Lithium Plus Valproate Combination Therapy Versus Monotherapy for Relapse Prevention in Bipolar I Disorder (BALANCE): A Randomised Open-Label Trial
Abstract
Background: Lithium carbonate and valproate semisodium are both recommended as monotherapy for prevention of relapse in bipolar disorder, but are not individually fully effective in many patients. If combination therapy with both agents is better than monotherapy, many relapses and consequent disability could be avoided. We aimed to establish whether lithium plus valproate was better than monotherapy with either drug alone for relapse prevention in bipolar I disorder. Methods: 330 patients aged 16 years and older with bipolar I disorder from 41 sites in the UK, France, USA, and Italy were randomly allocated to open-label lithium monotherapy (plasma concentration 0.4–1.0 mmol/L, n=110), valproate monotherapy (750–1250 mg, n=110), or both agents in combination (n=110), after an active run-in of 4–8 weeks on the combination. Randomisation was by computer program, and investigators and participants were informed of treatment allocation. All outcome events were considered by the trial management team, who were masked to treatment assignment. Participants were followed up for up to 24 months. The primary outcome was initiation of new intervention for an emergent mood episode, which was compared between groups by Cox regression. Analysis was by intention to treat. This study is registered, number ISRCTN 55261332. Findings: 59 (54%) of 110 people in the combination therapy group, 65 (59%) of 110 in the lithium group, and 76 (69%) of 110 in the valproate group had a primary outcome event during follow-up. Hazard ratios for the primary outcome were 0.59 (95% CI 0.42–0.83, p=0.0023) for combination therapy versus valproate, 0.82 (0.58–1.17, p=0.27) for combination therapy versus lithium, and 0.71 (0.51–1.00, p=0.0472) for lithium versus valproate. 16 participants had serious adverse events after randomisation: seven receiving valproate monotherapy (three deaths); five lithium monotherapy (two deaths); and four combination therapy (one death). Interpretation: For people with bipolar I disorder, for whom long-term therapy is clinically indicated, both combination therapy with lithium plus valproate and lithium monotherapy are more likely to prevent relapse than is valproate monotherapy. This benefit seems to be irrespective of baseline severity of illness and is maintained for up to 2 years. BALANCE could neither reliably confirm nor refute a benefit of combination therapy compared with lithium monotherapy. Funding: Stanley Medical Research Institute; Sanofi-Aventis.
(Reprinted with permission from Lancet 2010; 375:385–395)
Introduction
Bipolar disorder is a disabling mental illness that is characterised by episodes of both elevated or irritable mood and depression (1). Although acute episodes can be succeeded by a period of remission, most patients have a recurrent or chronic illness, making bipolar disorder one of the most important causes of disability at ages 15–44 years (2). Lithium carbonate was the standard maintenance treatment for more than four decades and reduces risk of relapse and suicide, but it is not helpful for all patients (3–8). It has a narrow therapeutic index and can cause adverse effects that some patients cannot tolerate, or can lead to suboptimum adherence. These limitations stimulated the search for alternative long-term treatments for bipolar disorder. Anticonvulsant and second-generation antipsychotic drugs have increasingly been proposed as alternatives, although their long-term safety and efficacy compared with lithium remains uncertain (4, 8).
Notwithstanding the scarcity of good comparative evidence, major shifts away from prescription of lithium have occurred, especially in North America (9–12). One widely used agent is sodium valproate, which is an effective antimanic agent and is probably effective in relapse prevention (13,14). In the USA, prescription of lithium for outpatients nearly halved between 1992 and 1996, and 1996 and 1999, whereas the rate of prescription of valproate almost tripled (12).
Many patients do not respond to monotherapy, and combinations of drugs are often recommended despite little evidence (4,5,15). Lithium plus valproate is often recommended after failure of first-line monotherapy (3–5). Should this combination have additive pharmacological effects and prove better than monotherapy, it could be an appropriate first-line therapy (16–18). We report here on BALANCE (Bipolar Affective disorder: Lithium/ANti-Convulsant Evaluation), a randomised trial that was designed to establish whether lithium plus valproate semisodium is better than monotherapy with either drug alone for prevention of relapse in bipolar I disorder.
Methods
Study design and participants
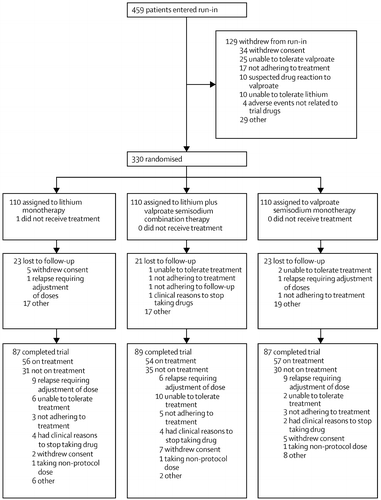
BALANCE was a randomised, open-label, three-group trial of maintenance therapy, with up to 24 months of follow-up (19–21). Patients initially entered an active run-in phase to confine randomisation to those who tolerated both drugs in the short term and were likely to take the randomly allocated study treatment for 2 years (22). The run-in was usually 4–8 weeks, but could be extended if clinically required. Recruitment took place at 41 sites in the UK, USA, Italy, and France between May 31, 2001, and Feb 22, 2007. The protocol was approved by the appropriate institutional review boards and ethics committees. Men and women aged 16 years and older, with a clinical diagnosis of bipolar I disorder (on the basis of a previous episode of mania meeting Diagnostic and Statistical Manual-IV criteria (23) were eligible for entry into the run-in provided that five further criteria were met: (1) written informed consent was given; (2) the patient was not having an acute episode, and long-term drug therapy was clinically indicated to prevent relapse; (3) combination therapy with lithium plus valproate was considered clinically reasonable for the patient by the clinician; (4) there was no medical disorder or condition contraindicating either of the investigational drugs (eg, pregnancy); and (5) the patient was normally resident in one country and had a residential address.
Participants were randomly assigned if: (1) no clear treatment preference for either investigational drug was apparent; (2) lithium plasma concentration was between 0.4 and 1.0 mmol/L; (3) valproate dose was at least 750 mg, or valproic acid serum concentration was at least 50 μg/mL; (4) the combination of lithium and valproate was tolerated at trial doses; and (5) adherence during the run-in phase was judged by the investigator to be at least 70%.
Randomisation and masking
The computerised randomisation program included a minimisation algorithm to ensure balanced allocation of participants across the intervention groups for six prognostic factors: (1) nature of most recent episode (mania or non-mania); (2) number of previous psychiatric admissions (<two or ≥two); (3) previous maintenance treatment (yes or no); (4) age (<35 years, or ≥35 years); (5) sex; and (6) region. Treatment allocation was via the 24-h telephone service at the Clinical Trial Service Unit, University of Oxford, UK. Investigators telephoned the service and logged the patient as eligible for randomisation. The investigator was then informed of the treatment allocation. Investigators and participants were aware of treatment allocation because of the complexities of masking of lithium therapy and the concern that concealment would restrict participation and generalisability (21). The consequent risk of performance and ascertainment biases was managed by restriction of randomisation to patients for whom there was no strong treatment preference on the part of the patient or clinician and by careful verification of outcomes. All outcome events were considered by the trial management team, who were masked to treatment assignment. In the case of any doubt, a description of the event was sent to an independent adjudicator.
Procedures
During the run-in, patients received 4–8 weeks of treatment with both lithium carbonate (Priadel, Sanofi-Aventis, Quetigny, France; Téralithe, Sanofi-Aventis, France; US patients received generic drug, manufactured by several drugmakers), at doses producing a serum concentration of 0.4–1.0 mmol/L, and valproate semisodium (Depakote, Sanofi-Aventis, Riells, Spain; Depakote, Abbott Laboratories, Abbott Park, IL, USA), at least 750 mg per day, with a target daily dose of 1250 mg or the highest dose tolerated. For participants starting one or both treatments, drugs were titrated to the target dose during 4 weeks. Participants taking doses of valproate that were higher than the target dose at study entry could continue at the same dose during the trial. Low doses of valproate were allowed if needed for tolerability and if the serum concentration was at least 50 μg/mL before randomisation. BALANCE was designed to be as straightforward as possible, with patients needing no appointments in addition to those for routine clinical practice.
After run-in, participants were randomly allocated to one of three groups: (1) combination therapy with lithium and valproate; (2) lithium monotherapy (valproate withdrawn and lithium continued); and (3) valproate monotherapy (lithium withdrawn, valproate continued). Allocated drugs were continued at the dose established during the run-in. In monotherapy groups, the discontinued drug was withdrawn over 4 weeks to reduce risk of relapse associated with abrupt discontinuation (24). Doses of the investigational drug could be increased if the serum concentration fell below the minimum threshold (measurement of serum concentrations after randomisation was at the discretion of the investigator) and decreased (within the trial ranges) if side-effects became troublesome. Participants remained on the allocated treatment for 2 years or until treatment failure. Non-investigational co-therapies could be continued during the trial.
The primary outcome was time to new intervention for an emerging mood episode, including drug treatment (commencement of a new drug, increase in dose of concurrent drug, restarting of a discontinued drug, or increasing the investigational drug dose in response to an emergent mood episode) or admission to hospital. Secondary outcomes were time to new intervention for an emerging depressive episode, time to new intervention for an emerging manic episode (including mixed and cycling), global assessment of functioning (25), episodes of deliberate self-harm, quality of life according to the EuroQol (EQ-5D) questionnaire (26), adverse events including both emergent serious adverse events and participant-reported adverse effects, withdrawal from study treatment, and adherence to study treatment estimated by investigator (27).
Statistical analysis
231 participants were needed to give 90% power at two-sided 5% significance level to detect a 40% relative reduction in the hazard from an expected value of 70% (6), on the assumption of a 20% drop-out rate from allocated treatment. A secondary objective was to establish whether study treatments had differential effects on prevention of depressive and manic episodes. To detect a 40% reduction in hazard ratio for a depressive episode needing new treatment with 80% power at 5% significance level needed 115 participants per group, with a sample size of 345.
Analysis was by intention to treat, and followed a detailed, prespecified plan. Time to first event during the scheduled follow-up was compared between the three groups. Follow-up was censored at the last available assessment in patients who were lost to follow-up without having an event. Time from randomisation to event was summarised by Kaplan-Meier curves, and compared with the log rank test. Hazard ratios (HRs) with 95% CIs were calculated with Cox's regression to estimate size of the treatment effect. The proportional hazards assumption was tested formally with analysis of Schoenfeld residuals. An analysis adjusting for minimisation factors was also done. Because BALANCE had very specific hypotheses and only one outcome of primary importance, we made no formal adjustment for multiple significance testing. We used Stata (version 10) for all power calculations and analyses.
Protocol changes
The protocol was approved for the UK by the South-West Multicentre Research Ethics Committee on April 4, 2001. In July, 2003, the protocol was changed to require blood samples at randomisation only. In October, 2003, an end-of-trial questionnaire was added to ask about participants' experiences. In August, 2005, there was a change in primary outcome and, consequently, sample size. Initially, the primary outcome was admission to hospital and the planned sample size was 1068 participants. During the early course of the trial, time to intervention became established as the primary outcome of choice in long-term trials in bipolar disorder. Change to this clinically meaningful primary outcome led to a revision of the planned sample size from 1068 to 345. All protocol changes were approved by the ethics committee, data monitoring and ethics committee, and the trial steering committee. After implementation of the EU Directive on Clinical Trials in 2004, the initial nominal academic principal investigators were replaced with principal investigators who met European Union Directive 2001/20/EC criteria.
This study is registered, number ISRCTN 55261332.
Role of the funding source
The funders of the study had no role in study design, data collection, data analysis, data interpretation, or writing of the report. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
Results
Participant characteristics at screening and randomisation were broadly similar between groups (Table 1). 459 patients entered the run-in (figure 1) and 330 were randomly allocated to treatment groups. About a quarter of patients who were allocated to treatment had not previously been prescribed maintenance treatment with mood stabilisers (Table 1). On entering run-in, 221 (53%) of 414 UK patients were given a lithium titration pack and 296 (71%) a valproate titration pack, suggesting that more patients were already taking lithium than valproate. Adherence to study drug was very high (Table 1). Plasma concentrations and drug doses at randomisation were mostly within the planned ranges and were similar between groups (Table 1). The participants allocated to treatment were followed up for 589.8 person-years (combination 201.1, lithium 191.6, and valproate 197.2), of which 452.6 person-years (combination 155.1, lithium 149.0, and valproate 148.5) were on the allocated therapy.
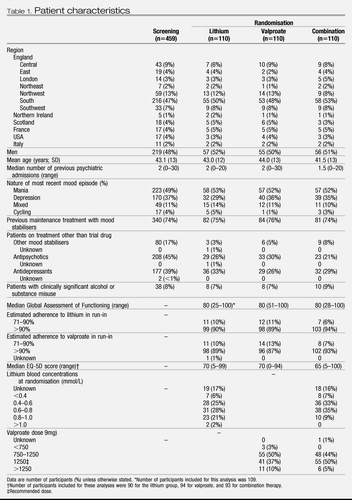 |
Table 1. Patient characteristics
During the follow-up period the primary outcome occurred in 59 of 110 (54%) of participants on combination therapy, 76 of 110 (69%) on valproate semisodium and 65 of 110 (59%) on lithium carbonate (table 2 and figure 2). The hazard of the primary outcome was significantly lower than for participants allocated to combination therapy than for those allocated to valproate monotherapy, but not lower than for those allocated to lithium monotherapy (Table 2). The hazard of the primary outcome was significantly lower for participants allocated to lithium monotherapy than for those given valproate (HR 0.71 [95% CI 0.51–1.00], p=0.0472). The proportional hazards assumption held for all analyses, and results were unchanged after adjustment for minimisation factors (figure 2).
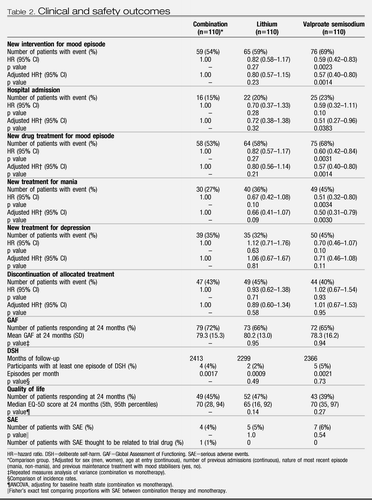 |
Table 2. Clinical and safety outcomes
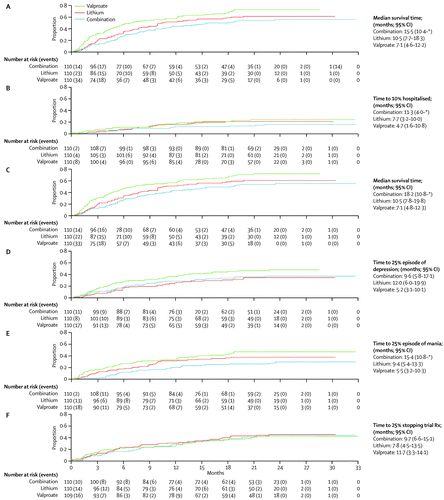
(A) First admission or adjuvant treatment for emerging mood episode. (B) First admission to hospital. (C) First added treatment. (D) First episode of depression. (E) First manic episode. (F) Stopping trial treatment. *Survival curve does not extend sufficiently far to allow calculation of upper limit of 95% CI.
Prespecified subgroup analyses taking into account baseline severity of disorder (measured as number of previous admissions) and nature of most recent mood episode did not show significantly different results (figure 3–5). Sensitivity analyses restricted to participants taking adequate doses of drugs and to those treated per protocol (data censored at the point when allocated trial drug was stopped) confirmed the robustness of the results (figure 3–5). Exclusion of events occurring within the first 3 months after randomisation resulted in broadly similar findings to those of the primary analysis (figure 3–5).
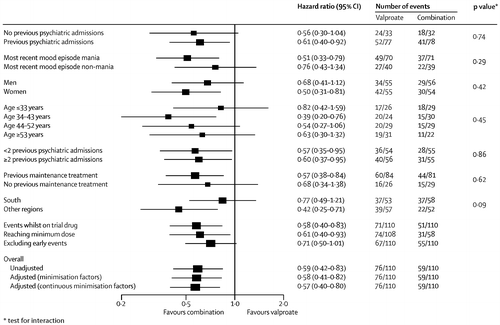
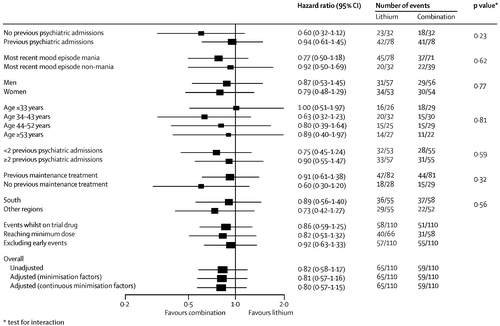
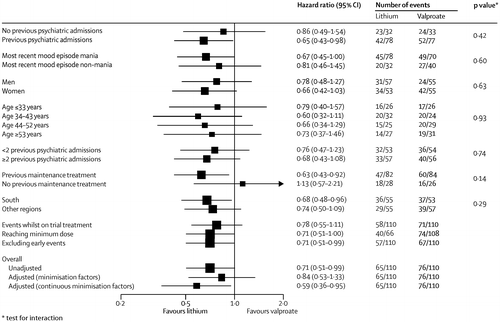
Separate analyses were done for the two components of the primary outcome and were consistent with results of the primary analysis (table 2 and figure 2). Notably, the adjusted risk of hospital admission for participants allocated to combination therapy was significantly lower than for patients allocated to valproate (Table 2), replicating the finding for the primary outcome. The benefit of combination therapy compared with valproate was most apparent for manic relapses (Table 2), whereas the advantage of lithium compared with valproate was most apparent for depressive relapses (HR 0.63 [95% CI 0.41– 0.96], p=0.0331). Discontinuation of allocated treatment, self-harm, quality of life, and global functioning did not differ between groups (Table 2).
Serious adverse events occurred in five participants during the run-in. After randomisation, 16 participants had serious adverse events: seven in the valproate group (three deaths, which were caused by stroke, peritonitis,and carcinoma bronchus); five in the lithium monotherapy group (two deaths, caused by respiratory failure and bronchopneumonia); and four in the combination therapy group (one death, caused by respiratory failure). One event (polycystic ovaries) was thought to have been revealed by weight gain caused by the trial drug. No suicides occurred. Five women became pregnant during the trial and stopped trial drugs (two during run-in and three after randomisation). No obstetric complications or congenital abnormalities were reported. Most participants who responded (lithium, 52 of 55 patients [95%]; valproate, 48 of 52 patients [92%]; combination, 57 of 57 patients [100%]) reported at least one non-serious adverse event during follow-up.
Discussion
The results of BALANCE show that for people with bipolar I disorder for whom long-term therapy is clinically indicated, combination therapy with lithium plus valproate is more likely to prevent relapse than is monotherapy with valproate. The 41% relative benefit is irrespective of baseline severity of illness, is maintained for up to 2 years, and is most apparent in prevention of manic relapse. BALANCE could neither confirm nor refute a benefit of combination therapy compared with lithium monotherapy. Since the trial was designed mainly to compare combination therapy with monotherapy, conclusions about the comparative efficacy of the two agents should be cautious. Nevertheless, lithium monotherapy was slightly more effective than was valproate. The smaller margin of difference reported after monotherapy than after combination therapy suggests an additive effect when the two drugs are used together. Previous trials (14, 28, 29) have not shown unequivocal differences between lithium and valproate.
We recorded a 15.5% difference in risk between combination therapy and valproate monotherapy (number need to treat [NNT]=7) (30), a 10% difference between valproate and lithium monotherapies (NNT=10), and a non-significant 5.5% difference between combination therapy and lithium monotherapy (NNT=19) during follow-up. The unequivocal and substantial effect of adding lithium to valproate is striking and could be even larger in highly adherent patients with optimum therapy. One previous randomised trial (17) compared lithium monotherapy with a combination of lithium plus valproate in patients with rapid-cycling disorder and comorbid substance misuse. Although this trial was quite small and so of restricted power, the estimate of the HR (0.72 [95% CI 0.32–1.65) in favour of combination therapy was similar to that recorded in BALANCE.
Although BALANCE did not have a no-treatment or placebo group, an approximate indirect estimate of the benefit of combination therapy compared with no treatment can be obtained from the product of the BALANCE hazard ratios for combination versus monotherapy and the relative risk estimated from results of previous trials of monotherapy versus placebo (6, 14). This calculation yields a relative risk reduction for combination therapy compared with placebo that is in the range of 45–64%.
The wide range of patients enrolled from various locations and clinical situations ensures that the BALANCE findings are broadly applicable to patients with bipolar I disorder. The proportion of participants with substance-misuse comorbidity (8%) was lower than that reported from population surveys (31). This finding probably mirrors differences in case ascertainment—in BALANCE the investigator was asked only to record whether they considered substance or alcohol use to be a clinically significant problem, whereas in population surveys, evidence of substance misuse is sought through structured interview. Use of an active run-in means that the randomised sample was a selected group, and hence the results are most applicable to patients who can tolerate treatment with lithium and valproate and who are largely adherent to therapy (21,32). Nonetheless, the active run-in would tend to increase the applicability of the results to the clinical setting, in which people who are prescribed long-term treatments are usually selected from those who have been able to tolerate the drug in the short term (32).
Several possible weaknesses in the study design should be considered. First, treatment allocation was not masked from the investigators or participants. Therefore, performance and ascertainment biases could have arisen if clinicians or participants had behaved systematically differently dependent on the treatment allocation, thereby affecting the recorded outcome. Our prospective strategy to avoid these biases was to limit trial entry to patients for whom there was explicit uncertainty about which treatment was likely to be best. Patients who had, or developed, a strong preference for an investigational therapy were excluded either at screening or before randomisation. Second, although the eligibility criteria required that participants were not acutely unwell, symptomatic status was not systematically assessed and some prerandomisation selection on the basis of response to treatment could have arisen. Third, although intervention for a new mood episode is, we believe, a meaningful clinical endpoint, it could also be subverted by, for example, the very early introduction of additional drugs into monotherapy groups. This effect does not seem to have occurred—the difference between treatments was constant up to 2 years and exclusion of events occurring in the first 3 months did not significantly change the results. Most importantly, the effect of combined therapy on risk of admission to hospital was similar to that on the combined primary outcome. The trial physician is unlikely to make the decision to admit a patient alone. Absence of equipoise and consequent bias is therefore unlikely to account for the difference in admissions between the study treatments. Finally, although around 21% of patients withdrew from the trial before 24 months, reasons for withdrawal did not differ between groups.
BALANCE was designed to imitate routine use of the agents in clinical practice. Hence, lithium plasma concentrations at randomisation were possibly suboptimum in some patients. This potential drawback did not prevent the trial from detecting a substantial effect of addition of lithium to valproate. Similarly, the dose of valproate that we used was lower than is recommended for treatment of acute mania, and increased doses might have improved its effectiveness. However, the dose that was used was agreed by the manufacturer and independent experts, and was shown during the pilot phase to achieve optimum tolerability.
Moreover, patients who dropped out during the run-in did so more often because of intolerance than for any other reason, so use of an increased dose would probably have reduced tolerability and overall effectiveness. Adverse events and tolerability did not differ between the treatments during the randomised phase.
Although BALANCE has provided reliable evidence to inform treatment of people with bipolar disorder, we emphasise that more than half of the participants who were given combination therapy needed additional treatment during follow-up. Depressive episodes in bipolar disorder are both prevalent and disabling, and available therapies show only little effectiveness in their treatment and prevention (6,33,34). Depressive relapses lead to disability that can jeopardise a person's ability to function at home or in work and contribute to the burden on caregivers (35). Other monotherapies, both pharmacotherapies and psychotherapies, exist that could reduce relapse, and further study of other possible combinations is needed (8,36). Quantification of the risk of specific adverse effects of both drugs, including the possibility of renal toxic effects with lithium and congenital abnormalities with valproate (37), was beyond the scope of BALANCE, but should be considered inclinical application of the results.
The main BALANCE findings have important implications for clinical decisions about long-term therapy for bipolar disorder. First, valproate monotherapy is recommended by clinical practice guidelines as a first-line option for long-term therapy (3–5). Our results suggest that patients should be advised that a better outcome would be likely with combination therapy with lithium plus valproate semisodium or lithium alone. Second, guidelines suggest that patients who have frequent relapses during treatment with lithium monotherapy could switch to valproate monotherapy (5). The results of BALANCE suggest that these patients would fare better if they changed to combination therapy.
1. Müller-Oerlinghausen B, Berghöfer A, Bauer M. Bipolar disorder. Lancet 2002;359:241–47.Crossref, Google Scholar
2. Murray CJL, Lopez AD. Global mortality, disability, and the contribution of risk factors: Global Burden of Disease Study. Lancet 1997;349:1436–42.Crossref, Google Scholar
3. American Psychiatric Association. Practice guideline for the treatment of patients with bipolar disorder (revision). Am J Psychiatry 2002;159 (4 suppl):1–50.Crossref, Google Scholar
4. Goodwin GM. Evidence-based guidelines for treating bipolar disorder: revised second edition—recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2009;23:346–88.Crossref, Google Scholar
5. National Institute for Health and Clinical Excellence. Bipolar disorder: the management of bipolar disorder in adults, children and adolescents, in primary and secondary care. http://guidance.nice.org.uk/CG38 (
6. Geddes JR, Burgess S, Hawton K, Jamison K, Goodwin GM. Long-term lithium therapy for bipolar disorder: systematic review and meta-analysis of randomized controlled trials. Am J Psychiatry 2004;161:217–22.Crossref, Google Scholar
7. Cipriani A, Pretty H, Hawton K, Geddes JR. Lithium in the prevention of suicidal behaviour and all-cause mortality in patients with mood disorders: a systematic review of randomised trials. Am J Psychiatry 2005;162:1805–19.Crossref, Google Scholar
8. Soares-Weiser K, Bravo Vergel Y, Beynon S, et al. A systematic review and economic model of the clinical effectiveness and cost-effectiveness of interventions for preventing relapse in people with bipolar disorder. Health Technol Assess 2007;11:1–226.Crossref, Google Scholar
9. Fenn HH, Robinson D, Luby V, et al. Trends in pharmacotherapy of schizoaffective and bipolar affective disorders: a 5-year naturalistic study. Am J Psychiatry 1996;153:711–13.Crossref, Google Scholar
10. Sanderson DR. Use of mood stabilizers by hospitalized geriatric patients with bipolar disorder. Psychiatr Serv 1998;49:1145–47.Crossref, Google Scholar
11. Citrome L, Levine J, Allingham B. Utilization of valproate: extent of inpatient use in the New York State Office of Mental Health. Psychiatr Q 1998;69:283–300.Crossref, Google Scholar
12. Blanco C, Laje G, Olfson M, Marcus SC, Pincus HA. Trends in the treatment of bipolar disorder by outpatient psychiatrists. Am J Psychiatry 2002;159:1005–10.Crossref, Google Scholar
13. MacRitchie K, Geddes JR, Scott J, Haslam D, de Lima M, Goodwin G. Valproate for acute mood episodes in bipolar disorder. Cochrane Database Syst Rev 2004;4:CD004052.Google Scholar
14. Macritchie KA, Geddes JR, Scott J, Haslam DR, Goodwin GM. Valproic acid, valproate and divalproex in the maintenance treatment of bipolar disorder. Cochrane Database Syst Rev 2001;3:CD003196.Google Scholar
15. Freeman MP, Stoll AL. Mood stabilizer combinations: a review of safety and efficacy. Am J Psychiatry 1998;155:12–21.Crossref, Google Scholar
16. Williams RS, Cheng L, Mudge AW, Harwood AJ. A common mechanism of action for three mood-stabilizing drugs. Nature 2002;417:292–95.Crossref, Google Scholar
17. Geddes J, Goodwin G. Bipolar disorder: clinical uncertainty, evidence-based medicine and large-scale randomised trials. Br J Psychiatry 2001; 178:S191–94.Crossref, Google Scholar
18. Zarate J, Singh J, Manji HK. Cellular plasticity cascades: targets for the development of novel therapeutics for bipolar disorder. Biol Psychiatry 2006;59:1006–20.Crossref, Google Scholar
19. Kemp DE, Gao K, Ganocy SJ, et al. A 6-month, double-blind, maintenance trial of lithium monotherapy versus the combination of lithium and divalproex for rapid-cycling bipolar disorder and co-occurring substance abuse or dependence. J Clin Psychiatry 2009;70:113–21.Crossref, Google Scholar
20. Geddes JR, Rendell JM, Goodwin GM. BALANCE: a large simple trial of maintenance treatment for bipolar disorder. World Psychiatry 2002;1: 48–51.Google Scholar
21. Rendell JM, Juszczak E, Hainsworth J, et al. Developing the BALANCE trial—the role of the pilot study and start-up phase. Bipolar Disord 2004; 6:26–31.Crossref, Google Scholar
22. Lang JM. The use of a run-in to enhance compliance. Stat Med 1990; 9:87–93.Crossref, Google Scholar
23. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edn. Washington, DC: American Psychiatric Association, 1994.Google Scholar
24. Suppes T, Baldessarini RJ, Faedda GL, Tohen M. Risk of recurrence following discontinuation of lithium treatment in bipolar disorder. Arch Gen Psychiatry 1991;48:1082–88.Crossref, Google Scholar
25. Jones SH, Thornicroft G, Coffey M, Dunn G. A brief mental health outcome scale—reliability and validity of the Global Assessment of Functioning (GAF). Br J Psychiatry 1995;166:654–59.Crossref, Google Scholar
26. Kind P. The Euroqol instrument: an index of health related quality of life. In: Spilker B, ed. Quality of life and pharmacoeconomics in clinical trials, 2nd edn. Philadelphia: Lippincott-Raven Publishers, 1996:191–201.Google Scholar
27. Stephenson BJ, Rowe BH, Haynes RB, Macharia WM, Leon G. Is this patient taking the treatment as prescribed? JAMA 1993;269:2779–81.Crossref, Google Scholar
28. Findling RL, McNamara NK, Youngstrom EA, et al. Double-blind 18-month trial of lithium versus divalproex maintenance treatment in pediatric bipolar disorder. J Am Acad Child Adolesc Psychiatry 2005;44: 409–17.Crossref, Google Scholar
29. Calabrese JR, Shelton MD, Rapport DJ, et al. A 20-month, double-blind, maintenance trial of lithium versus divalproex in rapid-cycling bipolar disorder. Am J Psychiatry 2005;162:2152–61.Crossref, Google Scholar
30. Cook RJ, Sackett DL. The number needed to treat: a clinically useful measure of treatment effect. BMJ 1995;310:452–54.Crossref, Google Scholar
31. Merikangas KR, Akiskal HS, Angst J, et al. Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey Replication. Arch Gen Psychiatry 2007;64:543–52.Crossref, Google Scholar
32. Pablos MA, Barr RG, Shea S. Run-in periods in randomized trials: implications for the application of results in clinical practice. JAMA 1998; 279:222–25.Crossref, Google Scholar
33. Goodwin GM, Bowden CL, Calabrese JR, et al. A pooled analysis of 2 placebo-controlled 18-month trials of lamotrigine and lithium maintenance in bipolar I disorder. J Clin Psychiatry 2004;65:432–41.Crossref, Google Scholar
34. Sachs GS, Nierenberg AA, Calabrese JR, et al. Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med 2007; 356:1711–22.Crossref, Google Scholar
35. Ostacher MJ, Nierenberg AA, Iosifescu DV, et al. Correlates of subjective and objective burden among caregivers of patients with bipolar disorder. Acta Psychiatr Scand 2008;118:49–56.Crossref, Google Scholar
36. Geddes JR, Calabrese JR, Goodwin GM. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry 2009; 194:4–9.Crossref, Google Scholar
37. Meador KJ, Baker GA, Browning N, et al. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. N Engl J Med 2009; 360:1597–605.Crossref, Google Scholar



