Whole-Body Heating: An Emerging Therapeutic Approach to Treatment of Major Depressive Disorder
Abstract
Major depressive disorder is the leading cause of disability worldwide. Currently available pharmacological approaches to the treatment of depression (which are the mainstay of treatment in the United States) suffer from important shortcomings, including limited efficacy, delayed onset of action, increased relapse risk upon withdrawal, and significant side effects that impair quality of life and promote treatment nonadherence and/or discontinuation. There is an emerging interest in the potential use of evolutionarily conserved interoceptive pathways (i.e., pathways that relay sensory information, related to the internal, physiologic state of the body, from the periphery to the central nervous system) as “gateways” to neural systems controlling affective and cognitive function relevant to the pathophysiology of depression. In support of the potential utility of this approach, we have shown in open and randomized, double-blind, sham-controlled trials that infrared whole-body heating has significant and long-lasting antidepressant effects relative to a sham condition. In this review, we explore the potential role of thermosensory pathways in the etiology, pathophysiology, and symptomatology of major depressive disorder, as well as its potential as a novel therapeutic approach to the treatment of major depressive disorder.
Major depressive disorder is the leading cause of disability worldwide (1). In the United States, it affects 16.2 million Americans, or 6.7% of the adult U.S. population. It is more prevalent among women (8.5%) than men (4.8%) (2). Among adults, 18- to 25-year-olds have the highest prevalence, at 10.9% (2). In the United States, major depressive disorder also affects an estimated 3.1 million adolescents, ages 12–17 years (2). In addition to being widespread, major depressive disorder is often recurrent and can be lethal. Estimates of lifetime risk of suicide among those with treated depression have been found to be as high as 15% (3).
Biopsychosocial Underpinnings of Major Depressive Disorder
Modern conceptualizations of depression have sought to incorporate several different approaches to understanding the disorder. Models that incorporate the role of biological, cognitive, social, and behavioral vulnerabilities often fall under the larger umbrella of stress-diathesis models, indicating that weak links in any of these categories can often lead to depression, especially in the context of stress (4).
Treatment and Outcomes
Reflecting widespread frustration in scientific and clinical circles, an international scientific body convened by the Royal Society in London concluded that “a fundamental change was needed in nearly every aspect of translational research in mental health” (5). Nowhere would such change be more welcome than in the treatment of depression, which is the third leading cause of overall global disease burden (6). Much of this burden comes from the fact that currently available pharmacological modalities (which are the mainstay of treatment in the United States) suffer from important shortcomings, including limited efficacy, delayed onset of action, increased relapse risk upon withdrawal, and significant side effects that impair quality of life and promote treatment nonadherence and/or discontinuation (7–13). Moreover, despite years of concerted effort, and some progress (14), the search for biomarkers that reliably predict antidepressant response has proven remarkably difficult (9, 15, 16), leaving pharmacological treatment decisions in a perpetual state of trial and error. In addition to reducing efficacy and lengthening average time to adequate response, this situation may actually contribute to the development of treatment resistance (17).
Future Directions
Recently, attention has been drawn to the possibility that interoceptive systems may be harnessed to treat major depressive disorder (18). These systems relay information related to the physiologic state of the body to the central nervous system, and include sensory systems relevant to non-painful and painful senses. Non-painful interoceptive signals include those related to air hunger, bladder, blood serum (pH, osmolality, glucose), cardiovascular, fatigue, genital sensation, itch, gastrointestinal (esophageal, gastric, intestinal, colorectal), hunger, muscle tension, respiratory, sensual touch, shudder, temperature, thirst, tickle, and vasomotor flush physiologic processes (18). One such interoceptive system is the thermosensitive system, with afferent fibers traveling within spinoparabrachial and spinothalamic pathways (19). In recent open (20) and randomized, double-blind, sham-controlled trials (21), we have shown that infrared whole-body heating (WBH) has potential for the treatment of major depressive disorder. WBH is a novel intervention that appears to hold promise as an alternative and/or adjunct to pharmacological therapies based on data that it has a rapid and sustained antidepressant effect, confers none of the central nervous system (CNS)-mediated side effects that bedevil other somatic therapies for depression, and may operate via a body-to-brain pathway that provides biomarkers that reliably predict who is likely to respond (19–21).
Harnessing Evolved Sensory Signaling Pathways to Circumvent Limitations Inherent in Whole-Brain Manipulations
With few exceptions, currently extant somatic treatments for depression (e.g., antidepressant medications, electroconvulsive therapy) share in common mechanisms of action that simultaneously affect multiple areas of brain functioning, only a portion of which are likely to confer antidepressant benefit (22). As noted earlier, it is increasingly recognized that the current pharmacological approach of indiscriminately targeting receptors and/or reuptake sites for neurotransmitter systems that impact widely distributed, and functionally divergent, brain regions may have reached the limit of its effectiveness (5). One very obvious reason for our inability to materially enhance efficacy with this approach is the fact that it fails to recognize the highly complex functional topology of the CNS and the corollary truth that the same neurotransmitter and/or receptor can have very different, and not infrequently opposing, physiologic and behavioral effects, depending on the anatomical pathway in which they operate (23). What is needed, therefore, is a methodology for enhanced anatomical (and, hence, functional) specificity in our interventions. In the present article, we explore the possibility of achieving this specificity by utilizing anatomical and functional specificity built into peripheral sensory signaling pathways by evolutionary processes to selectively affect functioning in specific brain regions linked in animal and human studies to depression and well-being (22). For example, spinal afferents innervate multiple brain regions implicated in the etiology and pathophysiology of major depressive disorder (24, 25).
Supporting this approach, prior studies by our group suggest that altering functional activity in such peripheral sensory pathways—including the innate immune inflammatory response—produces antidepressant effects without incurring the multiple disadvantages inherent in directly manipulating the CNS with either drugs or devices. For example, utilizing a randomized, placebo-controlled design in 60 patients with treatment-resistant depression, we have recently found that peripheral blockade of the proinflammatory cytokine tumor necrosis factor (TNF) with a biologic agent (infliximab) too large to cross the blood-brain barrier produces antidepressant effects that are similar in magnitude to those seen with standard antidepressants in individuals with elevated concentrations of peripheral inflammatory biomarkers (i.e., C-reactive protein [CRP] and TNF) while having no effect, or a detrimental effect compared with that of placebo, in patients with progressively lower levels of these same biomarkers (26). Importantly, treatment with infliximab was associated with an incidence of side effects no greater than those of placebo, consistent with the notion that modulating peripheral pathways that signal the CNS via specific, evolutionarily conserved, mechanisms induces antidepressant effects that are apparent primarily in those individuals with evidence of abnormal functioning in these same pathways, while avoiding the myriad adverse events incurred by the widespread alteration of neurotransmitter systems typically induced by antidepressants. It is to this possibility that we now turn, focusing on the use of WBH to sensitize (enhance activity) in one such peripheral signaling system.
Antidepressant Effects of WBH in Humans and Antidepressant-Like Effects in an Animal Model
Based on both the theoretical considerations discussed earlier and basic/clinical studies conducted by our group (as discussed later), we have conducted two studies examining the acute antidepressant effects of a single session of WBH (20, 21). In the first open trial, we found, in 16 medically healthy patients with major depressive disorder, that WBH induced a rapid, robust, and sustained reduction in depressive symptom scores (assessed with the German version of the Centers for Epidemiologic Studies Depression Scale [CES-D]) (27), based on CES-D mean±SD scores dropping from 29.9±10.6 pretreatment to 19.2±12.3 five days posttreatment, t=4.53, df=15, p<.001, effect size=1.13. Thirteen of these subjects received no other pharmacological or psychotherapeutic intervention during this period, whereas three subjects were on chronic treatment with a selective serotonin reuptake inhibitor (SSRI) antidepressant (with no change in dosage during the study period). When looked at separately, WBH appeared to have no effect in these three subjects and, in fact, worsened depressive symptoms in one of them. In animal models, acute administration of SSRIs increases extracellular serotonin concentrations in the dorsal raphe nucleus, resulting in inhibition of serotonergic neuronal excitability and firing rates (28). Meanwhile, studies in animals have shown that WBH activates brainstem serotonergic neurons, as measured by increased immediate-early gene expression (29). If therapeutic effects of WBH depend in part on activation of brainstem serotonergic neurons, SSRIs may interfere with this effect through inhibition of serotonergic neuronal excitability. With the three subjects receiving SSRIs removed from analysis, the effect size of WBH increased, t=5.15, df=12, p<.001, effect size=1.4. Interestingly, the antidepressant response observed five days posttreatment was actually improved six weeks later in nine patients for whom these longer term follow-up data were available (CES-D mean score, 13.9). Although these patients received additional treatment during this period, these findings, although preliminary, suggest—at the least—that WBH may induce a rapid antidepressant response that can be solidified by longer term modalities. Although this was the first study, to our knowledge, to examine WBH specifically for major depressive disorder, our findings were consistent with reports that WBH improves mood in patients with cancer and improves quality of life scores in patients with type II diabetes (30, 31).
We subsequently conducted a randomized, double-blind, sham-controlled study of the effects of WBH in 29 medically healthy individuals with major depressive disorder (21). When compared with the sham group, the active WBH group showed improved Hamilton Depression Rating Scale scores across the six-week postintervention study period (WBH versus sham; week 1: −6.53, 95% CI=−9.90 to −3.16, p<.001; week 2: −6.35, 95% CI=−9.95 to −2.74, p=.001; week 4: −4.50, 95% CI=−8.17 to −0.84, p=.02; and week 6: −4.27, 95% CI=−7.94 to −0.61, p=.02). In a subsequent study, Naumann and colleagues (32) found in a randomized, two-arm placebo-controlled study of medically healthy depressed individuals that an intervention consisting of two hyperthermic baths (40 °C) per week for four weeks improved depressive symptoms measured using the Hamilton Depression Rating Scale, relative to placebo, at the two-week time point. Although additional randomized, double-blind, placebo-controlled studies are needed, the studies discussed earlier suggest that infrared WBH or other thermal warming approaches hold promise as novel therapeutic approaches for the treatment of major depressive disorder.
Thermosensitive Systems and Major Depressive Disorder
Warm cutaneous thermal signals activate thermosensitive afferents that travel in spinal nerves and synapse in lamina I of the spinal cord (Figure 1). There, signals traveling in the lateral spinothalamic tract are relayed via the spinoparabrachial and spinothalamic pathways to the CNS. Spinoparabrachial pathways have been implicated in thermoregulatory cooling responses to warm temperature (33) and may be involved in the activation of brainstem serotonergic neurons (22). Spinothalamic pathways relay signals to somatosensory and limbic forebrain circuits (19, 34–36). Spinothalamic pathways originating in lamina I of the spinal cord include a lateral branch, which projects to the ventral posterior nucleus of the thalamus (including the thermosensitive ventromedial posterior nucleus [VMpo]); the ventral posterior nucleus of the thalamus in turn projects to the mid/posterior dorsal insula, which relays signals to the anterior insula (34, 35). This system has been proposed to play a central role in interoception (34–36). In addition, spinothalamic pathways originating in lamina I of the spinal cord include a medial branch, which projects to the ventral caudal subdivision of the mediodorsal nucleus (MDvc) (36). The MDvc in turn projects to the anterior cingulate cortex (36). Together, the spinothalamic pathways relaying signals to the insula and anterior cingulate cortex are thought to represent the affective aspects of thermosensation (34–36). In addition, spinothalamic afferents originating in deeper layers of the spinal cord, lamina IV-V, travel in the anterior spinothalamic tract to also innervate the ventral posterior nucleus of the thalamus (in this case, the ventral posterolateral nucleus [VPL] and VPI); the ventral posterior nucleus of the thalamus in turn projects to the S1 and S2 regions of the somatosensory cortex (Figure 2). The spinothalamic pathways relaying signals to the somatosensory cortex are thought to represent the discriminative aspect of thermosensation (36).
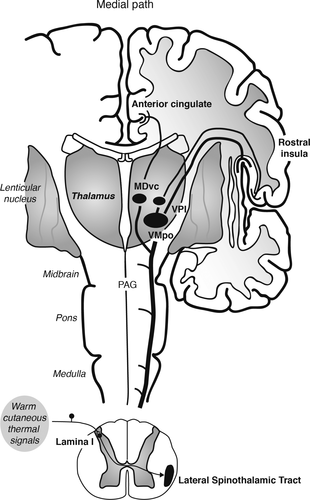
FIGURE 1. The Medial Path Taken by Thermosensitive Spinothalamic Afferentsa
aThe medial path taken by thermosensitive spinothalamic afferents originating in lamina I of the spinal cord represents the affective component of thermosensation. Spinothalamic afferents originating in lamina I of the spinal cord innervate the ventral posterior nucleus of the thalamus (including the thermosensitive ventromedial posterior nucleus [VMpo]); the ventral posterior nucleus of the thalamus in turn projects to the mid/posterior dorsal insula, which relays signals to the anterior insula (34–36). In addition, spinothalamic pathways originating in lamina I of the spinal cord include a medial branch, which projects to the ventral caudal subdivision of the mediodorsal nucleus (MDvc) (36). The MDvc in turn projects to the anterior cingulate cortex (36). Abbreviations: MDvc, ventral caudal subdivision of the mediodorsal nucleus; PAG, periaqueductal gray; VMpo, ventromedial posterior nucleus of the thalamus; VPI, ventral posterior inferior nucleus of the thalamus; STT, spinothalamic tract. Adapted from “Somatosensory System,” by S. Hendry and S. Hsiao, 2013, Fundamental Neuroscience, 4th ed., pp. 531–551, p. 545. Copyright 2013 by Elsevier. Adapted with permission.
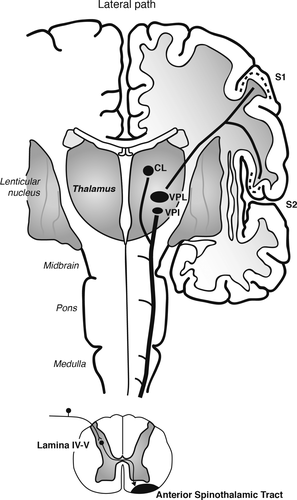
FIGURE 2. The Lateral Path Taken by Thermosensitive Spinothalamic Afferentsa
aThe lateral path taken by thermosensitive spinothalamic afferents originating in lamina IV-V of the spinal cord represents the discriminative components of thermosensation. Spinothalamic afferents originating in deeper layers of the spinal cord, lamina IV-V, travel in the anterior spinothalamic tract to innervate the ventral posterior nucleus of the thalamus (ventral posterolateral nucleus (VPL) and ventral posterior inferior nucleus (VPI)); the ventral posterior nucleus of the thalamus in turn projects to the S1 and S2 regions of the somatosensory cortex (36). Abbreviations: CL, central lateral nucleus of the intralaminar thalamus; VPI, ventral posterior inferior nucleus of the thalamus; VPL, ventral posterolateral nucleus of the thalamus; S1, primary somatosensory cortex; S2, secondary somatosensory cortex; STT, spinothalamic tract. Adapted from “Somatosensory System,” by S. Hendry and S. Hsiao, 2013, Fundamental Neuroscience, 4th ed., pp. 545. Copyright 2013 by Elsevier. Adapted with permission.
To study effects of WBH on brain serotonergic neurons and antidepressant-like behavioral responses in rodent models, we placed rats in incubators with temperature maintained at 37°C or room temperature for 85–105 minutes (29, 37) and monitored body temperature using rectal thermometry (29) or chronically implanted biotelemetric devices (37). Consistent with older animal studies suggesting that WBH affects mood-relevant serotonergic activity in the CNS (38), we have shown in rodent models that WBH acts synergistically with a subthreshold dose of the SSRI citalopram to produce an antidepressant-like behavioral effect (37). We have also shown that WBH activates serotonergic neurons in the midbrain (dorsal raphe nucleus, interfascicular part [DRI], and dorsal raphe nucleus, ventrolateral part [DRVL]) (29) that are implicated in antidepressant (22) and antipanic-like (39) behavioral effects, respectively.
Consistent with these preclinical findings, studies using functional magnetic resonance imaging have shown that warm (41°C) cutaneous stimuli applied to the hand of humans induces activations in the midorbitofrontal cortex, pregenual cingulate cortex, and the ventral striatum that are correlated with subjective pleasantness ratings (suggesting a central role of spinothalamic afferents originating in lamina I of the spinal cord, innervating the MDvc and subsequently the anterior cingulate cortex [Figure 1]) (40). In contrast, intensity ratings of the stimuli were associated with activation of the somatosensory cortex and ventral and anterior insular cortices (suggesting a central role of spinothalamic afferents innervating the ventral posterior nucleus of the thalamus and subsequently the somatosensory and insular cortices [Figure 1 and Figure 2]) (40). It is important to note that the brain areas associated with affective responses to warm cutaneous temperature constitute neural systems that are dysregulated in patients with depression (25, 41–43) and are projection targets for DRI serotonergic neurons (22, 44, 45). Indeed, DRI neurons project heavily to the orbitofrontal cortex and anterior cingulate cortex in primates (46) and to the medial prefrontal cortex and ventral striatum in rodents (47). Moreover, DRI neurons project to other nodes within neural circuits implicated in depression, including frontal and entorhinal cortices, hippocampus, and midline thalamus (25, 47).
The relevance of these findings and considerations for the pathophysiology and treatment of mood disorders is further highlighted by the fact that major depressive disorder has been repeatedly associated with physiologic changes that would be expected to result from suboptimal activity in thermoregulatory circuits (e.g., increased core body temperature as a result of suboptimal thermoregulatory cooling ability [48–54], including a decreased ability to sweat [52–55]). Moreover, effective treatments tend to reverse these abnormalities (49). In this regard, it is important to emphasize that a single session of WBH resulted in long-term lower core body temperature (potentially via increased thermoregulatory cooling) in our open trial in humans (20), raising the intriguing possibility that the procedure may actually normalize functioning of the thermosensory pathways and thus impact core depressive pathophysiology, as opposed to merely utilizing this pathway for therapeutic purposes. Furthermore, our finding that increased core body temperature prior to WBH predicted symptom improvement suggests that treatment response may identify a subgroup of patients with depression with some degree of biological homogeneity and that, therefore, biomarkers might be developed to predict who would and would not respond.
The primary conceptual shift in this review is to highlight the potential etiological and therapeutic relevance of peripheral sensory signaling pathways as “gateways” into the CNS functional circuitry implicated in the development of depression. The demonstration that WBH is superior to placebo in the acute treatment of depression, while conferring a very low side effect burden (20, 21), opens the door to entirely novel technologies and treatments that would be easy to administer and highly cost effective. In addition to reducing the time to therapeutic response when added as an “accelerator” strategy to standard treatments, the observation of a prolonged antidepressant effect from a single WBH session raises the possibility that this modality might hold promise as a “stand-alone” option for the chronic treatment of depression. Moreover, if our preliminary observation that increased body temperature at baseline predicts therapeutic response is confirmed, WBH may enhance psychiatry’s ability to offer personalized treatment strategies, which is an important—and persistently elusive—goal. Finally, future studies should examine the potential utility of WBH as a preventive strategy for the development or relapse of depression (56).
1 Depression [Fact Sheet]. Geneva: World Health Organization, 2017. http://www.who.int/en/news-room/fact-sheets/detail/depression. Accessed May 17, 2018Google Scholar
2 Major Depression. Bethesda, MD: National Institute of Mental Health, 2017. https://www.nimh.nih.gov/health/statistics/major-depression.shtml. Accessed May 17, 2018Google Scholar
3 : Suicide mortality among patients treated for depression in an insured population. Am J Epidemiol 1998; 147:155–160Crossref, Google Scholar
4 : A biopsychosocial model as a guide for psychoeducation and treatment of depression. Depress Anxiety 2006; 23:312–324Crossref, Google Scholar
5 : Drug research: a plan for mental illness. Nature 2012; 483:269Crossref, Google Scholar
6 : Grand challenges in global mental health. Nature 2011; 475:27–30Crossref, Google Scholar
7 : Ketamine for treatment-resistant unipolar depression: current evidence. CNS Drugs 2012; 26:189–204Crossref, Google Scholar
8 : Does the probability of receiving placebo influence clinical trial outcome? A meta-regression of double-blind, randomized clinical trials in MDD. Eur Neuropsychopharmacol 2009; 19:34–40Crossref, Google Scholar
9 : Review of pharmacological treatment in mood disorders and future directions for drug development. Neuropsychopharmacology 2012; 37:77–101Crossref, Google Scholar
10 : Acute and longer-term outcomes in depressed outpatients requiring one or several treatment steps: a STAR*D report. Am J Psychiatry 2006; 163:1905–1917Crossref, Google Scholar
11 : Blue again: perturbational effects of antidepressants suggest monoaminergic homeostasis in major depression. Front Psychol 2011; 2:159Crossref, Google Scholar
12 : The role of the primary care physician in patients’ adherence to antidepressant therapy. Med Care 1995; 33:67–74Crossref, Google Scholar
13 : Abrupt withdrawal of antidepressant treatment. Am J Psychiatry 2010; 167:886–888Crossref, Google Scholar
14 : Biomarkers to predict antidepressant response. Curr Psychiatry Rep 2010; 12:553–562Crossref, Google Scholar
15 : Using neuroimaging to predict treatment response in mood and anxiety disorders. Ann Clin Psychiatry 2006; 18:33–42Crossref, Google Scholar
16 : Laboratory tests to aid in psychiatric diagnosis: are we making progress? Arch Gen Psychiatry 1999; 56:405–406Crossref, Google Scholar
17 : Duration of untreated illness in major depressive disorder: a naturalistic study. Int J Clin Pract 2007; 61:1697–1700Crossref, Google Scholar
18 : Interoception and mental health: a roadmap. Biol Psychiatry Cogn Neurosci Neuroimaging (Epub ahead of print, Mar 26, 2018). doi:
19 : Somatic influences on subjective well-being and affective disorders: the convergence of thermosensory and central serotonergic systems. Front Psychol 2015; 5:1580Crossref, Google Scholar
20 : Whole-body hyperthermia for the treatment of major depression: associations with thermoregulatory cooling. Am J Psychiatry 2013; 170:802–804Crossref, Google Scholar
21 : Whole-body hyperthermia for the treatment of major depressive disorder: a randomized clinical trial. JAMA Psychiatry 2016; 73:789–795Crossref, Google Scholar
22 : Integrative physiology of depression and antidepressant drug action: implications for serotonergic mechanisms of action and novel therapeutic strategies for treatment of depression. Pharmacol Ther 2013; 137:108–118Crossref, Google Scholar
23 : Stress-related serotonergic systems: implications for symptomatology of anxiety and affective disorders. Cell Mol Neurobiol 2012; 32:695–708Crossref, Google Scholar
24 : Projections from the spinal cord to the brain; in The Spinal Cord. Edited by Watson C, Paxinos G, Kayalioglu G. London, Academic Press,2009, pp 148–167Crossref, Google Scholar
25 : Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 2008; 213:93–118Crossref, Google Scholar
26 : A randomized controlled trial of the tumor necrosis factor antagonist infliximab for treatment-resistant depression: the role of baseline inflammatory biomarkers. JAMA Psychiatry 2013; 70:31–41Crossref, Google Scholar
27 : Factorial and discriminant validity of the Center for Epidemiological Studies Depression (CES-D) scale. J Clin Psychol 1986; 42:28–33Crossref, Google Scholar
28 : Autoregulation of serotonin neurons: role in antidepressant drug action. Pharmacol Rev 1999; 51:533–591Google Scholar
29 : Evidence for in vivo thermosensitivity of serotonergic neurons in the rat dorsal raphe nucleus and raphe pallidus nucleus implicated in thermoregulatory cooling. Exp Neurol 2011; 227:264–278Crossref, Google Scholar
30 : Changes in mood state following whole-body hyperthermia. Int J Hyperthermia 1992; 8:305–307Crossref, Google Scholar
31 : The effects of repeated thermal therapy on quality of life in patients with type II diabetes mellitus. J Altern Complement Med 2010; 16:677–681Crossref, Google Scholar
32 : Effects of hyperthermic baths on depression, sleep and heart rate variability in patients with depressive disorder: a randomized clinical pilot trial. BMC Complement Altern Med 2017; 17:172Crossref, Google Scholar
33 : A thermosensory pathway mediating heat-defense responses. Proc Natl Acad Sci USA 2010; 107:8848–8853Crossref, Google Scholar
34 : Human feelings: why are some more aware than others? Trends Cogn Sci 2004; 8:239–241Crossref, Google Scholar
35 : How do you feel? Interoception: the sense of the physiological condition of the body. Nat Rev Neurosci 2002; 3:655–666Crossref, Google Scholar
36 : The somatosensory system; in Fundamental Neuroscience, 4th ed. Edited by Squire LR, Berg D, Bloom FE, . Amsterdam, Elsevier,2013, pp 531–551Crossref, Google Scholar
37 : Whole-body hyperthermia and a subthreshold dose of citalopram act synergistically to induce antidepressant-like behavioral responses in adolescent rats. Prog Neuropsychopharmacol Biol Psychiatry 2017; 79(Pt B):162–168Crossref, Google Scholar
38 : Specific responses of rat raphé neurones to skin temperature. J Physiol 1977; 273:277–293Crossref, Google Scholar
39 : Disruption of GABAergic tone in the dorsomedial hypothalamus attenuates responses in a subset of serotonergic neurons in the dorsal raphe nucleus following lactate-induced panic. J Psychopharmacol 2008; 22:642–652Crossref, Google Scholar
40 : Warm pleasant feelings in the brain. Neuroimage 2008; 41:1504–1513Crossref, Google Scholar
41 : The effects of antidepressants on human brain as detected by imaging studies. Focus on major depression. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35:1544–1552Crossref, Google Scholar
42 : Amygdala hyperactivation in untreated depressed individuals. Psychiatry Res 2009; 173:158–161Crossref, Google Scholar
43 : Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry 2009; 166:702–710Crossref, Google Scholar
44 : Topographical organization and chemoarchitecture of the dorsal raphe nucleus and the median raphe nucleus; in Serotonin and Sleep: Molecular, Functional and Clinical Aspects, 1st ed. Edited by Monti JM, Pandi-Perumal BL, Jacobs BL, . Basel, Birkhauser, 2008, pp 25–68Crossref, Google Scholar
45 : That warm fuzzy feeling: brain serotonergic neurons and the regulation of emotion. J Psychopharmacol 2009; 23:392–400Crossref, Google Scholar
46 : Brainstem innervation of prefrontal and anterior cingulate cortex in the rhesus monkey revealed by retrograde transport of HRP. J Comp Neurol 1982; 205:63–76Crossref, Google Scholar
47 : Topography of serotonin neurons in the dorsal raphe nucleus that send axon collaterals to the rat prefrontal cortex and nucleus accumbens. Brain Res 1993; 624:188–198Crossref, Google Scholar
48 : Effects of light therapy on sleep, mood, and temperature in women with nonseasonal major depression. Issues Ment Health Nurs 2005; 26:781–794Crossref, Google Scholar
49 : Electroconvulsive therapy increases circadian amplitude and lowers core body temperature in depressed subjects. Biol Psychiatry 1997; 42:1130–1137Crossref, Google Scholar
50 : Nocturnal sweating and temperature in depression. Acta Psychiatr Scand 1999; 100:295–301Crossref, Google Scholar
51 : Nocturnal temperature in affective disorder. J Affect Disord 1982; 4:61–71Crossref, Google Scholar
52 : Palmar digital sweating in women suffering from depression. Br J Psychiatry 1966; 112:1251–1255Crossref, Google Scholar
53 : Skin conductance: a potentially sensitive test for depression. Psychiatry Res 1983; 10:295–302Crossref, Google Scholar
54 : Skin conductance. A potentially sensitive and specific marker for depression. J Nerv Ment Dis 1986; 174:553–559Crossref, Google Scholar
55 : Electrodermal activity in euthymic unipolar and bipolar affective disorders. A possible marker for depression. Arch Gen Psychiatry 1983; 40:557–565Crossref, Google Scholar
56 . Rationale for, barriers to, and appropriate medication for the long-term treatment of depression. J Clin Psychiatry 2010; 71(Suppl E1):e02Crossref, Google Scholar



