Neurocircuitry of Anxiety Disorders
Abstract
A major focus in the field of anxiety in the past decade, and an area of intense ongoing interest, is the delineation of the basic neurocircuitry underlying normal and pathologic anxiety. Preclinical work defining the basic neurocircuitry responsible for fear responding has fueled neuroimaging investigations attempting to model the neurocircuitry of the anxiety disorders. Herewith, the authors review neuroimaging findings contributing to the development and refinement of neuroanatomic models for post-traumatic stress disorder, panic disorder, and social anxiety disorder.
Introduction
Recent progress in the development of neurocircuitry models of anxiety disorders has been made possible by major developments in the field of neuroimaging. Research in this area is critical to increasing understanding of the basic mechanisms of anxiety and also of the influence that pharmacologic and nonpharmacologic therapies have on modulating anxiety.
Many of the early neuroimaging studies were fueled by discoveries made in preclinical models of stress and conditioned fear by researchers such as Davis (1) and LeDoux et al. (2). Through the elegant work of these and other researchers, a basic model of normal fear responding was developed and refined, leading to the description of a “fear neurocircuitry” centered around the amygdala. It is this “fear neurocircuitry” that has served as the basis for the human neurocircuitry models being explored and refined through neuroimaging techniques today.
Herewith, the authors focus on neurocircuitry models of post-traumatic stress disorder (PTSD), panic disorder (PD), and social anxiety disorder (SAD), which have a common neuroanatomy emerging from the neuroimaging literature that converges with the preclinical “fear neurocircuitry.” Equally important, but outside the scope of this review, are obsessive-compulsive disorder (OCD), generalized anxiety disorder (GAD), and specific phobias. Neuroimaging studies in OCD have demonstrated a divergent neuroanatomy and neurocircuitry from PTSD, PD, and SAD involving frontal-striatal circuits (3•). Scant data are currently available for GAD, limiting potential neuroanatomic modeling of this disorder. Although intriguing neuroimaging studies have been reported in specific phobias, review of this area is excluded given space limitations, and the reader is referred elsewhere for comprehensive coverage of that topic (4, 5).
Preclinical models of stress, fear, and anxiety: focus on the amygdala
Because it serves as the foundation to understanding the neurocircuitry models of the anxiety disorders discussed herewith, a brief review of the fear neurocircuitry, synthesized from the work of several preclinical researchers, is presented.
It is now well established in preclinical models that the amygdala, located in the anterior part of the medial temporal lobe, is the central node for the coordination of autonomic and behavioral fear responding. Sensory information critical to threat assessment is processed via pathways running through the anterior thalamus to the amygdala (6). In preparation for action in response to threat, the amygdala activates ascending projections to motor areas and descending projections to brain stem nuclei that control autonomic responses and arousal. The amygdala also facilitates the acquisition of additional information regarding specific threats via reciprocal projections to subcortical and limbic cortical regions (7, 8). Critical feedback to the amygdala is provided by specific brain regions, including the following: 1) medial frontal cortex, 2) the hippocampus, and 3) cortico-striato-thalamic circuits, which mediate “gating” at the level of the thalamus, thereby regulating the flow of incoming information that reaches the amygdala. The medial frontal cortex, including anterior cingulate and medial and orbitofrontal cortex, is believed to provide critical “top-down” governance over the amygdala, curbing the fear response once danger has passed or when the import of a potentially threatening stimulus has changed. The hippocampus provides information about the context of a potentially threatening stimulus or situation, and draws on information about the environment retrieved from explicit memory caches. These structures compose the core neurocircuitry believed critical in fear responding.
Imaging approaches to neurocircuitry modeling of the anxiety disorders
Herewith, the authors discuss structural and functional neuroimaging studies used in investigating neuroanatomy and neurocircuitry of the anxiety disorders. Although neurochemical studies involving magnetic resonance spectroscopy and neuroreceptor mapping with positron emission tomography (PET) provide critical links among anatomy, neurochemistry, and neurobiology, the review of these neurochemistry studies is beyond the scope of this paper.
Morphometric magnetic resonance imaging (mMRI) is the current approach to structural neuroimaging (providing superior gray and white matter resolution compared with computed axial tomography scanning). By using semi-automated or fully automated schemes for segmenting defined brain structures, mMRI can calculate corresponding volumes with great precision. Although mMRI techniques enable the accurate assessment of brain structure volumes, they provide little information regarding regional white matter tract orientation and connectivity. Magnetic resonance diffusion tensor imaging is a complementary neuroimaging technique by which fiber tract orientation and tissue anisotropy are estimated in vivo, and then algorithms are used in three dimensions to determine white matter tract orientations (9). To the authors’ knowledge, this technique has not yet been applied to investigation of the anxiety disorders; however, it holds the promise of evaluating progressive changes in connectivity over time in a wide array of neuropsychiatric disorders (10, 11).
Functional imaging methods are at the forefront of research aimed at formulating neurocircuitry models of the anxiety disorders. There are currently three main methods of obtaining functional data, reflecting regional brain activity. These are 1) PET with tracers that measure blood flow (e.g., oxygen-15–labeled carbon dioxide, or oxygen-15–labeled water) or glucose metabolism (i.e., F-18-labeled fluorodeoxyglucose); 2) single photon emission tomography with tracers that measure correlates of blood flow (e.g., technetium-99–labeled hexamethyl propylene amine oxime; and 3) functional magnetic resonance imaging (fMRI), which measures blood oxygenation level–dependent (BOLD) signal changes.
Functional imaging studies reflect to some extent the state of the patient at the time of tracer distribution or image acquisition, and functional imaging paradigms are described in terms of the type of state manipulations used. “Neutral state” paradigms consist of imaging subjects during a nominal “resting” state, or while performing a nonspecific continuous task. “Symptom provocation” paradigms involve scanning subjects during a neutral (control) state, and then during a symptomatic state in which anxiety is intentionally induced through the use of specific pharmacologic or behavioral manipulations. “Cognitive activation” paradigms are used to study subjects while performing cognitive or behavioral tasks, often specially designed to activate specific brain regions of interest (Fig. 1).
Post-traumatic stress disorder
Neurocircuitry models of PTSD (12) focus on the central role of the amygdala in directing an individual’s response to perceived danger through its reciprocal connections with the hippocampus, medial prefrontal cortex, and other cortical areas associated with higher cognitive functions. This model emphasizes a lack of adequate top-down governance over the amygdala by medial prefrontal cortex (specifically, the rostral portion of anterior cingulate cortex [13] and the hippocampus, resulting in hyperresponsivity within the amygdala to threat-related stimuli. The lack of habituation to repetitive stimuli that do not signal imminent threat and that characterizes PTSD is attributed to inadequate influence of the anterior cingulate cortex on the amygdala. Another hallmark of PTSD, the overgeneralization of fear responding to nonthreatening stimuli, is associated with decreased hippocampal function.
Structural imaging findings
Morphometric MRI studies of PTSD have focused primarily on the hippocampus, in light of its known susceptibility to stress-induced damage via the action of glucocorticoids and excitatory amino acids (14, 15). Two studies have reported reduced hippocampal volumes in Vietnam combat veterans with PTSD (16, 17). In one study, total hippocampal volume was inversely correlated with the extent of combat exposure and PTSD symptom severity (17).
Three recent studies investigating potential brain alterations in children and adolescents with PTSD failed to find differences in hippocampal volumes between patients with PTSD and healthy control individuals (18–20). However, two of these studies reported that children with PTSD had smaller total brain and cerebral volumes (19, 20), suggesting a more generalized effect of traumatic stress in early development. De Bellis et al. (21) also demonstrated larger superior temporal gyrus volumes and a loss of the normal asymmetry pattern in a pediatric sample with PTSD. Abnormalities of the superior temporal gyrus, implicated in verbal and nonverbal auditory processing, may reflect abnormal developmental changes as the result of chronic childhood maltreatment resulting in PTSD.
Contrary to the pediatric findings, in mMRI studies of adults with PTSD resulting from childhood abuse, investigators have reported similar hippocampal volumetric differences to those studies examining samples with PTSD resulting from traumatic exposure in adulthood. Several studies now confirm that adult survivors of childhood sexual or physical abuse have smaller hippocampal volumes than healthy comparison samples (22–24, 25•). In summary, the results of studies of adults with PTSD with childhood or adult traumatic exposure support an association between reduced hippocampal volume and PTSD. However, the negative findings reported in pediatric studies suggest that hippocampal atrophy may develop in a progressive fashion over time. In a prospective study designed to test this hypothesis in an adult population, Bonne et al. (26) found that individuals who developed PTSD as a result of a traumatic exposure did not differ from those who did not develop PTSD in hippocampal volume at baseline, or at 6 months. The results of this study suggest that significant brain changes, particularly in the hippocampus, do not occur within 6 months of developing PTSD symptoms in response to an acute traumatic event. A recent study of adult male monozygotic twins discordant for combat exposure indicated that smaller hippocampal volumes may represent a risk factor for developing PTSD, rather than evolving as a consequence of the trauma (27•).
In an mMRI study using a new scheme for cortical parcellation, Rauch et al. (28) found selectively decreased rostral anterior cingulate and subcallosal cortical volumes in combat nurses with PTSD versus those without PTSD.
Functional imaging findings
Early functional studies in PTSD patients suggested abnormally elevated regional cerebral blood flow (rCBF) in the orbitofrontal cortex, insula, anterior cingulate cortex, and amygdala (29, 30). Later, controlled investigations with PTSD have implicated many of the same areas that came to light in these early studies. In a neutral state study, Sachinvala et al. (31) reported relatively greater rCBF in the bilateral anterior and posterior cingulate, the right temporal and parietal cortices, right basal ganglia, left hippocampus, and left orbitofrontal cortex. A limitation of this study is that the majority of the patients included were on psychotropic medications. When the five medication-free patients with PTSD only were compared with the healthy control group, increased rCBF was seen in the bilateral caudate and putamen, as well as the right orbitofrontal and bilateral anterior cingulate cortices. Semple et al. (32) measured rCBF in a sample of patients with PTSD and comorbid substance abuse and a sample of healthy comparison subjects at rest and during an auditory continuous performance task. They reported that the PTSD group had greater rCBF in the right amygdala and left parahippocampal gyrus, and lower rCBF in the rostral anterior cingulate during these conditions.
Several studies of PTSD using symptom provocation paradigms have been reported that suggest group differences involving the amygdala, anterior cingulate, and areas of the prefrontal cortex. Using a variety of symptom provocation paradigms, several investigators reported increased amygdala activity in PTSD, compared with control individuals, in response to the symptom provocation condition (33–35). The majority of studies have reported a relative failure of patients with PTSD to recruit the anterior cingulate cortex compared with healthy control individuals in response to symptom provocation (32, 36–38, 39•), although this finding is not completely consistent (34, 40). In order to look at the issue of anterior cingulate function in PTSD in greater depth, Shin et al. (39•) designed a study to specifically test the functional integrity of the anterior cingulate cortex in patients with PTSD using an Emotional Counting Stroop task (41), which is a reliable means of recruiting the anterior cingulate in healthy, nonpsychiatric individuals. The PTSD group failed to activate rostral anterior cingulate cortex in the combat versus general negative word conditions, whereas the non-PTSD group exhibited significant rostral anterior cingulate cortex activation. Similar results were later reported by Lanius et al. (42) using fMRI and a high field strength (4 Tesla) magnet. Using a symptom provocation paradigm with scripted imagery to study patients with non–combat-related PTSD, brain activity was again lower in patients with PTSD compared with control individuals in the anterior cingulate and medial prefrontal cortex. In addition, reduced activity in the thalamus was thought related to disruption in normal intrathalamic sensory processing relays. Studies using symptom provocation paradigms have also demonstrated significantly greater activity in patients with PTSD compared with control individuals in brain regions associated with motor preparedness in response to threat, such as the amygdala, periaqueductal gray, primary and supplementary motor cortices, and the cerebellar vermis (34).
Osuch et al. (43) suggested that some of the differences in reported findings in symptom provocation studies in PTSD may be attributable to the presence or absence of flashbacks. They reported positive correlations between flashback intensity and left hippocampal, left inferior frontal, left somatosensory, and cerebellar cortices, brainstem, right insula, and right putamen. Flashback intensity was inversely correlated with bilateral superior frontal, fusiform, and medial temporal cortices. These results are consistent with several other studies showing relatively decreased superior frontal rCBF in patients with PTSD compared with control individuals in response to symptom provocation paradigms (30, 38, 40). Lanius et al. (44) examined the issue of whether patients with PTSD who respond to script-driven imagery with dissociative responses (without physiologic activation) differ in patterns of brain activation from patients with PTSD with reexperiencing or anxiety responses associated with physiologic activation. Their findings suggest that the pattern of brain activity associated with dissociation differs from that associated with reliving the traumatic event; dissociated individuals demonstrate greater activity in the right anterior cingulate, right medial prefrontal cortex, inferior and middle frontal gyri, and middle temporal gyrus, whereas patients with reexperiencing responses typically demonstrate decreased anterior cingulate and medial prefrontal activity (36–38).
Most recently, Clark et al. (45) examined the pattern of brain activity in response to a trauma-neutral verbal working memory updating task. Compared with healthy control individuals, patients with PTSD demonstrated a lack of bilateral dorsolateral prefrontal cortex activation and a shift to bilateral superior parietal lobe activation. This finding, along with the earlier finding of decreased activation of Broca’s area (30), suggests that PTSD may have a distinct pattern of brain activation in response to the organization and processing of verbal working memory. This is consistent with earlier proposals (30, 37) that suggest a greater reliance in PTSD on nonverbal working memory and a shift away from verbal-based processing. Clark et al. (45) suggested that this shift toward nonverbal processing is “important to the quality, nature, and intrusiveness of traumatic memories.”
In summary, functional neuroimaging findings in PTSD suggest that in threatening situations, patients with PTSD reallocate neural resources to more primitive limbic regions that mediate fear responding at the expense of cortical areas mediating higher cognitive functions. Evidence suggests that difficulties with verbal memory often observed in PTSD may be the result of a failure to recruit appropriate frontal temporal regions involved in verbal processing, with a shift toward more posterior brain regions (parietal lobes) typically involved in visuospatial processing and encoding. Overall, the pathogenesis of PTSD may be conceptualized as a fear conditioning process that is superimposed over some diathesis, which may involve a predisposition to amygdala hyperresponsivity, lack of sufficient functional connectivity between the anterior cingulate and the amygdala, hippocampal deficiency, and inadequate function of brain regions involved in executive function, as well as verbal memory and processing.
Panic disorder
Although neurocircuitry models of PD are arguably less well developed than in PTSD, these models have the difficult task of accounting for the occurrence of spontaneous panic attacks. Attempts to explain these “out-of-the-blue” attacks have focused on spontaneous recruitment of the normal anxiety and fear circuitry. This may be the result of 1) abnormal regulation of homeostasis in the fear neurocircuitry, because of aberrant modulation by monoamine systems or aberrant processing of sensory or neurochemical information; and 2) failure in constraint by systems responsible for limiting anxiety responding. Thus, PD may be characterized by fundamental amygdala hyperresponsivity to subtle (even unconscious) environmental cues, triggering full-scale threat-related responding with insufficient top-down governance from higher cortical control areas.
Structural imaging findings
In an early qualitative study (46), a higher frequency of gross structural abnormalities, particularly in the temporal lobe, was reported in patients with PD when compared with healthy control individuals. In a later quantitative mMRI study focusing on the temporal lobe and hippocampus, patients with PD demonstrated decreased mean temporal lobe volumes bilaterally, despite normal hippocampal volumes (47). Most recently, Massana et al. (48•) reported a reduction in gray matter density in the left parahippocampal gyrus in PD compared with control individuals using a voxel-based mMRI approach.
Functional imaging findings
Five neutral state studies have reported alterations in hippocampal-parahippocampal activity in patients with PD compared with healthy control individuals, although the direction and laterality of these changes has not always been consistent (49–53). The majority of these studies have been interpreted as suggesting abnormally greater right-sided activity in the hippocampal-parahippocampal region in PD. In light of findings suggesting reduced left parahippocampal gyrus density, Massana et al. (48•) suggested that these functional neuroimaging studies indicate compensatory activity in the right parahippocampal region because of gray matter deficits in the left parahippocampal region. However, this is inconsistent with two functional studies that reported increased activity in the left parahippocampal region at rest (53, 54).
Six symptom provocation studies of PD have been published; four used pharmacologic challenges, one used a respiratory challenge, and one used anxiety situation imagery exposure. In the earliest of these studies, Stewart et al. (55) reported that patients with PD who experienced lactate-induced panic attacks displayed global cortical decreases in cerebral blood flow (CBF), whereas patients with PD and healthy control individuals who did not panic exhibited global cortical increases in CBF during lactate infusions, suggesting the possibility of abnormal cerebrovascular responsiveness in PD. A recent study using the carbon dioxide inhalation technique to examine changes in global CBF in patients with PD versus healthy control individuals also reported that patients with PD exhibited an abnormal change in CBF in response to respiratory challenge (56). Patients with PD demonstrated a decrease from baseline in arterial carbon dioxide–adjusted global CBF values in response to carbon dioxide inhalation, whereas control individuals demonstrated an increase in global CBF.
In a pharmacologic challenge study using yohimbine, an alpha-2 antagonist, patients with PD reported increased anxiety and demonstrated decreased rCBF in bilateral frontal cortex compared with control individuals in response to yohimbine administration (57). Using PET methods to measure rCBF in patients with PD and healthy comparison individuals during lactate infusions, patients with PD who panicked to lactate infusion exhibited rCBF increases in bilateral insular cortex, claustrum, and putamen relative to the normal control individuals and to the patients with PD who did not experience lactate-induced panic attacks (58). In a more recent PET study examining anticipatory anxiety in response to an impending pentagastrin challenge, patients with PD demonstrated greater activity in the parahippocampal gyrus, superior temporal lobe, hypothalamus, and anterior cingulate gyrus when compared with control individuals (54).
In a behavioral symptom provocation study using fMRI, patients were imaged during directed exposure blocks of neutral and high-anxiety situations. Results revealed increased activity during high-anxiety imagery compared with neutral-anxiety imagery in the patients with PD in the inferior frontal cortex, orbitofrontal cortex, hippocampus, and anterior and posterior cingulate when compared with control individuals (59).
In a study examining the effects of imipramine treatment on regional cerebral glucose metabolism (rCMRglu) in patients with PD using PET-fluorodeoxyglucose, Nordahl et al. (51) observed no change with treatment in the preexisting rightward shift in symmetry in hippocampus and posterior inferior frontal cortex, suggesting a lack of normalization of laterality with treatment. However, when compared with the untreated group, the imipramine-treated group exhibited rCMRglu decreases in posterior orbitofrontal cortex. These results suggest that the abnormal hippocampal and inferior frontal cortex asymmetries may be a trait marker for PD (51).
Summary
Neuroimaging data in PD suggest that functional abnormalities in the hippocampal-parahippocampal region may be a trait marker for this anxiety disorder. During symptom provocation, patients with PD exhibit activation of insular and motor striatal regions, and reductions are seen in widespread cortical regions, including the prefrontal cortex. Several studies suggest that abnormal cerebrovascular responsivity occurs in PD. There is some support for dysfunction in temporal lobe structures in PD, suggesting a potential role for hippocampal or parahippocampal involvement in this disorder. Although there is theoretic appeal to considering a role for the amygdala in PD, the scientific community await empiric data to support such a hypothesis.
Social anxiety disorder
Currently, neuroanatomically based models for phobias remain in the early stages of development (4, 5, 60, 61). However, in SAD, there is now increasing evidence for links between systems responsible for processing social cues and the fear neurocircuitry, suggesting that this may be an area of aberrant connectivity in pathologic social anxiety.
Structural imaging findings
Despite the high prevalence of SAD, only a single volumetric study in SAD has been reported. Using mMRI techniques, the investigators reported no significant differences between patients with SAD and healthy control individuals in any of the brain regions examined (62).
Functional imaging findings
In an early neutral state single photon emission computed tomography study of patients with social phobia and healthy control individuals, no significant between-group differences in rCBF were reported (63). In an exposure paradigm to human face stimuli, patients with SAD demonstrated greater responsivity within the amygdala compared with healthy control individuals (64). Subsequently, Schneider et al. (65) demonstrated increased activity within the amygdala and hippocampus in patients with social phobia in response to neutral faces linked with negative odors in a conditioning paradigm, whereas healthy comparison individuals had signal decreases in these same areas. In a more recent study, also using aversive conditioning with neutral faces using painful pressure as the unconditioned stimuli, patients with SAD demonstrated increased activity in the orbitofrontal cortex, anterior cingulate, insula, right amygdala, and left dorsolateral prefrontal cortex compared with control individuals during habituation and extinction in response to the faces stimuli (66). Patients with SAD were already exhibiting increased activation of right amygdala and bilateral orbitofrontal cortex during the habituation phase (before aversive conditioning).
In a symptom-provocation study, which required patients to speak in front of an audience, Tillfors et al. (67) reported that patients with SAD demonstrated significantly greater rCBF responses in the right amygdala and periamygdaloid cortex compared with control individuals in response to public versus private speaking. In addition, rCBF decreased in patients with SAD in the orbitofrontal and insular cortices and the temporal pole, whereas healthy comparison individuals demonstrated increases in rCBF in these same regions. Overall, the pattern of response seen in patients with SAD suggests relatively increased subcortical activity and relatively decreased frontal cortical activity; this is consistent with a failure of cortical processing and a shift to the phylogenetically older subcortical fear circuitry during social stress.
There are two reported imaging studies examining the effects of treatment on regional brain activity in SAD. Van der Linden et al. (68) used single photon emission computed tomography and rCBF methods to examine the effects of 8 weeks of treatment with citalopram. Patients with SAD demonstrated reductions in rCBF within the anterolateral left temporal cortex, left cingulate, and left midfrontal cortex. Those judged as nonresponders to treatment demonstrated greater rCBF at baseline in the anterolateral left temporal cortex and the lateral left midfrontal cortex when compared with responders. Interpretation of the results of this study is limited by the presence of comorbid anxiety disorders and other psychotropic medications at the time of scanning.
A more recent study examined the effects of two different anxiety treatments, pharmacotherapy with a selective serotonin reuptake inhibitor and cognitive-behavioral therapy (CBT) on rCBF in patients with SAD. Patients with SAD were scanned using PET techniques at baseline, and again after 9 weeks while performing public speaking (69•). During the interim 9 weeks, patients received citalopram or CBT, or remained on a waiting list (control group). Similar changes in rCBF were exhibited by responders in the treatment groups in response to public speaking, including the following: decreases bilaterally in rCBF in the amygdala, hippocampus, and related periamygdaloid, perihippocampal, and rhinal cortices. However, no significant changes in rCBF were observed in the wait-list control group. These findings suggest that pharmacotherapy and CBT reduce activity in brain regions associated with the fear neurocircuitry, and that this change in pattern of brain activity is not specific to treatment modality.
Summary
In SAD, in response to exposure to human face stimuli or to the stress of public speaking, patients demonstrate exaggerated activity in the amygdala and related medial temporal lobe areas. Social anxiety disorder is also associated with abnormal brain activity within medial temporal lobe structures during aversive conditioning with human face stimuli, suggesting that incorrect assignment of threat to human faces may be occurring.
Conclusions
A convergence of neuroimaging data suggest that among the anxiety disorders, PTSD, PD, and SAD share in common certain mediating neuroanatomy, including the paralimbic cortex, amygdala, hippocampus, and prefrontal cortical regions. Neuroanatomic models are now being refined to describe specific abnormalities of brain structure and function relevant to each of these anxiety disorders. Thus, neuroimaging studies of PTSD support a model of PTSD focusing on the amygdala and its relationship with the hippocampus and medial prefrontal cortex (particularly, anterior cingulate dysfunction). In SAD, exaggerated amygdala responsivity to human face stimuli is consistent with hypersensitivity in a specialized system for assessing a specific class of potentially threatening stimuli (i.e., threatening content within social cues). For PD, neuroimaging research has raised a wide range of possible neural substrates underlying this anxiety disorder. Regional abnormalities within the temporal lobe may reflect fundamental deficits in threat assessment similar to those found in SAD and PTSD. In particular, abnormalities in the parahippocampal gyrus have been identified in PD. However, more global abnormalities in homeostatic mechanisms related to lactate metabolism and vascular responses to carbon dioxide may prove specific to this anxiety disorder.
In summary, neuroimaging data are being used to formulate and test neuroanatomic models of anxiety disorders. Convergent data suggest many common neurocircuitry elements underlying models of PTSD, PD, and SAD, while reporting several important neuroanatomic distinctions between these disorders. The potential of neuroimaging to help delineate the pathophysiology of the anxiety disorders is now being realized. The combination of structural and functional imaging, along with neurochemical imaging techniques, are advancing the field toward achieving useful neurobiologic models to characterize the commonalities and distinctions among the anxiety disorders.
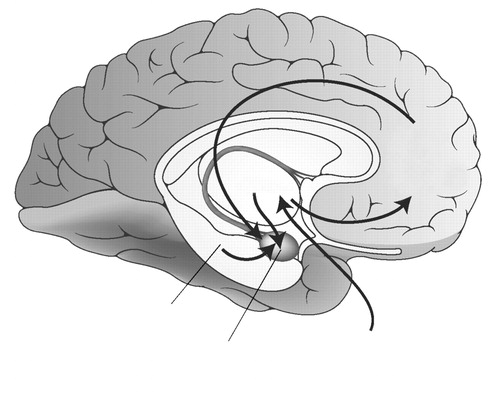
Figure 1. Schematized Diagram Indicating Critical Pathways in Threat Assessment and Responding, Relevant to Neurocircuitry Models in Post-traumatic Stress Disorder, Panic Disorder, and Social Anxiety Disorder
Papers of particular interest, published recently, have been highlighted as: •Of importance ••Of major importance
1 Davis M: The role of the amygdala in fear and anxiety. Ann Rev Neurosci 1992, 58(suppl):26–28.Google Scholar
2 LeDoux JE, Iwata J, Cicchetti P, et al.: Different projections of the central amygdaloid nucleus mediate autonomic and behavioral correlates of conditioned fear. J Neurosci 1988, 8:2517–2519.Crossref, Google Scholar
3 • Rauch SL, Whalen PJ, Curran T, et al.: Probing striato-thalamic function in OCD and TS using neuroimaging methods. In Tourette Syndrome. Edited by Cohen DJ, Jankovic J, Goetz CG. Philadelphia: Lippincott, Williams & Wilkins; 2000. A review of current neurocircuitry models of OCD based on neuroimaging findings.Google Scholar
4 Fyer AJ: Current approaches to etiology and pathophysiology of specific phobia. Biol Psychiatry 1998, 44:1295–1304.Crossref, Google Scholar
5 Rauch SL: Neuroimaging and the neurobiology of anxiety disorders. In Handbook of Affective Sciences. Edited by Davidson RJ, Scherer K, Goldsmith HH. New York: Oxford University Press; 2003:963–975.Google Scholar
6 LeDoux JE, Cicchetti P, Xagoraris A, et al.: The lateral amygdaloid nucleus: sensory interface of the amygdala in fear conditioning. J Neurosci 1990, 10:1062–1069.Crossref, Google Scholar
7 Aggleton JP: The Amygdala: Neurobiological Aspects of Emotion, Memory and Mental Dysfunction. New York: Wiley-Liss; 1992.Google Scholar
8 LeDoux JE: The Emotional Brain. New York: Simon and Schuster; 1996.Google Scholar
9 Jones DK, Simmons A, Williams SC, Horsfield MA: Noninvasive assessment of axonal fiber connectivity in the human brain via diffusion tensor MRI. Magn Reson Med 1999, 42:37–41.Crossref, Google Scholar
10 Sato T, Hasan K, Alexander AL, Minato K: Structural connectivity in white matter using the projected diffusion-tensor distance. Medinfo 2001, 10:929–932.Google Scholar
11 Makris N, Rauch SL, Kennedy DN: Diffusion imaging: Principles, methods, and applications. CNS Spectrums 2002, 7:486–546.Google Scholar
12 Rauch SL, Shin LM, Whalen PJ, Pitman RK: Neuroimaging and the neuroanatomy of PTSD. CNS Spectrums 1998, 3(suppl):30–41.Google Scholar
13 Whalen PJ, Bush G, McNally RJ, et al.: The Emotional Counting Stroop paradigm: an fMRI probe of the anterior cingulate affective division. Biol Psychiatry 1998, 44:1219–1228.Crossref, Google Scholar
14 Cameron HA, Hazel TG, McKay RD: Regulation of neurogenesis by growth factors and neurotransmitters. J Neurobiol 1998, 36:287–306.Crossref, Google Scholar
15 Sapolsky R: Glucocorticoids and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 2000, 57:825–835.Google Scholar
16 Bremner JD, Randall P, Scott TM, et al.: MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995, 152:973–981.Crossref, Google Scholar
17 Gurvits TV, Shenton ME, Hokama H, et al.: Magnetic resonance imaging study of hippocampal volume in chronic, combat-related posttraumatic stress disorder. Biol Psychiatry 1996, 40:1091–1099.Crossref, Google Scholar
18 De Bellis MD, Keshavan MS, Clark DB et al.: Developmental traumatology, part II: brain development. Biol Psychiatry 1999, 45:1271–1284.Crossref, Google Scholar
19 De Bellis MD, Hall J, Boring AM, et al.: A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry 2001, 50:305–309.Crossref, Google Scholar
20 Carrion VG, Weems CF, Eliez S, et al.: Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry 2001, 50:943–951.Crossref, Google Scholar
21 De Bellis MD, Keshavan MS, Frustaci K, et al.: Superior temporal gyrus volumes in maltreated children and adolescents with PTSD. Biol Psychiatry 2002, 51:544–552.Crossref, Google Scholar
22 Bremner JD, Randall P, Vermetten E, et al.: Magnetic resonance imaging-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse: a preliminary report. Biol Psychiatry 1997, 41:23–32.Crossref, Google Scholar
23 Stein MB, Koverola C, Hanna C, et al.: Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med 1997, 27:951–960.Crossref, Google Scholar
24 Driessen M, Herrmann J, Stahl K, et al.: Magnetic resonance imaging volumes of the hippocampus and amygdala in women with borderline personality disorder and early traumatization. Arch Gen Psychiatry 2000, 57:1115–1122.Crossref, Google Scholar
25 • Villarreal G, Hamilton DA, Petropoulos H, et al.: Reduced hippocampal volume and total white matter volume in posttraumatic stress disorder. Biol Psychiatry 2002:52:119–125. An mMRI study demonstrating reduced bilateral hippocampal volumes in patients with PTSD after correcting for intracranial volume.Crossref, Google Scholar
26 Bonne O, Brandes D, Gilboa A, et al.: Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry 2001, 158:1248–1251.Crossref, Google Scholar
27 • Gilbertson MW, Shenton ME, Ciszewski A, et al.: Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nat Neurosci 2002, 5:1242–1247. Results of this mMRI study of adult male monozygotic twins discordant for combat exposure indicate that smaller hippocampal volumes may represent a risk factor for developing PTSD, rather than evolving as a consequence of the trauma exposure.Crossref, Google Scholar
28 Rauch SL, Shin LM, Segal E, et al.: Selectively reduced regional cortical volumes in posttraumatic stress disorder. Neuroreport 2003, 14:913–916.Crossref, Google Scholar
29 Semple WE, Goyer P, McCormick R, et al.: Preliminary report: brain blood flow using PET in patients with posttraumatic stress disorder and substance-abuse histories. Biol Psychiatry 1993, 34:115–118.Crossref, Google Scholar
30 Rauch SL, van der Kolk BA, Fisler RE, et al.: A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry 1996, 53:380–387.Crossref, Google Scholar
31 Sachinvala N, Kling A, Suffin S, et al.: Increased regional cerebral perfusion by 99mTc hexamethyl propylene amine oxime single photon emission computed tomography in post-traumatic stress disorder. Mil Med 2000, 165:473–479.Crossref, Google Scholar
32 Semple WE, Goyer PF, McCormick R, et al.: Higher brain blood flow at amygdala and lower frontal cortex blood flow in PTSD patients with comorbid cocaine and alcohol abuse compared with normals. Psychiatry 2000, 63:65–74.Crossref, Google Scholar
33 Shin LM, Kosslyn SM, McNally RJ, et al.: Visual imagery and perception in posttraumatic stress disorder: a positron emission tomographic investigation. Arch Gen Psychiatry 1997, 54:233–241.Crossref, Google Scholar
34 Rauch SL, Whalen PJ, Shin LM, et al.: Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 2000, 47:769–776.Crossref, Google Scholar
35 Pissiota A, Frans O, Fernandez M, et al.: Neurofunctional correlates of posttraumatic stress disorder: a PET symptom provocation study. Eur Arch Psychiatry Clin Neurosci 2002, 252:68–75.Crossref, Google Scholar
36 Bremner JD, Narayan M, Staib LH, et al.: Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry 1999, 156:1787–1795.Google Scholar
37 Bremner JD, Staib LH, Kaloupek D, et al.: Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry 1999, 45:806–816.Crossref, Google Scholar
38 Shin LM, McNally RJ, Kosslyn SM, et al.: Regional cerebral blood flow during script-driven imagery in childhood sexual abuse-related posttraumatic stress disorder: a PET investigation. Am J Psychiatry 1999, 156:575–584.Google Scholar
39 • Shin LM, Whalen PJ, Pitman RK, et al.: An fMRI study of anterior cingulate function in posttraumatic stress disorder. Biol Psychiatry 2001, 50:932–942. Using a specific cognitive task known to recruit anterior cingulate, the investigators demonstrated abnormal anterior cingulate recruitment in the PTSD compared with the normal control group. This study demonstrates how cognitive tasks, which reliably recruit specific brain regions, can be used to test hypotheses regarding neurocircuitry in clinical anxiety samples.Crossref, Google Scholar
40 Liberzon I, Taylor SF, Amdur R, et al.: Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry 1999, 45:817–826.Crossref, Google Scholar
41 Whalen PJ, Bush G, McNally RJ, et al.: The Emotional Counting Stroop paradigm: an fMRI probe of the anterior cingulate affective division. Biol Psychiatry 1998, 44:1219–1228.Crossref, Google Scholar
42 Lanius RA, Williamson PC, Densmore M, et al.: Neural correlates of traumatic memories in posttraumatic stress disorder: a functional MRI investigation. Am J Psychiatry 2001, 158:1920–1922.Crossref, Google Scholar
43 Osuch EA, Benson B, Geraci M, et al.: Regional cerebral blood flow correlated with flashback intensity in patients with posttraumatic stress disorder. Biol Psychiatry 2001, 50:246–253.Crossref, Google Scholar
44 Lanius RA, Williamson PC, Boksman K, et al.: Brain activation during script-driven imagery induced dissociative responses in PTSD: a functional magnetic imaging investigation. Biol Psychiatry 2002, 52:305–311.Crossref, Google Scholar
45 Clark RC, McFarlane AC, Morris P, et al.: Cerebral function in posttraumatic stress disorder during verbal working memory updating: a positron emission tomography study. Biol Psychiatry 2003, 53:474–481.Crossref, Google Scholar
46 Fontaine R, Breton G, Dery R, et al.: Temporal lobe abnormalities in panic disorder: an MRI study. Biol Psychiatry 1990, 27:304–310.Crossref, Google Scholar
47 Vythilingam M, Anderson ER, Goddard A, et al.: Temporal lobe volume in panic disorder: a quantitative magnetic resonance imaging study. Psychiatry Res 2000, 99:75–82.Crossref, Google Scholar
48 • Massana G, Serra-Grabulosa JM, Salgado-Pineda P, et al.: Parahippocampal gray matter density in panic disorder: a voxel-based morphometric study. Am J Psychiatry 2003, 160:566–568. This recent morphometric study identified reduced parahippocampal gray matter density in patients with PD compared with healthy control individuals. This finding adds to the growing literature suggesting structural and functional parahippocampal-hippocampal abnormalities in PD.Crossref, Google Scholar
49 Reiman EM, Raichle ME, Robins E, et al.: The application of positron emission tomography to the study of panic disorder. Am J Psychiatry 1986, 143:469–477.Crossref, Google Scholar
50 Nordahl TE, Semple WE, Gross M, et al.: Cerebral glucose metabolic differences in patients with panic disorder. Neuropsychopharmacology 1990, 3:261–272.Google Scholar
51 Nordahl TE, Stein MB, Benkelfat C, et al.: Regional cerebral metabolic asymmetries replicated in an independent group of patients with panic disorder. Biol Psychiatry 1998, 44:998–1006.Crossref, Google Scholar
52 De Cristofaro MT, Sessarego A, Pupi A, et al.: Brain perfusion abnormalities in drug-naive, lactate-sensitive panic patients: a SPECT study. Biol Psychiatry 1993, 33:505–512.Crossref, Google Scholar
53 Bisaga A, Katz JL, Antonini A, et al.: Cerebral glucose metabolism in women with panic disorder. Am J Psychiatry 1998, 155:1178–1183.Crossref, Google Scholar
54 Boshuisen ML, Ter Horst GJ, Paans AMJ, et al.: rCBF differences between panic disorder patients and control subjects during anticipatory anxiety and rest. Biol Psychiatry 2002, 52:126–135.Crossref, Google Scholar
55 Stewart RS, Devous MD Sr, Rush AJ, et al.: Cerebral blood flow changes during sodium-lactate-induced panic attacks. Am J Psychiatry 1988, 145:442–449.Crossref, Google Scholar
56 Boles Ponto LL, Kathol RG, Kettelkamp R, et al.: Global cerebral blood flow after CO2 inhalation in normal subjects and patients with panic disorder determined with [15O]water and PET. J Anxiety Disorders 2002, 16:247–258.Crossref, Google Scholar
57 Woods SW, Koster K, Krystal JK, et al.: Yohimbine alters regional cerebral blood flow in panic disorder. Lancet 1988, 2:678.Crossref, Google Scholar
58 Reiman EM, Raichle ME, Robins E, et al.: Neuroanatomical correlates of a lactate-induced anxiety attack. Arch Gen Psychiatry 1989;46:493–500.Crossref, Google Scholar
59 Bystritsky A, Pontillo D, Powers M, et al.: Functional MRI changes during panic anticipation and imagery exposure. Neuroreport 2001, 12:3953–3957.Crossref, Google Scholar
60 Stein MB: Neurobiological perspectives on social phobia: from affiliation to zoology. Biol Psychiatry 1998, 44:1277–1285.Crossref, Google Scholar
61 Mathew SJ, Coplan JD, Gorman JM: Neurobiological mechanisms of social anxiety disorder. Am J Psychiatry 2001, 158:1558–1567.Crossref, Google Scholar
62 Potts NL, Davidson JR, Krishnan KR, Doraiswamy PM: Magnetic resonance imaging in social phobia. Psychiatry Res 1994, 52:35–42.Crossref, Google Scholar
63 Stein MB, Leslie WD: A brain SPECT study of generalized social phobia. Biol Psychiatry 1996, 39:825–828.Crossref, Google Scholar
64 Birbaumer N, Grodd W, Diedrich O, et al.: fMRI reveals amygdala activation to human faces in social phobics. Neuroreport 1998, 9:1223–1226.Crossref, Google Scholar
65 Schneider F, Weiss U, Kessler C, et al.: Subcortical correlates of differential classical conditioning of aversive emotional reactions in social phobia. Biol Psychiatry 1999, 45:863–871.Crossref, Google Scholar
66 Veit R, Flor H, Erb M, et al.: Brain circuits involved in emotional learning in antisocial behavior and social phobia in humans. Neurosci Let 2002, 328;233–236.Crossref, Google Scholar
67 Tillfors M, Furmark T, Marteinsdottir I, et al.: Cerebral blood flow in subjects with social phobia during stressful speaking tasks: a PET study. Am J Psychiatry 2001, 158:1220–1226.Crossref, Google Scholar
68 Van der Linden G, van Heerden B, Warwick J, et al.: Functional brain imaging and pharmacotherapy in social phobia: single photon emission computed tomography before and after treatment with the selective serotonin reuptake inhibitor citalopram. Prog Neuro-Psychopharmacol Biol Psychiat 2000, 24:419–438.Crossref, Google Scholar
69 • Furmark T, Tillfors M, Marteinsdottir I, et al.: Common changes in cerebral blood flow in patients with social phobia treated with citalopram or cognitive-behavioral therapy. Arch Gen Psychiatry 2002, 59:425–433. Results of this study suggest that heightened amygdala activity in response to the stress of public speaking can be reduced by treatment with a selective serotonin reuptake inhibitor or CBT. This suggests that modulation of the fear neurocircuitry as a result of successful treatment may not be dependent on treatment modality.Crossref, Google Scholar



