Overview of Antidepressant Treatment of Bipolar Depression
Abstract
Bipolar depression remains a major unresolved challenge for psychiatric therapeutics. It is associated with significant disability and mortality and represents the major proportion of the approximately half of follow-up time spent in morbid states despite use of available treatments. Evidence regarding effectiveness of standard treatments, particularly with antidepressants, remains limited and inconsistent. We reviewed available clinical and research literature concerning treatment with antidepressants in bipolar depression and its comparison with unipolar depression. Research evidence concerning efficacy and safety of commonly used antidepressant treatments for acute bipolar depression is very limited. Nevertheless, an updated meta-analysis indicated that overall efficacy was significantly greater with antidepressants than with placebo-treatment and not less than was found in trials for unipolar major depression. Moreover, risks of non-spontaneous mood-switching specifically associated with antidepressant treatment are less than appears to be widely believed. The findings encourage additional efforts to test antidepressants adequately in bipolar depression, and to consider options for depression in types I vs. II bipolar disorder, depression with subsyndromal hypomania and optimal treatment of mixed agitated-dysphoric states – both short- and long-term. Many therapeutic trials considered were small, varied in design, often involved co-treatments, or lacked adequate controls.
Reprinted with permission from the International Journal of Neuropsychopharmacology 2013; 16:1673–1685
Introduction
Background
Bipolar disorder (BD) is a disabling, recurrent or chronic condition associated with functional impairment, psychiatric and somatic co-morbidity and premature mortality (Angst et al., 2002; Krishnan, 2005; Kupfer, 2005; Roshanaei-Moghaddam and Katon, 2009; de Hert et al., 2011; Leboyer et al., 2012). Lifetime prevalence was recently estimated to exceed 4% of the general population (Merikangas et al., 2007). Depressive, dysthymic and mixed (dyphoric-agitated) states contribute importantly to the total illness-burden of type I (with mania) and II (with hypomania) BD, and these components of future morbidity are predicted by similar first lifetime episodes (Baldessarini et al., 2010a, b, c, 2012). Several longitudinal studies indicate that the proportion of total time in depressive components of BD (averaging 75% of a total of 54% of weeks ill) is far greater than in manic or hypomanic phases, despite use of available treatments (Judd et al., 2002; Post et al., 2003a; Joffe et al., 2005; Paykel et al., 2006; Kupka et al., 2007; Baldessarini et al., 2010a, c). Depressive components of BD are associated with high morbidity (Baldessarini et al., 2010a, b; Leboyer et al., 2012), co-morbidity (Kilbourne et al., 2004; Bauer et al., 2005a, b), disability (Keck et al., 2008; Rosa et al., 2010), mortality (Ösby et al., 2001; Angst et al., 2002; Roshanaei-Moghaddam and Katon, 2009; Baldessarini et al., 2010d) and high levels of clinical and economic burdens to patients and their families (Kupka et al., 2007; Baldessarini et al., 2010d). In addition, both types I and II BD are associated with very high rates of suicide (about 20 times above rates in the general population) and other violent causes of death especially among young patients (Ösby et al., 2001; Tondo et al., 2003, 2007; Novick et al., 2010; Undurraga et al., 2012b), as well as similar total annual numbers of deaths associated with medical co-morbidity in older patients, albeit at far-lower standardized mortality rates (2–3 times above rates in the general population) than for suicide (Ösby et al., 2001; Tondo et al., 2003, 2007; Novick et al., 2010).
Despite the high prevalence and the major public health, clinical and economic significance of bipolar depression, few treatments are proved to be highly and consistently effective in acute episodes or to provide major prophylactic protection from recurrences of this major component of BD. The prevalent, related, dysthymic and dysphoric mixed-states of BD are even less extensively studied. These circumstances leave the depressive components of BD among the most critical, unsolved challenges for contemporary psychiatric therapeutics (Baldessarini, 2013).
Status of Antidepressants for Bipolar Depression
Opinions concerning the value of antidepressants to treat bipolar depression vary markedly and reflect the strikingly limited and inconsistent state of available research-based information. Specific clinical recommendations from international academic guidelines and expert consensus statements differ substantially in their assessments of the value and safety of antidepressants for the treatment of acute episodes of bipolar depression (National Institute for Health and Clinical Excellence, 2006; Goodwin, 2009; Yatham et al., 2009; Grunze et al., 2010; Nivoli et al., 2011). Commonly, they call for caution in the use of antidepressants or give priority to various mood-stabilizing agents or second-generation antipsychotics, in contrast to the very prevalent and sustained use of antidepressants in clinical practice (Baldessarini et al., 2007a, 2008; Angst et al., 2011; Heeren et al., 2011; Pacchiarotti et al., 2011a; Post et al., 2011; Lorenzo et al., 2012).
Well-designed, controlled trials of antidepressants for acute bipolar depression are rare, vary in size and quality and their findings have been notably inconsistent (Gjisman et al., 2004; Frye et al., 2011; Vázquez et al., 2011; Sidor and MacQueen, 2011, 2012; Amit and Weizman, 2012; Bauer et al., 2012; Fountoulakis et al., 2012). Concerns about inducing mania surely contribute to the routine exclusion of patients with identified BD from controlled trials of antidepressant drugs, contributing to the dearth of investigations of bipolar depression. Evidence of long-term, prophylactic benefit of antidepressants is even more limited, with greater concerns about increased long-term risk of mania, emotional de-stabilization or more rapid cycling of mood-states (Ghaemi et al., 2003, 2008, 2010; Leverich et al., 2006; Post et al., 2006; Pacchiarotti et al., 2011b; Sussman et al., 2012; Valentí et al., 2012). One meta-analysis of 12 heterogeneous, short-term trials that included both BD I and II patients in acute depression found that antidepressants of various types yielded a rather large apparent superiority in pooled response-rates vs. placebo or other control treatments, averaging 86% [95% confidence intervals (CI) 49–130] and for remission rates, 41% (95% CI 11–80; Gjisman et al., 2004). A meta-analytic review that included more recent trials found only small and non-significant pooled differences in short-term outcomes of randomly assigned treatment with antidepressants vs. placebo, with respect either to responses (18%; 95% CI –1 to 44) or remission (20%; 95% CI –2 to 47; Sidor and MacQueen, 2011). Even more recent updates also found little evidence for efficacy of antidepressants in acute bipolar depression (Amit and Weizman, 2012; Sidor and MacQueen, 2012).
Of particular note, two of the largest, well-designed trials found no added benefit associated with treatment with a serotonin reuptake inhibitor (SRI) antidepressant or bupropion (Sachs et al., 2007; McElroy et al., 2010). The first involved adding bupropion, paroxetine or placebo to ongoing treatment with mood-stabilizing or antipsychotic agents for 6 months in BD patients recovering from an acute episode of depression; lack of superior attainment of sustained remission with the antidepressants suggested that their addition to mood-stabilizing treatments afforded little additional benefit. The second, short-term trial compared depressed BD subjects randomized to paroxetine or placebo in trial-arms of a larger comparison with quetiapine, which was non-dose-dependently superior to placebo, whereas paroxetine was superior to placebo only in some outcomes and less effective than quetiapine. These trials have been taken as evidence that antidepressants may be ineffective for many depressed BD patients and other controlled trials have yielded inconsistent and conflicting findings.
Despite the limited and largely inconclusive nature of evidence concerning the efficacy and safety of antidepressants for bipolar depression, they may well be the most common clinically employed treatments for BD (Baldessarini et al., 2007a, 2008). This practice probably arises from the highly questionable assumption that ‘major depressive episodes’, whether in bipolar or non-bipolar disorders, are similar in their characteristics and treatment responses. We propose that the inconclusive state of available evidence concerning antidepressants in bipolar depression justifies further effort to update the status of randomized, controlled trials (RCTs) of antidepressant treatment in acute bipolar depression. The primary aim of this report was to consider available reported RCTs in order to update the available evidence, and also to compare the findings in BD with a recent, comprehensive meta-analysis of RCTs for acute, unipolar major depression (Undurraga and Baldessarini, 2012). We also consider other aspects of antidepressant treatment of BD patients as well as noting alternative treatment-approaches that have emerged recently or remain under investigation.
Method
We performed a comprehensive literature search for reports on treatments for bipolar depression, focusing on RCTs of antidepressants in acute major depressive episodes in patients diagnosed with type I or II BD. To identify RCTs of antidepressants, we carried out a systematic search of several literature databases (PubMed, PsycINFO, EMBASE and ClinicalTrials.gov). Search terms included various combinations of ‘antidepressant’, ‘bipolar depression’, ‘bipolar disorder’, ‘controlled trial’, ‘major depression’, ‘efficacy’, ‘randomized controlled trial’ and ‘treatment’. In addition, we reviewed citations in identified reports and systematic reviews on this topic (Gjisman et al., 2004; Frye et al., 2011; Vázquez et al., 2011; Sidor and MacQueen, 2011, 2012; Amit and Weizman, 2012; Bauer et al., 2012; Fountoulakis et al., 2012). This search initially identified 1250 reports of potential interest, of which 160 were considered sufficiently relevant as to require detailed review. This process yielded a total of 25 reports of controlled trials. Of these, 10 fulfilled selection criteria: (1) acute phase of major depressive episodes in types I or II BD diagnosed by standard, internationally accepted diagnostic criteria, with ≥ 10 patients/trial-arm; (2) randomized treatment; (3) antidepressant as monotherapy or add-on to mood-stabilizing treatment; (4) placebo control (±other comparators); (5) double-blinded; (6) neither concerning special populations such as juvenile or geriatric patients nor limited by subject-count or weeks of treatment. Another 15 trials were considered separately and not included in the reported meta-analysis.
Findings from trials meeting the preceding selection criteria were then subjected to categorical, random-effects meta-analyses (Stata®; StataCorp, USA) to determine antidepressant/placebo response-rate (usually defined as ≥ 50% improvement in depression ratings) ratios (RR) and rate differences (RD) with their 95% CI; we also tested for publication-bias and estimated number-needed-to-treat (NNT, as 1/RD).
Results
A summary of findings from the 10 placebo-controlled antidepressant trials meeting inclusion criteria identified during November 2012 is provided in Table 1. They involved a total of 1432 patients diagnosed with bipolar depression. These trials are few, heterogeneous in patient characteristics, duration and in additional treatments allowed, making conclusions highly tentative. Nevertheless, the crude pooled response rate with antidepressant treatment was 44.8% (256/571) vs. 33.4% (288/861) with placebo (χ2=17.7, p<0.0001). Random-effects meta-analysis indicated a relative RR=1.43 (95% CI 1.11–1.84), indicating significant superiority of anti-depressants (z=2.76, p<0.006). The RD also was significant [0.164 (95% CI 0.049–0.280); z=2.79, p=0.005] and the corresponding NNT was 6.2 (95% CI 3.6–6.7). Publication bias was not found by inspection of a funnel-plot nor significant statistically [Egger’s coefficient=3.79 (95% CI –0.58 to 8.16); Fig. 1].
| Trial | Bipolar types | Duration (wk) | Dropouts (%) | Antidepressant dose [mg/d (Imi-eq)] | Treatments | Responders/cases [n/N(%)] | |
|---|---|---|---|---|---|---|---|
| Antidepressant | Placebo | ||||||
| Cohn et al. (1989) | I + II | 6 | 53.9 | 50 (250) | Flx vs. Pbo ± Li | 26/30 (86.7%) | 11/29 (37.9%) |
| Cohn et al. (1989) | I + II | 6 | 53.9 | 188 (188) | Imi vs. Pbo ± Li | 17/30 (56.7%) | 11/29 (37.9%) |
| Nemeroff et al. (2001) | I + II | 10 | 23.3 | 33 (165) | Li ± ACs: + Prx vs. Pbo | 15/33 (45.5%) | 15/43 (34.9%) |
| Nemeroff et al. (2001) | I + II | 10 | 23.3 | 167(167) | Li ± ACs: + Imi vs. Pbo | 14/31 (45.2%) | 15/43 (34.9%) |
| Tohen et al. (2003) | I + II | 8 | 33.9 | 39 (195) | OFC vs. Pbo | 46/82 (56.1%) | 108/355 (30.4%) |
| Shelton and Stahl (2004) | I + II | 12 | 40.2 | 28 (140) | MS + Prx ± Rsp vs. Pbo ± Rsp | 5/20 (25.0%) | 3/10 (30.0%) |
| Agosti and Stewart (2007) | II | 6 | 6.0 | 255 (255) | Imi vs. Pbo | 13/23 (56.5%) | 5/22 (22.7%) |
| Agosti and Stewart (2007) | II | 6 | 6.0 | 65 (162) | Pnz vs. Pbo | 13/25 (52.0%) | 5/22 (22.7%) |
| Sachs et al. (2007) | I + II | 12 | 56.8 | 300 (165) or 30 (150) | Bup or Prx + MS vs. Pbo + MS | 42/179 (23.5%) | 51/187 (27.3%) |
| McElroy et al. (2010) | I + II | 8 | 33.9 | 20(100) | Prx vs. Pbo | 65/118 (55.1%) | 64/121 (52.9) |
| Overall (n = 10 trials) | I + II | 8.4 ± 2.5 | 34.2 ± 18.4 | 179 ± 47 (Imi-eq) | Antidepressants ± Others vs. Pbo | 256/571 (44.8%) | 288/861 (33.4%) |
Table 1. Randomized, Placebo-Controlled Trials of Antidepressants in Acute Depression in Bipolar Disorders
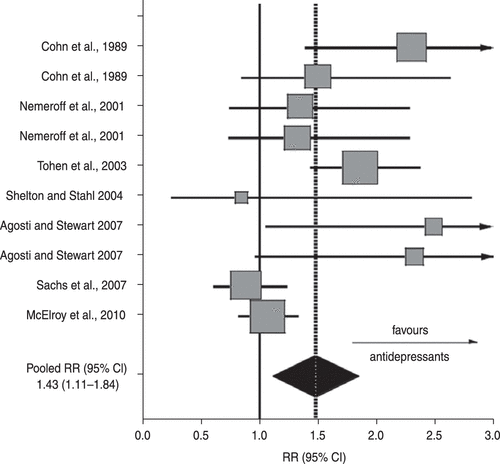
FIGURE. 1. Meta-Analytic Summary of 10 Randomized, Comparison Trials in Seven Studies of Short-Term Efficacy of Antidepressants vs. Placebo Controls in Acute Bipolar Depression (Reports Summarized in Table 1). Horizontal Bars=95 % Confidence Intervals (CI); Vertical Solid Bar=Null Value [Response-Rate Ratio (RR)=1.0] and Dotted Line=Pooled Drug/Placebo RR; Box Size is Proportional to the Weight of Each Trial. The Overall Drug/Placebo Response (Typically ≥ 50% Improvement in Depressive Symptom Ratings) Rate Ratio (RR; Black Diamond) was 1.43 (95 % CI 1.11–1.84), a Significant Difference (z = 2.76; p = 0.006), with an Estimated Number Needed to Treat (Reciprocal of Response-Rate Difference) of 6.1 (95% CI 3.6–20.5).
For comparison, the results of this meta-analysis for bipolar depression were compared with results of a comparable meta-analysis of findings from 142 placebo-controlled RCTs for antidepressants in unipolar major depressive disorder reported between 1980 and 2010 (Undurraga and Baldessarini, 2012). Although the outcomes in BD were inferior statistically (with much more limited power), the actual values of ratings of efficacy (RR and RD), as well as NNT estimates, were similar in both diagnostic groups (Table 2).
| Measure (95 %CI) | ||
|---|---|---|
| Measures | Bipolar | Unipolar |
| Trials | 10 | 142 |
| Antidepressant/control RR | 1.43 (1.11–1.84) | 1.42 (1.38–1.48) |
| Antidepressant/control RD | 0.164 (0.049–0.280) | 0.125 (0.111–0.141) |
| NNT | 6.2 (3.6–6.7) | 8.0 (7.1–9.1) |
Table 2. Meta-Analytic Findings from Placebo-Controlled Trials in Acute Bipolar vs. Unipolar Major Depression
We also considered reported rates of mood-switching into hypomania or mania in the studies analysed (not shown). These rates (with 95% CI) varied markedly by treatment-type, ranking: imipramine [26.7 (1.88–51.5)%]; modern antidepressants [SRIs or bupropion: 7.31 (2.79–11.8)%]; placebo [6.15 (3.47–8.83)%], with or without additional mood-stabilizing treatments, during an average exposure time of 2 months. This risk with imipramine was high, but with modern antidepressants it was only 1.16% greater than with placebo (spontaneous switching), although these differences were not statistically significant and need further study.
Findings from 15 other trials in depressed BD patients did not meet all study-inclusion criteria but several, involving an antidepressant vs. a comparison treatment, were of potential interest and are summarized as follows. Himmelhoch et al. (1991) found the monoamineoxidase (MAO) inhibitor tranylcypromine to be a significantly superior antidepressant to imipramine in bipolar depression (responders: 21/28 vs. 10/28; p=0.003), but lacked a placebo control. Tohen et al. (2003) found an olanzapine–fluoxetine combination to be superior to olanzapine monotherapy (responders: 46/82 vs. 137/351; p=0.005; this arm was excluded from meta-analysis as not comparing an antidepressant to placebo). Amsterdam and Shults (2005) compared three treatments to placebo in a small trial (eight or nine subjects/arm) involving types I and II, depressed BD patients: fluoxetine monotherapy; olanzapine monotherapy; their combination; placebo. Changes in depression ratings did not differ statistically but lacked statistical power. Brown et al. (2006) compared olanzapine–fluoxetine to lamotrigine without a placebo (responders: 141/205 vs. 122/205; p=0.05). Schaffer et al. (2006) compared citalopram to lamotrigine (responders: 4/10 vs. 2/10; p=0.33). Nolen et al. (2007) compared tranylcypromine [responders: 5/8 (62.5%)] to lamotrigine [4/11 (36.4%)] in treatment-refractory bipolar depressed patients (RR=1.72; not significant, but underpowered). Amsterdam and Shults (2008) compared venlafaxine to lithium (responders: 20/43 vs. 8/40; p=0.01). In six of these seven trials, treatments that included an antidepressant were more effective in bipolar depression than active comparators [by random-effects meta-analysis, pooled RR=1.49 (95% CI 1.17–1.89); z=3.22, p=0.001; six out of six trials, individually, involved significant differences; NNT=6.4 (95% CI 3.6–10.2)]. In addition, van der Loos et al. (2009) compared adding lamotrigine or placebo to lithium (responders: 33/64 vs. 19/60; p=0.025); non-responders also failed to respond to later, unblinded addition of paroxetine (van der Loos et al., 2010). Finally, several trials compared different antidepressants in bipolar depression and found no differences in responses, but lacked placebo controls (Bocchetta et al., 1993; Sachs et al., 1994; Grossman et al., 1999; Young et al., 2000; Silverstone, 2001; Post et al., 2006).
Discussion
Antidepressant Responses
The present primary meta-analysis indicated statistically significant overall efficacy of antidepressants vs. placebo in acute bipolar depression (Table 2), although we found only 10 trials meeting inclusion criteria, with variable conditions and findings across trials. There was also a rather low overall rate of response with antidepressant treatment (45%), but an unremarkable pooled response with placebo (33%; Table 1) that is similar to trials in unipolar depression (Undurraga and Baldessarini, 2012). This outcome may reflect some selection of BD patients who are clinically complex and may be experiencing depression despite mood-stabilizing treatments, although one would expect treatment-resistant sampling to yield low placebo- as well as drug-associated responses. Notably, the pooled antidepressant/placebo RR and differences in bipolar depression were not less than those found in a recent, comprehensive meta-analysis of RCTs in unipolar major depression [BD: RR=1.43 (95% CI 1.11–1.84) vs. unipolar: RR=1.42 (95% CI 1.38–1.48); Table 2; Undurraga and Baldessarini, 2012]. Our recent review of only 10 reports of direct comparisons of bipolar and unipolar depressed subjects also found very little difference in rates of response to antidepressant treatment by diagnosis (Vázquez et al., 2011). Moreover, the overall RR for mood-stabilizers vs. placebo in 18 RCTs in acute bipolar depression was only 1.30 (95% CI 1.16–1.44; van Lieshout and MacQueen, 2010), suggesting that mood-stabilizers may not be more effective than antidepressants and further underscoring the need for more effective treatments during depressive phases of BD.
The evidence of overall clinical efficacy of antidepressants in bipolar depression found in the present meta-analysis as well as in six out of six other RCTs comparing an antidepressant to various alternatives other than placebo-controls, if valid, raises questions about the striking dearth of trials of antidepressants in bipolar depression, in contrast to the large number of trials in patients diagnosed with unipolar major depression reported since the late 1950s (Undurraga and Baldessarini, 2012; Undurraga et al., 2012c). One possible basis of this striking research imbalance may be concern about the safety of antidepressant treatment in BD, especially type I, with risks of mania, psychosis and potentially dangerous behaviours associated with ‘mood- switches.’ Such concerns are suggested by the near-absence of RCTs in bipolar depression with corporate sponsorship identified in the present literature-search (a rare example being a study of the MAO inhibitor moclobemide by Silverstone, 2001) and the status of bipolarity as a routine exclusion criterion from most antidepressant trials. Such concerns probably also limit clinical use of antidepressants, particularly in type I BD patients (Heeren et al., 2011; Lorenzo et al., 2012; Undurraga et al., 2012a; Valentí et al., 2012).
Antidepressant treatment for BD patients is also encouraged by hoped-for, long-term prophylactic benefits against depressive recurrences, even though such effects remain unproved (Altshuler et al., 2003; Goldberg et al., 2007; Sachs et al., 2007; Ghaemi et al., 2008, 2010; Baldessarini et al., 2010d; Frye et al., 2011). Evidence for prophylactic effects of sustained antidepressant treatment, even in non-bipolar recurrent depressive illnesses, remains limited and ambiguous, with risk of confounding effects of discontinuing ongoing antidepressant treatment (Altshuler et al., 2003; Baldessarini et al., 2007a, 2008, 2010c; Vieta, 2008; Vöhringer and Ghaemi, 2011; Baldessarini, 2013).
Risks of Mood ‘Switching’
There has been concern about pathological activation of mood and behaviour during treatment with antidepressants, stimulants or other mood-elevating drugs for many years (Wehr and Goodwin, 1979; Peet, 1994; Wehr et al., 1988; Nemeroff et al., 2001; Vieta et al., 2002; Ghaemi et al., 2003, 2004, 2008, 2010; Post et al., 2006; Goldberg et al., 2007; Licht et al., 2008; Valenti et al., 2012). This concern arises mainly from treatment trials that are largely anecdotal and lack controls to test for a direct relationship between elevated mood and anti-depressant treatment, as opposed to spontaneous mood-shift characteristics of BD (Licht et al., 2008; Tondo et al., 2010). It is notable that risks of switching in the reports reviewed earlier were remarkably low when modern antidepressants were involved, and barely greater than during parallel treatment with a placebo. However, these samples include many type II BD patients, whose risk of readily identified and clinically dangerous switching appears to be limited and may be influenced further by prevalent use of mood-stabilizing co-treatments.
In a recent, comprehensive review of studies of spontaneous mood-switching and that associated with antidepressant treatment, rates of spontaneous hypomania or mania among patients diagnosed with a BD (type I or II), without exposure to an antidepressant were high (13.8% over an average of several months), but little-higher (15.3%) with antidepressants (Tondo et al., 2010). Another recent review (Sidor and MacQueen, 2012) reported pooled switch rates of 8% with either antidepressant or placebo treatments in RCTs for bipolar depression [RR=1.03 (95% CI 0.70–1.52)], indicating no difference between spontaneous and antidepressant-associated risks. These observations suggest that clinical concerns about risky mood-switching may be greater than is warranted.
It may be possible to predict risks of excessive mood-elevation during antidepressant treatment to some extent. Factors including previous mood-switching during antidepressant treatment, rapid-cycling (≥4 recurrences/yr), younger onset-age, presence of co-morbid anxiety or substance-use disorders, may contribute to risk of antidepressant-associated mood-switching but require further study (Ghaemi et al., 2010; Perlis et al., 2010; Angst et al., 2011; Biernacka et al., 2012; Cazard and Ferreri, 2012; Post et al., 2012; Tondo et al., 2012; Valentí et al., 2012). In addition, as suggested in the present meta-analysis, tricyclic antidepressants may have a higher risk of inducing mood-switches than most modern antidepressants, at least in adults, although SRI may have a higher risk among pre-pubertal juveniles (Martin et al., 2004; Baldessarini et al., 2005; Tondo et al., 2010). The impression that bupropion may have an especially low risk of inducing mood-switches may arise from its having been marketed, for safety, with dosing limits well below those found to be effective in its initial RCTs (Baldessarini, 2013). There is also some evidence that MAO inhibitors may have a relatively low risk of inducing mood-switching (Himmelhoch et al., 1991; Tondo et al., 2010).
The presence of unrecognized initial subsyndromal hypomanic symptoms may contribute to poor responses to antidepressant treatment, even in patients diagnosed with unipolar depression (Ghaemi et al., 2003; Post et al., 2003b; Sharma et al., 2005; Calabrese et al., 2006; Goldberg et al., 2007; Sachs et al., 2007; O’Donovan et al., 2008; Phelps et al., 2008; Frye et al., 2009; Baldessarini et al., 2010d; Correa et al., 2010; Angst et al., 2011; Pacchiarotti et al., 2011b; Perlis et al., 2011; Rihmer and Gonda, 2011; Rybakowski, 2012). This hypothesis parallels the view that bipolar depression is less treatment-responsive than unipolar depression – a conclusion that can be questioned based on the present findings (Table 2). Moreover, it is not clear to what extent unfavourable outcomes represent lack of response or actual worsening by induction of excessive activation. States with a mixture of agitation and dysphoria can sometimes be difficult to differentiate as a mixed-state vs. ‘worsening depression,’ and may occur only transiently, especially in early antidepressant treatment (Pompili et al., 2005; Tondo et al., 2012).
Many clinicians rely on plausibly expected, protective effects of ongoing mood-stabilizing treatments to limit risks of mood-switching when an antidepressant is given to a BD patient. However, evidence concerning putative protective effects of mood-stabilizers against mania or hypomania during treatment with antidepressants remains inconclusive and the point requires further, randomized-controlled trials (Licht et al., 2008; Tondo et al., 2010).
Additional treatments for bipolar depression are being considered, but most remain experimental (Table 3). Exceptions with regulatory approval include olanzapine–fluoxetine and quetiapine for acute bipolar depression, as well as lamotrigine for long-term prevention mainly of depressive recurrences, and quetiapine as a long-term adjunctive treatment with lithium or valproate (Table 3; Baldessarini, 2013). These approved treatments carry very low risks of mood-switching, but the effectiveness of lamotrigine is limited and the sedative and adverse metabolic effects of olanzapine and quetiapine can be substantial (Frye et al., 2011; Centorrino et al., 2012). Evidence to support use of anticonvulsants to treat acute bipolar depression is limited, and as noted earlier, may be even less effective than antidepressant treatment (Calabrese et al., 2008; van der Loos et al., 2009; van Lieshout and MacQueen, 2010; Muzina et al., 2011; Reinares et al., 2012).
| Agents | Indications in BD | Status for bipolar depression | References |
|---|---|---|---|
| Antidepressants | None for BD; indication for ‘major depression’ widely assumed to include bipolar depression | Clinically used | Amit and Weizman (2012), Sidor and MacQueen (2011, 2012) |
| Aripiprazole | Mania, mixed-states and recurrences | Mainly negative findings; not approved for bipolar depression | Thase et al. (2008); Cruz et al. (2010), De Fruyt et al. (2012) |
| Asenapine | Mania, mixed-states | Experimental; some evidence | Cruz et al. (2010), De Fruyt et al. (2012) |
| Carbamazepine | Mania or mixed-states | Not approved for bipolar depression | Reinares et al. (2012) |
| Deep brain stimulation | Treatment-resistant depression | Experimental; some evidence | Rizvi et al. (2011) |
| Dopamine agonistsa | Proposed for bipolar depression | Experimental; some evidence | Howland (2012) |
| Divalproex | Mania | Not approved for bipolar depression | Reinares et al. (2012) |
| Electroconvulsive treatment | Major and bipolar depression; mania | Clinically used; FDA Class III medical device | Dierckx et al. (2012) |
| Gabapentin | None for BD | Not approved for bipolar depression | Reinares et al. (2012) |
| Glutamate NMDA-antagonistsb | Treatment-resistant & bipolar depression | Experimental; some evidence | Owen (2012), Zarate et al. (2012) |
| Lamotrigine | Recurrences (mainly vs. depression) | Not approved for bipolar depression | Reinares et al. (2012) |
| Levetiracetam | None for BD | Not approved for bipolar depression | Saricicek et al. (2011) |
| Light therapy (intensive) | Depression, seasonal affective depression | Experimental | Poon et al. (2012) |
| Lithium salts | Mania, recurrences | Not approved for bipolar depression | Baldessarini (2013) |
| Lurasidone | Mania | Not approved for bipolar depression | De Fruyt et al. (2012) |
| Modafinil, R-modafinil | None for BD | Experimental; some evidence | Frye et al. (2007); Calabrese et al. (2010) |
| Olanzapine | Mania, mixed-states, maintenance | Some evidence; approved in Japan | Cruz et al. (2010), De Fruyt et al. (2012), Tohen et al. (2012) |
| Olanzapine+Fluoxetine | Treatment-resistant and acute bipolar depression | FDA-approved | Cruz et al. 2010, De Fruyt et al. 2012 |
| Omega-3 fatty acids and other ‘nutriceuticals’ | Proposed for bipolar depression | Experimental; some evidence | Keck et al. (2006), Sarris et al. (2011), Torrey and Davis (2012) |
| Pregabalin | None for BD | Experimental; some evidence | Reinares et al. (2012) |
| Psychostimulantsc | Proposed adjuncts | Experimental | Parker and Brotchie (2010); Howland (2012) |
| Psychosocial interventions | Major depression; maintenance treatment | Clinically used | Fountoulakis (2010), Lolich et al. (2012) |
| Quetiapine | Mania, mixed-states, acute bipolar depression; long-term with lithium or valproate | FDA-approved | Cruz et al. (2010), De Fruyt et al. (2012) |
| Repeated transcranial magnetic stimulation | Major depression | Experimental | George et al. (2013) |
| Risperidone, Paliperidone | Mania, mixed-states, maintenace (risperidone) with lithium or valproate | Experimental; some evidence | Cruz et al. (2010), De Fruyt et al. (2012) |
| Sleep deprivation | Major depression | Experimental | (Poon et al. 2012) |
| Thyroid hormones | Proposed for bipolar depression | Experimental | Bauer et al. (2005a, b) |
| Topiramate | None for BD | Not approved for bipolar depression | Reinares et al. (2012) |
| Vagal nerve stimulation | Chronic or recurrent depression | FDA-approved | Rizvi et al. (2011) |
| Ziprasidone | Mania, mixed-states, maintenance with lithium or valproate | Experimental; some evidence; not recommended for bipolar depression | Cruz et al. (2010), De Fruyt et al. (2012), Yatham et al (2013) |
Table 3. Treatments for Bipolar Disorder: Emphasis on Bipolar Depression
Conclusions
This overview was encouraged by the status of bipolar depression and related clinical states of dysthymia and dysphoria, as well as mixed-states of various types and levels of clinical severity, as major contributors to residual morbidity, disability and excess mortality of BD patients, even with application of available treatments aimed at mood-stabilization. It is remarkable that controlled trials for these clinically very important conditions remain limited and inadequate to support firm recommendations for treatment and optimal clinical management. The present review adds to the conclusion that placebo-controlled trials of antidepressants in acute bipolar depression remain rare. Nevertheless, trials that were identified yielded evidence of significant overall efficacy of antidepressants in bipolar depression that, remarkably, was not less than in unipolar depression. These findings, and the paucity of compellingly effective alternatives, encourage continued study of antidepressants in bipolar depression.
Further studies are required to advance therapeutic practices, despite difficulties that may be encountered, including fears of potentially dangerous activation during antidepressant trials, and uncertain commercial interest in the topic of bipolar depression as distinct from non-bipolar major depressive disorder. Needed is clarification of the efficacy and safety of antidepressants of different types and doses in various forms of depressive morbidity in bipolar or bipolar-like disorders. Specifically, head-to-head comparisons are needed to compare the short and long-term efficacy and safety of antidepressants vs. lithium, selected anticonvulsants and modern antipsychotic agents, given in monotherapy and in controlled combinations. Unresolved questions include whether some agents proposed for use in treating BD may worsen some aspects of mood, behaviour, or general health, including through excessive sedative effects or weight-gain and metabolic syndrome. The hypothesis that mood-stabilizing treatments can limit risk of antidepressant-associated mania also requires RCT to test this plausible but still-unproved concept. A major unresolved question is whether particular aspects of psychopathology, including mild, subsyndromal hypomanic features or elements of mixed-states that would not meet currently widely employed, but narrow, diagnostic criteria are indeed predictive of poor responses to antidepressants in bipolar depression, and whether such an effect is a reflection of a syndrome-type or an effect of current agitation.
(2007) Efficacy and safety of antidepressant monotherapy in the treatment of bipolar-II depression. Int Clin Psychopharmacol 22:309–311.Crossref, Google Scholar
(2003) Impact of antidepressant discontinuation after acute bipolar depression remission on rates of depressive relapse at one-year follow-up. Am J Psychiatry 160:1252–1262.Crossref, Google Scholar
(2012) Antidepressant treatment for acute bipolar depression: an update. Depress Res Treat. Advance online publication. doi:10.1155/2012/684725.Google Scholar
(2005) Comparison of fluoxetine, olanzapine, and the combination of fluoxetine and olanzapine in initial therapy of bipolar type I and type II major depression: lack of manic induction. J Affect Disord 87:121–130.Crossref, Google Scholar
(2008) Comparison of short-term venlafaxine versus lithium monotherapy for bipolar II major depressive episode: randomized open-label study. J Clin Psychopharmacol 28:171–181.Crossref, Google Scholar
(2002) Mortality of patients with mood disorders: follow up of 34–38 years. J Affect Disord 68:167–181.Crossref, Google Scholar
(2011) Prevalence and characteristics of undiagnosed bipolar disorders in patients with a major depressive episode. Arch Gen Psychiatry 68:791–799.Crossref, Google Scholar
(2008) Psychotropic medications for patients with bipolar disorder in the United States: polytherapy and adherence. Psychiatr Serv 59:1175–1183.Crossref, Google Scholar
(2013) Chemotherapy in psychiatry, 3rd edn. New York: Springer Press.Crossref, Google Scholar
(2005) Risk of mania with serotonin reuptake inhibitors vs. tricyclic antidepressants in children, adolescents and young adults. Arch Pediatr Adolesc Med 159:298–299.Crossref, Google Scholar
(2007a) Patterns of psychotropic drug prescription for US patients with diagnoses of bipolar disorders. Psychiatr Serv 58:85–91.Link, Google Scholar
(2010a) Morbidity in 303 first-episode bipolar I disorder patients. Bipolar Disord 12:264–270.Crossref, Google Scholar
(2010b) Dissimilar morbidity following initial mania versus mixed-states in type-I bipolar disorder. J Affect Disord 126:299–302.Crossref, Google Scholar
(2010c) Discontinuation rate vs. recurrence risk following long-term antidepressant treatment in major depressive disorder patients. Am J Psychiatry 167:934–941.Crossref, Google Scholar
(2012) Predominant recurrence polarity among 928 adult international bipolar I disorder patients. Acta Psychiatr Scand 125:293–302.Crossref, Google Scholar
(2010d) Bipolar depression: overview and commentary. Harv Rev Psychiatry 18:143–157.Crossref, Google Scholar
(2005a) Supraphysiological doses of levothyroxine alter regional cerebral metabolism and improve mood in bipolar depression. Mol Psychiatry 10:456–469.Crossref, Google Scholar
(2005b) Prevalence and distinct correlates of anxiety, substance, and combined comorbidity in a multi-site public sector sample with bipolar disorder. J Affect Disord 85:301–315.Crossref, Google Scholar
(2012) Treatment options for acute depression in bipolar disorder. Bipolar Disord 14 (Suppl. 2):37–50.Crossref, Google Scholar
(2012) Pharmacogenomics of antidepressant induced mania: review and meta-analysis of the serotonin transporter gene (5HTTLPR) association. J Affect Disord 136:e21–e29.Crossref, Google Scholar
(1993) Double-blind study of L-sulpiride versus amitriptyline in lithium-maintained bipolar depressives. Acta Psychiatr Scand 88:434–439.Crossref, Google Scholar
(2006) Seven-week, randomized, double-blind trial of olanzapine/fluoxetine combination versus lamotrigine in the treatment of bipolar I depression. J Clin Psychiatry 67:1025–1033.Crossref, Google Scholar
(2008) Lamotrigine in the acute treatment of bipolar depression: results of five double-blind, placebo-controlled clinical trials. Bipolar Disord 10:323–333.Crossref, Google Scholar
(2010) Adjunctive armodafinil for major depressive episodes associated with bipolar I disorder: randomized, multicenter, double-blind, placebo-controlled, proof-of-concept study. J Clin Psychiatry 71:1363–1370.Crossref, Google Scholar
(2006) Predictors of bipolar disorder risk among patients currently treated for major depression. Med Gen Med 8:38.Google Scholar
(2012) Bipolar disorders and comorbid anxiety: prognostic impact and therapeutic challenges (French). Encephale. Advance online publication. Retrieved 15 June 2012. doi: 10.1016/j.encep.2012.04.005.Google Scholar
(2012) Metabolic syndrome in psychiatrically hospitalized patients treated with antipsychotics and other psychotropics. Hum Psychopharmacol 27:521–526.Crossref, Google Scholar
(1989) Combination of fluoxetine, imipramine, and placebo in patients with bipolar depressive disorder. Int Clin Psychopharmacol 4:313–322.Crossref, Google Scholar
.(2010) Is unrecognized bipolar disorder a frequent contributor to apparent treatment resistant depression? J Affect Disord 127:10–18.Crossref, Google Scholar
(2010) Efficacy of modern antipsychotics in placebo-controlled trials in bipolar depression: meta-analysis. Int J Neuropsychopharmacol 13:5–14.Crossref, Google Scholar
(2012) Second generation antipsychotics in the treatment of bipolar depression: systematic review and meta-analysis. J Psychopharmacol 26:603–617.Crossref, Google Scholar
(2011) Physical illness in patients with severe mental disorders: prevalence, impact of medications and disparities in health care. World Psychiatry 10:52–77.Crossref, Google Scholar
(2012) Efficacy of electroconvulsive therapy in bipolar versus unipolar major depression: meta-analysis. Bipolar Disord 14:146–150.Crossref, Google Scholar
(2010) Update of evidence-based treatment of bipolar depression: where do we stand? Curr Opin Psychiatry 23:19–24.Crossref, Google Scholar
(2012) Efficacy of pharmacotherapy in bipolar disorder: report by the WPA section on pharmacopsychiatry. Eur Arch Psychiatry Clin Neurosci 262 (Suppl. 1) :1–48.Crossref, Google Scholar
(2007) Placebo-controlled evaluation of adjunctive modafinil in the treatment of bipolar depression. Am J Psychiatry 164:1242–1249.Crossref, Google Scholar
(2011) International consensus group on depression prevention in bipolar disorder. J Clin Psychiatry 72:1295–1310.Crossref, Google Scholar
(2009) Correlates of treatment-emergent mania associated with antidepressant treatment in bipolar depression. Am J Psychiatry 166:164–172.Crossref, Google Scholar
(2013) The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry 26:13–18.Crossref, Google Scholar
(2003) Antidepressants in bipolar disorder: the case for caution. Bipolar Disord 5:421–433.Crossref, Google Scholar
(2010) Antidepressant discontinuation in bipolar depression: systematic treatment enhancement program for bipolar disorder (STEP-BD) randomized clinical trial of long-term effectiveness and safety. J Clin Psychiatry 71:372–380.Crossref, Google Scholar
(2004) Antidepressant treatment in bipolar versus unipolar depression. Am J Psychiatry 161:163–165.Crossref, Google Scholar
(2008) Long-term antidepressant treatment in bipolar disorder: meta-analyses of benefits and risks. Acta Psychiatr Scand 118:347–356.Crossref, Google Scholar
(2004) Antidepressants for bipolar depression: systematic review of randomized, controlled trials. Am J Psychiatry 161:1537–1547.Crossref, Google Scholar
(2007) Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry 164:1348–1355.Crossref, Google Scholar
(2009) Evidence-based guidelines for treating bipolar disorder: revised second edition – recommendations from the British Association for Psychopharmacology. J Psychopharmacol (Oxford) 23:346–388.Crossref, Google Scholar
(1999) Double-blind study comparing idazoxan and bupropion in bipolar depressed patients. J Affect Disord 56:237–243.Crossref, Google Scholar
(2010) World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the biological treatment of bipolar disorders: update on treatment of acute bipolar depression. World J Biol Psychiatry 11:81–109.Crossref, Google Scholar
(2011) Psychotropic drug treatment preferences for Latin American bipolar disorder patients. Rev Psiquiatr Salud Ment 4:205–211.Crossref, Google Scholar
(1991) Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry 148:910–916.Crossref, Google Scholar
(2012) Use of dopaminergic and stimulant drugs for the treatment of depression. J Pyschosoc Nurs Ment Health Serv 50:11–14.Google Scholar
(2005) One-year outcome with antidepressant-treatment of bipolar depression. Acta Psychiatr Scand 112:105–109.Crossref, Google Scholar
(2002) Long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry 59:530–537.Crossref, Google Scholar
(2008) Clinical and economic effects of unrecognized or inadequately treated bipolar disorder. J Psychiatr Pract 14 (Suppl. 2) :31–38.Crossref, Google Scholar
(2006) Double-blind, randomized, placebo-controlled trials of ethyl-eicosopentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol Psychiatry 60:1020–1022.Crossref, Google Scholar
(2004) Burden of general medical conditions among individuals with bipolar disorder. Bipolar Disord 6:368–373.Crossref, Google Scholar
(2005) Psychiatric and medical comorbidities of bipolar disorder. Psychosom Med 67:1–8.Crossref, Google Scholar
(2005) Increasing medical burden in bipolar disorder. J Am Med Assoc 293:2528–2530.Crossref, Google Scholar
(2007) Three times more days depressed than manic or hypomanic in both bipolar I and bipolar II disorder. Bipolar Disord 9:531–535.Crossref, Google Scholar
(2012) Can bipolar disorder be viewed as a multisystem inflammatory disease? J Affect Disord 141 :1–10.Crossref, Google Scholar
(2006) Risk of switch in mood polarity to hypomania or mania in patients with bipolar depression during acute and continuation trials of venlafaxine, sertraline, and bupropion as adjuncts to mood stabilizers. Am J Psychiatry 163:232–239.Crossref, Google Scholar
(2008) Are antidepressants safe in the treatment of bipolar depression? Critical evaluation of their potential risk to induce switch into mania or cycle acceleration. Acta Psychiatr Scand 118:337–346.Crossref, Google Scholar
(2012) Psychosocial interventions in bipolar disorder: review. Acta Esp Psiquiatr 40:84–92.Google Scholar
(2012) Characteristics of bipolar disorder patients given antidepressants. Hum Psychopharmacol 27:486–491.Crossref, Google Scholar
(2004) Age effects on antidepressant-induced manic conversion. Arch Pediatr Adolesc Med 158:773–780.Crossref, Google Scholar
(2010) Double-blind, placebo-controlled study of quetiapine and paroxetine as monotherapy in adults with bipolar depression (EMBOLDEN II). J Clin Psychiatry 71:163–174.Crossref, Google Scholar
, Hirschfeld RMA, Petukhova M, Kessler RC (2007) Lifetime and 12-month prevalence of bipolar spectrum disorder in the National Comorbidity Survey replication. Arch Gen Psychiatry 64:543–552.Crossref, Google Scholar
(2011) Acute efficacy of divalproex sodium versus placebo in mood stabilizer-naive bipolar I or II depression: double-blind, randomized, placebo-controlled trial. J Clin Psychiatry 72:813–819.Crossref, Google Scholar
(2001) Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression. Am J Psychiatry 158:906–912.Crossref, Google Scholar
(2011) New treatment guidelines for acute bipolar depression: a systematic review. J Affect Disord 129:14–26.Crossref, Google Scholar
(2007) Tranylcypromine vs. lamotrigine in the treatment of refractory bipolar depression: a failed but clinically useful study. Acta Psychiatr Scand 115:360–365.Crossref, Google Scholar
(2010) Suicide attempts in bipolar I and bipolar II disorder: review and meta-analysis of the evidence. Bipolar Disord 12:1–9.Crossref, Google Scholar
(2008) Antidepressant monotherapy in pre-bipolar depression; predictive value and inherent risk. J Affect Disord 107:293–298.Crossref, Google Scholar
(2001) Excess mortality in bipolar and unipolar disorder in Sweden. Arch Gen Psychiatry 58:844–850.Crossref, Google Scholar
(2012) Glutamatergic approaches in major depressive disorder: focus on ketamine, memantine and riluzole. Drugs Today 48:469–478.Google Scholar
(2011a) Factors associated with initial treatment response with antidepressants in bipolar disorder. Eur Neuropsychopharmacol 21:362–369.Crossref, Google Scholar
(2011b) Differential outcome of bipolar patients receiving antidepressant monotherapy versus combination with an antimanic drug. J Affect Disord 129:321–326.Crossref, Google Scholar
(2010) Do the old psychostimulant drugs have a role in managing treatment-resistant depression? Acta Psychiatr Scand 121:308–314.Crossref, Google Scholar
(2006) Sub-syndromal and syndromal symptoms in the longitudinal course of bipolar disorder. Br J Psychiatry 189:118–123.Crossref, Google Scholar
(1994) Induction of mania with selective serotonin reuptake inhibitors and tricyclic antidepressants. Br J Psychiatry 164:549–550.Crossref, Google Scholar
(2010) Transition to mania during treatment of bipolar depression. Neuropsychopharmacology 35:2545–2552.Crossref, Google Scholar
(2011) Association between bipolar spectrum features and treatment outcomes in outpatients with major depressive disorder. Arch Gen Psychiatry 68:351–360.Crossref, Google Scholar
(2008) Validity and utility of bipolar spectrum models. Bipolar Disord 10:179–193.Crossref, Google Scholar
(2005) Suicidal risk emerging during antidepressant treatment: recognition and intervention. Clin Neuropsychiatry 2:66–72.Google Scholar
(2012) Evidence-based options for treatment-resistant adult bipolar disorder patients. Bipolar Disord 14:573–584.Crossref, Google Scholar
(2006) Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion and sertraline. Br J Psychiatry 189:124–131.Crossref, Google Scholar
(2003a) Morbidity in 258 bipolar outpatients followed for 1 year with daily prospective ratings on the NIMH life chart method. J Clin Psychiatry 64:680–690.Crossref, Google Scholar
(2011) Differential clinical characteristics, medication usage, and treatment response of bipolar disorder patients in the US versus Netherlands and Germany. Int Clin Psychopharmacol 26:96–106.Crossref, Google Scholar
(2012) Relationship of prior antidepressant exposure to long-term prospective outcome in bipolar I disorder outpatients. J Clin Psychiatry 73:924–930.Crossref, Google Scholar
(2003b) Re-evaluation of the role of antidepressants in the treatment of bipolar depression: data from the Stanley foundation bipolar network. Bipolar Disord 5:396–406.Crossref, Google Scholar
(2012) Systematic review on the role of anticonvulsants in the treatment of acute bipolar depression. Int J Neuropsychopharmacology 10:1–12.Google Scholar
(2011) Antidepressant-resistant depression and antidepressant-associated suicidal behavior: role of underlying bipolarity. Depress Res Treat. Advance online publication. doi:10.1155/2011/906462.Google Scholar
(2011) Neurostimulation therapies for treatment resistant depression: focus on vagus nerve stimulation and deep brain stimulation. Int Rev Psychiatry 23 :424–436.Crossref, Google Scholar
(2010) Functional impairment in bipolar II disorder: is it as disabling as bipolar I? J Affect Disord 127:71–76.Crossref, Google Scholar
(2009) Premature mortality from general medical illnesses among persons with bipolar disorder: review. Psychiatr Serv 60:147–156.Crossref, Google Scholar
(2012) Bipolarity and inadequate response to antidepressant drugs: clinical and psychopharmacological perspective. J Affect Disord 136:e13–e19.Crossref, Google Scholar
(1994) Double-blind trial of bupropion versus desipramine for bipolar depression. J Clin Psychiatry 55:391–393.Google Scholar
(2007) Effectiveness of adjunctive antidepressant treatment for bipolar depression. New Engl J Med 356:1711–1722.Crossref, Google Scholar
(2011) Levetiracetam in the management of bipolar depression: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 72:744–750.Crossref, Google Scholar
(2011) Adjunctive nutraceuticals with standard pharmacotherapies in bipolar disorder: systematic review of clinical trials. Bipolar Disord 13:454–465.Crossref, Google Scholar
(2006) Randomized, double-blind pilot trial comparing lamotrigine versus citaloporam for the treatment of bipolar depression. J Affect Disord 96:95–99.Crossref, Google Scholar
(2005) Closer look at treatment resistant depression: is it due to a bipolar diathesis? J Affect Disord 84:251–257.Crossref, Google Scholar
(2004) Risperidone and paroxetine given singly and in combination for bipolar depression. J Clin Psychiatry 65:1715–1719.Crossref, Google Scholar
(2011) Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry 72:156–167.Crossref, Google Scholar
(2012) An update on antidepressant use in bipolar depression. Curr Psychiatry Rep 14:696–704.Crossref, Google Scholar
(2001) Moclobemide vs. imipramine in bipolar depression: multicenter double-blind clinical trial. Acta Psychiatr Scand 104:104–109.Crossref, Google Scholar
(2012) The relationship between use of antidepressants and resource utilization among patients with manic or mixed bipolar disorder episodes: findings from a managed care setting. J Affect Disord 138:425–432.Crossref, Google Scholar
(2008) Aripiprazole monotherapy in nonpsychotic bipolar I depression: results of two randomized, placebo-controlled studies. J Clin Psychopharmacol 28:13–20.Crossref, Google Scholar
(2012) Randomised, double-blind, placebo-controlled study of olanzapine in patients with bipolar I depression. Br J Psychiatry 201:376–382.Crossref, Google Scholar
(2003) Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 60:1079–1088.Crossref, Google Scholar
(2012) Clinical responses to antidepressants among acutely depressed patients with bipolar-I, bipolar-II, or unipolar major affective disorder. Acta Psychiatr Scand 125:293–302.Crossref, Google Scholar
(2003) Suicide in bipolar disorder: risk and prevention. CNS Drugs 17:491–511.Crossref, Google Scholar
(2007) Suicidal risks among 2826 Sardinian major depressive disorder patients. Acta Psychiatr Scand 116:419–428.Crossref, Google Scholar
(2010) Mania associated with antidepressant treatment: comprehensive meta-analytic review. Acta Psychiatr Scand 121:404–414.Crossref, Google Scholar
(2012) Adjunct treatments for schizophrenia and bipolar disorder: what to try when you are out of ideas. Clin Schizophr Relat Psychoses 5:208–216.Crossref, Google Scholar
(2012) Randomized, placebo-controlled trials of antidepressants for acute major depression: thirty-year meta-analytic review. Neuropsychopharmacology 37:851–864.Crossref, Google Scholar
(2012a) Bipolar depression: clinical correlates of receiving antidepressants. J Affect Disord 139:89–93.Crossref, Google Scholar
(2012b) Suicidal risk factors in bipolar I and II disorder patients. J Clin Psychiatry 73:778–782.Crossref, Google Scholar
(2012c) Re-analysis of the earliest controlled trials of imipramine. J Affect Disord. Advance online publication. doi: 10.1016/j.jad.2012.10.032.Google Scholar
(2012) Risk factors for antidepressant-related switch to mania. J Clin Psychiatry 73:e271–276.Google Scholar
(2010) Efficacy and safety of two treatment algorithms in bipolar depression consisting of a combination of lithium, lamotrigine or placebo and paroxetine. Acta Psychiatr Scand 122:246–254.Crossref, Google Scholar
(2009) Efficacy and safety of lamotrigine as add-on treatment to lithium in bipolar depression: multicenter, double-blind, placebo-controlled trial. J Clin Psychiatry 70:223–231.Crossref, Google Scholar
(2010) Efficacy and acceptability of mood stabilizers in the treatment of acute bipolar depression: systematic review. Br J Psychiatry 196:266–273.Crossref, Google Scholar
(2011) Comparison of antidepressant responses in patients with bipolar vs. unipolar depression: meta-analytic review. Pharmacopsychiatry 44:21–26.Google Scholar
(2008) Antidepressants in bipolar depression. Acta Psychiatr Scand 118:335–336.Crossref, Google Scholar
(2002) A randomized trial comparing paroxetine and venlafaxine in the treatment of bipolar depressed patients taking mood stabilizers. J Clin Psychiatry 63:508–512.Crossref, Google Scholar
(2011) Solving the antidepressant efficacy question: effect sizes in major depressive disorder. ClinTher33:B49–B61.Google Scholar
(1979) Rapid cycling in manic-depressives induced by tricyclic antidepressants. Arch Gen Psychiatry 36:555–559.Crossref, Google Scholar
(1988) Rapid cycling affective disorder: contributing factors and treatment responses in 51 patients. Am J Psychiatry 145:179–184.Crossref, Google Scholar
. (2013) Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord. Advance online publication. doi: 10.1111/bdi.12025.Google Scholar
(2009) Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for bipolar disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder. Bipolar Disord 11:225–255.Crossref, Google Scholar
(2000) Double-blind comparison of addition of a second mood stabilizer versus an antidepressant to an initial mood stabilizer for treatment of patients with bipolar depression. Am J Psychiatry 157:124–126.Crossref, Google Scholar
(2012) Replication of ketamine’s antidepressant efficacy in bipolar depression: randomized controlled add-on trial. Biol Psychiatry 71:939–946.Crossref, Google Scholar



