Transcranial Electrical Stimulation for Psychiatric Disorders in Adults: A Primer
Abstract
Transcranial electrical stimulation (tES) comprises noninvasive neuromodulation techniques that deliver low-amplitude electrical currents to targeted brain regions with the goal of modifying neural activities. Expanding evidence from the past decade, specifically using transcranial direct current simulation and transcranial alternating current stimulation, presents promising applications of tES as a treatment for psychiatric disorders. In this review, the authors discuss the basic technical aspects and mechanisms of action of tES in the context of clinical research and practice and review available evidence for its clinical use, efficacy, and safety. They also review recent advancements in use of tES for the treatment of depressive disorders, schizophrenia, substance use disorders, and obsessive-compulsive disorder. Findings largely support growing evidence for the safety and efficacy of tES in the treatment of patients with resistance to existing treatment options, particularly demonstrating promising treatment outcomes for depressive disorders. Future directions of tES research for optimal application in clinical settings are discussed, including the growing home-based, patient-friendly methods and the potential pairing with existing pharmacological or psychotherapeutic treatments for enhanced outcomes. Finally, neuroimaging advancements may provide more specific mapping of brain networks, aiming at more precise tES therapeutic targeting in the treatment of psychiatric disorders.
Psychiatric disorders represent a public health concern, accounting for a significant portion of the general burden of disease—7.4% globally (1). Unfortunately, of those treated, only few patients respond to first-line pharmacological treatments (2). Also, both treatment resistance (3) and treatment-limiting adverse effects (4) are common, as well as high recurrence rates (5). Further, psychiatric disorders often occur comorbidly with other medical conditions, posing additional challenges for pharmacological treatments (6). Moreover, treatment challenges include overall access to providers and feasibility in treatment delivery, especially for psychotherapeutic options, and lack of treatment adherence (7). Together, these challenges underscore the critical need to identify alternative treatment approaches.
The current unmet need for treatment has led to increasing development and evaluation of noninvasive brain stimulation (NIBS), expanding the body of knowledge concerning the role of neural circuits that underlie psychiatric disorders (8), and highlighting the potential of NIBS as candidate interventions for these disorders (9, 10). Specifically, once a target neural circuitry has been identified as a related region of interest for a psychiatric disorder, NIBS could be used to selectively modify activities in the target region (11).
Among NIBS modalities, low-intensity transcranial electrical stimulation (tES) has been one of the most intensively investigated for both research and clinical applications, with particular promise for the treatment of psychiatric disorders (12). The most common tES technique is transcranial direct current stimulation (tDCS), in which a constant, low-amplitude current is delivered through electrodes placed on the scalp to the regions of interest. Related techniques in development include transcranial alternating current stimulation (tACS) and transcranial random noise stimulation (tRNS) (13). A strong record of safety and tolerability has been established for the use of tDCS, associated with only mild and transient side effects (e.g., tingling and redness at site of electrode, mild skin erythema [14]), and no serious adverse event has been reported across clinical trials to date. Skin lesions under the electrodes may occur but are rare and can be prevented by proper electrode preparation (14, 15).
Although each NIBS modality is uniquely advantageous over another, depending on the intended goal, tDCS presents some important advantages as treatment for psychiatric disorders. For example, compared with approaches that have shown high efficacy but that are in-clinic only, such as repetitive TMS (rTMS), tES devices can be portable and are relatively inexpensive and therefore suitable for use in protocols where treatment is self-administered in the home setting (9, 16–18).
The potential uses of tES as a novel treatment approach for psychiatric disorders have been discussed elsewhere (12, 17, 19, 20). Yet, because tES is an area of recent and rapid scientific and technological development, knowledge of and familiarity with its use in clinical settings remain limited among mental health professionals (21). Thus, in this review, recent research examining the use of tES and its implications for the treatment of common psychiatric disorders in adults are discussed, primarily focusing on clinical findings of relevant controlled trials and meta-analyses (Table 1) (22–26).
| Study, disorder, and measure | GRADEa | N of articles | N of participants | Testb | 95% CI |
|---|---|---|---|---|---|
| Meta-analysis | |||||
| Razza et al., 2020 (22) | Low | 25 | 1,092 | Hedges’ g=0.46 | 0.22, 0.7 |
| Depression | |||||
| Depression improvement | |||||
| Response rate | Low | 18 | 942 | OR=2.28 | 1.52, 3.42 |
| Remission rate | Low | 18 | 942 | OR=2.12 | 1.42, 3.16 |
| Cheng et al., 2020 (23) | |||||
| Schizophrenia | |||||
| Positive symptoms | Low | 14 | 566 | SMD=0.17 | 0.001, 0.33 |
| Negative symptoms | Low | 14 | 566 | SMD=0.43 | 0.11, 0.75 |
| Kim et al., 2021 (24) | |||||
| Alcohol use disorder | |||||
| Alcohol craving | Low | 17 | 820 | Hedges’ g=0.33 | 0.08, 0.58 |
| Alcohol consumption | Low | 5 | 321 | Hedges’ g=0.19 | 0.01, 0.37 |
| Kang et al., 2019 (25) | |||||
| Nicotine use disorder | |||||
| Cue-provoked craving | Low | 7 | 173 | SMD=0.42 | 0.13, 0.71 |
| Smoking intake | Low | 8 | 287 | SMD=0.56 | 0.17, 0.94 |
| Randomized controlled trialc | |||||
| Silva et al., 2021 (26) | |||||
| Obsessive-compulsive disorder (OCD) | |||||
| OCD symptoms | — | — | 43 | Cohen’s d=0.62 | 0.06, 1.18 |
| Depression symptoms | — | — | 43 | Cohen’s d=0.43 | –0.06, 0.92 |
| Anxiety symptoms | — | — | 43 | Cohen’s d=0.48 | –0.02, 0.97 |
TABLE 1. Summary of recent meta-analyses and a randomized controlled trial of studies of transcranial direct current stimulation as a treatment for psychiatric disorders
Basic Technical Aspects
A tES device is generally composed of the following items (27): a microprocessor-controlled current source, a battery compartment with single-use batteries or a power outlet electrical cable for recharging the device, connection cables most commonly leading to an anode (red cable) and a cathode (blue cable) electrode, conductive rubber pads for the electrodes (which are sites of electrochemical reactions and should not be in direct contact with the skin), saline-saturated sponges to cover the rubber pads (alternatively, the sponges or rubber or silicon pads can be coated in gel or conductive cream), and nonconductive headgear, such as straps (28), to fix the electrodes over desired cranial locations (13) (Figure 1). The number, size, and positions of the electrodes are called montage (29). Typically, the term “electrode” refers to the rubber electrodes and the sponge together (29).
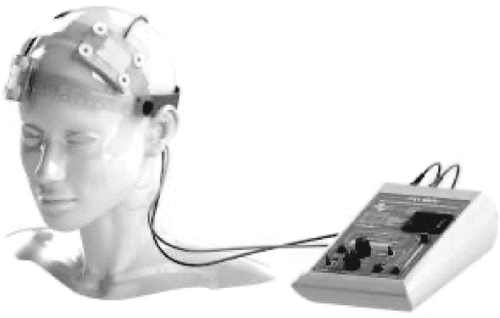
FIGURE 1. A transcranial direct current stimulation (tDCS) device applied to a phantom head model, with the anode over F3 and the cathode over the right supraorbital areaa
a Source: Soterix Medical Inc.
The anode is the electrode where positive current enters the body, and the cathode is the electrode where positive current exits the body (29, 30). In tDCS, the anode electrode and cathode electrode are fixed and defined, and these terms are used to describe the placement of each respective electrode. In tACS, because current regularly changes direction (with a timing defined by the stimulation frequency), the anode and cathode are not well defined—and so these terms are not used in tACS. In tRNS, the amplitude and direction fluctuate rapidly and irregularly (29).
Nonfocal brain current flow is an attribute of a larger electrode surface area, which in the most traditional montages, ranges from 25 cm2 to 35 cm2 per electrode (31), although the effects on the brain suggest electrode position-specific outcomes (32, 33). The alternate technique of high-definition tDCS or tACS (HD-tDCS, HD-tACS, respectively) uses electrodes with a smaller surface area (<1 cm in diameter) arranged in an array. For example, the 4×1 ring array increases the focality of the brain current, allowing noninvasive targeting of cortical regions (31, 34), whereas other HD-tDCS and HD-tACS configurations can target deeper regions simultaneously (35).
Current intensity is measured in amperes, which quantifies the rate of electric charge that passes through a specific point every second. In conventional tDCS, where two electrodes are used, the direction of current in the anode is the opposite of the current in the cathode, and the intensity is the same as both electrodes. If multiple electrodes are used, such as in HD-tDCS, the intensity is calculated by the sum of the current in all anodes, in opposition to all cathodes (14). Furthermore, electrode current density is calculated by dividing current intensity by the area of the electrode (e.g., 2 mA divided by 25 cm2 yields an electrode current density of 0.08 mA/cm2). The electrode montage, current, and duration of a tES session are the most important parameters that define stimulation dose (13, 36).
tES generally uses very weak electrical currents (in the order of a few milliamperes [mA]). Furthermore, only a small fraction of the current reaches the brain, with its greater part being deflected across the skin, connective tissue, muscles, skull, meninges, and cerebrospinal fluid, which all can divert current before it reaches the brain. The aggregate of all the resistance to current flow presented by the head is referred to as impedance or resistance (27, 37). Importantly, impedance (measured in ohms) then reflects the properties not only of the head but also of the electrodes in contact with the skin. In this way, when contact between the device and the head is poor at the electrodes, a high impedance may be detected by the tES device. High impedance levels are often associated with poor conductivity, reflecting a suboptimal electrode setup or humidification (38). To avoid high impedances, the sponges should always be properly humidified but never in excess, for if the solution spreads over the scalp, the current will likewise spread over a large superficial area and will not flow properly across the desired brain regions (13). The certified tES devices measure not only current intensity but also impedance and, for safety, warn the applicator or stop the current flow if too much impedance in the circuit is detected (13).
Definitive and optimal tDCS parameters for psychiatric research and clinical practice are still in development, as these parameters may depend on patient characteristics (39). Preliminary evidence suggests that increasing frequency of sessions, session duration, or current intensity might be associated with better clinical response (39), especially with concurrent and directed mental activity (i.e., “target engagement” [40]). Importantly, higher levels of total tDCS exposure (i.e., cumulative charge) have not been associated with greater adverse events (41).
Basic knowledge of these parameters is a prerequisite to operate a tES device. Although tES devices (especially for tDCS sessions) are typically straightforward and safe (14), it is important that certified equipment is used and that established protocols are followed by trained staff (13, 15). Some devices are designed to be especially portable and simple to use (42) and thus potentially adaptable for home use, provided there is proper patient training and remote supervision by clinical or research staff (18, 43).
Mechanisms of Action
One main distinction that separates the tES modalities from one another is the waveform (temporal pattern) in which the electrical current is applied (Figure 2). In tDCS, a continuous, direct electrical current of low intensity (e.g., typically up to 1–3 mA) passes through at least two electrodes (i.e., anode and cathode) that are applied noninvasively over the scalp (14). tACS, unlike tDCS, delivers an oscillating, sinusoidal electrical current of low intensity (i.e., typically spanning 1–2 mA or less) to the brain via two electrodes applied on to the scalp, whose position and size are determined based on the target region of the brain (29, 44). The method in applying conventional tACS is largely similar to tDCS, with the electrical current delivered via electrodes and saline-saturated sponges.
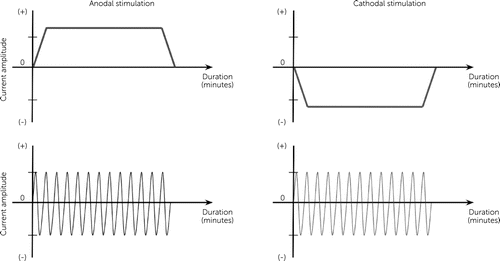
FIGURE 2. Current patterns of transcranial electrical stimulations
tDCS modulates neuronal activity on the network level by producing current flow around neurons, which results in an incremental shift in neuronal membrane potentials (e.g., polarization [45]) and which, in turn, leads to a host of neuronal function changes, such as a change in firing rates (20, 29, 46). These modulation effects may last after the stimulation period, potentially up to 0.5–2 hours, depending on the intensity and duration of stimulation due to changes in synaptic neuroplasticity (i.e., via mechanisms that resemble either long-term potentiation or depression) (47, 48). In addition, the direction of current flow (i.e., parallel or perpendicular to the underlying pyramidal neurons of the stimulation sites [49, 50]) is one of the key determinants of the desired neuromodulatory effects, along with dosage (i.e., current density and duration of stimulation), polarity, size, and placement of the electrodes (19, 20).
tACS, in contrast, is considered to entrain neuronal oscillations to the stimulation frequency by producing an oscillating polarization of neurons (51–53). tACS may also produce changes in brain function outlasting the stimulation duration (54–56). During the half cycle of an AC oscillation, one electrode acts as the anode while the other acts as the cathode, and vice-versa for the other half cycle (57).
Modulation achieved by tDCS and tACS is considered network based within the functionally connected regions beyond the superficial cortical regions, thus presenting more functionally specific stimulation, which can modulate task-relevant brain networks that may yield strong implications for clinical use (12, 17, 19, 20, 58).
In summary, tDCS and tACS present unique neuromodulation options whether the goal is a more functionally or spatially specific target. These techniques are capable of modulating or stimulating task-related neural networks, and as such, they may allow modulation or normalization of dysregulated neural activity that is associated with specific psychiatric disorders. This review focuses on the use of tDCS in treatment of psychiatric disorders (Figure 3) (59).
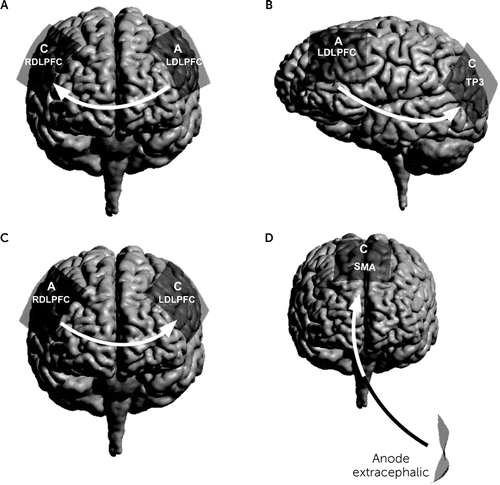
FIGURE 3. Transcranial direct current stimulation electrodes position and the current flow direction for each psychiatric disordera
a A, anode; C, cathode; RDLPFC and LDLPFC, right and left dorsolateral prefrontal cortex, respectively; SMA, supplementary motor area; TP3, left temporoparietal junction. Panel A, depression; panel B, schizophrenia; panel C, substance use disorders (alcohol and cigarettes); panel D, obsessive-compulsive disorder.
Clinical Uses of tDCS for Psychiatric Disorders
Depressive Disorders
Major depressive disorder is one of the main causes of disability and morbidity worldwide, besides being one of the most prevalent psychiatric disorders (60). First-line treatments, such as psychotherapy and antidepressant drugs, are, respectively, time-consuming and can present several adverse events (6). In such a scenario, tDCS can serve as an alternative treatment for major depressive disorder and could be provided as a first-line treatment for patients who do not wish to take medications or undergo psychotherapy.
The neurobiological rationale for using tDCS as the treatment for major depressive disorder is based on evidence of an interhemispheric functional asymmetry in depressive states that leads to a hypoactivation of the left dorsolateral prefrontal cortex (DLPFC) and hyperactivation of the right DLPFC (61). Therefore, the trials investigating the effects of tDCS for depression typically apply the anode over the left DLPFC (which corresponds to the F3 position of the 10–20 electroencephalogram [EEG] system), while the cathode is placed either over the right DLPFC (F4 in the 10–20 system) or the supraorbital area (22, 46).
In the first trial evaluating the clinical efficacy of tDCS for major depressive disorder, five sessions of tDCS over the left (anode) and right (cathode) DLPFC of 10 patients were conducted, and the study found significant symptom improvement in the active group (62). The initial positive results of tDCS for major depressive disorder subsequently led to several larger randomized controlled trials (RCTs) in unipolar and bipolar depression. A larger trial was conducted to investigate the safety and efficacy of tDCS alone and combined with sertraline 50 mg per day (SELECT-TDCS trial), in which tDCS was applied bilaterally (anode over F3 and cathode over F4) for 12 sessions (63). tDCS effects were similar to those of sertraline and superior to those of placebo or sham, whereas the combination of tDCS and medication led to a significantly greater effect, compared with placebo or sham (63). The largest trial to date of tDCS for unipolar depression (ELECT-TDCS trial), a noninferiority study that included 245 patients, assessed the efficacy of tDCS with the maximally effective dose (20 mg per day) of escitalopram (64). The participants were randomly assigned to three groups: active tDCS plus placebo medication, sham tDCS plus real medication, or sham tDCS plus placebo medication. The superiority analyses showed that tDCS was inferior to escitalopram in reducing depressive symptoms, although superior to sham.
Further studies performing secondary analyses with data derived from the ELECT-TDCS trial increased the understanding of the clinical aspects of both tDCS and pharmacotherapy for major depressive disorder. Overall, these results suggest that both interventions produce distinct effects in depressive symptoms and point to important directions that can optimize tDCS response. Studies applying machine learning, symptom clustering, and distinct-trajectory approaches showed that tDCS response might be improved in specific subgroups of patients. For instance, patients presenting insomnia or sleep symptoms and negative affect may benefit more from tDCS treatment, and older and more anxious patients may respond faster to the treatment (65–67).
Moreover, the effects of tDCS for bipolar depression were also assessed. First, an RCT with 59 participants who were allocated to a sham or an active group showed a significant improvement of depressive symptoms and sustained response for the tDCS group, compared with sham (68). However, a recent multicentric trial with 130 patients with unipolar and bipolar depression did not show significant differences between active and sham groups regarding symptom improvement and response rates (69). Nevertheless, these results might be justified by the sham method, which was found to elicit biological effects by itself (41), in addition to improvement in depressive symptoms (Hedges’ g=1.09, 95% confidence interval [CI]=0.8–1.38 [70]). Such effects are currently viewed as a possible hidden source of variability among tDCS studies (71). Although mixed effects were found in studies of tDCS for depression in past years, results of recent aggregate and individual patient data (IPD) meta-analyses corroborated tDCS as having a moderate effect for depression (22, 72). Both aggregate and IPD meta-analyses have found a medium effect size favoring active tDCS over sham for continuous outcomes after the acute treatment period (N=25, Hedges’ g=0.46, 95% CI=0.22–0.70 and N=9, β=0.31, 95% CI=0.15–0.47) and both response and remission rates favoring the active group (72). Moreover, subgroup analyses showed that tDCS presented similar efficacy for both unipolar and bipolar depression (22, 73).
The follow-up effects of tDCS for depression were less investigated to date, with high design heterogeneity observed among the trials, which involved mixed follow-up duration and number of sessions (from nine to 48). A recent meta-analysis conducted to investigate the evidence of the tDCS follow-up effects for depression identified that interventional studies (with tDCS during the follow-up period) might lead to greater symptom improvement after the acute treatment (74), compared with observational follow-ups.
Although research evidence suggests feasibility and efficacy of clinical implementation of tDCS in treatment of depression, official recommendations for tDCS in clinical practice vary from country to country, and the field has been urged to create formal, uniform guidelines. Recently, a guideline focusing on the therapeutic use of tDCS for both neurological and psychiatric disorders considered anodal tDCS on the left DLPFC to be effective (level A) in improving symptoms in major depressive disorder (17), without differentiating its efficacy for drug-resistant patients, as in a previous guideline (46). However, no specific recommendations were made regarding cathode position, which was either at F4 or right supraorbital in most studies. In this regard, and taking into consideration the favorable safety profile, the National Institute for Health and Care Excellence guidelines recommend tDCS for the treatment of major depressive disorder in the United Kingdom, with written consent by patients, and special arrangements for clinical governance and careful record of selected parameters.
Schizophrenia
Schizophrenia is a chronic neuropsychiatric disorder characterized by positive (e.g., hallucinations) and negative (e.g., anhedonia) symptoms (75). The first-line treatment consists of pharmacotherapy with antipsychotics. However, this class of drugs primarily targets positive symptoms, with modest effectiveness at best, and is limited in the management of negative and cognitive symptoms (76). Furthermore, poor adherence and intolerable side effects are common even with newer antipsychotics, often leading to discontinuation and poor clinical outcomes (77).
Functional neuroimaging studies in patients with schizophrenia demonstrate hyperactivity in the left temporoparietal junction (TP3), a cortical area relevant for language or speech perception that is related to auditory verbal hallucinations (78). Negative symptoms, however, have been linked to deficits in prefrontal activity and its connectivity to other cortical areas and also to subcortical structures (79). Thus most employed NIBS protocols are aimed at decreasing cortical activity in the left temporoparietal junction, increasing the activity of the left DLPFC, or both.
The first RCT investigating the effects of tDCS in schizophrenia recruited 30 subjects diagnosed as having auditory verbal hallucinations and applied 10 tDCS sessions (twice a day for 5 days), with the anode positioned at F3 and the cathode at TP3 (80, 81). At follow-up, clinical improvement both for positive and negative symptoms was superior in the active group, compared with sham. Subsequent studies with the same stimulation protocol further supported its efficacy (82, 83). On the other hand, RCTs that applied bilateral tDCS targeted at the frontal lobes, with anode at F3 and cathode either on F4 or Fp2, showed nonsignificant results for positive or negative symptoms (84, 85). Another trial that positioned the anode over the left DLPFC and the cathode over TP3 did not show reduction in the severity of positive symptoms (86). The delivery of a single daily session, contrary to two daily sessions in prior RCTs, might explain the null effects in that trial, because the other parameters of stimulation were similar. In a recent meta-analysis (10 RCTs, 338 participants), the superiority of active tDCS over sham regarding the reduction of auditory verbal hallucinations emerged only in a subgroup analysis that included studies that performed either twice daily sessions or 10 or more sessions overall, suggesting that the delivery of a higher number of sessions is associated with greater efficacy (standardized mean difference [SMD]=1.04, 95% CI=0.20–1.89, p=0.02 and SMD=0.86, 95% CI=0.22–1.51, p=0.009, respectively) (87).
A later meta-analysis (14 RCTs, 566 participants), however, showed a marginal superiority of active tDCS, compared with sham, in positive symptoms (SMD=0.17, 95% CI=0.001–0.33), with a greater improvement of negative symptoms (SMD=0.43, 95% CI=0.11–0.75) (23). In the largest RCT to date, 100 patients were recruited with stable negative symptoms (83); the study replicated the tDCS study originally conducted by Brunelin and colleagues (80). The anode and cathode positions were F3 and TP3, respectively, and the primary outcome was clinical evaluation 6 weeks after the end of the study. The investigators observed a greater improvement in negative symptoms in the active group, compared with sham, for both continuous outcomes and response rates. Of interest, tDCS sessions were delivered twice for only 5 days, but the improvement was greatest at the 6-week follow-up assessment and persisted for 12 weeks.
Although a few other trials have suggested that prefrontal tDCS might improve cognition in patients with schizophrenia (85, 88), findings in this domain are still preliminary. Furthermore, a recent meta-analysis indicated that active tDCS was not superior to sham regarding working memory performance in this population (89). The most current guideline on the topic specified that tDCS with the anode over F3 and the cathode over TP3 was probably effective (level B of evidence) for reducing positive symptoms (17).
Substance Use Disorders
Substance use disorders have a chronic and relapsing course, characterized by compulsive behavior aimed at drug consumption, difficulty in limiting its intake, and anxious or dysphoric symptoms if access to it is prevented (90). Nicotine and alcohol dependence rank among the most prevalent substance use disorders. For instance, in the United States, the prevalence of nicotine dependence is estimated at 14% (91, 92), with an estimated global prevalence of 100 million cases of alcohol use disorder in 2016 (93).
Management of substance use disorders aims at ceasing intake and preventing relapse, usually with multimodal approaches that include psychotherapy and pharmacological and psychoeducational interventions. Specific pharmacological treatments are currently available only for substance use disorders related to a limited number of substances, such as alcohol, nicotine, and opioids. Nonetheless, pharmacotherapy targets mainly withdrawal symptoms, having limited efficacy in preventing relapse (94).
It has been suggested that specific symptoms of substance use disorders occur as a consequence of abnormal activity in several neural circuits, which include overactivation of ascending meso-cortico-striatal dopaminergic projections that comprise the reward system and underactivation of multiple regions in the prefrontal cortex (90). Neuromodulatory strategies aim at improving executive function and top-down inhibitory control that the prefrontal cortex exerts at the overly hyperactive reward system (95). Alternatively, stimulation may be used to reduce the negative affect in the withdrawal phase and to diminish the craving intensity.
In the first published RCT to use tDCS to treat substance use disorders, 13 patients who were diagnosed with alcohol dependence received tDCS interventions (96). In this study, participants received three distinct tDCS montages, separated by a 48-hour interval (i.e., washout period). Active interventions (anode at F3 and cathode at F4 or anode at F4 and cathode at F3) led to a significant improvement in craving symptoms after cue provocation, compared with sham.
Subsequently, two larger RCTs were conducted to better investigate the effects of reversed polarities over the DLPFC in patients with alcohol dependence, applying anode and cathode over F4 and F3, respectively (97, 98). In the first trial, 31 patients received one session of 13-minute active or sham tDCS per day, during 5 consecutive days (97). The results showed three times less relapse in the active group, compared with sham, at the 6-month follow-up . In the second study, 49 participants were recruited and received tDCS once a day, every other day, in a total of 10 sessions, (98). After treatment, significant reduction in craving was observed only for the active group, and the relapse rate in the active group was almost three times lower (27.8%) than in the sham group (72.7%) in a 3-month follow-up assessment.
In turn, separate trials were conducted to compare different electrode positions (99). Among them, two larger RCTs investigated the effects of bilateral tDCS, having the anode over F3 and cathode over F4 (99). The first trial applied four sessions in 91 participants who were allocated to three groups: active tDCS during cognitive bias modification (CBM), sham tDCS during CBM, and active tDCS separate from CBM. The results showed no significant improvements in alcohol craving or relapse with either the online or the offline CBM at endpoint and subsequently in a 3-month and 1-year follow-up. Lastly, a recent meta-analysis also showed that bilateral tDCS over the DLPFC can improve symptoms of alcohol abuse disorder (N=11, Hedges’ g=0.44, 95% CI=0.10–0.79) (24).
Although few studies to date have examined tDCS as a treatment approach for nicotine dependence, several prior studies document favorable effects of tDCS for reduction in nicotine intake. A crossover trial, in which 24 patients underwent sham or active anodal tDCS over either the left or the right DLPFC, showed that both active interventions reduced craving, compared with the sham group (100). Subsequent trials also showed that both polarities (anode over the right DLPFC plus cathode over the left DLPFC and anode over the left DLPFC plus cathode over the right DLPFC) were significantly beneficial for the reduction in nicotine intake (101, 102). However, more recent large trials presented mixed results applying anodal tDCS over the left DLPFC.
Recently, a large RCT applying the anode over F3 and the cathode over the right supraorbital area in 106 participants failed to find significant differences in the daily nicotine intake rate or number of cigarettes smoked among two active tDCS groups (1 mA and 2 mA) and sham (103). Nevertheless, a recent meta-analysis (12 studies, 392 participants) investigating the effects of tDCS for nicotine dependence revealed that tDCS can decrease an individual's nicotine dependence symptoms for both cue-provoked craving (i.e., phasic increases in the urge for nicotine use induced by situational cues [104]) and nicotine intake, but positive effects on cue-provoked craving levels were driven by anodal tDCS on the right DLPFC (SMD=0.65, 95% CI=0.32–0.99; p<0.001) (25). Inconsistent findings across studies with opposite-polarity DLPFC stimulation suggest that samples might be heterogeneous regarding regional neural activity. Finally, a guideline (17) on the therapeutic use of tDCS has recommended that combining anodal tDCS of the right and cathodal tDCS of the left DLPFC is probably effective in alcohol addiction or craving symptoms (level B).
Obsessive-Compulsive Disorder
Obsessive-compulsive disorder (OCD) is a chronic psychiatric disorder characterized by the presence of obsessions (intrusive and unwanted thoughts), often associated with anxiety, and compulsions (e.g., repetitive behaviors rigidly performed in response to obsessions) (105, 106). The lifetime prevalence of OCD is 2%−3%, and it is often underdiagnosed and undertreated, despite the significant impairment it causes in daily functioning (106).
First-line interventions include psychotherapy (cognitive-behavioral therapy with exposure and response prevention) and pharmacotherapy (mainly antidepressants). However, a significant proportion of patients (40%−60%) do not respond satisfactorily to initial management strategies, prompting the need of novel interventions (107). Regarding more established neuromodulatory approaches, high frequency rTMS targeted at the dorsomedial prefrontal cortex with symptom provocation has been recently approved by the U.S. Food and Drug Administration (108).
Disruptions in the functioning of neural circuits in patients with OCD include multiple cortical regions and their related cortico-striato-thalamo-cortical connections, each associated with a distinctive cluster of symptoms. Most studies of tES interventions in OCD patients target the supplementary motor area (SMA) and pre-SMA, which are important components of the sensorimotor and dorsal cognitive circuits, respectively. Abnormal neuronal hyperactivity at the SMA has been specifically linked to symptomatic behavior (i.e., compulsions), and the pre-SMA, together with other prefrontal areas, is related to disruptions in executive function and emotion regulation (106).
The first RCT to use tDCS for OCD was conducted by applying anodal or cathodal tDCS for 10 days (daily consecutive 2-mA, 20 minutes) over the pre-SMA/SMA area, with an extracephalic return electrode (commonly over the left or right deltoid) (109). In this crossover study, cathodal stimulation over SMA, but not anodal, significantly improved OCD symptoms. A subsequent parallel RCT investigated the effects of bicephalic tDCS (anode over pre-SMA and cathode over the right supraorbital area) in 25 patients with treatment-resistant OCD (110). The results showed symptom improvement in the active group, compared with the sham group. The largest clinical trial was performed with cathode over the SMA and anode over the left deltoid muscle in 43 patients over 4 consecutive weeks (20 sessions, 2-mA, 30 minutes per session) (26). There was a significant reduction in OCD symptoms for patients in the active group, which was not observed for anxiety and depression symptoms. This study, however, was not included in the most recent tDCS guideline for OCD, which indicated a level C evidence (possibly effective) recommendation for anodal stimulation of the SMA (17).
Future Directions
Home Use
Although testing clinical efficacy of tES in treating psychiatric disorders has grown exponentially in the past few years, research examining the efficacy and accessibility of patient self-administered, home-use tES is still in its infancy (9, 18, 111). tES treatments have a cumulative effect and require daily application for optimal benefit. Most studies examining the efficacy of tES in mental health have been performed primarily in clinical and research settings, where patients are required to make multiple visits to the facilities to complete the trial; this arrangement, however, poses significant financial and logistical burdens in a real-world scenario (72). The development of portable, user-friendly tES devices for home use is a highly desirable goal for increasing accessibility and reducing health disparities, particularly for patients who have limited mobility or are immunocompromised (111, 112).
Few studies to date have examined the feasibility of remotely controlled and supervised tES for home-use intervention for psychiatric disorders (9, 16, 39, 111, 113). In one open-label study, 34 patients with depression self-administered 20 or 28 tDCS sessions (2 mA, 30 minutes per session) for 4 weeks, followed by a taper phase of four sessions with 1 week apart (113). The study showed a significant reduction in depressive symptoms (114), with transient and mild side effects, similar to the lab-based applications (113). Another pilot trial of home-use tDCS, in combination with app-based psychological interventions for major depressive disorder, has also shown promising results (16). Thus, early evidence supports the feasibility and initial efficacy of self-administered, at-home use of tES, and such development could lead to a breakthrough in the use of tES in the context of clinical practice, offering scalability and wide availability. Finally, as an approach that could be easily adapted to be used at home, this modality can be an alternative to investigate the follow-up effects of tDCS for psychiatric disorders.
Individualization Approach for tES
The concept of precision medicine in psychiatry describes how personalized treatment and prevention efforts targeting individual differences and transdiagnostic mechanisms can refine and improve prevention and intervention efforts, consistent with the Research Domain Criteria (RDoC) approach proposed by the National Institute of Mental Health (9, 10). Although precision medicine is in its nascent stage, approaches derived from precision medicine have the potential to enhance treatment outcomes by identifying treatment-relevant subgroups among target clinical populations (9, 115). tES is one such candidate, which can be used independently or combined with more traditional cognitive, behavioral, or pharmacological interventions to target specific treatment mechanisms that are best suited for each individual (19, 22, 26, 46).
Ideally, tES treatments could be built on the existing neuroimaging evidence for neural circuitry associated with specific psychiatric disorders, making it a unique approach to develop personalized medicine (10). For example, in transdiagnostic approaches that characterize psychiatric disorders based on their neurobiological underpinnings, tES has the advantage of being able to target specific brain regions or neural network (10). However, due to a broad electrical current field produced by tES, which may often result in networkwide stimulations, and the complexity in identification of neurobiological underpinnings of various psychiatric disorders (e.g., comorbidity), this goal may be more difficult to achieve.
With the current state of research, optimization of tES as an individualized treatment can be accomplished by other personalized indices. More specifically, tES allows for variability in dosage and stimulation techniques (e.g., tDCS and tACS), which can then be used to identify optimal parameters and type of stimulation that are individualized to each patient (52). Further, based on individual variabilities in brain activity, closed-loop systems (e.g., tES coupled to EEG to detect brain waves) might be used to dynamically control stimulation parameters in the pursuit of improved and personalized outcomes (116).
In sum, the idea of precision medicine using tES in combination with other biomarkers that are unique to different subgroups could enhance treatment outcomes and may accelerate progress of adaptation of neuromodulation approaches as an intervention method.
Combining tES With Other Interventions for Psychiatric Disorders
Although tES has shown efficacy in monotherapy (117), when combined with other treatment methods, it may lead to superior treatment outcomes (40, 63). In a study examining the efficacy of tDCS and pharmacological treatment for major depressive disorder, the combination of the two methods increased the efficacy of each treatment, compared with those treatments alone or sham (63). Further, several studies have demonstrated that a combination of cognitive therapy and tDCS yielded superior improvement in self-reported depressive symptoms (118, 119). Additionally, several studies that examined anxiety-related symptoms (e.g., attention bias to threat) showed that combination of tDCS and attention bias modification training reduced the attention bias to threat, compared with sham (120–122). Importantly, these multimodal therapies in psychiatry can maximize treatment outcomes when carried out carefully with the timing of delivery (e.g., simultaneous, sequential) (40). Thus, further investigation in the multimodal therapy approach is necessary for increasing treatment outcomes.
Beyond tDCS
Across clinical trials, tDCS has been more widely used for stand-alone or combined interventions. However, tACS has also been examined in recent RCTs. For major depressive disorder, 32 participants were randomly assigned to one of the three groups: tACS at 10 Hz (alpha range), tACS at 40 Hz (gamma range), or sham (123). Active electrodes were placed over the DLPFC bilaterally, with a return electrode at the vertex. Although the groups did not differ regarding depressive symptoms at 4-week follow-up (primary outcome), the group that underwent 10-Hz stimulation had a higher response rate at 2 weeks (77.8% versus 40% in 40-Hz tACS and 20% in sham). The 10-Hz stimulation protocol was also effective in engaging alpha oscillations (substitute outcome) in this feasibility study (123) and in the subsequent trial (124). In the first RCT to evaluate the effects of tACS in schizophrenia, 25 patients with medication-refractory auditory verbal hallucinations were randomly assigned to one out of three arms (tACS, tDCS, or sham) (125). Although the tACS group showed a higher symptom improvement for the primary outcome, the differences between groups were not significant, which may have been due to the small sample size of each subgroup.
With OCD, the only clinical study using tACS published to date was an open-label case series that included seven patients who received gamma stimulation (40 Hz) targeted at the DLPFC bilaterally, with improvement in all cases (126).
Conclusions
The goal of this review was to provide an overview of recent advancements in tES research, with an aim to identify its appropriate application in clinical settings as an intervention for psychiatric disorders. Research using tES in clinical samples has grown exponentially over the past decade. This body of evidence reflects substantial safety and modest efficacy of tES in clinical practice, along with its potential to help patients with resistance to existing treatment options, increasing its clinical relevance. This review suggests that most promising clinical outcomes have been documented in major depressive disorder research, along with growing evidence of its application to treat schizophrenia, OCD, and substance use disorders. Furthermore, the review has demonstrated promising technological and methodological improvements in tES that allow for greater specificity in treating specific psychiatric disorders. Future research, using a precision medicine framework, should leverage cutting-edge neuroimaging findings to improve the mapping of disruptions in specific brain networks associated with distinct psychiatric disorders to better personalize tES treatment protocols. In all, the clinical use of tES is particularly alluring for its mild side effects and its potential to be used as an independent or combined treatment method. With better understanding of its impact on and interaction with brain functions, future research can provide more standardized guidelines that are specific to each psychiatric disorder, leading to improved clinical outcomes.
1 : Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet 2012; 380:2197–2223Crossref, Google Scholar
2 : Treatment-resistant anxiety disorders. Mol Psychiatry 2006; 11:805–814Crossref, Google Scholar
3 : Protocol for a systematic review and meta-analysis of the placebo response in treatment-resistant depression: comparison of multiple treatment modalities. BMJ Open 2021; 11:e041349Crossref, Google Scholar
4 : Antidepressant efficacy of adjunctive aerobic activity and associated biomarkers in major depression: a 4-week, randomized, single-blind, controlled clinical trial. PLoS One 2016; 11:e0154195Crossref, Google Scholar
5 : Transcranial direct current stimulation (tDCS) for preventing major depressive disorder relapse: results of a 6-month follow-up. Depress Anxiety 2019; 36:262–268Crossref, Google Scholar
6 : Depression in the medically ill. Aust N Z J Psychiatry 2020; 54:346–366Crossref, Google Scholar
7 : Challenges and opportunities in global mental health: a research-to-practice perspective. Curr Psychiatry Rep 2017; 19:28Crossref, Google Scholar
8 : Identification of common neural circuit disruptions in emotional processing across psychiatric disorders. Am J Psychiatry 2020; 177:411–421Crossref, Google Scholar
9 : Precision non-implantable neuromodulation therapies: a perspective for the depressed brain. Br J Psychiatry 2020; 42:403–419Crossref, Google Scholar
10 : The intervention, the patient and the illness: personalizing non-invasive brain stimulation in psychiatry. Exp Neurol 2021; 341:113713Crossref, Google Scholar
11 : Toward a neurocircuit-based taxonomy to guide treatment of obsessive-compulsive disorder. Mol Psychiatry (Epub ahead of print, Jan 7, 2021)Crossref, Google Scholar
12 : Clinical research with transcranial direct current stimulation (tDCS): challenges and future directions. Brain Stimul 2012; 5:175–195Crossref, Google Scholar
13 : A technical guide to tDCS, and related non-invasive brain stimulation tools. Clin Neurophysiol 2016; 127:1031–1048Crossref, Google Scholar
14 : Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul 2016; 9:641–661Crossref, Google Scholar
15 : No risk of skin lesion or burn with transcranial direct current stimulation (tDCS) using standardized protocols. Brain Stimul 2021; 14:511–512Crossref, Google Scholar
16 : Use of app-based psychological interventions in combination with home-use transcranial direct current stimulation for the treatment of major depressive disorder: a case series. J Affect Disord 2021; 288:189–190Crossref, Google Scholar
17 : Evidence-based guidelines and secondary meta-analysis for the use of transcranial direct current stimulation in neurological and psychiatric disorders. Int J Neuropsychopharmacol 2021; 24:256–313Crossref, Google Scholar
18 : Supervised transcranial direct current stimulation (tDCS) at home: a guide for clinical research and practice. Brain Stimul 2020; 13:686–693Crossref, Google Scholar
19 : Transcranial direct current stimulation: a roadmap for research, from mechanism of action to clinical implementation. Mol Psychiatry 2020; 25:397–407Crossref, Google Scholar
20 : Transcranial direct current stimulation in psychiatric disorders: a comprehensive review. Psychiatr Clin North Am 2018; 41:447–463Crossref, Google Scholar
21 : Acceptability, attitudes and knowledge towards Transcranial Magnetic Stimulation (TMS) among psychiatrists in France. Encephale 2020; 46:88–95Crossref, Google Scholar
22 : A systematic review and meta-analysis on the effects of transcranial direct current stimulation in depressive episodes. Depress Anxiety 2020; 37:594–608Crossref, Google Scholar
23 : The effects of transcranial direct current stimulation (tDCS) on clinical symptoms in schizophrenia: a systematic review and meta-analysis. Asian J Psychiatr 2020; 53:102392Crossref, Google Scholar
24 : Bilateral transcranial direct current stimulation attenuated symptoms of alcohol use disorder: a systematic review and meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2021; 108:110160Crossref, Google Scholar
25 : Effects of transcranial direct current stimulation on symptoms of nicotine dependence: a systematic review and meta-analysis. Addict Behav 2019; 96:133–139Crossref, Google Scholar
26 : Efficacy and safety of transcranial direct current stimulation as an add-on treatment for obsessive-compulsive disorder: a randomized, sham-controlled trial. Neuropsychopharmacology 2021; 46:1028–1034Crossref, Google Scholar
27 : Physics of transcranial direct current stimulation devices and their history. J ECT 2018; 34:137–143Crossref, Google Scholar
28 : Electrode positioning and montage in transcranial direct current stimulation. J Vis Exp (Epub May 23, 2011)Crossref, Google Scholar
29 : Transcranial electrical stimulation nomenclature. Brain Stimul 2019; 12:1349–1366Crossref, Google Scholar
30 : Electrical stimulation of excitable tissue: design of efficacious and safe protocols. J Neurosci Methods 2005; 141:171–198Crossref, Google Scholar
31 : High-definition transcranial direct current stimulation induces both acute and persistent changes in broadband cortical synchronization: a simultaneous tDCS-EEG study. IEEE Trans Biomed Eng 2014; 61:1967–1978Crossref, Google Scholar
32 : Shaping the effects of transcranial direct current stimulation of the human motor cortex. J Neurophysiol 2007; 97:3109–3117Crossref, Google Scholar
33 : Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000; 527:633–639Crossref, Google Scholar
34 : Physiological and modeling evidence for focal transcranial electrical brain stimulation in humans: a basis for high-definition tDCS. Neuroimage 2013; 74:266–275Crossref, Google Scholar
35 : Optimized multi-electrode stimulation increases focality and intensity at target. J Neural Eng 2011; 8:046011Crossref, Google Scholar
36 : Fundamentals of transcranial electric and magnetic stimulation dose: definition, selection, and reporting practices. Brain Stimul 2012; 5:435–453Crossref, Google Scholar
37 : Noninvasive brain stimulation in psychiatric disorders: a primer. Br J Psychiatry 2019; 41:70–81Crossref, Google Scholar
38 : Transcranial direct current stimulation (tDCS): a beginner’s guide for design and implementation. Front Neurosci 2017; 11:641Crossref, Google Scholar
39 :
40 : The dynamic duo: combining noninvasive brain stimulation with cognitive interventions. Prog Neuropsychopharmacol Biol Psychiatry 2019; 89:347–360Crossref, Google Scholar
41 : Safety of repeated sessions of transcranial direct current stimulation: a systematic review. Brain Stimul 2018; 11:278–288Crossref, Google Scholar
42 : Updated technique for reliable, easy, and tolerated transcranial electrical stimulation including transcranial direct current stimulation. J Vis Exp (Epub Jan 3, 2020)Crossref, Google Scholar
43 : Remotely-supervised transcranial direct current stimulation (tDCS) for clinical trials: guidelines for technology and protocols. Front Syst Neurosci 2015; 9:26Crossref, Google Scholar
44 : Concurrent electroencephalography recording during transcranial alternating current stimulation (tACS). J Vis Exp (Epub Jan 22, 2016)Crossref, Google Scholar
45 : Role of cortical cell type and morphology in subthreshold and suprathreshold uniform electric field stimulation in vitro. Brain Stimul 2009; 2:215–228Crossref, Google Scholar
46 : Evidence-based guidelines on the therapeutic use of transcranial direct current stimulation (tDCS). Clin Neurophysiol 2017; 128:56–92Crossref, Google Scholar
47 : Comparing cortical plasticity induced by conventional and high-definition 4 × 1 ring tDCS: a neurophysiological study. Brain Stimul 2013; 6:644–648Crossref, Google Scholar
48 : Studying and modifying brain function with non-invasive brain stimulation. Nat Neurosci 2018; 21:174–187Crossref, Google Scholar
49 : Effects of uniform extracellular DC electric fields on excitability in rat hippocampal slices in vitro. J Physiol 2004; 557:175–190Crossref, Google Scholar
50 : Cellular effects of acute direct current stimulation: somatic and synaptic terminal effects. J Physiol 2013; 591:2563–2578Crossref, Google Scholar
51 : Mapping the mechanisms of transcranial alternating current stimulation: a pathway from network effects to cognition. Front Psychiatry 2014; 5:162Crossref, Google Scholar
52 : Transcranial electrical stimulation (tES) mechanisms and its effects on cortical excitability and connectivity. J Inherit Metab Dis 2018; 41:1123–1130Crossref, Google Scholar
53 : Effects of weak transcranial alternating current stimulation on brain activity-a review of known mechanisms from animal studies. Front Hum Neurosci 2013; 7:687Crossref, Google Scholar
54 : Lasting modulation of in vitro oscillatory activity with weak direct current stimulation. J Neurophysiol 2015; 113:1334–1341Crossref, Google Scholar
55 : Transcranial alternating current stimulation in the low kHz range increases motor cortex excitability. Restor Neurol Neurosci 2011; 29:167–175Google Scholar
56 : Comparatively weak after-effects of transcranial alternating current stimulation (tACS) on cortical excitability in humans. Brain Stimul 2008; 1:97–105Crossref, Google Scholar
57 : Transcranial alternating current stimulation (tACS): from basic mechanisms towards first applications in psychiatry. Eur Arch Psychiatry Clin Neurosci 2020; 271:135–156Crossref, Google Scholar
58 : Transcranial direct current stimulation in psychiatric disorders. World J Psychiatry 2015; 5:88–102Crossref, Google Scholar
59 : Field modeling for transcranial magnetic stimulation: a useful tool to understand the physiological effects of TMS? Annu Int Conf IEEE Eng Med Biol Soc 2015; 2015:222–225Google Scholar
60 : Major depressive disorder: new clinical, neurobiological, and treatment perspectives. Focus Am Psychiatr Publ 2016; 14:266–276Abstract, Google Scholar
61 : Imbalance between left and right dorsolateral prefrontal cortex in major depression is linked to negative emotional judgment: an fMRI study in severe major depressive disorder. Biol Psychiatry 2008; 63:369–376Crossref, Google Scholar
62 : Cognitive effects of repeated sessions of transcranial direct current stimulation in patients with depression. Depress Anxiety 2006; 23:482–484Crossref, Google Scholar
63 : The sertraline vs electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry 2013; 70:383–391Crossref, Google Scholar
64 : Repetitive transcranial magnetic stimulation for the acute treatment of major depressive episodes: a systematic review with network meta-analysis. JAMA Psychiatry 2017; 74:143–152Crossref, Google Scholar
65 : Effects of tDCS on neuroplasticity and inflammatory biomarkers in bipolar depression: results from a sham-controlled study. Prog Neuropsychopharmacol Biol Psychiatry 2021; 105:110119Crossref, Google Scholar
66 : Distinct trajectories of response to prefrontal tDCS in major depression: results from a 3-arm randomized controlled trial. Neuropsychopharmacology 2021; 46:774–782Crossref, Google Scholar
67 : Clinical patterns differentially predict response to transcranial direct current stimulation (tDCS) and escitalopram in major depression: a machine learning analysis of the ELECT-TDCS study. J Affect Disord 2020; 265:460–467Crossref, Google Scholar
68 : Efficacy and safety of transcranial direct current stimulation as an add-on treatment for bipolar depression: a randomized clinical trial. JAMA Psychiatry 2018; 75:158–166Crossref, Google Scholar
69 : International randomized-controlled trial of transcranial Direct Current Stimulation in depression. Brain Stimul 2018; 11:125–133Crossref, Google Scholar
70 : Understanding spatial variation in COVID-19 across the United States. J Urban Econ (Epub ahead of print, March 11, 2021) Crossref, Google Scholar
71 : Sham tDCS: a hidden source of variability? Reflections for further blinded, controlled trials. Brain Stimul 2019; 12:668–673Crossref, Google Scholar
72 : Efficacy and acceptability of non-invasive brain stimulation for the treatment of adult unipolar and bipolar depression: a systematic review and meta-analysis of randomised sham-controlled trials. Neurosci Biobehav Rev 2018; 92:291–303Crossref, Google Scholar
73 : Efficacy and acceptability of transcranial direct current stimulation (tDCS) for major depressive disorder: an individual patient data meta-analysis. Prog Neuropsychopharmacol Biol Psychiatry 2020; 99:109836Crossref, Google Scholar
74 : Follow-up effects of transcranial direct current stimulation (tDCS) for the major depressive episode: a systematic review and meta-analysis. Psychiatry Res 2021; 302:114024Crossref, Google Scholar
75 : Schizophrenia. Nat Rev Dis Primers 2015; 1:15067Crossref, Google Scholar
76 : Psychosis as a state of aberrant salience: a framework linking biology, phenomenology, and pharmacology in schizophrenia. Am J Psychiatry 2003; 160:13–23Crossref, Google Scholar
77 : Predictors of clozapine discontinuation in patients with schizophrenia. Int Clin Psychopharmacol 2011; 26:311–315Crossref, Google Scholar
78 : The neural mechanisms of hallucinations: a quantitative meta-analysis of neuroimaging studies. Neurosci Biobehav Rev 2016; 69:113–123Crossref, Google Scholar
79 : Hypofrontality in schizophrenia: distributed dysfunctional circuits in neuroleptic-naïve patients. Lancet 1997; 349:1730–1734Crossref, Google Scholar
80 : Examining transcranial direct-current stimulation (tDCS) as a treatment for hallucinations in schizophrenia. Am J Psychiatry 2012; 169:719–724Crossref, Google Scholar
81 : Efficacy and safety of bifocal tDCS as an interventional treatment for refractory schizophrenia. Brain Stimul 2012; 5:431–432Crossref, Google Scholar
82 : Anodal tDCS targeting the left temporo-parietal junction disrupts verbal reality-monitoring. Neuropsychologia 2016; 89:478–484Crossref, Google Scholar
83 : Efficacy and safety of transcranial direct current stimulation for treating negative symptoms in schizophrenia: a randomized clinical trial. JAMA Psychiatry 2020; 77:121–129Crossref, Google Scholar
84 : Effects of transcranial direct current stimulation (tDCS) on cognition, symptoms, and smoking in schizophrenia: a randomized controlled study. Schizophr Res 2015; 168:260–266Crossref, Google Scholar
85 : Adjunct transcranial direct current stimulation improves cognitive function in patients with schizophrenia: a double-blind 12-week study. Schizophr Res 2018; 197:378–385Crossref, Google Scholar
86 : Exploratory study of once-daily transcranial direct current stimulation (tDCS) as a treatment for auditory hallucinations in schizophrenia. Eur Psychiatry 2016; 33:54–60Crossref, Google Scholar
87 : A meta-analysis of transcranial direct current stimulation for schizophrenia: “Is more better?”. J Psychiatr Res 2019; 110:117–126Crossref, Google Scholar
88 : Using prefrontal transcranial direct current stimulation (tDCS) to enhance proactive cognitive control in schizophrenia. Neuropsychopharmacology 2020; 45:1877–1883Crossref, Google Scholar
89 : Non-invasive brain stimulation does not improve working memory in schizophrenia: a meta-analysis of randomised controlled trials. Neuropsychol Rev 2021; 31:115–138Crossref, Google Scholar
90 : Neurobiology of addiction: a neurocircuitry analysis. Lancet Psychiatry 2016; 3:760–773Crossref, Google Scholar
91 : Nicotine use and DSM-IV nicotine dependence in the United States, 2001–2002 and 2012–2013. Am J Psychiatry 2020; 177:1082–1090Crossref, Google Scholar
92 : Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and initiation among young people in 204 countries and territories, 1990–2019. Lancet Public Health 2021; 6:e472–e481Crossref, Google Scholar
93 : The associations between psychotic experiences and substance use and substance use disorders: findings from the World Health Organization World Mental Health surveys. Addiction 2018; 113:924–934Crossref, Google Scholar
94 : A case report illustrating the effects of repetitive transcranial magnetic stimulation on cue-induced craving in an individual with opioid and cocaine use disorder. Exp Clin Psychopharmacol 2020; 28:1–5Crossref, Google Scholar
95 : Ventral medial prefrontal cortex (vmPFC) as a target of the dorsolateral prefrontal modulation by transcranial direct current stimulation (tDCS) in drug addiction. J Neural Transm 2016; 123:1179–1194Crossref, Google Scholar
96 : Prefrontal cortex modulation using transcranial DC stimulation reduces alcohol craving: a double-blind, sham-controlled study. Drug Alcohol Depend 2008; 92:55–60Crossref, Google Scholar
97 : A randomized controlled trial of targeted prefrontal cortex modulation with tDCS in patients with alcohol dependence. Int J Neuropsychopharmacol 2014; 17:1793–1803Crossref, Google Scholar
98 : Multiple sessions of transcranial direct current stimulation (tDCS) reduced craving and relapses for alcohol use: a randomized placebo-controlled trial in alcohol use disorder. Front Pharmacol 2018; 9:716Crossref, Google Scholar
99 : A clinical trial with combined transcranial direct current stimulation and alcohol approach bias retraining. Addict Biol 2017; 22:1632–1640Crossref, Google Scholar
100 : Cortical stimulation of the prefrontal cortex with transcranial direct current stimulation reduces cue-provoked smoking craving: a randomized, sham-controlled study. J Clin Psychiatry 2008; 69:32–40Crossref, Google Scholar
101 : Modulation of smoking and decision-making behaviors with transcranial direct current stimulation in tobacco smokers: a preliminary study. Drug Alcohol Depend 2014; 140:78–84Crossref, Google Scholar
102 : Modulation of risk-taking in marijuana users by transcranial direct current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC). Drug Alcohol Depend 2010; 112:220–225Crossref, Google Scholar
103 : Lack of effect of transcranial direct current stimulation (tDCS) on short-term smoking cessation: results of a randomized, sham-controlled clinical trial. Drug Alcohol Depend 2019; 194:244–251Crossref, Google Scholar
104 : The relevance and treatment of cue-induced cravings in tobacco dependence. J Subst Abuse Treat 2009; 36:235–243Crossref, Google Scholar
105 : Obsessive-compulsive spectrum conditions in obsessive-compulsive disorder and other anxiety disorders. Depress Anxiety 2003; 18:118–127Crossref, Google Scholar
106 : Obsessive-compulsive disorder. Nat Rev Dis Primers 2019; 5:52Crossref, Google Scholar
107 : Treatment non-response in OCD: methodological issues and operational definitions. Int J Neuropsychopharmacol 2002; 5:181–191Crossref, Google Scholar
108 : Efficacy and safety of deep transcranial magnetic stimulation for obsessive-compulsive disorder: a prospective multicenter randomized double-blind placebo-controlled trial. Am J Psychiatry 2019; 176:931–938Crossref, Google Scholar
109 : Transcranial direct current stimulation for obsessive-compulsive disorder: a randomized, controlled, partial crossover trial. Depress Anxiety 2016; 33:1132–1140Crossref, Google Scholar
110 : Efficacy of pre-supplementary motor area transcranial direct current stimulation for treatment resistant obsessive compulsive disorder: a randomized, double blinded, sham controlled trial. Brain Stimul 2019; 12:922–929Crossref, Google Scholar
111 : Home use, remotely supervised, and remotely controlled transcranial direct current stimulation: a systematic review of the available evidence. Neuromodulation 2018; 21:323–333Crossref, Google Scholar
112 : Applications of non-invasive neuromodulation for the management of disorders related to COVID-19. Front Neurol 2020; 11:573718Crossref, Google Scholar
113 : Pilot trial of home-administered transcranial direct current stimulation for the treatment of depression. J Affect Disord 2019; 252:475–483Crossref, Google Scholar
114 : A comparison between the Beck Depression Inventory (BDI) and the self-rating version of the Montgomery Asberg Depression Rating Scale (MADRS). J Affect Disord 2001; 64:203–216Crossref, Google Scholar
115 : The new field of “precision psychiatry”. BMC Med 2017; 15:80Crossref, Google Scholar
116 : Surface EEG-transcranial direct current stimulation (tdcs) closed-loop system. Int J Neural Syst 2017; 27:1750026Crossref, Google Scholar
117 : Monotherapy with tDCS for schizophrenia: a case report. Brain Stimul 2013; 6:708–709Crossref, Google Scholar
118 : Working memory improvement with non-invasive brain stimulation of the dorsolateral prefrontal cortex: a systematic review and meta-analysis. Brain Cogn 2014; 86:1–9Crossref, Google Scholar
119 : Concurrent cognitive control training augments the antidepressant efficacy of tDCS: a pilot study. Brain Stimul 2014; 7:325–331Crossref, Google Scholar
120 : Impact of transcranial direct current stimulation on attentional bias for threat: a proof-of-concept study among individuals with social anxiety disorder. Soc Cogn Affect Neurosci 2017; 12:251–260Crossref, Google Scholar
121 : Impact of anodal and cathodal transcranial direct current stimulation over the left dorsolateral prefrontal cortex during attention bias modification: an eye-tracking study. PLoS One 2015; 10:e0124182Crossref, Google Scholar
122 : Transcranial direct current stimulation (tDCS) augments the effects of gamified, mobile attention bias modification. Front Neuroergonom (Epub May 19, 2021)Crossref, Google Scholar
123 : Double-blind, randomized pilot clinical trial targeting alpha oscillations with transcranial alternating current stimulation (tACS) for the treatment of major depressive disorder (MDD). Transl Psychiatry 2019; 9:106Crossref, Google Scholar
124 : Gamma transcranial alternating current stimulation in patients with negative symptoms in schizophrenia: a case series. Neurophysiol Clin 2020; 50:301–304Crossref, Google Scholar
125 : Randomized trial of transcranial alternating current stimulation for treatment of auditory hallucinations in schizophrenia. Eur Psychiatry 2018; 51:25–33Crossref, Google Scholar
126 : Case report: successful treatment of therapy-resistant ocd with application of transcranial alternating current stimulation (tACS). Brain Stimul 2016; 9:463–465Crossref, Google Scholar



