Antipsychotic-Induced Weight Gain in First-Episode Psychosis Patients: A Meta-Analysis of Differential Effects of Antipsychotic Medications
Abstract
Aim:
The first-episode psychosis (FEP) represents a critical period to prevent cardiovascular and metabolic morbidity decades later. Antipsychotic (AP)-induced weight gain is one modifiable factor in this period. The purpose of this study is to conduct a meta-analysis of AP-induced weight and body mass index (BMI) change in FEP.
Methods:
A comprehensive literature search identified 28 articles that reported data on AP-specific weight or BMI change in FEP. We conducted a meta-analysis of short- and long-term mean weight and BMI differences between placebo and AP medications. We also performed subgroup and meta-regression analysis to examine weight, BMI outcomes and their relationship with location (Asian vs. Western), sponsorship and baseline weight and BMIs.
Results:
Compared to placebo, AP-caused mean weight gain was 3.22 kg and 1.4 points BMI in the short-term, and 5.30 kg and 1.86 points BMI in the long term. Clinically significant weight gain risk increased about twofold with AP use. Weight gain was associated with duration of AP use. AP medications were associated with more weight gain in Western samples as opposed to Asian samples. Most AP medications were associated with significant body weight gain and BMI increase in FEP patients, except for ziprasidone. Olanzapine and clozapine caused the highest weight gain compared to placebo.
Conclusion:
Except for ziprasidone, most AP medications were associated with body weight gain and BMI increase in FEP patients. Early and continuing effects of various AP medications on weight gain and BMI increase should be taken into consideration by clinicians.
(Reprinted with permission from Early Intervention in Psychiatry 2016; 10:193–202)
Introduction
People with schizophrenia and related disorders die earlier than the general population.1,2 Although suicide is an important factor, the majority of early mortality can be explained by cardiovascular disorders that are unrelated to the primary mental illness.3 Main risk factors that increase the incidence of cardiovascular disorders in this population are excess rates of smoking and of obesity and its consequences, especially diabetes. Taken together, it is safe to assume that besides early mortality, these somatic problems also decrease health-related quality of life significantly after the third decade of life. We have previously shown that cardiovascular risk doubles in the first year of psychotic illness.4 This presents a rare opportunity for a ‘critical period’ of intervention to prevent morbidity and mortality and improve quality of life for psychosis early intervention programmes several decades later.
Our previous research has revealed that the impact of obesity on cardiovascular health of people with schizophrenia is about double as compared to general population.5 Prevention of rapid weight gain should be a sine qua non piece of early intervention in psychosis. In patients with chronic schizophrenia lifestyle, factors such as diet and exercise do contribute to obesity; however, most weight gain is mediated by antipsychotic (AP) medications as shown by studies of schizophrenia patients before AP medications were developed and of never medicated middle-aged patients.6,7 Still, it is possible to provide some improvement in weight and metabolic status with structured lifestyle interventions.8–11 In first-episode and psychosis prodrome patients, the lifestyle factors are more similar to their peers in the general population, which may explain contrary findings with lifestyle interventions to improve weight and metabolic diseases in this population.12,13 Although first-episode psychosis (FEP) patients may benefit from the lifestyle interventions, a recent negative study in a first-episode programme to improve weight and metabolic status13 underlines the importance of AP medication use and choice gain for prevention of weight gain.
A seminal paper by Allison et al.14 on AP-induced weight gain has provided a framework on AP-induced weight gain, whereby AP medications have differing effects, with second-generation drugs having a heavier impact than most first-generation APs. Based on animal and human studies, it was no surprise that most antihistaminic (H1) medications (clozapine, olanzapine, quetiapine, chlorpromazine etc.) were associated with most weight gain, closely followed by medications that block serotonin 2C (5HT2C) receptors (risperidone, paliperidone etc.). Blockade of both of these receptors increases appetite.15–18 What was surprising was the relatively low-risk weight profile of the pure dopamine 2 receptor blocker haloperidol that increases eating behaviour and weight in rodents. This is possibly due to the design of the research synthesis by Allison et al., which focused on studies of chronic schizophrenia patients who likely had been on dopamine blocking agents previously. Thus, if dopamine-induced weight gain is time limited to the first few years of use, and if the contribution of dopamine blockade to weight is relatively limited compared to blockade of histamine H1 and 5HT2c receptors, weight gain by haloperidol and similar agents would be statistically masked. Randomized clinical trials19,20 provided some support for this line of thinking as well as a recent meta-analysis which showed that medication-naïve patients, including first episode but mostly other diagnoses such as post-traumatic stress disorder, depression and dementia, gain weight on first-generation AP agents as well.19,21
Meta-analysis is a powerful technique for research synthesis to answer clinical questions such as AP-caused weight gain and to guide further leads research. On the other hand, mostly young prodrome and FEP patients present a special challenge when studying weight gain because a significant portion of them is still growing, thus some natural increase in weight is to be expected. Another challenge for a meta-analysis in this population is the dearth of studies that have utilized placebo control as it might be unethical for acutely psychotic patients. The only meta-analysis addressing AP-caused weight gain in FEP reported a 4.85 kg mean weight gain and 1.97 kg m–2 body mass index (BMI) increase.22 However, this study reported only overall weight and BMI changes, but not AP-specific outcomes. They also assumed that no weight change would occur without the AP medication use. Our group has previously conducted the only placebo-controlled randomized study in the psychosis prodrome reporting weight data, which gives us the opportunity to examine AP medications’ weight gain liability in this age group with psychotic symptoms.23
Identification of specific weight gain liabilities of various AP agents utilized in early intervention of psychotic disorders is the first step in developing strategies to take advantage of the critical period to decrease overall cardiovascular risk down the road. Therefore, the aim of this report is to conduct a meta-analysis of all AP studies in first-episode patients in terms of short-term (≤12 weeks) and long-term (>12 weeks) weight gain and BMI change.
Methods
Studies were identified searching the following databases: PubMed, PsycINFO and Web of Science (1950 to August 2014). Additionally, references of retrieved articles, related review articles and meta-analyses were manually searched to include relevant articles. Two reviewers (SG and SK) independently conducted literature search using search terms: antipsychotics, medication-naïve, first episode schizophrenia and weight (details were provided in the Supporting Information). All articles were independently reviewed, and data were extracted by two reviewers (SG and SK). Any conflicts were discussed with the third reviewer (CT).
Selection criteria
Inclusion criteria for this meta-analysis were as follows: (i) studies in FEP patients who were either AP-naïve or with prior AP treatment for less than 16 weeks during their lifetime; (ii) studies that were conducted in patients with minimum age of 15 years to reduce confounding effect of continuing growth on weight and BMI24; (iii) studies that were conducted in outpatient settings to limit restricted diet effects in inpatient settings; and (iv) studies that reported available weight and/or BMI data during the intervention phase.
The present meta-analysis was conducted and reported according to the PRISMA guidelines (Preferred Reporting Items for Systematic Reviews and Meta-analyses).25
Meta-analysis procedure
Study design, medication dose, funding source, study region, weight and BMI outcomes were extracted. To analyse short- and long-term effects of AP medications on weight gain independently, short-term and long-term weight, BMI change, and clinically significant weight gain (>7% weight gain from baseline) outcomes were additionally retrieved when data were available. In this meta-analysis, the period for the short-term AP treatment was defined up to 12 weeks in accordance with the previous research26; the period for the long-term AP treatment was defined from 13 to 52 weeks. Studies with a study period of more than 1 year were only included if weights or BMI data were provided for the follow-up period between 4 weeks and 1 year. Outcomes of overlapping samples from the same investigators were extracted from the more detailed report. To obtain the missing information, we contacted the authors to request relevant data. Correlation coefficients were used to calculate and impute the missing standard deviation of change from baseline applying the methods described in Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0.27,28
To the best of our knowledge, there is no placebo-controlled study of AP treatment in FEP patients, thereby the study sample consisting of prodromal patients23,29 was used to generate estimates of placebo-controlled group outcomes. This study sample was consisted of 12- to 45-year-old treatment-seeking outpatients with prodromal psychotic symptoms, who were randomized to placebo or olanzapine. In accordance with our age criterion, we analysed the short-term and the long-term weights, BMI changes, and clinical significant weight gain incidences for placebo in the subgroup of patients over 15 years old. In this subgroup, short-term mean weight change was 0.31 ± 1.99 kg, BMI change was −0.1 ± 0.72 kg m–2, and clinically significant weight gain estimate was 3.4%. Long-term mean weight change was 1.95 ± 7.03 kg, BMI change was 0.5 ± 2.34 kg m−2, and clinically significant weight gain estimate was 26.3%. For each AP medication, a placebo-controlled group with equal number of subjects was generated using these derived estimations.
We examined the difference between AP medications and placebo by calculating the mean difference using the software Comprehensive Meta-Analysis Version 2 (Biostat, Englewood, NJ, USA). A random-effects model was used in all analyses with the results for a fixed-effects model presented as a sensitivity analysis.
To avoid publication bias, we conducted a comprehensive search among published and unpublished studies. Publication bias was also assessed visually with funnel plots and statistically with Egger’s regression test.30 Heterogeneity was assessed using Q and I2 statistics.
As a secondary analysis, we performed subgroup and meta-regression analysis to examine weight, BMI outcomes and their relationship with study locations (Asian vs. Western), sponsorship bias (pharmaceutical companies vs. government/independent), and baseline weight and BMIs. For study location subgroup analysis, AP intervention studies from Japan (n = 3), China (n = 4), Korea (n = 2) and Singapore (n = 1) were considered as Asian studies, whereas interventions from other countries such as USA, UK and Spain were considered as Western studies. For all statistical analyses, the significant statistical threshold was P < 0.05.
Results
Our search strategy initially yielded 3059 articles excluding the duplicates. After screening the abstract and the title, 760 articles were selected for full-text screening (Fig. 1). After full-text review, 732 articles not fulfilling the selection criteria were excluded. Of the excluded studies, 451 were not conducted in a sample with only FEP patients, 220 provided no weight data, 9 did not specify prior AP use, 30 were conducted in inpatient settings, and 22 reported data from the samples that were already included in the analysis.
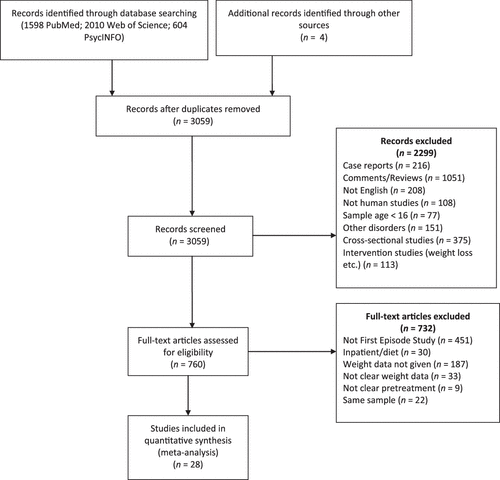
FIGURE 1. PRISMA Checklist Flow Diagram.25
Overall, we identified 28 eligible studies with 52 appropriate treatment arms for inclusion in this review31–55 (Supporting Information Table S1). The total number of patients in these studies was 4139 (2594 male, 1545 female). Five studies were double blinded,32–36 one was single blinded,37 and 22 were open label.20,24,31,38–52 Of these 28 studies, 12 (42.8%) were funded by pharmaceutical companies, 6 (21.4%) were funded by institutional or government agencies, and 3 (10.7%) were funded by pharmaceutical companies and institutional agencies. Six studies were carried out independently.
Short-term results (≤12 weeks)
For the short-term meta-analysis, 39 weight change outcomes were extracted from 21 unique intervention studies20,31–35,37–47,51–54 and 23 BMI change outcomes from 11 unique samples.20,33,37,41,42,44,51–55 Nine of these studies lasted less than 12 weeks.32,37,39–41,45–47,52
Weight change.
From analysis of the selected studies, the overall mean weight gain difference between AP medications and placebo was 3.22 kg (CI = 1.65–4.80, P < 0.001). Compared to the placebo, only ziprasidone did not cause significant weight gain in the short-term (Fig. 2).
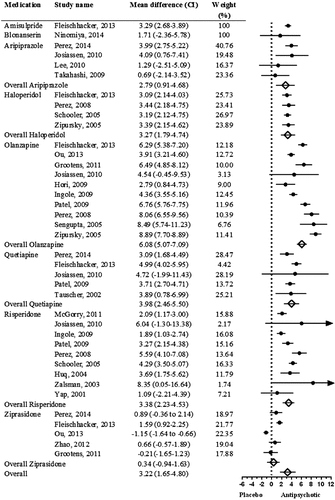
FIGURE 2. Meta-Analysis of Mean Weight Differences (kg) Between Placebo and Antipsychotic Medications in the Short-Term (<12 Weeks). Random-Effects Analysis: Q = 50.57, d.f. = 7, P = <0.001. Fixed-Effect Analysis: Q = 508.13, d.f. = 7, P = <0.001. I2 = 94.53, Tau2 = 5.53.
We found no evidence of publication bias in the short-term weight change analysis (Egger’s test intercept = 0.53, CI = –2.57 to 3.64). The short-term analysis showed considerable heterogeneity (Q = 50.57, d.f. = 7, P < 0.001).
Subgroup analysis showed no significant weight difference between pharmaceutical industry funding and other funding sources (e.g. governmental funding agencies) (Q = 0.06, d.f. = 1, P = 0.79). The mean weight difference was significantly higher in Western countries (4.17 kg, CI = 3.38–4.96) than in Asian countries (1.36 kg, CI = –0.25 to 2.99) (Q = 9.26, d.f. = 1, P = 0.002) (Supporting Information Fig. S1). Meta-regression analysis revealed that interventions with longer durations have a significant effect on weight change (PE = 1.23, CI = 0.09–2.37, P = 0.033). Baseline weight has a significant effect on weight gain in the short-term (PE = 0.12, CI = 0.000–0.24, P = 0.048).
BMI change.
Overall BMI change was 1.46 kg m–2 (CI = 0.90–2.03, P < 0.001) in AP treatment compared to that in placebo in the short-term (Supporting Information Fig. S2). We found evidence of publication bias in the short-term BMI change analysis (Egger’s test intercept = 4.20, CI = 0.11–8.29, P = 0.044). There was significant heterogeneity across interventions (Q = 18.09, d.f. = 6, P = 0.006).
Compared to placebo, interventions in Western (1.74 kg, CI = 1.24–2.24, P < 0.001) and Asian countries (1.45 kg, CI = 0.64–2.27, P < 0.001) showed significant BMI change in the short-term (Supporting Information Fig. S1). Subgroup analysis demonstrated no significant effect of study regions (Q = 0.34, d.f. = 1, P = 0.55) and funding source (Q = 0.14, d.f. = 1, P = 0.70) on BMI. No significant relationships were found between BMI differences with baseline BMI (PE = 0.02, CI = –0.23 to 0.27, P = 0.87) and intervention durations (PE = 0.04, CI = –0.06 to 0.15, P = 0.39).
Long-term results (>12 weeks)
For the long-term meta-analysis, we extracted 30 weight change outcomes from 13 unique samples20,24,31,33–36,48–50,54–56 and 17 BMI change outcomes from 8 unique samples.20,24,33,49,50,54–56 Of these studies, three of them lasted shorter than 52 weeks.34,49,56
Weight change.
Meta-analysis of the long-term mean weight differences is shown in Figure 3. A significant overall mean weight difference was found: 5.30 kg (CI = 2.87–7.74, P < 0.001) between AP medications and placebo. Olanzapine (n = 6, 9.34 kg (CI = 5.55–13.12), P < 0.001) and clozapine (n = 2, 7.19 kg (CI = 0.28–14.09), P = 0.041) were the interventions associated with the highest weight gain compared to placebo. The only one intervention with perphenazine reported weight loss (–0.41 kg).
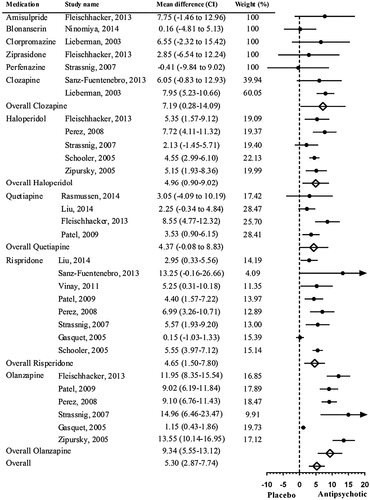
FIGURE 3. Meta-Analysis of Mean Weight Differences (kg) Between Placebo and Antipsychotic Medications in the Long Term (>12 Weeks). Random-Effects Analysis: Q = 8.73, d.f. = 9, P = <0.463. Fixed-Effect Analysis: Q = 35.98, d.f. = 9, P = <0.001. I2 = 87.31, Tau2 = 10.79.
We found significant evidence of publication bias in the long-term analysis (Egger’s test intercept = 2.96, CI = 1.48–4.45, P = 0.003). Long-term weight analysis revealed no significant heterogeneity across interventions (Q = 8.73, d.f. = 9, P = 0.46).
When the analysis was repeated after excluding three studies that lasted shorter than 52 weeks,34,49,56 order of weight gain liability across AP medications did not change. Weight gain with most AP medications remains similar to initial analysis with the exception of olanzapine, which was slightly higher (n = 5, 10.83 kg (CI = 8.83–12.83), P < 0.001). This analysis revealed significantly more long-term weight gain with olanzapine compared to other AP medications (Q = 39.542, d.f. = 9, P < 0.001) (Supporting Information Fig. S3).
Subgroup analysis showed that interventions lasting longer caused greater weight gain than those studies that lasted shorter (PE = 0.55, CI = 0.08–1.02, P = 0.02). Funding source (Q = 0.32, d.f. = 1, P = 0.569), Asian vs. Western source (Q = 0.788, d.f. = 1, P = 0.37) (Supporting Information Fig. S1) and baseline weights (PE = 0.03, CI = –0.20 to 0.28, P = 0.75) did not have significant effect on weight change.
BMI change.
Overall BMI change was 1.86 kg m–2 (CI = 0.89–2.83, P < 0.001) in AP treatment compared to that in placebo in the long term (Supporting Information Fig. S4). Compared to placebo, pooled BMI differences were significant with olanzapine (2.71 kgm–2 (CI = 1.12–4.29), P < 0.001), quetiapine (2.20 kgm–2 (CI = 0.37–4.02), P < 0.001) and risperidone (1.85 kg m−2 (CI = 0.88-2.83), P = 0.004). There was a trend toward increased BMI with haloperidol use (1.90 kg m−2 (CI = −0.31 to 4.08), P = 0.09).
For the long-term BMI analysis, we found significant publication bias (Egger’s test intercept = 3.12, CI = 1.06−5.18, P = 0.005). Heterogeneity between the studies was not significant (Q = 3.23, d.f. = 5, P = 0.66).
After excluding two studies that lasted shorter than 52 weeks,49,56 pooled BMI differences were similar with the initial analysis (olanzapine 3.46 kg m−2 (CI = 2.34−4.59), P < 0.001; risperidone 2.22 kg m−2 (CI= 1.43−3.01), P < 0.001; quetiapine 2.61 kg m−2 (CI = 1.47−3.75), P < 0.001). Heterogeneity between the studies was significant (Q = 13.08, d.f. = 5, P = 0.023).
Subgroup analysis demonstrated no significant effect of study regions (Q = 0.96, d.f. = 1, P = 0.32), funding source (Q = 3.15, d.f. = 1, P = 0.07) or baseline BMIs (PE = −0.11 (CI = −0.47 to 0.23), P = 0.51) on BMI change in the long term. Intervention duration significantly affects BMI change in the short-term (PE = 0.06 (CI = 0.02−0.09), P < 0.001).
Meta-analysis of percent weight change
For percent weight change analysis, we analysed the studies that reported the percentages of patients who gained more than 7% of their baseline weight. Our search revealed a limited number of studies that reported on such clinically significant weight gain estimates. More than 7% weight gain estimates of interventions were reported in three studies at the short-term32,46,47 and in four studies at the long term.20,31,33,50
Meta-analysis results showed a significant effect of APs on percent weight change compared to placebo (RR = 2.14 (CI = 1.58−2.91), P < 0.001; risk difference = 0.30 (CI = 0.11−0.49), P = 0.002). Of all the medications that were included in the analysis, the percent weight change was similar to placebo only with ziprasidone (RR = 1.05 (CI = 0.65−1.72), P = 0.82; RD = 0.30 (CI = 0.11−0.49), P = 0.52) (Supporting Information Fig. S5).
Regarding the percent weight change data, studies showed significant heterogeneity (Q = 12.65, d.f. = 5, P = 0.027). We also found evidence of publication bias (Egger’s test intercept = −1.90, CI = −3.59 to −0.22, P = 0.029).
Subgroup analysis showed no significant effect of funding source (Q = 0.96, d.f. = 1, P = 0.96), intervention duration (PE = 0.01, CI = −0.002 to 0.02, P = 0.12) or baseline weights (PE = 0.02, CI = −0.008 to 0.06, P = 0.13) on estimates of >7% weight gain.
Discussion
Most AP medications were associated with significant weight gain both in the short and long terms in early psychosis patients. Weight gain was associated with duration of AP use. In the short-term, weight gain with ziprasidone was similar to placebo; however, it was not clear if this advantage was carried over to the long term due to lack of studies. Haloperidol, unlike in studies of chronic schizophrenia patients, was associated with both short- and long-term weight gains. Similarly, aripiprazole, which appeared weight neutral in previous studies, was associated with short-term weight gain. Finally, AP medications were associated with higher weight gain in Western samples as opposed to Asian samples.
This meta-analytic study takes account of probable body growth in FEP population. The results are in line with and expand findings of a previous smaller meta-analysis of overall weight effects of APs in this population.22 The distribution of weight gain liabilities across APs appears to be similar to that in medication-naïve older adult patients of all diagnoses,21 but not to that in chronic schizophrenia samples.14,26 Notably, the selective and potent D2 blocker haloperidol was shown to be associated with very significant weight gain in FEP but not in chronic schizophrenia patients, which may point to a weight increasing effect that plateaus later. On the other hand, consistent results of weight gain with the worst offending agents olanzapine and clozapine, and to some extent quetiapine and risperidone, may suggest that weight gain with these agents remains high risk over time. The short-term advantage of ziprasidone may translate well to long term because this agent maintains its weight gain advantage in chronic schizophrenia populations.
The initial selection of medication in FEP may present an opportunity for preventing morbidity decades later. Ziprasidone appears to confer advantages of not causing as much weight gain as the other APs. Possible pharmacological explanations for this may be noradrenaline re-uptake blocking activity of ziprasidone, which differentiate this medication from others.57 It is important to note that individual responses to medications may differ as 7−16% of the patients on ziprasidone gained clinically significant weight gain in the registration studies.58 The weight gain advantage of aripiprazole in previous studies, an agonist/antagonist of both D2 and 5-HT2c receptors,59 did not emerge in the short-term in this study. Long-term studies investigating the weight effect of aripiprazole are lacking. It may still be advantageous to consider aripiprazole when switched from higher weight gain liability medications.60 Some previous studies20,34,61 reported the similar weight gain effects of haloperidol and second-generation AP medications including olanzapine and risperidone over the longer time in FEP. Our results revealed a significant weight gain with olanzapine compared to haloperidol and other second-generation AP medications in the short-term and long term.20,34 There does not seem to be a good reason to choose olanzapine as a first or second choice of AP from a prevention of future cardiovascular morbidity and mortality point of view. Clozapine, of course, represents a separate case with its unique action for treatment refractory schizophrenia. Regardless, it does not seem to confer any special advantage as a first trial AP in FEP.36
Our study underscores deficiencies in the literature, some of which represent limitations of this study. First, we have shown that studies from Asian countries report lower weight gain with AP as opposed to Western studies. Given the prevalence of Asian studies in the literature, this potential confound should be routinely addressed in future meta-analyses. Second, weight change is reported in a non-uniform manner across the studies, which create a difficulty for analyses such as ours. BMI takes height increase into account and BMI change should always be reported in FEP studies. We also advocate for baseline weights, weight change and clinically significant weight gain (>7% of total body weight) percentage being reported in every medication study. Third, studies do not consistently report on association between dose and weight change, which makes dose–weight gain analyses impossible without direct access to data. Relationship between weight gain and AP dose is not trivial from both clinical and research perspectives. Fourth, long-term studies with ziprasidone and aripiprazole in FEP are lacking, and thus some caution is required when interpreting our results. We also noted a dearth of studies with mid-potency first-generation AP such as perphenazine, which carried a weight advantage in the CATIE (the Clinical Antipsychotic Trials of Intervention Effectiveness) study and represented favourably with one small study in our meta-analysis. A limitation of the current study is the fact that the placebo-controlled group was consisted of prodrome patients,23,29 as we could not find any placebo-controlled studies in FEP. Generating outcomes from a small sample size can reduce the precision of outcome estimations. Compared to prodromal psychotic patients, growth/placebo effects could also differ in FEP. We also found evidence of publication bias in long-term weight and BMI change, which again calls for caution in interpretation of findings for the longer term weight gain.
FEP represents a critical period both for psychiatric and preventive medicine care.4,62 Lifestyle change incorporated in phase-specific FEP care could reap significant health and quality of life benefits several decades later for the FEP population. However, first and foremost, care in initial medication choice represents a relatively easy and inexpensive preventive health intervention for FEP. This meta-analysis sheds light on specific weight gain liabilities of various AP medications in early stages of psychosis. Except for ziprasidone, almost all AP medications were associated with body weight gain and BMI increase in FEP patients. Haloperidol and possibly other strong D2 receptor blockers are not weight neutral in FEP as previously had been thought from chronic schizophrenia studies. Early and continuing effects of various AP medications on weight gain and BMI increase should be taken into consideration by clinicians. Initial AP medication selection of a lower weight gain liability AP or switching to one later in the treatment is important in preventing future weight gain and cardiovascular risk several decades later.
1 . The unchanging mortality gap for people with schizophrenia. Lancet 2009; 374: 590–2.Crossref, Google Scholar
2 . 11-year follow-up of mortality in patients with schizophrenia: a population-based cohort study (FIN11 study). Lancet 2009; 374: 620–7.Crossref, Google Scholar
3 . Cardiovascular morbidity, mortality and pharmacotherapy in patients with schizophrenia. Psychol Med 2012; 42: 2275–85.Crossref, Google Scholar
4 . Cardiovascular mortality in schizophrenia: defining a critical period for prevention. Schizophr Res 2013; 146: 64–8.Crossref, Google Scholar
5 . Obese schizophrenia spectrum patients have significantly higher 10-year general cardiovascular risk and vascular ages than obese individuals without severe mental illness. Psychosomatics 2013; 54: 67–73.Crossref, Google Scholar
6 . A study of malnutrition in chronic schizophrenia. Am J Psychiatry 1943; 99: 534–41.Crossref, Google Scholar
7 . Low prevalence of obesity and metabolic syndrome in never-treated chronic schizophrenia. Schizophr Res 2010; 121: 199–202.Crossref, Google Scholar
8 . A behavioral weight-loss intervention in persons with serious mental illness. N Engl J Med 2013; 368: 1594–602.Crossref, Google Scholar
9 . A pilot study of a weight management program with food provision in schizophrenia. Schizophr Res 2007; 96: 198–205.Crossref, Google Scholar
10 . Contingency management for the treatment of antipsychotic-induced weight gain: a randomized controlled pilot study. Obes Facts 2012; 5: 919–27.Crossref, Google Scholar
11 . Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. N Engl J Med 2002; 346: 393–403.Crossref, Google Scholar
12 . Attenuation of antipsychotic-induced weight gain with early behavioral intervention in drug-naive first-episode psychosis patients: a randomized controlled trial. J Clin Psychiatry 2006; 67: 1253–60.Crossref, Google Scholar
13 . An exploratory randomized controlled study of a healthy living intervention in early intervention services for psychosis: the INTERvention to encourage ACTivity, improve diet, and reduce weight gain (INTERACT) study. J Clin Psychiatry 2014; 75: 498–505.Crossref, Google Scholar
14 . Antipsychotic-induced weight gain: a comprehensive research synthesis. Am J Psychiatry 1999; 156: 1686–96.Link, Google Scholar
15 . Metabolic side effects of antipsychotic drug treatment-pharmacological mechanisms. Pharmacol Ther 2010; 125: 169–79.Crossref, Google Scholar
16 . The role of histaminergic H1 and H3 receptors in food intake: a mechanism for atypical antipsychotic-induced weight gain? Prog Neuropsychopharmacol Biol Psychiatry 2010; 34: 1–4.Crossref, Google Scholar
17 . The role of hypothalamic H1 receptor antagonism in antipsychotic-induced weight gain. CNS Drugs 2013; 27: 423–34.Crossref, Google Scholar
18 . Association of prescription H1 antihistamine use with obesity: results from the National Health and Nutrition Examination Survey. Obesity (Silver Spring) 2010; 18: 2398–400.Crossref, Google Scholar
19 . Effectiveness of antipsychotic drugs in patients with chronic schizophrenia. N Engl J Med 2005; 353: 1209–23.Crossref, Google Scholar
20 . Weight gain induced by haloperidol, risperidone and olanzapine after 1 year: findings of a randomized clinical trial in a drug-naive population. Schizophr Res 2008; 99: 13–22.Crossref, Google Scholar
21 . Almost all antipsychotics result in weight gain: a meta-analysis. PLoS ONE 2014; 9: e94112, 1–19.Crossref, Google Scholar
22 . Weight gain in antipsychotic-naive patients: a review and meta-analysis. Psychol Med 2010; 40: 187–200.Crossref, Google Scholar
23 . Randomized trial of olanzapine versus placebo in the symptomatic acute treatment of the schizophrenic prodrome. Biol Psychiatry 2003; 54: 453–64.Crossref, Google Scholar
24 . Weight gain in newly diagnosed first-episode psychosis patients and healthy comparisons: one-year analysis. Schizophr Res 2007; 93: 90–8.Crossref, Google Scholar
25 . Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. BMJ 2009; 339: b2535.Crossref, Google Scholar
26 . Comparative efficacy and tolerability of 15 antipsychotic drugs in schizophrenia: a multiple-treatments meta-analysis. Lancet 2013; 382: 951–62.Crossref, Google Scholar
27 Higgins JPT, Green S (editors). Cochrane Handbook for Systematic Reviews of Interventions Version 5.1.0 [updated March 2011]. The Cochrane Collaboration, 2011. Available from www.cochrane-handbook.org.Google Scholar
28 . Meta-analysis of heterogeneously reported trials assessing change from baseline. Stat Med 2005; 24: 3823–44.Crossref, Google Scholar
29 . Randomized, double-blind trial of olanzapine versus placebo in patients prodromally symptomatic for psychosis. Am J Psychiatry 2006; 163: 790–9.Link, Google Scholar
30 . Bias in meta-analysis detected by a simple, graphical test. BMJ 1997; 315: 629–34.Crossref, Google Scholar
31 . Metabolic risk factors in first-episode schizophrenia: baseline prevalence and course analysed from the European First-Episode Schizophrenia Trial. Int J Neuropsychopharmacol 2013; 16: 987–95.Crossref, Google Scholar
32 . Ziprasidone vs. olanzapine in recent-onset schizophrenia and schizoaffective disorder: results of an 8-week double-blind randomized controlled trial. Schizophr Bull 2011; 37: 352–61.Crossref, Google Scholar
33 . Metabolic profiles of second-generation antipsychotics in early psychosis: findings from the CAFE study. Schizophr Res 2009; 111: 9–16.Crossref, Google Scholar
34 . Risperidone and haloperidol in first-episode psychosis: a long-term randomized trial. Am J Psychiatry 2005; 162: 947–53.Crossref, Google Scholar
35 . Course and predictors of weight gain in people with first-episode psychosis treated with olanzapine or haloperidol. Br J Psychiatry 2005; 187: 537–43.Crossref, Google Scholar
36 . Atypical and conventional antipsychotic drugs in treatment-naive first-episode schizophrenia: a 52-week randomized trial of clozapine vs. chlorpromazine. Neuropsychopharmacology 2003; 28: 995–1003.Crossref, Google Scholar
37 . Early intervention with second-generation antipsychotics in first-episode psychosis: results of an 8-week naturalistic study. Early Interv Psychiatry 2010; 4: 57–63.Crossref, Google Scholar
38 . A trial of low doses of risperidone in the treatment of patients with first-episode schizophrenia, schizophreniform disorder, or schizoaffective disorder. J Clin Psychopharmacol 2004; 24: 220–4.Crossref, Google Scholar
39 . Trial of aripiprazole in the treatment of first-episode schizophrenia. Psychiatry Clin Neurosci 2010; 64: 38–43.Crossref, Google Scholar
40 . Very low-dose risperidone in first-episode psychosis: a safe and effective way to initiate treatment. Schizophr Res Treatment 2011; 2011: 631690.Crossref, Google Scholar
41 . Comparison of metabolic effects of ziprasidone versus olanzapine treatment in patients with first-episode schizophrenia. Psychopharmacology (Berl) 2013; 225: 627–35.Crossref, Google Scholar
42 . Weight gain and lipid metabolic abnormalities induced by olanzapine in first-episode, drug-naive patients with psychotic disorders. Schizophr Res 2005; 80: 131–3.Crossref, Google Scholar
43 . Efficacy and tolerability of aripiprazole in first-episode drug naive patients with schizophrenia: an open-label trial. Clin Neuropharmacol 2009; 32: 149–50.Crossref, Google Scholar
44 . Quetiapine: an effective antipsychotic in first-episode schizophrenia despite only transiently high dopamine-2 receptor blockade. J Clin Psychiatry 2002; 63: 992–7.Crossref, Google Scholar
45 . Risperidone in the treatment of first episode psychosis. Singapore Med J 2001; 42: 170–3.Google Scholar
46 . Effectiveness, safety, and tolerability of risperidone in adolescents with schizophrenia: an open-label study. J Child Adolesc Psychopharmacol 2003; 13: 319–27.Crossref, Google Scholar
47 . Efficacy and safety of ziprasidone in the treatment of first-episode psychosis: an 8-week, open-label, multicenter trial. Int Clin Psychopharmacol 2012; 27: 184–90.Crossref, Google Scholar
48 . Randomized trial of clozapine vs. risperidone in treatment-naive first-episode schizophrenia: results after one year. Schizophr Res 2013; 149: 156–61.Crossref, Google Scholar
49 . Pharmacological treatment and other predictors of treatment outcomes in previously untreated patients with schizophrenia: results from the European Schizophrenia Outpatient Health Outcomes (SOHO) study. Int Clin Psychopharmacol 2005; 20: 199–205.Crossref, Google Scholar
50 . Effect of risperidone on metabolic parameters in antipsychotic-naïve schizophrenia: a prospective one year follow-up study. Asian J Psychiatr 2011; 4: 73–4.Crossref, Google Scholar
51 . Comparison of effects of olanzapine and risperidone on body mass index and blood sugar level in schizophrenic patients. Indian J Physiol Pharmacol 2009; 53: 47–54.Google Scholar
52 . Olanzapine orally disintegrating tablets (Zyprexa Zydis) rapidly improve excitement components in the acute phase of first-episode schizophrenic patients: an open-label prospective study. World J Biol Psychiatry 2009; 10: 741–5.Crossref, Google Scholar
53 . Comparison of metabolic effects of aripiprazole, quetiapine and ziprasidone after 12 weeks of treatment in first treated episode of psychosis. Schizophr Res 2014; 159: 90–4.Crossref, Google Scholar
54 . Long-term efficacy and safety of blonanserin in patients with first-episode schizophrenia: a 1-year open-label trial. Psychiatry Clin Neurosci 2014; 68: 841–9.Crossref, Google Scholar
55 . Randomized controlled trial comparing changes in serum prolactin and weight among female patients with first-episode schizophrenia over 12 months of treatment with risperidone or quetiapine. Shanghai Arch Psychiatry 2014; 26: 88–94.Google Scholar
56 . Neocortical serotonin2A receptor binding predicts quetiapine associated weight gain in antipsychoticnaive first-episode schizophrenia patients. Int J Neuropsychopharmacol 2014; 17: 1729–36.Crossref, Google Scholar
57 . Ziprasidone 80 mg/day and 160 mg/day in the acute exacerbation of schizophrenia and schizoaffective disorder: a 6-week placebo-controlled trial. Ziprasidone Study Group. Neuropsychopharmacology 1999; 20: 491–505.Crossref, Google Scholar
58 . Goedon Prescribing Information, 2014 [Updated 2014 January, 2014; cited 8 August 2014.] Available from URL: http://labeling.pfizer.com/ShowLabeling.aspx?id=584Google Scholar
59 . Distinct functional profiles of aripiprazole and olanzapine at RNA edited human 5-HT2C receptor isoforms. Biochem Pharmacol 2006; 71: 521–9.Crossref, Google Scholar
60 . A randomized trial examining the effectiveness of switching from olanzapine, quetiapine, or risperidone to aripiprazole to reduce metabolic risk: comparison of antipsychotics for metabolic problems (CAMP). Am J Psychiatry 2011; 168: 947–56.Crossref, Google Scholar
61 . Antipsychotic-induced weight gain in chronic and first-episode psychotic disorders: a systematic critical reappraisal. CNS Drugs 2008; 22: 547–63.Crossref, Google Scholar
62 . Early intervention in psychosis. The critical period hypothesis. Br J Psychiatry Suppl 1998; 172: 53–9.Crossref, Google Scholar



