Obsessive-Compulsive Disorder: Diagnosis, Epidemiology, Etiology, and Treatment
Abstract
Obsessive-compulsive disorder (OCD) has been given increased attention in DSM-5, receiving its own chapter. In this article, the authors discuss changes to the diagnostic criteria and symptoms differentiating OCD from other disorders. Two new specifiers, which have relevance in regard to treatment selection, appear in DSM-5: level of insight and presence of tic disorder. The epidemiology of OCD remains under investigation and may be modified based on the new DSM-5 criteria. Current data regarding age of onset and social and occupational functioning of patients with OCD are presented. The structural and functional neuroanatomy of OCD has been an area of increased research attention, and the authors summarize the research in this area. Treatment of OCD remains difficult, involving a combination of pharmacotherapy and cognitive-behavorial therapy, with a large percentage of nonresponders to initial treatment. The authors review pharmacotherapy recommendations from the APA practice guidelines and discuss evidence for pharmaceutical options. Surgical treatment is a promising option, and the authors review evidence for various procedures.
Once thought to be relatively rare, obsessive-compulsive disorder (OCD) is now believed to be one of the more common psychiatric disorders, with an estimated lifetime prevalence of approximately 2% (1–4). Increased understanding of this disorder has led to a modification of the structure of the most recent version of the DSM-5. In past editions of the DSM, OCD was considered an anxiety disorder. Although anxiety is still considered a central feature, OCD—along with the related disorders of trichotillomania, skin picking, body dysmorphic disorder, and hoarding—has been given its own section in DSM-5: Obsessive-Compulsive and Related Disorders (5). This significant modification distinguishes OCD as a unique mental disorder and will hopefully result in increased clinical attention.
In this issue of Focus, we provide an introduction to the changes in the understanding of OCD and related disorders and to the exciting new findings of research in these disorders. Effective treatment follows from an accurate diagnosis, and we begin by reviewing the diagnostic criteria for OCD as provided in DSM-5. We then examine findings of neuroimaging studies, followed by a discussion of pharmacotherapy strategies.
Diagnosis and Assessment
The diagnostic criteria for OCD in DSM-5 remain largely the same as in DSM-IV, requiring either obsessions or compulsions. Obsessions are defined as 1) “recurrent and persistent thoughts, urges, or images that are experienced, at some time during the disturbance, as intrusive and unwanted, and that in most individuals cause marked anxiety or distress” and 2) “the individual attempts to ignore or suppress such thoughts, urges, or images, or to neutralize them with some other thought or action (i.e., by performing a compulsion).” Compulsions are defined as “repetitive behaviors (e.g., hand washing, ordering, checking) or mental acts (e.g., praying, counting, repeating words silently) that the individual feels driven to perform in response to an obsession or according to rules that must be applied rigidly” that are “aimed at preventing or reducing anxiety or distress, or preventing some dreaded event or situation; however, these behaviors or mental acts are not connected in a realistic way with what they are designed to neutralize or prevent, or are clearly excessive.... The obsessions or compulsions are time-consuming, taking more than 1 hour per day, or cause clinically significant distress or impairment in social, occupational, or other important areas of functioning.” (5) The major change in the DSM-5 criteria for OCD is the addition of two specifiers for this diagnosis: insight and tic-related disorder.
The first specifier concerns the level of insight the patient possesses, defined as the degree to which individuals with OCD find their obsessions and related compulsions to be reasonable. DSM-5 recognizes that patients with OCD may have varying degrees of insight, ranging from absent insight/delusional beliefs at one extreme, with the individual “completely convinced that [OCD] beliefs are true,” and good or fair insight, with the individual recognizing that OCD beliefs “are definitely or probably not true.” DSM-5 also allows for the level of poor insight, in which “the individual thinks [OCD] beliefs are probably true.” (5) It has been recognized for some time that the degree of insight may vary during a single episode and across episodes (6), yet the etiology underlying these variations is unclear. Although research findings are mixed, poor insight has been associated with earlier age of onset (7, 8), longer illness duration (7, 8), greater severity of symptoms (9–12), and an increased comorbidity with major depression (8, 12).
The Yale-Brown Obsessive Compulsive Scale (YBOCS) (13) remains the gold standard for the assessment of OCD symptom severity and contains an item on degree of insight, measured on a 0–4 scale, to aid clinicians with the often difficult task of assessing the strength of patients’ conviction regarding the necessity of their obsessive-compulsive behavior [available in print from Goodman et al. (13)]. An additional assessment instrument, the Brown Assessment of Beliefs Scale (BABS), has been developed to provide a measure of insight (available for download at www.rhodeislandhospital.org/services/body-dysmorphic-disorder-program/questionnaires.html) (14). This semistructured interview includes seven items to assess the degree of conviction, perceptions of others’ views of the belief, degree of fixedness, and awareness that the obsessions and compulsions are caused by a mental disorder. The rating is on a 0–24 scale; only six items are used to calculate the total score. Using BABS, one study of 211 outpatients with OCD found 2.4% of these patients to have delusional beliefs (score >18) and 13.3% to have poor insight (score 13–17), whereas the remaining patients were approximately evenly split between fair (28.9%, score 8–12), good (26.5%, score 4–7), and excellent (28.9%, score 0–3) insight (15).
As mentioned earlier, the DSM-5 includes not one, but two new specifiers for OCD. The second specifier is for the presence of a tic disorder, which is coded as “tic-related” if “the individual has a current or past history of tic disorder.” (5) One study estimated that 30%–50% of children with tic disorder will go on to have OCD (16). Tic disorder and OCD appear to be genetically related disorders, as there is an increased incidence of tic disorder and OCD in first-degree relatives of patients with either of these disorders (17, 18). It has been suggested that tic disorder may actually be an alternate expression of genetically transmitted OCD (19). The addition of the tic specifier is useful, as the co-occurrence of OCD and tic disorder may have treatment implications. Patients with OCD and comorbid motor tics were less likely to respond to fluvoxamine in two studies (20, 21). The presence of a tic disorder has not been found to reduce the efficacy of clomipramine (22) or fluoxetine for OCD (23). In patients with Tourette’s disorder, selective serotonin reuptake inhibitors (SSRIs) appear to have little if any effect on tic symptoms. In patients with OCD and tic disorder, antipsychotic augmentation is a reasonable strategy, as it may treat both disorders simultaneously (24, 25).
Differentiating Symptoms of OCD From Those of Other Disorders
It is important to distinguish the absent insight and delusional beliefs of OCD from the delusions present in psychotic disorders, as OCD may closely resemble psychosis. The major feature distinguishing the absent insight/delusional beliefs of OCD from schizophrenia is that the OCD compulsion is performed in an effort to reduce anxiety provoked by a thought, image, or impulse. The presence of hallucinations, disorganized speech, and affective flattening should alert the clinician to the likelihood of schizophrenia rather than OCD. In addition, although patients with OCD may have difficulty fully verbalizing the phenomenology of their obsessions and compulsions, disorganized speech will not be present. There is some co-occurrence of OCD and schizophrenia, with one study estimating that approximately 12% of patients with schizophrenia have comorbid OCD (26).
Given that OCD with poor insight and delusional beliefs may closely resemble schizophrenia, some work has been done to distinguish these two disorders. One study found that patients with OCD and poor insight (operationalized as high scores on the Overvalued Ideas Scale) displayed executive dysfunction intermediate between patients with schizophrenia and patients without poor insight (27). A second study replicated these results, finding that patients with OCD and poor insight exhibited better performance compared with schizophrenia patients on some subscales of the Wisconsin Card Sorting Test and the Trail Making Test but exhibited worse performance compared with patients with OCD and fair or good insight. In addition, patients with OCD and poor insight exhibited worse performance on the verbal memory process than did patients without poor insight (28).
Another disorder that is important to differentiate from OCD is obsessive-compulsive personality disorder (OCPD). The main symptoms that should allude to OCPD are the patient’s egosyntonic thoughts about perfectionism and rigidity in behavior. According to the DSM-5 criteria, the OCPD individual is “1) preoccupied with details, rules, lists, order, organization, or schedules to the extent that the major point of the activity is lost, 2) shows perfectionism that interferes with task completion, 3) is excessively devoted to work and productivity to the exclusion of leisure activities and friendships, 4) is overconscientious, scrupulous, and inflexible about matters of morality, ethics, or values, 5) is unable to discard worn-out or worthless objects, even when they have no sentimental value, 6) is reluctant to delegate tasks or to work with others unless they submit to exactly their way of doing things, 7) adopts a miserly spending style toward both self and others, and 8) shows rigidity and stubbornness” (5).
Although DSM-5 indicates that social and occupational dysfunctions are necessary criteria for OCD, in contrast to OCPD, OCD does not include criteria specifically regarding the prominence of work in the self-identity of the patient with OCD. Lack of empathy and lack of intimacy are also not part of the criteria for OCD. However, OCD does appear to negatively affect romantic relationships, as it has been found to be more prevalent in divorced/separated individuals than in those who are married (2). It is important to note that DSM-5 no longer uses the five-axis diagnostic system; personality disorders are now considered a primary diagnosis.
A final disorder that should be differentiated from OCD is impulse control disorder. Patients with OCD do, by definition, have trouble controlling their compulsive behavior. In a study of 96 patients with OCD, 5.3% met criteria for compulsive gambling, 4.3% met criteria for intermittent explosive disorder, and 2.1% met criteria for pyromania (29). The important distinction between OCD and impulse control disorders is that in OCD, the compulsive behavior in response to a thought, image, or impulse is considered unacceptable to the patient. The thoughts, images, or impulses in impulse control disorder are egosyntonic, whereas the thoughts, images, and impulses of OCD are egodystonic (30).
Epidemiology
Although it remains to be seen whether the epidemiology of OCD will shift with the adoption of DSM-5 criteria, it appears that OCD typically has an onset during puberty. Overall, slightly more women than men exhibit symptoms of OCD, with an approximate female-to-male ratio of 1.5:1.0 (2, 31). It should be noted that men may have an earlier onset of the disorder than women, as epidemiological studies in adolescent samples have tended to find more boys than girls with OCD (32, 33). DSM-5 provides diagnostic criteria in an attempt to distinguish OCD with hoarding symptoms from the separate, but related, hoarding disorder. Some evidence suggests that this modification may shift the male-to-female ratio of OCD to be more equal across genders (29). In this issue of Focus, Dr. Steketee presents the current conceptualization of hoarding disorder as well as its treatment.
Pathophysiology
The pathophysiology of OCD from a genetic standpoint is discussed in this issue of Focus by Drs. Sobell, Pato, Pato, and Knowles. Results of studies of genetic differences in individuals with OCD have most consistently implicated polymorphisms of the glutamate receptor gene, specifically the SLC1A1 coding region located on chromosome 9 (34, 35). The SLC1A1 gene encodes the glutamate transporter known as the excitatory amino acid carrier 1, which is found in the cortex, thalamus, basal ganglia, hippocampus, and cerebellum (36–38).
Products of genes associated with OCD may affect specific brain regions. Despite the somewhat inconsistent findings of structural neuroimaging studies, it would appear that the brain regions most involved in OCD are those that assist with regulation of emotion and cognitive control, and the alterations in these structures potentially mediate the anxiety and misappraisal of threat exhibited by those with OCD. It remains to be determined whether these structural differences are the underlying cause of OCD symptoms or are products of persistent obsessive cognitions and compulsive behaviors. As noted below, the heterogeneity of OCD symptoms, as well as psychiatric illnesses comorbid with OCD, may mediate the discrepancies among the findings of the individual studies.
The neuroanatomy of OCD has been the focus of intense research. However, findings have been somewhat inconsistent, with studies reporting smaller (39–41), larger (42–44), or normal (45–47) volumes of overall, white, and gray matter in various regions of the brains of patients with OCD relative to control participants.
Meta-analytic research has synthesized the discrepant results, finding a smaller combined (white+gray matter) volume of the anterior cingulate cortex (ACC) (48, 49) and orbitofrontal cortex (OFC) (49), and an increased combined thalamic volume (49) in patients with OCD relative to control participants. Although data are very limited, increased combined thalamic volume has been found to correlate with an increased severity of obsessions and compulsions (49).
Gray matter density has been found in meta-analyses to be reduced in the dorsolateral prefrontal cortex (50), OFC (50), dorsomedial prefrontal cortex (DMPFC) (48), and ACC (48, 51) in patients with OCD relative to control participants. In contrast, gray matter density has been found to be greater in the putamen (50), caudate (51), and anterior prefrontal cortex (50, 51) in patients with OCD relative to control participants. In addition to gray matter reductions, white matter density was found to be reduced in the DMPFC and ACC in a recent mega-analysis (reanalysis of a random sampling of previously collected data) (48). Neuroimaging researchers have suggested that specific neuroimaging techniques may significantly affect study findings and may be partially responsible for discrepancies among studies (52).
As a visual aid, neuroanatomy imaging from a recent mega-analysis (48) is presented in Figure 1. In addition, Table 1 provides a summary of the findings from meta-analytic studies.
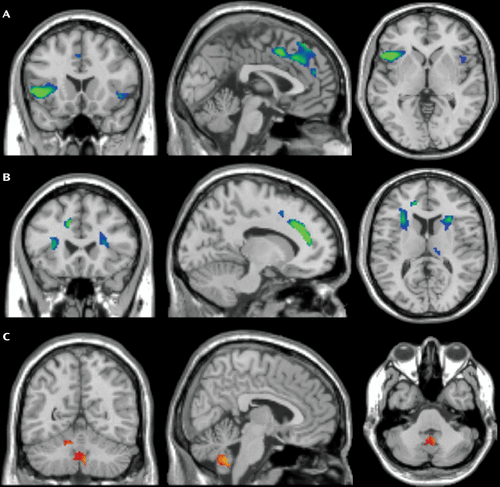
Figure 1. Regional Gray and White Matter Volume Differences Between OCD Patients (N=412) and Healthy Comparison Subjects (N=368)a
a Compared with healthy subjects, OCD patients exhibit smaller (blue/green) regional medial and inferior frontal gray (panel A) and white (panel B) matter volume and greater (red/yellow) regional cerebellar gray matter volume (panel C). Brain slices in panel A−C are in coronal (left), sagittal (middle), and axial (right) direction; left side of brain is on the left. MNI coordinates (x,y,z) of panel A, −1, 18, 0; of panel B, −11, 23, 12; of panel C, 6, −55, −40. All presented imaging results are T-statistic images overlain over the SPM8 single-subject T1 Standard brain with MRIcron (www.mccausland center.sc.edu/mricro/mricron) and thresholded at p<0.001 uncorrected with a minimum cluster extent of 100 voxels. T values are displayed from red/blue (T = 3.1) to yellow/green (T ≥ 4.0). Image obtained from de Wit SJ, Alonso P, Schweren L, et al: Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am J Psychiatry 2014; 171:340–349. Copyright © 2014 American Psychiatric Association. Used with permission.
| Characteristic | ACC | OFC | Thalamus | DLPFC | DMPFC | Putamen | Caudate | APFC |
|---|---|---|---|---|---|---|---|---|
| Total brain matter | Reduced | Reduced | Increased | |||||
| Gray matter density | Reduced | Reduced | Reduced | Reduced | Increased | Increased | Increased | |
| White matter density | Reduced | Reduced |
Table 1. Summary of Meta-Analytic Results of Volumetric Studies of Brain Regions in OCDa
Perhaps more important than the brain volumetric correlates of OCD may be the functional connections between the implicated neuroanatomical areas, referred to as the cortico-basal ganglia–thalamocortical circuitry and the corticostriatal-thalamocortical circuitry. (Note: The striatum consists, in part, of the caudate and putamen, which are also areas contained within the basal ganglia.) Through the use of functional MRI, an increased degree of coordinated activity, known as hyperconnectivity, has been demonstrated to occur between the ventral caudate/putamen/nucleus accumbens and the anterolateral and medial orbitofrontal cortices in patients with OCD, with the magnitude of coordinated activity predictive of symptom severity (53–58). Some evidence exists that serotonergic antiobsessional medications may improve OCD symptoms through a reduction of hyperconnectivity (58).
Two neuroanatomical areas appear to be of particular importance from a functional neuroimaging standpoint. The degree of hyperactivity in the OFC is considered by many researchers to be predictive of OCD global symptom severity (57–64). In the ACC, functional neuroimaging studies have shown elevated metabolism in individuals with OCD at rest and during symptom provocation (65–67).
Although differences in imaging techniques may partially explain the discrepant findings of neuroimaging studies, it seems possible for neuroanatomical findings in OCD to differ depending on specific OCD symptom type. Some researchers believe that OCD neuroanatomy may be better understood if OCD is considered a clinically related group of symptom dimensions rather than as a simple construct of obsessions and compulsions (68, 69). Consistent with this assessment, structural and functional neuroimaging studies have suggested that partially distinct neuroanatomy and neural pathways may be present depending on the specific OCD symptoms (54, 70–72).
Structural neuroimaging studies have suggested that reductions in temporolimbic (amygdala) volume may be associated with aggressive obsessions and checking compulsions (39, 73). Alterations in volumes of gray matter and white matter of various other regions of the brain have been associated with varying categories of obsessions in individual studies, but these findings await further substantiation through replication (74, 75). Somewhat more broadly, it has been suggested that the obsessional component of OCD may be associated with white matter reductions in the cingulate bundle and corpus callosum (76).
In terms of functional neuroimaging, aggressive obsessions have been positively associated with greater connectivity between the ventral caudate and ventral medial frontal cortex, whereas sexual and religious obsessions have been associated with greater connectivity between the ventral caudate connectivity and the mid- and anterobasal insular cortex (53).
Another variable that may mediate the discrepant neuroimaging findings is that of frequent comorbidity of OCD with other psychiatric disorders. Patients with OCD and comorbid disorders are sometimes the intended focus of research (77, 78), but inadvertent inclusion of individuals with comorbidities, or difficulty in parceling out the independent effects of comorbidities, may lead to the discrepancies among neuroimaging studies. For example, major depressive disorder shares many neuroanatomical findings with OCD (79, 80). However, neuroanatomical concordance of these disorders is likely less than complete, and inclusion of depressed patients in OCD imaging studies may be a variable leading to difficulty in the interpretation of results of many neuroimaging studies. Functional neuroimaging has been useful in distinguishing OCD from commonly comorbid major depressive disorder, finding that although both disorders share decreased activation of the prefrontal cortex during a task-switching exercise, only patients with OCD exhibited increased activation of the caudate, ACC, and insula (81).
Surgical Treatment
Several treatment strategies based on neuroanatomical correlates of OCD have been developed for patients whose condition is refractory to multiple trials of pharmacotherapy and psychotherapy. The first neurosurgical procedure specifically designed for OCD symptoms refractory to psychotropic medication and psychotherapy was stereotactic anterior cingulotomy, which was developed at Massachusetts General Hospital in the 1960s (82). This procedure continues to be an effective treatment (83, 84) and has been shown recently to have long-lasting positive effects, with a 35% reduction of YBOCS in 47% of patients at approximately 5 years in a recent study (85). Other surgical treatments of OCD include anterior capsulotomy, subcaudate tractotomy, and a combination of these two procedures known as limbic leukotomy (85). However, such procedures are invasive and irreversible. Gamma knife radiosurgery, also known as radioablative surgery, is a procedure that has been used since 1953 for the treatment of OCD, with mixed results (86). Until recently, the data on this procedure consisted of case reports, case series, and retrospective methods. However, a small randomized placebo-controlled trial of 16 patients recently found benefit of ventral capsulotomy with the gamma knife procedure (87).
A newer surgical treatment option that has yielded promising results thus far is stereotactic deep brain stimulation (DBS) (88–91), which was approved by the U.S. Food and Drug Administration (FDA) in 2008 for the treatment of OCD. The procedure involves the use of MRI to determine the location, the drilling of a small burr hole in the skull, and advancement of an electrode 1.27 mm in diameter (the approximate size of 7 strands of human hair) into the targeted structure (92). Studies have placed the electrode in various neuroanatomical locations, including the internal capsule/ventral striatum, nucleus accumbens, subthalamic nucleus, or inferior thalamic peduncle. All of these neuroanatomical placements have been associated with significant reductions of OCD symptoms (92). No single optimal location has yet been identified. This is, at least in part, because of the relatively small amount of data on the use of this procedure for OCD. It is estimated that DBS outcomes have been reported in the literature for only approximately 90 patients with OCD (92). It is possible that certain locations may be more or less effective for specific OCD symptoms. DBS for OCD appears to possess efficacy similar to that of ablative procedures, such as anterior cingulotomy (85). Effects of DBS appear to be long-lasting, with gains maintained at 2–3 years (84, 93).
DBS possesses the advantage of reversibility, by the ability to turn on and then turn off electrical stimulation. This reversibility provides an opportunity to study the neuroanatomical functioning of individuals with OCD with DBS “off” and “on,” allowing for demonstration of the efficacy of DBS in the subthalamic nucleus in double-blinded conditions (94). Positron emission tomography has shown a reduction of activity in the left cingulate gyrus and left frontal medial gyrus relative to baseline in patients with OCD when DBS is in the “on” mode. A decrease in metabolism in the prefrontal cortex, specifically the OFC, correlated with a decrease in YBOCS scores (95). DBS appears to be a very promising option for treatment-resistant patients. Given the invasive nature of DBS, this procedure is reserved for patients whose condition has failed adequate trials of cognitive-behavioral therapy (CBT) and at least three medication trials.
Nonpharmacological Treatment
CBT, specifically exposure and response prevention (ERP) therapy, is one of the most effective treatments of OCD and continues to be recommended as a first-line treatment and as an initial augmentation strategy (96, 97). The importance of this mode of therapy for patients with OCD cannot be overstated. We present evidence in support of pharmacological treatment of OCD in the next section but encourage the interested reader to become familiar with CBT-ERP techniques. An overview of these techniques is presented by Drs. Sassano, Sapp, and Van Noppen in this issue of Focus. Attention is given to the assessment of symptoms, case conceptualization, and design of a CBT treatment plan.
Pharmacological Treatment
Pharmacological treatment of OCD continues to involve SSRIs and clomipramine as mainstays (96, 97). APA has developed practice guidelines for OCD. At this time, five FDA-approved medications are available for treatment of OCD in adults: sertraline, fluoxetine, fluvoxamine, paroxetine, and clomipramine. A meta-analysis reports equal efficacy across the FDA-approved SSRIs (98). A new controlled-release formulation of fluvoxamine appears to allow a quicker dose titration than the immediate-release formulation (99).
Citalopram, although not approved by the FDA for the treatment of OCD, was found to have an efficacy equal to that of the FDA-approved SSRIs in a meta-analysis (98). Escitalopram, the S-enantiomer of citalopram, has shown efficacy in recent open-label trials (100, 101) as well as in randomized placebo-controlled trials (102, 103). The efficacy of escitalopram has been noted to be equal to that of paroxetine, with fewer medication side effects (102). On the basis of these findings, the APA practice guideline for OCD includes citalopram and escitalopram as potential first-line medications for the treatment of OCD (97). Because of concerns for QTc prolongation, the FDA maximal recommended dose of citalopram has been lowered to 40 mg/day (20 mg/day in those >60 years of age). However, recent research has challenged this recommendation, noting that the QTc prolongation associated with citalopram may be no worse than that seen with other SSRIs (104). Escitalopram does not carry the same warning regarding QTc prolongation in the United States, but the drug regulatory administration in the United Kingdom has applied this warning (105). The maximal FDA-recommended daily dose of escitalopram is not more than 20 mg.
Discontinuation rates because of undesirable side effects appear to be higher with clomipramine than with SSRIs (106–109), with weight gain from clomipramine being one of the main side effects (110). Therefore, treatment guidelines recommend a trial of the FDA-approved SSRIs before the use of clomipramine (96, 97). Although meta-analyses suggest that clomipramine may be superior to the SSRIs (111–117), direct-comparison trials of clomipramine and SSRIs indicate equal efficacy (109, 118, 119).
Patients with OCD have been found to often require medication doses beyond the maximum approved by the FDA (96, 97, 120), and it is recommended that the duration of a given medication trial be 8–12 weeks total, with 4–6 weeks at the maximal tolerable dose (96, 97, 101, 121, 122). In the case of symptom remission, current treatment guidelines recommend continuation of medications for 1–2 years before initiation of a taper during several months to discontinuation (96).
Although serotonergic/noradrenergic medications are an effective treatment for some patients, many individuals remain refractory to treatment, with some estimates as high as 50%−60% (96, 123). Before modifying therapy, it may be prudent to inquire about medication compliance and to consider whether a patient may possess CYP enzymes that rapidly metabolize medications. Before testing for CYP enzyme activity, the clinician can consider a trial of medication metabolized through an alternate CYP pathway.
We discussed the degree of patients’ insight earlier in this article. The prognostic value of insight is unclear at this time and requires more research. Some studies have found poorer insight to be associated with poorer treatment response to medication and psychotherapy [Matsunaga et al. (7), Ravi Kishore et al. (8), Jakubovski et al. (10), Erzegovesi et al. (124), Eisen et al. (125), Alonso et al. (126)]. This is potentially mediated by the degree of interest in, involvement in, and adherence to treatment. The clinician may be required to exert additional efforts to determine interest and involvement in pharmacological and psychotherapeutic treatment plans and adherence to medication recommendations when working with patients with absent insight or delusional beliefs. It is also worth noting that insight may vary in repeated episodes of illness. There has not been systematic study of the interplay between medication response and the degree of insight across multiple episodes. (See articles in this issue of Focus on body dysmorphic disorder, hoarding, and skin picking and trichotillomania for discussion of the role of insight and treatment response.)
OCD Refractory to Initial Pharmacological Management
The APA practice guidelines provide additional treatment strategies based on the response to the initial medication trial. For those with moderate response, defined as a clinically significant, but inadequate, response after a 12-week trial of the initial medication, augmentation with a second-generation antipsychotic drug is recommended. This recommendation is supported by meta-analytic data (127, 128), and approximately one-third to one-half of patients will respond to this augmentation strategy (25, 128). It is recommended that the length of a trial of augmentation with an atypical antipsychotic drug be in the range of 2–8 weeks.
Currently, insufficient data are available to determine which member of this class of medications possesses superior efficacy, although at least one randomized controlled trial (RCT) has shown efficacy for haloperidol, risperidone, quetiapine, olanzapine, and aripiprazole (129). No RCTs exist that have examined the efficacy of ziprasidone. Of the newer atypical antipsychotic drugs asenapine, lurasidone, iloperidone, and paliperidone, only paliperidone has been examined in a double-blind, placebo-controlled trial, with a nonsignificant trend toward improvement in OCD symptoms (130).
Head-to-head comparator studies are few, but quetiapine was found to be superior to ziprasidone in a study of 24 patients (131). Risperidone and haloperidol appear to have equal efficacy; however, haloperidol may be more useful in the presence of a comorbid tic disorder (20), whereas risperidone may be more useful in patients with comorbid depression (132). Risperidone was found to be superior to aripiprazole in a study of 41 patients (133) and to have an efficacy equal to that of olanzapine in a study of 50 patients (134).
The pharmacological mechanism through which the atypical antipsychotic drugs exert their anti-OCD effect is unclear but may be related to 5-hydroxytryptamine 1A (5-HT1A) partial agonism, which results in increased levels of serotonin. Given the efficacy of haloperidol, which has no serotonin receptor affinity, it is possible that dopamine blockade also mediates the efficacy of the antipsychotic medications. Some meta-analytic evidence supports this notion in that risperidone, the second-generation antipsychotic drug with the most potent D2 receptor blockade, may be the most efficacious of the class (127). It is possible that patients with OCD and absent insight or delusional beliefs may particularly benefit from augmentation with antipsychotic medication. However, this is a speculative assessment, and more research is thus needed to support it.
If little or no response is evident with the initial SSRI, recommended options are to conduct a trial with 1) an alternate SSRI, mirtazapine, venlafaxine, or clomipramine as monotherapy or 2) second-generation antipsychotic drugs in augmentation of the initial SSRI. Mirtazapine has been found to accelerate the response to citalopram in a single-blind randomized augmentation study (135) and showed some efficacy in a discontinuation trial (136). Venlafaxine has shown mixed results, with no difference from placebo in one double-blind, placebo-controlled study (137), yet its efficacy was relatively equal to that of paroxetine (138, 139) and clomipramine (140) in double-blind comparator studies.
Should the aforementioned treatment strategies result in an inadequate reduction of symptoms, the APA practice guidelines recommend a trial of augmentation with an alternate second-generation antipsychotic drug, a monotherapy trial of an alternate serotonin reuptake inhibitor, or augmentation with clomipramine, although clomipramine has limited supporting evidence.
Before concluding that a trial of clomipramine has been ineffective in monotherapy or in augmentation, the clinician can obtain a plasma level to determine that dosing is adequate. The clinician should be cognizant of the potential for cardiac complications when coadministering clomipramine with potent CYP 2D6 inhibitors, such as fluoxetine, paroxetine, and bupropion, as serum levels of clomipramine can become supratherapeutic, resulting in QTc prolongation. Thus, monitoring plasma levels of clomipramine is of considerable importance when such a strategy is implemented.
Additional Treatment Strategies
Once a practitioner has exhausted the previously discussed treatment strategies, options with less empirical evidence can be tested in trials. These options and supporting evidence are reviewed below.
Other medications exerting effects through 5-HT1A antagonism that may be useful as augmentation strategies in the treatment of OCD are buspirone and pindolol. Results of a double-blind, RCT found buspirone monotherapy to have an efficacy equal to that of clomipramine (141). However, studies have found poor efficacy of buspirone as an augmenting agent (21, 142, 143). Few studies of pindolol, a medication with potent presynaptic 5-HT1A antagonism, have been performed, but we found some evidence for efficacy in a recent meta-analysis (144). Although the overall results of this meta-analysis were statistically significant, results were equivocal when only placebo-controlled trials were examined (144).
In recent years, and parallel to the finding of a likely genetic influence of glutamate transporter polymorphisms, medications operating through the glutamate system have received increasing attention. One theory to explain the reduced volumes of cortical and subcortical structures reported on neuroimaging of OCD is that hypofunctioning glutamate transporters lead to increased extracellular glutamate concentrations, which in turn cause excitotoxicity and cell death in adjacent neurons (145, 146). Memantine is the best studied of the medications involving the glutamate system. To date, three observational studies (147–149) and one randomized placebo-controlled trial (150) have examined memantine for the treatment of OCD, all with positive results. Although its mechanism of action is not yet completely understood but likely reduces presynaptic release of glutamate (151), riluzole has shown promise in two open-label case studies (152, 153). N-acetylcysteine, an amino acid compound with complex actions via the glutamate neurotransmitter system, has shown some promise as a treatment of trichotillomania, and a recent case report suggests that this agent may be useful in OCD as well (154). Ketamine, a noncompetitive N-methyl-d-aspartic acid (NMDA) glutamate receptor antagonist, was associated with a small reduction of OCD symptoms in an open-label trial (155). Three studies have examined d-cycloserine, a partial agonist at the glycine unit of the NMDA receptor, as an agent to hasten response to ERP therapy (156–158). One of these studies found a positive effect, although this effect was not sustained to the end of therapy (158). Glycine itself was shown to have efficacy in a randomized, double-blind, placebo-controlled trial; however, it appeared to be poorly tolerated because of nausea and/or aversive taste (159). A full discussion of glutamate and glycine is beyond the scope of this article, and we refer the reader to chapters 2, 3, and 4 of Stahl (160) for a review.
Monoamine oxidase inhibitors have not been studied extensively as a treatment of OCD. Phenelzine was found to be effective in an open-label case series of patients with OCD and comorbid phobia and anxiety (161), yet it was shown to be inferior to fluoxetine in a randomized placebo-controlled study (162). An earlier double-blind study found equal efficacy for clomipramine and phenelzine (163).
Ondansetron, a 5-HT3 antagonist, showed some efficacy in a randomized, placebo-controlled trial of fluoxetine augmentation (164) and in an open-label study (165). However, as noted in the update of the APA practice guidelines (97), the result of a placebo-controlled trial sponsored by the manufacturer of ondansetron was negative. Ondansetron may be particularly useful as an augmentation strategy when nausea associated with serotonergic medication is present.
Many additional treatment strategies have been attempted. In brief, lamotrigine (166), topiramate (167, 168), oral morphine (169), tramadol (170), inositol (171), d-amphetamine (172), and caffeine (173, 174) are agents notable in having shown some amount of efficacy over placebo or active control in RCTs. Duloxetine has not been studied extensively but was found to reduce OCD symptoms in a case series of four patients whose condition had failed treatment with serotonin reuptake inhibitors (175). Bupropion also has not been studied extensively in OCD; however, an open-label trial did not find this medication to be effective (176).
Benzodiazepine Use in OCD
Benzodiazepines are considered a helpful adjunctive medication for many anxiety disorders; however, they are not currently recommended by APA for the treatment of OCD because of a lack of evidence for efficacy (97). Research on benzodiazepines in OCD is limited. Clonazepam is the best studied of this class of medication, and mixed results have been found for monotherapy of OCD (177, 178). Clonazepam augmentation yielded no additional benefit over sertraline monotherapy in a single RCT examining clonazepam as an augmentation strategy (179).
Also in This Issue
As mentioned earlier, OCD and related disorders have been given a separate chapter in DSM-5. One of the goals of this issue of Focus is to compare and contrast these disorders in order to assist clinicians with accurate diagnosis and treatment. Drs. Steketee and Bratiotis discuss hoarding, Dr. Phillips discusses body dysmorphic disorder, and Drs. Grant and Chamberlain discuss skin picking and trichotillomania.
The genetic effects underlying mental disorders have received increasing focus. In this issue, Drs. Sobell, Pato, Pato, and Knowles discuss intriguing findings from genetic studies of OCD.
Psychotherapy remains an important component of OCD treatment. This topic is given attention in this issue with a review of CBT as an effective singular treatment or in combination with pharmacotherapy. Psychoeducation is an important component of the psychotherapeutic process. Educational resources for patients and families are provided in the box at the end of this article.
OCD, hoarding, and body dysmorphic disorder: International OCD Foundation (iocdf.org)
OCD: Beyond OCD (beyondocd.org)
Trichotillomania and skin picking: Trichotillomania Learning Center (www.trich.org)
Tourette’s syndrome: International Tourette Syndrome Association (www.tsa-usa.org)
Conclusion
OCD is a disorder that has undergone significant revision and study since it was first recognized as “obsessive compulsive neurosis” in the first edition of DSM. This definition was modified to “obsessive-compulsive reaction” in DSM-II and became the more familiar “obsessive-compulsive disorder” DSM-III. Now, OCD and related disorders have been given their own chapter in DSM-5, reflecting mental health practitioners’ increasing comprehension of symptom dimensions and factors moderating treatment and prognosis. With our increasing understanding comes a greater ability to effectively diagnose and treat individuals with these disorders. As we further our studies, we are hopeful that new diagnostic features and treatment strategies will continue to develop. In this issue of Focus, we provide an overview of what is currently known and a view of what is on the horizon.
1 : Six-month prevalence of psychiatric disorders in three communities 1980 to 1982. Arch Gen Psychiatry 1984; 41:959–967Crossref, Google Scholar
2 : The epidemiology of obsessive-compulsive disorder in five US communities. Arch Gen Psychiatry 1988; 45:1094–1099Crossref, Google Scholar
3 : The cross national epidemiology of obsessive compulsive disorder. J Clin Psychiatry 1994; 55(Suppl):5–10Google Scholar
4 : Size and burden of mental disorders in Europe—a critical review and appraisal of 27 studies. Eur Neuropsychopharmacol 2005; 15:357–376Crossref, Google Scholar
5 Obsessive-compulsive and related disorders; in Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, Va, , 2013Google Scholar
6 : DSM-IV field trial: obsessive-compulsive disorder. Am J Psychiatry 1995; 152:90–96Crossref, Google Scholar
7 : Obsessive-compulsive disorder with poor insight. Compr Psychiatry 2002; 43:150–157Crossref, Google Scholar
8 : Clinical characteristics and treatment response in poor and good insight obsessive-compulsive disorder. Eur Psychiatry 2004; 19:202–208Crossref, Google Scholar
9 : Obsessive-compulsive disorder with poor insight: a three-year prospective study. Prog Neuropsychopharmacol Biol Psychiatry 2010; 34:323–330Crossref, Google Scholar
10 : Dimensional correlates of poor insight in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35:1677–1681Crossref, Google Scholar
11 : Clinical picture of obsessive-compulsive disorder with poor insight: a regression model. Psychiatry Res 2005; 136:223–231Crossref, Google Scholar
12 : Comparison of clinical characteristics in good and poor insight obsessive-compulsive disorder. J Anxiety Disord 2002; 16:413–423Crossref, Google Scholar
13 : The Yale-Brown Obsessive-Compulsive Scale. I. Development, use, and reliability. Arch Gen Psychiatry 1989; 46:1006–1011Crossref, Google Scholar
14 : The Brown Assessment of Beliefs Scale: reliability and validity. Am J Psychiatry 1998; 155:102–108Crossref, Google Scholar
15 : A comparison of insight in body dysmorphic disorder and obsessive-compulsive disorder. J Psychiatr Res 2012; 46:1293–1299Crossref, Google Scholar
16 : Obsessive-compulsive disorder comorbidity: clinical assessment and therapeutic implications. Front Psychiatry 2011; 2:70Crossref, Google Scholar
17 : A family study of obsessive-compulsive disorder. Arch Gen Psychiatry 2000; 57:358–363Crossref, Google Scholar
18 : The familial phenotype of obsessive-compulsive disorder in relation to tic disorders: the Hopkins OCD family study. Biol Psychiatry 2001; 50:559–565Crossref, Google Scholar
19 : Obsessive-compulsive disorder: a review of the diagnostic criteria and possible subtypes and dimensional specifiers for DSM-V. Depress Anxiety 2010; 27:507–527Crossref, Google Scholar
20 : Haloperidol addition in fluvoxamine-refractory obsessive-compulsive disorder. A double-blind, placebo-controlled study in patients with and without tics. Arch Gen Psychiatry 1994; 51:302–308Crossref, Google Scholar
21 : Limited therapeutic effect of addition of buspirone in fluvoxamine-refractory obsessive-compulsive disorder. Am J Psychiatry 1993; 150:647–649Crossref, Google Scholar
22 : How to treat OCD in patients with Tourette syndrome. J Psychosom Res 2003; 55:49–57Crossref, Google Scholar
23 : Effect of comorbid tics on a clinically meaningful response to 8-week open-label trial of fluoxetine in obsessive compulsive disorder. J Psychiatr Res 2007; 41:332–337Crossref, Google Scholar
24 : Comorbid obsessive-compulsive disorder and Tourette syndrome: therapeutic interventions. CNS Drugs 2000; 13:173–183Crossref, Google Scholar
25 : A systematic review: antipsychotic augmentation with treatment refractory obsessive-compulsive disorder. Mol Psychiatry 2006; 11:622–632Crossref, Google Scholar
26 : How prevalent are anxiety disorders in schizophrenia? A meta-analysis and critical review on a significant association. Schizophr Bull 2011; 37:811–821Crossref, Google Scholar
27 : Cognitive dysfunctions in patients with obsessive-compulsive disorder compared to the patients with schizophrenia patients: relation to overvalued ideas. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31:254–261Crossref, Google Scholar
28 : Schizophrenia with obsessive-compulsive disorder and obsessive-compulsive disorder with poor insight: a neuropsychological comparison. Psychiatry Res 2009; 165:38–46Crossref, Google Scholar
29 : Comorbidity in hoarding disorder. Depress Anxiety 2011; 28:876–884Crossref, Google Scholar
30 : Obsessive-compulsive spectrum disorders: a multidimensional approach. Psychiatr Clin North Am 2006; 29:343–351Crossref, Google Scholar
31 : Obsessive-compulsive disorder: prevalence, comorbidity, impact, and help-seeking in the British National Psychiatric Morbidity Survey of 2000. Am J Psychiatry 2006; 163:1978–1985Crossref, Google Scholar
32 : Treatment of childhood obsessive-compulsive disorder with clomipramine and desipramine in children and adolescents. A double-blind crossover comparison. Arch Gen Psychiatry 1989; 46:1088–1092Crossref, Google Scholar
33 : Juvenile-onset OCD: clinical features in children, adolescents and adults. Acta Psychiatr Scand 2008; 118:149–159Crossref, Google Scholar
34 : Isoforms of the neuronal glutamate transporter gene, SLC1A1/EAAC1, negatively modulate glutamate uptake: relevance to obsessive-compulsive disorder. Transl Psychiatry 2013; 3:e259Crossref, Google Scholar
35 : Glutamate transporter gene SLC1A1 associated with obsessive-compulsive disorder. Arch Gen Psychiatry 2006; 63:769–776Crossref, Google Scholar
36 : Localization of neuronal and glial glutamate transporters. Neuron 1994; 13:713–725Crossref, Google Scholar
37 : Differential synaptic localization of the glutamate transporter EAAC1 and glutamate receptor subunit GluR2 in the rat hippocampus. J Comp Neurol 2000; 418:255–269Crossref, Google Scholar
38 : Glutamate uptake. Prog Neurobiol 2001; 65:1–105Crossref, Google Scholar
39 : Mapping structural brain alterations in obsessive-compulsive disorder. Arch Gen Psychiatry 2004; 61:720–730Crossref, Google Scholar
40 : Gray matter structural alterations in obsessive-compulsive disorder: relationship to neuropsychological functions. Psychiatry Res 2008; 164:123–131Crossref, Google Scholar
41 : Gray matter structural alterations in psychotropic drug-naive pediatric obsessive-compulsive disorder: an optimized voxel-based morphometry study. Am J Psychiatry 2008; 165:1299–1307Crossref, Google Scholar
42 : Regional gray matter abnormalities in obsessive-compulsive disorder: a voxel-based morphometry study. Biol Psychiatry 2005; 58:479–487Crossref, Google Scholar
43 : Increased right caudate nucleus size in obsessive-compulsive disorder: detection with magnetic resonance imaging. Psychiatry Res 1992; 45:115–121Crossref, Google Scholar
44 : Volumetric MRI study of key brain regions implicated in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31:46–52Crossref, Google Scholar
45 : Reduced basal ganglia volumes in trichotillomania measured via morphometric magnetic resonance imaging. Biol Psychiatry 1997; 42:39–45Crossref, Google Scholar
46 : A short echo 1H spectroscopy and volumetric MRI study of the corpus striatum in patients with obsessive-compulsive disorder and comparison subjects. Am J Psychiatry 1998; 155:1584–1591Crossref, Google Scholar
47 : A manual and automated MRI study of anterior cingulate and orbito-frontal cortices, and caudate nucleus in obsessive-compulsive disorder: comparison with healthy controls and patients with schizophrenia. Psychiatry Res 2005; 138:99–113Crossref, Google Scholar
48 : Multicenter voxel-based morphometry mega-analysis of structural brain scans in obsessive-compulsive disorder. Am J Psychiatry 2014; 171:340–349Crossref, Google Scholar
49 : Meta-analysis of brain volume changes in obsessive-compulsive disorder. Biol Psychiatry 2009; 65:75–83Crossref, Google Scholar
50 : Gray matter alterations in obsessive-compulsive disorder: an anatomic likelihood estimation meta-analysis. Neuropsychopharmacology 2010; 35:686–691Crossref, Google Scholar
51 : Voxel-wise meta-analysis of grey matter changes in obsessive-compulsive disorder. Br J Psychiatry 2009; 195:393–402Crossref, Google Scholar
52 : White matter abnormalities in obsessive-compulsive disorder: a diffusion tensor imaging study. Arch Gen Psychiatry 2005; 62:782–790Crossref, Google Scholar
53 : Brain corticostriatal systems and the major clinical symptom dimensions of obsessive-compulsive disorder. Biol Psychiatry 2013; 73:321–328Crossref, Google Scholar
54 : Altered corticostriatal functional connectivity in obsessive-compulsive disorder. Arch Gen Psychiatry 2009; 66:1189–1200Crossref, Google Scholar
55 : Developmental alterations of frontal-striatal-thalamic connectivity in obsessive-compulsive disorder. J Am Acad Child Adolesc Psychiatry 2011; 50:938–948.e3Crossref, Google Scholar
56 : Corticostriatal functional connectivity in non-medicated patients with obsessive-compulsive disorder. Eur Psychiatry 2011; 26:463–469Crossref, Google Scholar
57 : Localization of cerebral functional deficits in patients with obsessive-compulsive disorder: a resting-state fMRI study. J Affect Disord 2012; 138:313–321Crossref, Google Scholar
58 : Abnormally high degree connectivity of the orbitofrontal cortex in obsessive-compulsive disorder. JAMA Psychiatry 2013; 70:619–629Crossref, Google Scholar
59 : Local cerebral glucose metabolic rates in obsessive-compulsive disorder. Patients treated with clomipramine. Arch Gen Psychiatry 1990; 47:840–848Crossref, Google Scholar
60 : Changes in metabolism of cerebral glucose after stereotactic leukotomy for refractory obsessive-compulsive disorder: a case report. J Neurol Neurosurg Psychiatry 1995; 58:502–505Crossref, Google Scholar
61 : Localized orbitofrontal and subcortical metabolic changes and predictors of response to paroxetine treatment in obsessive-compulsive disorder. Neuropsychopharmacology 1999; 21:683–693Crossref, Google Scholar
62 : Systematic changes in cerebral glucose metabolic rate after successful behavior modification treatment of obsessive-compulsive disorder. Arch Gen Psychiatry 1996; 53:109–113Crossref, Google Scholar
63 : Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Revisualization during pharmacotherapy. Arch Gen Psychiatry 1992; 49:690–694Crossref, Google Scholar
64 : Recurrent hyperperfusion in the right orbitofrontal cortex in obsessive-compulsive disorder. Prog Neuropsychopharmacol Biol Psychiatry 2008; 32:1082–1084Crossref, Google Scholar
65 : Regional cerebral blood flow measured during symptom provocation in obsessive-compulsive disorder using oxygen 15-labeled carbon dioxide and positron emission tomography. Arch Gen Psychiatry 1994; 51:62–70Crossref, Google Scholar
66 : Functional magnetic resonance imaging of symptom provocation in obsessive-compulsive disorder. Arch Gen Psychiatry 1996; 53:595–606Crossref, Google Scholar
67 : fMRI of neuronal activation with symptom provocation in unmedicated patients with obsessive compulsive disorder. J Psychiatr Res 2000; 34:317–324Crossref, Google Scholar
68 : A multidimensional model of obsessive-compulsive disorder. Am J Psychiatry 2005; 162:228–238Crossref, Google Scholar
69 : Symptom dimensions in obsessive-compulsive disorder: implications for the DSM-V. CNS Spectr 2007; 12:376–387, 400Crossref, Google Scholar
70 : To discard or not to discard: the neural basis of hoarding symptoms in obsessive-compulsive disorder. Mol Psychiatry 2009; 14:318–331Crossref, Google Scholar
71 : Neural responses to facial expressions of disgust but not fear are modulated by washing symptoms in OCD. Biol Psychiatry 2007; 61:1072–1080Crossref, Google Scholar
72 : Distinct neural correlates of washing, checking, and hoarding symptom dimensions in obsessive-compulsive disorder. Arch Gen Psychiatry 2004; 61:564–576Crossref, Google Scholar
73 : Amygdala activation and symptom dimensions in obsessive-compulsive disorder. Br J Psychiatry 2014; 204:61–68Crossref, Google Scholar
74 : The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain 2009; 132:853–868Crossref, Google Scholar
75 : Obsessive-compulsive symptom dimensions correlate to specific gray matter volumes in treatment-naïve patients. J Psychiatr Res 2012; 46:1635–1642Crossref, Google Scholar
76 : White matter structure and symptom dimensions in obsessive-compulsive disorder. J Psychiatr Res 2012; 46:264–270Crossref, Google Scholar
77 : Cerebellar morphology in Tourette syndrome and obsessive-compulsive disorder. Ann Neurol 2010; 67:479–487Crossref, Google Scholar
78 : Lateral frontal cortex volume reduction in Tourette syndrome revealed by VBM. BMC Neurosci 2012; 13:17Crossref, Google Scholar
79 : The neuropsychology of mood disorders. Curr Psychiatry Rep 2006; 8:458–463Crossref, Google Scholar
80 : Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 2008; 213:93–118Crossref, Google Scholar
81 : Cognitive inflexibility in obsessive-compulsive disorder and major depression is associated with distinct neural correlates. PLoS ONE 2013; 8:e59600Crossref, Google Scholar
82 : Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J Neurosurg 1967; 26:488–495Crossref, Google Scholar
83 : Prospective long-term follow-up of 44 patients who received cingulotomy for treatment-refractory obsessive-compulsive disorder. Am J Psychiatry 2002; 159:269–275Crossref, Google Scholar
84 : Cingulotomy for intractable obsessive-compulsive disorder. Prospective long-term follow-up of 18 patients. Arch Gen Psychiatry 1995; 52:384–392Crossref, Google Scholar
85 : Limbic system surgery for treatment-refractory obsessive-compulsive disorder: a prospective long-term follow-up of 64 patients. J Neurosurg 2013; 118:491–497Crossref, Google Scholar
86 : Radiosurgery for the treatment of psychiatric disorders: a review. World Neurosurg 2013; 80(S32):e1–e9Crossref, Google Scholar
87 : Gamma ventral capsulotomy for obsessive-compulsive disorder: a randomized clinical trial. JAMA Psychiatry 2014; 71:1066–1076Crossref, Google Scholar
88 : Three-year outcomes in deep brain stimulation for highly resistant obsessive-compulsive disorder. Neuropsychopharmacology 2006; 31:2384–2393Crossref, Google Scholar
89 : Deep brain stimulation of the ventral internal capsule/ventral striatum for obsessive-compulsive disorder: worldwide experience. Mol Psychiatry 2010; 15:64–79Crossref, Google Scholar
90 : Deep brain stimulation for intractable obsessive compulsive disorder: pilot study using a blinded, staggered-onset design. Biol Psychiatry 2010; 67:535–542Crossref, Google Scholar
91 : Deep brain stimulation for obsessive-compulsive disorder is associated with cortisol changes. Psychoneuroendocrinology 2013; 38:1455–1459Crossref, Google Scholar
92 : Deep brain stimulation in the treatment of obsessive-compulsive disorder. World Neurosurg 2013; 80:e245–e253Crossref, Google Scholar
93 : Deep brain stimulation for refractory obsessive-compulsive disorder. Biol Psychiatry 2005; 57:510–516Crossref, Google Scholar
94 : Subthalamic nucleus stimulation in severe obsessive-compulsive disorder. N Engl J Med 2008; 359:2121–2134Crossref, Google Scholar
95 : Decrease of prefrontal metabolism after subthalamic stimulation in obsessive-compulsive disorder: a positron emission tomography study. Biol Psychiatry 2010; 68:1016–1022Crossref, Google Scholar
96 : Practice guideline for the treatment of patients with obsessive-compulsive disorder. Am J Psychiatry 2007; 164(Suppl):5–53Google Scholar
97 : American Psychiatric Association. Guideline Watch (March 2013): Practice Guideline for the Treatment of Patients With Obsessive-Compulsive Disorder. Arlington, VA, American Psychiatric Association, 2013Google Scholar
98 : Selective serotonin re-uptake inhibitors (SSRIs) versus placebo for obsessive compulsive disorder (OCD). Cochrane Database Syst Rev 2008; 1:CD001765Google Scholar
99 : A double-blind, placebo-controlled study of the efficacy and safety of controlled-release fluvoxamine in patients with obsessive-compulsive disorder. J Clin Psychiatry 2003; 64:640–647Crossref, Google Scholar
100 : Open-label study of high (30 mg) and moderate (20 mg) dose escitalopram for the treatment of obsessive-compulsive disorder. Int Clin Psychopharmacol 2009; 24:306–311Crossref, Google Scholar
101 : High-dose escitalopram for the treatment of obsessive-compulsive disorder. Int Clin Psychopharmacol 2008; 23:49–53Crossref, Google Scholar
102 : Escitalopram in obsessive-compulsive disorder: a randomized, placebo-controlled, paroxetine-referenced, fixed-dose, 24-week study. Curr Med Res Opin 2007; 23:701–711Crossref, Google Scholar
103 : Escitalopram in obsessive-compulsive disorder: response of symptom dimensions to pharmacotherapy. CNS Spectr 2008; 13:492–498Crossref, Google Scholar
104 : Evaluation of the FDA warning against prescribing citalopram at doses exceeding 40 mg. Am J Psychiatry 2013; 170:642–650Crossref, Google Scholar
105 Medicines and Healthcare Products Regulatory Agency: Citalopram and escitalopram: QT interval prolongation — new maximum daily dose restrictions (including in elderly patients), contraindications, and warnings. Drug Safety Update. 2011; 5:A1 www.gov.uk/drug-safety-update/citalopram-and-escitalopram-qt-interval-prolongationGoogle Scholar
106 : Fluvoxamine versus clomipramine in the treatment of obsessive compulsive disorder: a multicenter, randomized, double-blind, parallel group comparison. J Clin Psychiatry 1994; 55:301–305Google Scholar
107 : Fluvoxamine versus clomipramine for obsessive-compulsive disorder: a double-blind comparison. J Clin Psychopharmacol 1996; 16:121–129Crossref, Google Scholar
108 : Multicentre, double-blind, comparison of fluvoxamine and clomipramine in the treatment of obsessive-compulsive disorder. Int Clin Psychopharmacol 2000; 15:69–76Crossref, Google Scholar
109 : Paroxetine versus clomipramine in the treatment of obsessive-compulsive disorder. Br J Psychiatry 1996; 169:468–474Crossref, Google Scholar
110 : Weight gain during long-term treatment of obsessive-compulsive disorder: a prospective comparison between serotonin reuptake inhibitors. J Clin Psychiatry 2004; 65:1365–1371Crossref, Google Scholar
111 : Efficacy and tolerability of serotonin transport inhibitors in obsessive-compulsive disorder. A meta-analysis. Arch Gen Psychiatry 1995; 52:53–60Crossref, Google Scholar
112 : Clomipramine versus fluoxetine in obsessive-compulsive disorder: a retrospective comparison of side effects and efficacy. J Clin Psychopharmacol 1990; 10:122–124Crossref, Google Scholar
113 : Efficacy of drug treatment in obsessive-compulsive disorder. A meta-analytic review. Br J Psychiatry 1995; 166:424–443Crossref, Google Scholar
114 : Meta-analysis of pharmacotherapy trials for obsessive-compulsive disorder. Int Clin Psychopharmacol 1995; 10:11–18Crossref, Google Scholar
115 : Effectiveness of psychological and pharmacological treatments for obsessive-compulsive disorder: a quantitative review. J Consult Clin Psychol 1997; 65:44–52Crossref, Google Scholar
116 : Behavioral versus pharmacological treatments of obsessive compulsive disorder: a meta-analysis. Psychopharmacology (Berl) 1998; 136:205–216Crossref, Google Scholar
117 : Multivariate meta-analysis of controlled drug studies for obsessive-compulsive disorder. J Clin Psychopharmacol 2002; 22:309–317Crossref, Google Scholar
118 : Fluvoxamine in obsessive-compulsive disorder: similar efficacy but superior tolerability in comparison with clomipramine. Hum Psychopharmacol 2001; 16:461–468Crossref, Google Scholar
119 : A double-blind comparison of fluvoxamine and clomipramine in OCD. Eur Neuropsychopharmacol 1998; 8:260–261Crossref, Google Scholar
120 : High-dose selective serotonin reuptake inhibitors in OCD: a systematic retrospective case notes survey. J Psychopharmacol 2010; 24:1439–1445Crossref, Google Scholar
121 : Meta-analysis of the dose-response relationship of SSRI in obsessive-compulsive disorder. Mol Psychiatry 2010; 15:850–855Crossref, Google Scholar
122 : High-dose sertraline strategy for nonresponders to acute treatment for obsessive-compulsive disorder: a multicenter double-blind trial. J Clin Psychiatry 2006; 67:15–22Crossref, Google Scholar
123 : A 2012 evidence-based algorithm for the pharmacotherapy for obsessive-compulsive disorder. Curr Psychiatry Rep 2012; 14:211–219Crossref, Google Scholar
124 : Clinical predictors of drug response in obsessive-compulsive disorder. J Clin Psychopharmacol 2001; 21:488–492Crossref, Google Scholar
125 : Insight and treatment outcome in obsessive-compulsive disorder. Compr Psychiatry 2001; 42:494–497Crossref, Google Scholar
126 : Clinical implications of insight assessment in obsessive-compulsive disorder. Compr Psychiatry 2008; 49:305–312Crossref, Google Scholar
127 : Antipsychotic augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a meta-analysis of double-blind, randomized, placebo-controlled trials. Int J Neuropsychopharmacol 2013; 16:557–574Crossref, Google Scholar
128 : Antipsychotic augmentation of serotonergic antidepressants in treatment-resistant obsessive-compulsive disorder: a meta-analysis of the randomized controlled trials. Eur Neuropsychopharmacol 2007; 17:79–93Crossref, Google Scholar
129 : Pharmacotherapy of obsessive-compulsive disorder: evidence-based treatment and beyond. Aust N Z J Psychiatry 2013; 47:121–141Crossref, Google Scholar
130 : Double-blind, placebo-controlled, pilot trial of paliperidone augmentation in serotonin reuptake inhibitor-resistant obsessive-compulsive disorder. J Clin Psychiatry 2013; 74:e527–e532Crossref, Google Scholar
131 , Ozen ME: Quetiapine and ziprasidone as adjuncts in treatment-resistant obsessive-compulsive disorder: a retrospective comparative study. Clin Drug Investig 2008; 28:439–442Crossref, Google Scholar
132 : Risperidone and haloperidol augmentation of serotonin reuptake inhibitors in refractory obsessive-compulsive disorder: a crossover study. J Clin Psychiatry 2005; 66:736–743Crossref, Google Scholar
133 : The comparison of aripiprazole and risperidone augmentation in selective serotonin reuptake inhibitor-refractory obsessive-compulsive disorder: a single-blind, randomised study. Hum Psychopharmacol 2011; 26:51–57Crossref, Google Scholar
134 : 8-week, single-blind, randomized trial comparing risperidone versus olanzapine augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder. Eur Neuropsychopharmacol 2008; 18:364–372Crossref, Google Scholar
135 : Response acceleration with mirtazapine augmentation of citalopram in obsessive-compulsive disorder patients without comorbid depression: a pilot study. J Clin Psychiatry 2004; 65:1394–1399Crossref, Google Scholar
136 : Mirtazapine for obsessive-compulsive disorder: an open trial followed by double-blind discontinuation. J Clin Psychiatry 2005; 66:515–520Crossref, Google Scholar
137 : Venlafaxine in obsessive-compulsive disorder. Arch Gen Psychiatry 1996; 53:653–654Crossref, Google Scholar
138 : A double blind comparison of venlafaxine and paroxetine in obsessive-compulsive disorder. J Clin Psychopharmacol 2003; 23:568–575Crossref, Google Scholar
139 : A double-blind switch study of paroxetine and venlafaxine in obsessive-compulsive disorder. J Clin Psychiatry 2004; 65:37–43Crossref, Google Scholar
140 : Venlafaxine versus clomipramine in the treatment of obsessive-compulsive disorder: a preliminary single-blind, 12-week, controlled study. J Clin Psychiatry 2002; 63:1004–1009Crossref, Google Scholar
141 : Controlled comparison of buspirone and clomipramine in obsessive-compulsive disorder. Am J Psychiatry 1991; 148:127–129Crossref, Google Scholar
142 : A double-blind study of adjuvant buspirone hydrochloride in clomipramine-treated patients with obsessive-compulsive disorder. J Clin Psychopharmacol 1992; 12:11–18Crossref, Google Scholar
143 : Double-blind study of adjuvant buspirone for fluoxetine-treated patients with obsessive-compulsive disorder. Am J Psychiatry 1993; 150:819–821Crossref, Google Scholar
144 SA, Pato MT: Pindolol augmentation of selective serotonin reuptake inhibitors and clomipramine for the treatment of obsessive-compulsive disorder: A meta-analysis. J Pharmacol Pharmacother 2015; 6:36–38Crossref, Google Scholar
145 : Excitatory amino acids as a final common pathway for neurologic disorders. N Engl J Med 1994; 330:613–622Crossref, Google Scholar
146 : Beyond the role of glutamate as a neurotransmitter. Nat Rev Neurosci 2002; 3:748–755Crossref, Google Scholar
147 : A single-blinded case-control study of memantine in severe obsessive-compulsive disorder. J Clin Psychopharmacol 2010; 30:34–39Crossref, Google Scholar
148 : Memantine augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial. J Clin Psychopharmacol 2009; 29:51–55Crossref, Google Scholar
149 : Differential efficacy of memantine for obsessive-compulsive disorder vs. generalized anxiety disorder: an open-label trial. Psychopharmacol Bull 2009; 42:81–93Google Scholar
150 : Memantine add-on in moderate to severe obsessive-compulsive disorder: randomized double-blind placebo-controlled study. J Psychiatr Res 2013; 47:175–180Crossref, Google Scholar
151 : Effects of riluzole on electrically evoked neurotransmitter release. Br J Pharmacol 2000; 130:1227–1234Crossref, Google Scholar
152 : Riluzole augmentation in treatment-refractory obsessive-compulsive disorder: a series of 13 cases, with long-term follow-up. J Clin Psychopharmacol 2008; 28:363–367Crossref, Google Scholar
153 : Riluzole augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial. Biol Psychiatry 2005; 58:424–428Crossref, Google Scholar
154 : N-acetylcysteine augmentation in serotonin reuptake inhibitor refractory obsessive-compulsive disorder. Psychopharmacology (Berl) 2006; 184:254–256Crossref, Google Scholar
155 : Effects of ketamine in treatment-refractory obsessive-compulsive disorder. Biol Psychiatry 2012; 72:964–970Crossref, Google Scholar
156 : D-cycloserine augmented exposure therapy for obsessive-compulsive disorder. Biol Psychiatry 2007; 62:835–838Crossref, Google Scholar
157 : D-cycloserine does not enhance exposure-response prevention therapy in obsessive-compulsive disorder. Int Clin Psychopharmacol 2007; 22:230–237Crossref, Google Scholar
158 : Augmentation of behavior therapy with D-cycloserine for obsessive-compulsive disorder. Am J Psychiatry 2008; 165:335–341, quiz 409Crossref, Google Scholar
159 : Adjunctive glycine in the treatment of obsessive-compulsive disorder in adults. J Psychiatr Res 2009; 43:664–670Crossref, Google Scholar
160 : Stahl’s Essential Pharmacology, 4th ed. New York, Cambridge University Press, 2013Google Scholar
161 : Monoamine oxidase inhibitors in obsessive-compulsive disorder. J Clin Psychiatry 1983; 44:131–132Google Scholar
162 : Placebo-controlled trial of fluoxetine and phenelzine for obsessive-compulsive disorder. Am J Psychiatry 1997; 154:1261–1264Crossref, Google Scholar
163 : Clomipramine versus phenelzine in obsessive-compulsive disorder. A controlled clinical trial. Br J Psychiatry 1992; 161:665–670Crossref, Google Scholar
164 : A double-blind, placebo-controlled pilot study of ondansetron for patients with obsessive-compulsive disorder. Hum Psychopharmacol 2010; 25:509–513Crossref, Google Scholar
165 : Ondansetron augmentation in patients with obsessive-compulsive disorder who are inadequate responders to serotonin reuptake inhibitors: improvement with treatment and worsening following discontinuation. Eur Neuropsychopharmacol 2014; 24:375–380Crossref, Google Scholar
166 : Lamotrigine augmentation of serotonin reuptake inhibitors in treatment-resistant obsessive-compulsive disorder: a double-blind, placebo-controlled study. J Psychopharmacol 2012; 26:1456–1462Crossref, Google Scholar
167 : Topiramate augmentation in resistant OCD: A double-blind placebo-controlled clinical trial. CNS Spectr 2010; 15:613–617Crossref, Google Scholar
168 : Double-blind, placebo-controlled trial of topiramate augmentation in treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry 2011; 72:716–721Crossref, Google Scholar
169 : Double-blind treatment with oral morphine in treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry 2005; 66:353–359Crossref, Google Scholar
170 : Open-label pilot study of tramadol hydrochloride in treatment-refractory obsessive-compulsive disorder. Depress Anxiety 1997; 6:170–173Crossref, Google Scholar
171 : Inositol treatment of obsessive-compulsive disorder. Am J Psychiatry 1996; 153:1219–1221Crossref, Google Scholar
172 : D-amphetamine in obsessive-compulsive disorder. Psychopharmacology (Berl) 1983; 80:231–235Crossref, Google Scholar
173 : Acute psychostimulant challenge in primary obsessive-compulsive disorder. J Clin Psychopharmacol 1991; 11:237–241Crossref, Google Scholar
174 : Double-blind study of dextroamphetamine versus caffeine augmentation for treatment-resistant obsessive-compulsive disorder. J Clin Psychiatry 2009; 70:1530–1535Crossref, Google Scholar
175 : Switching from serotonin reuptake inhibitors to duloxetine in patients with resistant obsessive compulsive disorder: a case series. J Psychopharmacol 2008; 22:210–213Crossref, Google Scholar
176 : Bupropion for patients with obsessive-compulsive disorder: an open-label, fixed-dose study. J Clin Psychiatry 2005; 66:228–230Crossref, Google Scholar
177 : A double-blind, placebo-controlled trial of clonazepam in obsessive-compulsive disorder. World J Biol Psychiatry 2003; 4:30–34Crossref, Google Scholar
178 : Clomipramine, clonazepam, and clonidine treatment of obsessive-compulsive disorder. J Clin Psychopharmacol 1992; 12:420–430Crossref, Google Scholar
179 : A double-blind combination study of clonazepam with sertraline in obsessive-compulsive disorder. Ann Clin Psychiatry 2004; 16:127–132Crossref, Google Scholar



