Premenstrual Dysphoric Disorder: Burden of Illness and Treatment Update
Abstract
Five percent of menstruating women have severe premenstrual symptoms and impairment of functioning defined as premenstrual dysphoric disorder (PMDD). Clinically significant premenstrual symptoms occur in at least an additional 20% of menstruating women. The diagnosis of PMDD should be confirmed by prospective symptom charting over 2 menstrual cycles to confirm the timing of the symptoms and to rule out other diagnoses. The burden of illness of PMDD includes disruption of parenting and partner relationships and decreased productivity in work roles. In addition, women with PMDD have increased use of health care services such as clinician visits and increased use of prescription medications and over-the-counter preparations. The etiology of PMDD is multifactorial. In particular, dysregulation of the serotonin and allopregnanolone systems is implicated. Several effective treatment options exist, including serotonergic antidepressant medications and an oral contraceptive that contains ethinyl estradiol and drosperinone. In addition, other hormones that suppress ovulation, anxiolytics, cognitive therapy, chasteberry and calcium may be helpful.
(Reprinted with permission from J Psychiatry Neurosci 2008; 33(4):291–301)
Diagnosis
About 80% of women report at least mild premenstrual symptoms, 20%–50% report moderate-to-severe premenstrual symptoms, and about 5% report severe symptoms for several days with impairment of functioning (1). The 5% of women with the severest premenstrual symptoms and impairment of social and role functioning often meet the diagnostic criteria for premenstrual dysphoric disorder (PMDD). The diagnostic criteria for PMDD are listed in the appendix of the Diagnostic and Statistical Manual of Mental Disorders, fourth edition, text revision (DSM-IV-TR) (1), and women who meet criteria for PMDD receive a DSM-IV-TR diagnosis code 311 (i.e., depressive disorder not otherwise specified). To meet the PMDD criteria, at least 5 of 11 possible symptoms must be present in the premenstrual phase, these symptoms should be absent shortly after the onset of menses, and at least 1 of the 5 symptoms must be depressed mood, anxiety, affective lability or irritability. Other symptoms include decreased interest in usual activities, difficulty concentrating, low energy, changes in appetite, changes in sleep, a sense of being overwhelmed or out of control, headaches, joint or muscle pain, breast tenderness or swelling and abdominal bloating (1). Women with fewer or less severe symptoms are considered to have premenstrual syndrome (PMS). The American College of Obstetrics and Gynecology Practice Guidelines (2) and the International Classification of Diseases, 10th revision (3) both suggest diagnostic criteria for PMS that require a minimum of 1 premenstrual symptom.
Prospective daily rating of the PMDD criteria symptoms over 2 menstrual cycles is required to confirm the PMDD diagnosis. The daily ratings should document the timing of the symptoms during the premenstrual phase and the absence of symptoms or a chronic underlying disorder during the follicular phase. The retrospective reporting of premenstrual symptoms may amplify the woman's recall of the severity and frequency of symptoms. Reporting of symptoms can be influenced by the phase of the menstrual cycle when queried, the phrasing of questions, expectations and cultural issues (4). Studies conducted over the past 2 decades have used various scoring methods and different instruments for daily ratings to measure the premenstrual increase of symptoms. Recent studies have used visual analog scales (5) and Likert scale daily rating forms such as the Daily Record of Severity of Problems (6) or the Penn Daily Symptom Report (7). Various scoring methods compare the average of symptom scores during the premenstrual days with the average of symptom scores postmenses. The DSM-IV-TR PMDD criteria state that the premenstrual symptoms should not be an exacerbation of an underlying disorder but that PMDD could be superimposed on an axis I or II disorder (1).
Epidemiology
Three studies in which subjects prospectively rated their symptoms reported PMDD prevalence rates of 4.6%–6.4% (8–10). Two studies of women who retrospectively rated their premenstrual symptoms according to PMDD criteria reported similar prevalence rates of 5.1%–6.7% (11, 12). These 2 studies reported that 18.6%–20.7% of women have “subthreshold PMDD,” in which the full criteria are not met because they have fewer than 5 symptoms or because they fail to meet the functional impairment criterion. Even though premenstrual symptoms are described in women from menarche to menopause, it is unclear whether symptoms remain stable or increase in severity with age (12, 13). Irritability has been identified as the most common premenstrual symptom in US and European samples (13–15). Some cultures emphasize somatic rather than emotional premenstrual symptoms (16). Symptom severity peaks on or just before the first day of menses (4, 10, 15). Studies examining age, menstrual cycle characteristics, cognitive attributions, socioeconomic variables, lifestyle variables and number of children have not identified these variables as predisposing factors (13, 17). An elevated lifetime prevalence of major depressive disorder (MDD) in women with PMDD has been reported in several studies, (18) as has an elevated lifetime prevalence of postpartum depression (19).
A recent study is the first to demonstrate allelic variation on the estrogen receptor α gene in women with PMDD when compared with control women (20). In addition, the allelic variation was only significant in women who had a valine/valine genotype for the catechol-O-methyltransferase enzyme. This significant study may identify a source of abnormal estrogen signalling during the luteal phase that leads to premenstrual affective, cognitive and somatic symptoms (20). Previous studies had failed to identify gene polymorphism differences between women with PMDD and control subjects in regard to the serotonin transporter (21, 22), the transcription factor activating protein 2 (23), tryptophan hydroxylase and monoamine oxidase A promoter (22) genes. Overlap of PMDD with genetic liability for MDD, seasonal affective disorder and personality characteristics has been suggested (24, 25).
The diagnosis of PMDD requires the confirmation of luteal-phase impairment of social and/or work functioning that is due to premenstrual symptoms. The functional impairment reported by women with PMDD is similar in severity to the impairment reported in MDD and dysthymic disorder (26, 27). One study identified anxiety, irritability and mood lability as the premenstrual symptoms most associated with functional impairment (28). The burden of illness of PMDD results from the severity of symptoms, the chronicity of the disorder and the impairment in work, relationships and activities (29). It has been estimated that women with PMDD cumulatively endure 3.8 years of disability over their reproductive years (26). A study of 1194 women who prospectively rated their symptoms reported that women with PMDD were more likely to endorse hours missed from work, impaired productivity, role limitations and less effectiveness (30). Borenstein and colleagues (31, 32) have published studies examining functioning and health service use in 436 women who prospectively charted their symptoms for 2 cycles. Women with confirmed PMS reported significantly lower quality of life, increased absenteeism from work, decreased work productivity, impaired relationships with others and increased visits to health providers, compared with control women. These authors also reported that, given a 14% absenteeism rate and a 15% reduction in productivity, PMDD was associated with US$4333 indirect costs per patient per year (33). The economic burden associated with PMDD is more related to self-reported decreased productivity than to direct health care costs (30, 33). However, women with PMDD do report increased health services use, with visits to health care providers and use of prescription medications and alternative therapies (30, 32). Small studies of women with prospectively confirmed PMDD have also reported decreased interpersonal and work functioning and reduced quality of life in comparison with women without PMDD (34–36). Larger studies of women diagnosed retrospectively according to PMDD criteria have also reported substantial functional impairment in work and interpersonal roles (11, 12, 37, 38).
Differential diagnosis
Because most PMDD symptoms are affective or anxiety-related, PMDD should generally not be diagnosed when an underlying depression or anxiety disorder is present; many such women are considered to have premenstrual exacerbation of their underlying depression or anxiety disorder. A recent study reported that 64% of the first 1500 women with MDD enrolled in the STAR*D study retrospectively reported premenstrual exacerbation of their depressive symptoms (39). Dysthymia, MDD, panic disorder and generalized anxiety disorder are the most common axis I psychiatric disorders that may be concurrent and exacerbated premenstrually, with less clear evidence for bipolar disorder, posttraumatic stress disorder, social phobia, eating disorders and substance abuse (12, 18, 40–43). Personality disorders do not have elevated prevalence in women with PMDD (19), but women with PMDD and a personality disorder may demonstrate premenstrual phase amplification of personality dysfunction (44). Schizophrenia may be an example of an underlying disorder that does not have premenstrual exacerbation of psychotic symptoms but may have superimposed affective and anxiety symptoms of PMDD (45). The prevalence of premenstrually exacerbated axis I disorders is unknown. It is generally recommended that clinicians treat the underlying disorder first, and if premenstrual symptoms persist, subsequent daily ratings can identify PMDD (46).
Symptoms of endometriosis, polycystic ovary disease, thyroid disorders, adrenal system disorders, hyperprolactinemia and panhypopituitarism may mimic symptoms of PMS. Medical disorders that may demonstrate a premenstrual increase in symptoms include migraines, asthma, epilepsy, irritable bowel syndrome, diabetes, allergies and autoimmune disorders (42, 47). It is presumed that the menstrual cycle fluctuations of gonadal hormones influence some of the symptoms of these medical conditions.
Etiology
Several reviews exist of pathophysiological hypotheses of PMS and PMDD and the evidence for them (48–52). Most studies do not identify consistent abnormalities in hormones of the hypothalamic-pituitary-gonadal (HPG) axis, although a few studies have suggested altered luteinizing hormone (LH) pulse (53). As well, studies have not identified clear abnormalities in thyroid hormones, cortisol, prolactin, glucose, prostaglandins, β-endorphins, vitamins or electrolytes (49, 50). Since specific abnormalities in the HPG axis have not been identified in women with PMDD when compared with control subjects, it is thought that premenstrual symptoms occur as a result of a differential sensitivity to the mood-perturbing effects of gonadal steroid fluctuations in women with PMS and PMDD (54). It is probable that the etiology of the “differential sensitivity” is multifactorial and in part genetically determined (55). The recently identified allelic variation in the estrogen receptor α gene in women with PMDD may underlie the neurotransmitter and neuropeptide differential sensitivity owing to estrogen receptor influence on synthesis, receptors, transporters and cell signalling (20).
Although the specific neurotransmitter, neuroendocrine and neurosteroid abnormalities in women with PMDD are not known, serotonin, norepinephrine, γ-aminobutyric acid (GABA), allopregnanolone (ALLO, an anxiolytic metabolite of progesterone that acts at the GABAA receptor), endorphins and factors involved in calcium homeostasis may all be involved. Women with PMDD are more sensitive to the anxiogenic properties of carbon dioxide inhalation, lactate infusion, cholecystokinin tetrapeptide and flumazenil (49). Increased adrenergic receptor binding may reflect abnormal noradrenergic function (49). Women with PMDD have abnormal melatonin secretion and other circadian system abnormalities (56). It has been proposed that women with PMDD have altered affective information processing and regulation during the luteal phase, with abnormal activation patterns in specific brain regions (55). Imaging studies have demonstrated altered functional magnetic resonance imaging responses to negative and positive stimuli in the orbitofrontal cortex, amygdala and ventral striatum in women with PMDD compared with control subjects (57). A recent study reported significantly increased negative bias in the recognition of emotional facial expressions during the luteal phase in women with PMDD when compared with control women (58).
Many studies have identified various abnormalities in the serotonin system in women with PMS and PMDD. These include abnormal levels of whole blood serotonin, serotonin platelet uptake and platelet tritiated imipramine binding; abnormal responses to serotonergic probes such as l-tryptophan, buspirone, metachlorophenylpiperazine and fenfluramine; and exacerbation of premenstrual symptoms after metergoline or tryptophan depletion (49, 51, 59). Several studies have also suggested that women with PMDD have decreased luteal-phase levels of GABA, abnormal ALLO levels and decreased luteal-phase sensitivity of the GABAA receptor, as shown by flumazenil challenge and the sedative and saccadic eye velocity responses to benzodiazepines and alcohol (48, 49, 52, 60). Imaging studies have reported altered serotonin function (61, 62) and altered GABAergic function (63, 64) in women with PMDD when compared with healthy control subjects. It is possible that the rapid efficacy of selective serotonin reuptake inhibitors (SSRIs) in PMDD may be due in part to their ability to increase ALLO levels in the brain and enhance GABAA receptor function (65). Alternative hypotheses for the fast action of SSRIs in PMDD include enhanced function of 5-HT2C receptors (66) and inhibition of the serotonin transporter with resulting decreased LH production (67). It has also been hypothesized that the increase in ALLO after SSRI administration underlies the improvement in depressive symptoms of MDD in both sexes (68–70).
Treatment
The most systematically studied treatments have been the elimination of hormonal fluctuations with ovulation suppression treatments or the “correction” of neurotransmitter dysregulation with antidepressant or anxiolytic medications. Other treatments have targeted putative vitamin or mineral deficiencies or the improvement of specific premenstrual symptoms (Box 1). Different treatment modalities may differentially treat specific symptom profiles, but treatment studies have rarely evaluated efficacy for specific anxiety, mood, behavioural and somatic symptom profiles (71).
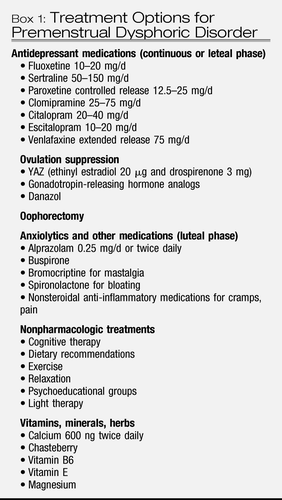 |
Box 1: Treatment Options for Premenstrual Dysphoric Disorder
Oral contraceptives
Oral contraceptives (OCs) have been commonly prescribed by gynecologists and primary care clinicians for the treatment of PMS even though there were few studies demonstrating their efficacy until recently. Two older randomized controlled trials (RCTs) in samples of women with prospectively confirmed PMS reported a lack of efficacy with monophasic and triphasic OCs (72, 73). Surveys of population cohorts without defined PMS or PMDD have reported that OCs do not alter mood in most women, but a subset of women report improvement of premenstrual symptoms, and another subset of women report the production of negative premenstrual symptoms (74, 75). After the introduction in the late 1990s of an OC containing ethinyl estradiol 30 μg and a unique progesterone, drospirenone 3 mg, improved mood and quality of life during the luteal phase began to be reported in nonclinical population cohorts (60, 76, 77). In addition, a 6-month extended regimen of this OC was reported to be associated with fewer premenstrual emotional and physical symptoms than the normal 21/7 monthly administration in women not seeking care for problematic premenstrual symptoms (78). An RCT comparing the OC containing ethinyl estradiol 30 μg and drospirenone 3 mg with placebo in 82 women with PMDD reported that both the OC and placebo improved most premenstrual symptoms and that the OC was significantly more effective than placebo in decreasing food cravings, increased appetite and acne only (79). However, 2 recent studies of YAZ (Bayer HealthCare), an OC containing ethinyl estradiol 20 μg and drospirenone 3 mg, administered as 24 days of active pills followed by a 4-day hormone-free interval (24/4), have reported superiority in reducing premenstrual emotional and physical symptoms when compared with placebo (80, 81).
Yonkers and colleagues (80) reported on a parallel design study in which YAZ or placebo was administered to 450 women with PMDD over 3 months. Pearlstein and colleagues (81) reported on a crossover design study in which YAZ or placebo was administered to 64 women with PMDD over 7 months with a middle washout cycle. Both studies reported that self-rated symptom, functioning and quality of life measures, along with clinician-rated symptom and functioning measures, all significantly improved with YAZ in comparison with placebo. In both studies, adverse effects that were more common with YAZ, compared with placebo, included nausea, intermenstrual bleeding and breast pain (80, 81). In 2006, YAZ received United States Food and Drug Administration (FDA) approval for the treatment of PMDD in women desiring oral contraception. The efficacy of this particular OC for reducing premenstrual symptoms may be due to its administration in a 24/4 regimen, which provides more stable hormone levels and reduces adverse symptoms that can occur during withdrawal bleeding (82, 83). Differential efficacy of this OC may also be due to the unique antimineralocorticoid and antiandrogenic properties of drospirenone (77, 80, 81).
Other ovulation suppression treatments
Gonadotropin-releasing hormone (GnRH) agonists suppress ovulation by downregulating GnRH receptors in the hypothalamus, leading to decreased follicle-stimulating hormone (FSH) and LH release from the pituitary resulting in decreased estrogen and progesterone levels. GnRH agonists are administered parenterally (e.g., subcutaneous monthly injections of goserelin, intramuscular monthly injections of leuprolide, daily intranasal buserelin). GnRH agonists have been reported to be superior to placebo in 8 of 10 published RCTs in women with PMS or PMDD (48, 84). A meta-analysis of 5 of these studies reported an odds ratio of 8.66 that GnRH agonists will lead to improvement in premenstrual emotional and physical symptoms, compared with placebo (85). “Add-back” hormone strategies have been investigated to counteract the undesirable medical consequences of hypoestrogenism resulting from prolonged anovulation induced by GnRH agonists. The meta-analysis concluded that add-back estrogen and progesterone did not reduce the efficacy of GnRH agonists (85) even though it had been reported that the add-back of estrogen and progesterone to goserelin (86) and leuprolide (54) led to the reappearance of mood and anxiety symptoms in some women with severe PMS. Since women with severe PMS and PMDD have an abnormal response to normal hormonal fluctuations (54), it is not a surprise that some women may experience the induction of mood and anxiety symptoms from the addition of gonadal steroids, which reduces the benefit of the replacement strategy. Suggested replacement strategies have also included low-dose pulsed progesterone (87) and tibolone, a synthetic steroid with estrogenic, androgenic and progestogenic properties (88). The safety of the long-term use of GnRH agonists and replacement hormones is unknown.
Oophorectomy and prolonged anovulation from danazol, estrogen or progesterone administered throughout the cycle are not common treatments, largely because of the medical risks attendant on a prolonged hypoestrogenic state, which leads to the same long-term health issues as those arising from use of GnRH agonists. Danazol, a synthetic steroid, alleviates premenstrual symptoms when administered at dosages that induce anovulation (200–400 mg/d) (89). When danazol 200 mg daily was administered during the luteal phase only, which did not lead to anovulation, breast tenderness improved, but other premenstrual symptoms did not improve (90). The few studies on estrogen or progesterone administered for most of the cycle have yielded mixed reports (48, 91). Two small studies reported relief of intractable PMS with hysterectomy and bilateral oophorectomy (92, 93). Hysterectomy with oophorectomy should be considered a last-resort treatment option for women with severe PMDD that has not responded to standard treatments (94, 95).
Progesterone
The administration of luteal-phase progesterone (oral micronized tablet, sublingual tablet, vaginal suppositories) is one of the older treatments studied for PMS and is largely due to the early hypothesis that PMS might be caused by a progesterone deficiency. However, abnormal hormone levels have not been demonstrated in women with PMS or PMDD. A recent systematic review concluded that, owing to the methodological shortcomings of previous studies, it is not possible to establish whether progesterone is helpful or not helpful for PMS or PMDD (96). A previous systematic review of published RCTs of luteal-phase progesterone and progestogens reported that there was no clinically meaningful difference between all progesterone forms and placebo, although progesterone had a small statistically significant superiority over placebo (97). A slight advantage for oral micronized progesterone was noted, and the authors postulated that this might be due to the increase in ALLO levels derived from this form of progesterone.
Antidepressant medication
Treatment studies of SSRIs and PMDD have suggested efficacy rates similar to those found in treatment studies of SSRIs and MDD, with 60%–70% of women responding to SSRIs in comparison with about 30% of women responding to placebo, depending on how response is defined (71, 98). The SSRI dosages that are effective for PMDD are similar to or slightly lower than the dosages recommended for the treatment of MDD. A systematic review of RCTs of SSRIs reported that, compared with placebo, women with severe PMS or PMDD were about 7 times more likely to respond to SSRIs (99, 100). This review included 12 trials with continuous dosing (daily for the full cycle) of fluoxetine (101–107), sertraline (108, 109), paroxetine (110), citalopram (111) and fluvoxamine (112) and 4 trials with intermittent dosing (daily during the luteal phase, i.e., from ovulation to menses only) of sertraline (113–115) and citalopram (111). The systematic review of these 15 studies concluded that continuous and intermittent dosing had equivalent efficacy (99). Since this systematic review, one study of 167 women with severe PMS and PMDD reported that continuous and intermittent dosing of sertraline had equivalent efficacy and that each was superior to placebo (116). However, another study of 167 women with PMDD recently reported that, although both continuous and intermittent paroxetine were both superior to placebo for irritability, affect lability and mood swings, intermittent paroxetine was less effective than continuous paroxetine for depressed mood, low energy, food cravings and somatic premenstrual symptoms (117). A recent meta-analysis of 29 SSRI studies in 2964 women concluded that continuous dosing is more effective than intermittent dosing for treatment of PMS and PMDD (118).
The seminal studies involving continuous dosing of SSRIs in PMDD involved relatively large samples. One RCT compared fluoxetine 20 mg daily, fluoxetine 60 mg daily and placebo in 277 women over 6 months (101). Both fluoxetine 20 mg daily and 60 mg daily were more efficacious than placebo in reducing premenstrual emotional, behavioural and physical symptoms; however, the 20-mg dosage was better tolerated than the 60-mg dosage. This study was one of the first to note a fast onset of action and improvement in physical premenstrual symptoms with an SSRI (101). Another large RCT involved flexible-dose sertraline, up to 150 mg daily, over 3 months, and the average dose of 110 mg daily was more effective than placebo in reducing premenstrual symptoms (108) and improving psychosocial functioning in 243 women (27). Since this review, 2 large RCTs have demonstrated that, when compared with placebo over 3 months, continuous dosing of paroxetine controlled release (CR) 12.5 mg or 25 mg daily had superior efficacy in reducing premenstrual mood and physical symptoms in 313 (119) and 359 (120) women with PMDD. In both studies, paroxetine CR 25 mg daily was more effective than 12.5 mg daily in the improvement of premenstrual functioning and the reduction of premenstrual physical symptoms.
RCTs conducted subsequent to the review by Dimmock and colleagues (99) have also further established the efficacy of intermittent dosing of fluoxetine, sertraline and paroxetine. Luteal-phase fluoxetine 20 mg daily was superior to placebo in reducing premenstrual emotional and physical symptoms in 252 women with PMDD, whereas fluoxetine 10 mg daily during the luteal phase only was superior to placebo in reducing emotional but not physical symptoms (121). An RCT in 200 women with PMDD demonstrated that luteal-phase sertraline 50–100 mg daily was superior to placebo for improving premenstrual emotional symptoms and impaired functioning but not premenstrual physical symptoms (122). Another RCT, in 366 women with PMDD, reported that luteal-phase dosing of both paroxetine CR 12.5 mg and 25 mg daily was superior to placebo in reducing premenstrual symptoms and improving functioning (123). Studies involving intermittent dosing have reported the absence of discontinuation symptoms when fluoxetine, sertraline and paroxetine CR at the given dosages were abruptly stopped on the first day of menses. It has been hypothesized that the lack of withdrawal effects when the SSRI is discontinued at menses may be due to the lack of prolonged exposure (98). Even when the SSRI is abruptly discontinued on the first day of menses, the therapeutic action of intermittent dosing continues to relieve symptoms that extend into the first few days of menses (124). The FDA has approved the use of both continuous and intermittent dosing of fluoxetine, sertraline and paroxetine CR for women with PMDD, with specific dosage recommendations (Box 1). The first placebo-controlled RCT conducted with escitalopram recently reported that intermittent dosing with escitalopram 20 mg daily was superior to placebo in 158 women with PMDD (125).
The improvement of symptoms within the first treatment cycle in most continuous-dosing SSRI trials, as well as the efficacy of intermittent dosing, suggests a more rapid and different mechanism of action for SSRIs in PMDD as compared with MDD, where SSRIs may take 3–6 weeks to be effective. An increase in ALLO is one hypothesis to explain the rapid improvement of premenstrual symptoms with SSRIs. If an increase in ALLO is what makes an SSRI helpful for reducing symptoms, it may not be necessary to start the SSRI before or at ovulation. The few studies that have examined SSRI administration during the luteal phase, but after ovulation, have yielded mixed results. One study suggested that the administration of an SSRI the second luteal week only was not sufficient for efficacy. Fluoxetine 90 mg administered once 1 week before the expected onset of menses was not superior to placebo, but fluoxetine 90 mg administered 2 weeks and 1 week before the expected onset of menses was reported to be superior to placebo in reducing premenstrual emotional symptoms and improving premenstrual functioning but not premenstrual physical symptoms in 257 women with PMDD (126). Recently published RCTs have reported the efficacy of SSRIs for symptom-onset dosing of SSRIs, that is, administering SSRIs from the postovulatory day that premenstrual symptoms appear until menses. Preliminary studies in women with PMDD have suggested the superiority of symptom-onset paroxetine when compared with placebo (127) and the efficacy of both symptom-onset and luteal-phase escitalopram dosing, although luteal-phase dosing was found to be more effective for women with severe symptoms (128). Symptom-onset dosing of low-dose sertraline (25–50 mg/d) was reported to be superior to placebo in reducing premenstrual symptoms in 296 women with prospectively confirmed PMS (129).
Other antidepressants have also been studied in women with PMS and PMDD. Early RCTs reported efficacy with clomipramine (a tricyclic antidepressant with largely serotonergic action) in daily dosing (130) and in luteal-phase dosing (131). The dosages of clomipramine reported to be effective for PMS (25–75 mg/d) are lower than the expected effective doses for MDD. Daily dosing of immediate-release venlafaxine was reported to be superior to placebo in the reduction of the emotional and physical symptoms of PMDD in 157 women (132). One RCT reported that nefazodone was not superior to placebo for PMS (133). However, in a small crossover study, increasing nefazodone in the luteal phase was reported to improve premenstrual exacerbation of MDD (134). Three RCTs have compared SSRIs to nonserotonergic antidepressants and placebo, and each has reported specific efficacy of the SSRI over both placebo and the nonserotonergic antidepressant. Sertraline was compared with desipramine and placebo in 167 women with severe PMS or PMDD (109). Two smaller studies compared paroxetine to maprotiline and placebo (110) and fluoxetine to bupropion and placebo (107). The selective superiority of serotonergic antidepressants for PMDD is compatible with the postulated serotonin dysfunction in PMDD.
Many women report the recurrence of premenstrual symptoms after SSRI discontinuation, and many women choose to take SSRIs over the long term. Most SSRI trials have been 3 months in duration, and although a few open studies suggest maintenance of SSRI efficacy over 1–2 years, evidence-based long-term treatment recommendations do not exist. Studies are needed to identify whether or not some women develop tolerance to the SSRI over time, necessitating increases in dosage or switch to another medication, and whether or not some women stay in remission for a period of time after SSRI discontinuation. The short duration of most treatment studies has also not yielded information about potential adverse effects with long-term SSRI use. Anecdotally, many women report weight gain and sexual dysfunction after long-term antidepressant use for PMDD. Intermittent dosing may improve sexual function during the weeks that the SSRI is not taken, but it is not clear whether or not there is less weight gain with intermittent dosing. Systematic studies are needed of semi-intermittent dosing (‘bumping up’) of SSRIs in women with premenstrual exacerbation of an underlying depressive or anxiety disorder. In these women, the SSRI dosage is increased during the luteal phase and decreased back to the usual dosage at menses (46). Future studies are also indicated to systematically compare the efficacy and predictors of response to symptom-onset, luteal-phase and continuous daily SSRI dosing in women with PMDD.
Other medications
Alprazolam 0.25 mg daily or twice daily (administered during the luteal phase only) has been reported to be more effective than placebo in most studies (135–138) but not all (139). It is primarily effective for premenstrual tension and irritability, and overall, it has a lower efficacy rate than the SSRIs. If alprazolam has been taken for more than 1 luteal-phase week, it should be tapered over the first couple of days of menses. Some efficacy has been reported with buspirone (133, 140). Studies reporting initial efficacy with nonsteroidal anti-inflammatory drugs (141), doxycycline (142), naltrexone (143) and atenolol (144) deserve replication. Bromocriptine has been reported to decrease premenstrual breast tenderness (145), and one study reported that spironolactone decreased premenstrual emotional symptoms as well as physical symptoms (146).
Lifestyle modifications and psychosocial treatments
Lifestyle modifications that may alleviate premenstrual symptoms can be obtained through self-help materials or professional-led psychoeducation programs. A 4-session group intervention over 18 weeks emphasizing diet, exercise and a positive reframing of a woman's perceptions of her menstrual cycle was superior to a control condition in reducing premenstrual symptoms (147). In another intervention, 4 weekly peer support and professional guidance groups that included diet and exercise changes, environment modification, self-monitoring and other cognitive techniques were reported to be superior to a waitlist control condition in reducing premenstrual symptoms (148). It is unclear which lifestyle modifications are most helpful because few studies have been conducted on specific lifestyle modifications or psychosocial treatments.
Common dietary recommendations include increased consumption of complex carbohydrates, frequent snacks or meals, reduced consumption of refined sugar and artificial sweeteners and decreased caffeine intake. It is hypothesized that the ingestion of complex carbohydrates may maintain a steady serum glucose level that may decrease premenstrual food cravings and increase the availability of tryptophan in the brain for serotonin synthesis (84). Two RCTs that compared a beverage containing simple and complex carbohydrates with an isocaloric placebo beverage that did not increase tryptophan availability reported that the beverage containing simple and complex carbohydrates was superior in reducing premenstrual dysphoria and other symptoms (149, 150). These 2 studies are the only published RCTs of a specific dietary regimen.
Cognitive therapy (CT) is consistently reported to be an effective treatment for women with PMS. Two studies in women with prospectively confirmed PMS reported superiority of individual CT for reducing premenstrual symptoms when compared with a waitlist control condition (151) and when compared with information-focused therapy (152). Another RCT examined the relative efficacy of fluoxetine 20 mg daily, 10 individual CT sessions or their combination in women with PMDD (153, 154). Efficacy rates at 6 months were comparable between the 3 treatments; however, at 1 year women who had received CT were coping better than those who had received fluoxetine alone. Relaxation has had little systematic study in PMS; a single RCT reported that relaxation therapy was superior to both symptom charting and leisure reading (155). Exercise has not yet been tested in a sample of women with PMS or PMDD, but it is a frequently recommended treatment. Negative affect and other premenstrual symptoms improve with regular exercise in women in general (156, 157). Both aerobic and nonaerobic exercise may be helpful.
Dietary supplementation
Calcium 600 mg twice daily was compared with placebo in 466 women with PMDD (158). Calcium was reported to have a 48% efficacy rate for reducing premenstrual emotional and physical symptoms (except for fatigue and insomnia), compared with 30% for placebo. However, women with concurrent psychiatric illness were not clearly excluded and concurrent medications were allowed, except for analgesics. The efficacy of calcium was somewhat reduced in women who were taking OCs. The results of this study were notable, and calcium deserves further study.
A review of early studies of vitamin B6 reported a lack of efficacy for PMS (159). A meta-analysis of 9 controlled trials that included 940 women with PMS indicated only weak support for the efficacy of vitamin B6 (50–100 mg/d) in reducing premenstrual symptoms (160). A recent study reported that 80 mg daily of pyridoxine was superior to placebo in relieving anxiety and mood premenstrual symptoms but not physical symptoms (161). Two small RCTs have suggested the efficacy of magnesium (162, 163), but other studies have failed to document magnesium deficiency in women with PMDD or improvement of premenstrual symptoms with supplemental magnesium when compared with placebo (164). An RCT reported superior efficacy for trytophan, compared with placebo, in women with PMDD (165), and an early study that suggested efficacy for vitamin E deserves replication (166). Some trials have been conducted with multinutrients containing several vitamin and mineral components. Several of the supplements contained quantities of vitamins that exceeded daily recommended levels, and as reviewed, results from these studies have been mixed (167, 168). Physical premenstrual symptoms have been reported to improve with fish oil (169) and soy isoflavones (170).
Herbal, complementary and other treatments
Reviews of complementary treatments have reported that the strongest evidence exists for the benefit of chasteberry, or V. agnus castus (168, 171–174). It has been hypothesized that the benefit of chasteberry for premenstrual symptoms could be due to its being a dopamine agonist that possibly reduces FSH or prolactin levels (174). Chasteberry was recently reported to reduce premenstrual symptoms in an open study of 118 women with PMDD (175). Chasteberry and fluoxetine were both reported to be effective in an RCT of 41 women with PMDD; however, fluoxetine was better for emotional symptoms and chasteberry was better for physical symptoms (176). The evidence-based reviews concluded that RCTs have not suggested consistent benefit for Ginkgo biloba, evening primrose oil and homeopathic treatments but that initial positive RCTs suggest further study of massage (177), reflexology (178), chiropractic manipulation (179) and biofeedback (180). Small RCTs have reported that saffron (181) and Qi therapy (182) were each superior to placebo in women with prospectively confirmed PMS. There have been positive open reports, but no RCTs, with Hypericum (183, 184), yoga (185), guided imagery (186), photic stimulation (187) and acupuncture (188). It has been proposed that sleep deprivation and light therapy may decrease premenstrual dysphoria by correcting abnormal circadian rhythms found in women with PMDD (56). Although a crossover study reported that evening bright light for 2 premenstrual weeks decreased depression and tension (189), a meta-analysis of the few existing trials suggested a small effect size for bright light therapy (190).
Conclusion
Five percent of menstruating women meet criteria for PMDD, and about 20% of menstruating women have “sub-threshold PMDD” or severe PMS. Thus, in each menstrual cycle, 1 in 4 women have emotional, behavioural and physical premenstrual symptoms that can lead to disruption in interpersonal relationships and role functioning. Recent studies of the burden of illness of PMDD identify a high economic indirect cost, mostly from reduced productivity and effectiveness at work, in addition to disturbed parenting and marital relationships. Once the diagnosis of severe PMS or PMDD is confirmed by prospective symptom charting for 2 menstrual cycles, lifestyle modifications, cognitive therapy, chasteberry and calcium are reasonable initial nonpharmacologic options. If medication is necessary, SSRI medication is considered the first-line treatment (2, 46, 191). SSRI medication may be effective when administered continuously or intermittently (from ovulation to menses). With the recent FDA approval of the oral contraceptive YAZ for women with PMDD desiring oral contraception, there may now be an alternative first-line treatment. Second-line pharmacologic treatment options include changing to a second SSRI, switching to YAZ, extended OC regimens, considering GnRH agonists or adding adjunctive anxiolytics or other medications targeted to specific symptoms (2, 77, 191). Future studies are needed to determine predictors that will identify which women will benefit from SSRIs versus hormonal strategies and to determine the optimal duration for medication treatment.
1 American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed, text revision. Washington: The Association; 2000.Google Scholar
2 American College of Obstetrics and Gynecology. Premenstrual syndrome. ACOG practice bulletin. No. 15. Washington: The College; 2000. p 1–9.Google Scholar
3 World Health Organization. International Classification of Diseases, version 10. Geneva: WHO; 1993.Google Scholar
4 Meaden PM, Hartlage SA, Cook-Karr J. Timing and severity of symptoms associated with the menstrual cycle in a community-based sample in the Midwestern United States. Psychiatry Res 2005;134:27–36.Crossref, Google Scholar
5 Steiner M, Streiner DL, Steinberg S, et al. The measurement of premenstrual mood symptoms. J Affect Disord 1999;53:269–73.Crossref, Google Scholar
6 Endicott J, Nee J, Harrison W. Daily Record of Severity of Problems (DRSP): reliability and validity. Arch Womens Ment Health 2006;9:41–9.Crossref, Google Scholar
7 Freeman EW, DeRubeis RJ, Rickels K. Reliability and validity of a daily diary for premenstrual syndrome. Psychiatry Res 1996;65:97–106.Crossref, Google Scholar
8 Rivera-Tovar AD, Frank E. Late luteal phase dysphoric disorder in young women. Am J Psychiatry 1990;147:1634–6.Crossref, Google Scholar
9 Cohen LS, Soares CN, Otto MW, et al. Prevalence and predictors of premenstrual dysphoric disorder (PMDD) in older premenopausal women. The Harvard Study of Moods and Cycles. J Affect Disord 2002;70:125–32.Crossref, Google Scholar
10 Sternfeld B, Swindle R, Chawla A, et al. Severity of premenstrual symptoms in a health maintenance organization population. Obstet Gynecol 2002;99:1014–24.Crossref, Google Scholar
11 Steiner M, Macdougall M, Brown E. The premenstrual symptoms screening tool (PSST) for clinicians. Arch Womens Ment Health 2003; 6:203–9.Crossref, Google Scholar
12 Wittchen HU, Becker E, Lieb R, et al. Prevalence, incidence and stability of premenstrual dysphoric disorder in the community. Psychol Med 2002;32:119–32.Crossref, Google Scholar
13 Endicott J, Amsterdam J, Eriksson E, et al. Is premenstrual dysphoric disorder a distinct clinical entity? J Womens Health Gend Based Med 1999;8:663–79.Crossref, Google Scholar
14 Angst J, Sellaro R, Stolar M, et al. The epidemiology of perimenstrual psychological symptoms. Acta Psychiatr Scand 2001;104:110–6.Crossref, Google Scholar
15 Pearlstein T, Yonkers KA, Fayyad R, et al. Pretreatment pattern of symptom expression in premenstrual dysphoric disorder. J Affect Disord 2005;85:275–82.Crossref, Google Scholar
16 Halbreich U, Alarcon RD, Calil H, et al. Culturally-sensitive complaints of depressions and anxieties in women. J Affect Disord 2007;102:159–76.Crossref, Google Scholar
17 Steiner M, Born L. Advances in the diagnosis and treatment of premenstrual dysphoria. CNS Drugs 2000;13:287–304.Crossref, Google Scholar
18 Endicott J. Differential diagnoses and comorbidity. In: Gold JH, Severino SK, editors. Premenstrual dysphorias: myths and realities. Washington: American Psychiatric Press; 1994. p 3–17.Google Scholar
19 Critchlow DG, Bond AJ, Wingrove J. Mood disorder history and personality assessment in premenstrual dysphoric disorder. J Clin Psychiatry 2001;62:688–93.Crossref, Google Scholar
20 Huo L, Straub RE, Schmidt PJ, et al. Risk for premenstrual dysphoric disorder Is associated with genetic variation in ESR1, the estrogen receptor alpha gene. Biol Psychiatry 2007;62:925–33.Crossref, Google Scholar
21 Melke J, Westberg L, Landen M, et al. Serotonin transporter gene polymorphisms and platelet [3H] paroxetine binding in premen-strual dysphoria. Psychoneuroendocrinology 2003;28:446–58.Crossref, Google Scholar
22 Magnay JL, Ismail KM, Chapman G, et al. Serotonin transporter, tryptophan hydroxylase, and monoamine oxidase A gene polymorphisms in premenstrual dysphoric disorder. Am J Obstet Gynecol 2006;195:1254–9.Crossref, Google Scholar
23 Damberg M, Westberg L, Berggard C, et al. Investigation of transcription factor AP-2 beta genotype in women with premenstrual dysphoric disorder. Neurosci Lett 2005;377:49–52.Crossref, Google Scholar
24 Praschak-Rieder N, Willeit M, Winkler D, et al. Role of family history and 5-HTTLPR polymorphism in female seasonal affective disorder patients with and without premenstrual dysphoric disorder. Eur Neuropsychopharmacol 2002;12:129–34.Crossref, Google Scholar
25 Treloar SA, Heath AC, Martin NG. Genetic and environmental influences on premenstrual symptoms in an Australian twin sample. Psychol Med 2002;32:25–38.Crossref, Google Scholar
26 Halbreich U, Borenstein J, Pearlstein T, et al. The prevalence, impairment, impact, and burden of premenstrual dysphoric disorder (PMS/PMDD). Psychoneuroendocrinology 2003;28(Suppl 3):1–23.Google Scholar
27 Pearlstein TB, Halbreich U, Batzar ED, et al. Psychosocial functioning in women with premenstrual dysphoric disorder before and after treatment with sertraline or placebo. J Clin Psychiatry 2000;61:101–9.Crossref, Google Scholar
28 Bloch M, Schmidt PJ, Rubinow DR. Premenstrual syndrome: evidence for symptom stability across cycles. Am J Psychiatry 1997; 154:1741–6.Crossref, Google Scholar
29 Freeman EW. Effects of antidepressants on quality of life in women with premenstrual dysphoric disorder. Pharmacoeconomics 2005;23:433–44.Crossref, Google Scholar
30 Chawla A, Swindle R, Long S, et al. Premenstrual dysphoric disorder: Is there an economic burden of illness? Med Care 2002;40:1101–12.Crossref, Google Scholar
31 Borenstein JE, Dean BB, Endicott J, et al. Health and economic impact of the premenstrual syndrome. J Reprod Med 2003;48:515–24.Google Scholar
32 Dean BB, Borenstein JE. A prospective assessment investigating the relationship between work productivity and impairment with premenstrual syndrome. J Occup Environ Med 2004;46:649–56.Crossref, Google Scholar
33 Borenstein J, Chiou CF, Dean B, et al. Estimating direct and indirect costs of premenstrual syndrome. J Occup Environ Med 2005;47:26–33.Crossref, Google Scholar
34 Kuan AJ, Carter DM, Ott FJ. Distress levels in patients with premenstrual dysphoric disorder. Can J Psychiatry 2002;47:888–9.Crossref, Google Scholar
35 Gallant SJ, Popiel DA, Hoffman DM, et al. Using daily ratings to confirm premenstrual syndrome/late luteal phase dysphoric disorder. Part II. What makes a “real” difference? Psychosom Med 1992;54:167–81.Crossref, Google Scholar
36 Kuczmierczyk AR, Labrum AH, Johnson CC. Perception of family and work environments in women with premenstrual syndrome. J Psychosom Res 1992;36:787–95.Crossref, Google Scholar
37 Robinson RL, Swindle RW. Premenstrual symptom severity: impact on social functioning and treatment-seeking behaviors. J Womens Health Gend Based Med 2000;9:757–68.Crossref, Google Scholar
38 Hylan TR, Sundell K, Judge R. The impact of premenstrual symp- .pb9 tomatology on functioning and treatment-seeking behavior: experience from the United States, United Kingdom, and France. J Womens Health Gend Based Med 1999;8:1043–52.Crossref, Google Scholar
39 Kornstein SG, Harvey AT, Rush AJ, et al. Self-reported premenstrual exacerbation of depressive symptoms in patients seeking treatment for major depression. Psychol Med 2005;35:683–92.Crossref, Google Scholar
40 Hendrick V, Altshuler LL, Burt VK. Course of psychiatric disorders across the menstrual cycle. Harv Rev Psychiatry 1996;4:200–7.Crossref, Google Scholar
41 Kim DR, Gyulai L, Freeman EW, et al. Premenstrual dysphoric disorder and psychiatric co-morbidity. Arch Womens Ment Health 2004; 7:37–47.Crossref, Google Scholar
42 Pearlstein T, Stone AB. Premenstrual syndrome. Psychiatr Clin North Am 1998;21:577–90.Crossref, Google Scholar
43 Hsiao MC, Hsiao CC, Liu CY. Premenstrual symptoms and premenstrual exacerbation in patients with psychiatric disorders. Psychiatry Clin Neurosci 2004;58:186–90.Crossref, Google Scholar
44 Berlin RE, Raju JD, Schmidt PJ, et al. Effects of the menstrual cycle on measures of personality in women with premenstrual syndrome: a preliminary study. J Clin Psychiatry 2001;62:337–42.Crossref, Google Scholar
45 Choi SH, Kang SB, Joe SH. Changes in premenstrual symptoms in women with schizophrenia: a prospective study. Psychosom Med 2001;63:822–9.Crossref, Google Scholar
46 Steiner M, Pearlstein T, Cohen LS, et al. Expert guidelines for the treatment of severe PMS, PMDD and comorbidities: the role of SSRIs. J Womens Health (Larchmt) 2006;15:57–69.Crossref, Google Scholar
47 Case AM, Reid RL. Menstrual cycle effects on common medical conditions. Compr Ther 2001;27:65–71.Crossref, Google Scholar
48 Backstrom T, Andreen L, Birzniece V, et al. The role of hormones and hormonal treatments in premenstrual syndrome. CNS Drugs 2003;17:325–42.Crossref, Google Scholar
49 Halbreich U. The etiology, biology, and evolving pathology of premenstrual syndromes. Psychoneuroendocrinology 2003;28(Suppl 3): 55–99.Google Scholar
50 Parry BL. Psychobiology of premenstrual dysphoric disorder. Semin Reprod Endocrinol 1997;15:55–68.Crossref, Google Scholar
51 Steiner M, Pearlstein T. Premenstrual dysphoria and the serotonin system: pathophysiology and treatment. J Clin Psychiatry 2000;61 (Suppl 12):17–21.Google Scholar
52 Sundstrom-Poromaa I, Smith S, Gulinello M. GABA receptors, progesterone and premenstrual dysphoric disorder. Arch Womens Ment Health 2003;6:23–41.Crossref, Google Scholar
53 Eriksson O, Backstrom T, Stridsberg M, et al. Differential response to estrogen challenge test in women with and without premenstrual dysphoria. Psychoneuroendocrinology 2006;31:415–27.Crossref, Google Scholar
54 Schmidt PJ, Nieman LK, Danaceau MA, et al. Differential behavioral effects of gonadal steroids in women with and in those without premenstrual syndrome. N Engl J Med 1998;338:209–16.Crossref, Google Scholar
55 Rubinow DR, Schmidt PJ. Gonadal steroid regulation of mood: the lessons of premenstrual syndrome. Front Neuroendocrinol 2006;27:210–6.Crossref, Google Scholar
56 Parry BL, Newton RP. Chronobiological basis of female-specific mood disorders. Neuropsychopharmacology 2001;25(Suppl 1):S102–8.Crossref, Google Scholar
57 Protopopescu X, Tuescher O, Pan H, et al. Toward a functional neuroanatomy of premenstrual dysphoric disorder. J Affect Disord 2008;108:87–94.Crossref, Google Scholar
58 Rubinow DR, Smith MJ, Schenkel LA, et al. Facial emotion discrimination across the menstrual cycle in women with premenstrual dysphoric disorder (PMDD) and controls. J Affect Disord 2007;104:37–44.Crossref, Google Scholar
59 Parry BL. The role of central serotonergic dysfunction in the aetiology of premenstrual dysphoric disorder: therapeutic implications. CNS Drugs 2001;15:277–85.Crossref, Google Scholar
60 Kurshan N, Epperson CN. Oral contraceptives and mood in women with and without premenstrual dysphoria: a theoretical model. Arch Womens Ment Health 2006;9:1–14.Crossref, Google Scholar
61 Eriksson O, Wall A, Marteinsdottir I, et al. Mood changes correlate to changes in brain serotonin precursor trapping in women with premenstrual dysphoria. Psychiatry Res 2006;146:107–16.Crossref, Google Scholar
62 Jovanovic H, Cerin A, Karlsson P, et al. A PET study of 5-HT(1A) receptors at different phases of the menstrual cycle in women with premenstrual dysphoria. Psychiatry Res 2006;148:185–93.Crossref, Google Scholar
63 Epperson CN, Haga K, Mason GF, et al. Cortical gamma-aminobutyric acid levels across the menstrual cycle in healthy women and those with premenstrual dysphoric disorder: a proton magnetic resonance spectroscopy study. Arch Gen Psychiatry 2002;59:851–8.Crossref, Google Scholar
64 Smith MJ, Adams LF, Schmidt PJ, et al. Abnormal luteal phase excitability of the motor cortex in women with premenstrual syndrome. Biol Psychiatry 2003;54:757–62.Crossref, Google Scholar
65 Eser D, Schule C, Baghai TC, et al. Neuroactive steroids and affective disorders. Pharmacol Biochem Behav 2006;84:656–66.Crossref, Google Scholar
66 Landen M, Thase ME. A model to explain the therapeutic effects of serotonin reuptake inhibitors: the role of 5-HT(2) receptors. Psychopharmacol Bull 2006;39:147–66.Google Scholar
67 Clayton AH, Keller AE, Leslie C, et al. Exploratory study of premenstrual symptoms and serotonin variability. Arch Womens Ment Health 2006;9:51–7.Crossref, Google Scholar
68 Eser D, Schule C, Baghai TC, et al. Neuroactive steroids in depression and anxiety disorders: clinical studies. Neuroendocrinology 2006;84:244–54.Crossref, Google Scholar
69 Kalueff AV, Nutt DJ. Role of GABA in anxiety and depression. Depress Anxiety 2007;24:495–517.Crossref, Google Scholar
70 Uzunova V, Sampson L, Uzunov DP. Relevance of endogenous 3alpha-reduced neurosteroids to depression and antidepressant action. Psychopharmacology (Berl) 2006;186:351–61.Crossref, Google Scholar
71 Halbreich U, O'Brien PMS, Eriksson E, et al. Are there differential symptom profiles that improve in response to different pharmacological treatments of premenstrual syndrome/premenstrual dysphoric disorder? CNS Drugs 2006;20:523–47.Crossref, Google Scholar
72 Backstrom T, Hansson-Malmstrom Y, Lindhe B-A, et al. Oral contraceptives in premenstrual syndrome: a randomized comparison of triphasic and monophasic preparations. Contraception 1992;46:253–68.Crossref, Google Scholar
73 Graham CA, Sherwin BB. A prospective treatment study of premenstrual symptoms using a triphasic oral contraceptive. J Psychosom Res 1992;36:257–66.Crossref, Google Scholar
74 Joffe H, Cohen LS, Harlow BL. Impact of oral contraceptive pill use on premenstrual mood: predictors of improvement and deterioration. Am J Obstet Gynecol 2003;189:1523–30.Crossref, Google Scholar
75 Sanders SA, Graham CA, Bass JL, et al. A prospective study of the effects of oral contraceptives on sexuality and well-being and their relationship to discontinuation. Contraception 2001;64:51–8.Crossref, Google Scholar
76 Schultz-Zehden B, Boschitsch E. User experience with an oral contraceptive containing ethinylestradiol 30mug and drospirenone 3mg (YASMIN®) in clinical practice. Treat Endocrinol 2006;5:251–6.Crossref, Google Scholar
77 Rapkin AJ. New treatment approaches for premenstrual disorders. Am J Manag Care 2005;11(Suppl):S480–91.Google Scholar
78 Coffee AL, Kuehl TJ, Willis S, et al. Oral contraceptives and premenstrual symptoms: comparison of a 21/7 and extended regimen. Am J Obstet Gynecol 2006;195:1311–9.Crossref, Google Scholar
79 Freeman EW, Kroll R, Rapkin A, et al. Evaluation of a unique oral contraceptive in the treatment of premenstrual dysphoric disorder. J Womens Health Gend Based Med 2001;10:561–9.Crossref, Google Scholar
80 Yonkers KA, Brown C, Pearlstein TB, et al. Efficacy of a new low-dose oral contraceptive with drospirenone in premenstrual dysphoric disorder. Obstet Gynecol 2005;106:492–501.Crossref, Google Scholar
81 Pearlstein TB, Bachmann GA, Zacur HA, et al. Treatment of premenstrual dysphoric disorder with a new drospirenone-containing oral contraceptive formulation. Contraception 2005;72:414–21.Crossref, Google Scholar
82 Sulak PJ. Ovulation suppression of premenstrual symptoms using oral contraceptives. Am J Manag Care 2005;11(Suppl):S492–7.Google Scholar
83 Mishell DRJ. Rationale for decreasing the number of days of the hormone-free interval with use of low-dose oral contraceptive formulations. Contraception 2005;71:304–5.Crossref, Google Scholar
84 Pearlstein TB. Hormonal and nonpharmacologic treatments for premenstrual syndrome and premenstrual dysphoric disorder. Prim Psychiatry 2004;11:48–52.Google Scholar
85 Wyatt KM, Dimmock PW, Ismail KM, et al. The effectiveness of GnRHa with and without ‘add-back’ therapy in treating premenstrual syndrome: a meta analysis. BJOG 2004;111:585–93.Crossref, Google Scholar
86 Leather AT, Studd JW, Watson NR, et al. The treatment of severe premenstrual syndrome with goserelin with and without ‘add-back’ estrogen therapy: a placebo-controlled study. Gynecol Endocrinol 1999;13:48–55.Crossref, Google Scholar
87 Mitwally MF, Gotlieb L, Casper RF. Prevention of bone loss and hypoestrogenic symptoms by estrogen and interrupted progestogen add-back in long-term GnRH-agonist down-regulated patients with endometriosis and premenstrual syndrome. Menopause 2002; 9:236–41.Crossref, Google Scholar
88 Di Carlo C, Palomba S, Tommaselli GA, et al. Use of leuprolide acetate plus tibolone in the treatment of severe premenstrual syndrome. Fertil Steril 2001;75:380–4.Crossref, Google Scholar
89 Hahn PM, Van Vugt DA, Reid RL. A randomized, placebo-controlled, crossover trial of danazol for the treatment of premen-.pb10 strual syndrome. Psychoneuroendocrinology 1995;20:193–209.Crossref, Google Scholar
90 O'Brien PM, Abukhalil IE. Randomized controlled trial of the management of premenstrual syndrome and premenstrual mastalgia using luteal phase-only danazol. Am J Obstet Gynecol 1999;180:18–23.Crossref, Google Scholar
91 Epperson CN, Wisner KL, Yamamoto B. Gonadal steroids in the treatment of mood disorders. Psychosom Med 1999;61:676–97.Crossref, Google Scholar
92 Casper RF, Hearn MT. The effect of hysterectomy and bilateral oophorectomy in women with severe premenstrual syndrome. Am J Obstet Gynecol 1990;162:105–9.Crossref, Google Scholar
93 Casson P, Hahn PM, Van Vugt DA, et al. Lasting response to ovariectomy in severe intractable premenstrual syndrome. Am J Obstet Gynecol 1990;162:99–105.Crossref, Google Scholar
94 Cronje WH, Vashisht A, Studd JW. Hysterectomy and bilateral oophorectomy for severe premenstrual syndrome. Hum Reprod 2004;19:2152–5.Crossref, Google Scholar
95 Studd J. Ovariotomy for menstrual madness and premenstrual syndrome-19th century history and lessons for current practice. Gynecol Endocrinol 2006;22:411–5.Crossref, Google Scholar
96 Ford O, Lethaby A, Mol B, et al. Progesterone for premenstrual syndrome. Cochrane Database Syst Rev 2006;(4):CD003415.Google Scholar
97 Wyatt K, Dimmock P, Jones P, et al. Efficacy of progesterone and progestogens in management of premenstrual syndrome: systematic review. BMJ 2001;323:776–80.Crossref, Google Scholar
98 Steiner M, Soares CN. Psychotropic therapies. In: O'Brien PM, Rapkin AJ, Schmidt PJ, editors. The Premenstrual syndromes: PMS and PMDD. London (UK): Informa Healthcare; 2008. p. 131–9.Google Scholar
99 Dimmock PW, Wyatt KM, Jones PW, et al. Efficacy of selective serotonin-reuptake inhibitors in premenstrual syndrome: a systematic review. Lancet 2000;356:1131–6.Crossref, Google Scholar
100 Wyatt KM, Dimmock PW, O'Brien PM. Selective serotonin reup-take inhibitors for premenstrual syndrome. Cochrane Database Syst Rev 2002;4:CD001396.Google Scholar
101 Steiner M, Steinberg S, Stewart D, et al. Fluoxetine in the treatment of premenstrual dysphoria. Canadian Fluoxetine/Premenstrual Dysphoria Collaborative Study Group. N Engl J Med 1995;332:1529–34.Crossref, Google Scholar
102 Stone AB, Pearlstein TB, Brown WA. Fluoxetine in the treatment of late luteal phase dysphoric disorder. J Clin Psychiatry 1991;52:290–3.Google Scholar
103 Wood SH, Mortola JF, Chan YF, et al. Treatment of premenstrual syndrome with fluoxetine: a double-blind, placebo-controlled, crossover study. Obstet Gynecol 1992;80:339–44.Google Scholar
104 Menkes DB, Taghavi E, Mason PA, et al. Fluoxetine's spectrum of action in premenstrual syndrome. Int Clin Psychopharmacol 1993;8: 95–102.Crossref, Google Scholar
105 Ozeren S, Corakci A, Yucesoy I, et al. Fluoxetine in the treatment of premenstrual syndrome. Eur J Obstet Gynecol Reprod Biol 1997; 73:167–70.Crossref, Google Scholar
106 Su TP, Schmidt PJ, Danaceau MA, et al. Fluoxetine in the treatment of premenstrual dysphoria. Neuropsychopharmacology 1997;16:346–56.Crossref, Google Scholar
107 Pearlstein TB, Stone AB, Lund SA, et al. Comparison of fluoxetine, bupropion, and placebo in the treatment of premenstrual dysphoric disorder. J Clin Psychopharmacol 1997;17:261–6.Crossref, Google Scholar
108 Yonkers KA, Halbreich U, Freeman E, et al. Symptomatic improvement of premenstrual dysphoric disorder with sertraline treatment. A randomized controlled trial. Sertraline Premenstrual Dysphoric Collaborative Study Group. JAMA 1997;278:983–8.Crossref, Google Scholar
109 Freeman EW, Rickels K, Sondheimer SJ, et al. Differential response to antidepressants in women with premenstrual syndrome/premenstrual dysphoric disorder: a randomized controlled trial. Arch Gen Psychiatry 1999;56:932–9.Crossref, Google Scholar
110 Eriksson E, Hedberg MA, Andersch B, et al. The serotonin reup-take inhibitor paroxetine is superior to the noradrenaline reuptake inhibitor maprotiline in the treatment of premenstrual syndrome. Neuropsychopharmacology 1995;12:167–76.Crossref, Google Scholar
111 Wikander I, Sundblad C, Andersch B, et al. Citalopram in premenstrual dysphoria: Is intermittent treatment during luteal phases more effective than continuous medication throughout the menstrual cycle? J Clin Psychopharmacol 1998;18:390–8.Crossref, Google Scholar
112 Veeninga AT, Westenberg HG, Weusten JT. Fluvoxamine in the treatment of menstrually related mood disorders. Psychopharmacology (Berl) 1990;102:414–6.Crossref, Google Scholar
113 Halbreich U, Smoller JW. Intermittent luteal phase sertraline treatment of dysphoric premenstrual syndrome. J Clin Psychiatry 1997; 58:399–402.Crossref, Google Scholar
114 Young SA, Hurt PH, Benedek DM, et al. Treatment of premenstrual dysphoric disorder with sertraline during the luteal phase: a randomized, double-blind, placebo-controlled crossover trial. J Clin Psychiatry 1998;59:76–80.Crossref, Google Scholar
115 Jermain DM, Preece CK, Sykes RL, et al. Luteal phase sertraline treatment for premenstrual dysphoric disorder. Arch Fam Med 1999;8:328–32.Crossref, Google Scholar
116 Freeman EW, Rickels K, Sondheimer SJ, et al. Continuous or intermittent dosing with sertraline for patients with severe premenstrual syndrome or premenstrual dysphoric disorder. Am J Psychiatry 2004;161:343–51.Crossref, Google Scholar
117 Landen M, Nissbrandt H, Allgulander C, et al. Placebo-controlled trial comparing intermittent and continuous paroxetine in premenstrual dysphoric disorder. Neuropsychopharmacology 2007;32:153–61.Crossref, Google Scholar
118 Shah NR, Jones JB, Aperi J, et al. Selective serotonin reuptake inhibitors for premenstrual syndrome and premenstrual dysphoric disorder: a meta-analysis. Obstet Gynecol 2008;111:1175–82.Crossref, Google Scholar
119 Cohen LS, Soares CN, Yonkers KA, et al. Paroxetine controlled release for premenstrual dysphoric disorder: a double-blind, placebo-controlled trial. Psychosom Med 2004;66:707–13.Crossref, Google Scholar
120 Pearlstein TB, Bellew KM, Endicott J, et al. Paroxetine controlled release for premenstrual dysphoric disorder: remission analysis following a randomized, double-blind, placebo-controlled trial. Prim Care Companion J Clin Psychiatry 2005;7:53–60.Crossref, Google Scholar
121 Cohen LS, Miner C, Brown EW, et al. Premenstrual daily fluoxetine for premenstrual dysphoric disorder: a placebo-controlled, clinical trial using computerized diaries. Obstet Gynecol 2002;100:435–44.Crossref, Google Scholar
122 Halbreich U, Bergeron R, Yonkers KA, et al. Efficacy of intermittent, luteal phase sertraline treatment of premenstrual dysphoric disorder. Obstet Gynecol 2002;100:1219–29.Crossref, Google Scholar
123 Steiner M, Hirschberg AL, Bergeron R, et al. Luteal phase dosing with paroxetine controlled release (CR) in the treatment of premenstrual dysphoric disorder. Am J Obstet Gynecol 2005;193:352–60.Crossref, Google Scholar
124 Yonkers KA, Pearlstein T, Fayyad R, et al. Luteal phase treatment of premenstrual dysphoric disorder improves symptoms that continue into the postmenstrual phase. J Affect Disord 2005;85:317–21.Crossref, Google Scholar
125 Eriksson E, Ekman A, Sinclair S, et al. Escitalopram administered in the luteal phase exerts a marked and dose-dependent effect in premenstrual dysphoric disorder. J Clin Psychopharmacol 2008;28: 195–202.Crossref, Google Scholar
126 Miner C, Brown E, McCray S, et al. Weekly luteal-phase dosing with enteric-coated fluoxetine 90 mg in premenstrual dysphoric disorder: a randomized, double-blind, placebo-controlled clinical trial. Clin Ther 2002;24:417–33.Crossref, Google Scholar
127 Yonkers KA, Holthausen GA, Poschman K, et al. Symptom-onset treatment for women with premenstrual dysphoric disorder. J Clin Psychopharmacol 2006;26:198–202.Crossref, Google Scholar
128 Freeman EW, Sondheimer SJ, Sammel MD, et al. A preliminary study of luteal phase versus symptom-onset dosing with escitalopram for premenstrual dysphoric disorder. J Clin Psychiatry 2005;66:769–73.Crossref, Google Scholar
129 Kornstein SG, Pearlstein TB, Fayyad R, et al. Low-dose sertraline in the treatment of moderate-to-severe premenstrual syndrome: efficacy of 3 dosing strategies. J Clin Psychiatry 2006;67:1624–32.Crossref, Google Scholar
130 Sundblad C, Modigh K, Andersch B, et al. Clomipramine effectively reduces premenstrual irritability and dysphoria: a placebo-controlled trial. Acta Psychiatr Scand 1992;85:39–47.Crossref, Google Scholar
131 Sundblad C, Hedberg MA, Eriksson E. Clomipramine administered during the luteal phase reduces the symptoms of premenstrual syndrome: a placebo-controlled trial. Neuropsychopharmacology 1993;9:133–45.Crossref, Google Scholar
132 Freeman EW, Rickels K, Yonkers KA, et al. Venlafaxine in the treatment of premenstrual dysphoric disorder. Obstet Gynecol 2001; 98:737–44.Crossref, Google Scholar
133 Landen M, Eriksson O, Sundblad C, et al. Compounds with affinity for serotonergic receptors in the treatment of premenstrual dysphoria: a comparison of buspirone, nefazodone and placebo. Psychopharmacology (Berl) 2001;155:292–8.Crossref, Google Scholar
134 Miller MN, Miller BE, Chinouth R, et al. Increased premenstrual dosing of nefazodone relieves premenstrual magnification of depression. Depress Anxiety 2002;15:48–51.Crossref, Google Scholar
135 Berger CP, Presser B. Alprazolam in the treatment of two subsamples of patients with late luteal phase dysphoric disorder: a double-blind, placebo-controlled crossover study. Obstet Gynecol 1994;84:379–85.Google Scholar
136 Freeman EW, Rickels K, Sondheimer SJ, et al. A double-blind trial of oral progesterone, alprazolam, and placebo in treatment of severe premenstrual syndrome. JAMA 1995;274:51–7.Crossref, Google Scholar
137 Harrison WM, Endicott J, Nee J. Treatment of premenstrual dysphoria with alprazolam. A controlled study. Arch Gen Psychiatry 1990;47:270–5.Crossref, Google Scholar
138 Smith S, Rinehart JS, Ruddock VE, et al. Treatment of premenstrual syndrome with alprazolam: results of a double-blind, placebo-controlled, randomized crossover clinical trial. Obstet Gynecol 1987;70:37–43.Google Scholar
139 Schmidt PJ, Grover GN, Rubinow DR. Alprazolam in the treatment of premenstrual syndrome. A double-blind, placebo-controlled trial. Arch Gen Psychiatry 1993;50:467–73.Crossref, Google Scholar
140 Rickels K, Freeman E, Sondheimer S. Buspirone in treatment of premenstrual syndrome. Lancet 1989;1:777.Crossref, Google Scholar
141 Mira M, McNeil D, Fraser IS, et al. Mefenamic acid in the treatment of premenstrual syndrome. Obstet Gynecol 1986;68:395–8.Crossref, Google Scholar
142 Toth A, Lesser ML, Naus G, et al. Effect of doxycycline on premenstrual syndrome: a double-blind randomized clinical trial. J Int Med Res 1988;16:270–9.Crossref, Google Scholar
143 Chuong CJ, Coulam CB, Bergstralh EJ, et al. Clinical trial of naltrexone in premenstrual syndrome. Obstet Gynecol 1988;72:332–6.Google Scholar
144 Rausch JL, Janowsky DS, Golshan S, et al. Atenolol treatment of late luteal phase dysphoric disorder. J Affect Disord 1988;15:141–7.Crossref, Google Scholar
145 Andersch B. Bromocriptine and premenstrual symptoms: a survey of double blind trials. Obstet Gynecol Surv 1983;38:643–6.Crossref, Google Scholar
146 Wang M, Hammarback S, Lindhe BA, et al. Treatment of premenstrual syndrome by spironolactone: a double-blind, placebo-controlled study. Acta Obstet Gynecol Scand 1995;74:803–8.Crossref, Google Scholar
147 Morse G. Positively reframing perceptions of the menstrual cycle among women with premenstrual syndrome. J Obstet Gynecol Neonatal Nurs 1999;28:165–74.Crossref, Google Scholar
148 Taylor D. Effectiveness of professional-peer group treatment: symptom management for women with PMS. Res Nurs Health 1999;22:496–511.Crossref, Google Scholar
149 Freeman EW, Stout AL, Endicott J, et al. Treatment of premenstrual syndrome with a carbohydrate-rich beverage. Int J Gynaecol Obstet 2002;77:253–4.Crossref, Google Scholar
150 Sayegh R, Schiff I, Wurtman J, et al. The effect of a carbohydrate-rich beverage on mood, appetite, and cognitive function in women with premenstrual syndrome. Obstet Gynecol 1995;86:520–8.Crossref, Google Scholar
151 Blake F, Salkovskis P, Gath D, et al. Cognitive therapy for premenstrual syndrome: a controlled trial. J Psychosom Res 1998;45:307–18.Crossref, Google Scholar
152 Christensen AP, Oei TP. The efficacy of cognitive behaviour therapy in treating premenstrual dysphoric changes. J Affect Disord 1995;33:57–63.Crossref, Google Scholar
153 Hunter MS, Ussher JM, Cariss M, et al. Medical (fluoxetine) and psychological (cognitive-behavioural therapy) treatment for premenstrual dysphoric disorder: a study of treatment processes. J Psychosom Res 2002;53:811–7.Crossref, Google Scholar
154 Hunter MS, Ussher JM, Browne SJ, et al. A randomized comparison of psychological (cognitive behavior therapy), medical (fluoxetine) and combined treatment for women with premenstrual dysphoric disorder. J Psychosom Obstet Gynaecol 2002;23:193–9.Crossref, Google Scholar
155 Goodale IL, Domar AD, Benson H. Alleviation of premenstrual syndrome symptoms with the relaxation response. Obstet Gynecol 1990;75:649–55.Google Scholar
156 Pearlstein T. Nonpharmacologic treatment of premenstrual syndrome. Psychiatr Ann 1996;26:590–4.Crossref, Google Scholar
157 Brosse AL, Sheets ES, Lett HS, et al. Exercise and the treatment of clinical depression in adults: recent findings and future directions. Sports Med 2002;32:741–60.Crossref, Google Scholar
158 Thys-Jacobs S, Starkey P, Bernstein D, et al. Calcium carbonate and the premenstrual syndrome: effects on premenstrual and menstrual symptoms. Premenstrual Syndrome Study Group. Am J Obstet Gynecol 1998;179:444–52.Crossref, Google Scholar
159 Kleijnen J, Ter Riet G, Knipschild P. Vitamin B6 in the treatment of the premenstrual syndrome-a review. Br J Obstet Gynaecol 1990,97:847–52.Crossref, Google Scholar
160 Wyatt KM, Dimmock PW, Jones PW, et al. Efficacy of vitamin B-6 in the treatment of premenstrual syndrome: systematic review. BMJ 1999;318:1375–81.Crossref, Google Scholar
161 Kashanian M, Mazinani R, Jalalmanesh S. Pyridoxine (vitamin B6) therapy for premenstrual syndrome. Int J Gynaecol Obstet 2007;96:43–4.Crossref, Google Scholar
162 Facchinetti F, Borella P, Sances G, et al. Oral magnesium successfully relieves premenstrual mood changes. Obstet Gynecol 1991;78:177–81.Google Scholar
163 Walker AF, De Souza MC, Vickers MF, et al. Magnesium supplementation alleviates premenstrual symptoms of fluid retention. J Womens Health 1998;7:1157–65.Crossref, Google Scholar
164 Khine K, Rosenstein DL, Elin RJ, et al. Magnesium (mg) retention and mood effects after intravenous mg infusion in premenstrual dysphoric disorder. Biol Psychiatry 2006;59:327–33.Crossref, Google Scholar
165 Steinberg S, Annable L, Young SN, et al. A placebo-controlled clinical trial of L-tryptophan in premenstrual dysphoria. Biol Psychiatry 1999;45:313–20.Crossref, Google Scholar
166 London RS, Murphy L, Kitlowski KE, et al. Efficacy of alpha-tocopherol in the treatment of the premenstrual syndrome. J Reprod Med 1987;32:400–4.Google Scholar
167 Bendich A. The potential for dietary supplements to reduce premenstrual syndrome (PMS) symptoms. J Am Coll Nutr 2000;19:3–12.Crossref, Google Scholar
168 Stevinson C, Ernst E. Complementary/alternative therapies for premenstrual syndrome: a systematic review of randomized controlled trials. Am J Obstet Gynecol 2001;185:227–35.Crossref, Google Scholar
169 Sampalis F, Bunea R, Pelland MF, et al. Evaluation of the effects of Neptune Krill Oil on the management of premenstrual syndrome and dysmenorrhea. Altern Med Rev 2003;8:171–9.Google Scholar
170 Bryant M, Cassidy A, Hill C, et al. Effect of consumption of soy isoflavones on behavioural, somatic and affective symptoms in women with premenstrual syndrome. Br J Nutr 2005;93:731–9.Crossref, Google Scholar
171 Dennehy CE. The use of herbs and dietary supplements in gynecology: an evidence-based review. J Midwifery Womens Health 2006; 51:402–9.Crossref, Google Scholar
172 Domoney CL, Vashisht A, Studd JW. Premenstrual syndrome and the use of alternative therapies. Ann N Y Acad Sci 2003;997:330–40.Crossref, Google Scholar
173 Fugh-Berman A, Kronenberg F. Complementary and alternative medicine (CAM) in reproductive-age women: a review of randomized controlled trials. Reprod Toxicol 2003;17:137–52.Crossref, Google Scholar
174 Girman A, Lee R, Kliger B. An integrative medicine approach to premenstrual syndrome. Am J Obstet Gynecol 2003;188(Suppl):S56–65.Crossref, Google Scholar
175 Prilepskaya VN, Ledina AV, Tagiyeva AV, et al. Vitex agnus castus: Successful treatment of moderate to severe premenstrual syndrome. Maturitas 2006;55(Suppl 1):S55–63.Crossref, Google Scholar
176 Atmaca M, Kumru S, Tezcan E. Fluoxetine versus Vitex agnus castus extract in the treatment of premenstrual dysphoric disorder. Hum Psychopharmacol 2003;18:191–5.Crossref, Google Scholar
177 Hernandez-Reif M, Martinez A, Field T, et al. Premenstrual symptoms are relieved by massage therapy. J Psychosom Obstet Gynaecol 2000;21:9–15.Crossref, Google Scholar
178 Oleson T, Flocco W. Randomized controlled study of premenstrual symptoms treated with ear, hand, and foot reflexology. Obstet Gynecol 1993;82:906–11.Google Scholar
179 Walsh MJ, Polus BI. A randomized, placebo-controlled clinical trial on the efficacy of chiropractic therapy on premenstrual syndrome. J Manipulative Physiol Ther 1999;22:582–5.Crossref, Google Scholar
180 Van Zak DB. Biofeedback treatments for premenstrual and premenstrual affective syndromes. Int J Psychosom 1994;41:53–60.Google Scholar
181 Agha-Hosseini M, Kashani L, Aleyaseen A, et al. Crocus sativus L. (saffron) in the treatment of premenstrual syndrom: a double-blind, randomised and placebo-controlled trial. BJOG 2008;115:515–9.Crossref, Google Scholar
182 Jang HS, Lee MS. Effects of qi therapy (external qigong) on premenstrual syndrome: a randomized placebo-controlled study. J Altern Complement Med 2004;10:456–62.Crossref, Google Scholar
183 Stevinson C, Ernst E. A pilot study of Hypericum perforatum for the treatment of premenstrual syndrome. BJOG 2000;107:870–6.Crossref, Google Scholar
184 Huang KL, Tsai SJ. St. John's wort (Hypericum perforatum) as a treatment for premenstrual dysphoric disorder: case report. Int J Psychiatry Med 2003;33:295–7.Crossref, Google Scholar
185 Sridevi K, Krishna Rao PV. Yoga practice and menstrual distress. JIAAP 1996;22:47–54.Google Scholar
186 Groer M, Ohnesorge C. Menstrual-cycle lengthening and reduction in premenstrual distress through guided imagery. J Holist Nurs 1993;11:286–94.Crossref, Google Scholar
187 Anderson DJ, Legg NJ, Ridout DA. Preliminary trial of photic stimulation for premenstrual syndrome. J Obstet Gynaecol 1997;17:76–9.Crossref, Google Scholar
188 Habek D, Habek JC, Barbir A. Using acupuncture to treat premenstrual syndrome. Arch Gynecol Obstet 2002;267:23–6.Crossref, Google Scholar
189 Lam RW, Carter D, Misri S, et al. A controlled study of light therapy in women with late luteal phase dysphoric disorder. Psychiatry Res 1999;86:185–92.Crossref, Google Scholar
190 Krasnik C, Montori VM, Guyatt GH, et al. The effect of bright light therapy on depression associated with premenstrual dysphoric disorder. Am J Obstet Gynecol 2005;193:658–61.Crossref, Google Scholar
191 Altshuler LL, Cohen LS, Moline ML, et al. The Expert Consensus Guideline Series. Treatment of depression in women. Postgrad Med 2001(Spec No):1–107.Crossref, Google Scholar



