Never Say Never: Successful Clozapine Rechallenge After Multiple Episodes of Neutropenia
Abstract
Clozapine is a second-generation antipsychotic with a superior efficacy for the management of treatment-resistant schizophrenia but underutilized because of potential side effects. A 59-year-old Caucasian male veteran was transferred from the long-term care unit to the acute psychiatry unit because of suicidality. He was noted as having a long-standing history of psychosis with significant referential and paranoid delusions. He had experienced two previous trials of clozapine; although he had significant response in the past, both trials ended in neutropenia and an absolute neutrophil count <500 cells per microliter, despite the second trial also including supplemental “as-needed” doses of pegfilgrastim to manage decline in neutrophil counts. This particular strategy of filgrastim use was determined to be a weakness of the second trial. A PubMed search identified recent literature that discussed preemptive dosing of filgrastim to prevent neutropenia. Thus, a protocol was established to administer 300 μg filgrastim subcutaneously, three times weekly, concurrently with clozapine initiation. This plan was discussed on local and national levels to achieve consensus before its initiation. Using a revised, patient-specific protocol led to successful initiation of clozapine and the ability to maintain the regimen for over 24 months without interruption or any further suicidal ideation.
Clozapine is a second-generation antipsychotic with superior efficacy for the management of treatment-resistant schizophrenia and evidence for the improvement of psychotic symptoms associated with other conditions, such as schizoaffective disorder or bipolar disorder (1, 2). Furthermore, it has a noted effect of decreasing suicidality (3). However, potential side effects include myocarditis, metabolic syndrome, and neutropenia (absolute neutrophil count [ANC] <1,500/μL) (4). Although agranulocytosis (ANC <500/μL; now replaced with the term “severe neutropenia”) was noted in less than 1% of all clozapine recipients after up to 1.5 years of treatment, the annual rate of neutropenia has been found to be 2.7% and highest within the first 18 weeks of therapy (5, 6). To address concerns of neutropenia, in September 2015, a Risk Evaluation and Mitigation Strategies program was established within the United States to ensure that a complete blood count (CBC) with differential is obtained weekly for the first 6 months of therapy and then every 2 weeks for an additional 6 months; at that point, monitoring occurs monthly thereafter for the duration of the clozapine regimen (7).
Patient History
In October of 2017, a 59-year-old Caucasian male veteran (post-Vietnam era) with a history of paranoid schizophrenia, depressive disorder not otherwise specified (NOS), cognitive disorder NOS, traumatic brain injury, anxiety, coronary artery disease, and cerebrovascular accident was transferred from the long-term care unit to the acute psychiatry unit because of suicidality with plans to hang himself by his pajama bottoms. He was noted as also having a long-standing history of psychosis with significant referential and paranoid delusions regarding staff and other veterans who, he felt, wanted him to be kicked off the unit and were stealing his belongings. At the time, his psychotropic regimen included 15 mg olanzapine at bedtime and 2.5 mg twice a day (BID) as needed, as well as 60 mg aripiprazole daily. The patient was also receiving 34 mg pimavanserin daily as an adjunct for psychotic symptoms. He was noted as having trialed numerous psychotropic agents in the past, with lack of sustained success, including aripiprazole, carbamazepine, citalopram, divalproex, fluphenazine, haloperidol, lurasidone, minocycline, mirtazapine, nefazodone, olanzapine, paroxetine, phenelzine, quetiapine, risperidone, and trifluoperazine. He refused electroconvulsive therapy, noting minimal response in the past. He and his providers noted that, in previous years, his symptoms had been less pronounced on clozapine, although not completely absent and not requiring admission to the acute psychiatry unit. Thus, clozapine was again considered after first factoring in the results of previous trials and current suicidal ideation.
Clozapine Trial 1 (2005–2014)
The veteran was previously maintained on clozapine successfully for 9 years, reaching a maintenance dose of 250 mg twice daily. However, upon routine monthly monitoring in April 2014, his ANC was noted to have decreased from a baseline range of 2,200–3,200/μL to 1,600/μL with no ascertained source, including medication changes. Monitoring of the CBC with differential increased to twice weekly, pursuant to the monitoring recommendations at the time. Three days later, the ANC was noted to be 1,300/μL. Clozapine was discontinued, and twice weekly monitoring of the CBC with differential was continued. After 8 days, including 2 consecutive days of ANC >2,000/μL, clozapine was restarted at 25 mg daily at bedtime, eventually reaching the previous maintenance dose of 250 mg twice daily. During that period, the veteran experienced occasional anxiety and persistent auditory hallucinations on a daily basis, both male and female. Voices were derogatory in content, but there was no evidence of command hallucinations, visual hallucinations, or suicidal ideation. Other medication changes were made to address current symptoms, including the addition of divalproex for mood stabilization and seizure prophylaxis, clonazepam for anxiety, and mirtazapine for depressive symptoms. Six months later (in October 2014), the ANC decreased from 2,800/μL to 1,700/μL. Mirtazapine was discontinued. Further reduction in neutrophil count persisted despite mirtazapine discontinuation, and clozapine and divalproex were also discontinued 3 days later. The ANC continued to decrease, and omeprazole was also discontinued; haloperidol and olanzapine were trialed for psychotic symptoms. Lithium was initiated, given data suggesting positive impact on circulating neutrophil counts (8). However, the decline in ANC persisted, reaching 300/μL 1 week after clozapine was discontinued. Olanzapine and lithium were discontinued the next day. The veteran reported feeling worried and anxious but, overall, stable. The hematology staff was consulted and advised to continue to hold clozapine. Haloperidol was replaced with trifluoperazine per patient preference and then titrated upward to manage psychotic symptoms. Mirtazapine was restarted for depressive symptoms, and pantoprazole was initiated for peptic ulcer disease. The ANC and white blood cell count began to trend upward. However, the veteran then began to decompensate and was transferred from the long-term care unit to the acute psychiatry unit because of worsening paranoia, auditory hallucinations, and thoughts of self-harm. Within the next 6 months (October 2014–April 2015), he required four separate acute admissions for similar symptoms, despite a regimen of lurasidone (replacing trifluoperazine), clonazepam, trazodone, olanzapine, lithium, and hydroxyzine.
Clozapine Trial 2 (April 2015–May 2015)
In April 2015, it was determined that the veteran would benefit from retrial of clozapine. Under the guidelines at that time, he was deemed “non-rechallengeable” (9). However, there was still evidence to support careful use of the medication in extenuating circumstances (6). The Department of Veterans Affairs (VA) allows for a special protocol, authorized at a national level. Previous neutropenic episodes were first reviewed to determine whether polypharmacy was contributory. Valproic acid and omeprazole were both identified as potential factors (10–15).
With the aforementioned information, the case was presented to the VA’s National Clozapine Coordinating Center (NCCC) and approved using a special protocol. The CBC was to be assessed thrice weekly for 1 month; if the ANC remained>1,000/μL, the plan was to decrease monitoring to twice weekly for 1 month and then weekly thereafter. If the ANC were to decrease, the plan was to increase monitoring frequency until again stable. Because of a history of neutropenia likely exacerbated by drug-drug interactions, it was requested that no new medications be administered to the veteran without first discussing this with both the psychiatrist and clinical pharmacist. This was emphasized by placing a note in the veteran’s chart describing the presence of a special protocol and restriction on new medications. Additionally, this document was scanned for patient records.
If the ANC <1,000/μL, the veteran was to be admitted to the acute medicine unit and continued on clozapine, receiving a 6-mg dose of pegfilgrastim. This threshold for the use of pegfilgrastim was originally set at <1,500/μL. However, it was lowered to minimize overall exposure to pegfilgrastim. This PEGylated formulation of filgrastim was chosen specifically because of its longer half-life and the hypothesis that this would allow for sustained protection against neutropenia (16). A meta-analysis of patients receiving cyclic doses of pegfilgrastim or daily doses of filgrastim for treatment of neutropenia found similar rates of efficacy between the two agents (17). However, one study within the meta-analysis found that pegfilgrastim was superior in terms of febrile neutropenia rates. If the ANC were to fall below 500/μL, the plan was to stop clozapine and not rechallenge.
This protocol was reviewed nationally and approved. Clozapine was initiated at 25 mg daily on April 27th and increased gradually, reaching a dose of 100 mg BID on May 15th. Other psychotropic medications at that time included 40 mg olanzapine daily, 300 mg lithium daily, 0.5 mg lorazepam by mouth every 6 hours as needed, 25 mg hydroxyzine BID, 0.5 mg benztropine daily, and 100 mg trazodone at bedtime. On day 22 of clozapine administration, the ANC began to decrease, reaching 900/μL on day 25. Per protocol, 6 mg pegfilgrastim was administered that day; however, the ANC continued to decrease below 500/μL on day 27. Clozapine was discontinued, and the ANC began to increase thereafter.
Clozapine Trial 3 (December 2017–Present)
It was determined that a weakness of the initial protocol in 2015 was the use of “as-needed” doses of pegfilgrastim. Recent literature was noted to discuss preemptive dosing of filgrastim to prevent neutropenia, a strategy under consideration for this veteran during the previous trial but not utilized (18). On the basis of further review of safety of dosing for as long as 48 months after clozapine initiation, and a lack of studies explicitly utilizing pegfilgrastim, the team planned to administer 300 μg filgrastim subcutaneously three times weekly, concurrently with clozapine initiation. Doses were to be adjusted up or down to maintain an ANC between 2,000/μL and 8,000/μL. Should the ANC decrease below 1,000/μL, the veteran was to be admitted to the acute medicine unit, and the hematology team was to be consulted for additional guidance. This plan was discussed on both local and national levels to achieve consensus (a specific protocol is available from the authors on request), and written informed consent was obtained from the patient after the treatment protocol had been fully explained to him.
Given the concern for continuity of care should the need arise to transfer the veteran to a higher or lower level of care, a chart flag was entered within the electronic health record apprising all providers of the protocol, including the request that no new medications be administered without first discussing with both the psychiatrist and clinical pharmacist. The protocol itself was also entered into the electronic health record for review by any provider.
Outcome and Follow-Up
In December 2017, clozapine was successfully initiated with the aforementioned protocol. Pimavanserin and olanzapine were eventually discontinued, and the clozapine dose was titrated to the previous maintenance dose of 250 mg BID; lithium was added for additional neutrophil stabilization, and the previous clonazepam regimen was continued. The veteran continued to report persecutory delusions, referential delusions, and paranoia regarding his being transferred off the unit. However, he remained able to complete activities of daily living, participate in daily yoga, and draw both for pleasure and to compete in art exhibitions at the facility. Throughout clozapine treatment, he denied suicidality, with no further acute hospitalizations required. On day 23 of clozapine treatment, the ANC decreased to 900/μL, and the filgrastim dose was adjusted to maintain the ANC within the desired range (Figure 1). The frequency of filgrastim injections and ANC monitoring decreased over time because of the ANC stabilization. Minor changes were made to the protocol, given concern for elevated ANC; as a result, the protocol was modified so that the filgrastim dose decreased to 300 μg twice weekly and was held if the ANC >3,000/μL. Lithium was subsequently discontinued, with no negative impact on neutrophil count. The veteran has not needed any doses of filgrastim since day 240 of clozapine treatment. Interestingly, the facility instituted a smoking ban that occurred after the patient had received approximately 22 months of clozapine treatment. As a result, his clozapine level increased from 646 to 1,166 ng/mL before the dose was adjusted to target a lower maintenance range. Again, no episodes of neutropenia or seizures were noted.
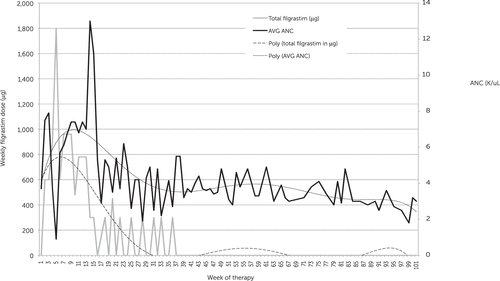
FIGURE 1. Absolute neutrophil count (ANC) and filgrastim dose versus time
Discussion
Clozapine is a highly effective antipsychotic and one that is underutilized within the VA setting (19). One factor that was found to influence clozapine utilization was the absence or presence of a multidisciplinary team to ensure safe and effective care. Through the involvement of a multidisciplinary team, the veteran in this case was able to be safely initiated on clozapine and maintained on a therapeutic dose, with no ANC excursions below 500/μL, despite 2 years of continuous treatment. However, this case may not be necessarily applicable to all hospital settings. Although behavioral flags and those related to patient confidentiality are often used within the VA setting, this was the first medication-specific flag to be implemented at our facility. Such a process is time sensitive and thus requires the support of a clozapine treatment team.
One other fact to note is that, in our case, the initiation of lithium did not produce any significant improvement in neutrophil count, nor did the discontinuation of lithium decrease the neutrophil count. Clozapine rechallenge has been shown to be less successful when patients are coprescribed both lithium and colony-stimulating factor (CSF), such as filgrastim (18). This is surprising, as there are multiple reports of successful use of lithium to treat clozapine-induced neutropenia, and given the concern for the precipitous drop in ANC upon its discontinuation (20). Our case demonstrates that further research may be necessary to quantify the effect of lithium on increasing ANC in clozapine recipients, particularly those who are coprescribed CSF.
Myles and colleagues, in a recent meta-analysis, have provided additional data regarding rates of neutropenia associated with clozapine use. Assuming a neutropenia threshold of 1,000/μL, they noted a 1.3% incidence over the course of the included studies (21). The rate decreased to 0.9% if neutropenia was more tightly defined as an ANC less than 500/μL, and 89% of neutropenia cases occurred within the first year of treatment, with the peak incidence occurring after 1 month. This is similar to the experience with the most recent clozapine titration (Figure 1). However, our case illustrates that there are still times at which neutropenia may occur even past this 1-year mark, given that the veteran was initially maintained on clozapine for many years before experiencing significant neutropenia. These findings are supported by a similar case, also reported from a VA facility, of a 55-year-old man who developed neutropenia after 20 years of clozapine therapy. He, too, responded well to filgrastim coadministration to maintain clozapine treatment (22).
Given that neutropenia may develop because of idiopathic reactions as well as drug-drug interactions, continued monitoring is still warranted throughout treatment with clozapine. The patient in our case is eligible for less frequent monitoring of ANC every 4 weeks. After discussion with the NCCC, the treating providers determined that continuing to monitor every 2 weeks would allow for more acute management of neutropenia should it occur again; at that point, the current protocol would allow for the resumption of filgrastim. Myles and colleagues (18) noted that, although prophylactic administration of filgrastim was the most common approach to prevent neutropenia on clozapine rechallenge, the strategies using “as-required” doses of filgrastim were the most successful. Utilizing a prophylactic strategy initially and then gradually adjusting or holding the dose to maintain a prespecified ANC range, as was utilized in this case, may provide a more flexible approach that requires further exploration. We hope that this case illustrates the risks and benefits of various strategies of CSF use and may serve as a potential model for successful clozapine use in the future, particularly in situations in which neutropenia is of concern for patients and providers.
1 : Clozapine for the treatment-resistant schizophrenic. A double-blind comparison with chlorpromazine. Arch Gen Psychiatry 1988; 45:789–796Crossref, Google Scholar
2 : Clozapine in severe mood disorders. J Clin Psychiatry 1995; 56:411–417Google Scholar
3 : Suicide in schizophrenia: risk factors and clozapine treatment. J Clin Psychiatry 1998; 59(Suppl 3):15–20Google Scholar
4 : Clozapine: balancing safety with superior antipsychotic efficacy. Clin Schizophr Relat Psychoses 2012; 6:134–144Crossref, Google Scholar
5 Clozapine and the Risk of Neutropenia: A Guide for Healthcare Providers. Phoenix, Clozapine REMS Program, 2019. https://www.clozapinerems.com/CpmgClozapineUI/rems/pdf/resources/Clozapine_REMS_A_Guide_for_Healthcare_Providers.pdf. Accessed Sept 4, 2020Google Scholar
6 : Restarting clozapine after neutropenia: evaluating the possibilities and practicalities. CNS Drugs 2007; 21:25–35Crossref, Google Scholar
7 Clozapine and the Risk of Neutropenia: A Guide for Healthcare Providers. Washington, DC, US Food and Drug Administration, 2015. Available: https://www.accessdata.fda.gov/drugsatfda_docs/rems/clozapine_2015-09-15_A_Guide_for_Healthcare_Providers.pdf. Accessed July 21, 2020Google Scholar
8 : Lithium and hematology: established and proposed uses. J Leukoc Biol 2009; 85:20–28Crossref, Google Scholar
9 Highlights of Prescribing Information [Clozaril; package insert]. East Hanover, NJ, Novartis Pharmaceuticals, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2020/019758s095lbl.pdf. Accessed July 21, 2020Google Scholar
10 : Adjunctive medication effects may increase risk for neutropenia. Am J Psychiatry 2008; 165:1611Crossref, Google Scholar
11 : Combined clozapine and valproic acid treatment-induced agranulocytosis. Eur Psychiatry 2002; 17:238–239Crossref, Google Scholar
12 : Increased risk of neutropaenia and agranulocytosis with sodium valproate used adjunctively with clozapine. Aust N Z J Psychiatry 2001; 35:544–545Crossref, Google Scholar
13 : Leukopenia in clozapine treated patients may be induced by other drugs: a case series. Eur Psychiatry 2004; 19:506–509Crossref, Google Scholar
14 : Review: drug-induced neutropenia--pathophysiology, clinical features, and management. Ann Clin Lab Sci 2004; 34:131–137Google Scholar
15 : Exploring the potential effect of polypharmacy on the hematologic profiles of clozapine patients. J Psychiatr Pract 2014; 20:50–58Crossref, Google Scholar
16 : Pharmacokinetics and pharmacodynamics of pegfilgrastim. Clin Pharmacokinet 2011; 50:295–306Crossref, Google Scholar
17 : Comparison of pegfilgrastim with filgrastim on febrile neutropenia, grade IV neutropenia and bone pain: a meta-analysis of randomized controlled trials. Curr Med Res Opin 2007; 23:2283–2295Crossref, Google Scholar
18 : Use of granulocyte-colony stimulating factor to prevent recurrent clozapine-induced neutropenia on drug rechallenge: a systematic review of the literature and clinical recommendations. Aust N Z J Psychiatry 2017; 51:980–989Crossref, Google Scholar
19 : Organizational characteristics of Veterans Affairs clinics with high and low utilization of clozapine. Psychiatr Serv 2016; 67:1189–1196Crossref, Google Scholar
20 : Mask off? Lithium augmentation for clozapine rechallenge after neutropenia or agranulocytosis: discontinuation might be risky. Prim Care Companion CNS Disord 2018; 20:
21 : Meta-analysis examining the epidemiology of clozapine-associated neutropenia. Acta Psychiatr Scand 2018; 138:101–109Crossref, Google Scholar
22 : Utilization of G-CSF and GM-CSF as an alternative to discontinuation in clozapine-induced neutropenia or leukopenia: a case report and discussion. Ment Health Clin 2018; 8:250–255Crossref, Google Scholar



