Causal Association Between Cannabis and Psychosis: Examination of the Evidence
Abstract
Background:
Controversy remains as to whether cannabis acts as a causal risk factor for schizophrenia or other functional psychotic illnesses. Aims: To examine critically the evidence that cannabis causes psychosis using established criteria of causality. Method: We identified five studies that included a well-defined sample drawn from population-based registers or cohorts and used prospective measures of cannabis use and adult psychosis. Results: On an individual level, cannabis use confers an overall twofold increase in the relative risk for later schizophrenia. At the population level, elimination of cannabis use would reduce the incidence of schizophrenia by approximately 8%, assuming a causal relationship. Cannabis use appears to be neither a sufficient nor a necessary cause for psychosis. It is a component cause, part of a complex constellation of factors leading to psychosis. Conclusions: Cases of psychotic disorder could be prevented by discouraging cannabis use among vulnerable youths. Research is needed to understand the mechanisms by which cannabis causes psychosis.
(Reprinted with permission from the British Journal of Psychiatry 2004; 184:110–117)
There is little dispute that cannabis intoxication can lead to acute transient psychotic episodes in some individuals (D'Souza et al, 2004) and that it can produce short-term exacerbation or recurrences of pre-existing psychotic symptoms (Thornicroft, 1990; Mathers & Ghodse, 1992; Hall & Degenhardt, 2004). However, controversy remains about whether cannabis use can actually cause schizophrenia or other functional psychotic illness in the long term (Johns, 2001). A previous review paper, published more than a decade ago, reached no firm conclusion regarding causality and stressed the importance of prospective longitudinal population-based cohort studies to elucidate a possible causal association (Thornicroft, 1990). Sixteen years after the publication of the first evidence that cannabis may be a causal risk factor for later schizophrenia (Andréasson et al, 1988), four recent prospective epidemiological studies have provided further evidence. We review the evidence from these studies within the framework of established criteria for determining causality.
METHOD
WHAT IS A CAUSE?
The precise definition of what constitutes a cause, and the elaboration of criteria for determining causality, have a long and contentious history. Causal criteria that deal with the exposure-disease relationship are often used as general guidelines for ascertaining causes. Hill (1965) listed the following criteria: strength, consistency, specificity, biological gradient, temporality, coherence and plausibility. Support for each criterion strengthens the case for a causal association but, as Rothman & Greenland (1998) point out, only one criterion, temporality, is a sine qua non for causality. Susser (1991) subsequently used the Hill criteria to distill three properties that may serve to define causes: association, temporal priority and direction.
Association is the requirement that a cause and an outcome appear together. When the putative cause is present, the outcome rate is higher than when the putative cause is absent. There is no requirement for the putative cause to be present in every case of the outcome, just that the rate of outcome is higher in those with it than without it. Temporal priority is the fundamental property that the putative cause be present before the outcome. Direction refers to the fact that changes in the putative cause will actually lead to a change in the outcome. In other words, the association of the putative cause with the outcome does not derive from a third factor associated with both. Epidemiologists refer to the latter phenomenon as ‘confounding'.
We examine the empirical evidence put forward to support the claim that cannabis is a causal factor in schizophrenia under these headings.
RESULTS
EVIDENCE FOR ASSOCIATION
Cross-sectional national surveys (from the USA, Australia and The Netherlands) have found that rates of cannabis use are higher (approximately twice as high) among people with schizophrenia than among the general population (Regier et al, 1990; Tien & Anthony, 1990; Robins & Regier, 1991; Hall & Degenhardt, 2000; van Os et al, 2002).
Local surveys have also found higher rates of cannabis use among patients with psychosis than among community controls. Surveys of patients with psychotic illnesses from London have found that between 20 and 40% report lifetime cannabis use (Menezes et al, 1996; Grech et al, 1998; Duke et al, 2001). Even higher rates of lifetime use of cannabis (51%) have been reported among patients detained under the 1983 Mental Health Act (Wheatley, 1998). Rates are lower in rural areas: 7% of patients with schizophrenia in Dumfries and Galloway, Scotland, reported problematic use of a drug, with 4% related specifically to cannabis use (McCreadie, 2002). However, irrespective of the setting of the study, rates of cannabis use seem to be about twice as high among patients with psychosis than among controls (Grech et al, 1998; McCreadie, 2002). These elevated rates of cannabis use among people with schizophrenia raise important questions about the reason for this association – is the cannabis use a consequence or a cause of the condition?
Two studies of clinical samples have examined retrospective reports of drug use in individuals who have developed schizophrenia. First, Hambrecht & Hafner (1996) reported on a retrospective study of 232 patients with schizophrenia. Data showed that one-third of the sample had used drugs at least 1 year before onset of the illness, another one-third had used drugs and subsequently developed the illness within a year and the remaining one-third had started using cannabis after the occurrence of schizophrenia symptoms. In a second study, Cantwell et al (1999) investigated a group of 168 patients with first-episode schizophrenia and found that 37% showed evidence of substance use and alcohol use before their presentation to services.
However, studies based on retrospective self-reports are prone to recall bias. To establish temporal priority (and hence causality) we need to examine prospective reports of cannabis use collected before the onset of schizophrenia, and therefore unbiased by later outcome. Ideally, we should also study population-based samples.
EVIDENCE FOR TEMPORAL PRIORITY AND DIRECTION
We included in this core section of the review those studies that fulfilled the following criteria: inclusion of a well-defined sample of cases drawn from population-based registers or cohorts; use of prospectively measured data on cannabis use and adult psychosis; and presentation of odds ratios as an indicator of the strength of association between cannabis and later psychosis, to allow calculation of an overall risk estimate of cannabis use for later psychosis. The research strategies used were: computerised Medline and PsycLIT searches; cross-referencing of original studies; and contact with other researchers in the field.
At the time of the search, five studies based on four samples (three cohort studies and one longitudinal population-based survey) fulfilled those criteria. These studies are reviewed in detail below and are summarised in Table 1. We used the evidence from these samples to establish temporal priority and direction for the association between cannabis use and schizophrenia. We calculated the overall risk of psychosis using adjusted odds ratios from all studies with the ‘meta' command of Stata 8.0 (StataCorp, 2003), which uses inverse-variance weighting to calculate fixed and random effects summary estimates (Sterne et al, 2001). Results across studies were not significantly heterogeneous.
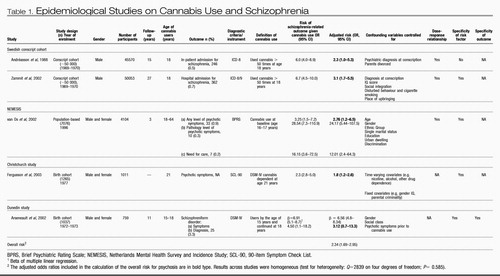 |
Table 1. Epidemiological Studies on Cannabis Use and Schizophrenia
The Swedish conscript cohort. For many years the only evidence that cannabis use might predispose to later psychosis came from a cohort study of Swedish conscripts who were followed up using record-linkage techniques based on in-patient admissions for psychiatric care (Andréasson et al, 1988). A dose-response relationship was observed between cannabis use at conscription (age 18 years) and schizophrenia diagnosis 15 years later. Self-reported ‘heavy cannabis users' (i.e. who had used cannabis more than 50 times) were six times more likely than non-users to have been diagnosed with schizophrenia 15 years later. However, more than half of these heavy users had a psychiatric diagnosis other than psychosis at conscription, and when this confound was controlled for the relative risk decreased to 2.3 (but none the less remained statistically significant). Very few heavy cannabis users (3%) went on to develop schizophrenia, suggesting that cannabis use may increase the risk for schizophrenia only among individuals already vulnerable to developing psychosis. The authors concluded that ‘Cannabis should be viewed as an additional clue to the still elusive aetiology of schizophrenia'.
Consistent with the previous findings, a follow-up study of the same Swedish conscript cohort showed that ‘heavy cannabis users' by the age of 18 years were 6.7 times more likely than non-users to be diagnosed with schizophrenia 27 years later (Zammit et al, 2002). This risk held when the analysis was repeated on a subsample of men who used cannabis only, as opposed to using other drugs as well. The risk was reduced but remained significant after controlling for other potential confounding factors such as disturbed behaviour, low IQ score, growing up in a city, cigarette smoking and poor social integration. The analysis was repeated on a subsample of individuals who developed schizophrenia only 5 years after conscription to control for the possibility that cannabis use is a consequence of prodromal manifestations of psychosis. Findings were similar to those for the entire cohort. The authors concluded that the findings are ‘consistent with a causal relationship between cannabis use and schizophrenia'.
The Dutch NEMESIS sample. An analysis of the Netherlands Mental Health Survey and Incidence Study (NEMESIS) (van Os et al, 2002) goes beyond the reliance on hospital discharge register data and examines the effect of cannabis use on self-reported psychotic symptoms among the general population. In this study, 4045 psychosis-free individuals and 59 who had a psychotic disorder were assessed at baseline and were administered follow-up assessments 1 year later and again 3 years after the baseline assessment. For those subjects who reported psychotic symptoms, an additional clinical interview was conducted by an experienced psychiatrist or psychologist (at baseline and at 3-year follow-up). Compared with non-users, individuals using cannabis at baseline were nearly three times more likely to manifest psychotic symptoms at follow-up. This risk remained significant after statistical adjustment for a range of factors, including ethnic group, marital status, educational level, urbanicity (population density) and discrimination. The authors also found a dose-response relationship with the highest risk (odds ratio=6.8) for the highest level of cannabis use. Further analysis revealed that lifetime history of cannabis use at baseline, as opposed to use of cannabis at follow-up, was a stronger predictor of psychosis 3 years later. This suggests that the association between cannabis use and psychosis is not merely the result of short-term effects of cannabis use leading to an acute psychotic episode. Although the use of other drugs was associated with psychosis outcomes, the effects were not significant after taking into account cannabis use. In this study, the short time-lag between baseline and follow-up assessments tends to provide more support for an association between cannabis use and psychosis, rather than verifying temporal priority. The authors concluded that their study confirmed that
‘cannabis use is an independent risk factor for the emergence of psychosis in psychosis-free persons and that those with an established vulnerability to psychotic disorders are particularly sensitive to its effects, resulting in a poor outcome’.
The Christchurch Health and Development Study. The Christchurch study is a general-population birth cohort from New Zealand that has examined the development of its participants for more than 20 years. The association between cannabis dependence disorder and the presence of psychotic symptoms at ages 18 and 21 years was examined, controlling for several potential confounding factors, including previous psychotic symptoms (Fergusson et al, 2003). Statistical control for previous psychotic symptoms clarified the temporal sequence by ruling out the alternative explanation suggesting that psychotic symptoms cause cannabis dependence. Findings indicated concurrent associations between cannabis dependence disorder and risk of psychotic symptoms both at ages 18 and 21 years. Individuals who met the diagnostic criteria for cannabis dependence disorder at age 18 years had a 3.7-fold increased risk of psychotic symptoms than those without cannabis dependence problems. The risk of psychotic symptoms was 2.3 times higher for those with cannabis dependence disorder at age 21 years. Moreover, after controlling for several confounding factors, including anxiety disorder, deviant peer affiliations, exposure to childhood sexual or physical abuse, educational achievement and, most importantly, psychotic symptoms at the previous assessment, the association remained strong and significant at age 21 years. The authors concluded that
‘the findings are clearly consistent with the view that heavy cannabis use may make a causal contribution to the development of psychotic symptoms since they show that, independently of pre-existing psychotic symptoms and a wide range of social and contextual factors, young people who develop cannabis dependence show an elevated rate of psychotic symptoms’.
Dunedin Multidisciplinary Health and Development Study. The Dunedin Multidisciplinary Health and Development Study (Silva & Stanton, 1996) is a study of a general-population birth cohort of individuals born in Dunedin, New Zealand (96% follow-up rate at age 26 years). Although small, this study has unique advantages: it has information on self-reported psychotic symptoms at age 11 years, before the onset of cannabis use; it allows the examination of the age of onset of cannabis use in relation to later outcome, because self-reports of cannabis use were obtained at ages 15 and 18 years; and it does not rely on treatment data for outcomes because the entire cohort was assessed at age 26 years using a standardised psychiatric interview schedule yielding DSM-IV (American Psychiatric Association, 1994) diagnoses (Poulton et al, 2000). This allowed the examination of schizophrenia outcome both as a continuum (by examination of symptoms) and as a disorder (DSM-IV schizophreniform disorder) in this population. In obtaining a schizophreniform diagnosis, the interview protocol ruled out psychotic symptoms occurring while under the influence of alcohol and drugs.
Individuals using cannabis at ages 15 and 18 years had higher rates of psychotic symptoms at age 26 years compared with non-users (Arseneault et al, 2002). This remained significant after controlling for psychotic symptoms pre-dating the onset of cannabis use. The effect was stronger with earlier use. In addition, onset of cannabis use by age 15 years was associated with an increased likelihood of meeting the diagnostic criteria for schizophreniform disorder at age 26 years. Indeed, 10.3% of cannabis users aged 15 years in this cohort were diagnosed with schizophreniform disorder at age 26 years, as opposed to 3% of the controls. After controlling for psychotic symptoms at age 11 years, the risk for adult schizophreniform disorder remained elevated (odds ratio=3.1) but was no longer statistically significant, possibly owing to power limitation.
Cannabis use by age 15 years did not predict depressive outcomes at age 26 years (indicating specificity of the outcome) and the use of other illicit drugs in adolescence did not predict schizophrenia outcomes over and above the effect of cannabis use (indicating specificity of the exposure). A significant exacerbation (or interaction) effect was found between cannabis use by age 18 years and psychotic symptoms at age 11 years (Fig. 1). This effect indicates that cannabis users at age 18 years had elevated scores on the schizophrenic symptom scale only if they had reported psychotic symptoms at age 11 years. The authors concluded that
‘using cannabis in adolescence increases the likelihood of experiencing symptoms of schizophrenia in adulthood’.
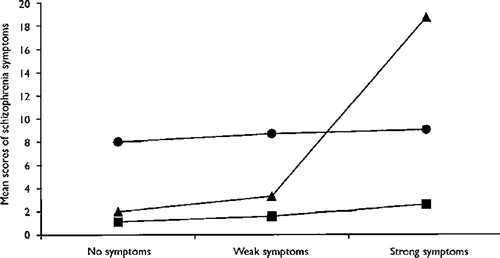
Figure 1. Interaction Between Cannabis Use at Age 18 Years and Psychotic Symptoms at Age 11 Years in Predicting Adult Schizophrenia Symptoms.
DISCUSSION
IS CANNABIS A CAUSAL RISK FACTOR FOR PSYCHOSIS?
In this review we have tried to determine whether cannabis is a cause of schizophrenia. We have shown that all the available population-based studies on the issue have found that cannabis use is associated with later schizophrenia outcomes (Table 1). All these studies support the concept of temporal priority by showing that cannabis use most probably preceded schizophrenia. These studies also provide evidence for direction by showing that the association between adolescent cannabis use and adult psychosis persists after controlling for many potential confounding variables such as disturbed behaviour, low IQ, place of upbringing, cigarette smoking, poor social integration, gender, age, ethnic group, level of education, unemployment, single marital status and previous psychotic symptoms. Further evidence for a causal relationship is provided by the presence of a dose-response relationship between cannabis use and schizophrenia (Andréasson et al, 1988; van Os et al, 2002; Zammit et al, 2002), specificity of exposure (Arseneault et al, 2002; van Os et al, 2002; Zammit et al, 2002; Fergusson et al, 2003) and specificity of the outcome (Arseneault et al, 2002). Overall, cannabis use appears to confer a twofold risk of later schizophrenia or schizophreniform disorder (pooled odds ratio=2.34; 95% CI 1.69–2.95).
Methodological issues. Before discussing further the issue of a causal association between cannabis use and schizophrenia, it is important to point out some methodological limitations in the literature reviewed.
First, various measures of schizophrenia outcome were used in these studies: hospital discharge, pathology level of psychosis, psychotic symptoms and schizophreniform disorder. The heterogeneity of the outcome makes it difficult to draw a firm conclusion on schizophrenia per se from the findings reported by these studies. However, all studies converge in showing an elevated risk for psychosis in later life among cannabis users.
Second, all measures of cannabis use in these studies were based on self-reports and were not supplemented by urine tests or hair analysis. In this situation, under-rather than over-reporting is possible. Therefore, the use of self-reported data would under-estimate the magnitude of the association between cannabis use and later schizophrenia, rather than giving rise to a spurious association. In fact, in the Dunedin study and the Christchurch study participants have learned after many years of involvement with the study that all information they provide remains strictly confidential and therefore their answers are likely to provide a good estimate of actual levels of drug use in those populations (Arseneault et al, 2002; Fergusson et al, 2003).
Third, there is limited information on other illicit drug use. It would be informative to gather more precise information about other illicit drugs used by young people to control more effectively for possible confounding effects of, for example, stimulant drug use. However, difficulties related to statistical power are likely to occur because of the small number of individuals reporting such use.
Fourth, most studies were unable to establish whether prodromal manifestations of schizophrenia preceded cannabis use, leaving the possibility that cannabis use may be a consequence of emerging schizophrenia rather than a cause of it. Findings have indicated that schizophrenia is typically preceded by psychological and behavioural changes years before the onset of diagnosed disease (Jones et al, 1994; Cannon et al, 1997; Malmberg et al, 1998). It is possible, therefore, that cannabis use may be consequent to an early emerging schizophrenia rather than predisposing to its development. Thus, it has become crucial to control for these early signs of psychosis to establish clearly the temporal priority between cannabis use and adult psychosis. Although the Christchurch study applied statistical control for previous psychotic symptoms, it is not clear whether the measure of psychotic symptoms at age 18 years preceded the onset of cannabis use. To date, the Dunedin study is the only study to demonstrate temporal priority by showing that adolescent cannabis users are at increased risk of experiencing schizophrenic symptoms in adult life, even after taking into account the childhood psychotic symptoms that preceded the onset of cannabis use.
Finally, there was limited statistical power in the studies using self-reports of schizophrenia outcomes (in the NEMESIS, the Christchurch and the Dunedin studies) for examining such a rare outcome. It will be important for future studies to examine larger population samples in order to assess a greater number of individuals with psychotic disorders.
Alternative explanations. One might speculate that cannabis is a ‘gateway drug' for the use of harder drugs (Kazuo & Kandel, 1984) and that individuals who use cannabis heavily might also be using other substances such as amphetamines, phenylcyclidine and lysergic acid diethylamide that are thought to be psychotogenic (Murray et al, 2003). Support for this explanation is provided by recent findings showing that the use of other drugs among young adults is almost always preceded by cannabis use (Fergusson & Horwood, 2000). This is especially true for heavy cannabis users (50 times or more per year), who were 140 times more likely to move on to other illicit drugs than people who had not used cannabis before. However, in the Dunedin, Christchurch, Dutch and Swedish studies, the association between cannabis and schizophrenia held even when adjusting for the use of other drugs (Arseneault et al, 2002; van Os et al, 2002; Zammit et al, 2002; Fergusson et al, 2003).
A second possibility is that individuals who use cannabis in adolescence continue to use this illicit substance in adulthood and because cannabis use intoxication can be associated with transient psychotic symptoms (Hall & Degenhardt, 2004; Verdoux, 2004) this could account for the observed association. However, the diagnostic interview used in the Dunedin study explicitly ruled out a diagnosis of schizophreniform disorder if psychotic symptoms occurred only following substance use.
A third possibility is that early-onset cannabis use is a proxy measure for poor premorbid adjustment, which is known to be associated with schizophrenia and other psychiatric outcomes (Cannon et al, 2002). Arseneault et al (2002) found that cannabis use was specifically related to schizophrenia outcomes, as opposed to depression, suggesting specificity in longitudinal association rather than general poor premorbid adjustment, although there is other evidence showing an association between cannabis use and depression (Patton et al, 2002).
What kind of cause is it? We have shown, on the basis of the best evidence currently available, that cannabis use is likely to play a causal role with regard to schizophrenia. However, further questions now arise. How strong is the causal effect and is cannabis use a necessary or sufficient cause of schizophrenia (Rothman & Greenland, 1998)?
The studies reviewed earlier show that cannabis use is clearly not a necessary cause for the development of psychosis, by failing to show that all adults with schizophrenia used cannabis in adolescence. It is also clear that cannabis use is not a sufficient cause for later psychosis because the majority of adolescent cannabis users did not develop schizophrenia in adulthood. Therefore, we can conclude that cannabis use is a component cause, among possibly many others, forming part of a causal constellation that leads to adult schizophrenia.
What might the other component causes be? Unfortunately, we get little insight on component causes other than cannabis from the studies reviewed in this article. Certainly, genes are likely to moderate the association between cannabis use and later psychosis by increasing the susceptibility of schizophrenic outcomes among early-onset cannabis users. However, no study yet has verified an interaction effect between candidate genes and cannabis use. Cannabis use appears to increase the risk of schizophrenia outcomes primarily among those individuals already vulnerable by virtue of pre-existing psychotic symptoms (Arseneault et al, 2002; van Os et al, 2002). A study of French undergraduate university students showed that the acute effects of cannabis were stronger among participants with high vulnerability for psychosis (by virtue of psychotic symptoms) (Verdoux et al, 2003). Such vulnerable participants reported an increased level of perceived hostility and unusual perceptions, and also a decreased level of pleasure associated with the experience of using cannabis. However, this mediator effect (Kraemer et al, 2001) is not a simple one.
Two studies explored the role of cannabis use in the development of psychotic symptoms in groups of young people considered to be at high risk of developing psychotic symptoms. An analysis of the Edinburgh High Risk Study found that both individuals at high genetic risk of schizophrenia (by virtue of two affected relatives) and individuals with no family history of schizophrenia were at increased risk of psychotic symptoms after cannabis use (Miller et al, 2001). An Australian study followed up a group of 100 individuals who asked for help from an early intervention service centre (Phillips et al, 2002). Cannabis use or dependence at entry to the study was not associated with the development of psychotic illness (transition to psychosis) over a 12-month period of follow-up after entry to the study. However, the low level of reported cannabis use among the group could indicate that the sample may not be representative of the population of ‘prodromal' individuals.
How strong is the causal effect? Can we say anything about the strength of the causal effect of cannabis for schizophrenia? We are somewhat hampered in this endeavour because the strength of any particular cause depends on the prevalence of the other component or interacting causes in the population (Rothman & Greenland, 1998). As discussed above, we do not know for certain, at present, any other component causes in the ‘schizophrenia constellation'. We can make some broad suggestions. A component cause, even if it is very common, will rarely cause a disorder if the other component causes in the causal constellation are rare. This will hold regardless of the prevalence of the component cause of interest in the population or its role in the pathophysiology of the disorder. On the other hand, the rarer a component cause relative to its partners in any sufficient cause, the stronger that component cause will appear. Because cannabis use is relatively common in the population but appears to cause schizophrenia rarely, it would follow that at least one of the other component causes in the causal constellation is rare. Indeed, calculation of the overall risk for schizophrenia associated with cannabis use revealed that cannabis use confers only a twofold increase in relative risk for schizophrenia. But does this mean that we should not worry about cannabis as a causal factor?
There is another way of looking at this issue. Once a direct causal relationship between exposure and outcome is assumed, the strength of a particular association from a public health point of view can be assessed with the population attributable fraction. This gives a measure of the number of cases of the disorder in the population that could be eliminated (i.e. would not occur) by removal of a harmful causal factor. The population-attributable fraction for the Dunedin study is 8%. In other words, removal of cannabis use from the New Zealand population aged 15 years would have led to an 8% reduction in the incidence of schizophrenia in that population. The NEMESIS group reported higher population-attributable fractions, possibly because the outcome measures that they used did not exclusively include clinical psychosis cases (i.e. the need for care). However, even 8% is not an insignificant figure from a public health point of view. Because the possibility of eliminating cannabis use totally from the population is rather remote, it may be advisable to concentrate on those for whom adverse outcomes are more common (vulnerable youths).
If cannabis use can cause psychosis, how can we explain that, despite steadily increasing rates of cannabis use over past decades, the incidence of schizophrenia in the population has remained stable? First, with a population-attributable fraction of 8% the causal influence of cannabis use on the incidence of schizophrenia is probably not easily visible in the general population. Second, the Dunedin study showed that cannabis use in early adolescence (first reported use at age 15 years) was associated with the strongest effects on schizophrenia outcomes. Trends of cannabis use among adolescents in the USA indicate that cannabis use under the age of 16 years is a fairly new phenomenon that has appeared only since the early 1990s (Johnston et al, 2002). One would therefore predict an increase in rates of schizophrenia in the general population over the next 10 years. Indeed, there is already some evidence that the incidence of schizophrenia is currently increasing in some areas of London, especially among young people (Boydell et al, 2003).
Although the majority of young people are able to use cannabis in adolescence without harm, a vulnerable minority experience harmful outcomes. The epidemiological evidence suggests that cannabis use among psychologically vulnerable young adolescents should be strongly discouraged by parents, teachers and health practitioners alike. Findings also suggest that the youngest cannabis users are most at risk (Arseneault et al, 2002), perhaps because their cannabis use becomes longstanding. This should encourage policy and law makers to concentrate their effort on delaying the onset of cannabis use. At the same time, further research is needed on the long-term impact of frequent cannabis use that begins at an early age and on the possible mechanisms by which cannabis use can lead to psychosis.
American Psychiatric Association (1994) Diagnostic and Statistical Manual of Mental Disorders (4th edn) (DSM-IV). Washington, DC: American Psychiatric Assocation.Google Scholar
Andréasson, S., Allebeck, P., Engström, A., et al (1988) Cannabis and schizophrenia: a longitudinal study of Swedish conscripts. Lancet, ii, 1483– 1485.Google Scholar
Arseneault, L., Cannon, M., Poulton, R., et al (2002) Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ. 325, 1212– 1213.Google Scholar
Boydell, J., van Os, J., Lambri, M., et al (2003) Incidence of schizophrenia in south-east London between 1965 and 1997. British Journal of Psychiatry, 182, 45– 49.Crossref, Google Scholar
Cannon, M., Jones, P., Gilvarry, C., et al (1997) Premorbid social functioning in schizophrenia and bipolar disorder: similarities and differences. American Journal of Psychiatry. 154, 1544– 1550.Google Scholar
Cannon, M., Caspi, A., Moffitt, T. E., et al (2002) Evidence for early, specific, pan-developmental impairment in schizophreniform disorder: results from a longitudinal birth cohort. Archives of General Psychiatry. 59, 449– 457.Crossref, Google Scholar
Cantwell, R., Brewin, J., Glazebrook, C., et al (1999) Prevalence of substance misuse in first-episode psychosis. British Journal of Psychiatry. 174, 150– 153.Crossref, Google Scholar
D'Souza, C., Cho, H.-S. Perry, E., et al (2004) A cannabinoid model psychosis, dopamine-cannabinoid interactions and implications for schizophrenia. In Marijuana and Madness (eds D. J. Castle & R. Murray). Cambridge: Cambridge University Press. In press.Google Scholar
Duke, P. J., Pantelis, C., McPhillips, M. A., et al (2001) Comorbid non-alcohol substance misuse among people with schizophrenia. Epidemiological study in central London. British Journal of Psychiatry. 179, 509– 513.Crossref, Google Scholar
Fergusson, D. M. & Horwood, L. J. (2000) Does cannabis use encourage other forms of illicit drug use? Addiction. 95, 505– 520.Crossref, Google Scholar
Fergusson, D. M., Horwood, L. J. & Swain-Campbell, N. R. (2003) Cannabis dependence and psychotic symptoms in young people. Psychological Medicine, 33, 15– 21.Crossref, Google Scholar
Grech, A., Takei, N. & Murray, R. (1998) Comparison of cannabis use in psychotic patients and controls in London and Malta. Schizophrenia Research. 29, 22.Google Scholar
Hall, W. & Degenhardt, L. (2000) Cannabis use and psychosis: a review of clinical and epidemiological evidence. Australian and New Zealand Journal of Psychiatry. 34, 26– 34.Crossref, Google Scholar
Hall, W. & Degenhardt, L. (2004) Is there a specific ‘cannabis psychosis'? In Marijuana and Madness (eds D. J. Castle & R. Murray). Cambridge: Cambridge University Press. In press.Google Scholar
Hambrecht, M. & Hafner, H. (1996) Substance abuse and the onset of schizophrenia. Biological Psychiatry. 40, 1155– 1163.Crossref, Google Scholar
Hill, A. B. (1965) The environment and disease: association or causation? Proceedings of the Royal Society of Medicine. 58, 295– 300.Google Scholar
Johns, A. (2001) Psychiatric effects of cannabis. British Journal of Psychiatry, 178, 116– 122.Crossref, Google Scholar
Johnston, L. D., O'Malley, P. M. & Bachman, J. G. (2002) Monitoring the Future National Results on Adolescent Drug Use: Overview of Key Findings 2001 (NIH Publication No. 02-5105). Bethesda. MD: National Institute of Drug Abuse.Google Scholar
Jones, P., Rodgers, B., Murray, R., et al (1994) Child developmental risk factors for adult schizophrenia in the British 1946 birth cohort. Lancet, 344, 1398– 1402.Crossref, Google Scholar
Kazuo, Y. & Kandel, D. B. (1984) Patterns of drug use from adolescence to young adulthood: II. sequences of progression. American Journal of Public Health. 74, 668– 672.Crossref, Google Scholar
Kraemer, H., Stice, E., Kazdin, A., et al (2001) How do risk factors work together? Mediators, moderators, and independent, overlapping, and proxy risk factors. American Journal of Psychiatry, 158, 848– 856.Crossref, Google Scholar
Malmberg, A., Lewis, G., David, A., et al (1998) Premorbid adjustment and personality in people with schizophrenia. British Journal of Psychiatry. 172, 308– 313.Crossref, Google Scholar
Mathers, D. C. & Ghodse, A. H. (1992) Cannabis and psychotic illness. British Journal of Psychiatry. 161, 648– 653.Crossref, Google Scholar
McCreadie, R. G. (2002) Use of drugs, alcohol and tobacco by people with schizophrenia: case-control study. British Journal of Psychiatry. 181, 321– 325.Crossref, Google Scholar
Menezes, P. R., Johnson, S., Thornicroft, G., et al (1996) Drug and alcohol problems among individuals with severe mental illnesses in south London. British Journal of Psychiatry. 168, 612– 619.Crossref, Google Scholar
Miller, P., Lawrie, S. M., Hodges, A., et al (2001) Genetic liability, illicit drug use, life stress and psychotic symptoms: preliminary findings from the Edinburgh study of people at high risk for schizophrenia. Social Psychiatry and Psychiatric Epidemiology, 36, 338– 342.Crossref, Google Scholar
Murray, R., Grech, A., Phillips, P., et al (2003) What is the relationship between substance abuse and schizophrenia? In The Epidemiology of Schizophrenia (eds R. Murray, P. Jones, E. Susser, et al, pp. 317– 342. Cambridge: Cambridge University Press.Google Scholar
Patton, G. C., Coffrey, C., Carlin, J. B., et al (2002) Cannabis use and mental health in young people: cohort study. BMJ. 325, 1195– 1198.Crossref, Google Scholar
Phillips, L. J., Curry, C., Yung, A. R., et al (2002) Cannabis use is not associated with the development of psychosis in an ‘ultra' high-risk group. Australian and New Zealand Journal of Psychiatry, 36, 800– 806.Crossref, Google Scholar
Poulton, R., Caspl, A., Moffitt, T. E., et al (2000) Children's self-reported psychotic symptoms and adult schizophreniform disorder: a 15-year longitudinal study. Archives of General Psychiatry, 57, 1053– 1058.Crossref, Google Scholar
Regier, D., Farmer, M. E., Rae, D. S., et al (1990) Comorbidity of mental disorders with alcohol and other drug abuse: results from the epidemiologic catchment area (ECA) study. Journal of the American Medical Association. 264, 2511– 2518.Google Scholar
Robins, L. N. & Regler, D. A. (1991) Psychiatric Disorders in America: the Epidemiologic Catchment Area Study. New York: The Free Press.Google Scholar
Rothman, K. J. & Greenland, S. (1998) Modern Epidemiology (2nd edn). Philadelphia, PA: Lippincott-Raven.Google Scholar
Silva, P. A. & Stanton, W. R. (1996) From Child to Adult: the Dunedin Multidisciplinary Health and Development Study. Auckland: Oxford University Press.Google Scholar
StataCorp (2003) Stata Statistical Software: Release 8.0. Texas: Stata Corporation.Google Scholar
Sterne, J. A. C., Bradburn, M. J. & Egger, M. (2001) Meta-analysis in StataTM. In Systematic Review in Health Care: Meta Analysis in Context (eds M. Egger, G. D. Smith & D. G. Altman), pp. 347– 369. London: BMJ Books.Google Scholar
Susser, M. (1991) What is a cause and how do we know one? A grammar for pragmatic epidemiology. American Journal of Epidemiology. 133, 635– 648.Crossref, Google Scholar
Thornicroft, G. (1990) Cannabis and psychosis. Is there epidemiological evidence for an association? British Journal of Psychiatry. 157, 25– 33.Google Scholar
Tien, A. Y. & Anthony, J. C. (1990) Epidemiological analysis of alcohol and drug use as risk factors for psychotic experiences. Journal of Nervous and Mental Disease. 178, 473– 480.Google Scholar
van Os, J., Bak, M., Bijl, R. V., et al (2002) Cannabis use and psychosis: a longitudinal population-based study. American Journal of Epidemiology, 156, 319– 327.Crossref, Google Scholar
Verdoux, H. (2004) Cannabis and psychosis proneness. In Marijuana and Madness (eds D.J. Castle & R. Murray). Cambridge: Cambridge University Press.Google Scholar
Verdoux, H., Gindre, C., Sorbara, F., et al (2003) Effects of cannabis and psychosis vulnerability in daily life: an experience sampling test study. Psychological Medicine. 33, 23– 32.Crossref, Google Scholar
Wheatley, M. (1998) The prevalence and relevance of substance use in detained schizophrenic patients. Journal of Forensic Psychiatry. 9, 114– 129.Crossref, Google Scholar
Zammit, S., Allebeck, P., Andréasson, S., et al (2002) Self-reported cannabis use as a risk factor for schizophrenia: further analysis of the 1969 Swedish conscript cohort. BMJ. 325, 1199– 1201.Crossref, Google Scholar




