Stimulants: Abuse and Performance Enhancement (or Lack Thereof)
Abstract
Since their discovery, psychostimulant drugs (amphetamine, dextroamphetamine, and methylphenidate) have been used to treat a wide a variety of conditions. The pharmacologic effects of these drugs include facilitation of monoaminergic neurotransmission. Although there is unequivocal evidence for their efficacy in treating attention-deficit hyperactivity disorder, the condition for which stimulants are most widely used, the evidence for benefit across a range of other conditions is less well established. Controversy has recently arisen regarding the widespread use of stimulants to enhance athletic and cognitive performance. This article briefly reviews the developmental history and pharmacology of stimulant medications and then outlines the increasing patterns of illicit use and the mixed evidence for enhancement.
Since the discovery of amphetamine in 1910 (1), stimulant drugs, including dextroamphetamine and methylphenidate, have been used to treat a wide range of ailments, from common colds to psychosis (2). Attention-deficit hyperactivity disorder (ADHD) is the primary clinical indication for stimulants today (3). ADHD is characterized by developmentally inappropriate levels of inattention, hyperactivity, and impulsivity (3, 4). Stimulant drugs facilitate central and peripheral monoamine activity, and deficits in this activity are hypothesized to underlie the clinical problems associated with ADHD (5). Although there is incontrovertible evidence for the efficacy of stimulant drugs in the treatment of ADHD (3), controversy has arisen regarding the illicit use of these drugs for performance enhancement. Amphetamines have been used for decades to boost aspects of athletic performance. In the last 20 years, the availability of stimulants to treat ADHD has also led to increased diversion for cognitive enhancement. This article briefly reviews the pharmacology and history of stimulant drug development and then describes current knowledge regarding illicit use for both physical and cognitive enhancement, with an emphasis on both patterns of use and evidence for true enhancement.
Pharmacology
Stimulants are synthetic psychoactive sympathomimetic compounds that act both centrally and peripherally. In general, stimulants increase monoaminergic (dopamine and norepinephrine) and, to a lesser extent, indolaminergic (serotonin) neurotransmission. Stimulants act to facilitate neurotransmission in the following four ways: they can 1) force the release of neurotransmitters from the presynaptic neuron, 2) block reuptake transporters, 3) inhibit monoamine oxidase (degradation enzyme), and 4) directly stimulate postsynaptic receptors (Figure 1). As a result of these mechanisms, the flow of information from monoamine presynaptic neurons to their postsynaptic neurons is enhanced. The downstream effect of stimulants is an increase in overall sympathetic tone centrally and peripherally, resulting in its physiological effects (5, 6).
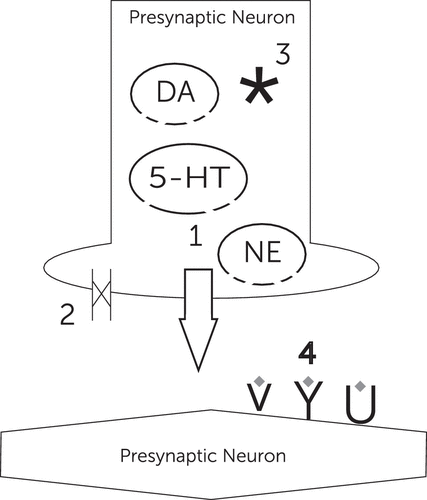
Figure 1. The Possible Effects of Stimulants on the Synapsea
a Possible effects include the following: 1) release of monoamines from their vesicles into the synaptic cleft, 2) blockage of presynaptic reuptake enzymes, 3) inhibition of monoamine oxidase inhibitors, and 4) direct action on postsynaptic neurons. 5-HT, 5-hydroxytryptamine; DA, dopamine; NE, norepinephrine.
History
In 1910, Barger and Dale (1) synthesized the first stimulant: amphetamine salts. However, this stimulant was not used therapeutically until 1929, when Gordon Alles studied it as a bronchodilator and a nasal decongestant. Alles patented the oral formulation of amphetamine under the brand name Benzedrine Sulfate and later sold it to Smith, Kline, and French. The company continued to research the use of amphetamine for multiple applications, and it received endorsements for use in narcolepsy, parkinsonism, weight loss, minor depression, and psychosis, among other conditions (2). In 1937, Charles Bradley theorized that amphetamine could be used to prevent lumbar puncture headaches by stimulating the choroid plexus. He tested this hypothesis during his studies on children with minimal brain dysfunction, and he serendipitously observed that these children’s behaviors improved (7). This study eventually led to the use of stimulants for ADHD. In 1955, methylphenidate was approved, along with amphetamine, for the treatment of hyperactivity (now a part of ADHD) (8, 9) and is currently the most prescribed stimulant in the United States (5). Although the U.S. Food and Drug Administration (FDA) has limited the indications for stimulants to ADHD and narcolepsy (8), stimulants continue to be studied for off-label usages such as treatment-resistant depression, cancer-related pain, neurocognitive deficits associated with pediatric cancer survival, HIV-related cognitive impairment, and traumatic brain injury, among other indications (5).
In the years since their first clinical use, stimulants have been used in a variety of ways to enhance performance across a range of functions in nonclinical samples. We loosely divide these applications into physical and cognitive domains below and provide an overview of the history and empirical support for stimulant enhancement.
Physical Enhancement
Patterns of Use
In one of the oldest accounts of taking a specialized food or drug for performance enhancement, Charmis believed that his special diet of dried figs helped him win the stade race in the 668 B.C. Olympic Games (10). Since their first synthesis, stimulants have been used to enhance athletic performance, with occasionally tragic results. British cyclist Tom Simpson famously collapsed and died in the 1967 Tour de France, and postmortem analysis showed that significant amounts of mixed amphetamines contributed to his death (11). Despite tragedies such as this one, the illicit use of stimulant medications in sports continues. A 2013 World Anti-Doping Agency report noted a 0.19% stimulant detection rate for that year (530 of 269,878) by professional athletes worldwide. Of the test results that came back positive, stimulants were the second most commonly identified sanctioned substance at 10% (behind anabolic agents at 63%) (12). Similarly, a National Collegiate Athletic Association survey of 13,914 collegiate-level athletes from 27 men’s and women’s sports reported a mean amphetamine use of 3.1% (13). When a sample of professional athletes was asked why they would use performance-enhancing drugs, the top two answers were improved performance (86%) and financial gains (74%) (14). Along with the athletes’ internal pressures, another survey found that 5.1% and 6.5% of athletes were encouraged to use drugs at the request of their coaches and their family and friends, respectively (15).
Experimental Investigation of Performance Enhancement
During World War II, the U.S., British, and German military forces used amphetamine as a physical performance enhancer to help servicemen and -women stay alert and awake as well as to augment fitness training (2, 16). Because the military forces reported benefits with stimulants, athletes began to adopt their use in increasing numbers. In 1957, the American Medical Association responded to the growing patterns of use by appointing an ad hoc committee to provide funding to study the physical effects of stimulant use (17). The first study of this application used a within-subject design and compared amphetamine (14 mg/70 kg) with placebo use by college athletes in their respective sporting events (running, swimming, or weight throwing). The study found that 73% of the runners, 85% of the weight throwers, and 67%−93% of the swimmers showed improvement with amphetamine compared with placebo. Performance measures showed that the weight throwers, runners, and swimmers had 3%−4%, 1.5%, and 0.59%−1.16% improvement with amphetamine, respectively (18). The investigators conducted a follow-up study with a similar design and found that 77% of the participants performed better while taking amphetamine (19). By contrast, a 1959 study compared the performance of amateur college students taking amphetamine (10 mg and 20 mg) versus placebo in either running (distance or endurance) or swimming. The study showed no statistical difference between amphetamine and placebo performance. Individually, four swimmers improved, whereas one endurance runner performed worse during the 20-mg trial (20).
Given the inconsistent results from these three studies, further studies were performed to clarify the data. For example, one study administered 5 mg of amphetamine to 12 untrained male swimmers 1.5 hours before a 100-yard swim test and found no time benefit (21). Another reported that 5, 10, and 15 mg/70 kg of amphetamine administered to amateur athletes 2 hours before a stationary bike test resulted in no difference in time to exhaustion (22). In 1963, a third study compared the effects of dextroamphetamine (10 mg) used by nonathletic men and women 17–43 years old with effects of placebo to assess how long participants could maintain compression on a hand dynamometer. The authors reported a significant improvement in time spent squeezing the device, 34.40 seconds in the active group compared with 19.40 seconds with placebo (23).
Subjective Benefit
Along with objective performance-based outcomes, researchers have investigated athletes’ subjective perception of their own performance while taking stimulants. In an earlier study investigating the effects of amphetamine on college athletes, participants reported that they felt “revved up,” had improved coordination and form, had increased strength and endurance, and had increased mental and physical activation when taking amphetamine compared with placebo (19). Another study using college students to examine the effects of 15 mg/70 kg of dextroamphetamine on a treadmill endurance test found similar results. Although the students did not show any distance benefit, they reported less overall subjective fatigue at the end of the dextroamphetamine trial. Furthermore, they had a 9% increase in total lactic acid levels at the end of their dextroamphetamine runs. The authors used these data to hypothesize that amphetamine did not influence physiological fatigue production; instead, it helped blunt the cognitive perception of fatigue (24). Two subsequent follow-up studies by another research group found consistent results; participants felt that they could do more on endurance-related tasks (e.g., step test and cycle ergometry task) while taking amphetamine, independent of actual performance (25, 26).
Adverse Effects for Athletes
As previously noted, there are potentially life-threatening adverse effects of stimulant drugs on physical performance. Subjectively, stimulants can create a sense of alertness that may mask other physiological drives such as pain. If pain is not accurately perceived, an athlete may not appreciate an injury and may continue his or her sport, resulting in an increased risk for further harm. Peripherally, stimulant use has particularly harmful effects on the cardiovascular system. Increased adrenergic tone leads to peripheral vasoconstriction and increased blood pressure. These vascular changes diminish blood flow from the core to the periphery, limiting the body’s ability to cool itself by conduction. This effect, along with increased body temperature during exercise, puts athletes at risk for heat stroke (27, 28). Furthermore, stimulant use has a risk for sudden cardiac death, and this risk is increased for those engaged in strenuous exercise (28). Stimulant use can also cause appetite suppression, which can be particularly harmful given the increased nutritional needs of athletes (8).
Physical Enhancement Summary
Multiple small trials have evaluated the use of stimulants to enhance athletic performance, and the results are mixed. Most trials have shown either no benefit in performance or minor improvements. Subjectively, studies have shown that athletes tended to feel like their performance was enhanced by the stimulant, regardless of their objective outcomes. Given the risks for an athlete taking stimulants and the possibility of a performance-enhancing effect, since 2004 the World Anti-Doping Agency (29) has considered stimulants as banned substances (unless there is a medical exemption for ADHD). Even with this ban and the marginal experimental reports of benefit, professional and collegiate athletes continue to illicitly use stimulants to gain a competitive edge.
Cognitive Enhancement
Pattern of Use
The illicit use of prescription drugs has increased during the past 2 decades in U.S. colleges and universities. Lifetime prevalence rates of nonmedical stimulant use on college campuses range from 5.3% to 35% (30), whereas use within the last month ranges from 5.4% to 7.5% (31, 32). Of the college students who have used stimulants illicitly, one-half report using stimulants once or twice during the past year, one-third report using them once or twice per month, and 15.5% report using them two to three times per week (33). One-half of college students surveyed agreed or strongly agreed when asked if prescription stimulants were “easy to get on campus” (31). For those who have been diagnosed with ADHD, one-third have reported misuse of the drugs either by misusing their own (i.e., taking more than prescribed) or taking someone else’s medications (34). In addition, high school students in the United States have increased their nonmedical use of stimulants. Approximately 2.7% of eighth graders, 4.6% of tenth graders, and 5.0% of 12th graders report illicit stimulant medication use during the past year (35). There are racial/ethnic differences with respect to illicit stimulant medication use, with Caucasians and Hispanics more likely to use compared with African Americans and Asians (36). With respect to motivations for use, a recent study reported that 73.8%−91.5% of college students used stimulants to assist with studying, whereas 6.2%−6.4% and 12.3%−14.5% took stimulants to get high and to stay awake to party, respectively (37).
Experimental Investigation of Performance Enhancement
Hundreds of well-controlled studies have demonstrated the efficacy of stimulant drugs for improving the core symptoms of ADHD in children, adolescents, and adults. In addition to effects on clinical symptoms, amphetamine and methylphenidate formulations have been shown to improve performance on a wide range of tasks in which patients with ADHD typically exhibit deficits when they are unmedicated (38). There have also been substantial increases in the rates of stimulant prescriptions and the subsequent availability of these drugs for misuse and diversion (39). As noted previously, the most widely reported motivation for illicit stimulant use is for performance enhancement, particularly among high school and college students. To address the question of whether these drugs are beneficial (i.e., truly performance enhancing) in academic activities, we consider the experimental evidence for drug effects on several tasks relevant to academic performance—namely, declarative memory, working memory, and cognitive control. It should be noted from the outset that to our knowledge, no studies have systematically evaluated whether stimulants improve cognitive performance in the actual settings in which they are illicitly used (i.e., evaluated whether illicit stimulant use results in better academic performance).
Declarative memory.
Single-exposure verbal learning tasks have been used to evaluate the effects of stimulant drugs on declarative memory. In these studies, participants were asked to memorize facts from a story or a list of words after they had taken a stimulant medication. Participants were then asked to spontaneously recall the information or recognize the presented information among distracters. If the testing occurred on a subsequent visit, participants were redosed before recall. Tasks such as this mimic academic situations, in which students may be required to commit factual information to memory that is later queried in a testing situation. A number of studies with methylphenidate or amphetamine have investigated whether stimulants improve outcomes compared with placebo on such tasks in nonclinical (i.e., non-ADHD) samples. In studies with methylphenidate, 20 mg did not improve recall after 20 minutes (40) or 2.5 hours (41), although 40 mg improved recognition 1 week after the task (42). In the dextroamphetamine studies, two studies showed no benefit at 20 minutes (43, 44) and another study showed no benefit at 30 minutes (45). On the other hand, one study showed improved recall after 10 minutes (46) and four studies showed benefit at longer recall times from 1 hour to 1 week (44, 45, 47, 48). Repeated-exposure verbal learning is another declarative memory task, which is similar to the single-exposure verbal learning task except that participants are given the information to memorize more than once. Two repeated-exposure task studies have compared performance while taking a stimulant versus placebo. One study used methylphenidate and the other used dextroamphetamine; neither study showed benefit on retention at 1 hour (41) and 25 minutes (49), respectively.
Working memory.
Working memory involves immediate holding and processing of information and is pivotal for academic subjects that require data manipulation (e.g., mathematics) (50). The Sternberg Short-Term Memory Scanning Paradigm (51) is one of the oldest working-memory paradigms. In the task, participants are given a set of items (letters or numbers) that they are to “hold” in their memory. Participants are then presented with a new “comparison” set of items and are asked to determine whether any of the “held” items are present. A number of studies have been completed to evaluate whether stimulants would improve performance on the Sternberg Short-Term Memory Scanning Paradigm compared with placebo in healthy participants. One study using methylphenidate (5, 10, or 20 mg) found no benefits (52), whereas a second study (0.3 mg/kg) found improvement in reaction time (53). Similarly, three studies that investigated the benefits of dextroamphetamine found no effect (47, 54, 55), whereas two studies found a benefit in reaction time (56, 57) and a third study found a benefit in the proportion of correct answers (58). The n-back task is another working-memory paradigm, in which participants are typically presented a series of letters and then are asked whether a comparator letter is identical to the n-back letter in the list. Three studies compared the use of dextroamphetamine and found no benefit on the n-back task compared with placebo (47, 59, 60), whereas a fourth found improved information-processing speed on 15 mg/70 kg but not on 7.5 mg/70 kg (61). The digit span task has also been used to test the effects of stimulants on working memory compared with placebo. In this task, participants are given a series of numbers and are asked to repeat them forward or backward. One of four studies showed benefit with dextroamphetamine on the digit span task (10 and 20 mg) (62). Two of the negative studies used dextroamphetamine (5 mg and 0.42 mg/kg) (63, 64), whereas the third study used methylphenidate (0.2 mg/kg) (65).
Cognitive control.
Cognitive control is the ability to inhibit natural, automatic actions and instead choose a less reflexive action that has better outcomes. Cognitive control is tested during every multiple-choice test, in which the correct answers are hidden among distracters. The stop-signal task is one example of a cognitive control task. In this task, the subject is asked to make rapid button presses when a target stimulus is present, except when a counterstimulus is also present. Three studies have investigated the benefit of dextroamphetamine in the stop-signal task compared with placebo in nonclinical samples. All three trials showed no benefit in their primary outcome (49, 61, 62). However, one of the trials found that the participants who performed in the bottom one-half on placebo had a statistically significant improvement on the secondary analysis (49). Another test of cognitive control is the go/no-go task. In this task, participants are asked to respond quickly by pressing a button when “go” stimuli are present and to not respond when “no-go” stimuli are present. One go/no-go study tested dextroamphetamine (10 or 20 mg) compared with placebo, and the study showed that there was an improvement in speed to button presses in the “go” scenario and a decrease in total incorrect presses during the “no-go” condition. The researchers also found that those who did worse during the placebo trial had the greatest improvement when they took dextroamphetamine (49). The Wisconsin Card Sorting Test assesses cognitive control as well. During the test, participants must identify the correct way to sort cards (e.g., by color or shape) based on prompts by the examiner (yes or no). Periodically, the criterion for sorting is changed, and performance is based on how many incorrect cards are placed. Three studies have been completed in healthy participants comparing dextroamphetamine (all 0.25 mg/kg) with placebo. All three studies found no statistically significant improvements on the test (43, 60, 66).
Cognitive Adverse Effects
Compared with the limited number of less rigorous studies done to study performance enhancement, the adverse effects of stimulants are well known through many large placebo-controlled trials. Of note, stimulants can produce a state of euphoria that mimics that produced by cocaine, especially at high dosages and by rapid delivery methods (i.e., injection) (8, 67). This euphoria can lead to dependence and abuse. Consequently, the FDA placed a black-box warning for abuse, dependence, and diversion of stimulants and gave them a schedule II classification. In addition, stimulants promote a sense of wakefulness, which can mask the cognitive effects of fatigue and intoxication. As a result, inappropriate stimulant use can lead to poor decision making as a result of impaired judgment. Other negative cognitive effects of stimulants include the potential to precipitate mania, psychosis, anxiety, and aggression (8).
Cognitive Enhancement Summary
The illicit use of stimulants as cognitive enhancers has increased during the past decade, with a lifetime prevalence of up to 35% in college students (30). Researchers have investigated the use of stimulants in this fashion by studying the effects of stimulants on different cognitive domains, including declarative memory, working memory, and cognitive control. The studies show inconclusive results, with a majority reporting no benefit. There are significant risks associated with illicit stimulus use, including a black-box warning for abuse and dependence. Although stimulants can be helpful for those with ADHD, nonclinical use is not advised at this time and is not approved by the FDA (8, 68).
Conclusions
There are multiple large randomized controlled studies showing definitive evidence that stimulants are effective for individuals with ADHD (38). On the other hand, the data for cognitive and physical enhancement in nonclinical populations are inconclusive. The two largest physical enhancement studies directly conflict (18, 20), and the studies on the different cognitive tasks have mixed results. There are several explanations for these inconsistencies. First, participant demographics varied across the studies. In the performance studies, the participants were college athletes or nonathletes. On the cognitive tasks, the study populations ranged in age, sex, baseline performance, and educational level. In addition, the actual tasks performed differed among the studies. In the cognitive enhancement studies, the different tests are theorized to study the same cognitive domain, but each task may involve different networks. For the physical enhancement studies, the sports varied from endurance running to distance weight throwing. The exercises that were used differed in muscle group, muscle type (fast or slow twitch), and coordination of movement. Also, a wide range of doses were tested across all of the studies. Finally, the studies tended to have small sample sizes. The studies may have not been powered enough to find differences when they existed, or the positive results could have been driven by a few outliers.
The use of performance enhancers in nonclinical populations leads to several ethical issues. First, if the use of stimulants to enhance performance were legalized or authorized, these drugs would need to be equally available to everyone. If individuals (or societies) with greater resources had preferential access to enhancement drugs, they could potentially use these drugs to further distance themselves from their competitors. This could perpetuate a vicious cycle of worsening disparity and disenfranchisement. Second, individuals would need to be protected from forced stimulant use from employers, governments, or other agencies. Otherwise, organizations could mandate that those they oversee use enhancement medications to improve the productivity of their workforce, military, or agenda, respectively. In addition, if regulatory bodies approve the use of stimulants for performance enhancement, there would be more stimulant availability, which could be diverted for recreational use and addiction. Finally, stimulants have side effects, which include risk of death. Regulations must consider the potential risks and benefits of stimulants together to ensure public safety (69).
In light of the lack of data, the side effect risk profile, and regulations by the World Anti-Doping Agency and the FDA, nonmedical use of stimulants as performance enhancers is increasing. Caution should be taken against this practice until further studies are available showing definitive benefit and safety. Regulation must also be put into place to address potential ethical issues.
1 : Chemical structure and sympathomimetic action of amines. J Physiol 1910; 41:19–59Crossref, Google Scholar
2 : America’s first amphetamine epidemic 1929-1971: a quantitative and qualitative retrospective with implications for the present. Am J Public Health 2008; 98:974–985Crossref, Google Scholar
3 : Attention-deficit/hyperactivity disorder: the past 50 years. J Paediatr Child Health 2015; 51:69–73Crossref, Google Scholar
4 : Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA, APA, 2013Google Scholar
5 : Methylphenidate: its pharmacology and uses. Mayo Clin Proc 2000; 75:711–721Crossref, Google Scholar
6 : Central nervous system stimulants. Best Pract Res Clin Endocrinol Metab 2000; 14:79–88Crossref, Google Scholar
7 : The history of attention deficit hyperactivity disorder. Atten Defic Hyperact Disord 2010; 2:241–255Crossref, Google Scholar
8 : The safety and efficacy of methylphenidate and dexmethylphenidate in adults with attention deficit/hyperactivity disorder. J Cent Nerv Syst Dis 2010; 2:15–30Crossref, Google Scholar
9 : [Studies on a new analeptic ritalin; synopsis of results]. Med Klin (Munich) 1954; 49:892–893Google Scholar
10 : Performance-Enhancing Substances in Sport and Exercise. Champaign, IL, Human Kinetics, 2002Google Scholar
11 : Science and Sporting Performance: Management or Manipulation? Oxford, UK, Clarendon Press, 1982Google Scholar
12 2013 Anti-Doping Testing Figures Laboratory Report. Montreal, World Anti-Doping Agency, 2013. Available at wada-main-prod.s3.amazonaws.com/resources/files/WADA-2013-Anti-Doping-Testing-Figures-LABORATORY-REPORT.pdfGoogle Scholar
13 : NCAA study of substance use and abuse habits of college student-athletes. Clin J Sports Med 2001; 11:51–56Crossref, Google Scholar
14 : Combating drug use in competitive sports. An analysis from the athletes’ perspective. J Sports Med Phys Fitness 2002; 42:354–359Google Scholar
15 : Doping in sport: a review of elite athletes’ attitudes, beliefs, and knowledge. Sports Med 2013; 43:395–411Crossref, Google Scholar
16 : The amphetamine margin in sports. Fed Proc 1981; 40:2689–2692Google Scholar
17 : Drugs and sport. Research findings and limitations. Sports Med 1997; 24:366–384Crossref, Google Scholar
18 : Amphetamine sulfate and athletic performance. I. Objective effects. J Am Med Assoc 1959; 170:542–557Crossref, Google Scholar
19 : Amphetamine, secobarbital, and athletic performance. II. Subjective evaluations of performance, mood states, and physical states. JAMA 1960; 172:1502–1514Crossref, Google Scholar
20 : Effect of amphetamine sulfate on athletic performance. J Am Med Assoc 1959; 170:558–561Crossref, Google Scholar
21 : Action of drugs on efficiency of swimmers. Res Q 1946; 17:96–101Google Scholar
22 : Effect of varient dosages of amphetamine upon endurance. Res Q 1973; 44:417–422Google Scholar
23 : The effects of stimulant and depressant drugs on physical persistence. Am J Psychol 1963; 76:698–700Crossref, Google Scholar
24 : The effect of amphetamines on selected physiological components related to athletic success. Med Sci Sports Exerc 1980; 12:65–69Crossref, Google Scholar
25 : The influence of amphetamine (Benzedrine) sulfate and caffeine on the performance of rapidly exhausting work by untrained subjects. J Lab Clin Med 1943; 28:601–603Google Scholar
26 : The influence of amphetamine (Benzedrine) sulfate, d-desoxyephedrine hydrochloride (pervitin), and caffeine upon work output and recovery when rapidly exhausting work is done by trained subjects. J Lab Clin Med 1943; 28:603–606Google Scholar
27 : Central nervous system stimulants and sport practice. Br J Sports Med 2006; 40(Suppl 1):i16–i20Crossref, Google Scholar
28 : Ergogenic risks elevate health risks in young athletes. Pediatr Ann 2003; 32:733–737Crossref, Google Scholar
29 World Anti-Doping Agency: World anti-doping Code. wada-main-prod.s3.amazonaws.com/resources/files/wada_anti-doping_code_2009_en_0.pdfGoogle Scholar
30 : Misuse of prescription stimulants among college students: a review of the literature and implications for morphological and cognitive effects on brain functioning. Exp Clin Psychopharmacol 2013; 21:385–407Crossref, Google Scholar
31 : Nonmedical prescription stimulant use among a sample of college students: relationship with psychological variables. J Atten Disord 2009; 13:284–296Crossref, Google Scholar
32 : Motives and perceived consequences of nonmedical ADHD medication use by college students: are students treating themselves for attention problems? J Atten Disord 2009; 13:259–270Crossref, Google Scholar
33 : Stimulant medication use, misuse, and abuse in an undergraduate and graduate student sample. J Am Coll Health 2006; 54:261–268Crossref, Google Scholar
34 : Perceived harmfulness predicts nonmedical use of prescription drugs among college students: interactions with sensation-seeking. Prev Sci 2008; 9:191–201Crossref, Google Scholar
35 : Prevalence and correlates of illicit methylphenidate use among 8th, 10th, and 12th grade students in the United States, 2001. J Adolesc Health 2004; 35:501–504Google Scholar
36 : Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration. Pharmacotherapy 2006; 26:1501–1510Crossref, Google Scholar
37 : Nonmedical use of prescription stimulants during college: four-year trends in exposure opportunity, use, motives, and sources. J Am Coll Health 2012; 60:226–234Crossref, Google Scholar
38 : Effects of methylphenidate on cognitive functions in children and adolescents with attention-deficit/hyperactivity disorder: evidence from a systematic review and a meta-analysis. Biol Psychiatry 2014; 76:603–615Crossref, Google Scholar
39 : National trends in the prevalence of attention-deficit/hyperactivity disorder and the prescribing of methylphenidate among school-age children: 1990-1995. Clin Pediatr (Phila) 1999; 38:209–217Crossref, Google Scholar
40 : Effects of the tranquillizer diazepam and the stimulant methylphenidate on alertness and memory. Neuropsychobiology 1997; 36:42–48Crossref, Google Scholar
41 : Cognitive effects of milacemide and methylphenidate in healthy young adults. Psychopharmacology (Berl) 1994; 115:46–52Crossref, Google Scholar
42 : Pharmacological manipulations of arousal and memory for emotional material: effects of a single dose of methylphenidate or lorazepam. J Psychopharmacol 2007; 21:673–683Crossref, Google Scholar
43 : Neuropsychological effects of amphetamine may correlate with personality characteristics. Psychopharmacol Bull 1995; 31:357–362Google Scholar
44 : Amphetamine enhances human-memory consolidation. Neurosci Lett 1993; 161:9–12Crossref, Google Scholar
45 : Verbal memory performance improved via an acute administration of D-amphetamine. Hum Psychopharmacol 2007; 22:279–287Crossref, Google Scholar
46 : Dextroamphetamine. Its cognitive and behavioral effects in normal and hyperactive boys and normal men. Arch Gen Psychiatry 1980; 37:933–943Crossref, Google Scholar
47 : A triazolam/amphetamine dose-effect interaction study: dissociation of effects on memory versus arousal. Psychopharmacology (Berl) 2007; 192:425–440Crossref, Google Scholar
48 , et al: Effect of amphetamine on long-term retention of verbal material. Psychopharmacology (Berl) 1995; 119:155–162Crossref, Google Scholar
49 : Acute administration of d-amphetamine decreases impulsivity in healthy volunteers. Neuropsychopharmacology 2002; 27:813–825Crossref, Google Scholar
50 : Brain activity during a visuospatial working memory task predicts arithmetical performance 2 years later. Cereb Cortex 2012; 22:1078–1085Crossref, Google Scholar
51 : High-speed scanning in human memory. Science 1966; 153:652–654Crossref, Google Scholar
52 : Presidential address, 1982. The pharmacology of human information processing. Psychophysiology 1983; 20:359–370Crossref, Google Scholar
53 : Effects of methylphenidate on stimulus evaluation and response processes: evidence from performance and event-related potentials. Psychophysiology 1988; 25:292–304Crossref, Google Scholar
54 : Differential effects of scopolamine and amphetamine on microcomputer-based performance tests. Aviat Space Environ Med 1990; 61:615–621Google Scholar
55 : Processing efficiency of a verbal working memory system is modulated by amphetamine: an fMRI investigation. Psychopharmacology (Berl) 2005; 180:634–643Crossref, Google Scholar
56 : Effects of d-amphetamine on task performance and social behavior of humans in a residential laboratory. Exp Clin Psychopharmacol 1997; 5:130–136Crossref, Google Scholar
57 : Dextroamphetamine causes a change in regional brain activity in vivo during cognitive tasks: a functional magnetic resonance imaging study of blood oxygen level-dependent response. Biol Psychiatry 2004; 56:284–291Crossref, Google Scholar
58 : Behavioral and subjective effects of d-amphetamine and modafinil in healthy adults. Exp Clin Psychopharmacol 2007; 15:123–133Crossref, Google Scholar
59 : Effects of dextroamphetamine on cognitive performance and cortical activation. Neuroimage 2000; 12:268–275Crossref, Google Scholar
60 : Catechol O-methyltransferase val158-met genotype and individual variation in the brain response to amphetamine. Proc Natl Acad Sci USA 2003; 100:6186–6191Crossref, Google Scholar
61 : Effects of d-amphetamine in human models of information processing and inhibitory control. Drug Alcohol Depend 2005; 77:151–159Crossref, Google Scholar
62 : Effects of d-amphetamine and ethanol on a measure of behavioral inhibition in humans. Behav Neurosci 2000; 114:830–837Crossref, Google Scholar
63 : Effects of scopolamine and dextroamphetamine on human performance. Aviat Space Environ Med 1988; 59:407–410Google Scholar
64 : The acute effects of d-amphetamine and methamphetamine on attention and psychomotor performance. Psychopharmacology (Berl) 2006; 187:154–169Crossref, Google Scholar
65 : Pharmacologically induced changes in arousal: effects on behavioral and electrophysiologic measures of alertness and attention. Electroencephalogr Clin Neurophysiol 1995; 95:359–371Crossref, Google Scholar
66 : Dextroamphetamine enhances “neural network-specific” physiological signals: a positron-emission tomography rCBF study. J Neurosci 1996; 16:4816–4822Crossref, Google Scholar
67 : Serum and brain concentrations of methylphenidate: implications for use and abuse. Neurosci Biobehav Rev 2003; 27:615–621Crossref, Google Scholar
68 Novartis: Ritalin Hydrochloride Package Insert. East Hanover, NJ, Novartis, 2013Google Scholar
69 : Neuroethical issues in cognitive enhancement. J Psychopharmacol 2011; 25:197–204Crossref, Google Scholar



