Schizophrenia Host Vulnerability and Risk of Metabolic Disturbances During Treatment with Antipsychotics
Abstract
People with schizophrenia die prematurely from comorbid physical diseases, particularly from cardiometabolic disturbances. Although some host vulnerability exists, there is also mounting evidence of a relationship between metabolic disturbances and antipsychotic medications. Clinicians must now make a careful appraisal of these risks when choosing an antipsychotic drug. Additionally, clinicians are required to undertake close monitoring for metabolic disturbances during antipsychotic therapy. Although switching antipsychotic medications is currently the preferred strategy if metabolic disturbances occur, there are other pharmacologic and nonpharmacologic approaches that might also prove beneficial for the individual patient. Metabolic disturbance and the detection and management thereof currently hold “center stage” in the psychopharmacology of schizophrenia.
Schizophrenia: A Life Shortening Illness
Above is the stark title of a seminal paper on schizophrenia—it “tells it all.” That patients with schizophrenia die prematurely is well known and antedates antipsychotic therapy (1–4). This is one of the well-replicated epidemiologic observations in schizophrenia. Although, of course, the higher rate of suicide in schizophrenia—most recently estimated to be 4.5% of patients—accounts substantially for this heightened morality, it is, by no means, the full story (5). Even before the advent of current antipsychotic medications, patients died prematurely, especially of cardiovascular diseases and respiratory effects of the medication, and, inevitably, there were sudden unexplained deaths (6). The latter were particularly apt to be attributed to antipsychotic medications, especially when used in high doses. This correlation was also fueled by studies showing the cardiotoxicity of antipsychotic drugs (7) as well as a high rate of respiratory deaths among patients receiving antipsychotic polypharmacy (8). Hence, the complexity of the current picture: patients with schizophrenia die prematurely, there seems to be something about the illness that contributes to this premature death, and there seems to be a heightened risk with antipsychotic therapy.
To put this information into even sharper relief, take the case of clozapine. Clozapine was initially hailed as a breakthrough (“life-saver”) drug for the most severely ill patients. Moreover, it was actually shown to reduce suicide in patients with schizophrenia (9). Now, it is associated with the greatest risk of metabolic disturbances, as well as with potentially fatal thromboembolism and myocarditis (10, 11). In a relatively short period of time, the profile of clozapine has changed substantially, as is also reflected by changes in practice and drug selection (12). In everyday practice we inevitably weigh the risk of illness with the risk-benefit profile of each drug as we undertake a commonplace, but yet inordinately complex, clinical decision-making process (13, 14) (Figure 1).
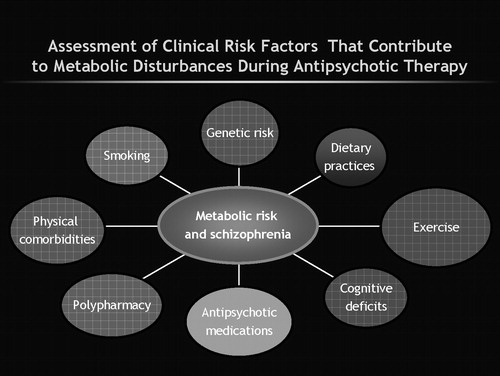
Figure 1. Complexity of Clinical Decision Making in Prescribing Antipsychotics for Schizophrenia
PREMATURE DEATH AND CARDIOVASCULAR RISK IN SCHIZOPHRENIA
Harris and Barraclough (1) provided an authoritative review of historical mortality studies in schizophrenia. Their conclusion is the same as that of Allebeck (6). Moreover, from this review emerges the fact that the heightened mortality is not just due to suicide nor is it “across the board,” but there is a particularly high risk of death from cardiovascular disorders. Two recent studies confirm this impression. Miller et al. (15) conducted an extensive evaluation of causes of death among inpatients in Ohio's public mental health facilities. They reported a disproportionately higher death rate from cardiovascular disorders. Colton and Manderscheid (16) have added fuel to the fire in reporting that patients in state facilities die at ages that are 25 years younger than the average age for the general population. This number goes well beyond the previously cited rate of 15 years less life for inpatients with schizophrenia (2). Not surprisingly, this recent finding has been a “call for arms” among policy makers. Also, this finding is not merely an American phenomenon, related to our society and the high prevalence of obesity and related conditions. In a British study, Osborn et al. (4) reported a rate of stroke that was 2.5 times higher in patients with schizophrenia. Another recent publication by Saha et al. (5) showed a similar pattern of premature death in other studies from several countries and reported overall an increasing trend in cardiovascular system deaths. The reasons for the heightened risk of cardiovascular and metabolic disturbances among patients with schizophrenia are complex, and they are not uniform across patients (13, 17–19). Hennekens et al. (18) described these clearly (Table 1).
 |
Table 1. Reasons for Heightened Risk of Cardiovascular Deaths in Patients with Schizophrenia
“IT IS ALL ABOUT THE MEDICINES—STUPID”
Although clinicians were well aware of the risk of agranulocytosis during clozapine therapy, the extent of weight gain and then reported diabetes mellitus during long-term therapy with clozapine caught practitioners in our field relatively unaware and has caused great concern. Allison et al. (20) showed that clozapine and other new antipsychotic medications were associated with weight gain. Henderson et al. (21) reported in their naturalistic follow-up study that one of four clozapine-treated patients had developed diabetes. There were also reports of episodes of pancreatitis and diabetic ketoacidosis—some fatal—early on in treatment with clozapine and now also with other second-generation antipsychotic (SGA) medications (22–24). Indeed, several pharmacoepidemiologic studies pointed to higher rates of diabetes mellitus in patients receiving risperidone, olanzapine, or quetiapine (13, 25–27). Ziprasidone and aripiprazole, as newer agents, appeared (at least at that time) to be less associated with this risk. The U.S. Food and Drug Administration (FDA) requested changes in the product labeling on each antipsychotic drug to reflect this risk (Table 2).
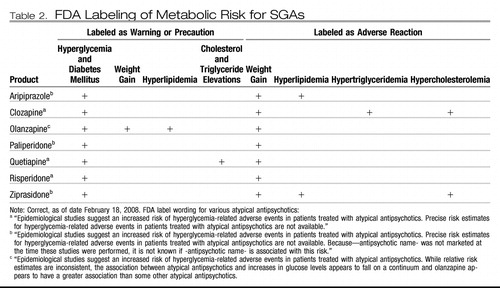 |
Table 2.
“A PERFECT STORM” —SCHIZOPHRENIA AND THE METABOLIC SYNDROME
It is important to recognize that the heightened risk of metabolic disturbance during antipsychotic therapy occurs in the context of an already existing national epidemic of obesity-related problems. In the general population, especially in the United States, there has been an alarming increase in obesity and raised triglyceride and cholesterol levels (19). The term “metabolic syndrome” (MS) has been given to this constellation of disturbances of fat, insulin, and cardiovascular integrity (Table 3). There are, however, differing definitions of MS in endocrinology and epidemiology research, and the reader is referred to an excellent recent review on MS (28).
 |
Table 3. Criteria for MS
However, irrespective of the definition used, it is already evident that MS is even more prevalent is patients with schizophrenia. McEvoy et al. (29) compared the baseline rate of MS in the seminal schizophrenia Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) and found that overall 40% of patients met the criteria for MS. The rate was higher in female than in male patients (51.6% versus 36.0%). Meyer et al. (30) found no clinical differences between those with and without MS. Goff et al. (31) indicated that this rate of MS was alarmingly high and likely to increase over time. In a Belgian study that applied similar diagnostic criteria for MS, DeHert et al. (32) reported a 26.8% rate. In another 1-year study similar to CATIE, but conducted early in the course of illness, Patel et al. (personal communication, 2008) reported a 13% rate of MS even in a first- episode of schizophrenia sample.
ATTRIBUTABLE RISK
Evidence that medications were (at least) a culprit is derived from several sources (Table 4). Newcomer (24) has provided an exhaustive review of this evidence. Nasrallah (38) provided a thoughtful synthesis of how, from a receptor-based profile, medications might contribute to the heightened risk for metabolic disturbances. However, it is not all about the medicines. In a thought-provoking study, Ryan et al. (39) reported elevated glucose levels and evidence of insulin resistance in first-episode schizophrenia patients who had never been exposed to antipsychotic medications (an important confounder is that these patients also had markedly raised cortisol levels). This finding has been confirmed. Kirkpatrick et al. (40) have offered an alternative explanation for this host vulnerability, citing evidence for widespread neurodevelopmental changes in schizophrenia that might leave the patient vulnerable to metabolic and adverse effects of medications. Mukherjee et al. (41) suggested that the host vulnerability for schizophrenia resided in a higher familial loading for diabetes. Fernandez-Egea et al. (42) have also recently replicated this finding. Moreover, Wright and Murray (43) reported a higher rate of autoantibodies associated with diabetes mellitus in patients with schizophrenia. Some have even questioned the impact of medications, citing flaws in studies that were largely retrospective in design. A recent systematic literature review by Bushe and Leonard (44) of prospective clinical trials found inconsistency in glucose levels over long-term trials. However, even this analysis of studies is incomplete and does not represent the entire breadth of studies—the majority of which at least point to some effect of medications in either causing unmasking and/or heightening the risk of metabolic disturbance.
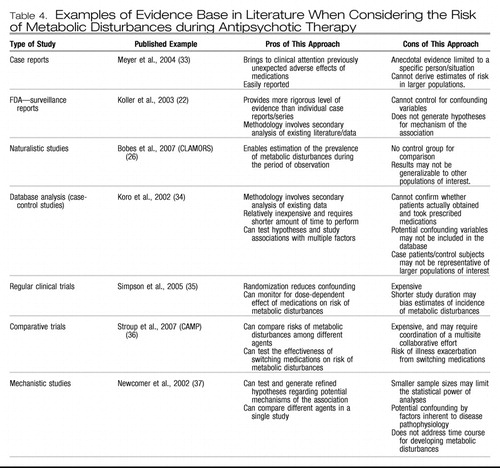 |
Table 4. Examples of Evidence Base in Literature When Considering the Risk of Metabolic Disturbances during Antipsychotic Therapy
And so the debate has raged on furiously as to the relative risk of metabolic disturbance with each of the SGAs (13). In CATIE, olanzapine was associated with the most weight gain and metabolic disturbance, whereas ziprasidone had the least (45). Aripiprazole was not included in the first two phases of CATIE. L'Italien and colleagues (46) presented data showing a greater likelihood of MS with olanzapine than with aripiprazole.
So what should I tell my patients? Well, that is a very complex problem. It does seem that the factors relevant to diabetes—e.g., smoking, family history of diabetes, and excessive weight gain—are also likely to be the contributory factors to metabolic disturbances alongside medications (17–19) (Figure 1). Nevertheless, it does also seem that antipsychotics can heighten the risk of metabolic disturbances (14, 24). It also seems that, at least on current incomplete and at times inconsistent evidence, the risk is not uniform across SGAs and some have a higher risk than others. The FDA-directed language for each of the agents is consistent with this nonuniformity (Table 1). Citrome and Stroup (47) offer an interesting way of considering this relative risk: number needed to harm (NNH). Ideally, the drug should have a large NNH. This is an indication that the risk of harm is low. Conversely, a drug should have a small number needed to treat (NNT). A low NNT indicates a robust clinical effect. In their analysis of the CATIE data, for example, Citrome and Stroup (47) found that olanzapine was the drug most likely to cause weight and metabolic disturbances in patients whereas, conversely, more patients could be treated with quetiapine before these effects were seen. Specifically, on the basis of CATIE data, they estimated that, for 100 patients treated for 18 months with either olanzapine or quetiapine, 18 more patients on olanzapine would complete treatment compared to those assigned to quetiapine. When they considered the data on weight gain, they estimated that 14 more patients receiving olanzapine would gain weight than with quetiapine. Of course, similar comparative metrics can be derived for each of the drugs. Although not without drawbacks, this is an interesting, evidence-based approach to comparing treatments (48).
WHAT ARE THE IMPLICATIONS FOR MY PRACTICE?
There are several up-front clinical implications. To effectively manage this problem a multifaceted approach is required (Figure 2). First, it is now incumbent upon clinicians to measure weight and metabolic parameters before starting antipsychotic treatment and also to reassess these regularly during treatment. This need emerges “loud and clear” from several guidelines (49–51). However, clinicians are slow to change practice habits and at least when looked at during 2001–2003, there was a low level of measuring for MS in practice (52, 53). It is likely, especially given the medicolegal specter now surrounding this topic, that the situation has changed. How best to implement widespread change in our clinical practice is also unclear (54). Henderson et al. (55) have shown that regular monitoring of hemoglobin A1C can aid in detection of diabetes during antipsychotic therapy.
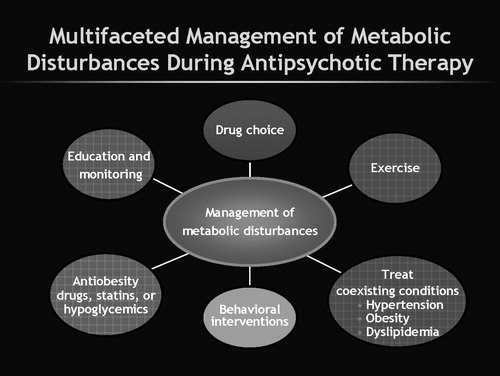
Figure 2. Multifaced Management of Metabolic Disturbances
Second, we need to encourage patients to improve their nutrition and to use available weight loss programs. There is evidence that these strategies work (56). Brar et al. (57) reported that 26.5% of patients receiving a (simple) behavior intervention lost 5% or more weight. This was in comparison with weight loss seen in 10.8% of patients in regular treatment when evaluated over a duration of 14 weeks. A recent Chinese 12-week study (58) tested a behavioral modification strategy and an antiobesity drug (metformin) in first-episode schizophrenia patients who had already gained weight from their antipsychotic therapy. Patients who got standard therapy alone (i.e., no intervention for weight gain) gained more weight, whereas significant reductions in weight and in measures of insulin functioning were seen in the other three groups, i.e., those who received some intervention, either pharmacologic, nonpharmacologic, or combined medication and behavioral treatments. The best results were seen in those patients who received both metformin and the lifestyle intervention.
Third, we need to develop more comprehensive and better integrated medical models for the long-term care of patients with serious mental illness (59). Collaborations with nursing and with primary care physicians offer advantages here (60, 61). Currently, medical management for patients with serious mental illness is below par (62–64). For example, in CATIE there was less than expected prescription of medications to address the extent of diabetes and hypertension that was reported in patients (62).
In terms of medication management, switching antipsychotics is probably the best option at present. Weiden (65) provided a review of this strategy and cited several studies showing benefits such as reduction in weight and metabolic disturbances when patients switch antipsychotics. It is also important to complete the switch as incomplete switches result in polypharmacy, which itself may be associated with a greater risk for diabetes (66). Another problem with the switching strategy is that it is unclear for any individual patient what benefit or risk the patient will actually incur until the switch is made (67). Additionally, current switch studies provide limited guidance as these studies are open labeled. A federally funded switch study, Comparing Antipsychotics for Metabolic Problems (CAMP), is currently underway (36). There are also a variety of add-on strategies that have been tried to reduce weight and metabolic disturbance. Recently, DeHert et al. (68) reported benefit from adding the lipid-lowering agent rosuvastatin in patients with schizophrenia. There is also interest in considering rimonabant, a cannabinoid-1 antagonist, which has been shown to be beneficial in obese, nonpsychiatric patients (69). However, this agent is still investigational, and it also seems to have increased incidence of psychiatric side effects. The use of a histamine-1 blocker has shown some benefit in patients with schizophrenia (70). Topiramate has also been used (71). There was even interest in considering fluoxetine as an add-on to help with weight gain during olanzapine therapy (72). It should be noted, however, that even in nonpsychiatric obese populations, the evidence that these agents are helpful is mixed at best (738). Moreover, the evidence for real benefit in patients with schizophrenia is even more sparse.
CONCLUDING REMARKS
The physical health of patients with schizophrenia is of great concern and requires careful and judicious treatment. It also spurs on the search for antipsychotic medications that might have a lesser metabolic side effect burden (74, 75). There is also some work examining predictors of risk for weight and metabolic disturbance. Ellingrod et al. (76) reported on a pharmacogenetic analysis suggesting that an allele of methylenetetrahydrofolate reductase might predict susceptibility for MS in patients with schizophrenia. Kaddurah-Daouk et al. (77) have shown how metabolomics—a new application of genetics and informatics—can assist in predicting risk. In the meantime, while we are waiting for such research approaches to be confirmed in later studies and mature out into practice, treatment decisions will need to be individualized, and clinicians will need to continue to rely on their judgment, careful assessment, and continued learning from the literature as they engage in complex decision making (13).
1 Harris EC, Barraclough B: Excess mortality of mental disorder. Br J Psychiatry 1998; 173:11–53Crossref, Google Scholar
2 Newman SC, Bland RC: Mortality in a cohort of patients with schizophrenia: a record linkage study. Can J Psychiatry. 1991; 36:239–245Crossref, Google Scholar
3 Osby U, Correia N, Brandt L, Ekbom A, Sparén P: Mortality and causes of death in schizophrenia in Stockholm County, Sweden. Schizophr Res 2000; 45:21–28Crossref, Google Scholar
4 Osborn DPJ, Levy G, Nazareth I, Petersen I, Islam A, King MB: Relative risk of cardiovascular and cancer mortality in patients with severe mental illness from the United Kingdom's general practice research database. Arch Gen Psychiatry 2007; 64:242–249Crossref, Google Scholar
5 Saha S, Chant D, McGrath J: A systematic review of mortality in schizophrenia: is the differential mortality gap worsening over time? Arch Gen Psychiatry 2007; 64:1123–1131Crossref, Google Scholar
6 Allebeck P: Schizophrenia: a life-shortening disease. Schizophr Bull 1989; 15:81–89Crossref, Google Scholar
7 Reilly JG, Ayis SA, Ferrier IN, Jones SJ, Thomas SH: QTc-interval abnormalities and psychotropic drug therapy in psychiatric patients. Lancet 2000; 355:1048–1052Crossref, Google Scholar
8 Waddington JL, Youssef HA, Kinsella A: Mortality in schizophrenia: antipsychotic polypharmacy and absence of adjunctive anticholinergics over the course of a 10-year prospective study. Br J Psychiatry 1998; 173:325–329Crossref, Google Scholar
9 Meltzer HY, Alphs L, Green AI, Altamura AC, Anand R, Bertoldi A, Bourgeois M, Chouinard G, Islam MZ, Kane J, Krishnan R, Lindenmayer JP, Potkin S (International Suicide Prevention Trial Study Group): Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Arch Gen Psychiatry 2003; 60:82–91Crossref, Google Scholar
10 Coodin S, Ballegeer T: Clozapine therapy and pulmonary embolism. Can J Psychiatry 2000; 45:395Google Scholar
11 Annamraju S, Sheitman B, Saik S, Stephenson A: Early recognition of clozapine-induced myocarditis. J Clin Psychopharmacol 2007; 27:479–483Crossref, Google Scholar
12 Moore TA, Buchanan RW, Buckley PF, Chiles JA, Conley RR, Crismon ML, Essock SM, Finnerty M, Marder SR, Miller del D, McEvoy JP, Robison DG, Schooler NR, Shon SP, Stroup TS, Miller AL: The Texas Medication Algorithm Project antipsychotic algorithm for schizophrenia: 2006 update. J Clin Psychiatry 2007; 68:1751–1762Crossref, Google Scholar
13 Newcomer JW: Antipsychotic medications: metabolic and cardiovascular risk. J Clin Psychiatry 2007; 68:(suppl 4)8–13Google Scholar
14 Citrome L: The effectiveness criterion: balancing efficacy against the risks of weight. J Clin Psychiatry 2007; 68:(suppl 12)12–17Google Scholar
15 Miller BJ, Paschall CB III, Svendsen DP: Mortality and medical comorbidity among patients with serious mental illness. Psychiatry Serv 2006; 57:1482–1487Crossref, Google Scholar
16 Colton CW, Manderscheid RW: Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis 2006; 3:A42Google Scholar
17 McCreadie RG: Diet, smoking and cardiovascular risk in people with schizophrenia: descriptive study. Br J Psychiatry 2003; 183:534–539Crossref, Google Scholar
18 Daumit GL, Goldberg RW, Anthony C, Dickerson F, Brown CH, Kreyenbuhl J, Wohlheifer K, Dixon L: Physical activity patterns in adults with severe mental illness. J Nerv Ment Dis 2005; 193:641–646Crossref, Google Scholar
19 Hennekens CH, Hennekens AR, Hollar D, Casey DE: Schizophrenia and increased risks of cardiovascular disease. Am Heart J 2005; 150:1115–1121Crossref, Google Scholar
20 Allison DB, Mentore JL, Heo M, Chandler LP, Cappelleri JC, Infante MC, Weiden PJ: Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999; 15:1686–1696Google Scholar
21 Henderson DC, Cagliero E, Gray C, Nasrallah RA, Hayden DL, Schoenfeld DA, Goff DC: Clozapine, diabetes mellitus, and weight gain, and lipid abnormalities: a five-year naturalistic study. Am J Psychiatry 2000; 157:975–981Crossref, Google Scholar
22 Koller EA, Cross JT, Doraiswamy PM, Malozowski SN: Pancreatitis associated with atypical antipsychotics: from the Food and Drug Administration's MedWatch surveillance system and published reports. Pharmacotherapy 2003; 23:1123–1130Crossref, Google Scholar
23 Koller EA, Weber J, Doraiswamy PM, Schneider BS: A survey of reports of quetiapine-associated hyperglycemia and diabetes mellitus. J Clin Psychiatry 2004; 65:857–863Crossref, Google Scholar
24 Newcomer JW: Second-generation (atypical) antipsychotics and metabolic effects: a comprehensive literature review. CNS Drugs 2005; 19:(suppl 1)1–932005Google Scholar
25 Sernyak MJ, Leslie DL, Alarcon RD, Losonczy MF, Rosenheck R: Association of diabetes mellitus wit use of atypical neuroleptics in the treatment of schizophrenia. Am J Psychiatry 2002; 159:561–566Crossref, Google Scholar
26 Bobes J, Arango C, Aranda P, Carmena R, Garcia-Garcia M, Rejass J, CLAMORS Study Collaborative Group: Cardiovascular and metabolic risk in outpatients with schizophrenia treated with antipsychotics: results of the CLAMORS study. Schizophr Res 2007; 90:162–173Crossref, Google Scholar
27 Lambert BL, Chang KY, Tafesse E, Carson W: Association between antipsychotic treatment and hyperlipidemia among California Medicaid patients with schizophrenia. J Clin Psycopharmacol 2005; 25:12–18Crossref, Google Scholar
28 Pi Sunyer FX: Metabolic syndrome. Med Clin North Am 2007; 91:1020–1040Crossref, Google Scholar
29 McEvoy JP, Meyer JM, Goff Dc, Nasrallah HA, Davis SM, Sullivan L, Meltzer HY, Hsiao J, Scott Stroup T, Lieberman JA: Prevalence of the metabolic syndrome in patients with schizophrenia: baseline results from the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial and comparison with national estimates from NHANES III. Schizophr Res 2005; 80:19–32Crossref, Google Scholar
30 Meyer JM, Nasrallah HA, McEvoy JP, Goff DC, Davis SM, Chakos M, Patel J, Keefe R, Stroup T, Lieberman J: The Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) schizophrenia trial: clinical comparison of subgroups wit and without the metabolic syndrome. Schizphr Res. 2005; 80:9–18Crossref, Google Scholar
31 Goff DC, Sullivan LM, McEvoy JP, Meyer JM, Nasrallah HA, Daumit GL, Lamberti S, D'Agostino RB, Stroup TS, Davis S, Lieberman JA: A comparison of ten-year cardiac risk estimates in schizophrenia patient study and matched controls. Schizophr Res 2005; 80:45–53Crossref, Google Scholar
32 DeHert MA, van Winkel R, Van Eyck D, Hanssens L, Wampers M, Scheen A, Peuskens J: Prevalence of the metabolic syndrome in patients with schizophrenia treated with antipsychotic medication. Schizophr Res 2006; 81:87–93Crossref, Google Scholar
33 Meyer JM, Leckband SG, Loh C, Moutier CY: Quetiapine-induced diabetes with metabolic acidosis. Int Clin Psychopharmacol 2004; 19:169–171Crossref, Google Scholar
34 Koro CE, Fedder DO, L'Italien GJ, Weiss S, Magder LS, Kreyenbuhl J, Revicki D, Buchanan RW: An assessment of the independent effects of olanzapine and risperidone exposure on the risk of hyperlipidemia in schizophrenic patients. Arch Gen Psychiatry 2002; 59:1021–1026Crossref, Google Scholar
35 Simpson GM, Weiden P, Pigott T, Murray S, Siu CO, Romano SJ: Six-month, blinded, multicenter continuation study of ziprasidone versus olanzapine in schizophrenia. Am J Psychiatry 2005; 162:1535–1538Crossref, Google Scholar
36 Stroup TS, McEvoy JP, Swartz MS, Hamer RM, Perkins DO, Lieberman JA: Comparison of antipsychotics for metabolic problems (CAMP): a NIMH schizophrenia trials network study. Clin Schizophr Relat Psychoses 2007; 4:69Google Scholar
37 Newcomer JW, Haupt DW, Fucetola R, Melson AK, Schweiger JA, Cooper BP, Selke G: Abnormalities in glucose regulation during antipsychotic treatment of schizophrenia. Arch Gen Psychiatry 2002; 59:337–345Crossref, Google Scholar
38 Nasrallah HA: Atypical antipsychotic-induced metabolic side effects: insights from receptor-binding profiles. Mol Psychiatry 2008; 13:27–35Crossref, Google Scholar
39 Ryan MC, Collins P, Thakore JH: Impaired fasting glucose tolerance in first-episode, drug-naïve patients with schizophrenia. Am J Psychiatry 2003; 160:284–289Crossref, Google Scholar
40 Kirkpatrick B, Messias E, Harvey PD, Fernandez-Egea E, Bowie CR: Is schizophrenia a syndrome of accelerated aging? Schizophr Bull 2007;Google Scholar
41 Mukherjee S, Schnur DB, Reddy R: Family history of type 2 diabetes in schizophrenia patients. Lancet 1989; 1:495Google Scholar
42 Fernandez-Egea E, Miller B, Bernardo M, Donner T, Kirkpatrick B: Parental history of type 2 diabetes in patients with nonaffective psychosis. Schizophr Res 2008; 98:302–306Crossref, Google Scholar
43 Wright P, Murray RM: Prenatal influenza, immunogenes and schizophrenia: a hypothesis and some recent findings, in The Neurodevelopmental Basis of Schizophrenia. Edited by Waddington JL, Buckley PF. Austin, TX, R.G. Landes Company, 1996Google Scholar
44 Bushe CJ, Leonard BE: Blood glucose and schizophrenia: a systematic review of prospective randomized clinical trials. J Clin Psychiatry 2007; 68:1682–1690Crossref, Google Scholar
45 Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, Keefe RS, Davis SM, Davis CE, Lebowitz BD, Severe J, Hsiao JK, Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Investigators: Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005; 353:1209–1223Crossref, Google Scholar
46 L'Italien GJ, Casey DE, Kan HJ, Carson WH, Marcus RN: Comparison of metabolic syndrome incidence among schizophrenia patents aripiprazole versus olanzapine or placebo. J Clin Psychiatry 2007; 68:1510–1516Crossref, Google Scholar
47 Citrome L, Stroup S: Schizophrenia, Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) and number needed to treat: how can CATIE inform clinicians? Int J Clin Pract. 2006; 60:933–940Crossref, Google Scholar
48 Smeeth C, Haines A, Elrahim S: Numbers need to treat derived from meta-analysis—sometimes informative, usually misleading. BMJ 1999; 318:1548–1551Crossref, Google Scholar
49 American Diabetes Association, American Psychiatric Association, American Association of Clinical Endocrinologists, North American Association for the Study of Obesity: Consensus development conference on antipsychotic drugs and obesity and diabetes. Diabetes Care 2004; 27:596–601Crossref, Google Scholar
50 Marder SR, Essock SM, Miller AL, Buchanan RW, Casey DE, Davis JM, Kane JM, Lieberman JA, Schooler NR, Covell N, Stroup S, Weissman EM, Wirshing DA, Hall CS, Pogach L, Pi-Sunyer X, Bigger JT Jr, Friedman A, Kleinberg D, Yevich SJ, Davis B, Shon S: Physical health monitoring of patients with schizophrenia. Am J Psychiatry 2004; 161:1334–1349Crossref, Google Scholar
51 Goff DC, Cather C, Evins AE, Henderson DC, Freudenreich O, Copeland PM, Bierer M, Duckworth K, Sacks FM: Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry 2005; 66:183–194Crossref, Google Scholar
52 Newcomer JW, Nasrallah HA, Loebel AD: The Atypical Antipsychotic Therapy and Metabolic Issues National Survey: practice patterns and knowledge of psychiatrists. J Clin Psychopharmacol 2004; 23:(5 suppl 1)S1–S6Google Scholar
53 Buckley PF, Miller DD, Singer B, Arena J, Stirewalt EM: Clinicians' recognition of the metabolic adverse effects of antipsychotic medications. Schizophr Res 2005; 79:281–288Crossref, Google Scholar
54 Shojania KG, Ranji SR, McDonald KM, Grimshaw JM, Sundaram V, Rushakoff RJ, Owens DK: Effects of quality improvement strategies for type 2 diabetes on glycemic control: a meta-regression analysis. JAMA 2006; 296:427–440Crossref, Google Scholar
55 Henderson DC, Cagliero E, Copeland PM, Louie PM, Borba CP, Fan X, Freudenreich O, Goff DC: Elevated hemoglobin A1c as a possible indicator of diabetes mellitus and diabetic ketoacidosis in schizophrenia patients receiving atypical antipsychotics. J Clin Psychiatry 2007; 68:533–541Crossref, Google Scholar
56 Strassnig M, Ganguli R: Weight loss interventions for patients with schizophrenia. Clin Schizophr Relat Psychoses 2007; 4:443Google Scholar
57 Brar JS, Ganguli R, Pandina G, Turkoz I, Berry S, Mahmoud R: Effects of behavioral therapy on weight loss in overweight and obese patients with schizophrenia or schizoaffective disorder. J Clin Psychiatry 2005; 66:205–212Crossref, Google Scholar
58 Wu RR, Zhao JP, Jin H,Shao P, Fang MS, Guo XF, He YQ, Liu YJ, Chen JD, Li LH: Lifestyle intervention and metformin for treatment of antipsychotic-induced weight gain. JAMA 2008; 299:185–193Google Scholar
59 Unützer J, Schoenbaum M, Druss BG, Katon WJ: Transforming mental health care at the interface with general medicine: report for the president's commission. Psychiatr Serv 2006; 57:37–47Crossref, Google Scholar
60 Manderscheid RW, Masi D, Rossignol CR, Masi DA: The integration of physical health and behavioral health services: three university case examples. Arch Psychiatr Nurs 2007; 21:141–149Crossref, Google Scholar
61 Dixon LB, Adler DA, Berlant JL, Dulit RA, Goldman, Hackman AL, Oslin DW, Siris SG, Sonis WA, Valenstein M: Psychiatrists and primary caring: what are our boundaries of responsibility? Psychiatr Serv 2007; 58:600–602Crossref, Google Scholar
62 Nasrallah HA, Meyer JM, Goff DC, McEvoy JP, Davis SM, Stroup TS, Lieberman JA: Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res 2006; 86:15–22Crossref, Google Scholar
63 Frayne SM, Halanych JH, Miller DR, Wang F, Lin H, Pogach L, Sharkansky EJ, Keane TM, Skinner KM, Rosen CS, Berlowotz DR: Disparities in diabetes care: impact of mental illness. Arch Intern Med 2005; 165:2631–2638Crossref, Google Scholar
64 Druss BG, Bradford WD, Rosenheck RA, Radford MJ, Krumholz HM: Quality of medical care and excess mortality in older patients with mental disorders. Arch Gen Psychiatry 2001; 58:565–572Crossref, Google Scholar
65 Weiden PJ: Switching antipsychotics as a treatment strategy for antipsychotic-induced weight gain and dyslipidemia. J Clin Psychiatry 2007; 6834–39Google Scholar
66 Correll CU, Frederickson AM, Kane JM, Manu P: Does antipsychotic polypharmacy increase the risk for metabolic syndrome? Schizophr Res 2007; 89:91–100Crossref, Google Scholar
67 Weiden PJ, Buckley PF: Reducing the burden of side effects during long term antipsychotic therapy: the role of switching medications. J Clin Psychiatry 2007; 68:14–23Crossref, Google Scholar
68 DeHert M, Kalnicka D, van Winkel R, Wampers M, Hanssens L, Van Eyck D, Scheen A, Peuskens J: Treatment with rosuvastatin for severe dyslipidemia in patients with schizophrenia and schizoaffective disorder. J Clin Psychiatry 2006; 67:1889–1896Crossref, Google Scholar
69 Pi-Sunyer FX, Aronne LJ, Heshmati HM, Devin J, Rosenstock J, RIO-North America Study Group: Effect of rimonabant, a cannabinoid-1 receptor blocker, on weight and cardiometabolic risk factors in overweight or obese patients: RIO-North America: a randomized controlled trial. JAMA 2006; 295:761–775Crossref, Google Scholar
70 Sacchetti E, Guarneri L: Bravi D: H antagonist nizatidine may control olanzapine-associated weight gain in schizophrenic patients. Biol Psychiatry 2000; 48:167–168Crossref, Google Scholar
71 Ko YH, Joe SH, Jung I, Kim SH: Topiramate as an adjuvant treatment with atypical antipsychotics in schizophrenic patients experiencing weight gain. Clin Neuropharmacol 2005; 28:169–175Crossref, Google Scholar
72 Bustillo JR, Lauriello J, Parker K, Hammond R, Rowland L, Bogenschutz M, Keith S: Treatment of weight gain with fluoxetine in olanzapine-treated schizophrenic patients. Neuropsychopharmacology 2003; 28:527–529Crossref, Google Scholar
73 Rucker D, Padwal R, Li SK, Curioni C, Lau DC: Long term pharmacotherapy for obesity and overweight: updated metaanalysis. BMJ 2007; 335:1194–1199Crossref, Google Scholar
74 Patil ST, Zhang L, Martenyl IF, Lowe SL, Jackson KA, Andreev BV, Avedisova AS, Bardenstein LM, Gurovich IY, Morozova MA, Mosolov SN, Neznanov NG, Reznik AM, Smulevich AB, Tochilov VA, Johnson BG, Monn JA, Schoepp DD: Activation of mGlu 2/3 receptors as a new approach to treat schizophrenia: a randomized phase 2 clinical trial. Nat Med 2007; 13:1102–1107Crossref, Google Scholar
75 Newman-Teacredi A, Depoortere R: Neuropharmacological profile of bifeprunox; merits and limitations in comparison with other third-generation antipsychotics. Curr Opin Investig Drugs 2007; 8:539–554Google Scholar
76 Ellingrod VL, Miller del D, Taylor SF, Moline J, Holman T, Kerr J: Metabolic syndrome and insulin resistance in schizophrenia patients receiving antipsychotics genotyped for the methylenetratrahydrofolate reductase (MTHFR) 677C/T and 1298 A/C variants. Schizophr Res 2008; 98:47–54Crossref, Google Scholar
77 Kaddurah-Daouk R, McEvoy J, Baillie RA, Lee D, Yao JK, Doraiswamy PM, Krishnan KR: Metabolomic mapping of atypical antipsychotic effects in schizophrenia. Mol Psychiatry 2007; 12:934–945Crossref, Google Scholar



