Interoception in Fear Learning and Posttraumatic Stress Disorder
Abstract
Posttraumatic stress disorder (PTSD) is a psychiatric condition characterized by sustained symptoms, including reexperiencing, hyperarousal, avoidance, and mood alterations, following exposure to a traumatic event. Although symptom presentations in PTSD are heterogeneous and incompletely understood, they likely involve interactions between neural circuits involved in memory and fear learning and multiple body systems involved in threat processing. PTSD differs from other psychiatric conditions in that it is a temporally specific disorder, triggered by a traumatic event that elicits heightened physiological arousal, and fear. Fear conditioning and fear extinction learning have been studied extensively in relation to PTSD, because of their central role in the development and maintenance of threat-related associations. Interoception, the process by which organisms sense, interpret, and integrate their internal body signals, may contribute to disrupted fear learning and to the varied symptom presentations of PTSD in humans. In this review, the authors discuss how interoceptive signals may serve as unconditioned responses to trauma that subsequently serve as conditioned stimuli, trigger avoidance and higher-order conditioning of other stimuli associated with these interoceptive signals, and constitute an important aspect of the fear learning context, thus influencing the specificity versus generalization of fear acquisition, consolidation, and extinction. The authors conclude by identifying avenues for future research to enhance understanding of PTSD and the role of interoceptive signals in fear learning and in the development, maintenance, and treatment of PTSD.
Posttraumatic stress disorder (PTSD) is defined clinically by elevated and sustained symptoms of reexperiencing, hyperarousal, avoidance, mood alterations, and dissociation following the experience of one or more traumatic events (1). The symptom presentations of PTSD are heterogeneous and have expanded considerably from DSM-IV to DSM-5 (2, 3). The individual differences, spanning genetic, environmental, physiological, and neurobiological factors that contribute to the unique symptom presentations, are not yet well understood but likely entail large-scale interactions across multiple body systems involved in threat processing (4).
PTSD differs from other psychiatric conditions in that it is a temporally specific disorder triggered by the experience of a traumatic or life-threatening event that elicits heightened physiological arousal and fear. PTSD can follow traumatic events that occur at any point across the human life span, although the temporal susceptibility window typically starts in infancy, and the greatest sensitivity occurs from early childhood through middle adulthood (Figure 1A) (5, 6). During the traumatic experience, external and internal environmental cues are encoded within the brain and integrated to form fear-related memories. Traumatic experiences are common, with estimates of 70% of civilians having experienced a criterion A event during their lifetime (7). However, only a subset of these individuals (approximately 6% of the general population) (8) will proceed to meet criteria for PTSD. In adaptive trauma recovery, the associations built between threat and neutral signals at the time of trauma exposure are not reinforced, and the symptoms dissipate with time. However, in PTSD, these associations persist even when a threat is no longer present, and the symptoms are reinforced and strengthened through the avoidance of traumatic memories and trauma reminders.
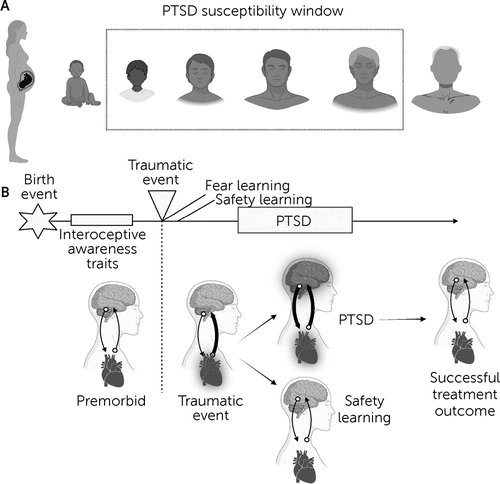
FIGURE 1. Temporal characteristics of posttraumatic stress disorder (PTSD)a
aA: PTSD can follow traumatic events at any point in the life span, but susceptibility is greatest from early childhood through middle adulthood. B: Traumatic events initiate a surge of afferent body-to-brain fear signaling, which, in the setting of PTSD development, is associated with abnormal interoceptive signaling throughout the brain-body feedback loop. Resilience to PTSD onset following a traumatic event involves safety learning and a lack of abnormal fear learning responses. Treatment for PTSD is focused on reducing learned fear responses and through modulating and reducing the sensitivity of fear neurocircuitry to nonfear signals.
Fear learning models (e.g., fear conditioning, fear extinction, fear reinstatement) are central to the leading theories regarding development, maintenance, and treatment of PTSD. However, these models are often considered separately from the role of internal physiological sensations in the affective processing of arousal and fear signals, representing a key gap. In this review, we propose that interoception, the process of sensing, interpreting, and integrating internal body signals, can offer valuable insight into the relationship between the experience of trauma and the fear learning processes that lead to PTSD. We start by reviewing the basic processes of fear learning and then consider interoception in relation to PTSD. We then focus our discussion on how interoception may intersect with current theoretical models of fear learning, beginning with the initial response to a traumatic event. We continue with a discussion of the effects of interoceptive signals on fear learning, extinction, and context processing. Finally, we identify avenues for further empirical and clinical research on interoception and its associated effects on fear learning among individuals with PTSD.
Fear Learning and PTSD: The Dominant Model
The processes considered most central to the development and maintenance of threat-related associations in PTSD include fear conditioning (9, 10) and fear extinction learning (11), respectively. Fear conditioning involves a process of learning to form associations between a neutral, conditioned stimulus (CS) and an unconditioned stimulus (US) that elicits an unconditioned response. In experimental paradigms, the repeated pairing of a CS with a US builds an association whereby the CS alone comes to elicit the unconditioned response, which is then called the conditioned response. A real-world example of conditioning could be the scent of a perpetrator’s cologne (i.e., CS) following a sexual assault (i.e., US) that may elicit a conditioned response (e.g., elevated heart rate, shallow breathing) when encountered in a safe setting. A life-threatening traumatic experience is usually considered a US that can become paired with various environmental and neurophysiological cues and contexts associated (or temporally bound) with the traumatic event. Because of the salience and intensity of a traumatic event, repeated pairing is not necessary to induce or condition a sustained fear response; PTSD is often observed after one traumatic event (e.g., a car crash or a sexual assault), as well as during situations in which the trauma has been repeatedly experienced over time (e.g., repeated combat-related exposure or sustained childhood abuse). Chronic stress from repeated traumatic experiences further reinforces fear conditioning through multiple pairings of the CS and US (12). Individuals with PTSD may also exhibit a generalization of their fear response to stimuli distinct from, but similar to, a CS (e.g., fear of a grizzly bear following an attack extending to dogs or other large animals; fear of any people who share the physical attributes of a perpetrator of assault) (13).
Fear extinction learning is a normative process that follows fear conditioning. It occurs when the CS is no longer reinforced by presence of the threat (US), and a new association between the CS and the experience of safety is formed. Given the sustained fear that characterizes PTSD, it has often been conceived as an inability to extinguish one’s fear after a trauma has ended, and deficits in laboratory-based extinction learning have been well-documented among individuals with PTSD. Compared with healthy trauma-exposed individuals, persons with PTSD frequently exhibit sustained fear responses (14), or increased fear reinstatement (i.e., return of conditioned response), when a CS is presented after a delay following extinction learning (15).
Context-dependent factors are thought to influence the acquisition and extinction of fear responses to specific stimuli or cues. Although conditioned cues predict the onset of a US, context is comprised of multiple internal and external factors (e.g., spatial, temporal, interoceptive, cognitive, social, and cultural) that convey information, which can influence the learning of associations about a CS (16). To elaborate, the same cue may be differentially associated with threat or safety on the basis of contextual information (e.g., a lion in the wild may be associated with threat, whereas a lion in the zoo may be associated with safety). Deficits in context processing have been documented among individuals with PTSD (17). In laboratory-based contextual fear paradigms, individuals with PTSD have shown persisting conditioned responses during extinction learning (18) when a CS is presented in a safe context during extinction recall (19). An example of context generalization is demonstrated when a combat veteran continues to display fear responses to cues previously associated with threat (e.g., rubble on the side of the road indicating presence of an improvised explosive device in a combat zone) when the cue is encountered in a safe context (e.g., rubble on the side of the road indicating presence of a construction site in the veteran’s neighborhood).
The neural mechanisms of exteroceptive fear conditioning are well established. During exteroceptive fear learning, multimodal perceptual inputs are relayed from the thalamus to the amygdala (20). Neural associations are formed via the repeated activation and strengthening of synapses in the basolateral complex of the amygdala, and expression of fear is modulated by connections between the basolateral complex and regions of the prefrontal cortex (PFC) (21). Contextual information is communicated through hippocampal inputs to the amygdala. The amygdala subsequently relays signals back to relevant body systems via the periaqueductal gray (PAG) (22). The PAG orchestrates a cascade of innate and learned physiological responses involving autonomic (i.e., heart rate, respiratory, skin conductance) and endocrine (e.g., blood pressure, adrenergic-cortisol) responses (23). The PAG is also thought to be involved in subsequent behavioral responses to threat, with the dorsal PAG involved in escape and contextual fear learning, and the ventrolateral PAG involved in freezing behavior or immobility (24, 25). Bidirectional connectivity between the PFC and the basolateral amygdala is also thought to be central to inhibition of fear (i.e., fear extinction), leading to a reduction in the conditioned fear response via regulation of central amygdala reactivity (26).
Previous research indicates that although the neural function underlying fear acquisition is similar for individuals with and without PTSD, there are differences observed in fear and memory circuity during extinction learning (17, 19). Successful fear extinction following fear conditioning relies on hippocampal-dependent memory and has been associated with inhibition of fear expression via the ventromedial PFC (vmPFC) (26, 27). Individuals with PTSD, compared with those without the condition, demonstrate decreased hippocampal activation, decreased within-network connectivity in the default mode network, and increased connectivity between the default mode and salience networks during fear extinction recall and fear renewal (28).
By comparison, the role of interoception in fear learning and interoceptive fear conditioning has received limited study, despite the significance of interoceptive processing in the implementation of basic emotional states in foundational theories of emotion. For more than a century, these theories have focused on the interactions between subjective appraisal of emotion and interoceptive and exteroceptive sensory input (29). The James-Lange theory of emotion (30, 31) relied on the premise that afferent physiological and interoceptive signals constitute the drivers of affective experience. Rebutting this notion, the Cannon-Bard theory (32, 33) posited that physiological and emotional experiences occur independently and are thus untethered to afferent interoceptive input. Attempting to integrate these perspectives, the Schachter-Singer theory (34) proposed that the physiological and cognitive appraisal of emotional signals occurs in tandem, with attributions of emotional states driven by both physiological arousal and environmental context cues. Despite their considerable differences, which continue to be debated (35), these theories underscore the firm recognition of the importance of interoceptive processing with respect to implementation of basic emotional states. However, only during the last several decades has a connection between interoceptive dysregulation and fear-related psychiatric disorders begun to be discussed (36–41). As we argue in the ensuing sections, investigating the role of interoception in fear learning and PTSD may provide essential insight into how differing profiles of PTSD symptoms are experienced by individuals.
Interoception: How the Nervous System Senses the Body’s Physiological Status
Interoception refers to the process by which the nervous system detects, interprets, and integrates information from the internal organs and tissues, serving as the basis for the conscious and unconscious experience of body sensations (42). Interoception is essential for maintaining homeostasis, the reactive regulatory process responsible for maintaining the internal balance of bodily functions (43), and for allostasis, the predictive regulatory process involved in adaptively controlling autonomic and endocrine responses in preparation for future internal and external stimuli (44). Interoception also plays a critical role in the sensation and perception of internal body sensations, such as hunger, thirst, heart palpitations, breathing, and body temperature (among others); in the regulation of emotional and social behavior; and in the development of emotions and conscious higher-order states, including self-awareness, empathy, and self-regulation (45). Interoception involves a network of neural pathways and brain regions that receive and process information from different organs and body systems. This network includes afferent fibers from sensory receptors located in peripheral organs, such as the heart, lungs, gut, and skin, which transmit signals to the spinal cord, brainstem, hypothalamus and thalamus, and then to higher cortical areas, such as the insular cortex, anterior cingulate cortex (ACC), and PFC (46).
The insular cortex is a key brain region for interoception, as it receives and integrates information from many different body systems and is involved in generating subjective feelings of body states, such as hunger, thirst, pain, heartbeat sensations, and emotional arousal (47, 48). The ACC is also involved in monitoring and regulating body states; it is reciprocally connected to the insula and has a role in the emotional and cognitive evaluation of interoceptive sensations (49, 50). The PFC is also involved in the conscious processing and modulation of interoceptive information, enabling individuals to monitor and potentially control their body responses, such as heart rate and breathing, and to use this information to adapt to environmental demands and social contexts (46, 51). In summary, interoception constitutes the nervous system’s monitoring of the internal state of the body, involving a complex network of neural pathways and brain regions that detect, interpret, and integrate information from different body systems, generating subjective feelings of body sensations, and enabling individuals to adapt to environmental demands and social contexts.
Interoception and PTSD: Empirical Evidence and Theoretical Models
Interoceptive dysregulation has been associated with a range of psychiatric disorders, such as anxiety, depression, eating disorders, schizophrenia, autism, somatic symptom disorders, and PTSD (52, 53). Unlike these other psychiatric disorders, PTSD is triggered following the experience of a traumatic or life-threatening event. These events kickstart complex communication between the brain and body, particularly between neural circuits focused on processing interoceptive signals and those focused on processing exteroceptive cues and environmental contexts specific to the trauma. Studies have demonstrated that childhood traumatic events, early life adversity, and sexual trauma are associated with lower subjective interoceptive accuracy (54), increased interoceptive accuracy on a heartbeat perception task (55), and greater dissociative symptoms (56). However, these recent findings are few in number, suggesting that substantial knowledge gaps remain.
Kearney and Lanius (57) recently introduced a theoretical model to describe how PTSD disrupts the hierarchical processing of brain and body signals, creating a distorted sense of self. They highlight that, during exposure to a traumatic event, individuals may exhibit or attend to different internal responses and focus more or less on their physical sensations and ongoing cognitions. The model suggests that when traumatic events disrupt the brainstem-level processing of physical sensations (i.e., at the level of the PAG), there are downstream breakdowns in the expression and subsequent integration of body signals and upstream exacerbations of arousal and affective signaling that can include numbing and dissociative responses. These signaling disruptions alter the basic sense of self as it relates to the experienced environment, which influences how limbic and other intrinsic brain networks (such as the default mode network) regulate self-referential processing and embodied self-consciousness. Neocortical modulations of this distorted self-representation perpetuate a less reflective, less embodied, and subordinate sense of self. This model of PTSD suggests that the sensing and processing of interoceptive sensations is in fact fundamental to the development of the disorder and could help to explain certain states of disembodiment, such as dissociation, which involves detachment from one’s sense of self (i.e., depersonalization), disconnection from surroundings or reality (i.e., derealization), or blunted emotional responsivity (i.e., numbing) following a trauma. Although the model focuses on dissociative symptoms, it also has relevance for nondissociative presentations of PTSD. Specifically, nondissociative presentations of PTSD have been characterized by exaggerated threat responses thought to relate to diminished top-down prefrontal control over the amygdala (58), whereas dissociative presentations of PTSD have been conceptualized as involving an overregulation of the amygdala, with increased activation in cortical regions (i.e., dorsolateral PFC, ACC, insula) involved in attentional control and interoception (58), and increased functional connectivity between these regions and the amygdala (59). As such, the integration of interoceptive, exteroceptive, affective, and self-referential processing likely lies on a continuum and contributes broadly to fear learning and extinction and the symptoms experienced by individuals with PTSD.
Fear Learning and Interoception: Key Role of the Body
Building on the previous discussion of the role of disrupted hierarchical processing of brain and body signals in PTSD, it is important to consider the interplay between fear learning and interoception in the consolidation of traumatic events and in the subsequent development and maintenance of the disorder. In addition, individual differences in interoceptive processing may subsequently contribute to—or perpetuate—experiences of dissociation and emotional numbing over longer time frames (such as those associated with racial discrimination) (60), which could then either facilitate or disrupt fear extinction processes. In the following sections we propose how interoceptive dysregulation could have an integral role in the processes of fear conditioning, fear extinction, and contextualization of these learning processes and could thereby influence development, maintenance, and treatment of PTSD (Figure 1B).
Fear Conditioning
There is currently limited evidence to suggest differences in neural function (61) or physiological response (15, 62) between individuals with and without PTSD during laboratory-based fear acquisition tasks. However, much of this research has lacked appreciation for how interoceptive body sensations serve as a core aspect of the unconditioned trauma response. During and following a traumatic event, a range of interoceptive responses may occur, including increased heart rate and heart palpitations; hyperventilation and shortness of breath; tightness in the chest or throat; muscle tension, aches, and pains; nausea or digestive issues; sweating or chills; dizziness or lightheadedness; numbness or tingling sensations; dissociation or feeling disconnected from the body; and hypersensitivity to physical sensations. There is also considerable individual variability in the quality and intensity with which interoceptive body sensations are experienced. In the context of sexual trauma, there may be other elements of interoceptive processing that become a core part of the trauma memory and/or have a greater link to one’s representation of the self—thus contributing to the observation that sexual trauma is associated with greater likelihood of experiencing dissociative symptoms (63).
Given the limited evidence for differences in neural function or physiological response between individuals with and without PTSD during laboratory-based fear acquisition paradigms, alternative fear learning explanations have begun to emerge. According to a predictive processing account of PTSD, links between interoceptive and exteroceptive sensory input inform the concordance between prior experiences and prospective predictions (64) and thus, interoceptive inferences in the wake of a trauma are likely to influence the saliency of a traumatic event, strengthening the magnitude and specificity of the trauma memory, and the predictive associations made. Heightened interoceptive arousal and attention to physiological sensations during a traumatic event can also provide an internal physiological context and/or serve as internal cues that are associated with the trauma, thereby modulating how the memory is subsequently recalled. This can lead to higher-order conditioning, in which the interoceptive signals themselves become a CS that can perpetuate further conditioning of associated cues (65, 66) (Figure 2). These factors can also result in avoidance of the interoceptive sensations themselves, although this desire for avoidance is challenging to meet, because avoidance of one’s own physiologic arousal-related signals is nearly impossible. This form of fear learning has not been well represented in standard models of fear learning, perhaps owing to the complexity of the learned associations and the inaccessibility of interoceptive signals for the purposes of modulation in laboratory settings.
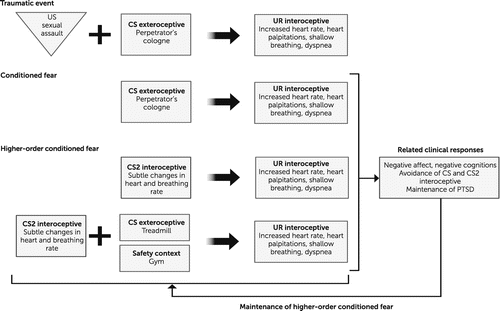
FIGURE 2. Interoception in higher-order fear learning and PTSDa
aDuring a traumatic event heightened interoceptive arousal and attention to physiological sensations (interoceptive unconditioned response; UR interoceptive) can serve as internal cues that are associated with the trauma and provide an accompanying internal physiological context, modulating how the memory is subsequently recalled. These factors can lead to higher-order conditioning, in which the interoceptive signals themselves become a new conditioned stimulus (CS2 interoceptive) that can perpetuate further conditioning of associated cues. US, unconditioned stimulus; UR, unconditioned response; CS, conditioned stimulus; CS2, new conditioned stimulus.
Interoceptive sensations have been used as the US or CS in fear conditioning experiments, and these studies may be useful in understanding the role of fear learning among individuals with PTSD, although these studies have primarily involved healthy individuals (67) or those with panic disorder (40) or drug dependence (68). Fear conditioning studies pairing neutral external cues (CS) with aversive internal sensations (US) report that healthy individuals rate the external cues as scarier after pairing with internal aversive sensations (e.g., rectal distension) and show increased brain activity in areas associated with fear (i.e., inferior, middle, and superior frontal gyrus; anterior insula; midcingulate cortex; inferior and superior parietal lobe; pallidum; putamen; and thalamus) when seeing the visual cue that had been paired with a visceral pain stimulus (rectal balloon distension) (69). This work demonstrates how interoceptive responses to a traumatic event may serve as part of an unconditioned response that is aversive, in and of itself, thus becoming a part of the US that can serve as the basis for further fear conditioning to external cues that become subsequently paired with these interoceptive responses—thus contributing to increased generalization of the fear.
Fewer laboratory-based studies have examined fear acquisition by using interoceptive signals as the CS. One study (70) demonstrated that when mild dyspnea (CS) was paired with an aversive shock (US), healthy individuals demonstrated higher expectancy ratings and a greater skin conductance response (SCR) to the CS, but no difference from the unpaired condition with respect to startle-induced eye blinks. Interestingly, studies (36, 67, 71) have shown that pairing harmless but aversive internal bodily sensations (e.g., brief partial breathing obstruction or CO2 inhalation as the CS) with painful or panic-inducing sensations (e.g., full occlusive breathing obstruction or longer duration CO2 inhalation as the US) creates strong associations that are hard to eliminate and may lead to avoidance behaviors. Healthy individuals show heightened physiological (e.g., skin conductance) responses and threat reactions to these pairings. Attending to body sensations before the pairing can make the associations even stronger (72). This phenomenon is thought to be particularly relevant for individuals with panic disorder or chronic pain, who may learn to fear and then avoid harmless bodily sensations that signal the onset of attacks or pain, thus reinforcing these associations and leading to further attempts to avoid experiencing these situations. However, this phenomenon may also be highly relevant to PTSD, in which distressing interoceptive sensations (e.g., feelings of hyperventilation or chest pain) that serve originally as unconditioned responses to an event become associated with milder or neutral interoceptive sensations (e.g., elevated heart rate and increased breathing rate, such as that experienced during exercise).
Although studies of interoceptive conditioning have largely probed fear acquisition by using explicit interoceptive cues, some evidence also suggests that interoceptive attention modulates the strength of associations between exteroceptive CS and US pairings. In one study, healthy participants completed a spatial cuing task with subliminal visual cues (i.e., angry face stimuli masked by neutral faces preceding a target image) paired with an auditory US. Participants assigned to complete a heartbeat detection task before conditioning showed slower response times for invalid CS– trials compared with CS+ trials, demonstrating a greater cue validity effect (73). This result raises the possibility that one’s acute state of interoceptive awareness—which could be a product of trait levels of interoceptive awareness in addition to situational attributes prior to the trauma—could influence rates of fear acquisition.
Extinction Learning
In adaptive trauma recovery, fear extinction often occurs naturally, when cues that may have been present during a traumatic experience are reencountered without a negative outcome occurring. However, in PTSD, posttraumatic symptoms are maintained by reinforcement of conditioned fear, likely through avoidance (preventing safety learning) and differences in appraisal of outcomes of encounters with conditioned cues. As noted previously, interoceptive sensations could serve as unconditioned responses to a trauma, a CS that is paired with external cues during the trauma, and subsequently as a US in further higher-order conditioning of other cues that are associated with these internal sensations. In addition, the role of interoception in making prospective predictions about the future would squarely implicate it in avoidance-based decision making; and the role of interoception in the signaling of prediction errors would implicate it in corrective learning, such as fear extinction (64).
Extinction learning relies on hippocampal-dependent memory processes needed to recall prior learning and to differentiate new learning (74). Evidence from a nonclinical sample (75) suggested that the hippocampus is recruited similarly during extinction learning for both interoceptive and exteroceptive cues. However, interoceptive cues may require more unreinforced presentations for extinction learning to occur and may elicit greater reinstatement of fear following extinction. In PTSD, reduced hippocampal volume and function has been strongly linked to deficits in extinction learning and context processing (76, 77). Moreover, the hippocampus has been implicated in the management of memory-based predictions in both interoception and PTSD (4). These findings suggest that an increased incorporation of interoceptive responses into the initial or high-order conditioned fear responses after a trauma may result in greater difficulties in fear extinction and could thus contribute to the maintenance of PTSD.
Interoceptive dysfunction associated with multiple forms of psychopathology has been proposed to relate to decreased awareness of prediction errors, where prior negative outcomes influence predictions about outcomes of future events, i.e., active inference (78). This framework of interoceptive dysregulation has particular relevance for threat and safety learning associated with PTSD: Heightened awareness of physiological reactivity and attention to interoceptive sensations may result in more negative predictions for outcomes of future encounters in the presence of conditioned cues, which in turn may result in greater avoidance of those cues and prevention of successful extinction learning. On the other hand, decreased awareness of internal sensations (e.g., in the case of dissociation or blunted physiological responses) and/or heightened baseline physiological arousal could result in a signal-to-noise problem in optimally interpreting prediction signals (79) (Figure 3). Thus, this dysfunction could result in less trust of one’s own gut instincts, difficulty in decision-making, and variability of engagement in risky versus inhibited behavior—a phenomenon often observed among individuals with PTSD (e.g., avoidance of some anxiety-provoking situations but engagement in behaviors such as reckless driving or risky sexual behavior) (80).
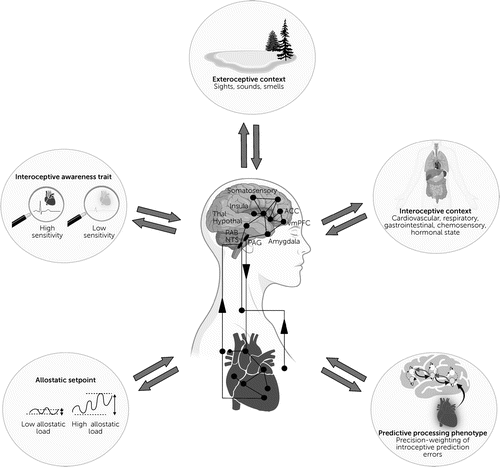
FIGURE 3. Factors that can influence brain-body communication in PTSDa
aThese factors include exteroceptive and interoceptive contexts, interoceptive awareness traits, allostatic load, and predictive processing phenotypes. Thal, thalamus; Hypothal, hypothalamus; PAB, parabrachial nucleus; NTS, nucleus tractus solitarius; PAG, periaqueductal gray matter; ACC, anterior cingulate cortex; vmPFC, ventromedial prefrontal cortex.
There is some evidence supporting the view that interoceptive sensitivity and regulation, prior to conditioning, may affect subsequent extinction learning. Both threat responsivity and heart rate variability (HRV, i.e., the temporal variance between heart beats) are modulated by the medial PFC, and higher HRV has been hypothesized as a proxy for greater inhibitory control (81); consistent with this hypothesis, one study (82) demonstrated that higher baseline HRV was associated with greater extinction learning. Studies conducted with healthy humans (83) and animals (84) have demonstrated that inflammation and sensitivity to interoceptive sensations (e.g., immobility during CO2 inhalation) during acquisition of conditioned fear may contribute to greater resistance to extinction learning. These findings are consistent with evidence that cardiac and respiratory changes acutely following a traumatic experience, and the ability to regulate these changes, may relate to one’s risk for developing PTSD symptoms—with the specific implication that cardiac responses may influence the specificity of encoding of the trauma (85). In addition, inhibition of the insular cortex in mice has even been associated with deficits in extinction memory (86), suggesting that altering neural circuits involved in interoception might disrupt extinction learning and thus contribute to the maintenance of PTSD.
Interoception in Context Processing: Neural Mechanisms and Behavioral Implications
From an interoceptive standpoint, the body may be viewed as a lens through which the world is experienced and remembered. Contextual factors surrounding the occurrence of conditioned cues can thus meaningfully inform whether these cues may or may not be predictive of threat, and these factors may be extended to include the state of the body at the time of the traumatic event. Individuals with PTSD have shown deficits in contextual processing (16, 17), contributing to impaired extinction learning when CSs are presented in safe contexts. Studies examining context processing in PTSD typically focus on external cues and contexts (e.g., rooms paired with colored lights as the CS) (19). However, interoceptive body sensations may also serve as negative internal contexts, conveying information to the nervous system that could reinforce threat conditioning to external cues or prevent safety learning through extinction. Conversely, external contexts (i.e., spatial or temporal) could inform when bodily symptoms become predictive of negative outcomes (Figure 3). For example, increased cardiovascular arousal in the gym may not be predictive of negative outcomes. However, the same responses when walking in a dark alley may be a response to help facilitate hypervigilance to potential threats. In turn, if one’s physiological arousal is blunted during a potential fear-related context (e.g., because of alcohol use or other drugs), this blunting may contribute to reductions in predicted negative outcomes to that context. The potentially important role of interoceptive signaling in fear learning was elegantly demonstrated in a study (87) reporting higher SCR responses when exteroceptive cues were paired with aversive shocks during cardiac systole compared with diastole. This finding suggests that differences in physiological activity during safe and threatening contexts, and awareness of these differences, may be useful for individuals when discriminating between safe and threatening contexts.
Few studies have used interoceptive cues in contextual conditioning paradigms among humans, and to our knowledge, no studies have been conducted among individuals with PTSD. In a sample of healthy participants (88), benign visceral sensations (CS) paired with painful stimulation (US) in a context connoting threat were associated with increased activation in the vmPFC, amygdala, and hippocampus. In the context connoting safety, greater hippocampal responsivity to the CS was related to less negative valence ratings of the context and greater expectancy ratings. Another study (89) demonstrated that suffocation fear moderated the persistence of startle eye blink responses to mild dyspnea (CS, induced via a small inspiratory breathing resistive load) that preceded a severe dyspnea (US, induced via maximally tolerable breathing load), in a context where presence of the US was unpredictable. Contextual conditioning was not reflected by explicit arousal and valence ratings nor was there evidence of declarative memories for the various experimental contingencies. In addition to providing evidence that context-specific conditioning to benign interoceptive cues can recruit fear neurocircuitry, these studies have illustrated how there can be discrepancies between conditioning and conscious awareness via verbal report.
Animal models have further corroborated the role of interoception in contextual processing. One study conducted with rats (90) reported evidence of context-dependent conditioning, with differences in acute startle responses to interoceptive CSs but not to exteroceptive CSs, in threatening compared with safe contexts. Another study (91) demonstrated disrupted hippocampal engagement and extinction learning in mice when internal, rather than external, context was manipulated through ethanol administration during fear acquisition. Further support for interoceptive involvement in context processing was evidenced by a study (92) in which inactivation of the insular cortex in mice contributed to reduced freezing behavior in response to predator odor, as well as reduced contextual learning.
Situating Interoception in PTSD Treatment and Outcomes
Given the varied role that interoceptive processing likely plays in fear learning, there are numerous ways in which one could imagine targeting interoception when attempting to improve behavioral and psychopharmacological treatment outcomes for patients with PTSD. These possibilities include facilitating extinction to interoceptive experiences associated with the trauma via exposure-based therapy, to break the cycle of avoidance of these signals and interrupt further higher-order conditioning to interoceptive cues; boosting the interoceptive signal-to-noise ratio in an effort to increase interoceptive awareness, which could potentially change the specificity of trauma memories or higher-order conditioning, increase confidence in one’s own internal sensations and predictive cues, and enhance awareness of and learning from prediction errors; and directly modulating interoceptive signaling, via pharmacologic interventions, to strengthen or weaken the salience of these cues and their associated predicted negative outcomes to modulate fear learning.
Evidence-based behavioral interventions (e.g., prolonged exposure therapy) use inhibitory learning to target avoidance surrounding trauma reminders and memories through repeated exposure. These interventions often target exteroceptive trauma reminders (i.e., through in vivo exposures), whereas interoceptive exposures specifically focused on eliciting feared internal sensations (e.g., running in place to increase heart rate) are often reserved primarily for panic disorder (93). However, preliminary evidence has suggested that interoceptive exposure therapies for PTSD are effective in reducing trauma-related symptoms (94–96). Interoceptive exposures for PTSD often involve inducing the interoceptive sensations (e.g., elevated heart rate, shallow breathing) that were experienced during the traumatic event, but in a safe context (i.e., therapy setting), to target expectancy of predicted negative outcomes (e.g., experiencing further trauma or expectancies about not being able to tolerate the distress). Additionally, interoceptive exposures for PTSD may be combined with imaginal or in vivo exposures to optimize inhibitory learning (97). Inducing interoceptive sensations may elicit memories of the traumatic event that could be elaborated on through a combined imaginal exposure. Exposure to combinations of interoceptive and external contextual cues, in tandem, may also maximize safety learning by enhancing prediction error and awareness of these errors.
Given previous evidence that sensitivity to interoceptive state may influence extinction learning and serve as a meaningful internal context that could influence outcomes, it has been argued that behavioral interoceptive interventions focused on enhancing interoceptive awareness could enhance learning (53). Several interventions may be useful for targeting enhanced interoceptive awareness and regulation in the treatment of PTSD. For example, there is evidence that mindfulness-based and mind-body interventions (e.g., yoga, tai chi) may be effective for some individuals with PTSD (98, 99). Potential mechanisms for these benefits include increased attentional control, openness to experience, nonjudgmental acceptance of cognitions, as well as greater connection and awareness of interoceptive sensations. Neuroimaging research to date suggests that mindfulness may exert its effects by influencing brain activation and connectivity within and across the default mode network (e.g., posterior cingulate cortex, medial PFC), salience network (e.g., insula cortex, dorsal ACC), and executive network (e.g., lateral PFC) (98). In addition, there is some evidence that mindfulness interventions may increase extinction retention (100) and have an impact on hippocampal-dependent contextual retrieval (101). Floatation-Reduced Environmental Stimulation Therapy is an intervention that increases interoceptive awareness in part by attenuating exteroceptive sensory input (102, 103), but thus far no studies have focused exclusively on individuals with PTSD. Further research is warranted to identify whether interventions directly targeting interoceptive awareness may be beneficial for individuals with PTSD, to identify strategies for optimizing the impact of mind-body interventions on interoceptive awareness, and to delineate how targeting interoceptive awareness may affect specific fear learning processes.
Pharmacologic manipulations may be used to directly enhance or dampen physiological responsivity in ways that could affect interoceptive awareness, fear learning, or PTSD symptoms. Prior research (104) has also suggested that extinction learning during behavioral interventions may be enhanced through combination with pharmacological approaches. For example, propranolol has been paired with trauma recollections as a way of disrupting the reconsolidation of trauma memories by reducing sympathetic arousal and has been found in some studies to lead to a reduction in PTSD symptoms although the findings have been mixed (105), and benefits may be more likely if propranolol is combined with exposure therapy soon after a traumatic event (106). Interestingly, a review (107) of pretreatment biomarkers for PTSD found that greater heart rate reactivity (e.g., change in heart rate in response to external stressor), but not SCR, to trauma reminders at the onset of PTSD treatment predicted posttreatment symptom reduction. The beneficial effects observed with propranolol may in part be due to increases in prediction error when the interoceptive signals one expects to experience during trauma recall are not experienced. Pharmacologic manipulations that enhance sympathetic arousal (e.g., isoproterenol) have been used as a way of assessing interoceptive awareness across states of homeostatic perturbation (51, 108). This method may potentially also be used to train interoceptive awareness or to enhance interoceptive exposure-based therapy. In an intriguing pilot study (109), the application of methylphenidate, a central nervous system stimulant that augments cerebral dopaminergic and noradrenergic function, was well tolerated, and found to reduce PTSD symptoms of reexperiencing, avoidance, and hyperarousal. Although mechanistically unclear, the possibility that modulating dopaminergic and noradrenergic arousal might help in improving interoceptive awareness, by increasing the interoceptive signal-to-noise ratio and by reducing the unpredictability of the internal world, appears worthy of follow-up. Overall, further work is needed to identify how interoceptive perturbations may influence fear learning processes in PTSD and to understand how to optimize the use of interoceptive processing to enhance prediction error or other fear extinction processes.
Conclusions
PTSD is a complex and heterogeneous disorder that develops after exposure to one or more traumatic events. Although fear learning processes have been at the core of leading theories regarding the development, maintenance, and treatment of PTSD, the role of interoception in affective processing, and its intersection with fear learning, remains largely unexplored. Future research that examines the role of interoception in the development, maintenance, and treatment of PTSD has the potential to offer unique insight and to advance our understanding of this disorder. Further empirical and clinical research integrating the study of interoception and associative learning in animal models of trauma, as well as among individuals with PTSD, may inform new treatment approaches for the disorder.
1 Diagnostic and Statistical Manual of Mental Disorders, 5th ed. American Psychiatric Association, Arlington, VA, 2013 Google Scholar
2 : Considering PTSD for DSM-5. Depress Anxiety 2011; 28:750–769Crossref, Google Scholar
3 : 636,120 ways to have posttraumatic stress disorder. Perspect Psychol Sci 2013; 8:651–662Crossref, Google Scholar
4 : Deconstructing the gestalt: mechanisms of fear, threat, and trauma memory encoding. Neuron 2019; 102:60–74Crossref, Google Scholar
5 : Age of trauma onset and HPA axis dysregulation among trauma-exposed youth. J Trauma Stress 2015; 28:572–579Crossref, Google Scholar
6 : Post-traumatic stress disorder across the adult lifespan: findings from a nationally representative survey. Am J Geriatr Psychiatry 2016; 24:81–93Crossref, Google Scholar
7 : The epidemiology of traumatic event exposure worldwide: results from the World Mental Health Survey Consortium. Psychol Med 2016; 46:327–343Crossref, Google Scholar
8 : The epidemiology of DSM-5 posttraumatic stress disorder in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions–III. Soc Psychiatry Psychiatr Epidemiol 2016; 51:1137–1148Crossref, Google Scholar
9 : Molecular mechanisms of fear learning and memory. Cell 2011; 147:509–524Crossref, Google Scholar
10 : Fear conditioning and extinction across development: evidence from human studies and animal models. Biol Psychol 2014; 100:1–12Crossref, Google Scholar
11 : Mechanisms of fear extinction. Mol Psychiatry 2007; 12:120–150Crossref, Google Scholar
12 : Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learn Mem 2006; 85:213–218Crossref, Google Scholar
13 : Fear generalization and anxiety: behavioral and neural mechanisms. Biol Psychiatry 2015; 78:336–343Crossref, Google Scholar
14 : From Pavlov to PTSD: the extinction of conditioned fear in rodents, humans, and anxiety disorders. Neurobiol Learn Mem 2014; 113:3–18Crossref, Google Scholar
15 : Neurobiological basis of failure to recall extinction memory in posttraumatic stress disorder. Biol Psychiatry 2009; 66:1075–1082Crossref, Google Scholar
16 : The contextual brain: implications for fear conditioning, extinction and psychopathology. Nat Rev Neurosci 2013; 14:417–428Crossref, Google Scholar
17 : Context processing and the neurobiology of post-traumatic stress disorder. Neuron 2016; 92:14–30Crossref, Google Scholar
18 : Behavioral and central correlates of contextual fear learning and contextual modulation of cued fear in posttraumatic stress disorder. Int J Psychophysiol 2015; 98:584–593Crossref, Google Scholar
19 : Impaired contextual modulation of memories in PTSD: an fMRI and psychophysiological study of extinction retention and fear renewal. J Neurosci 2014; 34:13435–13443Crossref, Google Scholar
20 : Topographic organization of neurons in the acoustic thalamus that project to the amygdala. J Neurosci 1990; 10:1043–1054Crossref, Google Scholar
21 : Trace fear conditioning differentially modulates intrinsic excitability of medial prefrontal cortex–basolateral complex of amygdala projection neurons in infralimbic and prelimbic cortices. J Neurosci 2015; 35:13511–13524Crossref, Google Scholar
22 : Functional interplay between central and autonomic nervous systems in human fear conditioning. Trends Neurosci 2022; 45:504–506Crossref, Google Scholar
23 : Orchestration of innate and conditioned defensive actions by the periaqueductal gray. Neuropharmacology 2023; 228:109458Crossref, Google Scholar
24 : The periaqueductal gray and primal emotional processing critical to influence complex defensive responses, fear learning and reward seeking. Neurosci Biobehav Rev 2017; 76:39–47Crossref, Google Scholar
25 : Integrated cardio-behavioral responses to threat define defensive states. Nat Neurosci 2023; 26:447–457Crossref, Google Scholar
26 : Neuronal circuits for fear expression and recovery: recent advances and potential therapeutic strategies. Biol Psychiatry 2015; 78:298–306Crossref, Google Scholar
27 : Recall of fear extinction in humans activates the ventromedial prefrontal cortex and hippocampus in concert. Biol Psychiatry 2007; 62:446–454Crossref, Google Scholar
28 : A review of hippocampal activation in post-traumatic stress disorder. Psychophysiology 2019; 57:e13357Google Scholar
29 : Appraisal theories of emotion: state of the art and future development. Emot Rev 2013; 5:119–124 Crossref, Google Scholar
30 : Discussion: the physical basis of emotion. Psychol Rev 1894; 1:516–529 Crossref, Google Scholar
31 : The varieties of emotional experience: a meditation on James-Lange theory. Psychol Rev 1994; 101:211–221Crossref, Google Scholar
32 : A diencephalic mechanism for the expression of rage with special reference to the sympathetic nervous system. Am J Physiol 1928; 84:490–515 Crossref, Google Scholar
33 : The Cannon–Bard thalamic theory of emotions: a brief genealogy and reappraisal. Emot Rev 2014; 6:13–20 Crossref, Google Scholar
34 : Cognitive, social, and physiological determinants of emotional state. Psychol Rev 1962; 69:379–399Crossref, Google Scholar
35 : What is an emotion? Curr Biol 2019; 29:R1060–R1064Crossref, Google Scholar
36 : Interoceptive fear conditioning and panic disorder: the role of conditioned stimulus–unconditioned stimulus predictability. Behav Ther 2012; 43:174–189Crossref, Google Scholar
37 : An insular view of anxiety. Biol Psychiatry 2006; 60:383–387Crossref, Google Scholar
38 : Initial evidence of a failure to activate right anterior insula during affective set shifting in posttraumatic stress disorder. Psychosom Med 2009; 71:373–377Crossref, Google Scholar
39 : Clinical implications of neuroscience research in PTSD. Ann N Y Acad Sci 2006; 1071:277–293Crossref, Google Scholar
40 : Interoception, conditioning, and fear: the panic threesome. Psychophysiology 2019; 56:e13421Crossref, Google Scholar
41 : Interoceptive exposure therapy combined with trauma‐related exposure therapy for post‐traumatic stress disorder: a case report. Cogn Behav Ther 2005; 34:34–40Crossref, Google Scholar
42 : Interoception and mental health: a roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging 2018; 3:501–513Crossref, Google Scholar
43 : A physiologist’s view of homeostasis. Adv Physiol Educ 2015; 39:259–266Crossref, Google Scholar
44 : Allostasis: a model of predictive regulation. Physiol Behav 2012; 106:5–15Crossref, Google Scholar
45 : Interoception and the origin of feelings: a new synthesis. BioEssays 2021; 43:2000261Crossref, Google Scholar
46 : Neural circuits of interoception. Trends Neurosci 2021; 44:17–28Crossref, Google Scholar
47 : How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002; 3:655–666Crossref, Google Scholar
48 : The insular cortex dynamically maps changes in cardiorespiratory interoception. Neuropsychopharmacology 2018; 43:426–434Crossref, Google Scholar
49 : Conjoint activity of anterior insular and anterior cingulate cortex: awareness and response. Brain Struct Funct 2010; 214:535–549Crossref, Google Scholar
50 : Saliency, switching, attention and control: a network model of insula function. Brain Struct Funct 2010; 214:655–667Crossref, Google Scholar
51 : Association of generalized anxiety disorder with autonomic hypersensitivity and blunted ventromedial prefrontal cortex activity during peripheral adrenergic stimulation: a randomized clinical trial. JAMA Psychiatry 2022; 79:323–332Crossref, Google Scholar
52 : Can interoception improve the pragmatic search for biomarkers in psychiatry? Front Psychiatry 2016; 7:121Crossref, Google Scholar
53 : Interventions and manipulations of interoception. Trends Neurosci 2021; 44:52–62Crossref, Google Scholar
54 : Childhood trauma affects stress-related interoceptive accuracy. Front Psychiatry 2019; 10:750Crossref, Google Scholar
55 : A multi-method assessment of interoception among sexual trauma survivors. Physiol Behav 2020; 226:113108Crossref, Google Scholar
56 : Trauma-related dissociation and altered states of consciousness: a call for clinical, treatment, and neuroscience research. Eur J Psychotraumatol 2015; 6:27905Crossref, Google Scholar
57 : The brain-body disconnect: a somatic sensory basis for trauma-related disorders. Front Neurosci 2022; 16:1015749Crossref, Google Scholar
58 : Neurobiology of PTSD: a review of neuroimaging findings. Psychiatr Ann 2009; 39:370–381Crossref, Google Scholar
59 : Unique insula subregion resting-state functional connectivity with amygdala complexes in posttraumatic stress disorder and its dissociative subtype. Psychiatry Res Neuroimaging 2016; 250:61–72Crossref, Google Scholar
60 : The role of racial discrimination in dissociation and interoceptive dysfunction. Neuropsychopharmacology 2023; 48:225–227Crossref, Google Scholar
61 : The functional neuroanatomy of PTSD: a critical review; in Stress Hormones and Post Traumatic Stress Disorder: Basic Studies and Clinical Perspectives. Edited by De Kloet RE, Oitzl MS. Amsterdam, Elsevier, 2007 Crossref, Google Scholar
62 : Fear conditioning in posttraumatic stress disorder: evidence for delayed extinction of autonomic, experiential, and behavioural responses. Behav Res Ther 2007; 45:2019–2033Crossref, Google Scholar
63 : In-the-moment dissociation, emotional numbing, and sexual risk: the influence of sexual trauma history, trauma symptoms, and alcohol intoxication. Psychol Violence 2016; 6:586–595Crossref, Google Scholar
64 : Rethinking post-traumatic stress disorder—a predictive processing perspective. Neurosci Biobehav Rev 2020; 113:448–460Crossref, Google Scholar
65 : Reduced amygdala responsivity during conditioning to trauma-related stimuli in posttraumatic stress disorder. Psychophysiology 2016; 53:1460–1471Crossref, Google Scholar
66 : Learning models of PTSD: theoretical accounts and psychobiological evidence. Int J Psychophysiol 2015; 98:594–605Crossref, Google Scholar
67 : Learning to fear suffocation: a new paradigm for interoceptive fear conditioning. Psychophysiology 2012; 49:821–828Crossref, Google Scholar
68 : The role of interoception in addiction: a critical review. Neurosci Biobehav Rev 2012; 36:1857–1869Crossref, Google Scholar
69 : Temporal dynamics of fMRI signal changes during conditioned interoceptive pain-related fear and safety acquisition and extinction. Behav Brain Res 2022; 427:113868Crossref, Google Scholar
70 : Interoceptive cues predicting exteroceptive events. Int J Psychophysiol 2016; 109:100–106Crossref, Google Scholar
71 : Interoceptive fear conditioning as a learning model of panic disorder: an experimental evaluation using 20% CO(2)-enriched air in a non-clinical sample. Behav Res Ther 2007; 45:2280–2294Crossref, Google Scholar
72 : The role of “interoceptive” fear conditioning in the development of panic disorder. Behav Ther 2012; 43:203–215Crossref, Google Scholar
73 : Interoceptive awareness and unaware fear conditioning: are subliminal conditioning effects influenced by the manipulation of visceral self-perception? Conscious Cogn 2011; 20:1393–1402Crossref, Google Scholar
74 : Molecular specificity of multiple hippocampal processes governing fear extinction. Rev Neurosci 2010; 21:1–17Crossref, Google Scholar
75 : Associative learning and extinction of conditioned threat predictors across sensory modalities. Commun Biol 2021; 4:553Crossref, Google Scholar
76 : Configural cue performance in identical twins discordant for posttraumatic stress disorder: theoretical implications for the role of hippocampal function. Biol Psychiatry 2007; 62:513–520Crossref, Google Scholar
77 : Smaller hippocampal volume in posttraumatic stress disorder: a multisite ENIGMA-PGC study: subcortical volumetry results from posttraumatic stress disorder consortia. Biol Psychiatry 2018; 83:244–253Crossref, Google Scholar
78 : An active inference approach to interoceptive psychopathology. Annu Rev Clin Psychol 2019; 15:97–122Crossref, Google Scholar
79 : Interoception in anxiety and depression. Brain Struct Funct 2010; 214:451–463Crossref, Google Scholar
80 : PTSD’s risky behavior criterion: relation with DSM-5 PTSD symptom clusters and psychopathology. Psychiatry Res 2017; 252:215–222Crossref, Google Scholar
81 : A meta-analysis of heart rate variability and neuroimaging studies: implications for heart rate variability as a marker of stress and health. Neurosci Biobehav Rev 2012; 36:747–756Crossref, Google Scholar
82 : Resting heart rate variability predicts safety learning and fear extinction in an interoceptive fear conditioning paradigm. PLoS One 2014; 9:e105054Crossref, Google Scholar
83 : Inflammation shapes neural processing of interoceptive fear predictors during extinction learning in healthy humans. Brain Behav Immun 2023; 108:328–339Crossref, Google Scholar
84 : High behavioral sensitivity to carbon dioxide associates with enhanced fear memory and altered forebrain neuronal activation. Neuroscience 2020; 429:92–105Crossref, Google Scholar
85 : Psychophysiology in the study of psychological trauma: where are we now and where do we need to be? Curr Top Behav Neurosci 2014; 21:157–183Crossref, Google Scholar
86 : Insular cortical circuits as an executive gateway to decipher threat or extinction memory via distinct subcortical pathways. Nat Commun 2022; 13:5540Crossref, Google Scholar
87 : Interoceptive cardiac signals selectively enhance fear memories. J Exp Psychol Gen 2021; 150:1165–1176Crossref, Google Scholar
88 : When gut feelings teach the brain to fear pain: context-dependent activation of the central fear network in a novel interoceptive conditioning paradigm. NeuroImage 2021; 238:118229Crossref, Google Scholar
89 : Cue and context conditioning to respiratory threat: effects of suffocation fear and implications for the etiology of panic disorder. Int J Psychophysiol 2018; 124:33–42Crossref, Google Scholar
90 : Mild interoceptive stressors affect learning and reactivity to contextual cues: toward understanding the development of unexplained illnesses. Neuropsychopharmacology 2005; 30:1483–1491Crossref, Google Scholar
91 : Hippocampal encoding of interoceptive context during fear conditioning. Transl Psychiatry 2017; 7:e991Crossref, Google Scholar
92 : Interoceptive insular cortex mediates both innate fear and contextual threat conditioning to predator odor. Front Behav Neurosci 2020; 13:283Crossref, Google Scholar
93 : Origins and outlook of interoceptive exposure. J Behav Ther Exp Psychiatry 2016; 53:41–51Crossref, Google Scholar
94 : Posttraumatic stress disorder and chronic pain arising from motor vehicle accidents: efficacy of interoceptive exposure plus trauma-related exposure therapy. Cogn Behav Ther 2010; 39:104–113Crossref, Google Scholar
95 : Efficacy of interoceptive exposure therapy combined with trauma-related exposure therapy for posttraumatic stress disorder: a pilot study. J Anxiety Disord 2007; 21:1050–1060Crossref, Google Scholar
96 : Responses to interoceptive exposure in people with posttraumatic stress disorder (PTSD): a preliminary analysis of induced anxiety reactions and trauma memories and their relationship to anxiety sensitivity and PTSD symptom severity. Cogn Behav Ther 2008; 37:90–100Crossref, Google Scholar
97 : Maximizing exposure therapy: an inhibitory learning approach. Behav Res Ther 2014; 58:10–23Crossref, Google Scholar
98 : Mindfulness-based treatments for posttraumatic stress disorder: a review of the treatment literature and neurobiological evidence. J Psychiatry Neurosci 2018; 43:7–25Crossref, Google Scholar
99 : Mindfulness and yoga for psychological trauma: systematic review and meta-analysis. J Trauma Dissociation 2020; 21:536–573Crossref, Google Scholar
100 : The effect of mindfulness training on extinction retention. Sci Rep 2019; 9:19896Crossref, Google Scholar
101 : Strengthened hippocampal circuits underlie enhanced retrieval of extinguished fear memories following mindfulness training. Biol Psychiatry 2019; 86:693–702Crossref, Google Scholar
102 : The elicitation of relaxation and interoceptive awareness using floatation therapy in individuals with high anxiety sensitivity. Biol Psychiatry Cogn Neurosci Neuroimaging 2018; 3:555–562Crossref, Google Scholar
103 : Reduced environmental stimulation in anorexia nervosa: an early-phase clinical trial. Front Psychol 2020; 11:567499Crossref, Google Scholar
104 : Can fear extinction be enhanced? A review of pharmacological and behavioral findings. Brain Res Bull 2014; 105:46–60Crossref, Google Scholar
105 : Effects of propranolol on the modification of trauma memory reconsolidation in PTSD patients: a systematic review and meta-analysis. J Psychiatr Res 2022; 150:246–256Crossref, Google Scholar
106 : Revisiting propranolol and PTSD: memory erasure or extinction enhancement? Neurobiol Learn Mem 2016; 130:26–33Crossref, Google Scholar
107 : Pretreatment biomarkers predicting PTSD psychotherapy outcomes: a systematic review. Neurosci Biobehav Rev 2017; 75:140–156Crossref, Google Scholar
108 : Bolus isoproterenol infusions provide a reliable method for assessing interoceptive awareness. Int J Psychophysiol 2009; 72:34–45Crossref, Google Scholar
109 : Randomized placebo-controlled trial of methylphenidate or galantamine for persistent emotional and cognitive symptoms associated with PTSD and/or traumatic brain injury. Neuropsychopharmacology 2016; 41:1191–1198Crossref, Google Scholar



