Ethics Commentary: Ethics in Psychopharmacology Practice
The practice of psychopharmacology has profoundly influenced the evolution of clinical psychiatry from the first use of chlorpromazine in 1952 for treatment of psychosis. Since then, a flurry of psychotropic medications have been introduced into the field, driven by the hypothesis that interventions in brain physiology can treat disorders defined by symptoms of mood, thought, or behavior. Because many of these symptoms are perceived and expressed through the lens of subjective experience, it is not surprising that the discussion of psychopharmacological treatment generates multiple ethical considerations. Weighing the risks and benefits of treatment and nontreatment, for example, against a backdrop of patient and societal values generates multiple tensions that the ethical practitioner must consider. A medically ill patient who is at high risk of adverse treatment effects is one salient example, as is the case of a healthy, functioning individual seeking cognitive enhancement.
The primary focus of this article is on ethical considerations arising from psychopharmacological practice. We begin with an overview of the prescribing process, and discuss the practical components of informed consent. Next, we examine four clinical situations with unique ethical challenges, namely, patients who are at heightened risk for adverse treatment effects, patients receiving long-acting medications, patients who are prescribed medications for off-label use, and patients desiring enhancement pharmacology (Figure 1).
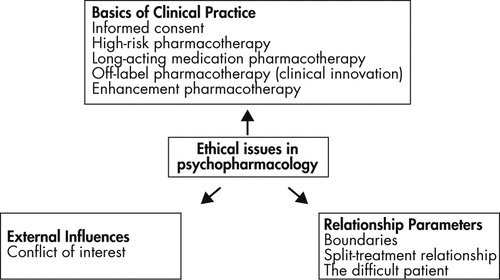
Ethical issues in psychopharmacology extend well beyond prescribing practice, into questions surrounding the parameters of therapeutic relationships themselves––for example, how to maintain professional boundaries with patients receiving pharmacotherapy, how to manage relationships in patients undergoing “split-treatment” (care from multiple mental health providers), and how to build therapeutic alliances with patients who are perceived as difficult (1). External influences on the prescribing process, such as role conflicts and potential conflicts of interest, constitute another key source of ethical tension that will not be discussed here, but are nonetheless significant (1, 2).
Basics and Ethics of Clinical Practice
The prescription of psychotropic medications should be grounded in best practices in clinical diagnosis, which guides the selection of evidence-based treatment. An accurate diagnosis is key, because much of our knowledge about psychopharmacological efficacy derives from studying patient populations typically defined by diagnostic criteria. There is often too little evidence for symptom-based prescribing, i.e., using medications to target various symptoms without consideration of the underlying disorder––an approach that may lead to poor outcomes and is not in keeping with appropriate standards of care (2). With the publication of the DSM-5 this past year, psychiatrists are especially aware of the complex diagnostic issues in our field. As psychiatric diagnoses evolve, it becomes important to know when prescribing for a new or revised diagnosis extends beyond the evidence base. Consistent with this idea, the introduction of the National Institute of Mental Health’s Research Domain Criteria (RDoC) framework, which aims to categorize disorders by neural circuit-defined symptomatology for research purposes, was accompanied by clear precaution against direct application to patient care at this stage (3).
Once candidate pharmacotherapy regimens have been selected, in the case of an individual patient, the process of comparing the risks and benefits of various treatments (and nontreatment) can be difficult. A thorough evaluation of the literature is warranted, and questions such as the generalizability and applicability of clinical trial findings should be carefully considered. The risks and benefits of psychopharmacological alternatives, of available nonpsychopharmacological interventions, and of nontreatment should be presented as well (1). In some psychiatric disorders, a combination of pharmacotherapy and psychotherapy options may offer the greatest clinical benefit, as in chronic depression (4, 5). In other cases, nonpharmacologic therapy may be the only available intervention fully supported by evidence, for example, in acute treatment of anorexia (6).
In fulfilling the aim of patient-centered care, it is essential that clinicians work with their patients to define the goals of treatment (7), even in the context of limited decisional capacity. These goals can assist the patient and clinician in promoting therapeutic alliance and the patient’s engagement with treatment, as well as anticipating the potential effectiveness of a proposed treatment regimen. Exploring the patient’s hopes and concerns is an important part of this process. It is helpful for clinicians to compare these goals to the outcomes defined in clinical trials (for example, remission of symptom scores, relapse of behaviors) to help guide expectations in the course of treatment. As the therapeutic process unfolds, the practitioner should remain vigilant for adverse effects or any other changes in a patient’s condition (such as pregnancy) that could affect the balance of psychopharmacological risks and benefits.
Informed Consent
Informed consent is the cornerstone of ethical clinical practice. Defined as the process by which patients make “free, knowledgeable decisions” about treatment, informed consent can be conceptualized as three components: the sharing of information with the patient, the patient’s decisional capacity, and the ability of a patient to make a decision voluntarily (Figure 2) (8, 9). This framework, in combination with careful evaluation of the evidence base behind psychopharmacological treatments, can be a useful guide in many clinical situations.
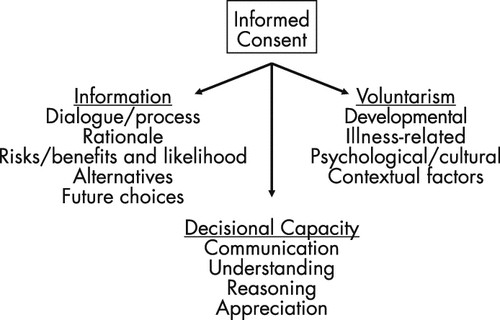
Sharing of information is a dialogue that involves disclosure of the rationale, risks, and benefits of treatment, in addition to possible alternative and future therapeutic strategies (Figure 2) (8). A discussion of the risks and benefits of nontreatment, or the expected natural course of illness, is also essential. An accurate presentation of treatment risk can be complicated—most practitioners would agree that it is essential to disclose the most common adverse effects (e.g., akathisia) in addition to those bearing the worst consequences (e.g., neuroleptic malignant syndrome). A conversation covering these topics inviting concerns and questions from the patient and, when appropriate, loved ones, can take time, but also can prevent later problems with treatment adherence. Addressing these and other frequently encountered concerns about treatment, such as the recommended duration of psychotropic administration, helps patients make fully informed decisions. Last, clinicians should also consider legal guidelines defining the amount of information to be provided to patients, such as the professional standard (the information most physicians would disclose) and the reasonable person standard (the information a reasonable person would request) (9).
The decisional capacity of a patient is commonly assessed in relation to four cardinal questions (Figure 2) (10): 1) Can the patient communicate a treatment choice? 2) Does the patient understand the information provided by the physician about his or her illness and treatments? 3) Does the patient appreciate how this information applies to him/her? 4) Can the patient arrive at his or her decision in a reasoned manner? These criteria delineate essential abilities that a patient should possess in order to make autonomous decisions. Involuntary treatment, for example, hinges on the notion that some patients lack decisional capacity, most commonly a lack of insight into their illness (question 3) (10).
The involuntary administration of psychopharmacological agents is a topic of extensive ethical discussion and legal statutes, as it bypasses the informed consent process (for a fuller discussion, see references 9, 11). It has been argued as a rare case where the physician’s duty of beneficence (the obligation to act in the patient’s best interest) supersedes patient autonomy—for example, in a patient with bipolar depression who is in imminent danger of harming himself, or a patient with schizophrenia who is hearing voices to harm others. Most states recognize this predicament and provide legal pathways for surrogate decision-making, separately for emergent and nonemergent situations (12).
Voluntarism, the third component of informed consent, represents the ability of a patient to make a free decision that is most congruent with his or her personal values and circumstances (13). Multiple types of influences may affect the voluntariness of a patient’s decision to give or withdraw consent, such as the developmental age of the patient, psychiatric or medical illness-related factors (including symptoms and experiences), psychological or cultural considerations, and contextual pressures (Figure 2). These influences can create conditions of subtle or obvious coercion that the ethical practitioner should anticipate and be prepared to explore or mitigate with a patient.
In practice, the process of informed consent varies widely. While there is no absolute “rule” for how rigorous the standard of informed consent should be, a commonly recommended approach is a “sliding scale” relationship between the stringency of consent and the risk-benefit ratio of treatment (14). As the risk of treatment increases relative to benefit, the rigorousness of the informed consent standard should be higher. The acceptance of antipsychotic treatment, for example, by acutely ill patients with schizophrenia on an inpatient unit carries a different risk-benefit calculation than acceptance by working patients seeking remission of depression in an outpatient setting. In practice, one would expect the informed consent standard to be more stringent in the second case. The same scale applies to consent for refusal of care: a suicidal patient seeking discharge from hospitalization typically faces a higher standard of consent than an asymptomatic patient seeking discharge from residential care.
High-Risk Pharmacotherapy
The practice of psychopharmacology invariably involves situations where patients are at a high risk of adverse events from treatment—pregnant or medically ill patients, for example, for whom concerns of harm from psychotropic medications extend beyond the usual risk considerations. Another ethical dilemma is when the medication itself constitutes a form of lethal means for a suicidal or impulsive patient, increasing the risk of self-harm by overdose. In either case, balancing the risks and benefits of treatment, nontreatment, and alternative interventions becomes more complicated.
Starting with the basics of ethical clinical practice, it would be best to begin with a thorough understanding and re-evaluation of diagnosis, risks and benefits of all possible treatment options, and likely clinical outcomes. This reappraisal should originate from the evidence base, although knowledge gaps and limitations of evidence should be considered, and expert opinion sought when possible. The above information, especially the estimated risks and benefits of all treatment possibilities, should be discussed comprehensively with the patient, and a rigorous standard of informed consent for accepting (or refusing) further psychopharmacological care should be applied. The clinician should also anticipate illness-related factors and other aspects affecting voluntarism to play a role in a patient’s decision. Once treatment proceeds, the practitioner may need to consider additional ways to “safeguard” a patient, such as increased frequency of clinical monitoring, seeking consent from the patient to engage the patient’s family more fully in treatment, or considering alternative treatments with lower risk (11). Revisiting the informed consent process during the course of treatment may also be warranted.
Long-Acting Medication Pharmacotherapy
A unique aspect of psychopharmacological practice is the prescription of long-acting medications (injectable antipsychotic depot formulations) for patients whose behavioral adherence with treatment may be problematic. Although long-acting antipsychotics are efficacious and safe to use, direct comparisons to oral antipsychotic therapy have yielded different conclusions with regard to superiority (15, 16). Many limitations exist in this literature, however, including selection bias in randomized controlled trials and expectation bias in naturalistic studies (16). In a way, long-acting antipsychotics are used for an indication—psychotic disorder in patients with difficulty in adhering to treatment—that may not exist in a research setting where adherence is monitored. The true comparative benefits of long-acting versus oral antipsychotic therapy, therefore, are unknown. This drawback of the evidence base complicates information provision to a patient; if research results are disclosed then the caveats of the studies should be mentioned as well. The safety and tolerability of long-acting antipsychotics compared with their oral counterparts may or may not be similar (15). Furthermore, patients may undervalue future risks, such as recurrent hospitalization and symptom relapse, compared with daily risks of adverse treatment effects. Thus, the sharing of information during the consent process can be challenging.
The use of long-acting medications has also raised concerns about voluntarism and possible coercion, because patients who authentically decide to stop antipsychotic therapy may need to wait weeks until the medication effects wear off (11, 17). It is unclear if long-acting medications lead to longer periods of decisional capacity in patients, but the idea that a patient with the capacity to refuse treatment must continue to experience a medication’s effects raises ethical concerns (17). On the other hand, an inability to appreciate the clinical importance of medication adherence may signify an illness-related and/or developmental factor (e.g., in first-break psychosis) that affects a patient’s degree of voluntarism, such that the decision to refuse long-acting medications is not truly authentic (17). Last, beneficent duty would support use of long-acting pharmacotherapy in the case of severely ill patients with a high risk of unsafe behaviors when nonadherent (17). As the evidence base continues to expand and additional long-acting psychotropics enter the market, continued ethical discussions regarding use of these medications are needed.
Off-Label Pharmacotherapy and Clinical Innovation
In psychopharmacological practice today, medications are sometimes prescribed for off-label use. Frequently encountered examples are found in pediatric psychopharmacology, where very few psychotropic medications are approved by the Federal Drug Administration (FDA) for use in children. (1). An ethical framework for proceeding with this kind of clinical innovation involves the following components: 1) identifying a serious clinical need of the patient, 2) checking that approved treatments have been adequately trialed without success, 3) finding evidence to support the possibility of benefit from the innovation, and 4) ensuring that the innovation carries less risk than the risk of living without treatment (11). In all circumstances of off-label prescribing, it is imperative that the potential side effects and adverse consequences of the intervention be monitored with additional scrutiny.
Although we are currently in the midst of an exciting acceleration in neurobiological research, caution must be taken before directly applying many preliminary findings toward clinical innovation. The brain is amazingly complex on molecular, cellular, and systems-level scales, and biological processes on these levels somehow underpin complex functions such as emotion, cognition, and behavior. The reality is that we still have an inadequate understanding of how the brain works, or the pathophysiology of many psychiatric disorders we treat. The validity of many animal models in psychiatry, for example, is unclear and often questioned (18). Also, psychotropic medications operate through mechanisms that are not completely understood; in fact, many of these medications affect alternative molecular targets and/or organ systems outside of the brain that might contribute to clinical response. Bringing preliminary mechanistic insights into clinical practice should only be done with extreme caution, and only when all other clinical innovation criteria mentioned above are rigorously met.
Informed consent for off-label prescribing involves special considerations for information sharing, decisional capacity assessment, and voluntarism, especially in pediatric cases. Information provision should include a clear description of off-label use, the risks and benefits of nontreatment, and the best estimates of risks and benefits expected with off-label treatment. In treating children, practitioners must engage parents (or other surrogate decision-makers) in the informed consent process, but should also inform the patients, and when appropriate, attain assent (1). As recommended for high-risk pharmacotherapy, off-label prescribing should proceed with adequate “safeguards” and opportunities to revisit a patient’s clinical need for continued medication use.
Enhancement Pharmacology
Prescribing psychotropic medications for enhancement (or “cosmetic” effects) remains a subject of wide debate, and there is need for broader societal engagement in addressing the ethics posed by enhancement pharmacology (19, 20, 21, 22). The basic premise is to enhance the functioning of a client (technically, not a patient) who does not carry a disabling diagnosis. On the one hand, individual autonomy includes the ability to seek improvement of one’s self, and psychotherapies are widely offered and practiced for this type of role. For example, cognitive behavioral therapy is often used to improve athlete performance (1). On the other hand, whether a physician’s duty of beneficence is fulfilled is less clear in these cases. Perhaps enhancement pharmacotherapy could benefit a patient in the short-term, but at what possible long-term costs? Using the sports analogy, would this practice be similar to performance steroid use (21, 22)? Enhancement is a societal question and needs greater societal consensus if the physician’s role is to change substantively.
Psychopharmacology for enhancement purposes would be an off-label prescription practice, providing medication for a clinical need that is not truly present. Thus, the framework of clinical innovation does not apply here (1). There are real risks involved (for example, stimulant use to enhance cognitive function) that may exceed the risk of nontreatment (19). One can argue that the risks of psychotherapy for this purpose are significantly less than the anticipated risks of psychopharmacological treatment. The possible benefits are questionable as well, because psychotropics are not widely studied for enhancement purposes. Would an antidepressant, for example, be “effective” in someone who is not clinically depressed?
If there is indeed a competitive advantage to be offered with enhancement pharmacotherapy, then fairness in access becomes an important question. If enhancement falls within a physician’s role, then there is a duty of justice—ensuring fairness in the distribution of medical resources to the community (9). At this time, enhancement pharmacotherapy raises many more questions than answers. Further ethical debate on the issue, and on the physician’s role in this context (19, 20), is warranted before enhancement pharmacotherapy becomes routine practice.
Conclusion
Practitioners of psychopharmacology can be faced with many ethical dilemmas. A careful evaluation of the risks and benefits of psychotropic treatment, nontreatment, and the alternatives for each clinical situation is vital. A wise and ethical clinician will rely on the existing evidence base but will also be cognizant of the limitations of our knowledge. This risk-benefit analysis should be subsumed in a core ethical framework of informed consent, which in turn rests on core values of patient autonomy and physician’s beneficence. Together, these concepts can be applied to anticipate and guide psychopharmacological treatment decisions.
1 : Ethical issues in psychopharmacology. Psychiatric Times 2011; 28 (http://www.psychiatrictimes.com/articles/ethical-issues-psychopharmacology).Google Scholar
2 : Reflections on ethical issues in psychopharmacology: an American perspective. Int J Law Psychiatry 2012; 35:387–391Crossref, Google Scholar
3 : Toward the future of psychiatric diagnosis: the seven pillars of RDoC. BMC Med 2013; 11:126–133Crossref, Google Scholar
4 : A comparison of nefazodone, the cognitive behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med 2000; 342:1462–1470Crossref, Google Scholar
5 : Chronic depression: update on classification and treatment. Curr Psychiatry Rep 2008; 10:458–464Crossref, Google Scholar
6 : Update on the treatment of anorexia nervosa: review of clinical trials, practice guidelines and emerging interventions. Psychol Med 2013; 43:2477–2500Crossref, Google Scholar
7 : Handbook of Psychiatric Drug Therapy. Philadelphia, PA, Lippincott Williams & Wilkins, 2010Google Scholar
8 : A Concise Guide to Ethics in Mental Health Care. Arlington, VA, American Psychiatric Publishing, 2004Google Scholar
9 : An overview for mental health clinicians, researchers, and learners, in Professionalism and Ethics Q&A Self-Study Guide for Mental Health Professionals. Edited by Roberts LWHoop JG. Arlington, VA, American Psychiatric Publishing, 2008, pp 3–72Google Scholar
10 : Clinical practice. Assessment of patients’ competence to consent to treatment. N Engl J Med 2007; 357:1834–1840Crossref, Google Scholar
11 : Ethical considerations in psychopharmacological treatment and research, in The American Psychiatric Publishing Textbook of Psychopharmacology, 4th ed. Edited by Schatzberg AFNemeroff CB. Arlington, VA, American Psychiatric Publishing, 2009, pp 1477–1496Crossref, Google Scholar
12 : The right to refuse psychiatric treatment. Psychiatr Clin North Am 1999; 22:173–182, viiiCrossref, Google Scholar
13 : Informed consent and the capacity for voluntarism. Am J Psychiatry 2002; 159:705–712Crossref, Google Scholar
14 : Competency to give an informed consent. A model for making clinical assessments. JAMA 1984; 252:925–927Crossref, Google Scholar
15 ;
16 : Long-acting injectable versus oral antipsychotics in schizophrenia: a systematic review and meta-analysis of mirror-image studies. J Clin Psychiatry 2013; 74:957–965Crossref, Google Scholar
17 : Ethical use of long-acting medications in the treatment of severe and persistent mental illnesses. Compr Psychiatry 2004; 45:161–167Crossref, Google Scholar
18 : Revitalizing psychiatric therapeutics. Neuropsychopharmacology 2014; 39:220–229Crossref, Google Scholar
19 : Cosmetic neurology: the controversy over enhancing movement, mentation, and mood. Neurology 2004; 63:968–974Crossref, Google Scholar
20 : Neurocognitive enhancement: what can we do and what should we do? Nat Rev Neurosci 2004; 5:421–425Crossref, Google Scholar
21 : Towards responsible use of cognitive-enhancing drugs by the healthy. Nature 2008; 456:702–705Crossref, Google Scholar
22 : Beyond therapy: biotechnology and the pursuit of happiness. Washington, DC, President’s Council on Bioethics, 2003Google Scholar



