Preclinical Development and Clinical Implementation of Treatments for Substance Abuse Disorders
Abstract
Substance abuse continues to be a major source of morbidity and mortality around the world. Preclinical research on substance abuse continues to focus on determining the neurobiological substrates underlying this disorder and using this information to develop new and more efficacious treatment strategies. Studying drug self-administration in laboratory animals is a powerful approach with clear-cut and dynamic links between preclinical findings and substance abuse in humans. It has been and will continue to be a valuable procedure for developing pharmacological and nonpharmacological treatment modalities for substance abuse. Currently, pharmacological treatment of substance abuse disorders includes drugs for replacement therapy, drugs that produce aversive effects, or drugs that alter or modulate neurotransmission in systems involved in the rewarding effects of abused drugs. Pharmacological agents are in various stages of testing or are now available for the treatment of nicotine, opioid, ethanol, and stimulant dependence. The most effective treatments of substance abuse disorders also include psychosocial interventions. Cognitive behavior therapy has been found to be efficacious and comparable to other psychosocial intervention approaches, including 12-step facilitation and motivational enhancement therapy. Family therapy and 12-step involvement appear to have positive incremental effects on treatment outcomes, and motivational interventions promote improvement in substance use largely through their effects on treatment adherence. The development and implementation of more successful treatments for substance abuse disorders will involve new discoveries on the fundamental mechanisms underlying substance abuse combined with the refinement of psychosocial interventions.
PRECLINICAL MODELS AND MEDICATION DEVELOPMENT
PRECLINICAL STUDIES OF SUBSTANCE ABUSE
In terms of assessing the abuse liability of new drugs, and developing treatment modalities for substance abuse, drug self-administration studies in laboratory animals have been and will continue to be a valuable strategy. Studying drug self-administration is a powerful approach with clear-cut and dynamic links between preclinical findings and human conditions. Discoveries in preclinical studies can rapidly proceed to potential clinical interventions. One of the problems is translating terms used in substance abuse in humans to behavioral responses obtained in laboratory animals. For example, substance abusers speak about “craving,” “wanting,” or “liking” a drug. These are internal states inferred by verbal reports provided by the user and cannot be applied directly to any animal model. Because a goal of pharmacotherapy of substance abuse is to reduce craving, wanting, and liking, this limitation is not trivial.
VALIDITY OF DRUG SELF-ADMINISTRATION PROCEDURES IN LABORATORY ANIMALS
When viewed in isolation, drug self-administration in laboratory animals is not a model of a behavior, it is a phenomenon in itself that can be quantified and studied. The relevant issue is how well it models drug self-administration in humans. In this respect, as with all animal models, drug self-administration studies are subject to the issues of validity. Three types of validity have been defined: face, predictive, and construct validity (1). Face validity reflects the degree of similarity between the behaviors modeled and the symptoms of the disease, i.e., substance abuse. There is a high level of face validity between drug self-administration in laboratory animals and humans. At the most basic level, the frequency and amount of drug intake can be measured in laboratory animals as well as in humans. Predictive validity describes the extent to which the model responds appropriately to drugs compared with the responses in humans. For drugs that have abuse liability in humans, self-administration should be maintained in animal models and vice versa. With some exceptions, self-administration procedures have been demonstrated to have very high predictive validity for assessing the abuse liability of drugs in humans (2, 3). In addition, substances or treatments that reduce drug self-administration in laboratory animals should reduce drug use in humans. Construct validity indicates that the theoretical rationale underlying the model is analogous to the pathophysiological process that occurs in the human. Because the pathophysiological processes underlying substance abuse in humans remain unknown, construct validity has not been established. An ideal animal model would resemble the human condition in terms of etiology, symptomatology, and treatment response, thus displaying construct, face, and predictive validity.
STRATEGIES USING DRUG SELF-ADMINISTRATION PROCEDURES
One common feature of many drugs of abuse is that they produce positive subjective effects in humans and can serve as positive reinforcers in laboratory animals. Drug self-administration procedures are well established, they have been standardized among laboratories to varying degrees, and they are used widely for studying the reinforcing effects of a variety of drugs (4–7). Rats and nonhuman primates are most commonly used, but the development of knockout and transgenic mice has stimulated investigators to develop techniques to study drug self-administration in mice. In most studies the drug is administered by intravenous infusion. With some drugs this is the same route of administration used in human substance abusers (e.g., heroin). With other drugs intravenous administration mimics the pharmacokinetic profile of the drug in humans with respect to absorption and distribution. For example, an intravenous route of administration is a valid approach for studying nicotine self-administration in the rat because the absorption of nicotine and delivery to the brain is rapid after smoking in humans, as after intravenous administration (8). Oral drug self-administration is more appropriate with some drugs of abuse (e.g., ethanol).
Typically, for a self-administration session the animal is placed into an operant apparatus and upon successful completion of a task (e.g., pressing a lever or poking their nose into a hole), the animal receives drug (the reinforcement or “reward”). The experiment can be designed so that the number of times the animal has to make the appropriate response to get the reward is fixed. If the animal has to press the lever only one time to get the reward, this is called a fixed ratio 1 schedule of reinforcement. In most cases, the ratio is held constant within a given session; however, the response criteria can be raised across sessions. For example, the ratio could be increased to 5 (i.e., fixed ratio 5). The type of information obtained during a fixed ratio drug self-administration session is the latency to the first reinforcement, the total number of reinforcements earned per session, and the total intake of drug per session.
When multiple fixed ratio sessions are conducted over time, this type of procedure can be used to study individual components of the phenomenon of substance abuse. For example, the acquisition of drug self-administration in laboratory animals has obvious analogies with the development of drug dependence in humans. The genetic and environmental risk factors that lead to the acquisition of drug self-administration can be studied. Once drug self-administration is stable (maintenance in laboratory animals; addiction/dependence in humans), saline can be delivered in place of drug (extinction). The animal eventually stops responding, and the behavior during this phase has been used as a model of withdrawal/cessation of drug use in humans. Following extinction, drug can be delivered again after the appropriate behavioral response, and reinstatement of drug self-administration (a model of relapse; see below) can be studied.
A complementary approach to using a fixed ratio schedule is the progressive ratio schedule of reinforcement (9). When this schedule is used within a session, the number of times the animal has to make the appropriate response to get the reward (the ratio) is increased after each reinforcement. The sequence usually is derived from a mathematical formula; typical response requirements for a progressive ratio schedule within a session could be 1, 5, 10, 17, 24, 32, 56, 73, 95, 124, 161, 208, 270, etc (9, 10). With this type of schedule, eventually the animal gives up responding, and a “breakpoint,” defined as the final ratio completed by the end of the session, is determined. The breakpoint has been used as a measure of how hard an animal is willing to “work” to get the reinforcer, which might have some analogy to “liking” and “wanting” a drug in humans.
Many factors can be manipulated in drug self-administration sessions, including: session length, the amount of time that must elapse between successive reinforcements (time out), and the use of cues (e.g., tone and/or lights). Usually specific cues are associated with the beginning and end of the session, the administration of drug, and the time-out period. All of these variables can have profound effects of the behavioral responses. Complex behavioral paradigms can be constructed in which the animal can choose between drug and other nondrug reinforcers (e.g., food or water). These can provide information on the relative value of a drug compared with alternate reinforcers and indicate the “response cost” and what the animal is “willing to give up” to receive drug (11–13).
USING DRUG SELF-ADMINISTRATION TO DISCOVER POTENTIAL THERAPEUTIC AGENTS
Drug self-administration paradigms can be used to test medications that might be useful as replacement agents or antagonists or might produce aversive effects that could reduce drug use (14). For example, if the animal is trained to self-administer heroin, then pretreatment with an opioid antagonist (see below) should reduce responding in a fixed ratio self-administration session. This reduction would indicate that heroin is less reinforcing in the presence of the antagonist and could translate to an addict getting less “high” at a given dose of heroin or having to take more heroin to get an equivalent “high” in the presence of the drug. In progressive ratio studies, an effective antagonist would produce a decrease in the breakpoint. Drugs can be tested during different components of drug self-administration (i.e., acquisition, maintenance, extinction, and reinstatement) to determine whether they have special utility during any of these phases. Experiments can be constructed to determine whether potential therapeutic agents can affect the choice between drug reinforcement and alternative reinforcers.
THE REINSTATEMENT MODEL OF RELAPSE
Many of the techniques described above can be used to discover medications that might be useful for treating ongoing drug use. However, the high vulnerability to relapse presents a formidable challenge. It is clear that more than the reinforcing properties of a drug are involved in relapse. In addition, symptoms of withdrawal are not consistently related to relapse and cannot explain relapse that occurs after weeks or months of abstinence (15). Developing pharmacological interventions aimed at reducing relapse might be the most effective approach to treating substance abuse.
The reinstatement model has been used to study the factors involved in drug relapse and to investigate and define the neuroanatomical and neurochemical substrates underlying drug-seeking behavior (14, 16–22). There is good correspondence between some of the events that induce reinstatement of drug-seeking behavior in laboratory animals and those that provoke relapse in humans, such as stress, re-exposure to drug (drug priming), and drug-associated cues (23, 24). Because the mechanisms underlying reinstatement of drug-seeking behavior are different from those mediating the direct reinforcing actions of drugs, the reinstatement model might be useful in developing drugs that specifically reduce relapse in abstinent individuals.
In the reinstatement model of relapse, the animal is trained to self-administer drug and then the drug-reinforced behavior is extinguished by withholding the delivery of drug (i.e., extinction). During reinstatement testing, individual factors that reinitiate drug-seeking behavior can be studied; however, it is important to point out that during testing the drug remains unavailable (i.e., lever pressing does not result in the infusion of drug). It is well known that stressful situations can cause relapse in human substance abusers. In the reinstatement model, stress can reinitiate responding after extinction even in the absence of drug reward. Therefore, this model can be used to study the factors underlying stress-induced reinitiation and test for drugs that might be efficacious in treating this relapse. Although it might take months or years for a substance abuser to reach a certain level of dependence, this same level of dependence is reachieved very rapidly after relapse, even in individuals who have remained abstinent for long periods of time. In the reinstatement model, a single “priming” injection of drug can reinstate robust responding, again in the absence of drug reward contingent upon responding. Thus, priming-induced reinstatement might be a useful model to study relapse after reexposure to drug in humans.
In substance abusers, cues can elicit subjective states that can trigger craving, drug-seeking behavior and the resumption of drug use. Relapse is often associated with exposure to drug-related environmental stimuli. The conditioned responses elicited by drug-associated cues might be one of the most important factors underlying the high rates of relapse after abstinence. This is especially true with regard to nicotine abuse. For instance, both abstinent and nonabstinent smokers have increased desire to smoke (craving) in response to cues such as a lit cigarette resting in an ashtray (25). The power of smoking-associated cues is demonstrated by a study showing that smoking de-nicotinized cigarettes (i.e., “cue alone”) produced a high level of satisfaction, whereas the intravenous administration of nicotine (i.e., “nicotine alone”) had no effect (26). With respect to the treatment of nicotine addiction, Tiffany et al. (27) found that transdermal nicotine patches attenuated only withdrawal-induced craving but not the craving induced by nicotine cues. Reexposure to environmental stimuli and cues previously associated with drug self-administration can produce robust and reproducible reinstatement of extinguished drug-seeking behavior in animal models, and procedures have been developed to study cue-induced reinstatement of nicotine-seeking (28, 29). These procedures might be useful in identifying pharmacological strategies to reduce cue-induced craving for nicotine and subsequent relapse.
HUMAN ADDICTION PSYCHOPHARMACOLOGY
ADDICTION IS A BRAIN DISEASE
Although the initial use of any substance is a voluntary choice, with continued administration of large amounts of that substance, that choice becomes progressively less voluntary and more influenced by the brain's adaptation to the chronic presence of the substance. This adaptation appears to create a drive to maintain the substance-induced homeostasis; a drive that uses brain mechanisms to see, hear, smell, or otherwise identify a cue that may lead to obtaining the substance. Numerous studies have shown the impact of chronic, long-term administration of drugs of abuse in both human and animal models (30). Changes in the quantity and functionality of neurotransmitter receptor systems and the quantity of the neurotransmitters themselves eventually yield a state of physiological dependence, a state marked by a biologically based drive for the exogenous substance of abuse to maintain this new homeostasis. Failure to fulfill that drive can cause the organism to experience withdrawal, an unpleasant state marked by specific symptoms that vary according to substance but are relieved by that drug or a close substitute; thus, in conditioning terms, the alleviation of withdrawal symptoms provides negative reinforcement (reinforced behavior due to the removal of a negative stimulus) for continued use of a drug. Physiological dependence is one of the key factors driving nicotine, alcohol, and opioid dependence. Even when an individual is detoxified from a substance, craving for that substance can lead to a return to drug use. Craving can be triggered by stress, environmental cues (31), or small amounts of the drug itself (32). Thus, another goal of addiction psychopharmacology is to minimize the craving that can lead to relapse.
REPLACEMENT THERAPY—FULL AGONISTS
The withdrawing brain does not know what chemical may be binding to and stimulating its receptors—e.g., in the case of heroin withdrawal, little distinction is made between heroin and methadone—both will relieve the body aches, nausea, diarrhea, anxiety, and elevated pulse that comprise opioid withdrawal in general. That is, the pharmacodynamics of heroin and methadone are similar: they have similar activity at the μ-opioid receptor. However, they are different in several important ways. Methadone is less potent, has a higher affinity, and its effects last longer at the μ-opioid receptor than heroin (its half-life is 36 hours compared with 1 hour for heroin). This is the basis of replacement therapy: a drug of abuse can be replaced with a medication that has a longer duration of action, less abuse potential, and a better safety profile to prevent drug withdrawal and craving. Indeed, many studies support the use of methadone treatment for heroin and other opioid dependence because it reduces incidence of HIV infection (33), intravenous drug use (34), unsafe sex (35), and crime (36). An active heroin user costs society roughly 10 times that of a person in a methadone treatment program (37).
Similarly, replacement therapy has been used with success in tobacco dependence as well, in the form of nicotine replacement therapy (38). In this case, the active chemical in both the substance of abuse and the medication being taken is the same: nicotine. The key difference between tobacco and nicotine replacement therapy is the exposure to carcinogens and other aromatic hydrocarbons that carry with them risks of developing cancer, lung disease, heart disease, and many other serious illnesses. The most common form of nicotine replacement therapy is the nicotine patch, which, analogous to methadone, ensures the delivery of a constant supply of the chemical the dependent brain craves, while eliminating most of the risk associated with the drug of abuse, in this case, tobacco (39). Other, more rapidly acting forms of nicotine replacement therapy, include nicotine gum, lozenges, nasal spray, and inhalers.
REPLACEMENT THERAPY—PARTIAL AGONISTS
Opioid dependence. In contrast to full agonists such as methadone and nicotine, which are chemicals that bind to a class of receptors and stimulate them fully to produce their effects, there exists a class of medications that both stimulate and block brain receptors. These partial agonists, newly approved by the U.S. Food and Drug Administration (FDA), are available to treat opioid and nicotine dependence (see discussion below). The effects of full agonists such as methadone are the same as those typically seen with opioids, i.e., sedation, euphoria, constipation, analgesia, tolerance, and, unfortunately, respiratory depression. The latter effect means that use of methadone carries with it a real risk of overdose, especially when it is combined with other sedating drugs, such as benzodiazepines (40). Because of this and other risks (e.g., diversion of prescribed methadone for sale on the street), methadone can only be legally prescribed for the treatment of opioid dependence by federally licensed methadone clinics (it can, however, be prescribed on an outpatient basis for the treatment of pain).
Buprenorphine is a partial agonist at the μ-opioid receptor—i.e., it binds to the same receptors as other opioids but only partially stimulates the receptors. In the literature, this action is sometimes referred to as an agonist-antagonist effect. The partial μ-opioid receptor stimulation does treat craving for opioids. However, because it is only partial stimulation, there is a limit to its opioid effect, called the “ceiling effect” (41), even at 100% μ-opioid receptor saturation (Table 1). This limit to its μ-opioid activity allows buprenorphine to have a much better safety profile, and patients report better functioning because of the absence of sedation seen with methadone and other full agonists. The high affinity of buprenorphine for μ-opioid receptors also diminishes the positive reinforcement that taking any additional opioids will provide (42). As a result of this improvement in safety profile and abuse liability, the federal government designated buprenorphine as the first medication available to treat opioid dependence on an outpatient basis. By taking an 8-hour course, physicians can qualify for a waiver, under the Drug Addiction Treatment Act of 2000 (DATA 2000), which allows them buprenorphine prescription privileges.
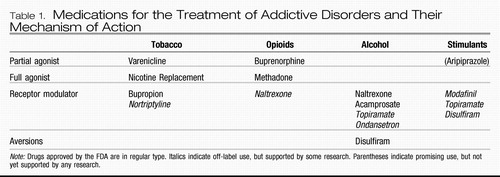 |
Table 1. Medications for the Treatment of Addictive Disorders and Their Mechanism of Action
An important note about buprenorphine is that it should only be administered initially when the patient is in withdrawal, so that it will improve withdrawal symptoms. If given to an opioid-dependent person who has enough full-agonist opioids on the receptors, the higher-affinity buprenorphine will displace the full agonist, immediately precipitating withdrawal (42). Therefore, it is recommended that induction onto buprenorphine be done in a physician's office with frequent assessment of withdrawal using a validated scale such as the Clinical Opioid Withdrawal Scale.
The availability of several preparations of buprenorphine is a frequent point of confusion among the lay public and physicians alike. The trade names for buprenorphine (sometimes called “bup”) preparations are Subutex and Suboxone. Both are pills taken sublingually that come in 2- and 8-mg sizes, and the dose given is usually between 2 and 32 mg/day. Subutex contains only buprenorphine; Suboxone also contains naloxone, a medication that is only active parenterally. The naloxone is included to prevent Suboxone from being ground up, dissolved, and injected, a method sometimes used by those seeking to maximize the speed of onset and effects of a drug. When taken sublingually, as directed, the naloxone has no activity; however, if injected, the naloxone will block the effects of buprenorphine and, in an opioid-dependent individual, will precipitate an immediate and unpleasant opioid withdrawal (43).
Stimulant dependence. Aripiprazole is an FDA-approved medication indicated for use in schizophrenia and bipolar disorder, which, as a partial D2 receptor agonist, has attracted some interest as a potential treatment for cocaine and methamphetamine dependence. Although it has been shown to reduce some of the pleasurable effects of d-amphetamine (45), there have not as yet been any studies showing a decrease in methamphetamine use. There is also no evidence of any efficacy for decreasing cocaine use.
RECEPTOR MODULATORS
Nicotine dependence. Receptor modulators are the other mainstay of addiction psychopharmacology. The first medication to gain FDA approval using the mechanism of receptor modulation was bupropion. The antidepressant efficacy of bupropion is thought to occur via its inhibition of norepinephrine and dopamine reuptake. Because of the importance of dopamine in the maintenance of addictive behaviors, inhibition of dopamine uptake is also thought to be a key mechanism in its action as an anti-nicotine craving agent (46). Interestingly, it also exhibits some antagonism at the α4β2 nicotinic acetylcholine receptors, which may also play some role in its anticraving properties (46). Dosing for bupropion for addiction is equivalent to that for its use in depression.
Alcohol dependence. Several treatments are available for the treatment of alcohol dependence. The first to be approved by the FDA was disulfiram in 1948. Disulfiram is an aldehyde dehydrogenase inhibitor, which allows the buildup of acetaldehyde, the by-product of alcohol metabolism responsible for the symptoms of hangover with which most drinkers are familiar: nausea, headache, flushing, and so on. The psychological effect at play is punishment, and, consistent with learning theory, it is not a very good way to extinguish drinking behavior. Better results are obtained with disulfiram with supervision by a spouse (47) and motivated populations (48). The serious side effects of hepatotoxicity and cardiovascular effects can be mitigated by using lower doses, such as 125–250 mg daily (48).
Almost 50 years later, in December 1994, the second medication for the treatment of alcohol dependence was approved by the FDA. This medication is the oral form of naltrexone which is usually started and maintained at 50 mg/day. Starting the medication at a half-dose and then titrating up to the full dose can reduce its minor gastrointestinal side effects (49). In April 2006, a monthly depot injectable form of naltrexone was approved. This was an exciting development because poor treatment response to oral naltrexone is usually due to poor medication adherence (49). Depot naltrexone is given at a dose 380 mg i.m. per month and is safe and effective, even in the 1st month (50). Naltrexone is an antagonist at the μ-opioid receptor and is thought to reduce drinking by blocking the craving for and euphoria from alcohol consumption (51). Several double-blind, placebo-controlled trials of naltrexone have demonstrated that naltrexone increases the time to relapse and decreases alcohol consumption and the quantity and frequency of drinking among alcoholics who relapse (52).
Acamprosate gained FDA approval for the treatment of alcohol dependence in July 2004, although it has been used in Europe for more than 10 years. Acamprosate is thought to have its anticraving efficacy as a partial agonist at the N-methyl-d-aspartate receptor (53), a key receptor system that is dysregulated in alcohol dependence (54). The effectiveness of acamprosate in helping patients maintain abstinence from alcohol is supported by several randomized, controlled, clinical trials, reviewed in a meta-analysis by Mann et al. (55). Unfortunately, in a recent large multisite trial comparing it to naltrexone and the combination, acamprosate did not separate from placebo (56), although questions have been posed as to whether the study design allowed the effectiveness of acamprosate to be recognized (57). It is given at a dose of 666 mg three times daily. Side effects are mainly mild gastrointestinal symptoms, such as nausea, diarrhea, and flatulence. Other receptor modulators showing some positive data include topiramate (58) and ondansetron (59), although these have not yet been approved by the FDA.
Stimulant dependence. No medications are FDA-approved for the treatment of dependence on stimulants, e.g., cocaine and methamphetamine. However, modafinil (60), topiramate (61), and disulfiram (62) did show some positive results in pilot trials in cocaine dependence. Because the mechanism of action of the drugs and withdrawal phenomena are similar, results from cocaine trials are hopeful for methamphetamine dependence. Whereas cocaine inhibits reuptake of dopamine and norepinephrine, methamphetamine inhibits reuptake and directly stimulates the release of these catecholamines.
Modafinil is an FDA-approved treatment for somnolence associated with narcolepsy, obstructive sleep apnea, and shift-work sleep disorder. It is thought to blunt cocaine euphoria and increase abstinence via its glutamate-enhancing action and dopamine reuptake inhibition. In their study of 62 cocaine-dependent patients, Dackis et al. (60) found that subjects randomly assigned to modafinil 200 mg b.i.d. had significantly more clean urine drug screens and were more likely to have prolonged abstinence than those taking placebo. A larger study is currently underway to confirm these results.
Topiramate is an anticonvulsant medication that has γ-aminobutyric acid-agonist activity and inhibits glutamatergic neurotransmission, neurotransmitter systems thought to be involved in the rewarding effects of stimulants. Kampman et al. (63) have published pilot data on the efficacy of 200 mg/day of topiramate versus placebo in 40 cocaine-dependent patients. Topiramate-treated patients were more likely to be abstinent from cocaine and more likely to sustain three continuous weeks of abstinence than patients treated with placebo. Kampman et al. are also conducting a larger randomized, placebo-controlled trial of topiramate for the treatment of patients with both cocaine and alcohol dependence.
In addition to its properties as an aldehyde dehydrogenase inhibitor, disulfiram increases dopamine levels through an indirect mechanism, which could explain its efficacy in reducing cocaine use (62).
BEHAVIORAL THERAPIES
PSYCHOSOCIAL INTERVENTIONS
The psychosocial component of treatment is thought to be essential to optimize clinical outcomes. As such, most studies of pharmacological interventions for substance-related disorders include a psychosocial intervention component. Among the most commonly used approaches are cognitive and behavioral therapies, motivational interventions, 12-step programs, and family therapy. Although intervention techniques vary according to the approach, many of these modalities overlap in terms of content and therapeutic objectives. Common objectives include engaging and retaining the patient in the treatment process, facilitating awareness of the negative consequences of substance use, and supporting lifestyle and psychological changes consistent with the goal of reducing or eliminating substance use. Treatment can vary by intensity, setting (i.e., inpatient or outpatient), and modality (i.e., group or individual).
COGNITIVE BEHAVIORAL THERAPIES
Cognitive behavior therapy was initially developed for the treatment of depression (64), and its use was subsequently extended and formalized as an approach to treating substance use disorders by Marlatt and Gordon (65). From the cognitive behavior perspective, substance dependence emerges from a process of social learning and/or through an operant conditioning mechanism whereby the individual learns, through experience, about the reinforcement value of the substance (66). Anticipated reinforcement is thought to drive continued and problematic behavioral patterns of substance use, particularly in individuals with coping skills deficits. Cognitive behavior therapy therefore focuses on coping skills training with the goal of facilitating abstinence from substance use. To achieve this, the therapist helps the substance user to identify maladaptive cognitions and behaviors that promote substance use and to modify them. The key components of this approach include
| 1. | identifying the cognitive, emotional, and behavioral antecedents and consequences of substance use | ||||
| 2. | teaching the substance user cognitive and behavioral coping skills to alter his or her response to typical antecedents to substance use (i.e., “triggers”) | ||||
| 3. | rehearsing these skills in treatment, and | ||||
| 4. | giving assignments between session exercises (i.e., “homework”) to monitor and evaluate implementation of new coping skills (67). | ||||
Cognitive behavior therapy has also been extended to understand the relapse process. As such, the cognitive behavior therapy relapse prevention therapist helps the substance user to identify conditions surrounding an initial return to substance use (i.e., “lapse”) following a period of abstinence, with the goals of:
| 1. | reducing the likelihood of subsequent lapses and | ||||
| 2. | preventing the continuation of a lapse into an extended episode of substance use or full-blown “relapse.” | ||||
To achieve this, relapse prevention emphasizes
| 1. | identification of risky situations in which temptation to use substances is likely | ||||
| 2. | coping skills to manage urges or cravings for substances | ||||
| 3. | assertiveness skills to manage risky situations and to refuse offers of alcohol and/or drugs, and | ||||
| 4. | coping with substance lapses. | ||||
Among individuals with stimulant (68) and alcohol use disorders (69), cognitive behavior therapy has been shown to be comparable to other behavioral and motivational psychosocial treatment approaches in facilitating reductions in substance use during treatment. Studies of cognitive behavior therapy relapse prevention similarly confirm its effectiveness in reducing substance use and improving psychosocial functioning, although it is not consistently superior to other intervention techniques (70). In a meta-analysis of 26 clinical trials, the effects of cognitive behavior therapy relapse prevention were found to be significantly better in the treatment of alcohol and polydrug use relative to cocaine use (71).
CONTINGENCY MANAGEMENT
Like the cognitive behavior therapy model, the theoretical basis for contingency management is rooted in principles of operant learning. According to this framework, operant behavior is largely formed by its reinforcement contingencies (i.e., its immediate pleasurable consequences, e.g., substance use → positive affective experience). The intervention therefore focuses on forming new reinforcement contingencies to increase the likelihood of adaptive, treatment-oriented behaviors and to reduce the likelihood of substance use. This is achieved by establishing predetermined tangible reinforcers for abstinence and other desirable behaviors (e.g., vouchers for goods or services) to be provided in the treatment context. Abstinence is assessed at regular intervals using urine toxicology assays, and reinforcers are typically provided immediately upon attaining the test result. The efficacy of contingency management in reducing cocaine use during initial treatment has been well established in a number of trials (72). As has been observed in studies of cognitive behavior therapy, contingency management has sustained effects in reducing cocaine use 6 and 12 months after treatment (73, 74), a finding that has been replicated in populations of methadone-maintained cocaine users (75). More recently, the use of contingency management was extended to methamphetamine abusers in a randomized clinical trial (68) and was found to be superior to cognitive behavior therapy during active treatment, producing lower rates of stimulant use and better treatment retention. As in other studies, contingency management was comparable to cognitive behavior therapy with respect to sustaining longer-term outcomes. In addition to reinforcing abstinence, contingency management has been used successfully to promote treatment attendance (76) and compliance with pharmacotherapy, such as naltrexone (77).
The community reinforcement approach is an individualized form of contingency management. Using the community reinforcement approach, the therapist helps the substance user to identify individualized nondrug alternative reinforcers to strive for (e.g., increased quality of family relationships, vocational training, employment, social support network, or hobbies). The goal of this intervention is to optimize the individual's satisfaction with a substance-free life by establishing “competing” reinforcers that are incompatible with substance use. Often delivered in conjunction with vouchers, the community reinforcement approach typically provides relationship counseling, vocational training, and assertiveness, social, and drug refusal skills. The combination of voucher-based contingency management and the community reinforcement approach has been shown to be superior to vouchers alone (78) and 12-step drug counseling (79) in facilitating treatment retention and abstinence in cocaine users. Moreover, although a recent meta-analysis revealed strong evidence for the efficacy of the community reinforcement approach in reducing alcohol use (80), this intervention has not been widely implemented in alcohol-abusing populations.
CUE EXPOSURE
According to principles of classical conditioning, cues associated with substance use (e.g., substance-using friends, environmental contexts in which the individual frequently used substances, and drug paraphernalia) are thought to elicit conditioned psychological and/or physiological craving responses and may in turn trigger substance use relapse. As such, the objective of cue exposure treatment is to modify the individual's subjective and behavioral responses to substance-related cues, i.e., to extinguish substance-seeking and consumption behaviors in the presence of these cues. Cue exposure treatment studies have been conducted with individuals addicted to alcohol, opiates, and cocaine. Collectively, these studies have shown little evidence for the effectiveness of this intervention in preventing relapse among substance-dependent individuals (81). Moreover, a recent randomized, controlled trial of cue exposure treatment in heroin-dependent inpatients showed higher rates of dropout and substance relapse among those treated with cue exposure treatment relative to psychotherapy (82).
MOTIVATIONAL INTERVENTIONS
Motivational interviewing is an intervention approach designed to help individuals to work through ambivalence and make or strengthen a commitment to change (83). Drawing on client-centered therapy techniques, motivation interviewing combines an empathic, supportive therapist style with directive elicitation of change statements to resolve ambivalence. The rationale for this approach is straightforward: targeting substance abusers' degree of motivation to quit using substances should increase treatment efficacy. Substance-using populations enter treatment with considerable variability in motivation, as evidenced by high treatment dropout rates (84).
Motivational interventions are now widely used, with demonstrated efficacy in reducing alcohol and stimulant abuse (84–86). The strongest support for motivational interviewing comes from studies of its efficacy in reducing alcohol use in abusing and dependent populations, of which there have been at least 32 trials. Collectively, this work has shown that motivational interviewing effectively increases adherence to alcoholism treatment and improves drinking outcomes, particularly when added to other standard treatments (87). Relatively fewer studies have examined its application to the treatment of substance abuse, and results of existing studies have not been consistent. Rohsenow et al. (84) found that two motivational interviewing sessions in conjunction with intensive treatment for substance abuse were beneficial in reducing cocaine and alcohol use among cocaine-dependent patients with low motivation to change. On the other hand, Miller et al. (88) reported no impact of a single session of motivational interviewing on drug use outcomes after substance abuse treatment.
Recent studies have shown that the effects of motivational interviewing are greater when it is used in combination with other treatments than when used as a stand-alone intervention (89), and these outcomes appear to be attributable to its effects on treatment retention and adherence (87). Likewise, recent reviews of psychosocial interventions trials for stimulant users have concluded that combined cognitive-motivational strategies may be a promising treatment approach for this population (84, 90, 91). Moreover, several pilot studies have adapted motivational interviewing for use in substance users with comorbid axis I disorders and demonstrated its feasibility in this population (92).
TWELVE-STEP PROGRAMS
The 12-step approach is a common element of substance abuse treatment in the United States, whether in the form of self-help groups incorporated into the treatment plan or therapist-guided interventions. According to the 12-step philosophy, addiction is viewed as an incurable disease of the body, mind, and spirit that can be managed on a day-to-day basis through a consistent commitment to abstinence and by working the 12 steps. Although traditionally practiced in the form of community self-help groups, this approach has been adapted and standardized as a therapist-led approach known as 12-step facilitation in order to facilitate research into its effectiveness. Controlled studies of 12-step facilitation have yielded mixed findings. The largest randomized trial incorporating 12-step facilitation in the treatment of alcohol users, Project MATCH, found 12-step facilitation to be comparable to the other interventions studied, cognitive behavior therapy and motivational enhancement therapy (69), in reducing alcohol use during treatment and more effective than these techniques in facilitating long-term abstinence (93). Among stimulant users with concomitant alcohol use disorders, Twelve-step facilitation has comparable in-treatment (94) and long-term (95) effects through 1 year posttreatment on reducing cocaine use, when compared with cognitive behavior therapy. Although 12-step facilitation has not been tested in populations other than alcohol and stimulant users, a wealth of evidence suggests that community 12-step affiliation has a positive impact on substance use outcomes after formal treatment in both alcohol- and drug-using populations (96).
FAMILY THERAPY
Family involvement in treatment has been formalized into several intervention approaches for alcohol- and drug-abusing individuals. Among these techniques are behavioral marital and family therapy (97) and network therapy (98). The aim of the former is to increase marital and family reinforcement and improve communication skills, whereas the latter focuses on using the patient's family and peers to form a therapeutic network that participates in the treatment process. Similarly, a modified community reinforcement approach incorporating family and the social network, community reinforcement, and family training, has the aim of engaging substance users with poor motivation for treatment through work with concerned family members and friends (99). Behavioral family therapy has been found to increase abstinence rates and compliance with naltrexone in male opioid-dependent patients (100) relative to individual treatment. Similarly, according to a recent review, behavioral marital therapy in alcoholism treatment is more effective than individual treatment in increasing abstinence and improving relationship functioning (100). Although there are few randomized, controlled trials of network therapy, limited evidence suggests that this technique, developed for use in office-based practice has utility in reducing heroin use among individuals taking buprenorphine (101). A larger evidence base for community reinforcement and family training suggests that it is an efficacious technique for engaging substance users in treatment and facilitating improved substance use outcomes, particularly in patients who participate in aftercare (102).
AFTERCARE
Aftercare is considered an essential aspect of treatment for substance-abusing populations, given the relapsing nature of substance abuse disorders. Aftercare, also known as continuing care, is particularly critical during the 6-month period following intensive treatment. Posttreatment relapse commonly occurs within this time frame and often alters the clinical course in a manner that undermines the overall maintenance of treatment gains (103). Randomized aftercare studies to date have been largely limited to populations with substance abuse disorders, excluding those with comorbid psychopathology. Twelve-step intervention, based on the principles of Alcoholics Anonymous, has been cited as the most prevalent aftercare approach (96). Although there have been few randomized trials of aftercare interventions, existing evidence suggests that outcomes of 12-step aftercare programs are comparable to those with other approaches, including cognitive behavior therapy and motivational enhancement therapy (104–107). Nevertheless, treatment adherence in 12-step aftercare is associated with better outcomes (96), highlighting the importance of the providers' emphasis on compliance.
1 Willner P: Animal models of depression: validity and applications, in Depression and Mania: From Neurobiology to Treatment. Edited by Fratta W, Pani L Gessa GL, Serra G. New York, Raven Press, 1995, pp 19– 41Google Scholar
2 Johanson CE, Woolverton WL, Schuster CR: Evaluating laboratory models of drug dependence, in Psychopharmacology: The Third Generation of Progress. Edited by Meltzer HY. New York, Raven Press, 1987, pp 1617– 1625Google Scholar
3 Epstein DH, Preston KL, Jasinski DR: Abuse liability, behavioral pharmacology, and physical-dependence potential of opioids in humans and laboratory animals: lessons from tramadol. Biol Psychol 2006; 73: 90– 99Crossref, Google Scholar
4 Young, AM, Herling S: Drugs as reinforcers: studies in laboratory animals, in Behavioral Analysis of Drug Dependence, Edited by Stolerman IP, Goldberg IP. New York, Academic Press, 1986, pp 9– 67Google Scholar
5 Woolverton, WL, Nader MA: Experimental evaluation of the reinforcing effects of drugs, in Modern Methods in Pharmacology, vol 6: Testing and Evaluation of Drugs of Abuse. New York, Wiley-Liss, 1990, pp 165– 192Google Scholar
6 Mello, NK, Negus SS: Preclinical evaluation of pharmacotherapies for treatment of cocaine and opioid abuse using drug self-administration procedures. Neuropsychopharmacology 1996; 14: 375– 424Crossref, Google Scholar
7 O'Brien, CP, Gardner EL: Critical assessment of how to study addiction and its treatment: human and non-human animal models. Pharmacol Ther 2005; 108: 18– 58Crossref, Google Scholar
8 Corrigall WA: Nicotine self-administration in animals as a dependence model. Nicotine Tob Res 1999; 1: 11– 20Crossref, Google Scholar
9 Richardson, NR, Roberts DC: Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods 1996; 66: 1– 11Crossref, Google Scholar
10 Paterson NE, Markou A: The metabotropic glutamate receptor 5 antagonist MPEP decreased break points for nicotine, cocaine and food in rats. Psychopharmacology (Berl) 2005; 179: 255– 261Crossref, Google Scholar
11 Nader MA, Hedeker D, Woolverton WL: Behavioral economics and drug choice: effects of unit price on cocaine self-administration by monkeys. Drug Alcohol Depend 1993; 33: 193– 199Crossref, Google Scholar
12 Bickel WK, Marsch LA, Carroll ME: Deconstructing relative reinforcing efficacy and situating the measures of pharmacological reinforcement with behavioral economics: a theoretical proposal. Psychopharmacology (Berl) 2000; 153: 44– 56Crossref, Google Scholar
13 Johnson, MW, Bickel WK: Replacing relative reinforcing efficacy with behavioral economic demand curves. J Exp Anal Behav 2006; 85: 73– 93Crossref, Google Scholar
14 Vocci FJ, Acri J, Elkashef A: Medication development for addictive disorders: the state of the science. Am J Psychiatry 2005; 162(8): 1432– 40Crossref, Google Scholar
15 Piasecki TM, Fiore MC, Baker TB: Profiles in discouragement: two studies of variability in the time course of smoking withdrawal symptoms. J Abnorm Psychol 1998; 107: 238– 251Crossref, Google Scholar
16 See RE: Neural substrates of conditioned-cued relapse to drug-seeking behavior. Pharmacol Biochem Behav 2002; 71: 517– 529Crossref, Google Scholar
17 Stewart J: Stress and relapse to drug seeking: studies in laboratory animals shed light on mechanisms and sources of long-term vulnerability. Am J Addict 2003; 12: 1– 17Crossref, Google Scholar
18 Kalivas, PW, McFarland K: Brain circuitry and the reinstatement of cocaine-seeking behavior. Psychopharmacology (Berl) 2003; 168: 44– 56Crossref, Google Scholar
19 Shaham Y, Shalev U, Lu L, De Wit H, Stewart J: The reinstatement model of drug relapse: history, methodology and major findings. Psychopharmacology (Berl) 2003; 168: 3– 20Crossref, Google Scholar
20 Le Foll B, Goldberg SR: Control of the reinforcing effects of nicotine by associated environmental stimuli in animals and humans. Trends Pharmacol Sci 2005; 26: 287– 293Crossref, Google Scholar
21 Epstein DH, Preston KL, Stewart J, Shaham Y: Toward a model of drug relapse: an assessment of the validity of the reinstatement procedure. Psychopharmacology (Berl) 2006; 189: 1– 16Crossref, Google Scholar
22 Koob GF: The neurobiology of addiction: a neuroadaptational view relevant for diagnosis. Addiction 2006; 101(suppl 1): 23– 30Crossref, Google Scholar
23 Katner SN, Magalong JG, Weiss F: Reinstatement of alcohol-seeking behavior by drug-associated discriminative stimuli after prolonged extinction in the rat. Neuropsychopharmacology 1999; 20: 471– 479Crossref, Google Scholar
24 Ciccocioppo R, Sanna PP, Weiss F: Cocaine-predictive stimulus induces drug-seeking behavior and neural activation in limbic brain regions after multiple months of abstinence: reversal by D1 antagonists. Proc Natl Acad Sci USA 2001; 98: 1976– 1981Crossref, Google Scholar
25 Perkins KA, Grobe JE, C. Fonte, J. Goettler, Caggiula AR, Reynolds WA, Stiller RL, A. Scierka, Jacob RG: Chronic and acute tolerance to subjective, behavioral and cardiovascular effects of nicotine in humans. J Pharmacol Exp Ther 1994; 270: 628– 638Google Scholar
26 Rose JE, Behm FM, Westman EC, Johnson M: Dissociating nicotine and nonnicotine components of cigarette smoking. Pharmacol Biochem Behav 2000; 67: 71– 81Crossref, Google Scholar
27 Tiffany ST, Cox LS, Elash CA: Effects of transdermal nicotine patches on abstinence-induced and cue-elicited craving in cigarette smokers. J Consult Clin Psychol 2000; 68: 233– 240Crossref, Google Scholar
28 Liu X, Caggiula AR, Yee SK, Nobuta H, Poland RE, Pechnick RN: Reinstatement of nicotine-seeking behavior by drug-associated stimuli after extinction in rats. Psychopharmacology (Berl) 2006; 184: 417– 425Crossref, Google Scholar
29 Liu X, Caggiula AR, Yee SK, Nobuta H, Sved AF, Pechnick RN, Poland RE: Mecamylamine attenuates cue-induced reinstatement of nicotine-seeking behavior in rats. Neuropsychopharmacology 2007; 32: 710– 718Crossref, Google Scholar
30 Nestler, EJ, Aghajanian GK: Molecular and cellular basis of addiction. Science 1997; 278: 58– 63Crossref, Google Scholar
31 Childress AR, Mozley PD, McElgin W, Fitzgerald J, Reivich M, O'Brien CP: Limbic activation during cue-induced cocaine craving. Am J Psychiatry 1999; 156: 11– 18Crossref, Google Scholar
32 Weiss F: Neurobiology of craving, conditioned reward and relapse. Curr Opin Pharmacol 2005; 5: 9– 19Crossref, Google Scholar
33 Camacho LM, Bartholomew NG, Joe GW, Cloud MA, Simpson DD: Gender, cocaine and during-treatment HIV risk reduction among injection opioid users in methadone maintenance. Drug Alcohol Depend 1996; 41: 1– 7Crossref, Google Scholar
34 Center for Disease Control and Prevention: Selected data from the HIV/AIDS Surveillance Report through Dec 1995. Pediatr AIDS HIV Infect 1996; 7: 287– 304Google Scholar
35 Strathdee SA, Galai N, Safaiean M, Celentano DD, Vlahov D, Johnson L, Nelson KE: Sex differences in risk factors for HIV seroconversion among injection drug users: a 10-year perspective. Arch Intern Med 2001; 161: 1281– 1288Crossref, Google Scholar
36 Yoast R, Williams MA, Deitchman SD, Champion HC: Report of the Council on Scientific Affairs: methadone maintenance and needle-exchange programs to reduce the medical and public health consequences of drug abuse. J Addict Dis 2001; 20: 15– 40Crossref, Google Scholar
37 Kraft MK, Rothbard AB, Hadley TR, McLellan AT, Asch DA: Are supplementary services provided during methadone maintenance really cost-effective? Am J Psychiatry 1997; 154: 1214– 1219Crossref, Google Scholar
38 Silagy C, Lancaster T, Stead L, Mant D, Fowler G: Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev 2004; (3):CD000146Google Scholar
39 Joseph AM, Norman SM, Ferry LH, Prochazka AV, Westman EC, Steele BG, Sherman SE, Cleveland M, Antonnucio DO, Hartman N, McGovern PG: The safety of transdermal nicotine as an aid to smoking cessation in patients with cardiac disease. N Engl J Med 1996; 335: 1792– 1798Crossref, Google Scholar
40 White JM, Irvine RJ: Mechanisms of fatal opioid overdose. Addiction 1999; 94: 961– 972Crossref, Google Scholar
41 Walsh SL, Preston KL, Stitzer ML, Cone EJ, Bigelow GE: Clinical pharmacology of buprenorphine: ceiling effects at high doses. Clin Pharmacol Ther 1994; 55: 569– 580Crossref, Google Scholar
42 Walsh SL, Preston KL, Bigelow GE, Stitzer ML: Acute administration of buprenorphine in humans: partial agonist and blockade effects. J Pharmacol Exp Ther 1995; 274: 361– 372Google Scholar
43 Stoller KB, Bigelow GE, Walsh SL, Strain EC: Effects of buprenorphine/naloxone in opioid-dependent humans. Psychopharmacology (Berl) 2001; 154: 230– 242Crossref, Google Scholar
44 Gonzales D, Rennard SI, Nides M, Oncken C, Azoulay S, Billing CB, Watsky EJ, Gong J, Williams KE, Reeves KR: Varenicline, an α4β2 nicotinic acetylcholine receptor partial agonist, vs sustained-release bupropion and placebo for smoking cessation: a randomized controlled trial. JAMA 2006; 296: 47– 55Crossref, Google Scholar
45 Lile JA, Stoops WW, Vansickel AR, Glaser PE, Hays LR, Rush CR: Aripiprazole attenuates the discriminative-stimulus and subject-rated effects of d-amphetamine in humans. Neuropsychopharmacology 2005; 30: 2103– 2114Crossref, Google Scholar
46 Warner C, Shoaib M: How does bupropion work as a smoking cessation aid? Addict Biol 2005; 10: 219– 231Crossref, Google Scholar
47 O'Farrell TJ, Allen JP, Litten RZ: Disulfiram (Antabuse) contracts in treatment of alcoholism. NIDA Res Monogr 1995; 150: 65– 91Google Scholar
48 Suh JJ, Pettinati HM, Kampman KM, O'Brien CP: The status of disulfiram: a half of a century later. J Clin Psychopharmacol 2006; 26: 290– 302Crossref, Google Scholar
49 Pettinati HM, Volpicelli JR, Pierce JD Jr, O'Brien CP: Improving naltrexone response: an intervention for medical practitioners to enhance medication compliance in alcohol dependent patients. J Addict Dis 2000; 19: 71– 83Crossref, Google Scholar
50 Garbutt JC, Kranzler HR, O'Malley SS, Gastfriend DR, Pettinati HM, Silverman BL, Loewy JW, Ehrich EW: Efficacy and tolerability of long-acting injectable naltrexone for alcohol dependence: a randomized controlled trial. JAMA 2005; 293: 1617– 1625Crossref, Google Scholar
51 O'Brien TP, Metallinos DL, Chen H, Shin MK, Tilghman SM: Complementation mapping of skeletal and central nervous system abnormalities in mice of the piebald deletion complex. Genetics 1996; 143: 447– 461Google Scholar
52 Anton RF, Swift RM: Current pharmacotherapies of alcoholism: a U.S. perspective. Am J Addict, 2003; 12(suppl 1): S53– S68Crossref, Google Scholar
53 Naassila M, Hammoumi S, Legrand E, Durbin P, Daoust M: Mechanism of action of acamprosate. Part I. Characterization of spermidine-sensitive acamprosate binding site in rat brain. Alcohol Clin Exp Res 1998; 22: 802– 809Crossref, Google Scholar
54 Krystal JH, Petrakis IL, Limoncelli D, Webb E, Gueorgueva R, D'Souza DC, Boutros NN, Trevisan L, Charney DS: Altered NMDA glutamate receptor antagonist response in recovering ethanol-dependent patients. Neuropsychopharmacology 2003; 28: 2020– 2028Crossref, Google Scholar
55 Mann K, Lehert P, Morgan MY: The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcohol Clin Exp Res 2004; 28: 51– 63Crossref, Google Scholar
56 Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A: Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 2006; 295: 2003– 2017Crossref, Google Scholar
57 Kranzler HR: Evidence-based treatments for alcohol dependence: new results and new questions. JAMA 2006; 295: 2075– 2076Crossref, Google Scholar
58 Johnson BA, Ait-Daoud N, Bowden CL, DiClemente CC, Roache JD, Lawson K, Javors MA, Ma JZ: Oral topiramate for treatment of alcohol dependence: a randomised controlled trial. Lancet 2003; 361: 1677– 1685Crossref, Google Scholar
59 Johnson BA, Roache JD, Javors MA, DiClemente CC, Cloninger CR, Prihoda TJ, Bordnick PS, Ait-Daoud N, Hensler J: Ondansetron for reduction of drinking among biologically predisposed alcoholic patients: a randomized controlled trial. JAMA 2000; 284: 963– 971Crossref, Google Scholar
60 Dackis CA, Kampman KM, Lynch KG, Pettinati HM, C, O'Brien CP: A double-blind, placebo-controlled trial of modafinil for cocaine dependence. Neuropsychopharmacology 2005; 30: 205– 211Crossref, Google Scholar
61 Kampman KM, Pettinati H, Lynch KG, Dackis C, Sparkman T, Weigley C, O'Brien CP: A pilot trial of topiramate for the treatment of cocaine dependence. Drug Alcohol Depend 2004; 75: 233– 240Crossref, Google Scholar
62 Carroll KM, Fenton LR, Ball SA, Nich C, Frankforter TL, Shi J, Rounsaville BJ: Efficacy of disulfiram and cognitive behavior therapy in cocaine-dependent outpatients: a randomized placebo-controlled trial. Arch Gen Psychiatry 2004; 61: 264– 272Crossref, Google Scholar
63 Kampman O, Kiviniemi P, Koivisto E, Vaananen J, Kilkku N, Leinonen E, Lehtinen K: Patient characteristics and diagnostic discrepancy in first-episode psychosis. Compr Psychiatry 2004; 45: 213– 218Crossref, Google Scholar
64 Beck AT: Thinking and depression. I. Idiosyncratic content and cognitive distortions. Arch Gen Psychiatry 1963; 14: 324– 333Crossref, Google Scholar
65 Marlatt GA, Gordon JR (eds): Relapse Prevention: Maintenance Strategies in the Treatment of Addictive Behaviors. New York, Guilford, 1985Google Scholar
66 Monti PM, Abrams DB, Kadden RM, Rohsenow DJ, Cooney NL: Treating Alcohol Dependence: A Coping Skills Therapy Guide. New York, Guilford, 1989Google Scholar
67 Carroll KM, Integrating psychotherapy and pharmacotherapy to improve drug abuse outcomes. Addict Behav 1997; 22: 233– 245Crossref, Google Scholar
68 Rawson RA, McCann MJ, Flammino F, Shoptaw S, Miotto K, Reiber C, Ling W: A comparison of contingency management and cognitive-behavioral approaches for stimulant-dependent individuals. Addiction 2006; 101: 267– 274Crossref, Google Scholar
69 Matching alcoholism treatments to client heterogeneity: treatment main effects and matching effects on drinking during treatment. Project MATCH Research Group. J Stud Alcohol 1998; 59: 631– 639Crossref, Google Scholar
70 Shearer J: Psychosocial approaches to psychostimulant dependence: a systematic review. J Subst Abuse Treat 2007; 32: 41– 52Crossref, Google Scholar
71 Irvin JE, Bowers CA, Dunn ME, Wang MC: Efficacy of relapse prevention: a meta-analytic review. J Consult Clin Psychol 1999; 67: 563– 570Crossref, Google Scholar
72 Lussier JP, Heil SH, Mongeon JA, Badger GJ, Higgins ST: A meta-analysis of voucher-based reinforcement therapy for substance use disorders. Addiction 2006; 101: 192– 203Crossref, Google Scholar
73 Higgins ST, Budney AJ, Bickel WK, Foerg FE, Donham R, Badger GJ: Incentives improve outcome in outpatient behavioral treatment of cocaine dependence. Arch Gen Psychiatry 1994; 51: 568– 576Crossref, Google Scholar
74 Higgins ST, Wong CJ, Badger GJ, Ogden DE, Dantona RL: Contingent reinforcement increases cocaine abstinence during outpatient treatment and 1 year of follow-up. J Consult Clin Psychol 2000; 68: 64– 72Crossref, Google Scholar
75 Rawson RA, Huber A, McCann M, Shoptaw S, Farabee D, Reiber C, Ling W: A comparison of contingency management and cognitive-behavioral approaches during methadone maintenance treatment for cocaine dependence. Arch Gen Psychiatry 2002; 59: 817– 824Crossref, Google Scholar
76 Ersner-Hershfield SM, Connors GJ, Maisto SA: Clinical and experimental utility of refundable deposits. Behav Res Ther 1981; 19: 455– 457Crossref, Google Scholar
77 Iguchi MY, Belding MA, Morral AR, Lamb RJ, Husband SD: Reinforcing operants other than abstinence in drug abuse treatment: an effective alternative for reducing drug use. J Consult Clin Psychol 1997; 65: 421– 428Crossref, Google Scholar
78 Higgins ST, Sigmon SC, Wong CJ, Heil SH, Badger GJ, Donham R, Dantona RL, Anthony S: Community reinforcement therapy for cocaine-dependent outpatients. Arch Gen Psychiatry 2003; 60: 1043– 1052Crossref, Google Scholar
79 Higgins ST, Budney AJ, Bickel WK, Hughes JR, Foerg F, Badger G: Achieving cocaine abstinence with a behavioral approach. Am J Psychiatry 1993; 150: 763– 769Crossref, Google Scholar
80 Roozen HG, Boulogne JJ, van Tulder MW, van den Brink W, De Jong CA, Kerkhof AJ: A systematic review of the effectiveness of the community reinforcement approach in alcohol, cocaine and opioid addiction. Drug Alcohol Depend 2004; 74: 1– 13Crossref, Google Scholar
81 Conklin CA, Tiffany ST: Cue-exposure treatment: time for change. Addiction 2002; 97: 1219– 1221Crossref, Google Scholar
82 Marissen MA, Franken IH, Blanken P, van den Brink W, Hendriks VM: Cue exposure therapy for the treatment of opiate addiction: results of a randomized controlled clinical trial. Psychother Psychosom 2007; 76: 97– 105Crossref, Google Scholar
83 Miller NN: Village victories: new motivational techniques in Kenya and Zimbabwe. UFSI Rep 1983; (13): 1– 8Google Scholar
84 Rohsenow DJ, Monti PM, Martin RA, Colby SM, Myers MG, Gulliver SB, Brown RA, Mueller TI, Gordon A, Abrams DB, Motivational enhancement and coping skills training for cocaine abusers: effects on substance use outcomes. Addiction 2004; 99: 862– 874Crossref, Google Scholar
85 Miller WR: Motivational interviewing: research, practice, and puzzles. Addict Behav 1996; 21: 835– 842Crossref, Google Scholar
86 Stotts AL, Schmitz JM, Rhoades HM, Grabowski J: Motivational interviewing with cocaine-dependent patients: a pilot study. J Consult Clin Psychol 2001; 69: 858– 862Crossref, Google Scholar
87 Hettema J, Steele J, Miller WR: Motivational Interviewing. Annu Rev Clin Psychol 2005; 1: 91– 111Crossref, Google Scholar
88 Miller WR, Yahne CE, Tonigan JS: Motivational interviewing in drug abuse services: a randomized trial. J Consult Clin Psychol 2003; 71: 754– 763Crossref, Google Scholar
89 Burke BL, Arkowitz H, Menchola M: The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol 2003; 71: 843– 861Crossref, Google Scholar
90 Baker A, Lee NK: A review of psychosocial interventions for amphetamine use. Drug Alcohol Rev 2003; 22: 323– 335Crossref, Google Scholar
91 Baker A, Lee NK, Claire M, Lewin TJ, Grant T, Pohlman S, Saunders JB, Kay-Lambkin F, Constable P, Jenner L, Carr VJ: Brief cognitive behavioural interventions for regular amphetamine users: a step in the right direction. Addiction 2005; 100: 367– 378Crossref, Google Scholar
92 Van Horn DH, Bux DA: A pilot test of motivational interviewing groups for dually diagnosed inpatients. J Subst Abuse Treat 2001; 20: 191– 195Crossref, Google Scholar
93 Matching alcoholism treatments to client heterogeneity: Project MATCH three-year drinking outcomes. Alcohol Clin Exp Res 1998; 22: 1300– 1311Crossref, Google Scholar
94 Carroll KM, Nich C, Ball SA, McCance E, Rounsavile BJ: Treatment of cocaine and alcohol dependence with psychotherapy and disulfiram. Addiction 1998; 93: 713– 727Crossref, Google Scholar
95 Carroll KM, Nich C, Ball SA, McCance E, Frankforter TL, Rounsaville BJ: One-year follow-up of disulfiram and psychotherapy for cocaine-alcohol users: sustained effects of treatment. Addiction 2000; 95: 1335– 1349Crossref, Google Scholar
96 Morgenstern J, Labouvie E, McCrady BS, Kahler CW, Frey RM: Affiliation with Alcoholics Anonymous after treatment: a study of its therapeutic effects and mechanisms of action. J Consult Clin Psychol 1997; 65: 768– 777Crossref, Google Scholar
97 McCrady BS: Extending relapse prevention models to couples. Addict Behav 1989; 14: 69– 74Crossref, Google Scholar
98 Galanter M: Network therapy for addiction: a model for office practice. Am J Psychiatry 1993; 150: 28– 36Crossref, Google Scholar
99 Meyers RJ, Smith JE, Lash DN: The community reinforcement approach. Recent Dev Alcohol 2003; 16: 183– 195Google Scholar
100 O'Farrell TJ, Fals-Stewart W: Alcohol abuse. J Marital Fam Ther 2003; 29: 121– 146Crossref, Google Scholar
101 Galanter M, Dermatis H, Glickman L, Maslansky R, Sellers MB, Neumann E, Rahman-Dujarric C: Network therapy: decreased secondary opioid use during buprenorphine maintenance. J Subst Abuse Treat 2004; 26: 313– 318Crossref, Google Scholar
102 Meyers RJ, Miller WR, Smith JE, Tonigan JS: A randomized trial of two methods for engaging treatment-refusing drug users through concerned significant others. J Consult Clin Psychol 2003; 70: 1182– 1185Crossref, Google Scholar
103 Foster JH, Marshall EJ, Peters TJ: Outcome after in-patient detoxification for alcohol dependence: a naturalistic comparison of 7 versus 28 days stay. Alcohol Alcohol 2000; 35: 580– 586Crossref, Google Scholar
104 Wells EA, Peterson PL, Gainey RR, Hawkins JD, Catalano RF: Outpatient treatment for cocaine abuse: a controlled comparison of relapse prevention and twelve-step approaches. Am J Drug Alcohol Abuse 1994; 20: 1– 17Crossref, Google Scholar
105 Ouimette PC, Finney JW, Moos RH: Twelve-step and cognitive–behavioral treatment for substance abuse: a comparison of treatment effectiveness. J Consult Clin Psychol 1997; 65: 230– 240Crossref, Google Scholar
106 Matching Alcoholism Treatments to Client Heterogeneity: Project MATCH posttreatment drinking outcomes. J Stud Alcohol 1997; 58: 7– 29Crossref, Google Scholar
107 Brown TG, Seraganian P, Tremblay J, Annis H: Process and outcome changes with relapse prevention versus 12-Step aftercare programs for substance abusers. Addiction 2002; 97: 677– 689Crossref, Google Scholar



