Nature and Nurture in Personality
Abstract
An interaction between individual and contextual factors is the central theme in development of personality. Molecular genetics offers the most valuable opportunity for increasing our understanding of the joint effect of nature and nurture. Sensitivity to environmental adversities and benefits may be conditional on genetic background, and the nature-nurture interactions may be of greater importance than direct gene-trait associations. In our recent series of studies, we have shown that different variants of serotonergic and dopaminergic genes may moderate the influence of environmental conditions on a range of psychological outcomes, at least on temperament, depression, and hostility. These studies suggest that, depending on their genotype, people may be differentially sensitive to the environmental conditions they encounter. In light of these results, it seems highly plausible that the effects of genes may become evident only when studied in the context of environmental factors.
It has recently been generally accepted that complex mental entities such as temperament and personality are the results of an interplay between genetic and environmental factors. The way to this balanced view emphasizing the joint effect of genes and environment has, however, not been easy in personality psychology. Environmentalism that attributes all human behaviors to an environment (“we are what we learn”) captivated developmental psychologists in the 1950s and dominated the research of personality over the two following decades. In the 1970s, there was still a debate about whether personality exists at all, and behavior was an indication of the environment only. During the last 15 years, a change toward a general acceptance of genetic factors in personality development has been so rapid that it is easily forgotten how “environmentalistic” the psychological explanations have been. Even in the 1960s, the major explanation for all personality traits and their associated clinical syndromes, such as autism or schizophrenia, was inappropriate or abnormal parenting.
Quantitative genetic research, i.e., animal behavior, family, twin, and adoption studies, demonstrated the importance of the genetic influence on personality (1). Most work to understand the roles of nature and nurture in human development has relied on molecular genetics and technologies for assessing brain metabolism.
Behavioral genetics has provided evidence of genetic influences on temperament and personality but, perhaps more importantly, has provided strong evidence for the significance of environmental influences. Early environmental information can considerably strengthen and even uncover associations between genes and traits. This has been initially demonstrated in animal studies that showed the contribution of rearing environment (types of maternal nurturance) and genetic background (5HTTLPR) and their interaction to a development of temperament (2).
Given that, behavioral genetics suggests a focus on the joint effect of genes and environment and especially on the developmental interplay between nature and nurture over the course of a person's life. Genes may have a direct effect on the development of personality traits but, more important, they may moderate environmental effects on personality development and explain individual differences in vulnerability and resilience to environmental hazards. Vulnerability to environmental adversities and sensitivity to environmental benefits may be conditional, i.e., the same genotypes may be associated with different outcomes in different environments (3, 4). This fact has been convincingly demonstrated, for instance, by Caspi et al. (5) in the Dunedin study and by Foley et al. (6) in the Virginia study, which showed a moderating effect of the MAO-A genotype in the relation between severe childhood maltreatment and later antisocial behavior.
BEHAVIORAL GENETICS IN PERSONALITY
Despite high initial expectancy, studies of behavioral genetics have not provided satisfactory results in personality psychology. The failure to identify the specific genetic underpinnings of behavior could be related to its multifactorial nature and the lack of biological validity of the personality concept and the important influence of the environmental factors on personality. A promising way to solve this problem is to look at the early biological roots of personality. Here, research has been concentrated on temperament, because an understanding of neuroregulatory systems underlying temperament may increase our knowledge of the interplay of nature and nurture in a development of normal personality and, in particular, may highlight mechanisms through which unfavorable and adverse environmental effects turn into personality disorders (7).
MONOAMINE TRANSMITTERS UNDERLIE TEMPERAMENT
Temperament consists of those components of personality that are heritable, developmentally stable, emotion based, or uninfluenced by sociocultural learning (8). Recent literature offers extensive evidence to show that interindividual differences in neuroregulatory systems, especially in the activities of the brain dopamine and serotonin systems, explain temperamental variability. This suggestion was originally made by Cloninger in his temperament and character theory (9). Cloninger conceptualizes personality as the combination of heritable, neurobiologically based temperament traits reflecting behavioral conditioning, and character traits reflecting both neurobiological and sociocultural mechanisms of semantic and self-aware learning. According to him, temperamental dimensions are related to heritable variation in responses to environmental stimuli and characterized by novelty seeking (NS) (a tendency toward exploratory activity and intense excitement in response to novel stimuli), harm avoidance (HA) (a tendency to respond intensely to aversive stimuli and to avoid punishment and novelty), and reward dependence (RD) (a tendency to respond intensely to reward and to learn to maintain rewarded behavior). NS is suggested to be linked with low basal dopaminergic activity, HA with high serotonergic activity, and RD with low basal noradrenergic activity (9). The extreme variants of these basic stimulus-response characteristics closely correspond to the traditional descriptions of personality disorders. This correspondence implies that the underlying structure of the normal adaptive traits is basically the same as that of the maladaptive personality traits.
The molecular genetics of temperament started in 1996 with two articles (10, 11) that showed an association between NS and the dopamine 4 receptor (DRD4). This finding was shortly replicated in many studies (12, 13), although some further studies questioned this association (14, 15). These two first articles on NS were shortly followed by investigations linking HA with the allelic variation of the serotonergic genes, first with the serotonin transporter 5HTTLPR. This link was also replicated (16, 17), even though the number of inconsistent findings was high (18).
In the 2000s, single gene effects as well as coeffects of several genes on temperament were increasingly documented, whereas studies using traditional personality traits were few in number. Gene × environment interactions in the development of temperament and personality have received less attention. The first molecular gene × environment interaction studies of human behavior were carried out by Berman and Noble (19) and Ozkaragoz and Noble (20), who showed that in adolescent boys, the minor alleles of the DRD2 gene interacted with familial alcoholism, resulting in high extraversion.
Later, it was demonstrated that the 5HTTLPR gene moderates parental influences on early temperament development. It has been shown that infants carrying the short allele of 5HTTLPR developed poorer behavioral self-regulation than their counterparts carrying only long alleles if they were insecurely attached to their caregivers but not when they were securely attached (21). Likewise, Fox et al. (22) showed that the short allele increased behavioral inhibition in children who had low parental support.
FINDINGS OF THE “CARDIOVASCULAR RISK IN YOUNG FINNS” SAMPLE
Our series of studies has capitalized on the population-based, longitudinal birth cohort study of the Cardiovascular Risk in Young Finns (Table 1). In this study, a representative sample of 3,600 healthy subjects from six age cohorts (aged 3, 6, 9, 12, 15, and 18 years at the baseline) have been followed over 27 years since their childhood and monitored in eight study waves resulting in a huge reservoir of somatic, psychological, behavioral, and environmental predictors and somatic and psychological outcomes (23) (Figure 1).
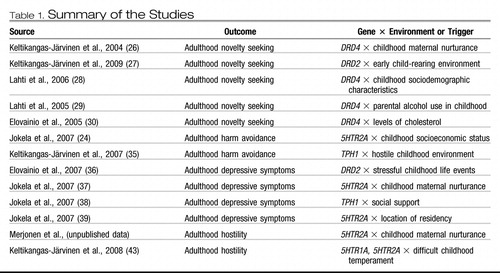 |
Table 1. Summary of the Studies

a Boxes in gray represent assessment waves discussed in the present article.
Nature versus nurture in temperament
We found both genetic and environmental main effects on temperament (24–27) (Figure 2). An interplay between genetic background and childhood environmental circumstances in the development of adult NS and HA existed, too. First, our findings suggest that the polymorphic variations of two central candidate genes of dopaminergic system, i.e., in DRD2 and DRD4, may moderate the influence of childhood environment on adulthood NS. This effect was first shown in a group of oversampled subjects (subjects with constantly extremely high or low NS) derived from the Cardiovascular Risk in Young Finns. In a hostile childhood environment, characterized by an emotional distance between the child and the mother and the mother's strict disciplinary style, the individuals carrying two- and five-repeat alleles of the DRD4 gene had a significantly greater risk to constantly score extremely high in NS compared with the individuals not carrying any of these alleles. A more favorable childhood environment, instead, predicted a low level of NS in adulthood, even in the presence of this very genotype (26).
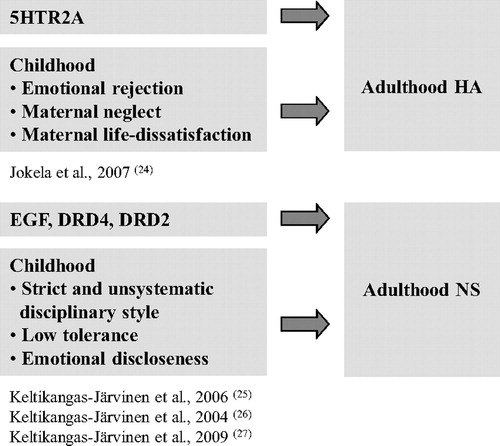
Figure 2. Main Genetic and Environmental Effects. Childhood Environment and Serotonergic and Dopaminergic Genes Have the Main Effects on Adulthood HA and NS, respectively.
This finding was repeated in the total Cardiovascular Risk in Young Finns sample and with a variant of another gene polymorphism related to dopaminergic transmission, i.e., DRD2. In a highly hostile child-rearing environment, especially in terms of disciplinary style, the A1 allele carriers of the Taq1A variant of the DRD2 gene had higher scores for NS in adulthood than A2/A2 genotype carriers. When the childhood environment was emotionally positively tuned, the genotype had no effect on NS (27) (Figure 3).

Figure 3. DRD2 and Childhood Environment Interact in Development of Adulthood NS.
In addition to emotional atmosphere, the DRD4 two- or five-repeat alleles interacted with childhood socioeconomic circumstances, residential setting, and parental alcohol use. In the carriers of this gene variant, high maternal education, high annual household income, and an urban residential setting in one's childhood increased the likelihood of scoring high on NS in adulthood. This was true after controlling for the effects of emotional relationships between the child and the mother. When maternal education and household income were low or the family resided in a rural setting, no differences between extreme high and extreme low NS groups were found (28). Further, in the presence of high paternal alcohol consumption, the two- or five-repeat alleles of the DRD4 were associated with a high level of NS, whereas no association was found in the presence of low alcohol consumption (29).
Somatic parameters modified an effect of the DRD4 polymorphism on NS, too. The two- or five-repeat alleles were associated with high NS in subjects with high levels of cholesterol but not in those with low cholesterol levels. This finding remained after adjustment for the apolipoprotein E polymorphism (30).
Second, we studied gene × environment interactions in the development of HA by using the T102C variant of the serotonin receptor 2A (5HTR2A), and A218C and A799C haplotypes of the tryptophan hydroxylase 1 gene (TPH1) as markers of the serotonin functioning. The T102C variant of the 5HT2RA polymorphism has been associated with the expression of the gene and with the binding potential of serotonin 2A receptors (31), and it has been considered as a candidate gene polymorphism for many psychiatric disorders, e.g., depression (32) and anxiety (33).
TPH1 is the rate-limiting enzyme for serotonin biosynthesis. It regulates serotonin levels and influences behaviors that are controlled by serotonin. Neurogenetic studies have mostly focused on A218C and A799C haplotypes of TPH1, and those polymorphisms have been shown to be associated with depression and suicidal behavior (34).
We showed that the T102C polymorphism of the HTR2A gene was directly associated with HA (24) and moderated the effect of childhood familial socioeconomic status (SES) on adulthood HA. The carriers of the T allele scored low on HA in adulthood if their familial SES was high in childhood or adolescence, whereas no association was observed among those with low familial childhood SES. This finding suggests that the T allele carriers may be more sensitive to environmental effects than the C/C carriers (24).
The joint effect of environment and genetic background was true with THP1 also. The A/A haplotypes of the TPH1 intron 7 A218A and A779C polymorphisms predicted a high level of adulthood HA in the presence of a hostile childhood environment as defined in terms of emotional rejection and inconsistent discipline, whereas no environmental effect was identified among C/C carriers (35) (Figure 4).

Figure 4. TPH1 and Childhood Environment Interact in Development of Adulthood HA.
All the above gene × environment interactions effects on NS and HA were repeated at two or three different study phases of the Cardiovascular Risk in Young Finns study (samples highly overlapping but not identical) 4 or 5 years apart.
Our most recent unpublished finding suggests an interaction of childhood nurturing environment and the T102C polymorphism of 5HTR2A on social attachment, as measured with the attachment subscale of RD and related measures of adult attachment styles. T/T genotype carriers were found to benefit from a favorable maternal nurturance in childhood and adolescence by showing greater temperamental attachment in adulthood, whereas an unfavorable maternal nurturance in childhood induced a lower attachment security in adolescence among them. In C allele carriers, a quality of maternal nurturance was not associated with later social attachment.
Nature versus nurture in depression
Our findings have also suggested moderating roles for the DRD2, TPH1, and 5HTR2A in a development of depressive symptoms. We found a weak overall association between childhood negative life events and adulthood depressive symptoms. This association was stronger among those who carried the A/A genotype of the Taq1A polymorphism of DRD2 and weaker among those with other genotypes (36).
We also found that the T allele carriers of the T102C polymorphism of 5HT2RA who had experienced high maternal nurturance in childhood or adolescence expressed lower levels of depressive symptoms in adulthood, compared with their C/C genotype counterparts, suggesting that the T allele carriers were more able to benefit from the protective aspects of the environment (37). Further, we found that low social support predicted depressive symptoms more strongly in individuals carrying A alleles of the TPH1 than in those with other alleles (38).
Finally, the T102C polymorphism of the 5HT2RA was shown to moderate the effect of urban/rural living conditions on depressive symptoms. In T allele carriers, urban or suburban residency was associated with lower depressive symptoms, whereas living in remote rural areas predicted a high level of depressive symptoms in them. No correlation was observed among individuals carrying the C/C genotype (39).
Nature versus nurture in hostility
A nonsupportive and conflictual childhood family environment has been shown to contribute to development of hostility (40), whereas twin studies suggest that hostility also has a genetic component (41). Hostility and difficult temperament share the same elements (7), and hostility has been suggested to be mediated by the serotonergic system (42).
Given these previous findings, we studied the modifying effects of the 5HTR1A and 5HTR2A gene polymorphisms in the association between childhood maternal behavior (quality of nurturance) and adulthood hostility. Among the carriers of the T/T and T/C genotypes of 5HT2RA, nurturance predicted hostility, whereas no association among C/C carriers was observed (unpublished data).
In addition, the T102C polymorphism was also shown to moderate the association between childhood hyperactive temperament and adulthood hostility in men (43). T/T genotype carriers, who were rated as hyperactive in childhood by their mothers, expressed higher levels of hostility in adulthood. The same was not true among C allele carriers. A child's hyperactive temperament might create a hostility-prone environment, i.e., a child's own behavior contributes to an unfavorable parent-child relationship and the T/T gene variant selects the children who are most sensitive to this detrimental influence.
CONCLUSIONS
Together with other studies of gene-environment interactions, our series of studies suggest that depending on their genotype, people may be differentially sensitive to the environmental conditions they encounter. In addition, it seems that a certain genetic background may sensitize a person both to the positive and the negative aspects of the environment suggesting that a gene variant may not be acting solely as a “vulnerability factor” nor a pure “opportunity gene” that would be entirely good or bad for its carrier (44). For example, the allelic variance of the HTR2A gene was shown to be associated with both an ability to use positive aspects of the environment, when offered, and with a heightened vulnerability to negative aspects of the environment, this way affecting the overall responsivity to environmental conditions. In addition to current environmental effects, certain gene variants may influence the duration of early experiences. In light of these results it seems highly plausible that the effects of genes may become evident only when studied in the context of environmental factors, whereas ignoring environmental variability may lead to inconsistent replications of findings, as has often been observed for multifactorial traits.
It is known that any extreme temperamental bias may predispose a person to personality disorders (45). Genetic background not only delineates early temperament but also determines how malleable these dispositions are in response to the environment. In some individuals, high anxiousness (which is strongly correlated with HA) may be the result of genetics and insensitivity to environmental influences that would decrease anxiousness in more sensitive individuals. In others, high anxiousness may reflect a combination of high susceptibility and exposure to environmental influences increasing anxiousness. The study of gene-environment interactions helps us to understand the origins and consequences of temperament and personality traits that stem from these different developmental pathways.
Despite high consistency of the findings on the joint effect of genes and environment, one weakness needs to be recognized. This weakness limits our findings as well as most previous research. “Environment” usually refers to parental parameters that at least partly indicate a shared genetic background, too. Thus, the environment may be partly genetic. Nevertheless, adoption studies and other separation experiments indicate that both nature and nurture need to be considered carefully in assessing the development of temperament, personality, and their disorders. Neither genes nor environmental influences alone can be expected to explain the development of personality across the lifespan.
1 Cloninger CR: Genetics of personality disorders, in Psychiatric Genetics. Edited by Nurnberger J, Wade B. New York, Cambridge University Press, in press Google Scholar
2 Ladd CO, Huot RL, Thrivikraman KV, Nemeroff CB, Meaney MJ, Plotsky PM: Long-term behavioral and neuroendocrine adaptations to adverse early experience. Prog Brain Res 2000; 122:81–103 Crossref, Google Scholar
3 Kendler KS: Twin studies of psychiatric illness: an update. Arch Gen Psychiatry 2001; 58:1005–1014 Crossref, Google Scholar
4 Rutter M, Silberg J: Gene-environment interplay in relation to emotional and behavioral disturbance. Annu Rev Psychol 2002; 53:463–490 Crossref, Google Scholar
5 Caspi A, McClay J, Moffitt TE, Mill J, Martin J, Craig IW, Taylor A, Poulton R: Role of genotype in the cycle of violence in maltreated children. Science 2002; 297:851–854 Crossref, Google Scholar
6 Foley DL, Eaves LJ, Wormley B, Silberg JL, Maes HH, Kuhn J, Riley B: Childhood adversity, monoamine oxidase a genotype, and risk for conduct disorder. Arch Gen Psychiatry 2004; 61:738–744 Crossref, Google Scholar
7 Plomin R, DeFries JC, McClearn GE, McGuffin P: Behavioral Genetics, 4th ed. New York, Worth Publishers, 2001 Google Scholar
8 Cloninger CR: Temperament and personality. Curr Opin Neurobiol 1994; 4:266–273 Crossref, Google Scholar
9 Cloninger CR, Svrakic DM, Przybeck TR: A psychobiological model of temperament and character. Arch Gen Psychiatry 1993; 50:975–990 Crossref, Google Scholar
10 Benjamin J, Li L, Patterson C, Greenberg BD, Murphy DL, Hamer DH: Population and familial association between the D4 dopamine receptor gene and measures of novelty seeking. Nat Genet 1996; 12:81–84 Crossref, Google Scholar
11 Ebstein RP, Novick O, Umansky R, Priel B, Osher Y, Blaine D, Bennett ER, Nemanov L, Katz M, Belmaker RH: Dopamine D4 receptor (D4DR) exon III polymorphism associated with the human personality trait of novelty seeking. Nat Genet 1996; 12:78–80 Crossref, Google Scholar
12 Noble EP, Ozkaragoz TZ, Ritchie TL, Zhang X, Belin TR, Sparkes RS: D2 and D4 dopamine receptor polymorphisms and personality. Am J Med Genet 1998; 81:257–267 Crossref, Google Scholar
13 Ono Y, Manki H, Yoshimura K, Muramatsu T, Mizushima H, Higuchi S, Yagi G, Kanba S, Asai M: Association between dopamine D4 receptor (D4DR) exon III polymorphism and novelty seeking in Japanese subjects. Am J Med Genet 1997; 74:501–503 Crossref, Google Scholar
14 Gelernter J, Kranzler H, Coccaro E, Siever L, New A, Mulgrew CL: D4 dopamine-receptor (DRD4) alleles and novelty seeking in substance-dependent, personality-disorder, and control subjects. Am J Hum Genet 1997; 61:1144–1152 Crossref, Google Scholar
15 Herbst JH, Zonderman AB, McCrae RR, Costa PT Jr: Do the dimensions of the temperament and character inventory map a simple genetic architecture? Evidence from molecular genetics and factor analysis. Am J Psychiatry 2000; 157:1285–1290 Crossref, Google Scholar
16 Rybakowski F, Slopien A, Dmitrzak-Weglarz M, Czerski P, Rajewski A, Hauser J: The 5-HT2A-1438 A/G and 5-HTTLPR polymorphisms and personality dimensions in adolescent anorexia nervosa: association study. Neuropsychobiology 2006; 53:33–39 Crossref, Google Scholar
17 Samochowiec J, Rybakowski F, Czerski P, Zakrzewska M, Stepien G, Pelka-Wysiecka J, Horodnicki J, Rybakowski JK, Hauser J: Polymorphisms in the dopamine, serotonin, and norepinephrine transporter genes and their relationship to temperamental dimensions measured by the Temperament and Character Inventory in healthy volunteers. Neuropsychobiology 2001; 43:248–253 Crossref, Google Scholar
18 Sen S, Burmeister M, Ghosh D: Meta-analysis of the association between a serotonin transporter promoter polymorphism (5-HTTLPR) and anxiety-related personality traits. Am J Med Genet B Neuropsychiatr Genet 2004; 127B:85–89 Crossref, Google Scholar
19 Berman SM, Noble EP: The D-2 dopamine receptor (DRD2) gene and family stress; interactive effects on cognitive functions in children. Behav Genet 1997; 27:33–43 Crossref, Google Scholar
20 Ozkaragoz T, Noble EP: Extraversion—interaction between D2 dopamine receptor polymorphisms and parental alcoholism. Alcohol 2000; 22:139–146 Crossref, Google Scholar
21 Kochanska G, Philibert RA, Barry RA: Interplay of genes and early mother-child relationship in the development of self-regulation from toddler to preschool age. J Child Psychol Psychiatry 2009; 50:1331–1338 Crossref, Google Scholar
22 Fox NA, Nichols KE, Henderson HA, Rubin K, Schmidt L, Hamer D, Ernst M, Pine DS: Evidence for a gene-environment interaction in predicting behavioral inhibition in middle childhood. Psychol Sci 2005; 16:921–926 Crossref, Google Scholar
23 Raitakari OT, Juonala M, Rönnemaa T, Keltikangas-Järvinen L, Räsänen L, Pietikäinen M, Hutri-Kähönen N, Taittonen L, Jokinen E, Marniemi J, Jula A, Telama R, Kähönen M, Lehtimäki T, Åkerblom HK, Viikari JS: Cohort profile: the cardiovascular risk in Young Finns Study. Int J Epidemiol 2008; 37:1220–1226 Crossref, Google Scholar
24 Jokela M, Lehtimäki T, Keltikangas-Järvinen L: The serotonin receptor 2A gene moderates the influence of parental socioeconomic status on adulthood harm avoidance. Behav Genet 2007; 37:567–574 Crossref, Google Scholar
25 Keltikangas-Järvinen L, Puttonen S, Kivimaki M, Rontu R, Lehtimaki T: Cloninger's temperament dimensions and epidermal growth factor A61G polymorphism in Finnish adults. Genes Brain Behav 2006; 5:11–18 Crossref, Google Scholar
26 Keltikangas-Järvinen L, Räikkönen K, Ekelund J, Peltonen L: Nature and nurture in novelty seeking. Mol Psychiatry 2004; 9:308–311 Crossref, Google Scholar
27 Keltikangas-Järvinen L, Pulkki-Råback L, Elovainio M, Raitakari OT, Viikari J, Lehtimäki T: DRD2 C32806T modifies the effect of child-rearing environment on adulthood novelty seeking. Am J Med Genet B Neuropsychiatr Genet 2009; 150B:389–394 Crossref, Google Scholar
28 Lahti J, Räikkönen K, Ekelund J, Peltonen L, Raitakari OT, Keltikangas-Järvinen L: Socio-demographic characteristics moderate the association between DRD4 and novelty seeking. Pers Individ Differences 2006; 40:533–543 Crossref, Google Scholar
29 Lahti J, Räikkönen K, Ekelund J, Peltonen L, Raitakari OT, Keltikangas-Järvinen L: Novelty seeking: interaction between parental alcohol use and dopamine D4 receptor gene exon III polymorphism over 17 years. Psychiatr Genet 2005; 15:133–139 Crossref, Google Scholar
30 Elovainio M, Kivimäki M, Puttonen S, Heponiemi T, Keltikangas-Järvinen L, Viikari J: Does the level of LDL cholesterol moderate a relationship between DRD4 and novelty seeking? Biol Psychol 2005; 68:79–86 Crossref, Google Scholar
31 Polesskaya OO, Sokolov BP: Differential expression of the “C” and “T” alleles of the 5-HT2A receptor gene in the temporal cortex of normal individuals and schizophrenics. J Neurosci Res 2002; 67:812–822 Crossref, Google Scholar
32 Du L, Bakish D, Lapierre YD, Ravindran AV, Hrdina PD: Association of polymorphism of serotonin 2A receptor gene with suicidal ideation in major depressive disorder. Am J Med Genet 2000; 96:56–60 Crossref, Google Scholar
33 Yoon HK, Yang JC, Lee HJ, Kim YK: The association between serotonin-related gene polymorphisms and panic disorder. J Anxiety Disord 2008; 22:1529–1534 Crossref, Google Scholar
34 Rujescu D, Giegling I, Sato T, Hartmann AM, Moller HJ: Genetic variations in tryptophan hydroxylase in suicidal behavior: analysis and meta-analysis. Biol Psychiatry 2003; 54:465–473 Crossref, Google Scholar
35 Keltikangas-Järvinen L, Puttonen S, Kivimaki M, Elovainio M, Rontu R, Lehtimaki T: Tryptophan hydroxylase 1 gene haplotypes modify the effect of a hostile childhood environment on adulthood harm avoidance. Genes Brain Behav 2007; 6:305–313 Crossref, Google Scholar
36 Elovainio M, Jokela M, Kivimäki M, Pulkki-Råback L, Lehtimäki T, Airla N, Keltikangas-Järvinen L: Genetic variants in the DRD2 gene moderate the relationship between stressful life events and depressive symptoms in adults: cardiovascular risk in young Finns study. Psychosom Med 2007; 69:391–395 Crossref, Google Scholar
37 Jokela M, Keltikangas-Järvinen L, Kivimäki M, Puttonen S, Elovainio M, Rontu R, Lehtimäki T: Serotonin receptor 2A gene and the influence of childhood maternal nurturance on adulthood depressive symptoms. Arch Gen Psychiatry 2007; 64:356–360 Crossref, Google Scholar
38 Jokela M, Räikkönen K, Lehtimäki T, Rontu R, Keltikangas-Järvinen L: Tryptophan hydroxylase 1 gene (TPH1) moderates the influence of social support on depressive symptoms in adults. J Affect Disord 2007; 100:191–197 Crossref, Google Scholar
39 Jokela M, Lehtimäki T, Keltikangas-Järvinen L: The influence of urban/rural residency on depressive symptoms is moderated by the serotonin receptor 2A gene. Am J Med Genet B Neuropsychiatr Genet 2007; 144B:918–922 Crossref, Google Scholar
40 Matthews KA, Woodall KL, Kenyon K, Jacob T: Negative family environment as a predictor of boys' future status on measures of hostile attitudes, interview behavior, and anger expression. Health Psychol 1996; 15:30–37 Crossref, Google Scholar
41 Rose RJ: Genetic and environmental variance in content dimensions of the MMPI. J Pers Soc Psychol 1988; 55:302–311 Crossref, Google Scholar
42 Staner L, Mendlewicz J: Heredity and role of serotonin in aggressive impulsive behavior. Encephale 1998; 24:355–364 Google Scholar
43 Keltikangas-Järvinen L, Puttonen S, Kivimäki M, Elovainio M, Pulkki-Råback L, Koivu M, Rontu R, Lehtimäki T: Serotonin receptor genes 5HT1A and 5HT2A modify the relation between childhood temperament and adulthood hostility. Genes Brain Behav 2008; 7:46–52 Google Scholar
44 Belsky J, Pluess M: Beyond diathesis stress: differential susceptibility to environmental influences. Psychol Bull 2009; 135:885–908 Crossref, Google Scholar
45 Cloninger CR: Feeling Good: The Science of Well Being. New York, Oxford University Press, 2004 Google Scholar



