Safety and Efficacy of Adjunctive Second-Generation Antidepressant Therapy With A Mood Stabiliser or an Atypical Antipsychotic in Acute Bipolar Depression: Randomised Systematic Review and Meta-Analysis of A Placebo-Controlled Trials
Abstract
Background:
Although mania and hypomania define bipolar disorder, depressive episodes are more common and impairing, with few proven treatments. Adjunctive therapy with second-generation antidepressants is widely used to treat acute bipolar depression, but their efficacy and safety remain controversial.
Methods:
In this systematic review and meta-analysis, we searched MEDLINE, Embase, the Cochrane Central Register of Controlled Trials, and ClinicalTrials.gov from inception to Jan 31, 2016, for randomised, double-blind, placebo-controlled trials of second-generation antidepressants adjunctive to a mood stabiliser or an antipsychotic in patients with acute bipolar depression. We extracted data from published reports. The primary outcome was change in clinician-rated depressive symptom score; secondary outcomes were clinical response, clinical remission, treatment-emergent mania or hypomania, and tolerability (using dropout rates as a proxy). We used pooled random-effects models, subgroup comparisons, and meta-regression for analyses. We made subgroup comparisons on the basis of mood stabiliser or antipsychotic treatment and did meta-regression examining trial duration. This study is registered with PROSPERO, number CRD#42015016024.
Findings:
We identified six trials representing 1383 patients with bipolar depression. Second-generation antidepressants were associated with a small but significant improvement in clinician-rated depressive symptom score (standardised mean differences 0·165 [95% CI 0·051–0·278], p=0·004). However, clinical response and remission rates did not differ significantly between patients receiving adjunctive antidepressants and those receiving placebo (1·158 [0·840–1·597], p=0·371 for clinical response; 1·220 [0·874–1·703], p=0·243 for remission). Acute treatment was not associated with an increased risk of treatment-emergent mania or hypomania (0·926 [0·576–1·491], p=0·753), but 52 week extension periods were associated with an increase in risk (1·774 [1·018–3·091], p=0·043).
Interpretation:
Adjunctive second-generation antidepressants are associated with reduced symptoms of acute bipolar depression, but the magnitude of benefit is small because they do not increase clinical response or remission rates. However, these medications should be used only in the short term because prolonged use is associated with an increased risk of treatment-emergent mania or hypomania.
(Reprinted with permission from Lancet Psychiatry 2016; 3: 1138–46)
Introduction
Bipolar disorder is a common condition and cause of global disability (1). Although it is defined by episodes of mania or hypomania, it is also characterised by chronic and recurring depressive episodes that account for a substantial proportion of disability (2, 3). With an early age of onset (4), patients are reported to spend as much as half of their lives with mood symptoms (2).
There are several level 1 pharmacological strategies for the management of acute mania, but few level 1 treatments exist for acute bipolar depression (5). Quetiapine, fluoxetine plus olanzapine, and lurasidone alone or in conjunction with lithium or valproate are the only treatments approved by the US Food and Drug Administration (FDA) for bipolar depression. Many patients with bipolar depression either do not respond to these medications or have difficulty tolerating the side-effects. Thus, treatment for bipolar depression remains a substantial unmet need.
In view of the dearth of proven treatments, clinicians widely use antidepressants to treat acute bipolar depression (6, 7). However, the effectiveness of these medications for the relief of depressive symptoms remains controversial because of the small evidence base. Previous meta-analyses that included data from studies of first-generation and second-generation antidepressants reported on the efficacy of antidepressants; of these, two suggest that antidepressants are more efficacious than placebo (8, 9) whereas the third did not (10). Overall, antidepressants were no more likely to switch patients into mania or hypomania (ie, affective switch) compared with placebo (8, 10); however, additional analyses suggested that tricyclic antidepressants and SNRIs were more likely to induce mania than were SSRIs or bupropion (8, 10, 11). Furthermore, little evidence suggests that non-SNRI second-generation antidepressants increase the risk of manic switch 8, 10, 12, 13.
The International Society for Bipolar Disorders (ISBD) expert consensus statement discourages the use of antidepressant monotherapy for the treatment of acute bipolar depression (14). However, previous meta-analyses have not distinguished between antidepressant monotherapy and antidepressants as adjuncts to adequate mood stabilisation, and therefore this important clinical question remains unaddressed. Additionally, previous meta-analyses included studies of tricyclic anti depressants and inhibitors of monoamine oxidase, classes that carry an increased risk of affective switch and cycle acceleration (15). Therefore, we aimed to determine whether second-generation antidepressant adjunctive therapy is efficacious and safe in the treatment of acute bipolar depression, a crucial consideration in clinical practice.
BOX 1. Research in context
Evidence before this study
The use of antidepressants in the treatment of bipolar depression is a controversial area in psychiatry. We searched MEDLINE from inception to Jan 31, 2016, for meta-analyses using the search term “(bipolar disorder OR bipolar depression) AND antidepressive agents AND meta-analysis”. We found four previous meta-analyses of antidepressants in bipolar depression that included agents and interventions that are discouraged— notably, antidepressant monotherapy, tricyclic antidepressants, and monoamine oxidase inhibitors. Therefore, the clinical relevance of existing evidence is unclear, and additional examination restricted to trials of second-generation antidepressants adjunctive to adequate mood stabilisation, a strategy now widely used by clinicians, is necessary.
Added value of this study
Our systematic review identified six randomised placebo-controlled trials representing a total of 1383 patients treated with a second-generation antidepressant in addition to a mood stabiliser or an antipsychotic. Random-effect analyses showed a small benefit of antidepressant treatment in reducing depressive symptoms, and no evidence of acute risk of treatment-emergent affective switch. However, treatment with second-generation antidepressants over 52 week extension periods was associated with an increased risk of affective switch despite adequate mood stabilisation.
Implications of all the available evidence
Concomitant to adequate mood stabilisation, time-limited use of second-generation antidepressants is associated with a small clinical benefit and no acute risk of treatment-emergent mania or hypomania in the short term. However, prolonged treatment should be avoided.
Methods
Search strategy and selection criteria.
In this systematic review and meta-analysis, we searched MEDLINE, Embase, and the Cochrane Central Register of Controlled Trials from their inception until Jan 31, 2016, using the search term “(bipolar disorder OR bipolar depression) AND antidepressive agents AND (clinical trial OR randomized controlled trial”; see appendix for search syntaxes, parameters, and results). We reviewed references and citations of articles retained in this study for additional unidentified studies. We also searched ClinicalTrials.gov using the search term “bipolar depression”.
Studies reported in English were included if they were double-blind, placebo-controlled trials with random allocation and allocation concealment, and if they included patients aged 18–75 years with a major depressive episode and a primary diagnosis of bipolar I disorder or bipolar II disorder according to DSM-III-R, DSM-IV, DSM-5, or ICD-9 criteria. Furthermore, patients had to have treatment with a mood stabiliser (lithium, valproate, or carbamazepine) or an atypical antipsychotic agent, to which a second-generation antidepressant was added as adjunctive treatment. We defined second-generation antidepressants as SSRIs, SNRIs, norepinephrine dopamine reuptake inhibitors, and melatonergic antidepressants. Studies were excluded if they used tricyclic or tetracyclic antidepressants, inhibitors of monoamine oxidase A or B, antipsychotics, NMDA receptor antagonists, stimulants, hormones, or natural supplements. Additionally, studies were excluded if they did not report raw data and such data could not otherwise be obtained.
Data analysis.
For each published study, summary estimates were extracted independently by AM and LNY, with subsequent verification (by PAV), review, and consensus. The following variables were extracted in a structured manner: patient characteristics (mean age, sex, and primary diagnosis), mood stabiliser or atypical antipsychotic used, second-generation antidepressant (dose and titration schedule) used, the placebo condition, treatment-emergent hypomania or mania episodes, and dropouts. For unpublished studies identified from ClinicalTrials.gov, we contacted study authors to obtain summary estimates. We assessed study quality using the Cochrane Collaboration’s Tool for Assessing Risk of Bias (16).
The primary outcome was change in clinician-rated depressive symptom score at study end, calculated as standardised mean differences (SMDs) (17). Secondary outcomes were clinical response, clinical remission, episodes of treatment-emergent mania or hypomania, and tolerability. For analyses of secondary outcomes, we used odds ratios (ORs, with 95% CIs), as well as risk differences (RDs) to calculate number needed to treat or number needed to harm to provide clinical significance. Clinical response was defined by the study authors (except for one study for which we defined clinical response) as at least a 50% reduction in primary efficacy measure (Montgomery–Åsberg Depression Rating Scale [MADRS] (18), Hamilton depression rating scale [HDRS] (19), or clinical monitoring form for mood disorders) (20) or Clinical Global Impressions—global improvement [CGI-I] of 2 or less. Clinical remission was defined as MADRS (18) of 12 or less, HDRS of 7 or less, or two or fewer manic or depressive symptoms for 1–7 weeks on the clinical monitoring form. We used dropout rates as a proxy for tolerability, although they might also reflect the absence of efficacy.
Since true treatment effects are likely to vary between studies because of different methodological characteristics—including patient selection, primary diagnosis, mood stabiliser or antipsychotic used, antidepressant used, and trial duration—we used pooled random-effects models (21). Only intention-to-treat data were analysed (22). We also did subgroup analyses to test the effect of mood stabilisers versus antipsychotics. Although some trials allowed both mood stabilisers and antipsychotics, we dichotomised trials on the basis of the predominant intervention to which second-generation antidepressants were adjuncts. We also did meta-regression to test the effect of study duration on SMDs and RDs.
We used Q statistics and I (2) to assess heterogeneity between trials (ie, clinical and methodological diversity), with the threshold for heterogeneity defined as p value for the Q statistic less than 0·1 or I (2) greater than 35% (23). We used funnel plots and Egger’s regression intercept (24) to test for publication bias (23, 25). Comprehensive Meta-Analyses version 2.0 (Biostat, Englewood, NJ, USA) was used for statistical analyses.
This study is registered with PROSPERO, number CRD#42015016024.
Role of the funding source.
There was no funding source for this study.
Results
We initially identified 587 potentially eligible studies (figure 1). Removal of duplicates and screening of titles and abstracts left 21 records, of which 14 were excluded after full-text review; all trials that were excluded because of the nature of the antidepressant also did not meet at least one other inclusion criterion (appendix). One trial identified from ClinicalTrials.gov (NCT00464191) was also excluded because the trial was terminated owing to insufficient recruitment. We included the remaining five published studies (12, 13, 26–28) and an additional trial from ClinicalTrials.gov (the CAPE-BD trial) (29) in the meta-analysis. Assessment of risk of bias of the included studies is shown in the appendix.
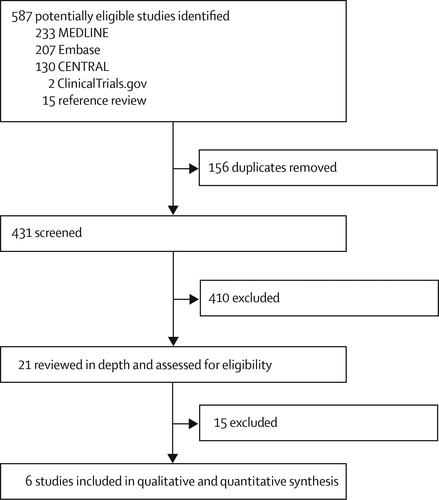
FIGURE 1. Study selection
CENTRAL=Cochrane Central Register of Controlled Trials.
Our meta-analysis included 1383 patients with bipolar disorder (1127 with bipolar I disorder, 166 with bipolar II disorder, 78 with DSM-III-R bipolar disorder, and 12 for whom the exact diagnosis was unspecified; table). Three trials (12, 26, 28) included only patients with bipolar disorder I, whereas three trials (13, 27, 29) included both types of the condition. The mean age of patients was 41·93 years (SD 12·21), and the overall patient population consisted of 559 men and 822 women (sex of two patients was unspecified).
| Diagnosis | Mood stabiliser or antipsychotic | Antidepressant | Number of patients receiving antidepressant | Number of patients receiving placebo | Mean age (range or SD), years | Primary outcome instrument | Duration of treatment | |
|---|---|---|---|---|---|---|---|---|
| Nemeroff et al (2001) (12) | DSM-III-R bipolar disorder | Lithium | Paroxetine 20–50 mg | 35 (19 men, 16 women) | 43 (23 men, 20 women) | 42·5 (24–66) in antidepressant group, 40·4 (21–66) in placebo group | HDRS-21 | 10 weeks |
| Tohen et al (2003) (26) | Bipolar I disorder | Olanzapine (5–20 mg) | Fluoxetine 25–50 mg | 86 (28 men, 58 women) | 370 (139 men, 231 women) | 41·84 (12·59) | MADRS | 8 weeks |
| Shelton et al (2004) (27) | Bipolar I disorder (n=12) and bipolar II disorder (n=8) | Risperidone (1–4 mg) in addition to lithium, valproate, or carbamazepine | Paroxetine 20–40 mg | 10 (5 men, 5 women) | 10 (5 men, 5 women) | 35·6 (10·7) | HDRS-17 | 12 weeks |
| STEP-BD (2007) (13) | Bipolar I disorder (n=240) and bipolar II disorder (n=114); 12 patients unspe cified | Lithium, valproate, carbamazepine, or other FDA-approved antimanic medications | Bupropion 150–375 mg or paroxetine 10–40 mg | 179 (75 men, 102 women, 2 unspecified) | 187 (82 men, 105 women) | 40·0 (11·7) | Clinical monitoring form | 26 weeks, of which the first 6 weeks were double blind without additions of subsequent antidepressants allowed by the protocol |
| Yatham et al (2016) (28) | Bipolar I disorder | Lithium or valproate | Lithium or valproate | Agomelatine 25–50 mg | 172 (67 men, 105 women) | 45·15 (12·64) | MADRS | 8 weeks |
| CAPE-BD (29). | Bipolar I disorder (n=75) and bipolar II disorder (n=44) | Lithium, valproate, carbamazepine, antipsychotic, or lamotrigine, or a combination of these medications | Citalopram 10–50 mg | 60 (22 men, 38 women) | 59 (27 men, 32 women) | 41·49 (11·93) | MADRS | 6 weeks |
TABLE 1. Characteristics of included randomised trials
Five trials (12, 13, 27–29) involved patients using lithium, valproate, or carbamazepine for the duration of the trial, although the STEP-BD trial (13) allowed patients to be on any FDA-approved antimanic agent in the final 2 years of recruitment (table). One study (27) involved a three-group design and used risperidone in addition to lithium, valproate, or carbamazepine for mood stabilisation; of the three study groups, we included the groups receiving risperidone plus placebo and risperidone plus paroxetine. One trial used olanzapine (26). SSRIs were the most studied class of modern antidepressants (paroxetine (12, 13, 27), fluoxetine (26), citalopram) (29), in addition to 5-HT2B and 5-HT2C antagonist and melatonergic agonist (agomelatine) (28) and norepinephrine–dopamine reuptake inhibitor (bupropion) (13). In one trial (13), patients were randomly assigned to receive either paroxetine or bupropion. We did not identify any placebo-controlled trials of SNRI adjunctive therapy. The duration of the trials were 6 weeks (29), 8 weeks (26, 28), 12 weeks (27), and 26 weeks (13).
For sensitivity analyses, we excluded the STEP-BD trial (13) because it used the clinical monitoring form for mood disorders to define remission, whereas other studies used MADRS or HDRS. Furthermore, open-label treatment allowed psychiatrists to add antidepressants at their discretion after 6 weeks of placebo-controlled treatment. We also excluded the olanzapine–fluoxetine trial (26) because this was the only study with findings supporting the efficacy of adjunctive antidepressant therapy.
Depressive symptom scores were available for five trials (12, 26–29), and we obtained data for the remaining trial (13) from the controlled access datasets distributed from the National Database for Clinical Trials (NCT00012558) supported by the US National Institute of Mental Health, which allowed us to extract clinician-rated depressive symptoms from the first 6 weeks (the double-blind period) of the trial without the confounding effect of subsequent antidepressants allowed by the protocol. However, symptoms were measured with different instruments, including MADRS (26, 28, 29) HDRS (12, 27), and clinical monitoring form (13). We conservatively assumed a correlation coefficient of 0·7 in SMD calculation because we could not retrieve the correlations between scores before and after treatment for all trials (30). Compared with placebo, adjunctive antidepressants were associated with a small but significant improvement in clinician-rated depressive symptoms (SMD 0·165 [95% CI 0·051–0·278], p=0·004; figure 2). Between-sample heterogeneity was not significant, with a Q statistic of 3·30 and I (2) of 0·00 (degree of freedom [df]=5; p=0·65). In sensitivity analyses, effect sizes remained small when excluding the STEP-BD trial (13) (0·171 [0·035–0·306], p=0·014) or the olanzapine–fluoxetine trial (26) (0·134 [0·005–0·263], p=0·041). Heterogeneity between trials using a mood stabiliser and those using an antipsychotic was not significant (Q=0·60, df=1; p=0·43). Findings of the funnel plot (appendix) and Egger’s intercept (–0·75, t(4)=0·84; p=0·44) suggested a low possibility of publication bias.
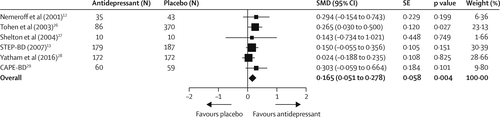
FIGURE 2. Clinician-rated depressive symptoms
STEP-BD (13) data are presented at 6 weeks. SMD=standardised mean difference.
Data for response rates were available for all six trials. For the STEP-BD trial (13), we used published outcomes for secondary analyses because the clinical monitoring form was used. By study end, 259 (48%) of 542 patients receiving antidepressants and 360 (43%) of 841 patients receiving placebo achieved response criteria. Pooled OR was 1·158 (95% CI 0·840–1·597, p=0·371; figure 3A) and RD was 0·035 (–0·043 to 0·113, p=0·377). Exclusion of the STEP-BD trial (13) enhanced the clinical response of antidepressants to a small degree (199 [55%] of 363 vs 286 [44%] of 654; OR 1·332 [1·010–1·756], p=0·042), corresponding to a number needed to treat of 15 (95% CI 8–250 000; RD 0·068 [0·000–0·137], p=0·046). There was heterogeneity between these trials, with a Q statistic of 8·35 and I (2) of 40·12 (df=5; p=0·13). We found heterogeneity between trials using mood stabilisers and those using antipsychotics (Q=6·13, df=1, p=0·013). Antidepressants were associated with increased clinical response in antipsychotic trials (OR 1·881 [1·188–2·979], p=0·007; RD 0·151 [0·040–0·263]; number needed to treat 7 [4–25]) but not in mood stabiliser trials (OR 0·963 [0·736–1·259], p=0·783; RD 0·029 [–0·026 to 0·084]). In patients receiving an antipsychotic, 49 (51%) of 96 patients receiving adjunctive antidepressants achieved clinical response, compared with 140 (37%) of 380 patients receiving placebo. By contrast, clinical response was achieved in similar proportions of patients given an antidepressant plus a mood stabiliser (47% [210 of 446]) and those given placebo plus a mood stabiliser (48% [220 of 461]). Additionally, we used meta-regression to test the relation between response RD and study duration, and found decreasing efficacy with increasing trial duration (slope –0·01 [95% CI –0·00 to –0·02]; Q=3·86, df=1, p=0·049). Examination of the funnel plots revealed one outlier (26) (appendix), but Egger’s intercept (0·30, t(4)=0·30, p=0·77) did not suggest publication bias.
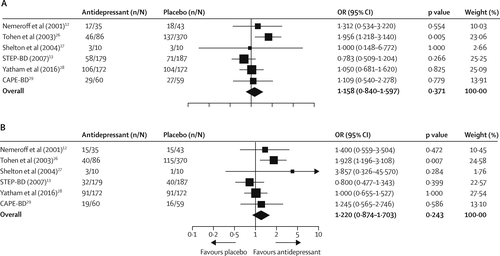
FIGURE 3. (A) Clinical response and (B) clinical remission
OR=odds ratio.
Data for remission rates were available for all six studies. By the study end, 200 (37%) of 542 patients receiving an antidepressant and 278 (33%) of 841 patients receiving placebo achieved clinical remission. Pooled OR was 1·220 (0·874 to 1·703, p=0·243; figure 3B) and RD was 0·044 (–0·029 to 0·118, p=0·235). Sensitivity analyses revealed a marginal difference in favour of antidepressants when the STEP-BD trial (13) was excluded (168 [46%] of 363 vs 238 [36%] of 654; OR 1·365 [0·985–1·891], p=0·061; RD 0·073 [0·003–0·144], p=0·042), with a number needed to treat of 14 (7–334). Overall, there was evidence of heterogeneity between the studies (Q=8·42, I2=40·67, df=5, p=0·13). Heterogeneity between trials using a mood stabiliser and those using an antipsychotic was significant (Q=6·01, df=1, p=0·014), an effect that persisted when the STEP-BD trial (13) was excluded (Q=3·96, df=1, p=0·046). This finding suggested that the efficacy of antidepressants was greater in antipsychotic trials (OR 1·977 [1·237–3·159], p=0·004; RD 0·159 [0·050–0·268], number needed to treat 7 [4–20]), but not in mood stabiliser trials (OR 0·993 [0·745–1·324], p=0·964; RD 0·030 [–0·021 to 0·081]). 43 (45%) of 96 receiving an antipsychotic plus an antidepressant achieved remission criteria, compared with 116 (31%) of 380 patients treated with an antipsychotic and placebo. By contrast, in patients given a mood stabiliser, remission rates were similar in those receiving an antidepressant (35% [157 of 446]) and in those receiving placebo (35% [162 of 461]). Although Egger’s intercept (1·75, t(4)=1·43, p=0·22) did not suggest publication bias, visual inspection of funnel plots indicated a skewed distribution (appendix).
The rates of acute treatment-emergent mania or hypomania were available for all six studies; however, in one trial (29), such data were available for only half the participants because these data were not collected at one of the participating sites. Two studies (28, 29) had data for 52 week extensions of treatment. At the end of the acute phase of treatment, 33 (6%) of 521 patients given antidepressants and 52 (6%) of 817 patients given placebo had episodes of treatment-emergent mania or hypomania, and antidepressant therapy was not associated with an increased risk of affective switch (OR 0·926 [0·576 to 1·491], p=0·753; RD 0·000 [–0·025 to 0·026], p=0·971; figure 4A). Sensitivity analyses did not reveal effects of either the STEP-BD trial (13) (p=0·91) or the olanzapine–fluoxetine trial (26) (p=0·73) on the risk of affective switch. Metaregression did not show a significant relation between risk and study duration (Q=2·51, df=1, p=0·11). Heterogeneity between studies was not significant (Q=3·77, I2=0·00, df=5, p=0·58), and subgroup analyses showed no statistical difference between studies using a mood stabiliser or an antipsychotic (Q=0·09, df=1, p=0·75), although interpretability of both is limited by the small number of studies. The asymmetrical funnel plot (appendix) and Egger’s intercept (–1·48, t(4)=2·33, p=0·079) suggested publication bias. When considering the two studies with 52 week treatment extensions (28, 29), adjunctive antidepressant therapy was associated with an increased risk of treatment-emergent affective switch (39 [17%] of 232 vs 24 [10%] of 231; OR 1·774 [1·018–3·091], p=0·043; RD 0·053 [–0·004 to 0·111], p=0·070; number needed to harm 19; figure 4B).
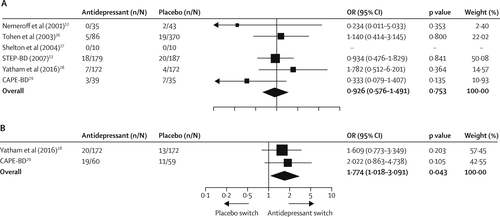
FIGURE 4. Treatment-emergent affective switches
(A) Acute treatment. (B) 52 week extensions. OR=odds ratio.
Overall, 164 (30%) of 542 patients given antidepressants and 323 (38%) of 841 those given placebo were lost to follow-up (OR 0·897 [0·637–1·262], p=0·532; appendix). There was evidence of heterogeneity between studies, with a Q statistic of 7·89 and I (2) of 36·68 (df=5, p=0·16). In subgroup analysis, dropout rates were higher in antipsychotic trials (49% [231 of 476]) than in mood stabiliser trials (28% [256 of 907]; Q=6·17, df=1, p=0·013), mostly driven by dropouts in patients given placebo plus antipsychotic (196 [52%] of 380). Funnel plots revealed one outlier (26) (appendix), but Egger’s intercept (0·23, t(4)=0·16, p=0·87) did not suggest publication bias.
Discussion
Although previous meta-analyses have examined the safety and efficacy of antidepressants in bipolar depression, the clinical relevance of their findings is limited by the inclusion of studies of antidepressant monotherapy as well as tricyclic antidepressants and inhibitors of monoamine oxidase. We restricted our analyses to randomised, placebo-controlled trials that tested the efficacy of second-generation antidepressants versus placebo adjunctive to a mood stabiliser or an atypical antipsychotic. Therefore, our analyses provide data to inform not only efficacy but also the risk of treatment-emergent affective switches when following ISBD recommendations (14). Our findings show that second-generation antidepressants, when used in combination with a mood stabiliser or an atypical antipsychotic, have a significant but small effect size and are not associated with notable acute mania or hypomania switch risks. The small effect size did not translate into increased clinical response or remission; however, we noted statistical separation for the subgroup of trials using an antidepressant as an adjunct to an antipsychotic.
With the exception of the STEP-BD trial (13), clinical trials of acute bipolar depression are typically 6–8 weeks in duration. Therefore, to compute the mean change in depressive symptoms for inclusion in our metaanalysis as a primary outcome, we extracted data from the 6 week double-blind period from the 26 week STEP-BD trial (13). Another important consideration when examining data from these trials is the high rates of clinical remission and response in the placebo groups. Indeed, in one 8 week randomised controlled trial (28), remission rate in the placebo group was as high as 53% (28). Remission rates of patients given placebo in trials of schizophrenia and depression have been increasing (31, 32). Although placebo responses are relatively stable in acute mania trials and do not seem to interfere with detection of the efficacy of antimanic treatments, placebo responses have been steadily increasing in bipolar depression trials (similar to the situation seen in trials of major depression) (28, 33, 34), which substantially interfere with the detection of efficacy signals. Therefore, strategies to address increasing placebo response rates are urgently needed in trials in psychiatry.
The risk of short-term treatment-emergent affective switches with antidepressant treatment in bipolar depression is one of the most contentious issues in the mood disorder literature. As has been highlighted (14), the fluctuant nature of mood in bipolar disorder makes the assignment of causality difficult. Yet, the use of antidepressant monotherapy is clearly associated with an increased risk of mania (6) and is discouraged by expert recommendations (14). Moreover, several agents—notably, tricyclics, tetracyclics, and SNRIs—have been associated with an increased risk of short-term treatment-emergent affective switch (8, 10, 11, 35). Although concern and caution have spread to all antidepressants, other classes of antidepressants such as SSRIs and other antidepressants, such as bupropion, have not separated from placebo in meta-analyses (10, 36).
Although additional trials are needed because the assumption of a class effect might ultimately prove erroneous, we did not find an increased risk of short-term treatment-emergent affective switches with adjunctive second-generation antidepressant therapy during acute treatment of bipolar depression in this population with predominantly bipolar I disorder. However, definitions of treatment-emergent mania differed among the trials, and more sensitive measures could reveal changes and be best suited to identify cycle acceleration (37). Accordingly, although we did not identify a risk with time-limited use of adjuntive second-generation antidepressants, the risk of treatment-emergent mania or hypomania increased with prolonged use (in 52 week placebo-controlled extensions) despite adequate mood stabilisation (number needed to harm 19) (28, 29). Thus, prolonged treatment might be detrimental, as reflected in international guidelines (5) and recommendations from the British Association for Psychopharmacology (38). However, this conclusion is provisional and based on only two trials, and additional investigation is needed.
A limitation of our meta-analysis was the multiple sources of diversity between the trials included (different antidepressants, mood stabilisers, and antipsychotics, outcome metrics, and durations of study). Indeed, statistical heterogeneity was noted in analyses of clinical response and remission, which seemed attributable to the use of mood stabilisers versus antipsychotics. Unfortunately, the small number of studies limited our exploration of clinical or methodological causes of heterogeneity beyond trials using mood stabilisers and those using antipsychotics. The study with the greatest antidepressant effect size (26) driving heterogeneity involved a washout period followed by initiation of the study intervention, whereas the remainder trials all required stable psychotropics before trial commencement. One study (29) had incomplete data with respect to treatment-emergent affective switches in the acute phase. Our findings should be interpreted in light of both type I and type II errors, and the possible effects of multiple testing in secondary outcomes. Differential remission rates between antipsychotic and mood stabiliser trials should be interpreted in light of the high dropout rate (>50%) in antipsychotic plus placebo trials.
The literature itself is subject to limitations, including a small number of randomised controlled trials, elevated placebo effects, and methodological exclusion of patients with rapid cycling courses or comorbid substance use disorders. Furthermore, trials have not yet systematically addressed other important questions with a double-blind design, such as the risk of depressive relapse with antidepressant discontinuation in patients who respond to second-generation antidepressants as an adjunct to mood stabilisers or antipsychotics. Such data might modify the risk-benefit assessment of prophylactic treatment.
Our meta-analysis of randomised placebo-controlled trials of second-generation antidepressants in conjunction with a mood stabiliser or an antipsychotic in acute bipolar depression suggests that they have a small antidepressant effect, and short-term use of these medications is not associated with an increased risk of treatment-emergent affective switches. This supports first-line recommendations by the joint guidelines of ISBD and the Canadian Network for Mood and Anxiety Treatments (CANMAT) (5). Antidepressants are well tolerated acutely, although subthreshold manic symptoms have not been adequately characterised. However, antidepressants are accompanied by an increased risk for manic or hypomanic episodes with prolonged use and therefore only time-limited use is supported by our analysis.
1 . The global burden of disease: 2004 update. Geneva: World Health Organization, 2008.Google Scholar
2 Psychosocial disability and work role function compared across the long-term course of bipolar I, bipolar II and unipolar major depressive disorders. J Aff ect Disord 2008; 108: 49–58.Crossref, Google Scholar
3 Three times more days depressed than manic or hypomanic in both bipolar I and bipolar II disorder. Bipolar Disord 2007; 9: 531–35.Crossref, Google Scholar
4 Long-term implications of early onset in bipolar disorder: data from the first 1000 participants in the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol Psychiatry 2004; 55: 875–81.Crossref, Google Scholar
5 Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) collaborative update of CANMAT guidelines for the management of patients with bipolar disorder: update 2013. Bipolar Disord 2013; 15: 1–44.Crossref, Google Scholar
6 The risk of switch to mania in patients with bipolar disorder during treatment with an antidepressant alone and in combination with a mood stabilizer. Am J Psychiatry 2014; 171: 1067–73.Crossref, Google Scholar
7 International prescribing patterns for mood illness: the International Mood Network (IMN). J Affect Disord 2014; 167: 136–39.Crossref, Google Scholar
8 . Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry 2004; 161: 1537–47.Crossref, Google Scholar
9 Vazquez GH, Tondo L, Undurraga J, Baldessarini RJ. Overview of antidepressant treatment of bipolar depression. Int J Neuropsychopharmacol 2013; 16: 1673–85.Google Scholar
10 . Antidepressants for the acute treatment of bipolar depression: a systematic review and meta-analysis. J Clin Psychiatry 2011; 72: 156–67.Crossref, Google Scholar
11 Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion and sertraline. Br J Psychiatry 2006; 189: 124–31.Crossref, Google Scholar
12 Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression. Am J Psychiatry 2001; 158: 906–12.Crossref, Google Scholar
13 Effectiveness of adjunctive antidepressant treatment for bipolar depression. N Engl J Med 2007; 356: 1711–22.Crossref, Google Scholar
14 The International Society for Bipolar Disorders (ISBD) task force report on antidepressant use in bipolar disorders. Am J Psychiatry 2013; 170: 1249–62.Crossref, Google Scholar
15 . Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry 1991; 148: 910–16.Crossref, Google Scholar
16 The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011; 343: d5928.Crossref, Google Scholar
17 . Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med 2002; 21: 1575–600.Crossref, Google Scholar
18 . A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134: 382–89.Crossref, Google Scholar
19 . A rating scale for depression. J Neurol Neurosurg Psychiatry 1960; 23: 56–62.Crossref, Google Scholar
20 . A clinical monitoring form for mood disorders. Bipolar Disord 2002; 4: 323–27.Crossref, Google Scholar
21 . Interpretation of random effects meta-analyses. BMJ 2011; 342: d549.Crossref, Google Scholar
22 . Post-randomisation exclusions: the intention to treat principle and excluding patients from analysis. BMJ 2002; 325: 652–54.Crossref, Google Scholar
23 . Introduction to meta-analysis. West Sussex: Wiley & Sons, 2009.Crossref, Google Scholar
24 . Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–34.Crossref, Google Scholar
25 . The handbook of research synthesis and meta-analysis. New York: Russell Sage Foundation Publications, 2009.Google Scholar
26 Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 2003; 60: 1079–88.Crossref, Google Scholar
27 . Risperidone and paroxetine given singly and in combination for bipolar depression. J Clin Psychiatry 2004; 65: 1715–19.Crossref, Google Scholar
28 Agomelatine or placebo as adjunctive therapy to a mood stabilizer in bipolar I depression: randomised double-blind placebo controlled trial. Br J Psychiatry 2016; 208: 78–86.Crossref, Google Scholar
29 . Antidepressants in bipolar depression: an update. 168th Annual Meeting of the American Psychiatric Association; Toronto, ON, Canada; May 16–20, 2015.Google Scholar
30 Meta-analytic procedures for social research. Newbury Park, CA: Sage Publications, 1993.Google Scholar
31 . Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. Am J Psychiatry 2009; 166: 42–49.Crossref, Google Scholar
32 Meta-regression analysis of placebo response in antipsychotic trials, 1970–2010. Am J Psychiatry 2013; 170: 1335–44.Crossref, Google Scholar
33 . Two 6-week, randomized, double-blind, placebo-controlled studies of ziprasidone in outpatients with bipolar I depression: did baseline characteristics impact trial outcome? J Clin Psychopharmacol 2012; 32: 470–78.Crossref, Google Scholar
34 An 8-week randomized, double-blind, placebo-controlled evaluation of the safety and efficacy of cariprazine in patients with bipolar I depression. Am J Psychiatry 2016; 173: 271–81.Crossref, Google Scholar
35 A double-blind trial of bupropion versus desipramine for bipolar depression. J Clin Psychiatry 1994; 55: 391–93.Google Scholar
36 . Induction of mania with selective serotonin re-uptake inhibitors and tricyclic antidepressants. Br J Psychiatry 1994; 164: 549–50.Crossref, Google Scholar
37 . Do we need to flick the switch? The need for a broader conceptualization of iatrogenic course aggravation in clinical trials of bipolar disorder. Psychiatry Clin Neurosci 2010; 64: 367–71.Crossref, Google Scholar
38 Evidence-based guidelines for treating bipolar disorder: revised third edition recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2016; 30: 495–553.Crossref, Google Scholar



