Delirium and Dementia
Abstract
Delirium, dementia, and other cognitive disorders represent an increasingly important focus of clinical research and practice. In this review, the authors survey recent developments in our understanding of these disorders. Delirium is now more frequently recognized as an important contributor to morbidity and as a factor in prognosis for patients in a number of treatment settings, particularly in the geriatric population. Early detection and aggressive treatment of delirium contribute to shorter hospital stays and lower treatment costs. Delirium is often confused with other disorders of cognitive function, such as dementia. The four most common dementia syndromes, Alzheimer’s disease, vascular dementia, Lewy body dementia, and frontotemporal dementia, are explored in this review. A greater understanding of the genetic risks and the neuropathological findings of these syndromes has led to novel therapeutic strategies involving interventions at critical points along the cascade to neuronal cell death. These approaches have opened new avenues of inquiry for future developments.
Delirium, dementia, and other cognitive disorders represent an important and growing area of clinical research and practice. These disorders can masquerade as other psychiatric disorders, as patients may present with mood problems, psychosis, or anxiety. Delirium and dementia must also be distinguished from one another (Table 1). As the population ages, an increasing portion of the general psychiatrist’s practice will involve geriatric patients. Although delirium, dementia, and other cognitive disorders are of particular interest in geriatric psychiatry, this review is designed to provide an overview and synthesis of the literature on these vast areas as they pertain to general psychiatric practice.
Ddelirium
Definition and clinical features
According to the Oxford English Dictionary, the word “delirium” is derived from the Latin word “delire,” which means to leave one’s furrow or deviate from a straight path. Over the years, the word evolved to mean “go crazy.” This nonspecific term for a deviation in mental status is a testament to the myriad ways that delirium can present as a clinical condition.
The American Psychiatric Association Practice Guideline for the Treatment of Patients With Delirium (1), citing DSM-IV diagnostic criteria, defines delirium as “disturbances of consciousness, attention, cognition, and perception.” The disturbance “develops over a short period of time (usually hours to days) and tends to fluctuate during the course of the day.” Disturbances in consciousness refer to waxing and waning levels of alertness.
In a retrospective study of hospitalized patients, psychotic symptoms occurred in 42.7% of patients with delirium. The most common psychotic symptoms are visual hallucinations and delusions (2). Patients may believe, for example, that they are incarcerated rather than hospitalized, and they may misinterpret caregivers’ actions as hostile. Also, perceptual disturbances may be simple misperceptions of actual stimuli—for example, IV tubes may be perceived as snakes. An important way to distinguish the psychotic symptoms of delirium from those of a primary psychotic disorder is that the former may vary throughout the day during the course of an episode of delirium.
Sleep-wake disturbances, such as insomnia or daytime somnolence, are a common sign of delirium and may be the initial presenting symptom. In fact, for older patients scheduled for surgery, regulation of the sleep-wake cycle has been studied as a preventive measure against postoperative delirium (3, 4).
Delirious patients also exhibit considerable neuropsychiatric disturbances, such as mood lability, apathy, and anxiety. A consulting psychiatrist who has been called to evaluate a general medical patient for depression may find that the symptoms of depressed mood and disturbed sleep actually represent delirium. Obtaining a good history and assessing the variability of symptoms are important in differentiating delirium from a primary psychiatric process.
The clinical features of delirium described above can be separated into “hyperactive” and “hypoactive” delirium (5). Hyperactive delirium is characterized by the more commonly known symptoms of hallucinations, delusions, and agitation, and hypoactive delirium by somnolence, depressed mood, and confusion. Differences in melatonin levels have been implicated in the distinction between these subtypes. Hyperactive delirium has been found to be correlated with lower levels of melatonin metabolites, and hypoactive delirium with higher levels (6). These phenomenological distinctions are relevant to outcomes for patients with delirium. In a study of the severity of postoperative delirium after hip fracture repair (7), hypoactive delirium was found to be more common than hyperactive delirium and was associated with better outcomes as measured by rates of nursing home placement, mortality, and ambulatory decline.
Epidemiology
A number of studies have examined the incidence and prevalence of delirium in a variety of settings and patient populations. Estimates of the prevalence of delirium in the general hospital setting range from 10% to 30% (5). A study of delirium in older patients on an intensive care unit found a prevalence of 31.4% at initial assessment and an incidence of 31.1% during the course of hospitalization (8). In a sample of elderly (65 years of age and older) patients who presented in an emergency department, the prevalence of delirium was 9.6%; this figure may underestimate the true prevalence, however, because the study excluded patients who were too ill to be assessed (9). Postoperative delirium is particularly common among elderly patients. Several intraoperative factors may contribute to delirium, such as hypoxia, the duration of surgery, and the type of anesthesia used (10). In a prospective cohort study of elderly patients undergoing hip surgery, postoperative delirium was present in 23.8% (11). The authors of the study also found that significant risk factors for developing delirium included advanced age, a history of cognitive impairment, depression, and visual or hearing impairment. A review of risk factors for delirium found that advanced age, presence of dementia, presence of a medical illness, and male gender were the most common (12).
Etiology
The etiology of delirium is multifactorial. Common causes of delirium include polypharmacy, intoxication, withdrawal, and infection, as well as metabolic derangements, vascular disorders, and trauma (Table 2). Although there are many potential causes for delirium, a “final common pathway” has been hypothesized involving particular neurotransmitter systems in specific brain regions. Trzepacz (13) has proposed that delirium may result from a concomitant decrease in cholinergic tone and increase in dopaminergic tone in relevant brain regions, such as the prefrontal cortex, the anterior and right thalamus, and the right basilar mesial temporoparietal cortex.
Assessment
A number of tools are available for use in assessing and screening for delirium. The confusion assessment method (CAM) is a useful screening tool that focuses on four salient features of delirium: acute onset/fluctuating course, inattention, disorganized thinking, and altered level of consciousness (14). An instrument that is frequently mentioned in the literature is the 10-item, 32-point Delirium Rating Scale (DRS) (Table 3) (15). The DRS has been shown to have a sensitivity of 91%–100% and a specificity of 85%–100% in distinguishing delirium from other diagnoses, such as dementia, depression, and schizophrenia (16).
Additional important tools for assessment include a thorough history, with particular attention to past alcohol or substance use and previous episodes of delirium. A physical examination can be helpful in identifying or ruling out possible causes of delirium. Laboratory studies are important for detecting underlying causes of delirium, such as electrolyte abnormalities, toxicity, and infection. Imaging studies, with particular attention to central nervous system lesions such as tumors or infarcts, may also be useful in determining the cause of delirium.
Treatment and management
The primary goal in treating delirium is to treat the underlying cause. Treatment may involve a variety of interventions, ranging from initiation of antibiotic therapy for a urinary tract infection to removing deliriogenic agents from a patient’s medication regimen. Behavioral management can be accomplished with both environmental and pharmacological interventions. Environmental interventions have been shown to be effective in the treatment and prevention of delirium. Inouye and colleagues found that interventions targeting specific risk factors in a cohort of hospitalized elderly patients led to significant decreases in the number of episodes of delirium (17). Such interventions include improved sleep hygiene, range-of-motion exercises, ambulation, reorientation, and cognitive stimulation. Clocks, calendars, and familiar objects from home, such as photographs of loved ones, are all important environmental cues that may be helpful in the prevention of delirium (1).
A key component of treatment is the avoidance of pharmacological agents that can precipitate or exacerbate delirium. With this in mind, anticholinergic agents, such as narcotic analgesics, benzodiazepines, and diphenhydramine, should be avoided in elderly patients who are at risk of delirium. A careful review of the delirious patient’s medication history is valuable, as many medications that are often prescribed for elderly patients have significant anticholinergic actions (18). The importance of anticholinergic load in cognition was demonstrated with a sample of elderly patients in a study that found increased serum anticholinergic activity to be associated with lower cognitive performance as indicated by Mini-Mental Status Examination (MMSE) score (19). Anticholinergic medications have also been shown to increase the severity of delirium independently of preexisting or coexisting dementia (20).
When behavioral and environmental modifications do not suffice for the acute management of delirium, haloperidol has traditionally been the gold standard in medication treatment. For elderly patients, the APA guideline recommends a dosage of 0.25–0.5 mg every 4 hours as needed (1).
Atypical antipsychotics have been shown in recent studies to be just as effective as haloperidol in treating delirium, with less risk of extrapyramidal side effects, which are of particular concern in the elderly population (21). In an open-label prospective trial of olanzapine for the treatment of delirium in hospitalized cancer patients, delirium symptoms resolved in 76% of the study sample (22). Similar results have been reported in case series with other atypical antipsychotics, such as risperidone (average dose, 1.7 mg/day) (23) and quetiapine (average dose, 93.75 mg/day) (24, 25). One case report described the effective use of ziprasidone in treating delirium (maximum dose, 100 mg/day) (26), although the drug had to be discontinued in this case because of electrolyte abnormalities and QT prolongation.
Cholinergic agents have long been hypothesized to be useful in the treatment of delirium, and several case reports as well as animal studies suggest that they offer a promising new direction in the treatment of delirium. A number of case reports have demonstrated the effectiveness of cholinomimetics in reversing delirium caused by anticholinergic agents such as benztropine, atropine, ranitidine, pheniramine (27), amitriptyline (28), and meperidine (29). More recent evidence from animal studies suggests that cholinergic agents may be helpful in treating delirium from nonanticholinergic causes. In an animal model of delirium, Nakamura and colleagues found that the cholinergic agent aniracetam reversed attentional deficits in rats treated with the dopamine agonist apomorphine (30). A case report described the successful use of the cholinesterase inhibitor rivastigmine (at a dose of 1.5 mg twice daily) for the treatment of delirium secondary to lithium toxicity (31). Common side effects of cholinesterase inhibitors include nausea, vomiting, diarrhea, abdominal pain, dizziness, and headache.
Outcomes
The consequences of delirium during hospitalization are associated with significant costs and poor outcomes. Postoperative delirium has been associated with longer hospital stays and higher costs (32). In a meta-analysis of delirium outcomes, Cole and Primeau found that hospitalized elderly patients with delirium had poorer outcomes and higher rates of mortality (14.2 %) and institutional placement (46.5%) as compared with unmatched control patients one month after admission (33). Another study found that patients who suffer from delirium during their hospitalization have a significantly higher 12-month mortality rate, with an estimated mortality of 63.3%, compared with 17.4% in controls (34). Mortality is related to the severity of the underlying medical condition and the intractability of the delirium (35). Patients may also suffer from persistent cognitive problems up to 12 months after hospitalization (36, 37).
Dementia
General features
The dementias are characterized by a global decline in cognitive function resulting in significant social or functional impairment. This decline occurs in several domains, such as memory, language, and visuospatial and executive functions. Patients with dementia also may suffer from agnosia (the inability to recognize objects) or apraxia (the inability to carry out complex motor tasks despite intact motor function). Psychotic symptoms, such as delusions and hallucinations, are common in dementia. Patients with dementia often suffer from delusions of persecution, for example, believing that a family member is trying to harm them in some way. Other associated symptoms common to all of the dementias are neuropsychiatric disturbances, such as depression, apathy, agitation, disinhibition, and irritability. A recent study found that 80% of patients with dementia and 50% of patients with mild cognitive impairment had at least one neuropsychiatric symptom from the onset of illness, as assessed by the Neuropsychiatric Inventory (38). The most common symptoms in these patients were apathy, agitation or aggression (such as verbal outburst, physical aggression, and wandering), and depression.
Depression in dementia represents a diagnostic challenge because of a significant overlap of symptoms (for example, psychomotor retardation and memory problems). A National Institute of Mental Health workshop was conducted recently to outline a preliminary set of criteria for clarifying the diagnosis of depression in patients with Alzheimer’s disease (39). In addition to meeting DSM-IV-TR criteria for Alzheimer’s dementia, patients must present with three or more of 11 depressive symptoms. Unlike the DSM-IV-TR criteria for major depressive disorder, additional symptoms are irritability and social withdrawal. These symptoms must be present for 2 weeks, but they need not be present every day. In relation to these provisional criteria, it has been suggested that depression in patients with dementia (specifically those with Alzheimer’s disease) represents a heterogeneous syndrome composed of four subtypes: adjustment reaction to cognitive deficits, recurrent depression, depressive symptoms associated with vascular disease, and mood disorder due to general medical condition (40).
Dementia constitutes a broad category of cognitive disorders. The four most common types of dementia are dementia of the Alzheimer’s type or Alzheimer’s disease, vascular dementia, Lewy body dementia, and frontotemporal dementia. The general features of these subtypes are summarized in Table 4.
Alzheimer’s disease
Alzheimer’s disease is the most common form of dementia, constituting up to two-thirds of cases (41). It is estimated that 4.5 million Americans are afflicted with Alzheimer’s disease, and the number is expected to increase threefold in the next 50 years (42). Alzheimer’s disease is characterized by insidious onset and slow, gradual progression. Memory and language deficits are the early hallmarks of Alzheimer’s disease. Reisberg and colleagues, using a number of scales to assess the longitudinal course of Alzheimer’s disease, have shown that the progression of the illness reflects a reverse developmental sequence, including the emergence of primitive reflexes in terminal phases of the disease (43).
The neuropathological findings in Alzheimer’s disease are amyloid plaques and neurofibrillary tangles composed of hyperphosphorylated tau proteins. Amyloid plaques are made up of Aβ42 β-amyloid fragments derived from γ-secretase cleavage of the amyloid precursor protein (APP). Knowledge of the pathology involved in Alzheimer’s disease has led to the identification of genes implicated in the disease. The first one identified was the gene that encodes APP, located on chromosome 21. Mutations in the gene associated with Alzheimer’s disease cause abnormal processing of the protein to increase formation of the plaque-forming Aβ42 fragment. This gene and the presenilin genes (presenilin-1 on chromosome 14 and presenilin-2 on chromosome 1) are implicated in early-onset Alzheimer’s disease. The presenilins are thought to contribute to the formation of amyloid plaques by increasing production and oligomerization of the Aβ42 β-amyloid protein (44). Late-onset Alzheimer’s disease, which constitutes the majority of cases of the illness, involves the ε4 allele of the apolipoprotein E (apoE) gene (45). Patients who are homozygous for the ε4 allele have nearly double the risk of developing Alzheimer’s disease (46).
The genetic determinants of Alzheimer’s disease indicate the importance of amyloid in the pathogenesis of the disease. Mechanisms thought to link the neuropathological findings to the death of cholinergic neurons in the forebrain nuclei include oxidative damage (47), excitotoxicity, and abnormal processing of heavy metals (48). These mechanisms are targets for current and future treatments for Alzheimer’s disease (Figure 1).
Vascular dementia
Vascular dementia, the second most common form of dementia, is characterized by cognitive deficits associated with focal neurological signs and symptoms or radiological evidence of cerebrovascular pathology. In an autopsy study of patients with dementia, pure vascular dementia was found in 13% of the sample (49). Patients with vascular dementia often present with risk factors for stroke, such as hypertension, hyperlipidemia, and diabetes. Vascular dementia encompasses a constellation of disorders differentiated by the brain regions affected, the vascular pathology, and predisposing genetic factors. Multi-infarct dementia is a subtype of vascular dementia caused by multiple large-vessel infarcts in either cortical or subcortical regions. Strategic-infarct dementia, another type of large-vessel vascular dementia, involves an infarct in a single critical brain area, such as the thalamus or the basal forebrain (50). Small-vessel vascular dementia typically involves subcortical regions, such as in Binswanger’s disease, lacunar dementia, and cerebral autosomal-dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL).
The clinical presentation of vascular dementia depends on the location of the lesion. However, the nature of the specific deficits may be indistinct from the presentation of Alzheimer’s disease. In fact, both the clinical features and the neuropathological findings of vascular dementia and Alzheimer’s disease may coexist in patients with “mixed” dementia (51). However, unlike Alzheimer’s disease, which is characterized by a gradual progression of symptoms, vascular dementia often develops in a stepwise fashion.
Lewy body dementia
Lewy body dementia presents with gait/balance difficulties, hallucinations (typically visual), and fluctuating attention. Patients with Lewy body dementia are particularly sensitive to the extrapyramidal side effects of antipsychotics, a fact often used to distinguish Lewy body dementia from delirium, with which there is significant overlap of symptoms.
Neuropathological findings in Lewy body dementia combine features of Alzheimer’s disease and Parkinson’s disease. While amyloid plaques are found in Lewy body dementia, the distinguishing feature of this disorder is the extensive neocortical, subcortical, and limbic distribution of Lewy bodies, which are neuronal inclusions made up of α-synuclein (52). The Lewy bodies found in Parkinson’s disease, by contrast, are located predominantly in brainstem nuclei and in the substantia nigra.
Frontotemporal dementia
Frontotemporal dementia, or Pick’s disease, is characterized by atrophy of the frontal and temporal lobes, leading to a distinct pattern of behavioral changes, including poor social skills, disinhibition, impulsivity, apathy, aphasias, and hyperorality (53). Patients with frontotemporal dementia also show signs of frontal degeneration with primitive reflexes on physical examination. Memory deficits are not as prominent in frontotemporal dementia as in other dementia subtypes. Frontotemporal dementia is subdivided into three clinical categories: frontal variant, nonfluent aphasia, and semantic dementia (54). In frontal variant frontotemporal dementia, behavioral changes are predominant, including hyperorality, apathy, disinhibition, and perseveration. Patients with nonfluent aphasia frontotemporal dementia have more left-hemisphere involvement and present with expressive language disturbances, including word-finding difficulties and grammatical errors. Semantic dementia is distinguished by the loss of word and object meanings. As frontotemporal dementia progresses, these subtypes become less distinct. Some patients with frontotemporal dementia also develop parkinsonian symptoms and motor neuron involvement in addition to the behavioral syndromes outlined above.
The genetics of frontotemporal dementia primarily involve abnormalities in the microtubule-associated protein tau. A familial variant of frontotemporal dementia has been linked to a mutation of the tau gene on chromosome 17 (55). Frontotemporal dementia has also been linked to chromosomes 9 and 3 (56). Common neuropathological findings related to the genetic abnormalities seen in families with frontotemporal dementia include tau-positive neuronal and glial inclusions, suggesting the designation “tauopathy,” which is applied to frontotemporal dementia and other, similar types of dementia.
Miscellaneous dementias
A number of dementing syndromes are associated with parkinsonism. Dementia is often a late-occurring sequela of Parkinson’s disease. Other dementias include progressive supranuclear palsy, multiple-systems atrophy, and cortical-basal ganglionic degeneration. These conditions are often referred to as the “Parkinson’s plus” syndromes.
Other dementing illnesses that occur with less frequency than those described above include the prion diseases (such as Creutzfeldt-Jakob disease and kuru), Huntington’s disease, Wilson’s disease, and toxic and metabolic conditions.
Assessment
In the assessment of dementia, careful consideration must be given to distinguishing age-associated memory impairment, mild cognitive impairment, and frank dementia. Age-associated memory impairment tends to show increased latency with recall and poor memory for names, but overall memory function remains intact and within normative function for age. In mild cognitive impairment, while there may be both subjective report and objective evidence of memory loss, cognition and activities of daily living remain intact (57). The Clinical Dementia Rating (CDR) is a useful scale for assessing patients along a spectrum of cognitive impairment. Roughly, CDR scores of 0, 0.5, 1, 2, and 3 are associated with normal function, mild cognitive impairment, and mild, moderate, and severe dementia, respectively (58). This type of scale reinforces the concept of mild cognitive impairment as a harbinger of true dementia.
Mental status and cognitive examination
A crucial element of the assessment of dementia is the mental status examination. The MMSE is a useful screening tool for cognitive decline. Scores of less than 24 on this 30-point scale are suggestive of dementia. A thorough history is important in differentiating frank dementia from mild cognitive impairment or an undetected episode of delirium. Essential elements of the history include collateral information from family or caregivers about the onset of deficits, behavioral changes, mood or personality changes, psychotic symptoms such as hallucinations or delusions, and activities of daily living.
If cognitive screening tests and history do not yield enough information for diagnosis, a more comprehensive neuropsychological assessment may be warranted (4, 5).
Laboratory tests are helpful in screening for reversible causes of dementia as well as for possible causes of delirium and comorbid illness. Assays for thyroid function and vitamin B12 are particularly useful tests. The American Academy of Neurology’s practice guideline on the diagnosis of dementia (59) recommends syphilis screening only in high-incidence areas, such as the southern United States and parts of the Midwest.
Biomarkers
Several studies have shown elevated levels of CSF tau and lower levels of CSF Aβ1–42 in patients with Alzheimer’s disease (60, 61). A large study and meta-analysis conducted by Sunderland and associates suggests that measurement of both of these CSF markers, in conjunction with genetic analysis and neuroimaging, may be useful in identifying patients at risk of developing Alzheimer’s disease (62).
Neuroimaging
Imaging techniques such as magnetic resonance imaging are important for ruling out mass lesions or bleeding that may be responsible for changes in cognition and for diagnosing vascular dementia. Findings of hypometabolism in particular brain regions on positron emission tomography (PET) are helpful in differentiating dementia subtypes—frontotemporal dementia versus Alzheimer’s type dementia, for example. While patients with Alzheimer’s disease tend to show hypoperfusion in temporoparietal regions, more frontal areas are involved in frontotemporal dementia.
More advanced neuroimaging has been developed to visualize the pathology of Alzheimer’s disease by using specific markers for amyloid plaques and neurofibrillary tangle burden (63). Magnetic resonance spectroscopy (MRS), a technique useful in measuring brain metabolites, has been used in studies of Alzheimer’s disease. Although research has produced conflicting reports, the consistent findings include a decrease by about 15% in N-acetylaspartate throughout the cortex in early Alzheimer’s disease and a 20% increase in myo-inositol throughout white and gray matter in Alzheimer’s disease and in mild cognitive impairment (64). Molecular markers, functional neuroimaging, and MRS represent promising methods for detecting early changes in Alzheimer’s disease and for monitoring treatment response.
Treatment
Cholinesterase inhibitors
Treatment of the dementias has advanced considerably over the past decade with the advent of novel pharmacological agents. Medications approved by the Food and Drug Administration (FDA) for dementia have been limited to treatments for Alzheimer’s disease. The first of these address the cholinergic deficit implicated in the neuropathology of the disease by using the cholinesterase inhibitors—tacrine, donepezil, galantamine, and rivastigmine. These medications work by increasing the amount of acetylcholine in the synapse. They have been particularly useful in treating the behavioral disturbances associated with dementia as well as in delaying the decline in cognitive function. A meta-analysis of cholinesterase inhibitors in the treatment of Alzheimer’s disease indicated that they lead to modest improvements in behavioral symptoms as measured by the Neuropsychiatric Inventory (NPI) and the Alzheimer’s Disease Assessment Scale (ADAS) (65). In addition, these medications have a modest beneficial effect on functional outcomes as measured by ratings of activities of daily living.
While the cholinesterase inhibitors have FDA approval only for Alzheimer’s disease, they have been shown to be safe and effective in delaying the progression of vascular dementia as well as in managing the behavioral disturbances associated with vascular dementia (66, 67).
Antioxidants and anti-inflammatories
Because of the putative role of oxidative damage in the pathophysiology of Alzheimer’s disease, it has been hypothesized that antioxidants may be useful in treatment of the illness. The antioxidant vitamin E has been shown to be effective in slowing cognitive decline (68).
Although initial studies have suggested that the monoamine oxidase inhibitor selegiline might be effective in delaying cognitive decline (68), a recent review concluded that there is no evidence to support its use in the treatment of Alzheimer’s disease (69).
Proposed inflammatory mechanisms for Alzheimer’s disease have led to the investigation of nonsteroidal anti-inflammatory drugs (NSAIDs) as a treatment. In addition, retrospective studies have indicated that subjects with prolonged exposure to NSAIDs are less likely to develop Alzheimer’s disease (70). A recently published 1-year randomized, placebo-controlled clinical trial evaluated the effects of naproxen and the cyclooxygenase-2 (COX-2) inhibitor rofecoxib on dementia rating scales (71). The study found no significant effect of these medications as compared with placebo.
Use of the herb ginkgo biloba for improving memory function has attracted a great deal of popular interest. However, a randomized, placebo-controlled clinical trial with a population of nursing home residents with dementia or age-associated memory impairment (72) found no significant beneficial effect of ginkgo on memory, attention, or activities of daily living.
Hormonal therapies
Previous work from animal studies and epidemiological data have suggested that estrogen replacement might be a viable treatment option for Alzheimer’s disease. Estrogen has been shown in animal models to be neuroprotective by a variety of mechanisms, including modulation of APP processing (73). Zandi and colleagues’ Cache County Study demonstrated that women on hormone replacement therapy had a reduced risk of developing Alzheimer’s disease (74). However, a randomized, placebo-controlled trial from the Women’s Health Initiative Memory Study demonstrated that women receiving estrogen plus progestin had an increased risk of developing dementia (75). Given the conflicting evidence, further studies are needed to elucidate the role of estrogen in dementia.
Novel treatments and future directions
Many treatments are being developed on the basis of our growing understanding of the pathophysiology of dementia. The FDA recently approved memantine for the treatment of moderate to severe Alzheimer’s disease. This drug, which acts as an N-methyl-d-aspartic acid antagonist, has been shown to be effective in this patient group (76). Memantine can also improve cognition ratings in patients with vascular dementia (77). The chief side effect cited in the vascular dementia study was dizziness.
Memantine works on the terminal stages of the pathogenic pathway implicated in Alzheimer’s disease. Efforts to intervene at earlier points in the progression of Alzheimer’s disease focus on abnormal amyloid protein processing. For example, γ-secretase inhibitors would interfere with formation of the plaque-generating Aβ42. Another enzyme involved in γ-secretase cleavage of APP is glycogen synthase kinase-3α (GSK-3α). A recent study by Phiel and colleagues demonstrated that therapeutic concentrations of lithium reduce Aβ production by inhibiting GSK-3α (78). Thus, lithium, a drug long familiar to psychiatrists, may become an attractive treatment for Alzheimer’s disease.
Metal chelators are being studied for their ability to sequester the heavy metals implicated in the pathogenesis of Alzheimer’s disease. Copper and iron contribute to the aggregation of Aβ and, in conjunction with Aβ, can generate neurotoxic oxygen radicals (48). Clioquinol, a chelating agent with affinity for copper and zinc, has been shown to reduce Aβ aggregates in human tissue from patients with Alzheimer’s disease and to decrease Aβ in transgenic mouse models of Alzheimer’s disease (79).
Treatment of comorbid conditions
Behavioral disturbances such as agitation, aggression, and psychosis often precipitate hospitalization of patients with dementia. Usually atypical antipsychotics are the drug of choice for treating such behavior. Risperidone at 1 mg/day has been shown to be effective in reducing psychotic symptoms and aggressive behavior in patients with Alzheimer’s disease (80, 81), and the severity of extrapyramidal symptoms with risperidone has been found to be significantly less than with haloperidol (82). In two placebo-controlled, double-blind studies with dementia patients in a long-term care facility, olanzapine was shown to reduce behavioral disturbances as measured by the Clinical Global Impression scale and the Neuropsychiatric Inventory (83, 84). A general principle in administering these medications in the geriatric population is to start with a low dose and titrate upward slowly. Medication side effects to be aware of include orthostasis, extrapyramidal symptoms, and anticholinergic effects such as urinary retention and constipation. These side effects are more common in the geriatric population; one should also bear in mind that they occur more frequently with conventional antipsychotics such as haloperidol than with atypical antipsychotics (82).
Certain nonpharmacological or environmental interventions are also useful for the management of behavioral disturbances in dementia. Frequent reorientation, the maintenance of familiar objects or surroundings, and redirection techniques can often help in the management of patients with dementia (85).
Depression is one of the most common comorbid conditions seen in dementia. A significant improvement in depressive symptoms was observed in a randomized, placebo-controlled trial of sertraline for the treatment of depression in patients with Alzheimer’s disease (86). Moreover, improvement in mood had beneficial effects on activities-of-daily-living rating scales.
Outcomes
All of the subtypes of dementia described here are progressive illnesses characterized by cognitive decline. While the prognosis is inevitably poor for patients with dementia, several mitigating factors have been identified that may improve outcomes. For example, in a study of institutionalization rates of dementia patients, the presence of a “coresident carer,” defined as a family caregiver living in the same household as the patient, was found to exert a marked protective effect against institutionalization (87). This study highlights the importance of strong social support for patients suffering from dementia.
Conclusion
As the population ages, delirium and dementia are increasingly important clinical challenges for the general psychiatrist. The two commonly coexist, which complicates the differential diagnosis in patients who have cognitive impairment. When approaching the patient with cognitive dysfunction, it is important to sort out the potential causes of the impairment, because delirium, dementia, and other cognitive disorders have significant overlap of symptoms and require distinct treatment strategies.
| Delirium | Dementia | |
|---|---|---|
| Onset | Abrupt | Gradual |
| Course | Fluctuating | Progressive |
| Attention | Impaired | Intact |
| Psychomotor | Variable | Normal |
| Hallucinations | Common | Less common |
| Infection |
| Intoxication |
| Withdrawal |
| Metabolic causes (hypoxia, hypoglycemia, electrolyte imbalances) |
| Medications (typically anticholinergics) |
| Trauma |
| Postoperative complications |
| Temporal onset |
| Perceptual disturbances |
| Hallucinations |
| Delusions |
| Psychomotor behavior |
| Cognitive status |
| Physical disorder |
| Sleep-wake cycle disturbance |
| Lability of mood |
| Variability of symptoms |
| Onset | Course | Neuroimaging | Neuropathology | |
|---|---|---|---|---|
| Alzheimer’s disease | Insidious | Progressive | Hypometabolism/hypoperfusion in temporoparietal regions | Amyloid plaques, neurofibrillary tangles |
| Vascular dementia | Abrupt | Stepwise | White matter hyperintensities | Infarcts, arteriosclerosis |
| Lewy body dementia | Gradual | Progressive | Hypoperfusion in occipital regions | α-Synuclein inclusion bodies |
| Frontotemporal dementia | Gradual | Progressive | Hypometabolism in frontotemporal regions | Tau-positive inclusion |
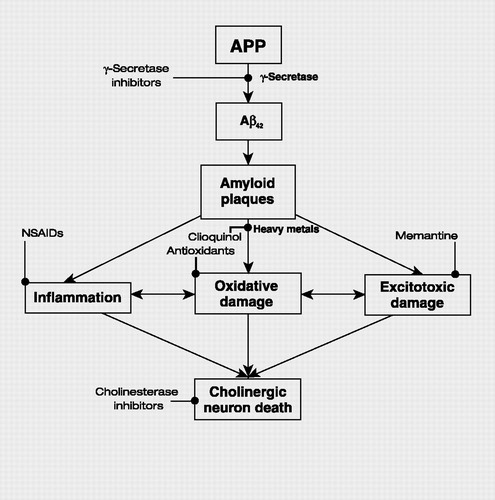
Figure 1. Simplified Schema of the Pathogenesis of Alzheimer’s Disease and Points of Interventions
APP, amyloid precursor protein
1 American Psychiatric Association: Practice Guideline for the Treatment of Patients With Delirium. Am J Psychiatry 1999; 156(May suppl):1–20Crossref, Google Scholar
2 Webster R, Holroyd S: Prevalence of psychotic symptoms in delirium. Psychosomatics 2000; 41:519–522Crossref, Google Scholar
3 Aizawa K, Kanai T, Saikawa Y, Takabayashi T, Kawano Y, Miyazawa N, Yamamoto T: A novel approach to the prevention of postoperative delirium in the elderly after gastrointestinal surgery. Surg Today 2002; 32:310–314Crossref, Google Scholar
4 Hanania M, Kitain E: Melatonin for treatment and prevention of postoperative delirium. Anesth Analg 2002; 94:338–339Google Scholar
5 Lipowski ZJ: Delirium (acute confusional states). JAMA 1987; 258:1789–1792Crossref, Google Scholar
6 Balan S, Leibovitz A, Zila SO, Ruth M, Chana W, Yassica B, Rahel B, Richard G, Neumann E, Blagman B, Habot B: The relation between the clinical subtypes of delirium and the urinary level of 6-SMT. J Neuropsychiatry Clin Neurosci 2003; 15:363–366Crossref, Google Scholar
7 Marcantonio E, Ta T, Duthie E, Resnick NM: Delirium severity and psychomotor types: their relationship with outcomes after hip fracture repair. J Am Geriatr Soc 2002; 50:850–857Crossref, Google Scholar
8 McNicoll L, Pisani MA, Zhang Y, Ely EW, Siegel MD, Inouye SK: Delirium in the intensive care unit: occurrence and clinical course in older patients. J Am Geriatr Soc 2003; 51:591–598Crossref, Google Scholar
9 Elie M, Rousseau F, Cole M, Primeau F, McCusker J, Bellavance F: Prevalence and detection of delirium in elderly emergency department patients. CMAJ 2000; 163:977–981Google Scholar
10 Parikh S, Chung F: Postoperative delirium in the elderly. Anesth Analg 1995; 80:1223–1232Google Scholar
11 Galanakis P, Bickel H, Gradinger R, Von Gumppenberg S, Forstl H: Acute confusional state in the elderly following hip surgery: incidence, risk factors, and complications. Int J Geriatr Psychiatry 2001; 16:349–355Crossref, Google Scholar
12 Elie M, Cole MG, Primeau FJ, Bellavance F: Delirium risk factors in elderly hospitalized patients. J Gen Intern Med 1998; 13:204–212Crossref, Google Scholar
13 Trzepacz PT: Is there a final common neural pathway in delirium? Focus on acetylcholine and dopamine. Semin Clin Neuropsychiatry 2000; 5:132–148Google Scholar
14 Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Horwitz RI: Clarifying confusion: the confusion assessment method: a new method for the detection of delirium. Ann Intern Med 1990; 113:941–948Crossref, Google Scholar
15 Trzepacz PT: The Delirium Rating Scale: its use in consultation-liaison research. Psychosomatics 1999; 40:193–204Crossref, Google Scholar
16 Trzepacz PT, Mittal D, Torres R, Kanary K, Norton J, Jimerson N: Validation of the Delirium Rating Scale-revised-98: comparison with the Delirium Rating Scale and the Cognitive Test for Delirium. J Neuropsychiatry Clin Neurosci 2001; 13:229–242Crossref, Google Scholar
17 Inouye SK, Bogardus ST Jr, Charpentier PA, Leo-Summers L, Acampora D, Holford TR, Cooney LM Jr: A multicomponent intervention to prevent delirium in hospitalized older patients. N Engl J Med 1999; 340:669–676Crossref, Google Scholar
18 Tune L, Carr S, Hoag E, Cooper T: Anticholinergic effects of drugs commonly prescribed for the elderly: potential means for assessing risk of delirium. Am J Psychiatry 1992; 149:1393–1394Crossref, Google Scholar
19 Mulsant BH, Pollock BG, Kirshner M, Shen C, Dodge H, Ganguli M: Serum anticholinergic activity in a community-based sample of older adults: relationship with cognitive performance. Arch Gen Psychiatry 2003; 60:198–203Crossref, Google Scholar
20 Han L, McCusker J, Cole M, Abrahamowicz M, Primeau F, Elie M: Use of medications with anticholinergic effect predicts clinical severity of delirium symptoms in older medical inpatients. Arch Intern Med 2001; 161:1099–1105Crossref, Google Scholar
21 Sipahimalani A, Masand PS: Olanzapine in the treatment of delirium. Psychosomatics 1998; 39:422–430Crossref, Google Scholar
22 Breitbart W, Tremblay A, Gibson C: An open trial of olanzapine for the treatment of delirium in hospitalized cancer patients. Psychosomatics 2002; 43:175–182Crossref, Google Scholar
23 Horikawa N, Yamazaki T, Miyamoto K, Kurosawa A, Oiso H, Matsumoto F, Nishimura K, Karasawa K, Takamatsu K: Treatment for delirium with risperidone: results of a prospective open trial with 10 patients. Gen Hosp Psychiatry 2003; 25:289–292Crossref, Google Scholar
24 Kim KY, Bader GM, Kotlyar V, Gropper D: Treatment of delirium in older adults with quetiapine. J Geriatr Psychiatry Neurol 2003; 16:29–31Crossref, Google Scholar
25 Al-Samarrai S, Dunn J, Newmark T, Gupta S: Quetiapine for treatment-resistant delirium. Psychosomatics 2003; 44:350–351Crossref, Google Scholar
26 Leso L, Schwartz TL: Ziprasidone treatment of delirium. Psychosomatics 2002; 43:61–62Crossref, Google Scholar
27 Mendelson G: Pheniramine aminosalicylate overdosage: reversal of delirium and choreiform movements with tacrine treatment. Arch Neurol 1977; 34:313Crossref, Google Scholar
28 Noyan MA, Elbi H, Aksu H: Donepezil for anticholinergic drug intoxication: a case report. Prog Neuropsychopharmacol Biol Psychiatry 2003; 27:885–887Crossref, Google Scholar
29 Eisendrath SJ, Goldman B, Douglas J, Dimatteo L, Van Dyke C: Meperidine-induced delirium. Am J Psychiatry 1987; 144:1062–1065Crossref, Google Scholar
30 Nakamura K, Kurasawa M, Tanaka Y: Apomorphine-induced hypoattention in rats and reversal of the choice performance impairment by anirace-tam. Eur J Pharmacol 1998; 342:127–138Crossref, Google Scholar
31 Fischer P: Successful treatment of nonanticholinergic delirium with a cholinesterase inhibitor. J Clin Psychopharmacol 2001; 21:118Crossref, Google Scholar
32 Franco K, Litaker D, Locala J, Bronson D: The cost of delirium in the surgical patient. Psychosomatics 2001; 42:68–73Crossref, Google Scholar
33 Cole MG, Primeau FJ: Prognosis of delirium in elderly hospital patients. CMAJ 1993; 149:41–46Google Scholar
34 McCusker J, Cole MG, Abrahamowicz M, Primeau FJ, Belzile E: Delirium predicts 12-month mortality. Arch Intern Med 2002; 162:457–463Crossref, Google Scholar
35 Kelly KG, Zisselman M, Cutillo-Schmitter T, Reichard R, Payne D, Denman SJ: Severity and course of delirium in medically hospitalized nursing facility residents. Am J Geriatr Psychiatry 2001; 9:72–77Crossref, Google Scholar
36 Gruber-Baldini AL, Zimmerman S, Morrison R, Grattan LM, Hebel J, Dolan MM, Hawkes W, Magaziner J: Cognitive impairment in hip fracture patients: timing of detection and longitudinal follow-up. J Am Geriatr Soc 2003; 51:1227–1236Crossref, Google Scholar
37 McCusker J, Cole M, Dendukuri N, Han L, Belzile E: The course of delirium in older medical inpatients. J Gen Intern Med 2003; 18:696–704Crossref, Google Scholar
38 Lyketsos CG, Lopez O, Jones B, Fitzpatrick AL, Breitner J, DeKosky S: Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the Cardiovascular Health Study. JAMA 2002; 288:1475–1483Crossref, Google Scholar
39 Olin JT, Schneider LS, Katz IR, Meyers BS, Alexopoulos GS, Breitner JC, Bruce ML, Caine ED, Cummings JL, Devanand DP, Krishnan KR, Lyketsos CG, Lyness JM, Rabins PV, Reynolds CF 3rd, Rovner BW, Steffens DC, Tariot PN, Lebowitz BD: Provisional diagnostic criteria for depression of Alzheimer disease. Am J Geriatr Psychiatry 2002; 10:125–128Crossref, Google Scholar
40 Lee HB, Lyketsos CG: Depression in Alzheimer’s disease: heterogeneity and related issues. Biol Psychiatry 2003; 54:353–362Crossref, Google Scholar
41 Small GW, Rabins PV, Barry PP, Buckholtz NS, DeKosky ST, Ferris SH, Finkel SI, Gwyther LP, Khachaturian ZS, Lebowitz BD, McRae TD, Morris JC, Oakley F, Schneider LS, Streim JE, Sunderland T, Teri LA, Tune LE: Diagnosis and treatment of Alzheimer disease and related disorders. Consensus statement of the American Association for Geriatric Psychiatry, the Alzheimer’s Association, and the American Geriatrics Society. JAMA 1997; 278:1363–1371Crossref, Google Scholar
42 Hebert LE, Scherr PA, Bienias JL, Bennett DA, Evans DA: Alzheimer disease in the US population: prevalence estimates using the 2000 census. Arch Neurol 2003; 60:1119–1122Crossref, Google Scholar
43 Reisberg B, Franssen EH, Souren LE, Auer SR, Akram I, Kenowsky S: Evidence and mechanisms of retrogenesis in Alzheimer’s and other dementias: management and treatment import. Am J Alzheimers Dis Other Demen 2002; 17:202–212Crossref, Google Scholar
44 Xia W, Zhang J, Kholodenko D, Citron M, Podlisny MB, Teplow DB, Haass C, Seubert P, Koo EH, Selkoe DJ: Enhanced production and oligomerization of the 42-residue amyloid beta-protein by Chinese hamster ovary cells stably expressing mutant presenilins. J Biol Chem 1997; 272:7977–7982Crossref, Google Scholar
45 Selkoe DJ, Podlisny MB: Deciphering the genetic basis of Alzheimer’s disease. Annu Rev Genomics Hum Genet 2002; 3:67–99Crossref, Google Scholar
46 Nussbaum RL, Ellis CE: Alzheimer’s disease and Parkinson’s disease. N Engl J Med 2003; 348:1356–1364Crossref, Google Scholar
47 Pitchumoni SS, Doraiswamy PM: Current status of antioxidant therapy for Alzheimer’s disease. J Am Geriatr Soc 1998; 46:1566–1572Crossref, Google Scholar
48 Finefrock AE, Bush AI, Doraiswamy PM: Current status of metals as therapeutic targets in Alzheimer’s disease. J Am Geriatr Soc 2003; 51:1143–1148Crossref, Google Scholar
49 Knopman DS, Parisi JE, Boeve BF, Cha RH, Apaydin H, Salviati A, Edland SD, Rocca WA: Vascular dementia in a population-based autopsy study. Arch Neurol 2003; 60:569–575Crossref, Google Scholar
50 Roman GC, Erkinjuntti T, Wallin A, Pantoni L, Chui HC: Subcortical ischaemic vascular dementia. Lancet Neurol 2002; 1(7):426–436Crossref, Google Scholar
51 O’Brien JT, Erkinjuntti T, Reisberg B, Roman G, Sawada T, Pantoni L, Bowler JV, Ballard C, DeCarli C, Gorelick PB, Rockwood K, Burns A, Gauthier S, DeKosky ST: Vascular cognitive impairment. Lancet Neurol 2003; 2(2):89–98Crossref, Google Scholar
52 Barber R, Panikkar A, McKeith IG: Dementia with Lewy bodies: diagnosis and management. Int J Geriatr Psychiatry 2001; 16(suppl 1):S12–S18Crossref, Google Scholar
53 Perry RJ, Miller BL: Behavior and treatment in frontotemporal dementia. Neurology 2001; 56:46S–51SCrossref, Google Scholar
54 Greicius MD, Geschwind MD, Miller BL: Presenile dementia syndromes: an update on taxonomy and diagnosis. J Neurol Neurosurg Psychiatry 2002; 72:691–700Crossref, Google Scholar
55 Lendon CL, Lynch T, Norton J, McKeel DW Jr, Busfield F, Craddock N, Chakraverty S, Gopalakrishnan G, Shears SD, Grimmett W, Wilhelmsen KC, Hansen L, Morris JC, Goate AM: Hereditary dysphasic disinhibition dementia: a frontotemporal dementia linked to 17q21–22. Neurology 1998; 50:1546–1555Crossref, Google Scholar
56 Yancopoulou D, Crowther RA, Chakrabarti L, Gydesen S, Brown JM, Spillantini MG: Tau protein in frontotemporal dementia linked to chromosome 3 (FTD-3). J Neuropathol Exp Neurol 2003; 62:878–882Crossref, Google Scholar
57 Kawas CH: Clinical practice: early Alzheimer’s disease. N Engl J Med 2003; 349:1056–1063Crossref, Google Scholar
58 Petersen RC, Doody R, Kurz A, Mohs RC, Morris JC, Rabins PV, Ritchie K, Rossor M, Thal L, Winblad B: Current concepts in mild cognitive impairment. Arch Neurol 2001; 58:1985–1992Crossref, Google Scholar
59 Knopman DS, DeKosky ST, Cummings JL, Chui H, Corey-Bloom J, Relkin N, Small GW, Miller B, Stevens JC: Practice parameter: diagnosis of dementia (an evidence-based review). Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2001; 56:1143–1153Crossref, Google Scholar
60 Galasko D, Chang L, Motter R, Clark CM, Kaye J, Knopman D, Thomas R, Kholodenko D, Schenk D, Lieberburg I, Miller B, Green R, Basherad R, Kertiles L, Boss MA, Seubert P: High cerebrospinal fluid tau and low amyloid beta42 levels in the clinical diagnosis of Alzheimer disease and relation to apolipoprotein E genotype. Arch Neurol 1998; 55:937–945Crossref, Google Scholar
61 Andreasen N, Minthon L, Davidsson P, Vanmechelen E, Vanderstichele H, Winblad, B, Blennow K: Evaluation of CSF-tau and CSF-Abeta42 as diagnostic markers for Alzheimer disease in clinical practice. Arch Neurol 2001; 58:373–379Google Scholar
62 Sunderland T, Linker G, Mirza N, Putnam KT, Friedman DL, Kimmel LH, Bergeson J, Manetti GJ, Zimmermann M, Tang B, Bartko JJ, Cohen RM: Decreased beta-amyloid1–42 and increased tau levels in cerebrospinal fluid of patients with Alzheimer disease. JAMA 2003; 289:2094–2103Crossref, Google Scholar
63 Shoghi-Jadid K, Small GW, Agdeppa ED, Kepe V, Ercoli LM, Siddarth P, Read S, Satyamurthy N, Petric A, Huang SC, Barrio JR: Localization of neurofibrillary tangles and beta-amyloid plaques in the brains of living patients with Alzheimer disease. Am J Geriatr Psychiatry 2002; 10:24–35Crossref, Google Scholar
64 Valenzuela MJ, Sachdev P: Magnetic resonance spectroscopy in AD. Neurology 2001; 56:592–598Crossref, Google Scholar
65 Trinh NH, Hoblyn J, Mohanty S, Yaffe K: Efficacy of cholinesterase inhibitors in the treatment of neuropsychiatric symptoms and functional impairment in Alzheimer disease: a meta-analysis. JAMA 2003: 289:210–216Crossref, Google Scholar
66 Roman GC: Vascular dementia: distinguishing characteristics, treatment, and prevention. J Am Geriatr Soc 2003; 51(5 Suppl Dementia):S296–S304Crossref, Google Scholar
67 Black S, Roman GC, Geldmacher DS, Salloway S, Hecker J, Burns A, Perdomo C, Kumar D, Pratt R; Donepezil 307 Vascular Dementia Study Group: Efficacy and tolerability of donepezil in vascular dementia: positive results of a 24-week, multicenter, international, randomized, placebo-controlled clinical trial. Stroke 2003; 34:2323–2330Crossref, Google Scholar
68 Sano M, Ernesto C, Thomas RG, Klauber M, Schafer K, Grundman M, Woodbury P, Growdon J, Cotman CW, Pfeiffer E, Schneider LS, Thal LJ; the Members of the Alzheimer’s Disease Cooperative Study: A controlled trial of selegiline, alpha-tocopherol, or both as treatment for Alzheimer’s disease. N Engl J Med 1997; 336:1216–1222Crossref, Google Scholar
69 Birks J, Flicker L: Selegiline for Alzheimer’s disease. Cochrane Database Syst Rev 2003: CD000442Google Scholar
70 Rich JB, Rasmusson DX, Folstein MF, Carson KA, Kawas C, Brandt J: Nonsteroidal anti-inflammatory drugs in Alzheimer’s disease. Neurology 1995; 45:51–55Crossref, Google Scholar
71 Aisen PS, Schafer KA, Grundman M, Pfeiffer E, Sano M, Davis KL, Farlow MR, Jin S, Thomas RG, Thal LJ: Effects of rofecoxib or naproxen vs placebo on Alzheimer disease progression: a randomized controlled trial. JAMA 2003; 289:2819–2826Crossref, Google Scholar
72 van Dongen M, van Rossum E, Kessels A, Sielhorst H, Knipschild P: Ginkgo for elderly people with dementia and age-associated memory impairment: a randomized clinical trial. J Clin Epidemiology 2003; 56:367–376Crossref, Google Scholar
73 Goodenough S, Schafer M, Behl C: Estrogen-induced cell signalling in a cellular model of Alzheimer’s disease. J Steroid Biochem Mol Biol 2003; 84:301–305Crossref, Google Scholar
74 Zandi PP, Carlson MC, Plassman BL, Welsh-Bohmer KA, Mayer LS, Steffens DC, Breitner JC; Cache County Memory Study Investigators: Hormone replacement therapy and incidence of Alzheimer disease in older women: the Cache County Study. JAMA 2002; 288:2123–2129Crossref, Google Scholar
75 Shumaker SA, Legault C, Rapp SR, Thal L, Wallace RB, Ockene JK, Hendrix SL, Jones BN 3rd, Assaf AR, Jackson RD, Kotchen JM, Wassertheil-Smoller S, Wactawski-Wende J; WHIMS Investigators: Estrogen plus progestin and the incidence of dementia and mild cognitive impairment in postmenopausal women: the Women’s Health Initiative Memory Study: a randomized controlled trial. JAMA 2003; 289:2651–2662Crossref, Google Scholar
76 Reisberg B, Doody R, Stoffler A, Schmitt F, Ferris S, Mobius HJ; Memantine Study Group: Memantine in moderate-to-severe Alzheimer’s disease. N Engl J Med 2003: 348:1333–1341Crossref, Google Scholar
77 Orgogozo JM, Rigaud AS, Stöffler A, Möbius HJ, Forette F: Efficacy and safety of memantine in patients with mild to moderate vascular dementia: a randomized, placebo-controlled trial (MMM 300). Stroke 2002; 33:1834–1839Crossref, Google Scholar
78 Phiel CJ, Wilson CA, Lee VM, Klein PS: GSK-3alpha regulates production of Alzheimer’s disease amyloid-beta peptides. Nature 2003; 423:435–439Crossref, Google Scholar
79 Cherny RA, Atwood CS, Xilinas ME, Gray DN, Jones WD, McLean CA, Barnham KJ, Volitakis I, Fraser FW, Kim Y, Huang X, Goldstein LE, Moir RD, Lim JT, Beyreuther K, Zheng H, Tanzi RE, Masters CL, Bush AI. Treatment with a copper-zinc chelator markedly and rapidly inhibits beta-amyloid accumulation in Alzheimer’s disease transgenic mice. Neuron 2001; 30:665–676Crossref, Google Scholar
80 Katz IR, Jeste DV, Mintzer JE, Clyde C, Napolitano J, Brecher M: Comparison of risperidone and placebo for psychosis and behavioral disturbances associated with dementia: a randomized, double-blind trial. Risperidone Study Group. J Clin Psychiatry 1999; 60:107–115Crossref, Google Scholar
81 Brodaty H, Ames D, Snowdon J, Woodward M, Kirwan J, Clarnette R, Lee E, Lyons B, Grossman F: A randomized placebo-controlled trial of risperidone for the treatment of aggression, agitation, and psychosis of dementia. J Clin Psychiatry 2003; 64:134–143Crossref, Google Scholar
82 De Deyn PP, Rabheru K, Rasmussen A, Bocksberger JP, Dautzenberg PL, Eriksson S, Lawlor BA: A randomized trial of risperidone, placebo, and haloperidol for behavioral symptoms of dementia. Neurology 1999; 53:946–955Crossref, Google Scholar
83 Fontaine CS, Hynan LS, Koch K, Martin-Cook K, Svetlik D, Weiner MF: A double-blind comparison of olanzapine versus risperidone in the acute treatment of dementia-related behavioral disturbances in extended care facilities. J Clin Psychiatry 2003; 64:726–730Crossref, Google Scholar
84 Street JS, Clark WS, Gannon KS, Cummings JL, Bymaster FP, Tamura RN, Mitan SJ, Kadam DL, Sanger TM, Feldman PD, Tollefson GD, Breier A: Olanzapine treatment of psychotic and behavioral symptoms in patients with Alzheimer disease in nursing care facilities: a double-blind, randomized, placebo-controlled trial. The HGEU Study Group. Arch Gen Psychiatry 2000; 57:968–976Crossref, Google Scholar
85 Cohen-Mansfield J: Nonpharmacologic interventions for inappropriate behaviors in dementia: a review, summary, and critique. Am J Geriatr Psychiatry 2001; 9:361–381Crossref, Google Scholar
86 Lyketsos CG, DelCampo L, Steinberg M, Miles Q, Steele CD, Munro C, Baker AS, Sheppard JE, Frangakis C, Brandt J, Rabins PV: Treating depression in Alzheimer disease: efficacy and safety of sertraline therapy, and the benefits of depression reduction: the DIADS. Arch Gen Psychiatry 2003; 60:737–746Crossref, Google Scholar
87 Banerjee S, Murray J, Foley B, Atkins L, Schneider J, Mann A: Predictors of institutionalisation in people with dementia. J Neurol Neurosurg Psychiatry 2003: 74:1315–1316Crossref, Google Scholar



