TV or computer to 1 or 2 hours a day, as recommended by the American Academy of Pediatrics.
Complications of weight-management programs
Medical complications associated with weight loss include inadequate nutrient intake and slow linear growth (Barlow and Dietz, 1998). Eliminating only high-calorie foods and encouraging well-balanced eating minimizes the risk of inadequate nutrient intake. Slow linear growth is a limited risk because the impact on adult stature appears to be minimal.
Psychological complications and self-esteem problems may occur if the patient is not succeeding with treatment. Supportive therapy and regular assessment of the child’s emotional state should minimize the risk. If conflicts arise within the family over weight loss, family therapy approaches are indicated.
Maintenance
Obesity is a chronic disease and improvement decreases once treatment stops. Children and parents must work actively to maintain behaviors that promote weight loss (Barlow and Dietz, 1998). Regular contact with the clinician is essential to reinforce treatment goals and skills.
Beyond diet and exercise
Pharmacological approaches
There are no pharmacological approaches approved by the U.S. Food and Drug Administration to treat childhood obesity. Clinical trials with sibutramine and orlistat in children are under way. Research on the use of leptin is in its preliminary stages, although far from clinical trials (Lustig, 2001). Little is known about pharmacotherapy in children. Therefore, this section will review current and past drug treatments of obesity in adults, because off-label use in children is common.
Typically, medications used for weight control are prescribed only to individuals with a BMI greater than 30 or to individuals with comorbid conditions who have a BMI greater than 27 (Devlin et al., 2000). Drug treatment, on average, results in a weight loss of about 5 to 20 pounds over behavioral treatment alone. Most drug-induced weight loss occurs during the first 6 months of treatment and remains stable during this time. Few studies have evaluated the effect of long-term treatment on weight (Devlin et al., 2000). Generally, treatment is limited to less than 1 year and weight gain ensues when it is discontinued, indicating that the underlying causes of obesity are still present. Only two drugs, sibutramine and orlistat, are approved for long-term use in adults (Devlin et al., 2000). Sibutramine, a 5-HT and NE reuptake inhibitor, suppresses appetite and elicits a feeling of satiety. Adverse side effects, including elevation in pulse and blood pressure, are rarely significant. Orlistat prevents the absorption of approximately half of ingested fat. Side effects are loose stools and oily spotting.
Medications that act on the dopamine system are being investigated. Doknic et al. (2002) targeted the dopamine system in 23 obese adults who had prolactinomas (resulting from high levels of prolactin, a side effect of obesity). The study administered bromocriptine, a dopamine receptor agonist, and found a significant weight loss in patients after 6 months that persisted after 2 years. These results indicate the need for research on the role of dopamine in weight regulation. The risk-to-benefit ratio of long-term treatment is largely unknown, as is true of many weight-loss drugs.
Phentermine-fenfluramine, a combination of a sympathomimetic and a serotonin-releasing agent and reuptake blocker, was used in the 1990s before its adverse side effect of valvular insufficiency, which causes cardiovascular problems, forced its removal from the market (Lustig, 2001). Historically, different classes of amphetamines, such as Dexedrine (dextroamphetamine), were also used for weight loss. These drugs increase intrasynaptic dopamine levels, thereby exerting an anorexigenic effect and posing risks for addiction in adults (Towell et al., 1988). We found no studies on the long-term effects of amphetamine treatment for obesity, and its use is highly discouraged. Topiramate, zonisamide, felbamate, and bupropion have been reported to cause weight loss in adults, but no published research in children has been reported (Anderson et al., 2002; Asconapé, 2002).
Intensive therapies
Yanovski (2001) reviews the indications and complications of bariatric procedures (banding and bypass of the stomach) and “very-low-calorie diets,” such as the protein-sparing modified fast, in children. Rand and Macgregor (1994) conducted a 6-year follow-up study on the physical and psychiatric status of adolescents who underwent obesity surgery. Patients experienced improved self-esteem, social relationships, and appearance but reported poor compliance with exercise and dietary instructions, and only a handful took the vitamins and calcium as directed. Suffice it to say that decisions to attempt higher-risk interventions should be made with great caution, given the high complication rates and minimal pediatric experience. Presurgical psychiatric consultation is logical.
Psychotropic medication: ramifications for weight change
Weight gain is an adverse side effect of many psychiatric medications and a leading cause of noncompliance in adults taking psychotropics. Psychiatric disorders usually require long-term treatment. Hence, medications that cause weight gain can lead to the serious adverse effects of obesity (Malhi et al., 2001). Little research is available on the ramifications of psychiatric treatment and weight gain in children. However, studies on adults reveal findings that may apply to children.
Psychiatric drugs that cause weight gain include certain classes of antidepressants, antipsychotics, and mood stabilizers. Lithium leads to weight gain in up to two thirds of adults, especially those who are already overweight (Devlin et al., 2000). Other antidepressants associated with weight gain are tricyclics and monoamine oxidase inhibitors (MAOIs). The antiepileptic drugs valproate, gabapentin, carbamazepine, and vigabatrin have been shown to cause weight gain, while lamotrigine, levetiracetam, and phenytoin have no effect on weight (Asconapé, 2002). Clozapine, olanzapine, and risperidone are antipsychotics associated with significant weight gain (Bryden and Kopala, 1999; Martin et al., 2000; Ratzoni et al., 2002). Although few studies document the effects of these medications on children, Bryden and Kopala (1999) reported a 58% increase in BMI in one adolescent patient treated with olanzapine, while Horrigan et al. (2001) described an adolescent boy with attention-deficit/hyperactivity disorder (ADHD) who experienced weight gain of approximately 0.4 kg/week from olanzapine treatment. The patient lost weight by taking Adderall before meals. Other medications associated with weight gain are drugs that block histamine H1, serotonin 5-HT2, and dopamine D2 receptors (Devlin et al., 2000). Gothelf et al. (2002) found that BMI increased significantly in schizophrenic adolescent inpatients treated with olanzapine, but not those given haloperidol. A recent study by Morrison et al. (2002) assessed metformin, an antidiabetic therapy, as a treatment for weight gain in children taking olanzapine, risperidone, quetiapine, or valproate. The majority of the subjects lost weight, and the mean changes in weight and BMI at 12 weeks were highly significant. In a letter to the editor, Lessig et al. (2001) describe patients who were given topiramate to counteract weight gain caused by atypical antipsychotics with significant success.
Clinicians should consider the implications of psychiatric medications for weight gain. According to Devlin et al. (2000) and Masand (1999), patients should be informed that weight gain is possible, monitored closely, and given a prevention and weight-management program. If weight gain occurs, one must consider alternative medications not associated with weight gain (for alternative medications see Cheskin et al., 1999). In addition, in adults “the use of weight loss medications must be carefully considered in populations already being treated with psychotropic (and perhaps other) drugs” (Masand, 1999, p. 3). As noted above, weight-loss medications are not approved by the Food and Drug Administration and cannot be considered for routine use in children.
Ethical dilemmas for child and adolescent psychiatrists
Child and adolescent psychiatrists face a range of ethical dilemmas. Some are specific to certain genetic conditions (e.g., Prader-Willi syndrome). Others are more general and pertain to the classification and proper treatment of obesity, which can be especially complicated in cases involving comorbid psychological disorders. Five recent publications address ethical issues and pediatric obesity; two of these (Holland and Wong, 1999; Kodish and Cuttler, 1996) focus on the issues surrounding Prader-Willi syndrome, a genetic condition associated with a known mutation and mental retardation. Fatal overeating conditions in Prader-Willi individuals present difficult management issues.
More germane to clinicians seeing idiopathic obesity are ethical/legal issues raised by several other authors. Kuss (1996) raises medical and legal arguments for including obesity as a disability under the Americans With Disabilities Act.
One ethical issue confronting clinicians is “cosmetic psychopharmacology,” in which parents pressure providers to prescribe anorectic agents. Another common ethical decision occurs in treating seriously depressed adolescents or psychotic teenagers who resist medication because of concerns over weight gain. Is it ethical to minimize or not mention the weight-gain effects of taking psychotropic medications if noncompliance may have lethal consequences?
Financial conflicts of interest should be avoided with unproven weight-loss approaches. Practitioners should steer their patients away from therapies that could be expensive, prone to failure, or physically harmful.
Finally, psychiatrists should understand and deal with countertransference. “Fat-ism,” like sexism and racism, may contaminate the development of appropriate therapeutic relationships. This is particularly true in working with younger obese patients, where parent-bashing or blaming is common.
Wigton and Mcgaghie (2001) document medical students’ increased pessimism and decreased expectations of compliance while working with obese versus nonobese simulated patients, although the same medical tests and treatments were recommended for both. Clinicians need to confront their own belief systems in order to treat or refer these patients.
Ethical issues are common for researchers studying obese children. Considerable debate exists in academic circles about whether obese children without medical complications have a “condition” that would allow them to participate in studies where no direct benefit may result. Finally, does having merely a risk factor (e.g., two obese parents) permit more invasive or preventive/proactive types of intervention in research settings?
Future trends and research goals in understanding and treating child and adolescent obesity
Targeted research to combat childhood obesity will need to focus on several specific areas. Few empirical data exist regarding prevention. For a comprehensive and extensive prevention literature review, see the Cochrane Review by Campbell et al. (2002). School-based intervention programs have been shown to be ineffective (Story, 1999). Approaches to avoid first episodes of obesity must be developed. High-risk and minority populations need to be oversampled and much earlier interventions need to be used. Public policy must support environmental changes (Devlin et al., 2000).
A second important area that needs research is the involvement of parents in the treatment of preadolescents. Collaborations among pediatricians, behaviorally oriented psychologists, and child and adolescent psychiatrists are needed to target behavior change beyond “diet and exercise.” Research is needed on how to modify activity levels and food choices permanently before trying to impose reduced-calorie regimens. Overeating should be conceptualized as a result of combined behaviors that can be targeted for individual study. Behavioral therapy should be integrated with pharmacological interventions and identification of genetic predispositions (Epstein et al., 2001). Long-term studies on the maintenance phase are needed to avoid the relapses seen among adults. Research is also needed on the factors that promote long-term maintenance and the best ways parents can help a child progress successfully (Jelalian and Saelens, 1999; Robinson, 1999a).
Many questions remain regarding effective styles of treatment for short- and long-term success. Research must explore speculation that group treatment for teens is the most effective method because it facilitates social skills and reduces psychosocial morbidity. Practitioners, parents, and investigators must consider the psychosocial outcomes of treatment—and its possible failure. Will weight-loss intervention improve a child’s overall quality of life (Jelalian and Saelens, 1999)?
A final area for research efforts is molecular biology and neuroendocrinolgy. Although a safe and effective long-term pharmacological treatment is the goal of intense industry-based research, a more pressing and realistic goal is the search for biological markers that confer risk. The discovery of a single gene responsible for obesity is unlikely, but discoveries in animal models eventually will prove clinically useful. In humans, imaging studies of receptors (e.g., dopamine and serotonin) that regulate hunger and satiety will be particularly informative. Only through sustained research efforts in the neurobiology of feeding and satiation will the neural circuitry of this behavior eventually become clear enough to inform more successful treatment strategies.
Conclusion
It has been proposed that we abandon traditional weight-loss goals based on tables in favor of “reasonable weight” (Devlin et al., 2000, p. 862). Significant health benefits are associated with modest weight losses that fall short of healthy and aesthetic ideals. Despite the lack of genetic markers or predictors of who will be successful candidates for significant weight-loss maintenance, features to maximize success are known. Obesity will remit in some individuals. Few empirical data exist from rigorously controlled research studies on the psychiatric (as opposed to pediatric) management of obese children with psychiatric disorders. Child and adolescent psychiatrists play a critical role in treating obese children with classic psychiatric disorders. Care and ingenuity must be used to avoid exacerbating weight problems in children with psychiatric disorders.
Note added in proof
In September 2003, the Food and Drug Administration formally requested that the makers of all atypical antipsychotic medications change the “Warnings” of the product labeling of antipsychotic medications to include baseline and periodic glucose monitoring. The letter stated, “epidemiological studies suggest an increased risk of treatment emergent hyperglycemia-related adverse events in patients treated with the atypical antipsychotics” (Rosack, 2003).
| Hormonal causes |
| Hypothyroidism |
| Hypercortisolism |
| Primary hyperinsulinism |
| Pseudohypoparathyroidism |
| Acquired hypothalamic |
| Genetic syndromes |
| Prader-Willi |
| Laurence-Moon/Bardet-Biedl |
| Alström |
| Börjeson-Forssman-Lehmann |
| Cohen |
| Turner |
| Familial lipodystrophy |
| Beckwith-Wiedemann |
| Sotos |
| Weaver |
| Ruvalcaba |
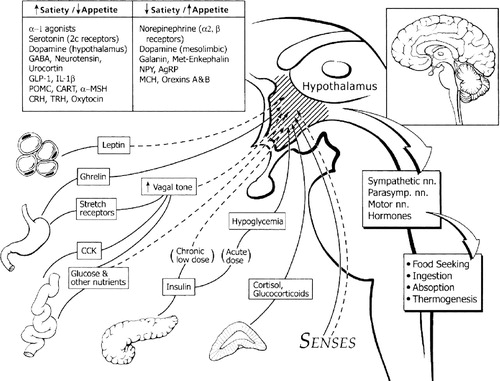
Figure 1. Proposed Neurobiological and Neuroendocrinological Signals of Weight Regulation
Box upper left: Central signals involved in weight regulation. Main figure: Dotted lines represent peripheral signals causing increased satiety/decreased appetite; solid lines indicate signals causing decreased satiety/increased appetite. GABA=γ-aminobutyric acid; GLP-1=glucagon-like peptide; IL-1β=interleukin-1β; POMC=pro-opiomelanocortin; CART=cocaine-and-amphetamine-regulated transcript; α-MSH=α-melanocyte stimulating hormone; CRH=corticotropin-releasing hormone; TRH=thyrotropin-releasing hormone; NPY=neuropeptide Y; AgRP=agouti-related peptide; MCH=melanin-concentrating hormone
Asterisks indicate the most seminal references.Google Scholar
Achenbach TM (1991), Manual for the Child Behavior Checklist/4-18 and 1991 Profile. Burlington: University of Vermont Department of PsychiatryGoogle Scholar
Achenbach TM, Ruffle TM (2000), The Child Behavior Checklist and related forms for assessing behavioral/emotional problems and competencies. Pediatr Rev 21:265–271Crossref, Google Scholar
American Psychiatric Association (1994), Diagnostic and Statistical Manual of Mental Disorders, 4th edition (DSM-IV). Washington, DC: American Psychiatric AssociationGoogle Scholar
Anderson JW, Greenway FL, Fujioka K, Gadde KM, McKenney J, O’Neil PM (2002), Bupropion SR enhances weight loss: a 48-week double-blind, placebo-controlled trial. Obes Res 10:633–641Crossref, Google Scholar
Archer LA, Rosenbaum PL, Streiner DL (1991), The Children’s Eating Behavior Inventory: reliability and validity results. J Pediatr Psychol 16:629–642Crossref, Google Scholar
Asconapé JJ (2002), Some common issues in the use of antiepileptic drugs. Semin Neurol 22:27–39Crossref, Google Scholar
*Barlow SE, Dietz WH (1998), Obesity evaluation and treatment: expert Committee recommendations. The Maternal and Child Health Bureau, Health Resources and Services Administration and the Department of Health and Human Services. Pediatrics 102:E29Crossref, Google Scholar
Berg-Smith SM, Stevens VJ, Brown KM et al. (1999), A brief motivational intervention to improve dietary adherence in adolescents. The Dietary Intervention Study in Children (DISC) Research Group. Health Educ Res 14:399–410Crossref, Google Scholar
Boschi V, Iorio D, Margiotta N, D’Orsi P, Falconi C (2001), The Three-Factor Eating Questionnaire in the evaluation of eating behavior in subjects seeking participation in a dietotherapy programme. Ann Nutr Metab 45:72–77Crossref, Google Scholar
Braet C, Van Strien T (1997), Assessment of emotional, externally induced and restrained eating behavior in nine to twelve-year-old obese and non-obese children. Behav Res Ther 35:863–873Crossref, Google Scholar
Brenner JS, Kelly CS, Wenger AD, Brich SM, Morrow AL (2001), Asthma and obesity in adolescents: is there an association? J Asthma 38:509–515Crossref, Google Scholar
Britz B, Siegfried W, Ziegler A et al. (2000), Rates of psychiatric disorders in a clinical study group of adolescents with extreme obesity and in obese adolescents ascertained via a population based study. Int J Obes Relat Metab Disord 24:1707–1714Crossref, Google Scholar
Bryden KE, Kopala LC (1999), Body mass index increase of 58% associated with olanzapine. Am J Psychiatry 156:1835–1836Google Scholar
Buddeburg-Fisher B, Klaghofer R, Reed V (1999), Associations between body weight, psychiatric disorders and body image in female adolescents. Psychother Psychosom 68:325–332Crossref, Google Scholar
Burrows A, Cooper M (2002), Possible risk factors in the development of eating disorders in overweight pre-adolescent girls. Int J Obes Relat Metab Disord 26:1268–1273Crossref, Google Scholar
Campbell K, Waters E, O’Meara S, Kelly S, Summerbell C (2002), Interventions for the preventing of obesity in children (Cochrane Review). The Cochrane Library Issue 3Google Scholar
Cheskin LJ, Bartlett SJ, Zayas R, Twilley CH, Allison DB, Contoreggi C (1999), Prescription medications: a modifiable contributor to obesity. South Med J 92:898–904Crossref, Google Scholar
Chinn S, Rona RJ (2001), Can the increase in body mass index explain the rising trend in asthma in children? Thorax 56:845–850Crossref, Google Scholar
Christoffel KK, Forsyth BWC (1989), Mirror image of environmental deprivation: severe childhood obesity of psychosocial origin. Child Abuse Negl 13:249–256Crossref, Google Scholar
Cooper Z, Cooper PJ, Fairburn CG (1989), The validity of the eating disorder examination and its subscales. Br J Psychiatry 154:807–812Crossref, Google Scholar
Corbett JJ, Savino PJ, Thompson S et al. (1982), Visual loss in pseudotumor cerebri. Arch Neurol 39:461–474Crossref, Google Scholar
Csabi G, Tenyi T, Molnar D (2000), Depressive symptoms among obese children. Eat Weight Disord 5:43–45Crossref, Google Scholar
Davison KK, Birch LL (2001), Weight status, parent reaction, and self-concept in five-year-old girls. Pediatrics 107:46–53Crossref, Google Scholar
Deslypere JP (1995), Obesity and cancer. Metabolism 44(suppl 3):24–27Google Scholar
Devlin MJ, Yanovski SZ, Wilson GT (2000), Obesity: what mental health professionals need to know. Am J Psychiatry 157:854–866Crossref, Google Scholar
Dietz WH (1997), Periods of risk in childhood for the development of adult obesity—what do we need to learn? J Nutr 127:1884S–1886SGoogle Scholar
Dietz WH, Gross WL, Kirkpatrick JA (1982), Blount disease (tibia vara): another skeletal disorder associated with childhood obesity. J Pediatr 101:735–737Crossref, Google Scholar
Doknic M, Pekic S, Zarkovic M et al. (2002), Dopaminergic tone and obesity: an insight from prolactinomas treated with bromocriptine. Eur J Endocrinol 147:77–84Crossref, Google Scholar
Drewnowski A, Kurth CL, Rahaim JE (1991), Taste preferences in human obesity: environmental and familial factors. Am J Clin Nutr 54:635–641Google Scholar
Durcan FJ, Corbett JJ, Wall M (1988), Incidence of pseudotumor cerebri: population studies in Iowa and Louisiana. Arch Neurol 45:875–877Crossref, Google Scholar
Ebbeling CB, Pawlak DB, Ludwig DS (2002), Childhood obesity: public health crisis, common sense cure. Lancet 360:473–482Crossref, Google Scholar
Epstein LH, Klein KR, Wisniewski L (1994a), Child and parent factors that influence psychological problems in obese children. Int J Eat Disord 15:151–158Crossref, Google Scholar
Epstein LH, Myers MD, Anderson K (1996), The association of maternal psychopathology and family socioeconomic status with psychological problems in obese children. Obes Res 4:65–73Crossref, Google Scholar
Epstein LH, Myers MD, Raynor HA, Saelens BE (1998), Treatment of pediatric obesity. Pediatrics 101:554–570Google Scholar
*Epstein LH, Roemmich JN, Raynor HA (2001), Behavioral therapy in the treatment of pediatric obesity. Pediatr Clin North Am 48:981–993Crossref, Google Scholar
Epstein LH, Valoski A, Wing RR, McCurley J (1990), Ten-year follow-up of behavioral family-based treatment for obese children. JAMA 264:2519–2523Crossref, Google Scholar
Epstein LH, Valoski A, Wing RR, McCurley J (1994b), Ten-year outcomes of behavioral family-based treatment of childhood obesity. Health Psychol 13:373–383Crossref, Google Scholar
Erickson SJ, Robinson TN, Haydel KF, Killem JD (2000), Are overweight children unhappy? Body mass index, depressive symptoms, and overweight concerns in elementary school children. Arch Pediatr Adolesc Med 154:931–935Crossref, Google Scholar
Fairburn CG, Cooper Z (1993), The eating disorder examination, 12th ed. In: Binge Eating: Nature, Assessment, and Treatment, Fairburn CG, Wilson GT, eds. (pp 317–360). London: GuilfordGoogle Scholar
Farooqi IS, O’Rahilly S (2000), Recent advances in the genetics of severe childhood obesity. Arch Dis Child 83:31–34Crossref, Google Scholar
Fellitti VJ (1993), Childhood sexual abuse, depression, and family dysfunction in adult obese patients. South Med J 86:732–736Crossref, Google Scholar
French SA, Story M, Perry CL (1995), Self-esteem and obesity in children and adolescents: a literature review. Obes Res 3:479–490Crossref, Google Scholar
Friedman MA, Brownell KD (1995), Psychological correlates of obesity: moving to the next research generation. Psychol Bull 117:3–20Crossref, Google Scholar
Friesen CA, Roberts CC (1989), Cholelithiasis: clinical characteristics in children. Clin Pediatr 7:294–298Crossref, Google Scholar
Goodman E, Whitaker RC (2002), A prospective study of the role of depression in the development and persistence of adolescent obesity. Pediatrics 109:497–504Crossref, Google Scholar
Gortmaker SL (1993), Social and economic consequences of overweight in adolescence and young adulthood. N Engl J Med 329:1008–1012Crossref, Google Scholar
Gothelf D, Falk B, Singer P et al. (2002), Weight gain associated with increased food intake and low habitual activity levels in male adolescent schizophrenic inpatients treated with olanzapine. Am J Psychiatry 159:1055–1057Crossref, Google Scholar
Grilo CM, Wilfley DE, Brownell KD, Rodin J (1994), Teasing, body image, and self-esteem in a clinical sample of obese women. Addict Behav 19:443–450Crossref, Google Scholar
Halcomb GW, O’Neill JA, Halcomb GW (1980), Cholecysitis, cholelithiasis and common duct stenosis in children and adolescents. Ann Surg 191:626–635Crossref, Google Scholar
Harter S (1988), The Perceived Competence Scale for Children. Child Dev 59:87–97Crossref, Google Scholar
Holland AJ, Wong J (1999), Genetically determined obesity in Prader-Willi syndrome: the ethics and legality of treatment. J Med Ethics 25:230–236Crossref, Google Scholar
Honore LH (1980), Cholesterol cholelithiasis in adolescent females. Arch Surg 115:62–64Crossref, Google Scholar
Horrigan JP, Barnhill LJ, Kohli RR (2001), Adderall, the atypicals, and weight gain. J Am Acad Child Adolesc Psychiatry40:620Crossref, Google Scholar
Irving LM, Neumark-Sztainer D (2002), Integrating the prevention of eating disorders and obesity: feasible or futile? Prev Med 34:299–309Crossref, Google Scholar
Israel AC, Ivanova MY (2002), Global and dimensional self-esteem in preadolescent and early adolescent children who are overweight: age and gender differences. Int J Eat Disord 31:424–429Crossref, Google Scholar
Janz KF, Golden JC, Hansen JR, Mahoney LT (1992), Heart rate monitoring of physical activity in the Muscatine study. Pediatrics 89:256–261Google Scholar
Jelalian E, Saelens BE (1999), Empirically supported treatments on pediatric psychology: pediatric obesity. J Pediatr Psychol 24:223–248Crossref, Google Scholar
Kelsey JL, Acheson RM, Keggi KJ (1972), The body builds of patients with slipped capital epiphyses. Am J Dis Child 124:276–281Google Scholar
Kim SYS, Glynn NW, Kriska AM et al. (2002), Decline in physical activity in black girls and white girls during adolescence. N Engl J Med 347:709–715Crossref, Google Scholar
*Klish WJ (1998), Childhood obesity. Pediatr Rev 19:312–315Crossref, Google Scholar
Knight D, Hensley VR, Waters B (1988), Validation of the Children’s Depression Scale and the Children’s Depression Inventory in a prepubertal sample. J Child Psychol Psychiatry 29:853–863Crossref, Google Scholar
Kodish E, Cuttler L (1996), Ethical issues in emerging new treatments such as growth hormone therapy for children with Down syndrome and Prader-Willi syndrome. Curr Opin Pediatr 8:401–405Crossref, Google Scholar
Kovacs M (1985), The Children’s Depression, Inventory (CDI). Psychopharmacol Bull 21:995–998Google Scholar
Kuss CL (1996), Absolving a deadly sin: a medical and legal argument for including obesity as a disability under the Americans With Disabilities Act. J Contemp Health Law Policy 12:563–605Google Scholar
Lessig MC, Shapira NA, Murphy TK (2001), Topiramate for reversing atypical antipsychotic weight gain. J Am Acad Child Adolesc Psychiatry 40:1364Google Scholar
Levine JA, Eberhardt NL, Jensen MD (1999), Role of nonexercise activity thermogenesis in resistance to fat gain in humans. Science 283:212–214Crossref, Google Scholar
Lissau I, Sorensen TIA (1994), Parental neglect during childhood and increased risk of obesity in young adulthood. Lancet 343:324–327Crossref, Google Scholar
Lubrano-Berthelier C, Durand E, Dubern B et al. (2003), Intracellular retention is a common characteristic of childhood obesity-associated MC4R mutations. Hum Mol Genet 12:145–153Crossref, Google Scholar
*Lustig RH (2001), Childhood and adolescent obesity: the neuroendocrinology of childhood obesity. Pediatr Clin North Am 48:909–930Crossref, Google Scholar
Maes HHM, Neale MC, Eaves LJ (1997), Genetic and environmental factors in relative body weight and human adiposity. Behav Genet 27:325–350Crossref, Google Scholar
Maffeis C, Tato L (2001), Long-term effects of childhood obesity on morbidity and mortality. Horm Res 55(suppl 1):42–45Crossref, Google Scholar
Malhi GS, Mitchell PB, Caterson I (2001), “Why getting fat, Doc?” Weight gain and psychotropic medications. Aust N Z J Psychiatry 35:315–321Crossref, Google Scholar
Mallory GB Jr, Fiser DH, Jackson R (1989), Sleep-associated breathing disorders in morbidly obese children and adolescents. J Pediatr 115:892–897Crossref, Google Scholar
Maloney MJ, McGuire JB, Daniels SR (1988), Reliability testing of a children’s version of the Eating Attitude Test. J Am Acad Child Adolesc Psychiatry 27:541–543Crossref, Google Scholar
Manus HE, Killeen MR (1995), Maintenance of self-esteem by obese children. J Child Adolesc Psychiatr Nurs 8:17–27Crossref, Google Scholar
Marcus CL, Curtis S, Koerner CB, Joffe A, Serwint JR, Loughlin GM (1996), Evaluation of pulmonary function and polysomnography in obese children and adolescents. Pediatr Pulmonol 21:176–183Crossref, Google Scholar
Martin A, Landau J, Leebens P et al. (2000), Risperidone-associated weight gain in children and adolescents: a retrospectic chart review. J Child Adolesc Psychopharmacol 10:259–268Crossref, Google Scholar
Masand PS (1999), Weight gain with psychotropics: size does matter. J Clin Psychiatry 60(suppl 21):3–4Crossref, Google Scholar
Moore LL, Lombardi DA, White MJ, Campbell JL, Oliveria SA, Ellison RC (1991), Influence of parents’ physical activity levels on activity levels of young children. J Pediatr 118:215–219Crossref, Google Scholar
Moran R (1999), Evaluation and treatment of childhood obesity. Am Fam Phys 59:861–868Google Scholar
Morgan CM, Yanovski SZ, Nguyen TT et al. (2002), Loss of control over eating, adiposity, and psychopathology in overweight children. Int J Eat Disord 31:430–431Crossref, Google Scholar
Morrison JA, Cottingham EM, Barton BA (2002), Metformin for weight loss in pediatric patients taking psychotropic drugs. Am J Psychiatry 159:655–657Crossref, Google Scholar
Mossberg H (1989), 40-year follow-up of overweight children. Lancet 2:491–493Crossref, Google Scholar
Muris P, Merckelbach H, Ollendick T, King N, Bogie N (2002), Three traditional and three new childhood anxiety questionnaires: their reliability and validity in a normal adolescent sample. Behav Res Ther 40:753–772Crossref, Google Scholar
National Center for Health Statistics (1999), Prevalence of Overweight Among Children and Adolescents: United States, 1999 (http://www.cdc.gov/nchs/products/pubs/pubd/hestats/overwght99.htm) (accessed August 2001)Google Scholar
Neumark-Sztainer D, Story M, Falkner NH, Beuhring T, Resnick MD (1999), Sociodemographic and personal characteristics of adolescents engaged in weight loss and weight/muscle gain behaviors: who is doing what? Prev Med 28:40–50Crossref, Google Scholar
Neumark-Sztainer D, Story M, French SA, Hannan PJ, Resnick MD, Blum RW (1997), Psychosocial concerns and health-compromising behaviors among overweight and nonoverweight adolescents. Obes Res 5:237–249Crossref, Google Scholar
Ogden CL, Flegal KM, Carroll MD, Johnson CL (2002), Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA 288:1728–1732Crossref, Google Scholar
Papay JP, Hedl JJ Jr (1978), Psychometric characteristics and norms for disadvantaged third and fourth grade children on the state-trait anxiety inventory for children. J Abnorm Child Psychol 6:115–120Crossref, Google Scholar
Parsons TJ, Power C, Logan S, Summerbell CD (1999), Childhood predictors of adult obesity: a systematic review. Int J Obes Relat Metab Disord 23(suppl 8):S1–107Crossref, Google Scholar
Pérusse L, Bouchard C (1999a), Role of genetic factors in childhood obesity and in susceptibility to dietary variation. Ann Med 31(suppl 1): 19–25Google Scholar
Pérusse L, Bouchard C (1999b), Genotype-environment interaction in human obesity. Nutr Rev 57:S31–S38Crossref, Google Scholar
Pesa JA, Syre TS, Jones E (2000), Psychosocial differences associated with body weight among female adolescents: the importance of body image. J Adolesc Health 26:330–337Crossref, Google Scholar
Pine DS, Cohen P, Brook J, Coplan JD (1997), Psychiatric symptoms in adolescence as predictors of obesity in early adulthood: a longitudinal study. Am J Public Health 87:1303–1310Crossref, Google Scholar
Pinhas-Hamiel O, Dolan LM, Daniels SR, Standiford D, Khonry PR, Zeitler P (1996), Increased incidence of non-insulin-dependent diabetes mellitus among adolescents. J Pediatr 128:608–615Crossref, Google Scholar
Rand CS, Macgregor AM (1994), Adolescents having obesity surgery: a 6-year follow-up. South Med J 87:1208–1213Crossref, Google Scholar
Ratzoni G, Gothelf D, Brand-Gothelf A et al. (2002), Weight gain associated with olanzapine and risperidone in adolescent patients: a comparative prospective study. J Am Acad Child Adolesc Psychiatry 41: 337–343Crossref, Google Scholar
Renman C, Engström I, Silfverdal S-A, Åman J (1999), Mental health and psychosocial characteristics in adolescent obesity: a population-based case-control study. Acta Paediatr 88:998–1003Crossref, Google Scholar
Resnick MD, Bearman PS, Blum RW et al. (1997), Protecting adolescents from harm: findings from the National Longitudinal Study of Adolescent Health. JAMA 278:823–832Crossref, Google Scholar
Rhodes SK, Shimoda KC, Waid LR et al. (1995), Neurocognitive deficits in morbidly obese children with obstructive sleep apnea. J Pediatr 127:741–744Crossref, Google Scholar
Rizvi SL, Peterson CB, Crow SJ, Agras WS (2000), Test-retest reliability of the eating disorder examination. Int J Eat Disord 28:311–316Crossref, Google Scholar
Robinson TN (1999a), Behavioural treatment of childhood and adolescent obesity. Int J Obes Relat Metab Disord 23(suppl 2):S52–S57Crossref, Google Scholar
Robinson TN (1999b), Reducing children’s television viewing to prevent obesity: a randomized control trial. JAMA 282:1561–1567Crossref, Google Scholar
Rosack J (2003), FDA to require diabetes warning on antipsychotics. Psychiatr News 38(20):1-a-27Google Scholar
Rumpel C, Harris TB (1994), The influence of weight on adolescent self-esteem. J Psychom Res 38:547–556Crossref, Google Scholar
Schwartz MW (2001), Brain pathways controlling food intake and body weight. Exp Biol Med 226:978–981Google Scholar
Scott IU, Siatkowski RM, Eneyni M, Brodsky MC, Lam BL (1997), Idiopathic intracranial hypertension in children and adolescents. Am J Ophthalmol 124:253–255Crossref, Google Scholar
Sheslow D, Hassink S, Wallace W et al. (1993), The relationship between self-esteem and depression in obese children. Ann N Y Acad Sci 699:289–291Crossref, Google Scholar
Silvesti JM, Weese-Mayer DE, Bass MT, Kenny AS, Hauptman SA, Pearsall SM (1993), Polysomnography in obese children with a history of sleep-associated breathing disorders. Pediatr Pulmonol 16:124–129Crossref, Google Scholar
Smucker MR, Craighead WE, Craighead LW, Green BJ (1986), Normative and reliability data for the Children’s Depression Inventory. J Abnorm Child Psychol 14:25–39Crossref, Google Scholar
Spielberger C (1973), STAIC Preliminary Manual. Palo Alto, CA: Consulting Psychologist PressGoogle Scholar
Spitzer RL, Yanovski S, Wadden T et al. (1993), Bingeeating disorder: its further validation in a multisite study. Int J Eat Disord 13:137–153Crossref, Google Scholar
Srinivasan SR, Bao W, Wattigney WA, Berenson GS (1996), Adolescent overweight is associated with adult overweight and related multiple cardiovascular risk factors: the Bogalusa Heart Study. Metabolism 45:235–240Crossref, Google Scholar
Story M (1999), School-based approaches for preventing and treating obesity. Int J Obes Relat Metab Disord 23:S43–S51Crossref, Google Scholar
Stradmeijer M, Bosch J, Koops W, Seidell J (2000), Family functioning and psychosocial adjustment in overweight youngsters. Int J Eat Disord 27:110–114Crossref, Google Scholar
*Strauss RS (1999), Childhood obesity. Curr Prob Pediatr 29:1–29Google Scholar
Strauss RS (2000), Childhood obesity and self-esteem. Pediatrics 105:1–5Crossref, Google Scholar
Strauss RS, Mir HM (2001), Smoking and weight loss attempts in overweight and normal-weight adolescents. Int J Obes Relat Metab Disord 25:1381–1385Crossref, Google Scholar
Stubbs RJ, Goldberg GR, Margatroyd PR, Prentice AM (1994), The effect of covert changes in dietary fat and energy density on ad libitum food intake in humans (abstract). Proc Nutr Soc 52:35AGoogle Scholar
Stunkard AJ, Messick S (1985), The Three-Factor Eating Questionnaire to measure dietary restraint, disinhibition and hunger. J Psychosom Res 29:71–83Crossref, Google Scholar
Styne DM (2001), Childhood and adolescent obesity: prevalence and significance. Pediatr Clin North Am 48:823–854Crossref, Google Scholar
Sugerman HG, DeMaria EJ, Felton WL, Nakatsuka M, Sismanis A (1997), Increased intra-abdominal pressure and cardiac filling pressure in obesity-associated pseudotumor cerebri. Neurology 49:507–511Crossref, Google Scholar
Tershakovec AM, Watson MH, Wenner WJ, Marx AL (1999), Insurance reimbursement for the treatmet of obesity in children. J Pediatr 134:535–536Crossref, Google Scholar
Towell A, Muscat R, Willner P (1988), Behavioural microanalysis of the role of dopamine in amphetamine anorexia. Pharmacol Biochem Behav 30:641–648Crossref, Google Scholar
Tucker LA, Friedman GM (1989), Television viewing and obesity in adult males. Am J Public Health 79:516–518Crossref, Google Scholar
Von Kries R, Hermann M, Grunert VP, Von Mutius E (2001), Is obesity a risk factor for childhood asthma? Allergy 56:318–322Crossref, Google Scholar
Von Mutius E, Schwartz J, Neas LM, Dockery D, Weiss ST (2001), Relation of body mass index to asthma and atopy in children: the National Health and Nutrition Examinations Study III. Thorax 56:835–838Crossref, Google Scholar
Wallace WJ, Sheslow D, Hassink S (1993), Obesity in children: a risk for depression. Ann N Y Acad Sci 699:301–303Crossref, Google Scholar
Wang G, Dietz W (2002), Economic burden of obesity in youths aged 6 to 17 years: 1979–1999. Pediatrics 109:E81Crossref, Google Scholar
Wang GJ, Volkow ND, Logan J et al. (2001), Brain dopamine and obesity. Lancet 357:354–357Crossref, Google Scholar
Weisburger JH (1997), Dietary fat and risk of chronic disease:mechanistic insights from experimental studies. J Am Diet Assoc 97:S16–S32Crossref, Google Scholar
Whitaker RC, Wright JA, Pepe MS, Seidel KD, Dietz WH (1997), Predicting obesity in young adulthood from childhood and parental obesity. N Engl J Med 337:869–873Crossref, Google Scholar
Wichstrom L (1995), Social, psychological and physical correlates of eating problems. A study of the general adolescent population in Norway. Psychol Med 25:567–579Crossref, Google Scholar
Wigton RS, McGaghie WC (2001), The effect of obesity on medical students’ approach to patients with abdominal pain. J Gen Intern Med 16:262–265Crossref, Google Scholar
Wolf AM, Colditz GA (1998), Current estimates of the economic cost of obesity in the United States. Obes Res 6:97–106Crossref, Google Scholar
Yager J (2000), Weighty perspectives: contemporary challenges in obesity and eating disorders. Am J Psychiatry 157:851–853Crossref, Google Scholar
Yanovski JA (2001), Intensive therapies for pediatric obesity. Pediatr Clin North Am 48:1041–1053Crossref, Google Scholar
Yanovski SZ, Nelson JE, Dubbert BK, Spitzer RL (1993), Association of binge eating disorder and psychiatric comorbidity in obese subjects. Am J Psychiatry 150:1472–1479Crossref, Google Scholar



