Psychiatric Applications of Repetitive Transcranial Magnetic Stimulation
Abstract
Transcranial magnetic stimulation (TMS) is an increasingly popular noninvasive brain stimulation modality. In TMS, a pulsed magnetic field is used to noninvasively stimulate a targeted brain region. Repeated stimulation produces lasting changes in brain activity via mechanisms of synaptic plasticity similar to long-term potentiation. Local application of TMS alters activity in distant, functionally connected brain regions, indicating that TMS modulates activity of cortical networks. TMS has been approved by the U.S. Food and Drug Administration for the treatment of major depressive disorder, obsessive-compulsive disorder, and smoking cessation, and a growing evidence base supports its efficacy in the treatment of other neuropsychiatric conditions. TMS is rapidly becoming part of the standard of care for treatment-resistant depression, where it yields response rates of 40%−60%. TMS is generally safe and well tolerated; its most serious risk is seizure, which occurs very rarely. This review aims to familiarize practicing psychiatrists with basic principles of TMS, including target localization, commonly used treatment protocols and their outcomes, and safety and tolerability. Practical considerations, including evaluation and monitoring of patients undergoing TMS, device selection, treatment setting, and insurance reimbursement, are also reviewed.
In 1985, Anthony Barker and his team (1) developed transcranial magnetic stimulation (TMS) when they produced a motor response by noninvasively applying a magnetic field to the human motor cortex. The potential effects of TMS on mood were serendipitously discovered just 2 years later (2). Substantial research efforts over the ensuing decades confirmed the antidepressant efficacy of TMS (3, 4), and the modality has gained widespread use following the 2008 Food and Drug Administration (FDA) approval of repetitive transcranial magnetic stimulation (rTMS) for treatment of depression. Familiarity with this treatment modality is increasingly essential as rTMS becomes part of the standard of care for treatment-resistant depression and other indications. The purpose of this review is to advance understanding of this emerging treatment modality and to empower practicing psychiatrists to incorporate TMS into their clinical practice.
Principles of TMS
Each TMS device consists of a stimulator connected to an electromagnetic coil. The stimulator generates a strong electric current that is passed through the windings in the coil, generating a strong but transient magnetic field that is referred to as a pulse (5). TMS may be applied one pulse at a time (single-pulse TMS), in pairs (paired-pulse TMS), or in trains, with hundreds or thousands of magnetic pulses applied in rapid succession (rTMS). rTMS is of greatest clinical utility in psychiatry and is the focus of this review.
When the electromagnetic coil is placed at the scalp during TMS, the magnetic field passes unimpeded through the scalp and skull. On reaching the cortex, the time-varying magnetic field induces a low amplitude electric current, causing depolarization of local neurons (6). Effects vary by location: a single TMS pulse delivered to the motor cortex produces a motor response, whereas a single pulse delivered to the visual cortex produces phosphenes. rTMS causes repeated neuronal depolarization, which produces lasting changes in cortical activity by triggering synaptic plasticity mechanisms similar to long-term potentiation (7, 8). Functional imaging studies (9–12) have demonstrated that rTMS modulates brain activity not only locally within the field of stimulation, but also in distant, functionally connected regions, most likely through transsynaptic signaling. Additionally, rTMS is hypothesized to normalize network and overall brain functioning by entraining oscillatory neuronal activity and restoring normal intrinsic cerebral rhythms (13).
Whether the overall effect of stimulation is excitatory or inhibitory depends on the parameters of stimulation, such as the pulse frequency and the duration of stimulation (14, 15). Changes in cortical excitability outlast the period of stimulation, and repeated stimulation sessions exert cumulative effects (16). For most clinical applications, rTMS is administered once daily, 5 days per week, across multiple weeks.
Stimulation Target
The location of stimulation determines the effects of TMS. The left dorsolateral prefrontal cortex (DLPFC) is commonly targeted in the treatment of depression, whereas the medial prefrontal cortex and anterior cingulate cortex are targeted in the treatment of obsessive-compulsive disorder (OCD).
Target localization is an active area of research, in part because treatment nonresponse is thought to be related to errors in target localization. The field is shifting from population-based targeting to more individualized approaches. The “5-cm rule,” or standard procedure, was used by the initial studies (3) demonstrating clinical efficacy of TMS in depression. This method localizes the treatment target for depression (left DLPFC) on the basis of its probabilistic distance from the hand region of the motor cortex in the parasagittal plane (17). The updated 5.5- and 6-cm rules are in common use today (4, 18), and a similar rule is used to identify the treatment target in OCD (19). These targeting methods have demonstrated clinical efficacy but fail to account for variation among skull sizes and prefrontal anatomy, which may lead to inaccurate target localization (20–23). Because of these limitations, many clinicians have adopted targeting methods that are based on the International 10-20 system for electroencephalogram electrode placement. The Beam F3 method is one such strategy, which demonstrates better inter- and intra-rater reliability in localizing left DLPFC compared with the 5.5-cm rule (24) and approximates DLPFC well when compared with anatomical magnetic resonance imaging (MRI) targeting (25). However, both methods are vulnerable to operator error.
Anatomical MRI with neuronavigation is another targeting approach that avoids many of the limitations of scalp-based strategies. However, findings on the efficacy of structural MRI targeting compared with the standard procedure have been mixed (26, 27), and cost and availability limit its use in clinical practice. Furthermore, this method does not account for interindividual variability in structural-functional relationships. Targeting strategies based on resting state functional MRI (rsfMRI) offer a promising approach. These strategies allow for targeting that is based on individual or group functional connectivity maps of brain regions and networks, in line with the current view that psychiatric disorders are largely disorders of large-scale functional networks in the brain (28). A commonly studied rsfMRI strategy targets the subregion of the DLPFC that is most anticorrelated with the subgenual anterior cingulate cortex—a structure that has numerous connections with frontal and limbic circuits implicated in the pathophysiology of depression (29). This approach may lead to better outcomes (30, 31) compared with traditional anatomical MRI or skull-based approaches (32), but additional research is needed.
Stimulation Parameters
Stimulation parameters include the frequency at which pulses are delivered, the number of pulses administered in a single train, the intertrain interval (the wait time between pulse trains), the total number of pulses delivered, and the intensity of the stimulation. Stimulation frequency determines whether stimulation is excitatory or inhibitory. Frequencies greater than 5 Hz (5 pulses per second) are termed “high-frequency” and are generally excitatory (14), whereas frequencies of 1 Hz (1 pulse per second) or less are termed “low-frequency” and are generally inhibitory (15). Different patterns of stimulation are available. The most common patterns of stimulation include 10-Hz stimulation, 1-Hz stimulation, intermittent theta burst stimulation (iTBS), and continuous theta burst stimulation (cTBS) (Figure 1).
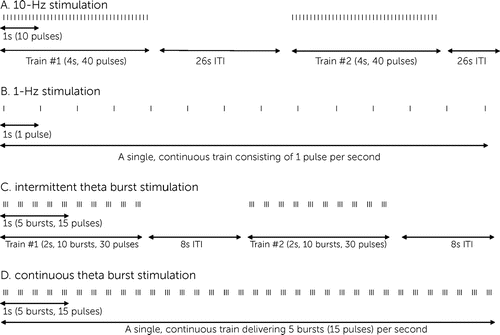
FIGURE 1. Common stimulation patterns of transcranial magnetic stimulationa
a Each vertical line represents one magnetic pulse. A: 10-Hz stimulation, which is excitatory, typically provides stimulation in 4-second trains (a frequency of 10 Hz indicates that 10 pulses are administered per second, meaning a 4-second train delivers a total of 40 pulses). This is followed by a 26-second intertrain interval (or wait time). This pattern repeats 75 times (75 trains), delivering a total of 3,000 pulses in a session lasting 37.5 minutes. B: 1-Hz stimulation, which is inhibitory, involves administering one pulse per second in a continuous train, often for a total of 500 to 1,200 pulses (requiring a session of only 8 to 20 minutes, respectively). C: Theta burst stimulation applies transcranial magnetic stimulation (TMS) pulses in a pattern mimicking the endogenous firing activity of hippocampal neurons. “Bursts” consist of three TMS pulses applied in extremely short succession (at a frequency of 50 Hz). These “bursts” are then administered at a frequency of 5 Hz (five bursts per second). Intermittent theta burst stimulation (iTBS) is excitatory and is generally administered as 10 bursts (30 pulses) during a 2-second train, followed by an 8-second waiting period. This pattern is often repeated 20 times (20 trains), delivering a total of 600 pulses in a session lasting just 3 minutes. D: Continuous theta burst stimulation (cTBS) is inhibitory and is generally administered in a single, uninterrupted train of bursts administered at a frequency of 5 Hz, often for a total of 600 to 1,800 pulses (requiring a session of only 40 seconds to 2 minutes, respectively). ITI, intertrain interval.
The intensity of treatment is determined by the patient’s motor threshold, which reflects his or her individual degree of cortical excitability. The motor threshold (MT) is established on the first day of treatment (and subsequently when indicated) by applying single TMS pulses to the motor cortex and monitoring for thumb or finger movement in the contralateral hand. The motor threshold is defined as the minimum stimulation intensity at which at least 50% of applied pulses elicit a visible motor response. The intensity of treatment stimulation is then measured as a percentage of the patient’s motor threshold (often 120%).
Indications, Protocols, and Outcomes
Overview
At present, rTMS has been approved by the FDA for three psychiatric conditions: major depressive disorder, OCD, and smoking cessation. For each FDA indication, one or more specific protocol(s) utilizing one or more specific device(s) is cleared for treatment of that condition. Notably, other devices capable of delivering the same treatment—and other off-label treatment protocols—are also in common clinical use. FDA-cleared rTMS protocols for the treatment of depression, OCD, and smoking cessation (3, 19, 32–42) are summarized in Table 1. The 2014 evidence-based guidelines and the 2020 update published by Lefaucheur et al. (43, 44) provide a more detailed review of the available rTMS protocols and of the evidence supporting their use in the treatment of various neurological and psychiatric conditions.
| Target | Protocol | Typical course | Cleared system(s) (33) | Outcomes |
|---|---|---|---|---|
| Major depressive disorder | ||||
| Left DLPFC | Commonly termed “10 Hz” or “HFL” (high-frequency left). 3,000 pulses are delivered at a frequency of 10 Hz in 75 trains (4-second duration, 40 pulses per train, 26-second ITI). Goal intensity is 120% MT (3). | Sessions last 37.5 minutes. Standard course is 36 sessions: 5 sessions per week for 6 weeks, followed by a 3-week taper (3, then 2, then 1 weekly session). | Apollo TMS, CloudTMS, Magstim, MagVenture, NeuroStar, Nexstim | Response rate of 47%, remission rate of 27% (N=192) in a controlled setting (32). In a naturalistic setting, response rate of 58%, remission rate of 37% (N=307) (34). About 63% of responders (N=120) maintain benefit 12 months later (35). |
| Left DLPFC | Identical to HFL, except ITI is reduced to between 11 and 25 seconds (36). | Identical to HFL, except session time is reduced to 19–36 minutes, depending on the selected ITI. | Apollo TMS, CloudTMS, Magstim, MagVenture, NeuroStar, Nexstim | Comparably safe and effective to standard 10 Hz treatment (36). |
| Left DLPFC | Commonly termed “theta burst” or “iTBS.” 600 pulses of iTBS delivered as 200 bursts (each consisting of 3 pulses delivered at 50 Hz). Bursts delivered at a frequency of 5 Hz in 2-second trains separated by an 8-second ITI. Goal intensity is 120% MT (32). | Each session is 3 minutes. Standard course of 36 sessions: 5 sessions per week for 6 weeks, followed by a 3-week taper (3, then 2, then 1 weekly session). | BrainsWay with H1-coil, Magstim, MagVenture, NeuroStar, Nexstim | Noninferior to standard 10 Hz treatment; response rate of 49%, remission rate of 32% (N=193) (32). |
| Left DLPFC | 1,980 pulses are delivered at a frequency of 18 Hz in 55 trains (2-second duration, 36 pulses per train, 20-second ITI). Goal intensity is 120% MT (37). | Each session is 20 minutes. Typical course involves 4 weeks of acute treatment (5 treatments per week) followed by up to 12 weeks of maintenance treatment (2 treatments per week) (37). | BrainsWay with H1-coil | Response rate of 38%, remission rate of 33% (N=89) with acute treatment (38). Response rates up to 73%, remission rates up to 64% with continued treatment of non-responders (N=33) (39). |
| Obsessive-compulsive disorder | ||||
| Bilateral DMPFC and ACC | 2,000 pulses delivered at a frequency of 20 Hz in 50 trains (2-second duration, 40 pulses per train, 20-second ITI). Goal intensity is 100% of the leg motor threshold (19, 40). | Each 18-minute session is preceded by 3–5 minutes of symptom provocation. A typical course administers 5 treatments per week for a total of 6 weeks (19, 40). | BrainsWay with H7-coil, MagVenture with Cool D-B80 coil | Response rate of 45%, partial response rate of 60% (N=42) 1 month posttreatment (19). |
| Smoking cessation | ||||
| Prefrontal cortex and insula | 1,800 pulses delivered at a frequency of 10 Hz in 60 trains (3-second duration, 30 pulses per train, 15-second ITI). Goal intensity is 120% MT (41). | Each 20-minute session is preceded by a brief craving provocation. A typical course of 5 daily sessions for 3 weeks, followed by 1 weekly session for another 3 weeks (41). | BrainsWay with H4-coil | Of highly addicted smokers who completed treatment (N=169), 28% achieved 4 consecutive weeks of abstinence (42). |
TABLE 1. Summary of FDA-approved rTMS treatment protocolsa
Major Depressive Disorder
Major depressive disorder is the primary condition treated with rTMS. In general, rTMS treatment for depression involves excitatory treatment delivered to the left DLPFC. This target is based in part on prior functional imaging studies (45) demonstrating left prefrontal hypometabolism among patients with depression. In addition, the left DLPFC is an important component of the central executive network and is functionally connected with nodes of the default mode network, both of which have been implicated in the pathophysiology of depression (46). The first depression treatment protocol, 10-Hz stimulation, was approved by the FDA in 2008. The FDA approval of iTBS in 2018 was a notable development, as this protocol reduces treatment time from 37.5 to 3 minutes. There are currently four treatment protocols (3, 32, 36, 37) FDA-cleared for the treatment of depression (Table 1).
Several non-FDA approved protocols are also commonly used for depression. Low-frequency right-sided stimulation (LFR) administers inhibitory 1-Hz rTMS treatment to the right DLPFC (43). Meta-analyses (47, 48) have reported response rates between 38% (N=131) and 43% (N=112) following LFR, suggesting the efficacy of LFR is likely equivalent to that of high-frequency left-sided treatment (HFL). Patients who fail to respond to HFL may benefit from LFR, and vice versa (43, 49). Also, patients unable to tolerate HFL treatment may benefit from a trial of LFR rTMS; there is some evidence that the latter is more tolerable (44). Some centers have combined sequential HFL and LFR stimulation into a single treatment protocol, termed sequential bilateral stimulation. Although direct comparisons between sequential bilateral stimulation and unilateral stimulation have reported mixed results, two recent network meta-analyses (50, 51) have suggested that sequential bilateral stimulation may be superior to unilateral approaches.
Inhibitory, 1-Hz rTMS has also been applied to the right orbitofrontal cortex in the treatment of depression, with a response rate of 30% (N=30) among patients who did not respond to other TMS approaches (52). Bilateral TBS (iTBS to the left DLPFC followed by cTBS to the right DLPFC) is superior to sham stimulation and was more effective than unilateral approaches in a recent network meta-analysis (51). Finally, some treatment centers perform “priming” TMS, a protocol that administers a brief, often low intensity, excitatory stimulus (e.g., ≥5 Hz or iTBS) immediately prior to another rTMS protocol in order to augment the effects, presumably by leading to greater neuroplastic effects. Priming enhances the efficacy of both LFR and HFL treatment protocols (53, 54). Further optimization of the efficacy and efficiency of rTMS treatment for depression is an area of active, ongoing research.
OCD
The neurobiology of OCD is well understood and neuromodulation approaches (i.e., deep brain stimulation) have already proven effective in its treatment. TMS treatment of OCD targets various components of the cortical-striatal-thalamocortical circuit. In 2018, a TMS protocol using the H7-coil by BrainsWay (Jerusalem) became the first TMS protocol to receive FDA clearance for adjunctive OCD treatment (Table 1). The coil used in this protocol was designed to target the medial prefrontal cortex and anterior cingulate cortex (19). The FDA approved a second OCD treatment protocol using the Cool D-B80 coil by MagVenture (Farum, Denmark) in 2020.
Several non-FDA approved protocols are also in use for the treatment of OCD. Inhibitory, 1-Hz stimulation administered to the right DLPFC appears effective in the treatment of OCD (55, 56). Inhibitory, 1-Hz stimulation administered to the presupplementary motor area (pre-SMA) also appears to be effective; in a recent meta-analysis (57), this target yielded the greatest reductions in patients’ Yale-Brown Obsessive Compulsive Scale (YBOCS) scores of all cortical targets. One-Hz rTMS of the orbitofrontal cortex also appears effective in the treatment of OCD. In a recent trial (58), 1-Hz stimulation of the left orbitofrontal cortex produced response rates (defined as ≥35% reduction in YBOCS) of 43% (N=33) and was comparably effective to pre-SMA stimulation. Sequential stimulation approaches treating both the DLPFC and the SMA also appear promising (59). Although the optimal stimulation parameters and targets in the treatment of OCD are not yet clear, rTMS is clearly effective in treating OCD. A recent meta-analysis (57) of 18 clinical trials found that rTMS reduced OCD symptomatology with a moderate-to-large effect size of 0.79, and the benefits appeared durable, with greater symptom reductions observed at the 12-week follow-up compared with the 4-week follow-up.
Smoking Cessation
In 2020, a TMS protocol using the BrainsWay H4-coil was approved by the FDA as a short-term smoking cessation aid among adults with smoking addiction (Table 1). The H4-coil is designed to stimulate the insula and prefrontal cortex, which have been implicated in the pathophysiology of tobacco use disorder and other substance use disorders (42, 60). Other rTMS protocols have been studied for smoking cessation, with promising results. High-frequency (10- or 20-Hz) rTMS to the left DLPFC appears particularly promising for reducing both cigarette craving and consumption (44). High-frequency rTMS and excitatory iTBS administered to right DLPFC also appear potentially effective for promoting abstinence (44). However, studies have used heterogeneous treatment protocols, and results have rarely been replicated. Beyond the FDA-cleared protocol, the optimal rTMS stimulation parameters for smoking cessation are not clear.
Safety Considerations
Electromagnetic Forces
rTMS is a safe procedure, but there are several important safety considerations. For in-depth review of this subject, three iterations of expert guidelines (61–63) provide detailed guidance on the safety of rTMS in clinical use and research. rTMS exposes patients to an intense magnetic field. The magnetic field poses no risk of ionizing radiation but poses risks when implanted objects (e.g., shrapnel, electrodes, aneurysm clips, cochlear implants) are present in the head and neck. Risks include displacement, heating, or induced voltages (62). However, rTMS is generally considered safe in the presence of implants below the neck (including cardiac pacemakers) and during pregnancy (63, 64).
When implants are present in the head and neck, MRI-compatible implants are generally safer than MRI-incompatible implants (63). Titanium implants, dental fillings and implants, and cervical spine hardware are generally safe. In all other cases of metal or implanted devices in the head and neck, a careful risk analysis must be conducted, with consideration of possible displacement, tissue heating, unintended stimulation, or device malfunction. In the presence of cochlear implants or deep brain or cortical stimulators with electrodes close to the intended magnetic field, TMS should be considered contraindicated (63). As an additional precaution, jewelry, eyeglasses, hearing aids, or other potentially conductive or ferromagnetic objects near the magnetic field should be removed during treatment (62, 63). Scalp or facial tattoos and permanent makeup with ferromagnetic particles in the ink carry theoretical risks of the ink overheating or becoming displaced (65).
Seizure Risk
Seizure is the most serious and most feared potential side effect of rTMS. Fortunately, the overall risk of seizure is low, with one report estimating a standardized seizure risk of 7 in 100,000 treatment sessions (66). Any neurological or general medical condition that heightens the risk of seizure may increase the risk of TMS-induced seizure. Other potential provoking factors include stress, anxiety, sleep deprivation, menses, alcohol use or withdrawal, use of cannabis, and use of caffeine or other psychostimulants (63). Concomitant use of medications that lower seizure threshold (e.g., bupropion or clozapine) or withdrawal from antiepileptic agents (e.g., mood stabilizers or benzodiazepines) may also increase seizure risk (63, 65). However, even in these situations, risk of seizure remains low, at approximately 33 in 100,000 treatment sessions (66). TMS is not contraindicated in the setting of these risk factors, but the risks and benefits of treatment should be carefully considered and discussed during the informed consent process. TMS may be postponed until risk factors are addressed, or a TMS protocol with a lower risk of seizure (i.e., low-frequency stimulation) may be selected.
Although TMS-induced seizure is rare, individuals or clinics administering TMS should have a protocol in place for managing treatment-induced seizure and should be trained to render appropriate care (65). Recommended management includes immediately stopping treatment, removing the coil from the patient’s head, placing the patient in the lateral decubitus position to reduce risk of aspiration or accidental injury, and calling for emergency medical assistance in case of injury, aspiration, cardiac arrest, or prolonged seizure activity (65). Fortunately, most rTMS-induced seizures are brief—often lasting less than 1 minute—and do not produce serious complications (65).
Side Effects
A major advantage of rTMS is that, as a local and anatomically focused treatment, it avoids the systemic adverse effects associated with pharmacotherapy. The most common side effects of rTMS are headache and local discomfort (67). Discomfort can occur at the site of stimulation, elsewhere on the scalp, or around the ipsilateral eye, ear, nose, jaw, or teeth (62). Neck pain can occur, likely caused by positioning in the chair and immobilization of the head during treatment (62). Patients may also experience transient brow, eyelid, or jaw twitching. Although some discomfort is common, few patients discontinue treatment because of pain (63).
Most discomfort rapidly resolves after the treatment session is completed, and most patients will habituate to the discomfort associated with treatment within the first few sessions (62). Strategies for managing local discomfort include gradual titration of the treatment intensity over the first few days of treatment, application of ice prior to treatment, use of a topical local anesthetic at least 30 minutes prior to treatment, or premedication with analgesics such as ibuprofen or acetaminophen (65). Headaches may be managed conservatively or with over-the-counter analgesics.
TMS can be loud, and transient increases in auditory thresholds have been reported following TMS (63). However, when hearing protection is used, there is no documented risk of permanent hearing loss (63). Patients and TMS operators alike should wear well-fitting earplugs or earmuffs that provide protection up to 30 dB at a minimum (65). Hyperacusis and development of tinnitus are also potential side effects (62). The risks and benefits of TMS should be carefully weighed among patients with preexisting auditory symptoms who may be at higher risk for these side effects.
Induction of hypomania or mania is a possible side effect of TMS treatment. The risk appears low, with one study (68) reporting a pooled occurrence rate of 0.84% (N=356). In naturalistic settings, mood cycling or a “sub-manic activation syndrome” can also occur, featuring agitation, anxiety, and insomnia (69). Because TMS involves daily patient contact, early signs of hypomania or mania can be readily detected and managed with adjustments to stimulation parameters, cessation of rTMS treatment, or adjustments to pharmacotherapy.
Finally, patients are often anxious about possible cognitive side effects of TMS treatment. Although TMS may decrease cognitive performance during or immediately after stimulation, multiple systematic reviews show no evidence of sustained adverse cognitive effects from TMS treatment (63). Patients may be reassured that, in contrast to- electroconvulsive therapy, rTMS does not appear to cause lasting adverse effects on cognition.
Practical Considerations
Evaluation and Monitoring
A comprehensive evaluation for the safety and appropriateness of rTMS is necessary before beginning TMS treatment. The evaluation should review medical, neurological, and surgical history and specifically screen for conditions that increase the risk of seizure, concomitant use of medications that may raise or lower seizure threshold, concomitant substance use, the presence of auditory complaints such as hearing loss or tinnitus, and the presence of implanted metal or medical devices (65). The evaluation should include discussion of the risks and benefits of treatment and a detailed informed consent process, with particular attention to any individual risk factors.
Throughout the course of TMS, patients should be monitored for any changes in their medical status, changes to their medication regimen (particularly with respect to medications that alter seizure threshold), or changes in patterns of substance use, including alcohol, caffeine, and cannabis (65). Sleep should be monitored, because significant reductions in sleep can increase the risk of seizure (65). Changes in these clinical factors may alter cortical excitability and may necessitate a new motor threshold determination in order to safely proceed with treatment. Patients should also be monitored for the development of side effects, in accordance with the 2018 consensus guidelines by McClintock et al. (65).
Response to treatment should be monitored regularly. Weekly symptom monitoring with validated symptom rating scales is the standard of care and is often required for insurance reimbursement. The Patient Health Questionnaire-9, Hamilton Depression Rating Scale, Beck Depression Inventory, Inventory of Depressive Symptomatology, or Clinical Global Impression rating scale may be used to monitor treatment of major depressive disorder, and the YBOCS may be used to monitor treatment of OCD.
Concomitant Treatment
It is estimated that most of the hundreds of thousands of patients who have received rTMS treatment for depression since 2008 were taking psychotropic medications during rTMS treatment, and no specific safety concerns have been identified (63). These findings provide compelling data that TMS is safe in combination with psychotropic medication, even with medications that lower seizure threshold (e.g., bupropion, clozapine). However, it remains critical to document and monitor concomitant medication use during TMS treatment. The initiation, discontinuation, or dosage adjustment of a medication that affects seizure threshold may necessitate a redetermination of the motor threshold before continuing with treatment.
Concomitant medication use may have an impact on treatment outcome. Multiple retrospective studies (70–72) have found that concomitant benzodiazepine use is linked to poorer TMS response, but it is not clear how these findings should influence clinical practice. Preliminary, retrospective evidence (71) suggests that concomitant use of psychostimulants may accelerate and enhance rTMS response among patients with depression. No other medication has been demonstrated to improve rTMS outcomes, but this is an active area of investigation.
Device Selection
Seven manufacturers of TMS devices have at least one FDA-cleared system available in the United States (Table 1). Six of these manufacturers use the traditional figure-eight or “butterfly” coils and one uses the Hesed (H) TMS coils. When choosing a TMS system, careful consideration should be given to the compatible coil type(s), because this will determine which treatment protocols can be offered. In addition, there is a trade-off between focality of stimulation and depth of stimulation (73). The figure-eight coil produces a more focal electrical field, meaning that errors in target localization are of greater concern, whereas the H-coils provide broader and deeper cortical stimulation, which can lead to modulation of nontarget sites along with the target site. Both figure-eight and H-coil systems have been shown to be safe and effective, and naturalistic studies and meta-analyses have suggested similar response and remission rates for both (18, 34, 74). One randomized controlled trial (75) comparing the efficacy of the H-coil and figure-eight coil in treating depression reported a higher response rate with the H-coil. However, it is difficult to directly compare efficacy of the two systems because the FDA-approved treatment protocols for the H-coil versus the figure-eight coil systems use different stimulation parameters. Other considerations in selecting a TMS device include presence of a cooling method to prevent coil overheating; compatibility with neuronavigation, electroencephalography, and electromyography; and ease-of-use options, such as software to store patient information.
Treatment Setting
As patient and provider interest in TMS has grown, the use of TMS has expanded beyond a few specialized research centers into outpatient offices in the public and private sectors. Models for TMS delivery include traditional private practice, device partnership, networked practice, dedicated TMS center, and institution (76).
Following the initial FDA clearance, the majority of TMS treatments have been delivered in the private practice setting. In this setting, psychiatrists may obtain a TMS device solely to treat their current patients or may accept external referrals to achieve and maintain full utilization of the TMS device, making device ownership maximally cost-effective. Depending on patient volume, additional staff (i.e., a TMS technician) may be needed to operate the device and monitor treatments. Expanding a private practice to include TMS may have great financial benefit if the business and administrative aspects are managed successfully. However, any psychiatrist considering this model should carefully consider the challenge of balancing clinical, administrative, and business elements of offering TMS (76).
In the partnership model, several practices jointly acquire and operate a TMS machine. This model distributes the financial burdens of device ownership—such as device acquisition, additional office space, and additional staff—across each participating practice, thereby reducing capital outlay (76).
In the networked practice model, a management company handles the administrative and business aspects of TMS delivery, while representing multiple practices as a single entity providing TMS. Although this model creates efficiencies of scale, there are significant legal complexities surrounding this model, and legal counsel is advisable (76).
The dedicated TMS center provides TMS exclusively and employs dedicated clinicians who evaluate patients for TMS and manage the course of TMS treatment; all other care (e.g., pharmacotherapy, psychotherapy) is managed by the referring provider (76). These practices may be simpler to manage and operate, given the single-service line. Additionally, the clinicians focus solely on TMS delivery and may develop special expertise in this area.
Finally, the institutional model is becoming more prevalent as TMS is increasingly available in institutional settings, such as hospital systems and academic medical centers. Advantages of developing a TMS service in this setting include the pre-existing staff, infrastructure, patient volume, and market presence offered by these institutions (76).
In any of these practice models, consideration must be given to the design and layout of the office space, because there are specific requirements for installation of a TMS device. The treatment room should be large enough to accommodate the TMS device and to allow room for patients and staff to maneuver around it. It is recommended (76) that the space be sufficient to accommodate a paramedic gurney as well. To accommodate a NeuroStar device, Neuronetics (Malvern, PA) (77) recommends a space of at least 12 feet by 15 feet and requires a minimum space of 8.5 feet by 12 feet. BrainsWay (78) requires a treatment space of at least 10 feet by 8 feet. Treatment rooms should be air conditioned to avoid overheating; device manufacturers specify acceptable ambient temperature ranges. Offices are required to maintain an automated external defibrillator on site. Finally, patient comfort should be prioritized, because patients may sit in the treatment chair for up to an hour or longer. A well-positioned television may help distract patients from any discomfort associated with treatment.
Personnel and Training
TMS is prescribed by a licensed clinician (e.g., psychiatrist, neurologist, nurse practitioner, physician assistant) with TMS-specific training. TMS sessions may be administered by the clinician or, more commonly, by a TMS technician. There are no specific licensing or credentialing requirements for TMS technicians, but medical assistants, nurses, or psychiatric technicians often fill the technician role. TMS technicians must have thorough training in all aspects of TMS delivery, including device operation, patient monitoring, and response to adverse events, such as seizure. TMS technicians administer each treatment session and monitor the patient throughout the session, ensuring that the coil remains in good contact with the patient’s scalp and monitoring for any sign of hand movement, which may indicate an impending seizure. TMS technicians may make minor adjustments to treatment (coil repositioning, adjustments to intensity) but should have clear guidelines describing when to contact the prescribing clinician for assistance. The prescribing clinician is ultimately responsible for each treatment session and must remain available (although not necessarily onsite) during TMS treatments. For details on recommended training for TMS clinicians and technicians, the International Federation of Clinical Neurophysiology has recently published guidelines (79). In addition, TMS device manufactures offer training programs that cover general principles of TMS as well as device-specific information.
Insurance Coverage
TMS for the treatment of major depressive disorder is covered by most commercial insurance carriers, Medicare, and in some states, by Medicaid. A course of 30–36 treatments is generally covered for adults with moderate-to-severe depression who have no contraindications to TMS. Generally, treatment is covered only after patients have failed a course of evidence-based psychotherapy and a certain number of medication trials (ranging from one to six, depending on the insurance carrier). If a treatment course is successful, many insurers will cover a second or third treatment course after a period of 2–3 months. Maintenance rTMS treatment (less frequent treatment intended to maintain response or remission) is considered experimental and is generally not covered. In addition to depression treatment, several insurance carriers cover TMS treatment for OCD, and treatment for smoking cessation may sometimes be covered on a single case agreement basis (60).
The provider should always refer to the patient’s insurance plan for accurate and updated information. It is important to verify benefits and eligibility for each individual patient and to obtain prior authorization when required. Because insurance may deny coverage even if prior authorization has been obtained, it is critical to discuss possible out-of-pocket costs with the patient before beginning treatment. To assist patients who must self-pay, many practices offer payment plans, including sliding scale rates.
Billing
An initial evaluation for TMS is generally billed by using an evaluation and management code, such as 90792, 99205, or 99245. TMS delivery is generally billed by using Current Procedural Terminology (CPT) codes. The three billable CPT codes for the delivery of TMS treatment include 90867 (therapeutic rTMS treatment; initial, including cortical mapping, motor threshold determination, delivery, and management); 90868 (therapeutic rTMS treatment; subsequent delivery and management, per session); and 90869 (therapeutic rTMS treatment; subsequent motor threshold redetermination with delivery and management) (80).
Regular follow-up during TMS may occur daily, weekly, or as infrequently as every other week. TMS follow-up is generally not billed separately unless distinct services (e.g., medication management) are performed.
Future Directions
TMS is a rapidly evolving area and there are several emerging rTMS paradigms of note. Individualized treatment protocols that use a personalized target (e.g., based on fMRI) or personalized stimulation parameters (e.g., based on individual peak alpha frequency) are promising (81, 82). Accelerated treatment protocols, which provide multiple TMS sessions per day to compress many weeks of treatment into a matter of days, are also promising. After only 5 days of treatment, the Stanford accelerated intelligent neuromodulation therapy protocol produced a remission rate of 79% (N=14) with active treatment in a sham-controlled trial (83). If these findings can be replicated in larger trials, this protocol may revolutionize the treatment of depression. TMS also holds promise for the treatment of other neuropsychiatric conditions beyond depression, OCD, and smoking cessation. TMS has demonstrated efficacy in treatment of posttraumatic stress disorder, schizophrenia, substance use disorders, mild cognitive impairment, Alzheimer’s disease, and other psychiatric and neurological conditions (44).
Conclusions
There have been three fundamental revolutions in the field of psychiatry: the psychotherapeutic revolution, which began with the advent of psychoanalysis in the late 19th century; the psychopharmacological revolution, which started with the development of chlorpromazine in the mid-20th century; and the ongoing neuromodulation revolution, brought about by the emergence of TMS in the early 21st century. TMS is at the forefront of this revolution and is an increasingly important part of the modern psychiatrist’s therapeutic armamentarium.
1 : Non-invasive magnetic stimulation of human motor cortex. Lancet 1985; 1:1106–1107Crossref, Google Scholar
2 : Magnetic stimulation of human peripheral nerve and brain: response enhancement by combined magnetoelectrical technique. Neurosurgery 1987; 20:110–116Crossref, Google Scholar
3 : Efficacy and safety of transcranial magnetic stimulation in the acute treatment of major depression: a multisite randomized controlled trial. Biol Psychiatry 2007; 62:1208–1216Crossref, Google Scholar
4 : Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry 2010; 67:507–516Crossref, Google Scholar
5 : The Clinical TMS Society consensus review and treatment recommendations for TMS therapy for major depressive disorder. Brain Stimul 2016; 9:336–346Crossref, Google Scholar
6 : Basic mechanisms of TMS. J Clin Neurophysiol 2002; 19: 322–343Crossref, Google Scholar
7 : TMS induced plasticity in human cortex. Rev Neurosci 2004; 15:253–266Crossref, Google Scholar
8 : Unraveling the cellular and molecular mechanisms of repetitive magnetic stimulation. Front Mol Neurosci 2013; 6:50Crossref, Google Scholar
9 : Unilateral left prefrontal transcranial magnetic stimulation (TMS) produces intensity-dependent bilateral effects as measured by interleaved BOLD fMRI. Biol Psychiatry 2001; 50:712–720Crossref, Google Scholar
10 : Opposite effects of high and low frequency rTMS on regional brain activity in depressed patients. Biol Psychiatry 2000; 48:1133–1141Crossref, Google Scholar
11 : Elevated prefrontal cortex GABA in patients with major depressive disorder after TMS treatment measured with proton magnetic resonance spectroscopy. J Psychiatry Neurosci 2016; 41:E37–E45Crossref, Google Scholar
12 : Dorsolateral prefrontal γ-aminobutyric acid in patients with treatment-resistant depression after transcranial magnetic stimulation measured with magnetic resonance spectroscopy. J Psychiatry Neurosci 2019; 44:386–394Crossref, Google Scholar
13 : Rhythms and blues: modulation of oscillatory synchrony and the mechanism of action of antidepressant treatments. Ann N Y Acad Sci 2015; 1344:78–91Crossref, Google Scholar
14 : Responses to rapid-rate transcranial magnetic stimulation of the human motor cortex. Brain 1994; 117:847–858Crossref, Google Scholar
15 : Depression of motor cortex excitability by low-frequency transcranial magnetic stimulation. Neurology 1997; 48:1398–1403Crossref, Google Scholar
16 : Cumulative sessions of repetitive transcranial magnetic stimulation (rTMS) build up facilitation to subsequent TMS-mediated behavioural disruptions. Eur J Neurosci 2008; 27:765–774Crossref, Google Scholar
17 : Daily repetitive transcranial magnetic stimulation (rTMS) improves mood in depression. Neuroreport 1995; 6:1853–1856Crossref, Google Scholar
18 : Deep transcranial magnetic stimulation (DTMS) in the treatment of major depression: An exploratory systematic review and meta-analysis. J Affect Disord 2015; 187:73–83Crossref, Google Scholar
19 : Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry 2019; 176:931–938Crossref, Google Scholar
20 : Assessment of standard coil positioning in transcranial magnetic stimulation in depression. Psychiatry Res 2011; 186:232–238Crossref, Google Scholar
21 : Cytoarchitectonic definition of prefrontal areas in the normal human cortex: II. Variability in locations of areas 9 and 46 and relationship to the Talairach Coordinate System. Cereb Cortex 1995; 5:323–337Crossref, Google Scholar
22 : Transcranial magnetic stimulation in therapy studies: examination of the reliability of “standard” coil positioning by neuronavigation. Biol Psychiatry 2001; 50:58–61Crossref, Google Scholar
23 : Application of transcranial magnetic stimulation for major depression: coil design and neuroanatomical variability considerations. Eur Neuropsychopharmacol 2021; 45:73–88Crossref, Google Scholar
24 : Reliability of targeting methods in TMS for depression: beam F3 vs 5.5 cm. Brain Stimul 2020; 13:578–581Crossref, Google Scholar
25 : Concordance between beamF3 and MRI-neuronavigated target sites for repetitive transcranial magnetic stimulation of the left dorsolateral prefrontal cortex. Brain Stimul 2015; 8:965–973Crossref, Google Scholar
26 : A randomized trial of rTMS targeted with MRI based neuro-navigation in treatment-resistant depression. Neuropsychopharmacology 2009; 34:1255–1262Crossref, Google Scholar
27 : Antidepressant efficacy of prolonged intermittent theta burst stimulation monotherapy for recurrent depression and comparison of methods for coil positioning: a randomized, double-blind, sham-controlled study. Biol Psychiatry 2020; 87:443–450Crossref, Google Scholar
28 : Large-scale network dysfunction in major depressive disorder: a meta-analysis of resting-state functional connectivity. JAMA Psychiatry 2015; 72:603–611Crossref, Google Scholar
29 : Efficacy of transcranial magnetic stimulation targets for depression is related to intrinsic functional connectivity with the subgenual cingulate. Biol Psychiatry 2012; 72:595–603Crossref, Google Scholar
30 : Subgenual functional connectivity predicts antidepressant treatment response to transcranial magnetic stimulation: independent validation and evaluation of personalization. Biol Psychiatry 2019; 86:e5–e7Crossref, Google Scholar
31 : Stanford accelerated intelligent neuromodulation therapy for treatment-resistant depression. Am J Psychiatry 2020; 177:716–726Crossref, Google Scholar
32 : Effectiveness of theta burst versus high-frequency repetitive transcranial magnetic stimulation in patients with depression (THREE-D): a randomised non-inferiority trial. Lancet 2018; 391:1683–1692Crossref, Google Scholar
33 TMS devices. Fresno, CA, Clinical TMS Society, nd https://www.clinicaltmssociety.org/tms/devices. Accessed Nov 17, 2021Google Scholar
34 : Transcranial magnetic stimulation (TMS) for major depression: a multisite, naturalistic, observational study of acute treatment outcomes in clinical practice. Depress Anxiety 2012; 29:587–596Crossref, Google Scholar
35 : A multisite, naturalistic, observational study of transcranial magnetic stimulation for patients with pharmacoresistant major depressive disorder: durability of benefit over a 1-year follow-up period. J Clin Psychiatry 2014; 75:1394–1401Crossref, Google Scholar
36 : Comparison of clinical outcomes with two transcranial magnetic stimulation treatment protocols for major depressive disorder. Brain Stimul 2021; 14:173–180Crossref, Google Scholar
37 BrainsWay Deep TMS System 510k Number K122288, 510K summary. Silver Spring, MD, US Food and Drug Administration, Center for Devices and Radiological Health, Office of Device Evaluation, Division of Neurological and Physical Medicine Devices, 2013. https://www.accessdata.fda.gov/cdrh_docs/pdf12/K122288.pdfGoogle Scholar
38 : Efficacy and safety of deep transcranial magnetic stimulation for major depression: a prospective multicenter randomized controlled trial. World Psychiatry 2015; 14:64–73Crossref, Google Scholar
39 : 61% of unmedicated treatment resistant depression patients who did not respond to acute TMS treatment responded after four weeks of twice weekly deep TMS in the Brainsway pivotal trial. Brain Stimul 2017; 10:847–849Crossref, Google Scholar
40 MagVenture TMS Therapy—for adjunctive treatment of OCD, MagVenture TMS Therapy System 510k Number K193006 approval letter. Silver Spring, MD, US Food and Drug Administration, Center for Devices and Radiological Health, Office of Product Evaluation and Quality, Office of Neurological and Physical Medicine Devices, Division of Neurological and Physical Medicine Devices, 2020. https://www.accessdata.fda.gov/cdrh_docs/pdf19/K193006.pdfGoogle Scholar
41 Brainsway Deep (DTMS) System 510k Number K203616 approval letter. Silver Spring, MD, US Food and Drug Administration, Center for Devices and Radiological Health, Office of Product Evaluation and Quality, Office of Neurological and Physical Medicine Devices, Division of Neurological and Physical Medicine Devices, 2021. https://www.accessdata.fda.gov/cdrh_docs/pdf20/K203616.pdfGoogle Scholar
42 : Repetitive transcranial magnetic stimulation for smoking cessation: a pivotal multicenter double-blind randomized controlled trial. World Psychiatry 2021; 20:397–404Crossref, Google Scholar
43 : Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS). Clin Neurophysiol 2014; 125:2150–2206Crossref, Google Scholar
44 : Evidence-based guidelines on the therapeutic use of repetitive transcranial magnetic stimulation (rTMS): an update (2014–2018). Clin Neurophysiol 2020; 131:474–528Crossref, Google Scholar
45 : A review of functional neuroimaging in mood disorders: positron emission tomography and depression. Can J Psychiatry 1997; 42:467–475Crossref, Google Scholar
46 : Default mode network mechanisms of transcranial magnetic stimulation in depression. Biol Psychiatry 2014; 76:517–526Crossref, Google Scholar
47 : Clinically meaningful efficacy and acceptability of low-frequency repetitive transcranial magnetic stimulation (rTMS) for treating primary major depression: a meta-analysis of randomized, double-blind and sham-controlled trials. Neuropsychopharmacology 2013; 38:543–551Crossref, Google Scholar
48 : Left versus right repetitive transcranial magnetic stimulation in treating major depression: a meta-analysis of randomised controlled trials. Psychiatry Res 2013; 210:1260–1264Crossref, Google Scholar
49 : Improving the antidepressant efficacy of transcranial magnetic stimulation: maximizing the number of stimulations and treatment location in treatment-resistant depression. Depress Anxiety 2011; 28:973–980Crossref, Google Scholar
50 : Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis. JAMA Psychiatry 2017; 74:143–152Crossref, Google Scholar
51 : Comparative efficacy and acceptability of non-surgical brain stimulation for the acute treatment of major depressive episodes in adults: systematic review and network meta-analysis. BMJ 2019; 364:l1079Crossref, Google Scholar
52 : 1Hz rTMS of the right orbitofrontal cortex for major depression: safety, tolerability and clinical outcomes. Eur Neuropsychopharmacol 2018; 28:109–117Crossref, Google Scholar
53 : Priming stimulation enhances the effectiveness of low-frequency right prefrontal cortex transcranial magnetic stimulation in major depression. J Clin Psychopharmacol 2008; 28:52–58Crossref, Google Scholar
54 : Strategies for augmentation of high-frequency left-sided repetitive transcranial magnetic stimulation treatment of major depressive disorder. J Affect Disord 2020; 277:964–969Crossref, Google Scholar
55 : Repetitive transcranial magnetic stimulation in the treatment of obsessive-compulsive disorders: double blind randomized clinical trial. Psychiatry Res 2016; 238:264–269Crossref, Google Scholar
56 : Adjunctive low-frequency repetitive transcranial magnetic stimulation over the right dorsolateral prefrontal cortex in patients with treatment-resistant obsessive-compulsive disorder: a randomized controlled trial. Clin Psychopharmacol Neurosci 2016; 14:153–160Crossref, Google Scholar
57 : A meta-analysis of the effectiveness of different cortical targets used in repetitive transcranial magnetic stimulation (rTMS) for the treatment of obsessive-compulsive disorder (OCD). Psychiatr Q 2018; 89:645–665Crossref, Google Scholar
58 : Effectiveness and predictors of response to 1-Hz repetitive transcranial magnetic stimulation in patients with obsessive-compulsive disorder. J ECT 2019; 35:61–66Crossref, Google Scholar
59 : Sequential multi-locus transcranial magnetic stimulation for treatment of obsessive-compulsive disorder with comorbid major depression: a case series. Brain Stimul 2020; 13:1600–1602Crossref, Google Scholar
60 Smoking Addiction Treatment. Jerusalem, BrainsWay, nd https://www.brainsway.com/treatments/smoking-addiction/. Accessed Aug 1, 2021Google Scholar
61 : Risk and safety of repetitive transcranial magnetic stimulation: report and suggested guidelines from the International Workshop on the Safety of Repetitive Transcranial Magnetic Stimulation, June 5–7, 1996. Electroencephalogr Clin Neurophysiol 1998; 108:1–16Crossref, Google Scholar
62 : Safety, ethical considerations, and application guidelines for the use of transcranial magnetic stimulation in clinical practice and research. Clin Neurophysiol 2009; 120:2008–2039Crossref, Google Scholar
63 : Safety and recommendations for TMS use in healthy subjects and patient populations, with updates on training, ethical and regulatory issues: expert guidelines. Clin Neurophysiol 2021; 132:269–306Crossref, Google Scholar
64 : Transcranial magnetic stimulation (TMS) during pregnancy: a fetal risk factor. Australas Psychiatry 2021; 29:226–229Crossref, Google Scholar
65 : Consensus recommendations for the clinical application of repetitive transcranial magnetic stimulation (rTMS) in the treatment of depression. Journal of Clinical Psychiatry 2018; 79:16cs10905Crossref, Google Scholar
66 : Seizures from transcranial magnetic stimulation 2012–2016: results of a survey of active laboratories and clinics. Clin Neurophysiol 2019; 130:1409–1416Crossref, Google Scholar
67 : Safety, tolerability, and nocebo phenomena during transcranial magnetic stimulation: a systematic review and meta-analysis of placebo-controlled clinical trials. Neuromodulation 2020; 23:291–300Crossref, Google Scholar
68 : Treatment-emergent mania in unipolar and bipolar depression: focus on repetitive transcranial magnetic stimulation. Int J Neuropsychopharmacol 2008; 11:119–130Crossref, Google Scholar
69 : 5Hz repetitive transcranial magnetic stimulation to left prefrontal cortex for major depression. J Affect Disord 2015; 186:13–17Crossref, Google Scholar
70 : Concomitant lorazepam use and antidepressive efficacy of repetitive transcranial magnetic stimulation in a naturalistic setting. Eur Arch Psychiatry Clin Neurosci 2021; 271:61–67Crossref, Google Scholar
71 : Concomitant medication use and clinical outcome of repetitive transcranial magnetic stimulation (rTMS) treatment of major depressive disorder. Brain Behav 2019; 9:e01275Crossref, Google Scholar
72 : Trajectories of response to dorsolateral prefrontal rTMS in major depression: a THREE-D study. Am J Psychiatry 2019; 176:367–375Crossref, Google Scholar
73 : Electric field depth-focality tradeoff in transcranial magnetic stimulation: simulation comparison of 50 coil designs. Brain Stimul 2013; 6:1–13Crossref, Google Scholar
74 : Clinical outcomes in a large registry of patients with major depressive disorder treated with transcranial magnetic stimulation. J Affect Disord 2020; 277:65–74Crossref, Google Scholar
75 : Efficacy of repetitive transcranial magnetic stimulation using a figure-8-coil or an H1-coil in treatment of major depressive disorder; a randomized clinical trial. J Psychiatr Res 2019; 114:113–119Crossref, Google Scholar
76 : The clinical application of transcranial magnetic stimulation. Psychiatr Ann 2014; 44:305–309Crossref, Google Scholar
77 NeuroStar environmental requirements. Malvern, PA, Neuronetics, nd https://neurostar.com/wp-content/uploads/80-50101-002-NeuroStar-TMS-System-Version-1.7-Technical-Data-Sheet.pdf. Accessed Nov 16, 2021Google Scholar
78 Healthcare professional FAQs. Jerusalem, BrainsWay, nd https://www.brainsway.com/how-does-it-work/faqs/professional-faqs/. Accessed Nov 16, 2021Google Scholar
79 : Training in the practice of noninvasive brain stimulation: recommendations from an IFCN committee. Clin Neurophysiol 2021; 132:819–837Crossref, Google Scholar
80 BrainsWay deep TMS treatment—reimbursement info & help. Jerusalem, BrainsWay, nd https://www.brainsway.com/insurance/. Accessed Nov 16, 2021Google Scholar
81 : Functional magnetic resonance imaging—guided personalization of transcranial magnetic stimulation treatment for depression. JAMA Psychiatry 2021; 78:337–339Crossref, Google Scholar
82 : The relationship between individual alpha peak frequency and clinical outcome with repetitive transcranial magnetic stimulation (rTMS) treatment of major depressive disorder (MDD). Brain Stimul 2019; 12:1572–1578Crossref, Google Scholar
83 : Stanford neuromodulation therapy (SNT): a double-blind randomized controlled trial. Am J Psychiatry 2021; (Epub, March 31, 2021)Link, Google Scholar



