Structural Magnetic Resonance Imaging in Bipolar Disorder: An International Collaborative Mega-Analysis of Individual Adult Patient Data
Abstract
Background: There is substantial inconsistency in results of brain structural magnetic resonance imaging studies in adult bipolar disorder.This is likely consequent upon limited statistical power of studies together with their clinical and methodological heterogeneity. The current study was undertaken to perform an international collaborative mega-analysis of regional volumetric measurements of individual patient and healthy subject data, to optimize statistical power, detect case-control differences, assess the association of psychotropic medication usage with brain structural variation, and detect other possible sources of heterogeneity. Methods: Eleven international research groups contributed published and unpublished data on 321 individuals with bipolar disorder I and 442 healthy subjects. We used linear mixed effects regression models to evaluate differences in brain structure between patient groups. Results: Individuals with bipolar disorder had increased right lateral ventricular, left temporal lobe, and right putamen volumes. Bipolar patients taking lithium displayed significantly increased hippocampal and amygdala volume compared with patients not treated with lithium and healthy comparison subjects. Cerebral volume reduction was significantly associated with illness duration in bipolar individuals. Conclusions: The application of mega-analysis to bipolar disorder imaging identified lithium use and illness duration as substantial and consistent sources of heterogeneity, with lithium use associated with regionally specific increased brain volume.
(Reprinted with permission from Biological Psychiatry 2011; 69:326–335)
A number of studies assessing volumetric measurements of regional brain structures in bipolar disorder have been published in recent years, but results have been inconsistent with lateral ventricular enlargement being the most consistently reported finding (1–3). Markedly conflicting findings have been reported for some structures. For example, various studies reported increased (4–7), reduced (8–11), or no difference in amygdala volume (12). A previous meta-analysis of methodologically robust volumetric magnetic resonance imaging (MRI) studies of bipolar disorder based on published aggregate data by our group revealed right ventricular enlargement as the only brain region that differed significantly from healthy subjects (13). Extensive heterogeneity existed for several other brain regions, including the amygdala, subgenual prefrontal cortex, thalamus, and the third ventricle (13). A recent meta-analysis of neuroimaging studies of bipolar disorder that included computed tomography and qualitative magnetic resonance studies highlighted the low statistical power of most studies and identified lateral ventricular enlargement as the only consistent volumetric finding (14). This report included a metaregression analysis for sources of heterogeneity and found that those patients taking lithium were more likely to have increased gray matter volume. A further recent meta-analysis again identified lateral ventricular enlargement but also noted whole brain and prefrontal lobe reductions and globus pallidus enlargement in bipolar disorder (15).
Meta-analysis is the most widely used method for averaging statistical effect sizes from different studies (16) and is usually based on combining effect sizes from published literature rather than analyzing pooled individual patient data. Meta-analysis, however, is associated with several methodological problems, the most significant of which is the frequent inclusion of studies that are heterogenous in nature. Studies included in meta-analyses commonly differ in aspects of their design. Each study in a meta-analysis is drawn from somewhat different populations, depending on the patients chosen and the investigations and conditions unique to the study. Thus, even if each study has a large patient sample, the investigation effect would be expected to differ; these differences are known as random effects and describe the between-study variation with regard to an overall mean effect of all studies (17). This between study variance is difficult to control using conventional meta-analytical techniques.
However, analysis pooling individual patient data from different studies, or mega-analysis (18,19), helps to adjust for between-study differences. In MRI datasets, unique conditions to individual studies include MRI field strengths and pulse sequences and different techniques to delineate anatomical brain regions. These differences cannot be controlled for using meta-analysis; however, mega-analysis has the advantage of being specifically able to control for research center and thus control for differences in MRI techniques between research centers. Additionally, because reanalysis of pooled patient data requires collaboration of the investigators who conducted the studies, it enables better identification of relevant studies and the provision of a more balanced interpretation and wider endorsement of results (20).
In the present study, we employed mega-analytic techniques to analyze pooled individual patient data by obtaining raw regional brain volumes and associated demographic and clinical data from several international research groups who had published structural MRI research in adult bipolar disorder. By comparing regional brain measurements of individuals with bipolar disorder with those of healthy comparison subjects, while covarying for relevant confounds, we sought to maximize statistical power. We also sought to determine if regional brain volume in bipolar disorder was affected by certain clinical characteristics commonly measured by researchers, in particular medication use at time of scanning, as well as duration of illness and number of hospitalizations. In relation to pharmacotherapy, we were especially interested in associations of regional brain structures with lithium and valproate treatment, given their reported neurotrophic properties (14, 21–23).
Methods and Materials
Published brain volumetric studies in bipolar disorder were identified by a systematic search of the databases MedLine, PsychLIT, and EMBASE for articles published between 1980 and 2009 in any language using the following medical subject heading categories; magnetic resonance imaging or MRI and bipolar affective disorder, bipolar disorder, or mania. We cross-referenced all relevant original research and review articles relating to imaging of affective disorders to search for additional published articles. A systematic literature search using the medical subject heading categories magnetic resonance imaging or MRI and schizophrenia or schizoaffective disorder was also conducted, because some studies have compared bipolar disorder, schizophrenia, and other comparison groups.
Subjects
We invited research groups that had employed robust methodology in data acquisition by setting certain minimum requirements, and we used similar inclusion criteria to our previous aggregate data meta-analysis (13): 1) an MRI scanner strength of 1T or greater, 2) regional brain structure measurement based on contiguous slices, 3) included individuals aged 16 to 55 years, 4) brain structures independently examined by three or more research groups, and 5) patients with bipolar disorder only—data contributed by research groups including individuals with schizoaffective disorder, schizo-phrenia, or unipolar depression alone were not included.
We identified 22 research groups meeting these criteria. Senior representatives from each group were invited to participate and submit to us their relevant individual raw data for this mega-analysis. Data requested included participant diagnosis, relevant brain volumetric measurements, demographic details including age and gender, clinical details about the age of onset and course of illness, and medication use. Researchers from 11 groups submitted data on 321 individuals with bipolar I disorder and 442 healthy subjects. Data on a further 29 individuals with bipolar II disorder were submitted; however due to this limited data, we only examined individuals with bipolar I disorder. The majority of these data have been previously published (Table S1 in Supplement 1) (4–9, 24–43). Projects from each research center were approved by the respective ethical committees. Data from the different research centers were merged into a single spreadsheet and corrected for inaccuracies, duplications, and inclusion criteria (e.g., adolescents under 16 years and individuals with bipolar II disorder were excluded from further analysis).
Linear mixed effects regression models were used where the research center was incorporated as a random effect to account for the correlation due to individuals within each research center and possible heterogeneity between research centers. As the factor, gender, and covariates, age + brain slice thickness, could be potential confounders, these were forced into each model in addition to the primary diagnostic group variable. Summary statistics and point and 95% confidence interval (CI) estimates of the difference in the mean adjusted response variables across the levels of diagnostic group were reported. The level of statistical significance was set at α = .05, and effect sizes using Cohen's d were also reported. Model diagnostics were performed using suitable residual plots. Ventricular volume distributions were normalized through log transformation. All analysis was performed using the Statistical Package for Social Sciences 15.0 for Windows (SPSS 15.0 for Windows, SPSS, Inc., Chicago, Illinois) and R (version 2.6.1) (The R project for statistical computing; http://www.rproject.org). We corrected for multiple testing using the technique described by Holm (44).
Our principal analysis compared regional brain volumes between groups (bipolar I individuals vs. healthy subjects). We also performed analyses to ascertain the effect of medication between subgroups (e.g., bipolar disorder individuals treated with lithium vs. bipolar disorder individuals not treated with lithium vs. healthy subjects) and any effect on brain volume or duration of illness and number of hospitalizations.
Results
Demographic and clinical data
The sociodemographic details are provided in Table 1 and unadjusted regional brain volumes are provided in Table S2 in Supplement 1 for individuals with bipolar disorder and healthy subjects. All research centers used reliable and valid criteria for diagnosing bipolar disorder (Structured Clinical Interview for DSM or the Schedule for Affective Disorders [SADS]) and had appropriate mechanisms for drug coding. Age and gender were similar between the individuals with bipolar disorder and healthy subjects (Table 1). In individuals with bipolar disorder, the mean age of onset was 21 years (SD = 7), the mean duration of illness was 12 years (SD = 11), and the median number of hospitalizations was 3 (range 0–20). For bipolar individuals with information on medication use at the time of MRI acquisition, 141 subjects (50.4%) were prescribed lithium, 74 (30.6%) were prescribed valproate, 17 (8.9%) were prescribed carbamazepine, 217 (77.5%) were prescribed at least one mood stabilizing agent (lithium, valproate, or carbamazepine), 90 (44.3%) were prescribed an antipsychotic medication, and 26 (24.3%) were prescribed an antidepressant. Our sample included 50 (15.6%) firstepisode bipolar subjects.
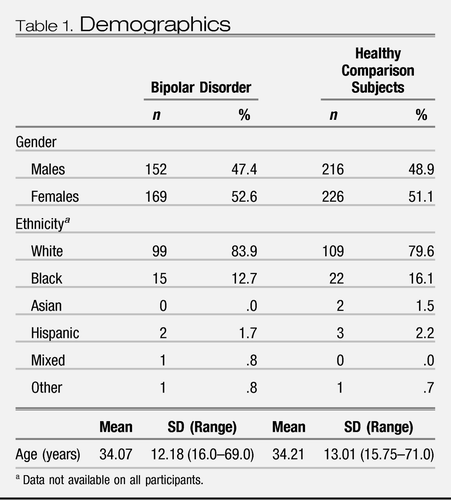 |
Table 1. Demographics
Bipolar disorder versus healthy subjects
Mean adjusted regional brain volumes (for research center, gender, and age) of individuals with bipolar disorder and healthy subjects are shown in Table 2. There was a significant difference in total temporal lobe volume between diagnostic groups (p = .007). The adjusted mean temporal lobe volume was estimated to be between 2.1 and 12.6 mm3 larger, on average, in bipolar compared with healthy individuals. Bipolar subjects also displayed significantly increased left temporal lobe volume, right putamen volume, and right lateral ventricular volume compared with healthy subjects. No significant differences were found between bipolar and healthy subjects for any other brain region. There was a differential effect of age for hippocampal volume with bipolar subjects having a greater decrease in hippocampal volume with age compared with healthy subjects (Z = 2.86, p = .001). This was not evident for other brain regions.
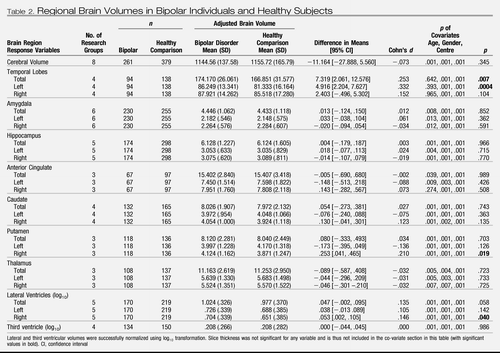 |
Table 2. Regional Brain Volumes in Bipolar Individuals and Healthy Subjects
Brain volume and lithium
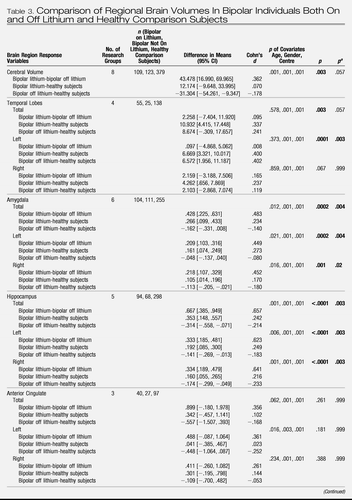
A comparison of regional brain volumes in bipolar patients taking lithium with those not taking lithium and with healthy subjects is provided in Table 3 Those not taking lithium had a younger mean age (32 years, SD = 12) than both those taking lithium (36 years, SD = 13) and healthy subjects (34 years, SD = 13) (F = 2.462, df = 2, 750, p = .037), and there was no significant difference in the duration of illness (F = 3.112, df = 1, 271, p = .079) between bipolar patients taking and not taking lithium. No interaction was found between lithium and research center for any brain region. There was evidence of a significant difference in regional brain volume across subject groups. Individuals with bipolar disorder treated with lithium had larger mean total, left, and right hippocampal volume (p < .001) and total, left, and right amygdala volume (p < .001) than both those patients not treated with lithium and healthy subjects. In Figure S1 in Supplement 1, we present a plot of hippocampal volume and amygdala volume versus age paneled by research center, and in Figure 1, we present a plot of predicted hippocampal volume and amygdala volume (from the linear mixed model) by the different levels of lithium factor (bipolar individuals taking lithium, bipolar individuals not taking lithium, and healthy subjects), while adjusting for gender, research center, and age. Global cerebral volume was also significantly different across subject groups (p = .003), with bipolar subjects not taking lithium having a smaller mean volume compared with both healthy subjects and bipolar subjects taking lithium. Bipolar individuals regardless of lithium use had larger total (p = .003) and left (p = .0001) temporal lobe volume compared with healthy subjects, although only left temporal lobe volume remained significantly increased in volume after correcting for multiple testing.
 |
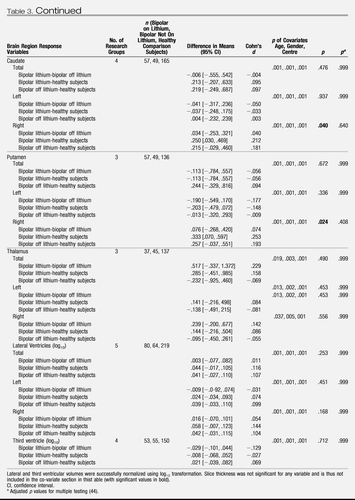 |
Table 3. Comparison of Regional Brain Volumes In Bipolar Individuals Both On and Off Lithium and Healthy Comparison Subjects
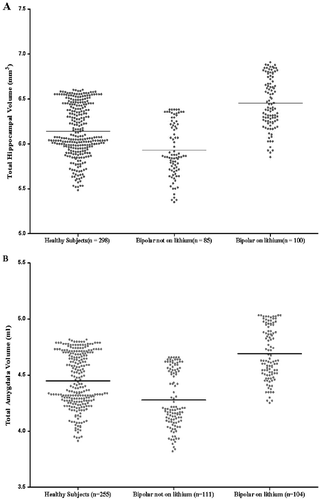
(A) Predicted hippocampal volumes. Predicted hippocampal volumes are presented from linear mixed models while adjusting for gender, research center, and age. Means for each group are represented by horizontal bars. A highly significant difference between the three groups (F = 11.32, p = .0001) is present, with individuals with bipolar disorder on lithium having a greater mean hippocampal volume compared with both those not on lithium (F = 9.53, p = .002) and healthy subjects (F = 12.96, p = .0004) and healthy subjects having a greater mean hippocampal volume than bipolar individuals not on lithium (F = 7.81, p = .005). (B) Predicted amygdala volumes. Predicted amygdala volumes are presented from linear mixed models while adjusting for gender, research center, and age. Means for each group are represented by horizontal bars. A highly significant difference between the three groups (F = 8.904, p = .0002) is present, with individuals with bipolar disorder on lithium having a greater mean amygdala volume compared with those both on lithium (F = 6.33, p = .013) and healthy subjects (F = 10.97, p = .001) and healthy subjects having a greater mean amygdala volume than bipolar individuals not on lithium (F = 5.13, p = .024).
Brain volume and other medications
No association was found for individuals with bipolar disorder prescribed valproate or carbamazepine alone at the time of scanning and any regional brain volume compared with individuals not taking these medications. Similarly, there was no difference in any regional brain volume in those patients taking antipsychotic medication compared with those not taking antipsychotic medication. As we only had data for 20 individuals on typical antipsychotic agents and 31 individuals on atypical antipsychotic agents for any brain region, we considered this too small a subset to carry out analysis comparing these individuals to healthy subjects or to each other.
Brain volume and clinical variables
We found an association between reduced mean cerebral volume (B = −4.064%, 95% CI −7.460, −.668; p = .019) and longer duration of illness in bipolar patients with research center, gender, age, and lithium treatment in our regression model. No other brain region demonstrated a significant positive or negative association with illness duration and no association was demonstrated between the number of hospitalizations and any regional brain volume. We similarly examined age of onset and regional brain volumes and found that an earlier age of onset of bipolar disorder was associated with reduced mean cerebral volume (B = 3.615%, 95% CI .304, 6.927; p = .033) and left thalamic volume (B = .054%, 95% CI. 004, .104; p = .035) and increased total (B = −.024%, 95% CI −.046, −.001; p = .039) and left amygdala volume (B = −.014%, 95% CI = −.025, −.002; p = .020). There were no significant two-way interactions identified between age and gender and any regional brain volume.
First episode and psychosis
We only had data from three or more research centers for cerebral (n = 48, six research centers) and amygdala volume (n = 47, six research centers) in relation to individuals with bipolar disorder in their first episode of illness. Cerebral volume (F = 5.731, p = .017) and total (F = 12.162, p = .001), left (F = 6.695, p = .010), and right amygdala volume (F = 14.552, p = .0002) were reduced in volume in first episode bipolar subjects compared with healthy comparison subjects. When we compared these brain regions for individuals in their first-episode bipolar disorder to those who had multiple episodes, we found no difference in cerebral volume (F = .170, p = .680) or total (F = .650, p = .421), left (F = .728, p = .395), and right amygdala volume (F = .471, p = .493). We only had sufficient data to examine the effect of psychosis for cerebral volume (n = 83, three research centers), and no difference was found between bipolar individuals who had or did not have psychosis (F = .011, p = .916).
MRI field strength
Two research sites used MRI field strengths other than 1.5 T and analyzed cerebral hemisphere and temporal lobe volume. When we excluded these research sites from our analysis, no significant differences were noted from our original findings, with increased total temporal lobe (F = 12.029, p = .001), left temporal lobe (F = 19.654, .00001), and right temporal lobe volume (F = 4.394, p = .037) in bipolar individuals but no difference in mean cerebral hemisphere volume between bipolar individuals and control subjects noted. Global cerebral volume remained significantly different across subject groups (F = 7.534, p = .001), with bipolar subjects not taking lithium having a smaller mean volume compared with both healthy subjects and bipolar subjects taking lithium.
Discussion
Similar to meta-analyses of aggregate data (13–15), we found that bipolar I disorder is associated with increased right lateral ventricular volume, which suggests a subtle anatomical laterality difference in pathogenesis compared with schizophrenia, where left-sided ventriculomegaly is more prominent (45). Our findings comparing the entire bipolar group with healthy subjects are also consistent with previous meta-analyses that found a preservation of global cerebral volume and hippocampal volume in bipolar disorder (13, 14), although a recent meta-analysis noted a global reduction in brain volume (15). However, the present study provides strong evidence for a substantial contribution of lithium treatment to medial temporal lobe enlargement in patients with bipolar disorder and reveals this phenomenon as a potential source of heterogeneity in published magnetic resonance bipolar studies. The apparent trophic effect of lithium was present across research centers and age and was anatomically specific, most prominently evident in the neuroplastic hippocampus and the amygdala. No such lithium effect was detected for anterior cingulate, lateral ventricular, thalamic, basal ganglia, or temporal lobe volume.
First-episode bipolar disorder patients had reduced cerebral volume and amygdala volume and individuals with bipolar disorder who were not taking lithium had reduction in cerebral and hippocampal volumes compared with healthy comparison subjects—findings consistently reported in the related syndromes of unipolar depression and schizophrenia (45–49). This finding raises the intriguing possibility that bipolar disorder may indeed share with schizophrenia and depression the pathogenetic mechanisms associated with medial temporal lobe volume reduction, perhaps induced by glucocorticoid excess (50, 51), but that these changes are reversed in patients with bipolar disorder alone by the differential use of lithium in this patient group.
Our finding of increased hippocampal volume in individuals treated with lithium compared with those bipolar individuals not treated with lithium (and healthy subjects) is consistent with some other cross-sectional studies not included in the mega-analysis (21–23, 52, 53) and two recent longitudinal studies of 1 to 8 weeks and 2 to 4 years duration (43, 54). Although the mechanism of action of lithium is poorly understood (55), an important role in neuroprotection and neurogenesis has been suggested (56, 57). The hippocampus is a particularly plastic brain region (58) and is one of the few areas to produce neurons postnatally (59); therefore, it may be more likely than other brain regions to be affected by medication induced neurogenesis (60). The mechanism of lithium-induced neurogenesis has not been fully elucidated; however, proposed mechanisms include an effect on signal pathways including its ability to upregulate the cytoprotective protein Bcl-2 (58, 60) and its effect on growth factors including nerve growth factor (57) and brain-derived neurotrophic factor (57, 61, 62). Our finding of increased amygdala volume in individuals treated with lithium is consistent with the findings from another recent study (21) and suggests that the amygdala may also be modified by lithium. Interestingly, these structures are closely interconnected and form part of the anterior limbic system known to be involved in mood regulation and that demonstrate task-related hyperactivity in functional imaging studies of bipolar disorder, possibly related to impaired prefrontal modulation of these regions (63, 64). In contrast, there was no apparent effect of lithium on striatum, temporal lobes, or lateral ventricular volumes. However, almost all patients in the sample were taking medications and it may well be that alternative mood stabilizing agents had differential effects on other brain volumes. For example, taking lithium or any other mood stabilizer could be related to the temporal lobe and putamen volume increase identified in the present study, as there were insufficient psychotropic naive patients in the sample to assess this. The inclusion of medication-free patients with bipolar disorder and careful longitudinal studies assessing homogenous samples of patients on different medications will be required to elucidate such propositions.
There are some other limitations to this study. It was only possible to acquire individual data from 11 of the 22 research groups identified who satisfied our inclusion and exclusion criteria. Due to this, we were unable to examine anterior cingulate gyrus and intracranial volume between treatment groups. Not all research sites had acquired data on sociodemographic or clinical variables of interest such as socioeconomic class, medication use (two sites did not supply data in relation to medication use; however, these two sites contributed the fewest individuals to the mega-analysis and did not analyze the amygdala or hippocampus), treatment adherence, severity of depression or mania at time of scanning (most individuals were euthymic), and psychotic symptoms. All research sites did, however, supply data in relation to subjects' age, gender, and illness duration. We did not have sufficient data to analyze the duration of medication exposure or medication levels and regional brain volumes. We were also unable to control for intracranial or cerebral volume in our analysis of brain subregions, as not all research groups had acquired these data. We did not have sufficient data to examine if a history of psychosis significantly affected regional brain volumes in bipolar disorder. The different MRI datasets analyzed used different MRI field strengths and pulse sequences and different techniques to delineate anatomical brain regions. While mega-analysis has the advantage of being specifically able to control for research center and thus control for differences in MRI techniques between research centers, it would, of course, be preferable if identical techniques were used by each research center.
Our finding of increased amygdala volume in individuals with bipolar disorder treated with lithium may help to explain some of the inconsistencies in the published literature. Previous studies demonstrating increased amygdala volume in individuals with bipolar disorder have predominantly included individuals treated with lithium (71% and 63%) (5, 6), whereas studies demonstrating a reduction in amygdala volume included fewer individuals treated with lithium (20%–40%) (8–11).
We noted increased temporal lobe volume in individuals with bipolar disorder, with left temporal lobe volume increased in bipolar individuals both on and off lithium. Although this is not a well documented finding, three of the five studies included in our analysis had nonsignificant increases in temporal lobe volume in bipolar individuals compared with healthy subjects (4–6). Other studies have noted an increase in left temporal lobe volume (65, 66). Wilke et al. (65) suggested that their finding of increased left temporal lobe volume was due to increased volume of the superior temporal gyrus, and an association between left temporal lobe volume and increasing duration of illness has been reported (66).
While we had fairly modest power in relation to the putamen, we detected an increased volume of this region in individuals with bipolar disorder, which is consistent with some (3, 7, 37) but not all previous findings (26, 67, 68). This increased volume was only noted for the right putamen and may reflect previous findings where shape differences (increased radii) in the right putamen but not left side were noted in drug-naive bipolar subjects compared with control subjects (69).
Our finding of a reduction in cerebral volume with duration of illness is consistent with a number of studies that have demonstrated reduced total gray matter volume with illness duration in bipolar disorder (69–72) and suggests that a neurodegenerative process may be involved in the pathophysiology of bipolar disorder. Further prospective longitudinal studies are necessary to comprehensively elucidate this proposition.
In this study, we demonstrated that adults with established bipolar I disorder display right lateral ventricle enlargement and increased mean left temporal lobe and right putamen volume. The main source of heterogeneity identified by this large individual level analysis of clinical and demographic factors was medication usage. Lithium treatment was differentially associated with a regionally specific increased mean volume of the hippocampus and amygdala. This increased mean brain volume associated with lithium may be due to the neuroprotective effects of lithium, which are possibly mediated by its effect on neurotrophic growth factors.
1. Bearden CE, Hoffman KM, Cannon TD (2001): The neuropsychology and neuroanatomy of bipolar disorder: A critical review. Bipolar Disord 3:106–150.Crossref, Google Scholar
2. Beyer JL, Krishnan KR (2002): Volumetric brain imaging findings in mood disorders. Bipolar Disord 4:89–104.Crossref, Google Scholar
3. Strakowski SM, Adler CM, DelBello MP (2002): Volumetric MRI studies of mood disorders: Do they distinguish unipolar and bipolar disorder? Bipolar Disord 4:80–88.Crossref, Google Scholar
4. Altshuler LL, Bartzokis G, Grieder T, Curran J, Mintz J (1998): Amygdala enlargement in bipolar disorder and hippocampal reduction in schizophrenia: An MRI study demonstrating neuroanatomic specificity. Arch Gen Psychiatry 55:663–664.Google Scholar
5. Altshuler LL, Bartzokis G, Grieder T, Curran J, Jimenez T, Leight K, et al. (2000): An MRI study of temporal lobe structures in men with bipolar disorder or schizophrenia. Biol Psychiatry 48:147–162.Crossref, Google Scholar
6. Brambilla P, Harenski K, Nicoletti M, Sassi RB, Mallinger AG, Frank E, et al. (2003): MRI investigation of temporal lobe structures in bipolar patients. J Psychiatr Res 37:287–295.Crossref, Google Scholar
7. Strakowski SM, DelBello MP, Sax KW, Zimmerman ME, Shear PK, Hawkins JM, Larson ER (1999): Brain magnetic resonance imaging of structural abnormalities in bipolar disorder. Arch Gen Psychiatry 56:254–260.Crossref, Google Scholar
8. Doty TJ, Payne ME, Steffens DC, Beyer JL, Krishnan KR, LaBar KS (2008): Age-dependent reduction of amygdala volume in bipolar disorder. Psychiatry Res 163:84–94.Crossref, Google Scholar
9. Rosso IM, Kilgore WD, Cintron CM, Gruber SA, Tohen M, Yurgelun-Todd DA (2007): Reduced amygdala volumes in first-episode bipolar disorder and correlation with cerebral white matter. Biol Psychiatry 61:743–749.Crossref, Google Scholar
10. Blumberg H, Kaufman J, Martin A, Whiteman R, Zhang J, Gore JC, et al. (2003): Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder. Arch Gen Psychiatry 60:1201–1208.Crossref, Google Scholar
11. Blumberg H, Fredericks C, Wang F, Kalmar JH, Spenser L, Papademetris X, et al. (2005): Preliminary evidence for persistent abnormalities in amygdala volumes in adolescents and young adults with bipolar disorder. Bipolar Disord 7:570–576.Crossref, Google Scholar
12. Frazier JA, Chiu S, Breeze JL, Makris N, Lange N, Kennedy DN, et al. (2005): Structural brain magnetic resonance imaging of limbic and thalamic volumes in pediatric bipolar disorder. Am J Psychiatry 162:1256–1265.Crossref, Google Scholar
13. McDonald C, Zanelli J, Rabe-Hesketh S, Ellison-Wright I, Sham P, Kalidindi S, et al. (2004): Meta-analysis of magnetic resonance imaging brain morphometry studies in bipolar studies. Biol Psychiatry 56:411–417.Crossref, Google Scholar
14. Kempton MJ, Geddes JR, Ettinger U, Williams SC, Grasby PM (2008): Meta-analysis, database, and meta-regression of 98 structural imaging studies in bipolar disorder. Arch Gen Psychiatry 65:1017–1032.Crossref, Google Scholar
15. Arnone D, Cavanagh J, Gerber D, Lawrie SM, Ebmeier KP, McIntosh AM (2009): Magnetic resonance imaging studies in bipolar disorder and schizophrenia: Meta-analysis. Br J Psychiatry 195:194–201.Crossref, Google Scholar
16. Lopez-Lee D (2002): Indiscriminate data aggregation in meta-analysis: A cause for concern among policy makers and social scientists. Eval Rev 26:520–544.Crossref, Google Scholar
17. Lau J, Ioannidis JPA, Schmid C (1997): Quantitative synthesis in systematic reviews. Ann Intern Med 127:820–826.Crossref, Google Scholar
18. Segurado R, Hamshere ML, Glaser B, Nikolov I, Moskvina V, Holmans PA (2007): Combining linkage data sets for meta-analysis and mega-analysis: The GAW15 rheumatoid arthritis dataset. BMC Prog 1(suppl 1):S104.Crossref, Google Scholar
19. Serretti A, Cusin C, Rausch JL, Bondy B, Smeraldi E (2006): Pooling pharmacogenic studies on the serotonin transporter: A mega-analysis. Psychiatry Res 145:61–65.Crossref, Google Scholar
20. Oxman AD, Clarke MJ, Stewart LA (1995): From science to practice: Meta-analyses using individual patient data are needed. JAMA 274:845–846.Crossref, Google Scholar
21. Foland LC, Altshuler LL, Sugar CA, Lee AD, Leow AD, Townsend J, et al. (2008): Increased volume of the amygdala and hippocampus in bipolar patients treated with lithium. Neuroreport 19:221–224.Crossref, Google Scholar
22. Bearden CE, Thompson PM, Dutton RA, Frey BN, Peluso MAM, Nicoletti M, et al. (2008): Three-dimensional mapping of hippocampal anatomy in unmedicated and lithium-treated patients with bipolar disorder. Neuropsychopharmacology 33:1229–1238.Crossref, Google Scholar
23. Sassi RB, Brambilla P, Hatch JP, Nicoletti MA, Mallinger AG, Frank E, et al. (2004): Reduced left anterior cingulate volumes in untreated bipolar patients. Biol Psychiatry 56:467–476.Crossref, Google Scholar
24. DelBello MP, Strakowski SM, Zimmerman ME, Hawkins JM, Sax KW (1999): MRI analysis of the cerebellum in bipolar disorder: A pilot study. Neuropsychopharmacology 21:63–68.Crossref, Google Scholar
25. Brambilla P, Harenski K, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, et al. (2001): Anatomical MRI study of basal ganglia in bipolar disorder patients. Psychiatry Res 106:65–80.Crossref, Google Scholar
26. Brambilla P, Harenski K, Nicoletti M, Mallinger AG, Frank E, Kupfer DJ, et al. (2001): MRI study of posterior fossa structures and brain ventricles in bipolar patients. J Psychiatr Res 35:313–322.Crossref, Google Scholar
27. Caetano SC, Sassi R, Brambilla P, Harenski K, Nicoletti M, Mallinger AG, et al. (2001): MRI study of thalamic volumes in bipolar and unipolar patients and healthy individuals. Psychiatry Res 108:161–168.Crossref, Google Scholar
28. McIntosh AM, Forrester A, Lawrie SM, Byrne M, Harper A, Kestelman JN, et al. (2001): A factor model of the functional psychoses and the relationship of factors to clinical variables and brain morphology. Psychol Med 31:159–171.Crossref, Google Scholar
29. Brambilla P, Nicoletti M, Harenski K, Sassi RB, Mallinger AG, Frank E, et al. (2002): Anatomical MRI study of subgenual prefrontal cortex in bipolar and unipolar subjects. Neuropsychopharmacology 27:792–799.Crossref, Google Scholar
30. Kieseppa T, van Erp TG, Haukka J, Partonen T, Cannon TD, Poutanen VP, et al. (2002): The volumetric findings in MRI brain study of bipolar twins and their healthy c-twins. Bipolar Disord 4(suppl 1):29–30.Crossref, Google Scholar
31. López-Larson MP, DelBello MP, Zimmerman ME, Schwiers ML, Strakowski SM (2002): Regional prefrontal gray and white matter abnormalities in bipolar disorder. Biol Psychiatry 52:93–100.Crossref, Google Scholar
32. Sassi RB, Nicoletti M, Brambilla P, Mallinger AG, Frank E, Kupfer DJ, et al. (2002): Increased grey matter volume in lithium-treated bipolar disorder patients. Neurosci Lett 329:243–245.Crossref, Google Scholar
33. Strakowski SM, DelBello MP, Zimmerman ME, Getz GE, Mills NP, Ret J, et al. (2002): Ventricular and periventricular structural volumes in firstversus multiple-episode bipolar disorder. Am J Psychiatry 159:1841–1847.Crossref, Google Scholar
34. Kieseppa T, van Erp TG, Haukka J, Partonen T, Cannon TD, Poutanen VP, et al. (2003): Reduced left hemispheric white matter volume in twins in bipolar I disorder. Biol Psychiatry 54:896–905.Crossref, Google Scholar
35. Sharma V, Menon R, Carr TJ, Densmore M, Mazmanian D, Williamson PC (2003): An MRI study of subgenual prefrontal cortex in patients with familial and non-familial bipolar I disorder. J Affect Disord 77:167–171.Crossref, Google Scholar
36. Chen BK, Sassi R, Axelson D, Hatch JP, Sanches M, Nicoletti M, et al. (2004): Cross-sectional study of abnormal amygdala development in adolescents and young adults with bipolar disorder. Biol Psychiatry 56: 399–405.Crossref, Google Scholar
37. DelBello MP, Zimmerman ME, Mills NP, Getz GE, Strakowski SM (2004): Magnetic resonance imaging analysis of amygdala and other subcortical brain regions in adolescents with bipolar disorder. Bipolar Disord 6:43–52.Crossref, Google Scholar
38. Kaur MD, Sassi RB, Axelon D, Nicoletti M, Brambilla P, Monkul ES, et al. (2005): Cingulate cortex anatomical abnormalities in children and adolescents with bipolar disorder. Am J Psychiatry 162:1637–1643.Crossref, Google Scholar
39. Mills NP, DelBello MP, Adler CM, Strakowski SM (2005): MRI analysis of cerebellar vermal abnormalities in bipolar disorder. Am J Psychiatry 162:1530–1532.Crossref, Google Scholar
40. McDonald C, Marshall N, Sham PC, Bullmore ET, Schulze K, Chapple B, et al. (2006): Regional brain morphometry in patients with schizophrenia or bipolar disorder and their unaffected relatives. Am J Psychiatry 163:478–487.Crossref, Google Scholar
41. Zimmerman ME, DelBello MP, Getz GE, Shear PK, Strakowski SM (2006): Anterior cingulate subregion volumes and executive function in bipolar disorder. Bipolar Disord 8:281–288.Crossref, Google Scholar
42. Fornito A, Mahli GS, Lagopoulos J, Ivanovski B, Wood SJ, Saling MM, et al. (2008): Anatomical abnormalities of the anterior cingulate and paracingulate cortex in patients with bipolar I disorder. Psychiatry Res 162:123–132.Crossref, Google Scholar
43. Yucel K, Taylor VH, McKinnon MC, MacDonald K, Alda M, Young LT, MacQueen GM (2008): Bilateral hippocampal volume increase in patients with bipolar disorder and short-term lithium treatment. Neuropsychopharmacology 33:361–367.Crossref, Google Scholar
44. Holm S (1979): A simple sequentially rejective multiple test procedure. Scand J Stat 6:65–70.Google Scholar
45. Videnbech P, Ravnkilde B (2004): Hippocampal volume and depression: A meta-analysis of MRI studies. Am J Psychiatry 161:1957–1966.Crossref, Google Scholar
46. Campbell S, Marriott M, Nahmias C, MacQueen GM (2004): Lower hippocampal volume in patients suffering from depression: A meta-analysis. Am J Psychiatry 161:598–607.Crossref, Google Scholar
47. Wright IC, Rabe-Hesketh S, Woodruff PWR, David AS, Murray RM, Bull-more ET (2000): Meta-analysis of regional brain volumes in schizophrenia. Am J Psychiatry 157:16–25.Crossref, Google Scholar
48. Steen GR, Mull C, McClure R, Hamer RM, Lieberman JA (2006): Brain volume in first-episode schizophrenia: Systematic review and meta-analysis of magnetic resonance imaging studies. Br J Psychiatry 188:510–518.Crossref, Google Scholar
49. Nelson MD, Saykin AJ, Flashman LA, Riordan HJ (1998): Hippocampal volume reduction in schizophrenia as assessed by magnetic resonance imaging: A meta-analysis study. Arch Gen Psychiatry 55:433–440.Crossref, Google Scholar
50. Sapolsky RM (2000): Glucocorticoid and hippocampal atrophy in neuropsychiatric disorders. Arch Gen Psychiatry 57:925–935.Crossref, Google Scholar
51. Webster MJ, Knable MB, O'Grady J, Orthmann J, Weickert CS (2002): Regional specificity of brain glucocorticoid mRNA alterations in subjects with schizophrenia and mood disorders. Mol Psychiatry 7:985–994.Crossref, Google Scholar
52. Moore GJ, Bebchuk JM, Wilds IB, Chen G, Manji HK (2000): Lithium-induced increase in human brain grey matter. Lancet 356:1241–1242.Crossref, Google Scholar
53. Beyer JL, Kuchibhatla M, Payne ME, Moo-Young M, Cassidy F, Macfall J, Krishnan KR (2004): Hippocampal volume measurement in older adults with bipolar disorder. Am J Geriatr Psychiatry 12:613–620.Crossref, Google Scholar
54. Yucel K, McKinnon MC, Taylor VH, MacDonald K, Alda M, Young LT, et al. (2007): Bilateral hippocampal volume increases after long-term lithium treatment in patients with bipolar disorder: A longitudinal MRI study. Psychopharmacology (Berl) 195:356–367.Crossref, Google Scholar
55. Bachmann RF, Schloesser RJ, Gould TD, Manji HK (2005): Mood stabilisers target cellular plasticity and resilience cascades: Implications for the development for novel therapeutics. Mol Neurobiol 32:173–202.Crossref, Google Scholar
56. Cadotte DW, Xu B, Raccine RJ, MacQueen GM, Wang JF, McEwen B, Young LT (2003): Chronic lithium treatment inhibits pilocarpine-induced mossy fiber sprouting in rat hippocampus. Neuropsychopharmacology 28:1448–1453.Crossref, Google Scholar
57. Frey BN, Andreazza AC, Cereser KM, Martins MR, Valvassori SS, Reus GZ, et al. (2006): Effects of mood stabilisers on hippocampus BDNF levels in an animal model of mania. Life Sci 79:281–286.Crossref, Google Scholar
58. Chen G, Manji HK (2006): The extracellular signal-regulated kinase path-way: An emerging promising target for mood stabilisers. Curr Opin Psychiatry 19:313–323.Crossref, Google Scholar
59. Becker S, Wojtowicz JM (2007): A model of hippocampal neurogenesis in memory and mood disorders. Trends Cogn Sci 11:70–76.Crossref, Google Scholar
60. Bauer M, Alda M, Priller J, Young LT (2003): Implications of the neuropro-tective effects of lithium for the treatment of bipolar and neurodegenerative disorders. Pharmacopsychiatry 36(suppl 3):S250–S254.Crossref, Google Scholar
61. Angelucci F, Aloe L, Jimenez-Vasquez P, Mathe AA (2003): Lithium treatment alters brain concentrations of nerve growth factor, brain-derived neurotrophic factor and glial cell line derived neurotrophic factor in a rat model of depression. Int J Neuropsychopharmacol 6:225–231.Crossref, Google Scholar
62. Jacobsen JP, Mork A (2004): The effect of escitalopram, desimipramine, electroconvulsive seizures and lithium on brain-derived neurotrophic factor mRNA and protein expression in the rat brain and the correlation to 5-HT and 5-HIAA levels. Brain Res 1024:183–192.Crossref, Google Scholar
63. Strakowski SM, Adler CM, Holland SK, Mills N, DelBello MP (2004): A preliminary fMRI study of sustained attention in unmedicated, euthymic bipolar disorder. Neuropsychopharmacology 29:1734–1740.Crossref, Google Scholar
64. Adler CM, DelBello MP, Strakowski SM (2006): Brain network dysfunction in bipolar disorder. CNS Spectr 11:312–320.Crossref, Google Scholar
65. Wilke M, Kowatch RA, DelBello NP, Mills NP, Holland SK (2004): Voxel based morphometry in adolescents with bipolar disorder: First results. Psychiatry Res 13:57–69.Crossref, Google Scholar
66. Ali OS, Denicoff KD, Altshuler LL, Hauser P, Li X, Conrad AJ, et al. (2001): Relationship between prior course of illness and neuroanatomic structures in bipolar disorder. Neuropsychiatry Neuropsychol Behav Neurol 14:227–232.Google Scholar
67. Brambilla P, Harenski K, Nicoletti, Mallinger AG, Frank E, Kupfer DJ, et al. (2001): Differential effects of age on brain gray matter in bipolar patients and healthy individuals. Neuropsychobiology 43:242–247.Crossref, Google Scholar
68. Swayne VW, Andreasen NC, Allider R, Yuh WT, Ehrhardt JC (1992): Subcortical and temporal structures in affective disorder and schizophrenia: A magnetic resonance imaging study. Biol Psychiatry 31:221–240.Crossref, Google Scholar
69. Aylward EH, Roberts-Twillie JV, Barta PE, Kumar AJ, Harris GJ, Geer M, et al. (1994): Basal ganglia volumes and white matter hyperintensities in patients with bipolar disorder. Am J Psychiatry 151:687–693.Crossref, Google Scholar
70. Hwang J, Lyoo IK, Dager SR, Friedman SD, Oh JS, Lee JY, et al. (2006): Basal ganglia shape alterations in bipolar disorder. Am J Psychiatry 163:276–285.Crossref, Google Scholar
71. Frey BN, Zunta-Soares GB, Caetano SC, Nicoletti MA, Hatch JP, Brambilla P, et al. (2008): Illness duration and total brain grey matter in bipolar disorder: Evidence for neurodegeneration? Eur Neuropsychopharmacol 18:717–722.Crossref, Google Scholar
72. Moorhead TW, McKirdy J, Sussmann JE, Hall J, Lawrie SM, Johnstone EC, McIntosh AM (2007): Progressive gray matter loss in patients with bipolar disorder. Biol Psychiatry 8:894–900.Crossref, Google Scholar



