The Emerging Neurobiology of Bipolar Disorder
Abstract
Bipolar disorder (BD) is a leading cause of global disability. Its biological basis is unknown, and its treatment unsatisfactory. Here, we review two recent areas of progress. First, the discovery of risk genes and their implications, with a focus on voltage-gated calcium channels as part of the disease process and as a drug target. Second, facilitated by new technologies, it is increasingly apparent that the bipolar phenotype is more complex and nuanced than simply one of recurring manic and depressive episodes. One such feature is persistent mood instability, and efforts are underway to understand its mechanisms and its therapeutic potential. BD illustrates how psychiatry is being transformed by contemporary neuroscience, genomics, and digital approaches.
(Reprinted with permission from Trends in Neurosciences, January 2018, Vol. 41, No. 1 )
Bipolar Disorder and the New Psychiatry
Psychiatry still relies largely on 19th-Century diagnostic categories. These are based on clusters of symptoms rather than biological markers, and are treated with drugs discovered serendipitously several decades ago. BD typifies this unsatisfactory state of affairs. Although its name has changed [it was formerly known as manic depression (see Glossary)], its cardinal features, and how it is assessed and treated (Box 1) have barely altered. An important reason for this stagnation has been the lack of any real traction on its causes and underlying biology, beyond its well-established high heritability (1). Although there is evidence for altered structural and functional brain connectivity (2–4), and changes in markers of oxidative stress (5), mitochondrial function (6), inflammation (7), circadian rhythms (8), and dopamine (9), it remains difficult to integrate these diverse findings, and to disentangle causative changes from those that are secondary to the disorder and its treatment.
BOX 1. Bipolar Disorder: A Clinical Primer
The classic picture of BD is like a modified sine wave, with mood fluctuating between episodes of mood elevation (mania) and depression, interspersed with periods of euthymia. The number of episodes in each mood phase, and their duration, varies markedly between individuals, but for most patients the depressive episodes are more prolonged and are responsible for much of the morbidity of the disorder.
The depressive episodes of BD are broadly similar in nature and severity to those of ‘ordinary’ depression. A manic episode includes not only significant elevation of mood, but also related changes in behaviour, such as a reduced need for sleep, increased energy, grandiose thoughts and beliefs, rapid speech, increased libido, and reckless behaviour (e.g., spending excessively). In severe episodes, psychotic symptoms (delusions and hallucinations) may also be present; for example, the person may believe, or hear voices telling them, that they have superpowers (and they may then act accordingly). ‘Hypomania’ refers to a milder and less prolonged form of mania. The exact criteria depend upon the classificatory system used (ICD-10 or DSM-5); the latter subdivides BD into bipolar I and bipolar II. Although not part of the diagnostic criteria, cognitive impairment is a notable aspect of BD; it is present at first episode and persists during euthymia. Attention, processing speed, and verbal learning and fluency are the domains most affected. At least half of patients with BD also have an anxiety disorder or substance use disorder. Patients are typically diagnosed during their 20s, following a long prodrome, and actual onset is often in adolescence. The lifetime prevalence of BD is approximately 1%, rising to 4% if a broader definition of bipolar spectrum disorder is used. The major risk factors are genetic (see the main text), but environmental factors, including childhood adversity, also have a role. Of patients with BD, 10% die by suicide and this, coupled with an excess mortality from natural causes, shortens average life expectancy by approximately 15 years.
The mainstay of BD treatment is pharmacological, with lithium salts or the antiepileptic drug sodium valproate used for prophylaxis. Antipsychotics, antidepressants, and antiepileptic drugs are given to treat the mood episodes. Psychoeducation and psychological treatments also have an important supporting role. All current treatments have limited efficacy, and the drugs can have serious adverse effects.
The situation is belatedly improving. While optimism must be tempered by appreciation of the many complexities, there are realistic prospects for a transformation in our understanding of BD and how it is diagnosed and treated. Here, we highlight two areas of current interest: the discovery of the first BD risk genes and their implications, and the application of novel technologies with the potential to refine, or redefine, the BD phenotype. These developments exemplify how genomics, neuroscience, and digital technologies are heralding a new era for psychiatry. For broader reviews of BD, see (10, 11).
The Genomics of Bipolar Disorder
A child of an affected parent has about a tenfold increased risk of developing BD, and twin studies estimate a heritability of 0.7–0.8 (1). There is no evidence for Mendelian inheritance or for genes of major effect. Instead, as with most psychiatric disorders, there are multiple susceptibility loci, each of small effect, which genome-wide association studies (GWAS) are beginning to identify. Several GWAS, and meta-analyses thereof, have been carried out since 2007; Table 1 lists the loci and implicated genes that have emerged to date. The combined sample sizes remain small by GWAS standards, and more loci remain to be identified; indeed, the forthcoming Psychiatric Genomics Consortium analysis, comprising over 20 000 BD cases and 30 000 controls, identifies 19 significant loci, including 12 novel ones. Initial exome and genome sequencing data suggest that rare deleterious variants also have a role in some BD cases, but their identity and overall contribution to the disorder remain unclear (12–14). Within BD, there is modest clinicogenetic heterogeneity, for example, based on the predominant symptoms, or between bipolar I and bipolar II subtypes (15). However, there is little evidence for BD-specific genes; joint GWAS analyses show substantial commonalities in risk loci for BD and schizophrenia (16), as well as significant overlap with other major psychiatric disorders (17) and with intermediate phenotypes, including circadian traits (18, 19). One distinction between schizophrenia and BD is that copy number variation is much less prominent in the latter (20).
| Locus | Implicated gene(s) |
|---|---|
| 2q11.2 | LMAN2L |
| 2q32.1 | ZNF804A |
| 3p22.2 | TRANK1 (LBA 1) |
| 5p15.31 | ADCY2 |
| 6q16.1 | MIR2113, POU3F2(OTF7) |
| 6q25.2 | SYNE1 |
| 7p22.3 | MAD1L1 |
| 9p21.3 | Intergenic |
| 10q21.2 | ANK3 |
| 11q14.1 | TENM4(ODZ4) |
| 12p13.3 | CACNA1C |
| 12q13.1 | DDN |
| 17q12 | ERBB2 |
TABLE 1. GWAS Hits for BDa
Although the genomics of BD are in their infancy, efforts have begun to understand the biological basis for the associations identified to date. Interest has centred on two genes (CACNA1C and ANK3) because of what was already known of their functions. CACNA1C is discussed in detail below. ANK3 encodes ankyrin G, which couples axonal voltage-gated sodium channels to the cytoskeleton and also has roles in dendrites and glia; another risk gene, TRANK1, contains multiple ankyrin repeat domains, suggesting some shared functions. Complementing the focus on specific risk genes, the first attempts have been made to identify the pathways that they influence. Using data from four of the GWAS, Nurnberger et al. (21) reported six pathways that showed replicable association with BD, involving glutamate and calcium signalling, second messengers, and hormones. Together, these findings support the possibility that BD is, at least in part, an ion channelopathy (22), in which aberrant calcium signalling is important (23).
Calcium Signalling in Bipolar Disorder: Linking Genetics, Pathophysiology, and Therapeutics
Calcium dysregulation has long been implicated in BD, based primarily on ex vivo studies in cells taken from patients and controls. The findings are disparate, but on balance indicate that measures of intracellular calcium signalling are increased in BD, especially after stimulation (reviewed in (23,24)). The abnormalities appear largely independent of current mood state (i.e., they are trait rather than state related). Moreover, they are attenuated by lithium, which is used in the treatment of the disorder (Box 1). Despite the many uncertainties, these findings led to L-type voltage-gated calcium channel (VGCC) antagonists, with existing indications in angina and hypertension, being evaluated for the treatment of BD (25). Antiepileptic drugs, such as pregabalin, which act via VGCC α2δ subunits (Box 2) have also been tested (26), and lamotrigine, another antiepileptic drug that may block calcium channels, among its various actions (27), is an effective treatment for bipolar depression (28).
BOX 2. VGCC Genes, Their Isoforms, and Relevance in Bipolar Disorder
Identifying the specific VGCCs most relevant for BD is a significant challenge, because their genes give rise to a vast diversity of functional channels (named Cav channels) [96,97]. VGCCs comprise multiple subunits, each encoded by one of a subfamily of separate genes. The properties of the ten distinct α1 subunits (encoded by the CACNA1- gene family) depend on the accessory subunits to which it is bound (Figure I). The main accessory subunits are the β (encoded by CACNB1–4) and α2δ (encoded by CACNA2D1–4) subunits, which are obligate in most cases [98]. Current VGCC antagonists block the L-type channels; the anticonvulsant/analgesic drugs pregabalin and gabapentin are α2δ ligands [30].
Further channel diversity arises because each gene gives rise to multiple isoforms. The human CACNA1C mRNA has at least 50 exons and over 40 predicted isoforms (arising from transcriptional and splicing mechanisms). CACNA1C splicing gives rise to channel isoforms that are differentially expressed in brain compared with heart, and which differ in their biophysical properties, including voltage-gating characteristics [97]. Another feature affected by splicing is the isoform sensitivity to existing VGCC antagonists [98]. This suggests that it might be possible to selectively target splice variants that mediate disease risk and/or are preferentially expressed in the brain, compared with peripheral tissues (particularly the cardiovascular system, where VGCCs are also abundant), thereby maximising their therapeutic potential and tolerability in BD [25].
Given these considerations, defining the repertoire of VGCCs present in different human tissues is important, as is identifying which ones are impacted by the BD-associated risk variants or by BD itself. However, information on the transcript diversity of human VGCC subunits is sparse, particularly in brain. Furthermore, because VGCC subunit genes are large (full-length CACNA1C mRNA, for instance, is over 10 kb long), the transcript structure of most isoforms remains unclear. Characterising the profile of full-length VGCCs isoforms in the human brain, compared with other tissues, and assessing which are altered in association with genetic risk for BD, are critical first steps in translating the VGCC genomic findings into pathophysiological insights and novel treatment targets. The availability of large, high-quality human postmortem brain series and technological advances in the field of RNA sequencing make this goal achievable.
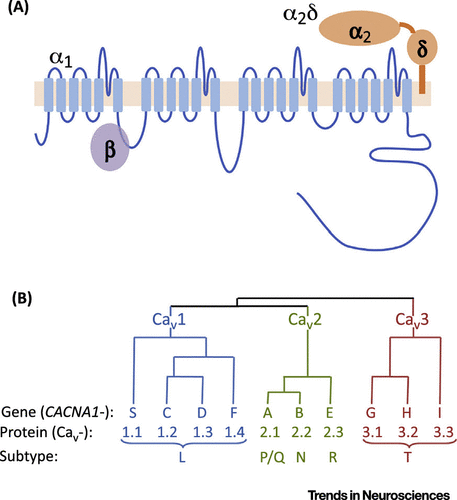
Figure I. The Voltage-Gated Calcium Channel (VGCC) Family of Proteins. (A) Structure of VGCCs, showing the transmembrane topology of the α1 subunit, its long intracellular C terminus and interactions with accessory (β and α2δ) subunits. (B) Dendrogram and nomenclature of the VGCC family.a
a A color version of the figure, as originally published, appears in the online version of this article (focus.psychiatryonline.org).
The results of the recent genomic studies strongly suggest that the involvement of calcium signalling in BD is at least partly causal (29), and have rekindled attempts to explain more precisely the nature of the alterations, not least because this may provide clues to more-effective and tolerable drug strategies to normalise them (30). However, the discovery of genetic variants is only the first step, and provides many more questions than answers. Calcium signalling offers an informative exemplar to highlight the opportunities and complexities associated with moving from psychiatric genomic discoveries to pathophysiological insights and therapeutic advances (31,32).
Genomic data provide a starting point to identify the molecules involved in the core ‘calcium pathophysiology’ of BD. They focus attention on the VGCCs, especially of the L-type, and their accessory subunits (encoded by the CACNx genes; Box 2). As indicated above, the best evidence is for CACNA1C (encoding the α1 subunit of Cav 1.2), but pathway analysis also suggests a role for CACNA1D and CACNB3 (29,33), and other VGCC genes are implicated in BD by rare variant studies (13). Of note, apart from BD, CACNA1C is associated with schizophrenia (34) and major depression (35), and CACNB2 confers susceptibility to multiple psychiatric disorders (17). Involvement of VGCC genes has also been reported in large-scale genomic studies of BD-relevant phenotypes, such as working memory performance and the associated patterns of brain activation (36), as well as in general cognitive functioning (37). There are also smaller candidate gene studies that suggest effects of CACNA1C genetic variation on brain imaging phenotypes (38,39) and on cognitive domains, such as reward responsiveness (40).
Identification of the molecular basis for disease associations is a key step in understanding the mechanisms linking VGCC genes with BD. The VGCC loci revealed by GWAS are noncoding, and while large-scale exome studies may identify rare variants that disrupt the coding sequence of VGCCs, it is unlikely that the BD-associated GWAS loci tag as-yet-unidentified, disease-causing mutations. Instead, they probably act by influencing aspects of gene expression, including methylation (41), alternative promoter usage, and RNA splicing (42). In the case of CACNA1C, the index risk polymorphism for BD (rs1006737) is located in the third intron. On balance, the available data indicate that the risk allele is associated with enhanced expression and activity of the gene product (43,44), but there are conflicting findings (45,46), precluding firm conclusions. Some of the variability in these results may be due to differences in the effect of rs1006737 on CACNA1C expression between brain regions. Inconsistencies may also result from the risk single nucleotide polymorphism (SNP) differentially altering the abundance of particular splice variants, as has been observed for other BD-relevant genes, including ANK3 (47) and ZNF804A (48). In the case of ANK3, there is evidence that the shift in isoform ratios has functional consequences for neuronal physiology (49). Efforts to identify and understand whether altered splicing is also relevant for CACNA1C and other VGCCs are hampered by the limited information about their isoform profile in the human brain (Box 2). This information is critical since splicing patterns are poorly conserved and the brain shows one of the greatest diversities of alternative splicing (50), meaning that the current data, which pertain to other species and tissues, are insufficient.
While the identification of specific VGCC subunits and isoforms that mediate BD risk is important, better understanding of the underlying biology is also crucial. This requires the use of appropriate cellular and animal model systems, as well as in vivo approaches in humans. For cellular analyses, in addition to standard cell lines, which are useful for studying the function of individual genes, induced pluripotent stem cells (iPSCs) may prove valuable. Indeed, iPSC-derived neurons have already provided intriguing data to support the presence of cellular BD-related phenotypes, including alterations in calcium signalling (Box 3).
BOX 3. Stem Cells and the Calcium Pathophysiology of Bipolar Disorder
The potential of iPSCs for studying cellular phenotypes of psychiatric disorders such as BD is considerable, and the approach has already been exploited in several studies. These have been of three designs: comparison of BD with healthy patients; contrasting lithium-responsive versus lithium-unresponsive BD cases; or evaluating the effect of BD risk genotypes (reviewed in [99]).
Mertens et al. [100] generated dentate gyrus-like neurons and showed that cells from patients with BD were hyperexcitable, with differences in several electrophysiological and transcriptional parameters; moreover, the excitability was normalised by lithium, but only in cells derived from patients who had responded clinically to the drug. Notably, these findings were largely replicated in a separate cohort and using a different methodology [101]. Taking a genetic strategy, Yoshimizu et al. [44] made induced neurons from 24 people, genotyped for the CACNA1C rs1006737 polymorphism, and showed that CACNA1C gene expression and calcium current density were greater in risk allele homozygotes compared with nonrisk carriers.
3D differentiation approaches are now being used to produce ‘brain spheroids’. Although no studies of this kind have yet been reported using iPSCs from patients with BD, Birey et al. [102] reported that, intriguingly, spheroids made with iPSCs from patients with Timothy syndrome (caused by a gain-of-function coding CACNA1C mutation) exhibited interneuron migratory abnormalities that could be normalised by a VGCC antagonist. The finding draws attention to a possible role for early developmental events and specific interneuron populations in mediating the role of calcium signalling in the pathogenesis of BD. Studies using spheroids in BD may be valuable in helping identify circuit-level phenotypes, while CRISPR techniques to selectively manipulate disease-relevant VGCC isoforms and variants could aid the identification of the key molecular mechanisms.
These findings, and others (e.g., [103]), illustrate how iPSCs are providing new clues regarding the neuronal phenotypes of BD, and support an involvement of calcium signalling in these processes. The results encourage further efforts to extend and scale up the work [104].
The molecular findings can also help guide the development of rodent models overexpressing or lacking specific VGCC genes (51–53) or splice variants thereof (54), in which their functional impact can be studied in the intact animal. For example, embryonic deletion of Cacna1c from forebrain glutamatergic neurons in mice produced BD-relevant behavioural and cognitive effects and an increased susceptibility to stress, whereas the same deletion during adulthood caused a lesser and, in some instances, opposite phenotype (51). This effect of developmental stage on the phenotype is intriguing, given the characteristic early adulthood age of BD onset, and its childhood antecedents (Box 1). In a separate study, the phenotype of Cacna1c-deficient mice could be rescued using a small-molecule inhibitor of the translation initiation factor eIF2α (52), giving clues as to the possible intervening biochemical mechanisms.
In parallel with these various genetically driven approaches, pharmacological investigations of VGCCs in humans are possible because of the existing L-type VGCC antagonists, which can be used as experimental tools and for proof-of-principle studies. Their availability is a distinct advantage compared with most other genetically supported therapeutic targets in BD, for which no such drugs are available. To date, the clinical trial data linking L-type VGCC blockade with therapeutic outcome in BD (and, indeed, their psychiatric effects more generally) are wholly inconclusive (25). Hence, a priority is to investigate in detail the impact of brain-penetrant VGCC blockers on BD-relevant phenotypes, including detailed measures of mood, cognition, sleep, and brain activity (55), and with the incorporation of genotype as a factor (56). The potential psychiatric effects of VGCC antagonists can also be assessed using routinely collected clinical data; for example, a study of electronic medical records in Scotland reported higher hospital admission rates for depression in patients given VGCCs compared with those prescribed other antihypertensives (57).
In summary, a range of methods are needed to make the most of the genomic discoveries in BD, to understand their molecular mechanisms and implications for cellular and systems functioning, and to evaluate their therapeutic potential. The conceptual approach is illustrated in Figure 1 (Key Figure), which uses VGCCs as the exemplar.
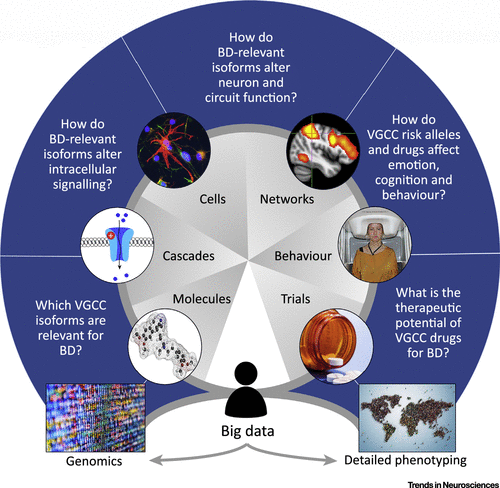
FIGURE 1. The study of voltage-gated calcium channels (VGCCs) in BD exemplifies the potential and the challenges of translating genomic discoveries into pathophysiology and therapeutics. Although sometimes portrayed as a one-way, bottom-up, process from genes to treatments, the wheel-like figure emphasises that it is iterative. Underpinning this approach is the increasing wealth of ‘big data’, since it is the scale of both the genomics and the contemporary digital approaches to phenotyping that have driven the developments discussed in this review. Looking ahead, an integrative, collaborative approach will be essential to link the power of big data with the more-focussed, hypothesis-driven studies that are required for most of the intervening segments.a
a A color version of the figure, as originally published, appears in the online version of this article (focus.psychiatryonline.org).
New Approaches to the Bipolar Phenotype
Digital Psychiatry
Complementing the developments from genomics-driven discovery science, novel methods are being applied to characterise the BD phenotype, and thereby provide new perspectives on its key elements.
Conventional psychiatric assessments rely on cross-sectional, retrospective analysis of the pattern of symptoms over weeks or months. Being based on a patient’s or informant’s recall, this approach is subject to inherent biases and unreliability, especially when, as in BD, the key feature is not simply a symptom (mood), but its profile of variation over time. Several approaches have been used to try to improve the reliability and validity of BD assessments (58,59). The main advances are emerging from developments in information technology, including the increasingly widespread use of connected and wearable devices: we are entering an era of ‘digital phenotyping’ in psychiatry (60), with BD, we would argue, at the forefront (61).
One early example is the True Colours platform, which allows patients to submit (by text, email, web, or app) their ratings of depression, mania, and other symptoms, in response to a weekly or daily prompt, resulting in a longitudinal and graphical representation of symptom course (62).
Importantly, smartphones and other devices allow the remote capture of not only contemporaneous self-reporting of symptoms, but also behavioural, cognitive and physiological measures, such as heart rate, activity, geolocation, speech, and environmental interactions (63–68). In turn, digital methods can facilitate a change from entirely symptom-based characterisation of psychiatric disorders, such as BD, towards a more multimodal, biologically informed one. Thus, they raise the prospect of more-objective, data-driven diagnoses, and ultimately personalised predictions of illness and treatment response. However, this promise has yet to be realised; the initial hype about digital phenotyping, including its application to BD, has been tempered by increasing awareness of the significant technical, analytical, and other issues involved (Box 4).
BOX 4. Digital and Mathematical Approaches to Bipolar Disorder
Collection of multidimensional data, as in the remote monitoring of symptoms, behaviours, and physiology, has considerable potential in the study and treatment of BD and other disorders, but also throws up several significant challenges [105,106].
First, there are many technical and practical issues to overcome, ranging from ensuring the compatibility of data collection between operating systems and software versions, to maintaining compliance over long time periods. There are also privacy, acceptability, and engagement issues raised by the recording and storage of personal data [107].
Second, the resulting data sets are large and complex, and the extraction, analysis, and interpretation of information are not straightforward [108]. One key question to consider is the mathematical approach chosen for analysing the data. Options include linear and nonlinear time series methods [109–111] as well as more-flexible and advanced techniques, such as relaxation oscillator frameworks [112] and machine-learning methods [90]. Another mathematical technique of interest is rough paths theory [113]. By taking account of lead-lag relationships, rough paths can reduce time-stamped data sets from complex interacting nonlinear systems to their critical information content or ‘signature’. This transformation provides a structured sequence of low-dimensional summaries of the primary data that completely characterise their complexity but are amenable to linear analysis. This greatly facilitates efforts to use the data for classification and prediction. For example, a rough path signature, based on daily measures of mood instability, can differentiate BD from borderline personality disorder [114].
Progress in this developing field will need to be interdisciplinary, combining advanced mathematical analyses with digital phenotyping technologies that produce robust data and that are sufficiently acceptable, and rewarding, to patients to secure their long-term engagement [115,116].
Persistent Mood Instability
Notwithstanding the many challenges, the longitudinal, long-term collection of data using digital approaches has already contributed to a renewed focus on the ‘real-world’ phenotype of BD. One example is the appreciation that many patients have chronic mood instability (also called affective lability), persisting during euthymia. This contrasts with the simplistic textbook description of BD as comprising periods of normal, stable mood in between the episodes of depression and mania (Box 1). Although this reality was already appreciated by experienced clinicians (69,70), it is the use of digital methods that has led to a greater awareness of mood instability, which in turn has encouraged research to understand its origins and significance. Here, we briefly review some of these implications.
Mood (in)stability is a continuous variable, and is present to varying extents in healthy individuals (71,72). Therefore, it is a useful phenotype for studies seeking to identify mechanisms underlying mood (dys)regulation per se, and a range of experimental approaches are being taken. For example, it can be viewed from a computational perspective, with models showing how mood instability interacts with reward sensitivity to alter performance (73). One can also ask how mood instability relates to variation in neural activity across a range of temporal resolutions, using functional MRI (74) and magnetoencephalography (75). In addition, mathematical analyses, such as those outlined in Box 4, can help explain the relationships between fluctuations in mood and other parameters, ranging from environmental exposures to behavioural variables (66,67).
Mood instability is prominent not only in BD, but also in several other psychiatric disorders, such as attention deficit disorder, borderline personality disorder, and schizophrenia (76,77). It is therefore a trans-diagnostic construct, compatible with the NIMH Research Domain Criteria project (78). Thus, understanding the origins and mechanisms of mood instability, and the biological and behavioural sequelae of interventions which modify it – is therefore likely to have value beyond BD. Indeed, mood instability is a moderately heritable trait in the general population, and the first genome-wide significant loci have been reported (79). Apart from studying the implicated molecules and pathways in their own right, it will be of interest to investigate the extent to which the risk loci for mood instability overlap with those for BD specifically, and other disorders involving mood instability. Moreover, the specific characteristics of mood instability (e.g., its frequency, amplitude, or impact on behaviour), while partially overlapping, may differ sufficiently between disorders (80) to help gain traction on the underlying neural mechanisms, providing further clues to the biological bases of these illnesses and leading to refinements in classification.
Finally, mood instability has potential immediate value as a therapeutic target. First, it may provide a way to screen novel treatments for BD more rapidly and cost-effectively than conventional clinical trials, in which treatment or prevention of depressive or manic episodes are the usual outcomes, requiring prolonged periods of observation. In this sense, early mood stabilisation could prove to predict therapeutic efficacy in the way that rapid effects on emotional processing predict subsequent therapeutic response in unipolar depression (81). Moreover, the rapid effects of antidepressants on emotional processing are seen in euthymic subjects as well as in those with depression; similarly, new treatments for BD, such as novel VGCC-acting drugs, could be screened in patients who have unstable mood but who do not have a formal diagnosis. Second, stabilisation of mood may be beneficial in its own right, above and beyond simply preventing clinical episodes of depression and mania, because mood instability independently predicts poor functional recovery in BD, and worse outcomes of various kinds (76,82–84). In addition, since mood instability often occurs before the onset of BD (85,86), such treatments might have a preventative role.
In summary, the emergence of persistent mood instability as a construct illustrates how digital methods can highlight neglected or hard-to characterise psychiatric phenomena, and stimulate a range of studies into their origins, mechanisms, and therapeutic implications. Comparable approaches are being taken to other components of BD, such as circadian dysregulation and reward sensitivity. Although the work is at an early stage, it is likely ultimately to alter the view of the core phenotype of BD, with a greater role for biological and quantitative data in BD diagnosis, prognosis, and management.
Concluding Remarks
Our understanding of BD remains frustratingly limited. It continues to be a descriptive syndrome, since we lack sufficient knowledge to allow its characterisation or conceptualisation based on aetiology or mechanism. Certainly, many questions remain (see Outstanding Questions). However, there are reasons for optimism. First, the discovery of some of the BD risk genes has the potential to revolutionise our understanding of its pathogenesis and neurobiology. Second, the use of digital technologies and remote sensors, coupled with advanced analyses of the resulting data, is already allowing a more-quantitative, longitudinal approach to the BD phenotype. This raises the potential for better prediction of an individual’s clinical course, and also provides a more-sophisticated phenotype for behavioural and biological studies. In both of these domains, a common feature is the increasing importance of ‘big data’, whether in terms of the genomic studies, or the multidimensional data streams captured by digital devices. Third, although not discussed here, structural and functional brain imaging is helping to identify the key neural circuits of BD and may also have diagnostic and prognostic value (2–4,87–90).
The ultimate goal of these contemporary approaches is to allow a more scientifically informed, evidence-based approach to how BD is classified, measured, and treated (91). Possible changes include redrawing or removing the diagnostic boundaries between BD and other disorders involving lability of mood, emotion, and behaviour; the inclusion of behavioural and physiological correlates of mood and mood instability (or other symptoms) into clinical practice; a focus on identifying interventions that can stabilise mood independent of the underlying diagnosis; and new, genetically informed treatments, such as those targeting VGCCs.
It is not always appreciated that BD causes a global health burden comparable with that of schizophrenia (92), yet it has attracted considerably less research funding and interest from policy makers (93). We would argue that, for various reasons, BD currently has a greater potential for transformative advances. More generally, BD is a useful case study for illustrating how psychiatric disorders are belatedly embarking on the journey from being descriptive syndromes towards more neurobiologically grounded, quantitative, and digital phenotypes (94). Despite the many difficulties this process entails, it is not unreasonable to hope that it will be successful, and accompanied by the development of more rational, effective, and personalised treatments.
1 (2013) Genetics of bipolar disorder. Lancet 381, 1654–1662Crossref, Google Scholar
2 (2014) A critical appraisal of neuroimaging studies of bipolar disorder: toward a new conceptualization of underlying neural circuitry and a road map for future research. Am. J. Psychiatry 171, 829–843Crossref, Google Scholar
3 (2016) Voxel-based meta-analytical evidence of structural disconnectivity in major depression and bipolar disorder. Biol. Psychiatry 79, 293–302Crossref, Google Scholar
4 (2017) Cortical abnormalities in bipolar disorder: an MRI analysis of 6503 individuals from the ENIGMA Bipolar Disorder Working Group. Mol. Psychiatry Published online May 2, 2017. http://dx.doi.org/10.1038/mp.2017.73Google Scholar
5 (2014) An updated meta-analysis of oxidative stress markers in bipolar disorder. Psychiatry Res. 218, 61–68Crossref, Google Scholar
6 (2017) Bipolar disorder as a mitochondrial disease. Biol. Psychiatry Published online September 28, 2017. http://dx.doi.org/10.1016/j.biopsych.2017.09.018Google Scholar
7 (2016) A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol. Psychiatry 21, 1696–1709Crossref, Google Scholar
8 (2017) Chronotype and circadian rhythm in bipolar disorder: a systematic review. Sleep Med. Rev. 34, 46–58Crossref, Google Scholar
9 (2017) The dopamine hypothesis of bipolar affective disorder: the state of the art and implications for treatment. Mol. Psychiatry 22, 666–679Crossref, Google Scholar
10 (2016) Bipolar disorder. Lancet 387, 1561–1572Crossref, Google Scholar
11 (2016) Innovative approaches to bipolar disorder and its treatment. Ann. N.Y. Acad. Sci. 1366, 76–89Crossref, Google Scholar
12 (2016) Exome sequencing of familial bipolar disorder. JAMA Psychiatry 73, 590–597Crossref, Google Scholar
13 (2015) Rare variants in neuronal excitability genes influence risk for bipolar disorder. Proc. Natl. Acad. Sci. U. S. A. 112, 3576–3581Crossref, Google Scholar
14 (2016) Exome sequencing for bipolar disorder points to roles of de novo loss-of-function and protein-altering mutations. Mol. Psychiatry 21, 885–893Crossref, Google Scholar
15 (2017) Evidence for genetic heterogeneity between clinical subtypes of bipolar disorder. Transl. Psychiatry 7, e993Crossref, Google Scholar
16 (2016) The implications of the shared genetics of psychiatric disorders. Nat. Med. 22, 1214–1219Crossref, Google Scholar
17
18 (2014) Multisystem component phenotypes of bipolar disorder for genetic investigations of extended pedigrees. JAMA Psychiatry 71, 375–387Crossref, Google Scholar
19 (2016) Genetic contributions to circadian activity rhythm and sleep pattern phenotypes in pedigrees segregating for severe bipolar disorder. Proc. Natl. Acad. Sci. U. S. A. 113, E754–E761Crossref, Google Scholar
20 (2016) Copy number variation in bipolar disorder. Mol. Psychiatry 21, 89–93Crossref, Google Scholar
21 (2014) Identification of pathways for bipolar disorder: a meta-analysis. JAMA Psychiatry 71, 657–664Crossref, Google Scholar
22 (2006) Ion channel functional candidate genes in multigenic neuropsychiatric disease. Biol. Psychiatry 60, 177–185Crossref, Google Scholar
23 (2014) Calcium signalling and psychiatric disease: bipolar disorder and schizophrenia. Cell Tissue Res. 357, 477–492Crossref, Google Scholar
24 (2004) Role of intracellular calcium signalling in the pathophysiology and pharmacotherapy of bipolar disorder: current status. Clin. Neurosci. Res. 4, 201–213Crossref, Google Scholar
25 (2016) A systematic review of calcium channel antagonists in bipolar disorder and some considerations for their future development. Mol. Psychiatry 21, 1324–1332Crossref, Google Scholar
26 (2017) Biological rationale and potential clinical use of gabapentin and pregabalin in bipolar disorder, insomnia and anxiety: protocol for a systematic review and meta-analysis. BMJ Open 7, e013433Crossref, Google Scholar
27 (2003) Potential mechanisms of action of lamotrigine in the treatment of bipolar disorders. J. Clin. Psychopharmacol. 23, 484–495Crossref, Google Scholar
28 (2016) Comparative evaluation of quetiapine plus lamotrigine combination versus quetiapine monotherapy (and folic acid versus placebo) in bipolar depression (CEQUEL): a 2 × 2 factorial randomised trial. Lancet Psychiatry 3, 31–39Crossref, Google Scholar
29 (2015) Genetic disruption of voltage-gated calcium channels in psychiatric and neurological disorders. Prog. Neurobiol. 134, 36–54Crossref, Google Scholar
30 (2015) The physiology, pathology, and pharmacology of voltage-gated calcium channels and their future therapeutic potential. Pharmacol. Rev. 67, 821–870Crossref, Google Scholar
31 (2015) Recent genetic findings in schizophrenia and their therapeutic relevance. J. Psychopharmacol. 29, 85–96Crossref, Google Scholar
32 (2016) Translating genome-wide association findings into new therapies for psychiatry. Nat. Rev. Neurosci. 19, 1392–1396Crossref, Google Scholar
33
34
35 (2016) Common variants in CACNA1C and MDD susceptibility: a comprehensive meta-analysis. Am. J. Med. Genet. Neuropsychiatr. Genet. 171, 896–903Crossref, Google Scholar
36 (2014) Converging genetic and functional brain imaging evidence links neuronal excitability to working memory, psychiatric disease, and brain activity. Neuron 81, 1203–1213Crossref, Google Scholar
37 (2017) Identification of genetic loci jointly influencing schizophrenia risk and the cognitive traits of verbal numerical reasoning, reaction time, and general cognitive function. JAMA Psychiatry 74, 1065–1075Crossref, Google Scholar
38 (2014) Identification of gene ontologies linked to prefrontal-hippocampal functional coupling in the human brain. Proc. Natl. Acad. Sci. U. S. A. 111, 9657–9662Crossref, Google Scholar
39 (2015) CACNA1C rs1006737 genotype and bipolar disorder: focus on intermediate phenotypes and cardiovascular comorbidity. Neurosci. Biobehav. Rev. 55, 198–201Crossref, Google Scholar
40 (2014) CACNA1C risk variant affects reward responsiveness in healthy individuals. Transl. Psychiatry 4, e461Crossref, Google Scholar
41 (2016) CACNA1C hypermethylation is associated with bipolar disorder. Transl. Psychiatry 6, e831Crossref, Google Scholar
42 (2017) Molecular mechanisms underlying noncoding risk variations in psychiatric genetic studies. Mol. Psychiatry 22, 497–511Crossref, Google Scholar
43 (2010) Genetic variation in CACNA1C affects brain circuitries related to mental illness. Arch. Gen. Psychiatry 67, 939–945Crossref, Google Scholar
44 (2015) Functional implications of a psychiatric risk variant within CACNA1C in induced human neurons. Mol. Psychiatry 20, 162–169Crossref, Google Scholar
45 (2014) A rare mutation of CACNA1C in a patient with bipolar disorder, and decreased gene expression associated with a bipolar-associated common SNP of CANA1C in brain. Mol. Psychiatry 19, 890–894Crossref, Google Scholar
46 (2016) Functional characterization of schizo-phrenia-associated variation in CACNA1C. PLoS One 11, e0157086Crossref, Google Scholar
47 (2016) A loss-of-function variant in a minor isoform of ANK3 protects against bipolar disorder and schizophrenia. Biol. Psychiatry 80, 323–330Crossref, Google Scholar
48 (2014) Expression of ZNF804A in human brain and alterations in schizophrenia, bipolar disorder, and major depressive disorder: a novel transcript fetally regulated by the psychosis risk variant rs1344706. JAMA Psychiatry 71, 1112–1120Crossref, Google Scholar
49 (2017) Ankyrin-G isoform imbalance and interneuronopathy link epilepsy and bipolar disorder. Mol. Psy-chiatry 22, 1464–1472Crossref, Google Scholar
50 (2012) The evolutionary landscape of alternative splicing in vertebrate species. Science 338, 1587–1593Crossref, Google Scholar
51 (2017) Cross-disorder risk gene CACNA1C differentially modulates susceptibility to psychiatric disorders during development and adulthood. Mol. Psychiatry Published online July 11, 2017. http://dx.doi.org/10.1038/mp.2017.133Google Scholar
52 (2017) Rescue of impaired sociability and anxiety-like behavior in adult cacna1c–deficient mice by pharmacologically targeting eIF2α. Mol. Psychiatry 22, 1096–1109Crossref, Google Scholar
53 (2017) Decreased nucleus accumbens expression of psychiatric disorder risk gene Cacna1c promotes susceptibility to social stress. Int. J. Neuropsychopharmacol. 20, 428–433Crossref, Google Scholar
54 (2013) Spinal morphine but not ziconotide or gabapentin analgesia is affected by alternative splicing of voltage-gated calcium channel Ca(V)2.2 pre-mRNA. Mol. Pain 9, 67Crossref, Google Scholar
55 (2016) L-type Ca2+ channels in mood, cognition and addiction: integrating human and rodent studies with a focus on behavioural endophenotypes. J. Physiol. 594, 5823–5837Crossref, Google Scholar
56 (2014) Pilot investigation of isradipine in the treatment of bipolar depression motivated by genome-wide association. Bipolar Disord. 16, 199–203Crossref, Google Scholar
57 (2016) Monotherapy with major antihypertensive drug classes and risk of hospital admissions for mood disorders. Hypertension 68, 1132–1138Crossref, Google Scholar
58 (2004) Using technology to improve longitudinal studies: self-reporting with ChronoRecord in bipolar disorder. Bipolar Disord. 6, 67–74Crossref, Google Scholar
59 (2016) Data-driven classification of bipolar I disorder from longitudinal course of mood. Transl. Psychiatry 6, e912Crossref, Google Scholar
60 (2017) Digital phenotyping. Technology for a new science of behavior. JAMA 318, 1215–1216Crossref, Google Scholar
61 (2015) Mobile apps for bipolar disorder: a systematic review of features and content quality. J. Med. Internet Res. 17, e198Crossref, Google Scholar
62 (2017) Longitudinal mood monitoring in bipolar disorder: course of illness as revealed through a short messaging service. J. Affective Disord. 223, 139–145Crossref, Google Scholar
63 (2015) Smartphone data as an electronic biomarker of illness activity in bipolar disorder. Bipolar Disord. 17, 715–728Crossref, Google Scholar
64 (2016) Voice analysis as an objective marker in bipolar disorder. Transl. Psychiatry 6, e856Crossref, Google Scholar
65 (2014) Advancing biomarker research: utilizing ‘Big Data’ approaches for the characterization and prevention of bipolar disorder. Bipolar Disord. 16, 531–547Crossref, Google Scholar
66 (2015) Smartphone-based recognition of state and state changes in bipolar disorder patients. IEEE J. Biomed. Health Inform. 19, 140–147Crossref, Google Scholar
67 (2017) Detecting bipolar depression from geographic location data. IEEE Trans. Biomed. Eng. 64, 1761–1771Crossref, Google Scholar
68 (2017) Dysregulation of objectively assessed 24- hour motor activity patterns as a potential marker for bipolar I disorder: results of a community-based family study. Transl. Psychiatry 7, e1211Crossref, Google Scholar
69 (2006) Affective instability as rapid cycling: theoretical and clinical implications for borderline personality and bipolar spectrum disorders. Bipolar Disord. 8, 1–14Crossref, Google Scholar
70 (2008) Affective lability and affect intensity as core dimensions of bipolar disorders during euthymic period. Psychiatr. Res. 159, 1–6Crossref, Google Scholar
71 (2015) Mood instability. Br. J. Psychiatry 207, 283–285Crossref, Google Scholar
72 (2012) The prevalence and clinical associations of mood instability in adults living in England: results from the Adult Psychiatric Morbidity Survey 2007. Psychiatr. Res. 205, 262–268Crossref, Google Scholar
73 (2017) Mood instability and reward dysregulation – a neurocomputational model of bipolar disorder. JAMA Psychiatry Published online October 11, 2017. http://dx.doi.org/ 10.1001/jamapsychiatry.2017.3163Google Scholar
74 (2015) Neurobiological and behavioural studies of affective instability in clinical populations: a systematic review. Neurosci. Biobehav. Rev. 51, 243–254Crossref, Google Scholar
75 (2014) Fast transient networks in spontaneous human brain activity. eLife 3, e01867Crossref, Google Scholar
76 (2015) Mood instability is a common feature of mental health disorders and is associated with poor clinical outcomes. BMJ Open 5, e007504Crossref, Google Scholar
77 (2015) Affective dynamics in psychopathology. Emotion Rev. 7, 355–361Crossref, Google Scholar
78 (2014) The NIMH Research Domain Criteria (RDoC) project. Am. J. Psychiatry 177, 395–397Crossref, Google Scholar
79 Ward, J. et al. Genome-wide analysis in UK Biobank identifies four loci associated with mood instability and genetic correlation with major depressive disorder, anxiety disorder and schizophrenia. Transl. Psychiatry (in press)Google Scholar
80 (2016) Daily longitudinal self-monitoring of mood variability in bipolar disorder and borderline personality disorder. J. Affect. Dis. 205, 225–233Crossref, Google Scholar
81 (2009) Why do antidepressants take so long to act? A cognitive neuropsychological model of antidepressant drug action. Br. J. Psychiatry 195, 102–108Crossref, Google Scholar
82 (2013) Mood instability and functional recovery in bipolar disorders. Acta Psychiatr. Scand. 128, 194–202Crossref, Google Scholar
83 (2017) Affect lability predicts occurrence of suicidal ideation in bipolar patients: a two-year prospective study. Acta Psychiatr. Scand. 135, 460–469Crossref, Google Scholar
84 (2015) Inter-episode affective intensity and instability: predictors of depression and functional impairment in bipolar disorder. J. Behav. Ther. Exp. Psychiatry 46, 14–18Crossref, Google Scholar
85 (2016) Toward the definition of a bipolar prodrome: dimensional predictors of bipolar spectrum disorders in at-risk youths. Am. J. Psychiatry 173, 695–704Crossref, Google Scholar
86 (2016) The bipolar prodrome: meta-analysis of symptom prevalence prior to initial or recurrent mood episodes. J. Am. Acad. Child Adolesc. Psychiatry 55, 543–555Crossref, Google Scholar
87 (2014) Multivariate analysis reveals genetic associations of the resting default mode network in psychotic bipolar disorder and schizophrenia. Proc. Natl. Acad. Sci. U. S. A. 111, E2066–E2075Crossref, Google Scholar
88 (2014) Neuropathological and neuromorpho metric abnormalities in bipolar disorder: view from the medial prefrontal cortical network. Neurosci. Biobehav. Rev. 42, 132–147Crossref, Google Scholar
89 (2017) Functional dysconnection of the inferior frontal gyrus in young people with bipolar disorder or at genetic high risk. Biol. Psychiatry 81, 718–727Crossref, Google Scholar
90 (2017) The impact of machine learning techniques in the study of bipolar disorder: a systematic review. Neurosci. Biobehav. Rev. 80, 538–554Crossref, Google Scholar
91 (2015) All the world’s a (clinical) stage: rethinking bipolar disorder from a longitudinal perspective. Mol. Psychiatry 20, 23–31Crossref, Google Scholar
92 (2015) The global burden of mental, neurological and substance use disorders: an analysis from the Global Burden of Disease Study 2010. PLoS One 10, e0116820Crossref, Google Scholar
93 (2007) What is the heartland of psychiatry? Br. J. Psychiatry 191, 189–191Crossref, Google Scholar
94 (2017) The WPA-Lancet Psychiatry Commission on the Future of Psychiatry. Lancet Psychiatry 4, 775–818Crossref, Google Scholar
95 (2017) Bipolar disorder. In Shorter Oxford Textbook of Psychiatry (7th edn) (Harrison, P.J., ed.), pp. 233–252, Oxford University PressCrossref, Google Scholar
96 (2016) Voltage-gated calcium channels and their auxiliary subunits: physiology and pathophysiology and phar-macology. J. Physiol. 594, 5369–5390Crossref, Google Scholar
97 (2014) L-type Ca2+ channels in heart and brain. WIREs Membr. Transp. Signal. 3, 15–38Crossref, Google Scholar
98 (2014) L-type CaV1.2 channels: from in vitro findings to in vivo function. Physiol. Rev. 94, 303–326Crossref, Google Scholar
99 (2016) Molecular neurobiological clues to the pathogenesis of bipolar disorder. Curr. Opin. Neurobiol. 36, 1–6Crossref, Google Scholar
100 (2015) Differential responses to lithium in hyperexcitable neurons from patients with bipolar disorder. Nature 527, 95–99Crossref, Google Scholar
101 (2017) Neurons derived from patients with bipolar disorder divide into intrinsically different sub-populations of neurons, predicting the patients’ responsiveness to lithium. Mol. Psychiatry Published online February 28, 2017. http://dx.doi. org/10.1038/mp.2016.260Google Scholar
102 (2017) Assembly of functionally integrated human forebrain spheroids. Nature 545, 54–59Crossref, Google Scholar
103 (2017) Probing the lithium-response pathway in hiPSCs implicates the phosphoregulatory set-point for a cytoskeletal modulator in bipolar pathogenesis. Proc. Natl. Acad. Sci. U. S. A. 114, E4462–E4471Crossref, Google Scholar
104 (2016) Reprogramming psychiatry: stem cells and bipolar disorder. Lancet 387, 823–825Crossref, Google Scholar
105 (2015) Big data are coming to psychiatry – a general introduction. Int. J. Bipolar Disord. 3, 21Crossref, Google Scholar
106 (2017) Big data for bipolar disorder. Int. J. Bipolar Disord. 4, 10Crossref, Google Scholar
107 (2017) Ethical perspectives on recommending digital technology for patients with mental illness. Int. J. Bipolar Disord. 5, 6Crossref, Google Scholar
108 (2016) Data science for mental health: a UK perspective on a global challenge. Lancet Psychiatry 3, 993–998Crossref, Google Scholar
109 (2014) Mood dynamics in bipolar disorder. Int. J. Bipolar Disord. 2, 11Crossref, Google Scholar
110 (2012) Nonlinear time-series approaches in characterizing mood stability and mood instability in bipolar disorder. Proc. R. Soc. B Biol. Sci. 279, 3632Crossref, Google Scholar
111 (2016) Applications of time-series analysis to mood fluctuations in bipolar disorder to promote treatment innovation: a case series. Transl. Psychiatry 6, e720Crossref, Google Scholar
112 (2015) Bipolar disorder dynamics: affective instabilities, relaxation oscillations and noise. J. R. Soc. Interface 12, 0670Crossref, Google Scholar
113 (2010) Uniqueness for the signature of a path of bounded variation and the reduced path group. Ann. Mathemat. 171, 109–167Crossref, Google Scholar
114 (2017) A signature-based machine learning model for bipolar disorder and borderline personality disorder. arXiv 1707.07124Google Scholar
115 (2017) Digital footprints. Mol. Psychiatry 22, 164–169Crossref, Google Scholar
116 (2017) A systematic review of the psychometric properties, usability and clinical impacts of mobile mood-monitoring applications in young people. Psychol. Med. Published online June 23, 2017. http://dx.doi.org/10.1017/S0033291717001659Google Scholar
117 (2016) Genome-wide association study of 40,000 individuals identifies two novel loci associated with bipolar disorder. Hum. Mol. Genet. 25, 3383–3394Crossref, Google Scholar



