Narcolepsy and Syndromes of Central Nervous System–Mediated Sleepiness
The complaints of tiredness, fatigue, sedation, and sleepiness are common among patients with psychiatric disorders. Many factors contribute to the experience of diminished alertness across individuals with such complaints. Potential contributing elements include presumed physiological epiphenomena of primary pathological conditions (e.g., “hypersomnia” in “atypical” depression), medication effects, use or abuse of sedating substances, sleep disruption, advanced or delayed circadian rhythm phase, voluntary or involuntary sleep restriction, and other sleep disorders (e.g., obstructive sleep apnea syndrome). The careful characterization of the complaint is of key importance in the evaluation of suboptimal alertness or fatigue for appropriate diagnosis and symptom management—primary treatment, when possible, or secondary amelioration.
Occasionally, patients will manifest somnolence or frank sleepiness—unrelated to factors such as those noted above or to the primary psychiatric condition they have—as a result of distinct central nervous system (CNS) dysfunction. Sometimes the CNS condition of excessive sleepiness is comorbid with a primary psychiatric disorder. However, not infrequently, the patient receives the diagnosis of a psychiatric illness when none is present. Comorbid disorders of somnolence or excessive daytime sleepiness (EDS) add frustration to the diagnostic workup and complexity to the treatment of individuals with psychiatric disorders. Sleep-related conditions that arise from CNS dysfunction tend to be less familiar to the clinician. Indeed, lack of an understanding of these conditions not only leads to their being misdiagnosed or overlooked, but at times results in gross mismanagement (e.g., the use of antipsychotic medications for narcolepsy-related hypnagogic hallucinations).
Narcolepsy is the most well known and extensively studied of the EDS conditions arising from CNS dysfunction, yet it, too, is often misunderstood and misdiagnosed. Idiopathic hypersomnia, the recurrent hypersomnias, and EDS associated with nervous system disorders also must be understood in order to provide appropriate evaluation and management. In this chapter, we summarize the distinguishing features of these clinical syndromes of primary EDS and provide a brief overview of their pharmacologic management. Additionally, we review the great advances in our understanding of the pathophysiology of narcolepsy that have occurred in the past few years.
Epidemiology of excessive daytime sleepiness
The epidemiology of EDS has been explored, using a variety of research methods, in many countries around the world. The largest and most comprehensive representative population survey was performed across four Western European countries (the United Kingdom, Germany, Spain, and Italy). Substantial EDS, defined as meeting three parameters of marked sleepiness during three or more days a week, was reported in 15% of this combined population (Ohayon et al. 2002). Smaller population surveys in the United States have been conducted. Two recent polls suggest that 15%–16% of the U.S. population may experience EDS that interferes with daily activities a few days a week or more (National Sleep Foundation 2002, 2003). These data do not indicate the percentage of sleepy individuals whose sleepiness results from insufficient sleep or environmental factors rather than from pathological causes of EDS.
The prevalence of syndromes of CNS-mediated sleepiness is much more difficult to assess than that of EDS in the general population and, with the exception of narcolepsy, it remains poorly characterized. Data gathered in U.S. and Western European narcolepsy studies demonstrate a fairly consistent prevalence of approximately 0.05%, although rates as low as 0.002% and as high as 0.16% have been reported for Israel and Japan, respectively (Mignot 1998). In general, the published literature on the epidemiology of CNS-mediated EDS syndromes indicates a total prevalence that ranges from as high as 2%–3% to much less than 1% of the population.
Insufficient sleep and primary syndromes of excessive daytime sleepiness
Fragmented sleep and insufficient sleep
Essential in the evaluation of patients presenting with EDS is the determination of the presence or absence of the two most common causes of EDS: 1) fragmented sleep due to extrinsic or intrinsic factors and 2) insufficient sleep. Fragmented sleep, predominantly microfragmentation resulting from such conditions as sleep-related breathing disorders (e.g., obstructive sleep apnea syndrome) and periodic limb movement disorder (PLMD), is an important consideration in the evaluation of EDS. These conditions are addressed in Chapter 3, “Sleep Apnea,” and Chapter 5, “Restless Legs Syndrome,” in this volume. The most common cause of daytime sleepiness is insufficient sleep, which may reflect poor sleep “hygiene” (behaviors negatively affecting sleep) or self-imposed or socially dictated sleep deprivation. Insufficient sleep is expected to lead to frank EDS, yet the more common subjective complaints are those of tiredness, lack of energy, or fatigue. Additionally, decrements in attention, learning capacity, short-term memory, and psychomotor performance, with or without EDS, may be present. Moreover, irritability, poor impulse control, or other forms of mood instability may exist alone or in concert with the above-noted features in individuals with insufficient sleep.
Primary disorders of excessive daytime sleepiness
Two diagnostic classification manuals are commonly used to specify diagnostic criteria for disorders of primary EDS. DSM-IV-TR (American Psychiatric Association 2000) subcategorizes these disorders into narcolepsy and primary hypersomnia; the latter includes all non-narcolepsy forms of primary EDS. The International Classification of Sleep Disorders (ICSD; American Sleep Disorders Association 1997) subcategorizes primary EDS into narcolepsy, idiopathic hypersomnia, recurrent hypersomnia, and posttraumatic hypersomnia. We have selected the ICSD format to organize this review, but we suggest the expansion of the posttraumatic category to include CNS pathology in addition to trauma.
As suggested by both DSM-IV-TR and ICSD, several entities may be regarded as primary disorders of EDS. Narcolepsy, the best known and the most completely understood disorder of this group, will be considered at greater length; other clinical syndromes will be reviewed more briefly. Patients with these CNS-mediated EDS syndromes commonly receive a misdiagnosis of a mood disorder and are treated with antidepressant therapy.
Narcolepsy
Narcolepsy is a syndrome of unknown etiology characterized by a profound degree of EDS. Narcolepsy usually occurs in association with cataplexy and other symptoms and signs, including hypnagogic or hypnopompic hallucinations, sleep paralysis, automatic behavior, and disrupted nocturnal sleep (American Sleep Disorders Association 1997). Symptoms most often begin during adolescence or young adulthood. However, narcolepsy may also occur earlier in childhood or not until the third or fourth decade of life. The impact of narcolepsy on quality of life is equal to that of other chronic neurologic disorders, such as Parkinson’s disease (Beusterien et al. 1999). No symptom or sign of narcolepsy is specific to narcolepsy; even cataplexy unrelated to narcolepsy occurs, rarely, either as an isolated symptom or in conjunction with other conditions. Unfortunately, the average duration from symptom onset to the conferring of an accurate diagnosis is greater than 10 years. This substantial delay in diagnosis is largely due to clinicians’ lack of education about and experience with this disorder.
Symptoms of narcolepsy include the following:
| • | Sleepiness or excessive daytime sleepiness. The EDS of narcolepsy presents as an increased propensity to fall asleep, nod, or doze easily in relaxed or sedentary situations, or a need to exert extra effort to avoid sleeping in these situations. Additionally, irresistible or overwhelming urges to sleep commonly occur from time to time during wakeful periods in untreated patients. These “sleep attacks” are not instantaneous lapses into sleep, as is often thought by the general public, but represent episodes of profound sleepiness similar to that caused by severe sleep deprivation or other severe sleep disorders. Many but not all patients with narcolepsy find that brief naps and longer sleep periods can be temporarily restorative or refreshing. This contrasts with the response to naps or sleep observed in idiopathic hypersomnia, wherein sleep of any duration is rarely restorative. In addition to frank sleepiness, the excessive daytime sleepiness of narcolepsy can cause or contribute to related symptoms, including poor memory, reduced concentration or attention, and irritability. | ||||
| • | Cataplexy. Cataplexy is the partial or complete loss of bilateral voluntary muscle tone in response to strong emotion. The reduced muscle tone may be minimal, occur in a few muscle groups, and cause minimal symptoms such as bilateral ptosis, head drooping, slurred speech, or dropping things from the hand. On the other hand, it may be so severe that total body paralysis occurs, resulting in complete collapse. Cataplectic events usually last from a few seconds to 2 or 3 minutes, but they occasionally continue longer (Honda 1988). The patient is usually alert and oriented during the event despite the inability to respond; thus, cataplectic episodes are distinct from sleep episodes. Positive emotions such as laughter trigger cataplexy more commonly than negative emotions. However, any strong emotion is a potential trigger (Gelb et al. 1994). Startling stimuli, stress, physical fatigue, and sleepiness may also be important triggers or factors that exacerbate cataplexy. According to epidemiologic studies, cataplexy is found in 60%–100% of patients with narcolepsy. The onset of cataplexy is most frequently simultaneous with or within a few months of the onset of EDS, but in some cases cataplexy may not develop until many years after initial onset of EDS (Honda 1988). | ||||
| • | Hypnagogic or hypnopompic hallucinations. These phenomena are visual (most common), tactile, auditory, or multisensory events, usually brief but occasionally continuing for a few minutes, that occur at the transition from wakeful-ness to sleep (hypnagogic) or from sleep to wakefulness (hypnopompic). Hallucinations may contain elements of dream sleep and consciousness combined, and they are often bizarre or disturbing to patients. Occasionally, patients who experience these episodes are misdiagnosed with a psychotic syndrome and inappropriately treated with antipsychotic medications. | ||||
| • | Sleep paralysis. Sleep paralysis is the inability to move, lasting from a few seconds to minutes, during the transition from sleep to wakeful-ness or from wakefulness to sleep. Episodes of sleep paralysis may alarm patients, particularly those who experience the sensation of being unable to breathe. Although accessory respiratory muscles may not be active during these episodes, diaphragmatic activity continues and air exchange remains adequate. | ||||
| • | Other symptoms. Other commonly reported symptoms in narcolepsy include automatic behavior—absent-minded behavior or speech that is often nonsensical and that the patient does not remember because of extreme sleepiness. In addition, many individuals with narcolepsy report fragmented nocturnal sleep—that is, frequent awakenings during the night. | ||||
Hypnagogic hallucinations, sleep paralysis, and automatic behavior are not specific to narcolepsy and may occur in other sleep disorders, as well as in healthy individuals. These symptoms are, however, far more common and occur with much greater frequency in narcolepsy.
The following tools are available for the evaluation of narcolepsy:
| • | Polysomnography. Nocturnal polysomnography (PSG) is not essential in the diagnostic workup when straightforward cataplexy accompanies EDS. However, it remains an important part of the evaluation process, primarily to exclude other conditions that occur in narcolepsy at a higher than normal rate, such as obstructive sleep apnea (see Chapter 3, “Sleep Apnea”), PLMD (see Chapter 5, “Restless Legs Syndrome”), and rapid eye movement (REM) sleep behavior disorder. These conditions may contribute to the sleepiness or nocturnal sleep disruption the patient may be experiencing (Overeem et al. 2001). Additionally, individuals with narcolepsy may demonstrate sleep-onset REM periods during nocturnal PSG. Normally, nocturnal sleep begins with a long period of non–rapid eye movement (NREM) sleep (see Chapter 1, “Introduction”). | ||||
| • | Daytime sleep studies. Daytime nap studies, in the form of the multiple sleep latency test (MSLT), usually demonstrate substantially reduced sleep latency coupled with sleep-onset REM periods (SOREMPs) in patients with narcolepsy. This test subjects the patient to four or five 20-minute nap opportunities, under PSG monitoring, at 2-hour intervals across the morning and afternoon. During testing, the patient is in bed in a dark room and is instructed to try to fall asleep. Average MSLT sleep latencies for normal control subjects are 12–15 minutes, but latencies for patients with narcolepsy with cataplexy average approximately 2–4 minutes (US Xyrem in Narcolepsy Multi-center Study Group 2002); however, there can be substantial variability across patients and within patients. SOREMPs are not specific for narcolepsy, but the occurrence of two or more of these events during the MSLT, in the setting of objective marked sleepiness and without another explanation for their occurrence (e.g., sleep deprivation, REM-suppressant medication rebound, altered sleep schedule, obstructive sleep apnea, or delayed sleep phase syndrome), is suggestive of narcolepsy. The presence of two or more SOREMPs can be found in a subset of patients with any of the other conditions just mentioned. These conditions are far more prevalent than narcolepsy. Therefore, the specificity for narcolepsy of two or more SOREMPs on the MSLT is low, but most patients with straightforward narcolepsy will manifest this finding. | ||||
| • | Cerebrospinal fluid (CSF) hypocretin assessment. As discussed below in the section on the pathophysiology of narcolepsy, many, but not all, patients with narcolepsy have very low or undetectable levels of the peptide neurotransmitter hypocretin in the CSF (Nishino et al. 2000b; Mignot et al. 2002). Such low levels of CSF hypocretin are not specific for narcolepsy. However, when used to assess patients for narcolepsy, low CSF hypocretin appears to be a much more specific test than the MSLT. Whether this test is more sensitive than the MSLT has yet to be determined. | ||||
| • | Histocompatibility human leukocyte antigen (HLA) testing. A very strong but incomplete correlation exists between narcolepsy (with cataplexy) and the HLA subtype DQB1* 0602. However, this subtype is also very common in the general population (occurring in about 20% in the United States) and is not at all specific nor sensitive for narcolepsy (Mignot 1998). HLA testing is therefore not useful in confirming or excluding the diagnosis of narcolepsy, and in fact may lead a clinician to inappropriate diagnostic conclusions. | ||||
Idiopathic hypersomnia
Idiopathic hypersomnia (previously labeled idiopathic CNS hypersomnia) is an incompletely defined disorder characterized by EDS. This diagnosis has been used as a nosologic haven for classifying individuals with excessive somnolence but without the classic features of narcolepsy or another disorder known to cause EDS, such as sleep apnea. Without doubt, many patients have received diagnoses of idiopathic hypersomnia who actually had other disorders, such as narcolepsy without cataplexy, delayed sleep phase syndrome, or upper airway resistance syndrome, a subtle variant of the obstructive sleep apnea syndrome (Guilleminault et al. 1993). Roth (1976) described monosymptomatic and polysymptomatic forms of idiopathic hypersomnia, the former characterized by EDS alone and the latter characterized by EDS, prolonged nocturnal sleep time, and marked difficulty with awakening. Others have suggested that the category of idiopathic hypersomnia is heterogeneous, including individuals with EDS with or without one or more of the other features of Roth’s polysymptomatic form (Aldrich 1996).
Idiopathic hypersomnia is believed to be less common than narcolepsy; estimation of prevalence, however, is elusive because strict diagnostic criteria are lacking and no specific biological marker has been identified. Typically, onset of symptoms occurs in adolescence or early adulthood. Symptoms generally persist for life, although a few patients with idiopathic hypersomnia have improved over time or attained complete remission of symptoms (Bassetti and Aldrich 1997; Billiard and Dauvilliers 2001; Bruck and Parkes 1996). The etiology of the disorder is not known, but viral illnesses, including Guillain-Barré syndrome, hepatitis, mononucleosis, and atypical viral pneumonia, may herald the onset of sleepiness in a subset of patients. EDS may occur as part of the acute illness, but it persists after the other symptoms subside. Familial cases are known to occur, with increased frequency of HLA-Cw2 and HLA-DR11 (Montplaisir and Poirier 1988). Some of these patients have associated symptoms suggesting autonomic nervous system dysfunction, including orthostatic hypotension, syncope, vascular headaches, and peripheral vascular complaints. Most patients with idiopathic hypersomnia have neither a family history nor an obvious associated viral illness. Little is known about the pathophysiology of idiopathic hypersomnia. No animal model is available for study. Neurochemical studies examining CSF have suggested that patients with idiopathic hypersomnia may have altered noradrenergic system function (Faull et al. 1983, 1986; Montplaisir et al. 1982).
The clinical picture of idiopathic hypersomnia varies among individual patients. The disorder may be mistaken for narcolepsy if a careful history is not taken. The two disorders share several common features, including similar age of onset, lifelong persistence after onset, EDS as the primary symptom, and familial clustering of some cases. However, essential differences between the disorders become apparent in the history and in diagnostic studies (see Table 1). Patients with idiopathic CNS hypersomnia present with EDS, but with neither cataplexy (although some patients have episodes of sleep paralysis or hypnagogic hallucinations) nor significant nocturnal sleep disruption (Billiard and Dauvilliers 2001). They complain of daytime sleepiness that interferes with normal activities. Occupational and social functioning may be severely affected by sleepiness. Nocturnal sleep time tends to be long, and patients are usually difficult to awaken in the morning, becoming irritable or even abusive in response to the efforts of others to rouse them. In some patients, this difficulty may be substantial and may include confusion, disorientation, and poor motor coordination, a condition sometimes termed “sleep drunkenness” (Roth et al. 1972). These patients often take naps, which may be prolonged but, in contrast to naps in narcolepsy, are usually nonrefreshing. No amount of sleep ameliorates the EDS. “Microsleeps,” with or without automatic behavior, may occur throughout the day.
Polysomnographic studies of patients with idiopathic CNS hypersomnia usually reveal shortened initial sleep latency, increased total sleep time, and normal sleep architecture, in contrast to narcoleptic patients, who exhibit significant sleep fragmentation. Using quantitative electroencephalography, Sforza et al. (2000) found reduced sleep pressure, as evidenced by decreased slow-wave activity during the first two NREM episodes of nocturnal sleep, in patients with idiopathic hypersomnia. Mean sleep latency on MSLT is usually reduced, often in the 8–10-minute range but sometimes dramatically shorter. Also in contrast to narcolepsy, SOREMPs are not typically seen.
As with narcolepsy, other disorders producing EDS (such as insufficient sleep, sleep-related breathing disorders, PLMD, other sleep-fragmenting disorders, psychiatric diseases, and circadian rhythm disorders) must be ruled out before the diagnosis of idiopathic hypersomnia is made.
Recurrent hypersomnias
Kleine-Levin syndrome.
This uncommon disorder is a form of recurrent hypersomnia that occurs primarily in adolescents (Critchley 1967), with a male preponderance. It is characterized by the occurrence of episodes of EDS and is usually, but not invariably, accompanied by hyperphagia, aggressiveness, and hypersexuality. These episodes last for days to weeks and are separated by asymptomatic periods of weeks or months. During symptomatic periods, individuals sleep up to 18 hours per day and are usually drowsy (often to the degree of stupor), confused, and irritable the remainder of the time. During symptomatic episodes, polysomnographic studies show long total sleep time with high sleep efficiency and decreased slow-wave sleep. MSLT studies demonstrate short sleep latencies and SOREMPs (Rosenow et al. 2000).
The etiology of this syndrome remains obscure. Symptomatic cases of Kleine-Levin syndrome associated with structural brain lesions have been reported, but most cases are idiopathic. Single-photon emission computed tomography studies have demonstrated hypoperfusion in the thalamus in one patient and in the nondominant frontal lobe in another (Arias et al. 2002).
Menstrual-related hypersomnia.
In this form of recurrent hypersomnia, EDS occurs during the several days prior to menstruation (Billiard et al. 1975; Sachs et al. 1982). The prevalence of this syndrome has not been well characterized. Likewise, the etiology is not known, but presumably the symptoms are related to hormonal changes. Some cases of menstrual-related hypersomnia have responded to the blocking of ovulation with estrogen and progesterone (birth control pills) (Bamford 1993).
Idiopathic recurring stupor.
Numerous cases have been reported in which individuals (predominantly middle-aged males) are subject to stuporous episodes lasting from hours to days, in the absence of obvious toxic, metabolic, or structural cause. Episodes occur unpredictably, and the individuals are normal between episodes. Electroencephalographic data collected during symptomatic episodes have shown fast background activity in the 13–16 Hz range. Several of these patients have been shown to have elevated plasma and CSF levels of endozepine-4, an endogenous ligand with affinity for the benzodiazepine recognition site at the γ-aminobutyric acid type A receptor (Rothstein et al. 1992). Administration of flumazenil, a benzodiazepine antagonist, has produced transient awakening with normalization of the EEG profile (Lugaresi et al. 1998). In some cases, the episodes resolved spontaneously after several years. Similar cases have been reported in children (Soriani et al. 1997).
Nervous system disorders and excessive daytime sleepiness
EDS is often associated with disorders of the central or peripheral nervous system. It is a clinical feature of many toxic or metabolic encephalopathic processes. These disorders often present with other symptoms and signs, but EDS may dominate the picture, particularly in chronic cases. Structural brain lesions, including strokes, tumors, cysts, abscesses, hematomas, vascular malformations, hydrocephalus, and multiple sclerosis plaques, are known to produce EDS. Somnolence may result either from direct involvement of discrete brain regions (especially the brainstem reticular formation or midline dien-cephalic structures) or from effects on sleep continuity (for example, nocturnal seizure activity or sleep-related breathing disorders such as sleep apnea).
EDS is a frequent sequela of encephalitis or head trauma. Victims of “encephalitis lethargica,” described by Von Economo in the early twentieth century, were found to have lesions in the midbrain, subthalamus, and hypothalamus. Additionally, post-traumatic narcolepsy with cataplexy is well documented (Francisco and Ivanhoe 1996). Epileptic patients may experience EDS as a consequence of medication effects or, less obviously, nocturnal seizure activity (Manni and Tartara 2000). EDS may be associated with numerous infectious agents affecting the CNS, including bacteria, viruses, fungi, and parasites. Perhaps the best known is trypanosomiasis, which is called “sleeping sickness” because of the prominent hypersomnia. Sleepiness may occur with acute infectious illness, even without direct invasion of the nervous system, and may be mediated by cytokines, including interferon, interleukins, and tumor necrosis factor (Toth and Opp 2002). EDS may also persist chronically after certain viral infections (Guilleminault and Mondini 1986).
Sleep disruption and EDS are also common in neurodegenerative disorders, including Parkinson’s disease, Alzheimer’s disease, and other dementias, as well as multiple system atrophy (Askenasy 1993; Chokroverty 1996; Trenkwalder 1998). Patients with neuromuscular disorders or peripheral neuropathies may also develop EDS due to associated central or obstructive sleep apnea, pain, or PLMD (George 2000). Patients with myotonic dystrophy often suffer from EDS, even in the absence of sleep-disordered breathing (Gibbs et al. 2002).
Finally, psychiatric illness, especially depression, is often accompanied by complaints of EDS. Indeed, tiredness, fatigue, or lack of energy are reported by an overwhelming majority of patients with major depression (Tylee et al. 1999). However, evaluation of true EDS with subjective rating scales and objective measures suggests that frank sleepiness or a high sleep propensity may be much less common than the complaint of fatigue or lack of energy. The few published studies evaluating objective measures of sleepiness (such as the MSLT) in depression suggest that only a minority of depressed patients have clinically important EDS and that the majority are in the normal range (Reynolds et al. 1982).
Management of disorders of excessive daytime sleepiness
Behavioral treatment
In addition to the use of medications, the effective treatment of primary EDS conditions requires instituting structured and regular nocturnal sleep as well as structured daytime naps. A period of 8 hours or longer should be established for nocturnal sleep, with a consistent bedtime and time of morning awakening. Inadequate sleep duration, poor sleep environment, and fluctuating sleep-wake schedules can contribute substantially to daytime sleepiness, even in patients with primary sleep disorders. Moreover, shift work in any form is usually problematic. Additionally, in many EDS disorders, such as narcolepsy, scheduling two or more brief naps at regular times during the day is almost always necessary to further enhance daytime function. The importance of regular and adequate nocturnal and daytime sleep must always be emphasized with the patient.
Pharmacologic treatment
Stimulants and other alerting medications
Alerting agents provide a critical component of treatment for most patients with primary disorders of EDS (Mitler et al. 1994) (Table 2). Occasionally, patients may wish to avoid medications and instead may attempt to take extra naps during the day. This approach can be successful but usually fails to provide enough daytime alertness to function adequately. For most patients with narcolepsy, alerting agents do not yield a normal degree of daytime alertness but will nonetheless produce substantial improvement (Mitler et al. 1994). Clinically, the practice of combining two alerting agents of different chemical classes has been employed when a single agent is insufficient. This technique can be useful and is undergoing controlled evaluation in a large international trial with modafinil and sodium oxybate in narcolepsy.
Modafinil.
Modafinil is a wake-promoting therapeutic somewhat comparable to traditional stimulants in promoting alertness, but with a different mechanism of action. This mechanism is unknown, but modafinil appears to act more locally at hypo-thalamic regions and less globally on the CNS than the traditional stimulants (US Modafinil in Narcolepsy Multicenter Study Group 2000). The clinical duration of effect of modafinil may be longer because its serum half-life (12–15 hours) is longer than that of traditional stimulants. Modafinil has been evaluated extensively in the treatment of the EDS of narcolepsy, and in other sleep disorders manifesting EDS, and has been found to be useful in ameliorating EDS in these conditions (US Modafinil in Narcolepsy Multicenter Study Group 1998).
Sodium oxybate (GHB).
Another agent that has been found to enhance alertness in narcolepsy is sodium oxybate, also known as γ-hydroxybutyrate (GHB). This agent has been explored extensively and found to be highly effective for the treatment of EDS in narcolepsy (Mamelak et al. 2004). The mechanism of action of the agent has been explored over many years, but how it effects improved alertness is unknown (Tunnicliff and Cash 2002). Data from multiple studies demonstrate that sodium oxy-bate imparts a degree of alertness similar to that of other agents and that its effect may be additive when used in combination with other stimulants (US Xyrem in Narcolepsy Multi-center Study Group 2002). Whether sodium oxybate will prove useful in other conditions of primary EDS is unknown.
Traditional stimulants.
Commonly used traditional stimulants include methylphenidate, dextroamphetamine, and methamphetamine (Mitler et al. 1994). Other sympathomimetic amines and sustained-release preparations are available. Patients may experience negative effects with any alerting agent. Some patients report rebound hypersomnia (exacerbation of sleepiness) as the dose wears off. Tolerance (tachyphylaxis) to the alerting effect may occur with time in some patients. In cases of tolerance, switching to a different class of medication or providing a “drug holiday” can be useful.
Medications for cataplexy (anticataplectics)
Medications useful in the treatment of cataplexy usually also improve hypnagogic/hypnopompic hallucinations and sleep paralysis.
Sodium oxybate (GHB).
In addition to its effect on EDS in narcolepsy, sodium oxybate is remarkably effective as an anticataplectic. It has been extensively evaluated as an anticataplectic agent over many years (US Xyrem in Narcolepsy Multi-center Study Group 2002). Additionally, GHB is effective in reducing nocturnal sleep disruptions and consolidating nocturnal sleep. Again, the mechanism of action of GHB in treating these symptoms is unknown (Tunnicliff and Cash 2002).
Antidepressants.
Very low doses of tricyclic anti-depressants (TCA) and typical antidepressant doses of selective serotonin reuptake inhibitors (SSRI), especially those with CNS noradrenergic activity, are also useful in treating cataplexy (Nishino and Mignot 1997). Tolerance to these traditional cataplexy medications can occur, requiring medication switch or drug holiday. Atomoxetine, venlafaxine, and other newer non-SSRI/non-tricyclic antide-pressants have been reported in individual cases to provide effective treatment for cataplexy, although they have not yet been rigorously studied.
Pathophysiology of narcolepsy
Narcolepsy symptoms
The similarity between cataplexy and REM sleep atonia, the presence of frequent episodes of hypnagogic hallucinations and of sleep paralysis, and the propensity for narcolepsy patients to go directly from wakefulness into REM sleep suggest that narcolepsy is primarily a “disease of REM sleep” (Dement et al. 1966). This hypothesis may, however, be too simplistic, and it does not explain the presence of sleepiness during the day and the short latency to both NREM and REM sleep during nocturnal and nap recordings. A complementary hypothesis is that narcolepsy results from the disruption of the control mechanisms of both sleep and wakefulness or, in other words, from vigilance-state boundary problems (Broughton et al. 1986). According to this hypothesis, a cataplectic attack represents an intrusion of REM sleep atonia during wakefulness, and the hypnagogic hallucinations appear as dreamlike imagery taking place in the waking state, especially at sleep onset in patients who frequently have SOREMPs.
Cataplexy is associated with an inhibition of the monosynaptic H-reflex and the polysynaptic deep tendon reflexes (Guilleminault et al. 1974). In healthy subjects, it is only during REM sleep that the H-reflex is totally suppressed. This finding highlights the relationship between the inhibition of motor processes during REM sleep and the sudden atonia and areflexia seen during cataplexy. Studies in canine narcolepsy, however, suggest that the mechanisms for induction of cataplexy are different from those for REM sleep (Nishino et al. 2000a). Furthermore, an extended human study confirmed that cataplexy correlates much more highly with hypocretin deficiency (discussed below) than do other REM sleep–related phenomena (Mignot et al. 2002). Cataplexy may thus be viewed as a hypocretin-deficiency pathological phenomenon somewhat distinct from other REM-related symptoms. Patients with other sleep disorders such as sleep apnea, and even healthy individuals, can manifest SOREMPs, hypnagogic hallucinations, and sleep paralysis when their sleep-wake patterns are sufficiently disturbed. However, these subjects never develop cataplexy, further supporting the proposal that cataplexy is unrelated to other REM-associated symptoms (Aldrich et al. 1997; Bishop et al. 1996; Fukuda et al. 1987; Ohayon et al. 1996). Although cataplexy and REM sleep atonia have great similarity and possibly share a common executive system, the regulatory mechanisms of the two states are not necessarily identical. The mechanism of emotional triggering of cataplexy remains undetermined.
Narcolepsy, human leukocyte antigen, and the immune system
A remarkably high association of HLA with narcolepsy was discovered in the early 1980s (Juji et al. 1984). Since the time of this initial finding, a variety of research across multiple ethnic groups has corroborated the existence of this strong HLA association. The most specific marker of narcolepsy in a number of different ethnic groups studied to date is DQB1*0602 (Mignot 1998). This association is seen in an average of approximately 90% of those with unequivocal cataplexy (Mignot et al. 1997). Importantly, this association is substantially lower (only approximately 40%) in individuals who have received the diagnosis of narcolepsy but do not have cataplexy.
The strong association between HLA type and narcolepsy with cataplexy raises the possibility that narcolepsy is an autoimmune disease (Mignot et al. 1992). There is, however, no evidence of inflammatory processes or immune abnormalities associated with narcolepsy (Mignot et al. 1992). Studies have found no classical autoantibodies and no increase in oligoclonal CSF bands in individuals with narcolepsy (Fredrikson et al. 1990). Results of typical autoimmune pathology measures (erythrocyte sedimentation rates, serum immunoglobulin levels, C-reactive protein levels, complement levels, and lymphocyte subset ratios) are apparently normal in narcoleptic patients (Matsuki et al. 1988). In contrast, a variety of serological tests performed in narcoleptic patients and in age- and sex-matched control subjects yielded higher levels of antistreptolysin 0 and anti-DNase antibodies in patients than in control subjects (Billiard et al. 1989; Montplaisir et al. 1989). In view of these preliminary data, further exploration of possible immune-related dysfunction in narcolepsy is warranted.
Deficiency in hypocretin (orexin) transmission in canine and human narcolepsy
Narcolepsy genes in animal models
Narcolepsy has been described in several animal species, including dogs, and most recently in genetically engineered mice models. Canine narcolepsy is a naturally occurring model, with both sporadic forms (17 breeds) and familial forms (Doberman, Labrador, and dachshund). In Doberman pinschers and Labrador retrievers, the disease is transmitted as a recessive autosomal trait with complete penetrance (Mignot et al. 1991).
In 1999, using positional cloning and gene targeting strategies, two groups independently revealed the pathogenesis of narcolepsy in animals. The lack of the hypothalamic neuropeptide hypocretin/orexin ligand (preprohypocretin/orexin gene knockout mice [Chemelli et al. 1999]) or mutations in one of the two hypocretin/orexin receptor genes (hypocretin receptor 2 [hcrtr 2] gene in autosomal recessive canine narcolepsy [Lin et al. 1999]) was observed to result in narcolepsy. Extensive screening in humans, especially in familial and early-onset narcolepsy, demonstrated that mutations in hypocretin-related genes are rare; only a single case with early onset (at age 6 months) was found to be associated with a single-point mutation in the preprohypocretin gene (Peyron et al. 2000).
Hypocretin deficiency in human narcolepsy
Despite the lack of genetic abnormalities in the hypocretin system, the large majority (85%–90%) of patients with narcolepsy-cataplexy have low or undetectable hypocretin-1 ligand in their cerebrospinal fluid (Figure 1) (Nishino et al. 2000b, 2001b). This hypocretin deficiency is tightly associated with occurrence of cataplexy and HLA-DQ1*0602 positivity (Kanbayashi et al. 2002; Krahn et al. 2002; Mignot et al. 2002). Postmortem human studies, although using few brains, have confirmed hypocretin ligand deficiency (both hypocretin-1 and -2) in the narcoleptic brain (Figure 1) (Peyron et al. 2000; Thannickal et al. 2000). Hypocretin deficiency has also been observed in sporadic cases of canine narcolepsy (7 of 7 animals studied; the results of 4 cases are reported), suggesting that the pathophysiology in these animals mirrors that of most human cases (Ripley et al. 2001a).
The establishment of CSF hypocretin measurement as a new diagnostic tool in human narcolepsy is encouraging. Although positive predictive value is not 100%, low CSF hypocretin-1 levels are very specific for narcolepsy when compared with other sleep or neurologic disorders (Figure 2) (Kanbayashi et al. 2002; Mignot et al. 2002; Ripley et al. 2001b). Previously, no specific and sensitive diagnostic test for narcolepsy based on the pathophysiology of the disease was available, and the final diagnosis was often delayed for several years after the onset (typically during adolescence) of the disease (Alaila 1992). Many patients with narcolepsy and related EDS disorders are therefore likely to obtain immediate benefit from this new specific diagnostic test. In addition, hypocretin agonists may be promising in the treatment of narcolepsy.
Hypocretin/orexin and sleep physiology
Hypocretins/orexins were identified by two independent research groups in 1998, one year prior to the cloning of the canine narcolepsy gene. One group called the peptides hypocretin because of their primary hypothalamic localization and similarities with the hormone secretin (De Lecea et al. 1998). The other group called the molecules orexin after observing that central administration of these peptides increased appetite in rats (Sakurai et al. 1998). Hypocretin-1 and -2 are produced exclusively by a well-defined group of neurons localized in the lateral hypothalamus. The neurons project to the olfactory bulb, cerebral cortex, thalamus, hypothalamus, and brainstem, particularly the locus coeruleus (LC), raphe nucleus, and cholinergic nuclei and cholinoceptive sites (such as pontine reticular formation), thought to be important for sleep regulation (Peyron et al. 1998).
The hypocretin system is a major excitatory system that affects the activity of monoaminergic (dopamine, norepinephrine, serotonin, and histamine) and cholinergic systems with major effects on vigilance states (Taheri et al. 2002; Willie et al. 2001). It is therefore likely that a deficiency in hypocretin neurotransmission induces an imbalance between these classical neurotransmitter systems, with primary effects on sleep-state organization and vigilance. Indeed, dopamine and/or nor-epinephrine contents have been reported to be high in several brain structures in narcoleptic Dobermans and in human narcolepsy postmortem brains (Nishino and Mignot 1997). These changes in humans are possibly due to compensatory mechanisms, because drugs that enhance dopaminergic neurotransmission (such as amphetamine-like stimulants and modafinil for EDS) and norepinephrine neurotransmission (such as noradrenaline uptake blockers for cataplexy) are commonly used (Nishino and Mignot 1997).
Histamine is another monoamine implicated in the control of vigilance, and the histaminergic system is also likely to indirectly mediate the wake-promoting effects of hypocretin (Eriksson et al. 2001; Huang et al. 2001; Yamanaka et al. 2002). Interestingly, brain histamine contents both in hcrtr-2 gene–mutated and in ligand-deficient narcoleptic dogs are dramatically reduced (Nishino et al. 2001a). The involvement of the histaminergic system in the pathophysiology of narcolepsy and therapeutic applications of histaminergic compounds (Tedford et al. 1999) should be further studied.
Studies of animal hypocretin system function suggest that basic hypocretin neurotransmission fluctuates across the 24-hour period and slowly builds up during the active period (Fujiki et al. 2001; Yoshida et al. 2001; Zeitzer et al. 2003). Adrenergic LC neurons are typical wake-active neurons involved in vigilance control, and it has been recently demonstrated that basic firing activity of wake-active LC neurons also significantly fluctuates across various circadian times (Aston-Jones et al. 2001). Several acute manipulations, such as exercise, low glucose utilization in the brain, and forced wakefulness, increase hypocretin levels (Willie et al. 2001; Wu et al. 2002; Yoshida et al. 2001). It is therefore hypothesized that a buildup or acute increase of hypocretin levels may counteract the homeostatic sleep propensity that typically increases during the daytime and during forced wakefulness (Yoshida et al. 2001; Zeitzer et al. 2003). Because of the lack of increase in hypocretin tone, narcoleptic subjects may not be able to stay awake for a prolonged period and do not respond to various alerting stimuli. Conversely, the reduction of the hypocretin tone at sleep onset may contribute to the profound deep sleep that normally inhibits REM sleep at sleep onset, and the lack of this system in narcolepsy may allow the occurrence of REM sleep at sleep onset.
Summary
Excessive daytime sleepiness is a prevalent problem in medical practice and in society in general. It exacts a great cost in terms of quality of life, personal and public safety, and productivity. The causes of EDS are myriad, and a careful evaluation is needed to determine the cause in an individual case. Although much progress has been made in discovering the pathophysiology of narcolepsy, much more remains to be understood, and far less is known about other primary conditions of EDS. Several methods have been developed to assess EDS, although each of them has limitations. Treatment is available for the great majority of individuals with excessive daytime sleepiness.
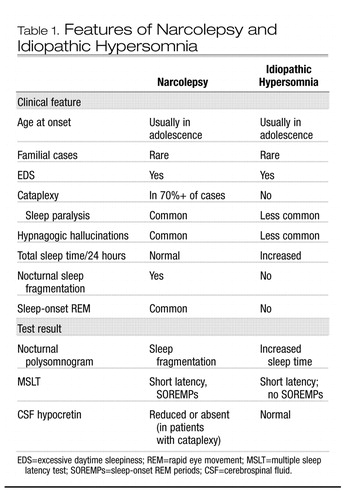 |
Table 1. Features of Narcolepsy and Idiopathic Hypersomnia
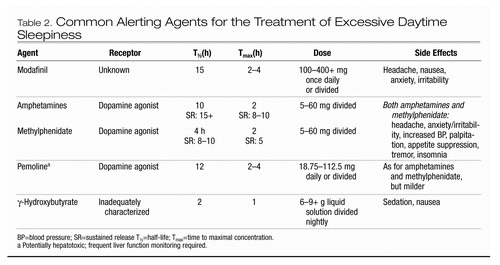 |
Table 2. Common Alerting Agents for the Treatment of Excessive Daytime Sleepiness
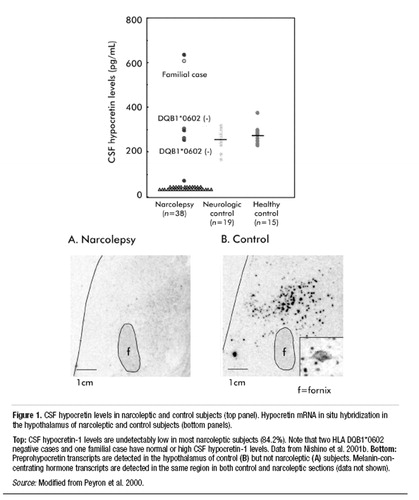
Figure 1. CSF hypocretin levels in narcoleptic and control subjects (top panel). Hypocretin mRNA in situ hybridization in the hypothalamus of narcoleptic and control subjects (bottom panels).
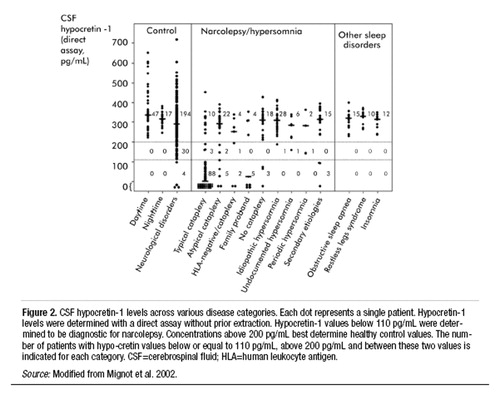
Figure 2. CSF hypocretin-1 levels across various disease categories. Each dot represents a single patient. Hypocretin-1 levels were determined with a direct assay without prior extraction. Hypocretin-1 values below 110 pg/mL were determined to be diagnostic for narcolepsy. Concentrations above 200 pg/mL best determine healthy control values. The number of patients with hypocretin values below or equal to 110 pg/mL, above 200 pg/mL and between these two values is indicated for each category. CSF=cerebrospinal fluid; HLA=human leukocyte antigen.
Alaila SL: Life effects of narcolepsy: measures of negative impact, social support and psychological well-being, in Psychosocial Aspects of Narcolepsy (Loss, Grief and Care Series, No. 3). Edited by Goswanmi M, Pollak CP, Cohen FL, et al. New York, Haworth, 1992, pp 1–22Google Scholar
Aldrich MS: The clinical spectrum of narcolepsy and idiopathic hypersomnia. Neurology 46:393–401, 1996Crossref, Google Scholar
Aldrich MS, Chervin RD, Malow BA: Value of the Multiple Sleep Latency Test (MSLT) for the diagnosis of narcolepsy. Sleep 20:620–629, 1997Google Scholar
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision. Washington, DC, American Psychiatric Publishing, 2000Google Scholar
American Sleep Disorders Association: International Classification of Sleep Disorders, Revised: Diagnostic and Coding Manual. Rochester, MN, American Sleep Disorders Association, 1997Google Scholar
Arias M, Crespo Iglesias JM, Perez J, et al: [Kleine-Levin syndrome: contribution of brain SPECT in diagnosis] (Spanish). Rev Neurol 35:531–533, 2002Google Scholar
Askenasy JJ: Sleep in Parkinson’s disease. Acta Neurol Scand 87:167–170, 1993Crossref, Google Scholar
Aston-Jones G, Chen S, Zhu Y, et al: A neural circuit for circadian regulation of arousal. Nature Neurosci 4:732–738, 2001Crossref, Google Scholar
Bamford CR: Menstrual-associated sleep disorder: an unusual hypersomniac variant associated with both menstruation and amenorrhea with a possible link to prolactin and metoclopramide. Sleep 16:484–486, 1993Crossref, Google Scholar
Bassetti C, Aldrich MS: Idiopathic hypersomnia: a series of 42 patients. Brain 120:1423–1435, 1997Crossref, Google Scholar
Beusterien KM, Rogers AE, Walsleben JA, et al: Health-related quality of life effects of modafinil for treatment of narcolepsy. Sleep 22:757–765, 1999Crossref, Google Scholar
Billiard M, Dauvilliers Y: Idiopathic hypersomnia. Sleep Med Rev 5:349–358, 2001Crossref, Google Scholar
Billiard M, Guilleminault C, Dement WC: A menstruation-linked periodic hypersomnia: Kleine-Levin syndrome or a new clinical entity? Neurology 25:436–443, 1975Crossref, Google Scholar
Billiard M, Laaberki MF, Reygrobellet C, et al: Elevated antibodies to streptococcal antigens in narcoleptic subjects (abstract). Sleep Res 18:201, 1989Google Scholar
Bishop C, Rosenthal L, Helmus T, et al: The frequency of multiple sleep onset REM periods among subjects with no excessive daytime sleepiness. Sleep 19:727–730, 1996Crossref, Google Scholar
Broughton R, Valley V, Aguirre M, et al: Excessive daytime sleepiness and pathophysiology of narcolepsy-cataplexy: a laboratory perspective. Sleep 9:105–215, 1986Google Scholar
Bruck D, Parkes JD: A comparison of idiopathic hypersomnia and narcolepsy-cataplexy using self report measures and sleep diary data. J Neurol Neurosurg Psychiatr 60:576–578, 1996Crossref, Google Scholar
Chemelli RM, Willie JT, Sinton CM, et al: Narcolepsy in orexin knockout mice: molecular genetics of sleep regulation. Cell 98:437–451, 1999Crossref, Google Scholar
Chokroverty S: Sleep and degenerative neurologic disorders. Neurol Clin 14:807–826, 1996Crossref, Google Scholar
Critchley M: [The syndrome of hypersomnia and periodical megaphagia in the adult male (Kleine-Levin): what is its natural course?] (French). Rev Neurol (Paris) 116:647–650, 1967Google Scholar
De Lecea L, Kilduff TS, Peyron C, et al: The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proc Natl Acad Sci USA 95:322–327, 1998Crossref, Google Scholar
Dement W, Rechtschaffen A, Gulevich G: The nature of the narcoleptic sleep attack. Neurology 16:18–33, 1966Crossref, Google Scholar
Eriksson KS, Sergeeva O, Brown RE, et al: Orexin/hypocretin excites the histaminergic neurons of the tuberomammillary nucleus. J Neurosci 21:9273–9279, 2001Crossref, Google Scholar
Faull KF, Guilleminault C, Berger PA, et al: Cerebrospinal fluid monoamine metabolites in narcolepsy and hypersomnia. Ann Neurol 13:258–263, 1983Crossref, Google Scholar
Faull KF, Thiemann S, King RJ, et al: Monoamine interactions in narcolepsy and hypersomnia: a preliminary report. Sleep 9:246–249, 1986Crossref, Google Scholar
Francisco GE, Ivanhoe CB: Successful treatment of posttraumatic narcolepsy with methylphenidate: a case report. Am J Phys Med Rehabil 75:63–65, 1996Crossref, Google Scholar
Fredrikson S, Carlander B, Billiard M, et al: CSF immune variables in patients with narcolepsy. Acta Neurol Scand 81:253–254, 1990Crossref, Google Scholar
Fujiki N, Yoshida Y, Ripley B, et al: Changes in CSF hypocretin-1 (orexin A) levels in rats across 24 hours and in response to food deprivation. Neuroreport 12:993–997, 2001Crossref, Google Scholar
Fukuda K, Miyasita A, Inugami M, et al: High prevalence of isolated sleep paralysis: Kanashibari phenomenon in Japan. Sleep 10:279–286, 1987Crossref, Google Scholar
Gelb M, Guilleminault C, Kraemer H, et al: Stability of cataplexy over several months: information for the design of therapeutic trials. Sleep 17:265–273, 1994Crossref, Google Scholar
George CFP: Neuromuscular disorders, in Principles and Practice of Sleep Medicine, 3rd Edition. Edited by Kryger MH, Roth T, Dement WC. Philadelphia, PA, WB Saunders, 2000, pp 1087–1092Google Scholar
Gibbs JW 3rd, Ciafaloni E, Radtke RA: Excessive daytime somnolence and increased rapid eye movement pressure in myotonic dystrophy. Sleep 25:672–675, 2002Google Scholar
Guilleminault C, Mondini S: Mononucleosis and chronic daytime sleepiness: a long-term follow-up study. Arch Intern Med 146:1333–1335, 1986Crossref, Google Scholar
Guilleminault C, Wilson RA, Dement WC: A study on cataplexy. Arch Neurol 31:255–261, 1974Crossref, Google Scholar
Guilleminault C, Stoohs R, Clerk A, et al: A cause of excessive daytime sleepiness: the upper airway resistance syndrome. Chest 104:781–787, 1993Crossref, Google Scholar
Honda Y: Clinical features of narcolepsy: Japanese experiences, in HLA in Narcolepsy. Edited by Honda Y, Juji T. Berlin, Springer-Verlag, 1988, pp 24–57Google Scholar
Huang ZL, Qu WM, Li WD, et al: Arousal effect of orexin A depends on activation of the histaminergic system. Proc Natl Acad Sci USA 98:9965–9970, 2001Crossref, Google Scholar
Johns MW: A new method for measuring daytime sleepiness: the Epworth Sleepiness Scale. Sleep 14:540–545, 1991Crossref, Google Scholar
Juji T, Satake M, Honda Y, et al: HLA antigens in Japanese patients with narcolepsy: all the patients were DR2 positive. Tissue Antigens 24:316–319, 1984Crossref, Google Scholar
Kanbayashi T, Inoue Y, Chiba S, et al: CSF hypocretin-1 (orexin-A) concentrations in narcolepsy with and without cataplexy and idiopathic hypersomnia. J Sleep Res 11:91–93, 2002Crossref, Google Scholar
Krahn LE, Pankratz VS, Oliver L, et al: Hypocretin (orexin) levels in cerebrospinal fluid of patients with narcolepsy: relationship to cataplexy and HLA DQB1*0602 status. Sleep 25:733–736, 2002Crossref, Google Scholar
Lin L, Faraco J, Li R, et al: The sleep disorder canine narcolepsy is caused by a mutation in the hypocretin (orexin) receptor 2 gene. Cell 98:365–376, 1999Crossref, Google Scholar
Lugaresi E, Montagna P, Tinuper P, et al: Endozepine stupor: recurring stupor linked to endozepine-4 accumulation. Brain 121:127–133, 1998Crossref, Google Scholar
Mamelak M, Black J, Montplaisir J, et al: A pilot study on the effects of sodium oxybate on sleep architecture and daytime alertness in narcolepsy. Sleep 27:1327–1334, 2004Crossref, Google Scholar
Manni R, Tartara A: Evaluation of sleepiness in epilepsy. Clin Neurophysiol 111 (suppl 2): S111–S114, 2000Crossref, Google Scholar
Matsuki K, Juji T, Honda Y: Immunological features of narcolepsy in Japan, in HLA in Narcolepsy. Edited by Honda Y, Juji T. Berlin, Springer-Verlag, 1988, pp 150–157Google Scholar
Mignot E: Genetic and familial aspects of narcolepsy. Neurology 50 (suppl 1):S16–S22, 1998Crossref, Google Scholar
Mignot E, Wang C, Rattazzi C, et al: Genetic linkage of autosomal recessive canine narcolepsy with a mu immunoglobulin heavy-chain switch-like segment. Proc Natl Acad Sci USA 88:3475–3478, 1991Crossref, Google Scholar
Mignot E, Guilleminault C, Grumet FC, et al: Is narcolepsy an autoimmune disease? In Proceedings of the Third Milano International Symposium, September 18–19, “Sleep, Hormones, and the Immune System.” Edited by Smirne S, Francesci M, Ferini-Strambi L, Zucconi M. Milan, Masson, 1992, pp 29–38Google Scholar
Mignot E, Hayduk R, Black J, et al: HLA Class II studies in 509 narcoleptic patients. Sleep Res 26:433, 1997Google Scholar
Mignot E, Lammers GJ, Ripley B, et al: The role of cerebrospinal fluid hypocretin measurement in the diagnosis of narcolepsy and other hypersomnias. Arch Neurol 59:1553–1562, 2002Crossref, Google Scholar
Mitler MM, Aldrich MS, Koob GF, et al: Narcolepsy and its treatment with stimulants: ASDA standards of practice. Sleep 17:352–371, 1994Google Scholar
Montplaisir J, Poirier G: HLA in disorders of excessive sleepiness without cataplexy in Canada, in HLA in Narcolepsy. Edited by Honda Y, Juji T. Berlin, Springer-Verlag, 1988, pp 186–190Google Scholar
Montplaisir J, De Champlain J, Young SN, et al: Narcolepsy and idiopathic hypersomnia: biogenic amines and related compounds in CSF. Neurology 32:1299–1302, 1982Crossref, Google Scholar
Montplaisir J, Poirier G, Lapierre O, et al: Streptococcal antibodies in narcolepsy and idiopathic hypersomnia. Sleep Res 18:271, 1989Google Scholar
National Sleep Foundation: Sleep in America Poll 2002. Available at: http://www.sleepfoundation.org/2002poll.cfm. Accessed March 2005.Google Scholar
National Sleep Foundation: Sleep in America Poll 2003. Available at: http://www.sleepfoundation.org/2003poll.cfm. Accessed March 2005.Google Scholar
Nishino S, Mignot E: Pharmacological aspects of human and canine narcolepsy. Prog Neurobiol 52:27–78, 1997Crossref, Google Scholar
Nishino S, Riehl J, Hong J, et al: Is narcolepsy REM sleep disorder? Analysis of sleep abnormalities in narcoleptic Dobermans. Neurosci Res 38:437–446, 2000aCrossref, Google Scholar
Nishino S, Ripley B, Overeem S, et al: Hypocretin (orexin) deficiency in human narcolepsy. Lancet 355:39–40, 2000bCrossref, Google Scholar
Nishino S, Fujiki N, Ripley B, et al: Decreased brain histamine contents in hypocretin/orexin receptor-2 mutated narcoleptic dogs. Neurosci Lett 313:125–128, 2001aCrossref, Google Scholar
Nishino S, Ripley B, Overeem S, et al: Low CSF hypocretin (orexin) and altered energy homeostasis in human narcolepsy. Ann Neurol 50:381–388, 2001bCrossref, Google Scholar
Ohayon MM, Priest RG, Caulet M, et al: Hypnagogic and hypnopompic hallucinations: pathological phenomena? Br J Psychiatry 169:459–467, 1996Crossref, Google Scholar
Ohayon MM, Priest RG, Zulley J, et al: Prevalence of narcolepsy symptomatology and diagnosis in the European general population. Neurology 58:1826–1833, 2002Crossref, Google Scholar
Overeem S, Mignot E, van Dijk JG, et al: Narcolepsy: clinical features, new pathophysiological insights, and future perspectives. J Clin Neurophysiol 18:78–105, 2001Crossref, Google Scholar
Peyron C, Tighe DK, van den Pol AN, et al: Neurons containing hypocretin (orexin) project to multiple neuronal systems. J Neurosci 18:9996–10015, 1998Crossref, Google Scholar
Peyron C, Faraco J, Rogers W, et al: A mutation in a case of early onset narcolepsy and a generalized absence of hypocretin peptides in human narcoleptic brains. Nat Med 6:991–997, 2000Crossref, Google Scholar
Reynolds CF 3rd, Coble PA, Kupfer DJ, et al: Application of the Multiple Sleep Latency Test in disorders of excessive sleepiness. Electroencephalogr Clin Neurophysiol 53:443–452, 1982Crossref, Google Scholar
Ripley B, Fujiki N, Okura M, et al: Hypocretin levels in sporadic and familial cases of canine narcolepsy. Neurobiol Dis 8:525–534, 2001aCrossref, Google Scholar
Ripley B, Overeem S, Fujiki N, et al: CSF hypocretin levels in various neurological conditions: low levels in narcolepsy and Guillain-Barre syndrome (abstract). Sleep 24:A322, 2001bGoogle Scholar
Rosenow F, Kotagal P, Cohen BH, et al: Multiple Sleep Latency Test and polysomnography in diagnosing Kleine-Levin syndrome and periodic hypersomnia. J Clin Neurophysiol 17:519–522, 2000Crossref, Google Scholar
Roth B: Narcolepsy and hypersomnia: review and classification of 642 personally observed cases. Schweiz Arch Neurol Neurochir Psychiatr 119:31–41, 1976Google Scholar
Roth B, Nevsimalova S, Rechtschaffen A: Hypersomnia with “sleep drunkenness.” Arch Gen Psychiatry 26:456–462, 1972Crossref, Google Scholar
Rothstein JD, Guidotti A, Tinuper P, et al: Endogenous benzodiazepine receptor ligands in idiopathic recurring stupor. Lancet 340:1002–1004, 1992Crossref, Google Scholar
Sachs C, Persson H, Hagenfeldt K: Menstruation-associated periodic hypersomnia: a case study with successful treatment. Neurology 32:1376–1379, 1982Crossref, Google Scholar
Sakurai T, Amemiya A, Ishii M, et al: Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell 92:573–585, 1998Crossref, Google Scholar
Sforza E, Gaudreau H, Petit D, et al: Homeostatic sleep regulation in patients with idiopathic hypersomnia. Clin Neurophysiol 111:277–282, 2000Crossref, Google Scholar
Soriani S, Carrozzi M, De Carlo L, et al: Endozepine stupor in children. Cephalalgia 17:658–661, 1997Crossref, Google Scholar
Taheri S, Zeitzer JM, Mignot E: The role of hypocretins (orexins) in sleep regulation and narcolepsy. Annu Rev Neurosci 25:283–313, 2002Crossref, Google Scholar
Tedford CE, Edgar DM, Seidel WF, et al: Effects of a novel, selective, and potent histamine H3 receptor antagonist, GT-2332, on rat sleep/wakefulness and canine cataplexy (abstract). Abstr Soc Neurosci 25:1134, 1999Google Scholar
Thannickal TC, Moore RY, Nienhuis R, et al: Reduced number of hypocretin neurons in human narcolepsy. Neuron 27:469–474, 2000Crossref, Google Scholar
Toth LA, Opp MR: Sleep and infection, in Sleep Medicine. Edited by Lee-Chiong TL, Sateia MJ, Carskadon MA. Philadelphia, PA, Hanley and Belfus, 2002, pp 77–83Google Scholar
Trenkwalder C: Sleep dysfunction in Parkinson’s disease. Clin Neurosci 5:107–114, 1998Google Scholar
Tunnicliff G, Cash CD: Gamma-hydroxybutyrate: Molecular, Functional and Clinical Aspects. New York, Taylor and Francis, 2002Google Scholar
Tylee A, Gastpar M, Lepine JP, et al: DEPRES II (Depression Research in European Society II): a patient survey of the symptoms, disability and current management of depression in the community. DEPRES Steering Committee. Int Clin Psychopharmacol 14:139–151, 1999Google Scholar
US Modafinil in Narcolepsy Multicenter Study Group: Randomized trial of modafinil for the treatment of pathological somnolence in narcolepsy. Ann Neurol 43:88–97, 1998Crossref, Google Scholar
US Modafinil in Narcolepsy Multicenter Study Group: Randomized trial of modafinil as a treatment for the excessive daytime somnolence of narcolepsy. Neurology 54:1166–1175, 2000Crossref, Google Scholar
US Xyrem in Narcolepsy Multi-center Study Group: A randomized, double blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep 25:42–49, 2002Crossref, Google Scholar
Willie JT, Chemelli RM, Sinton CM, et al: To eat or to sleep? Orexin in the regulation of feeding and wakefulness. Annu Rev Neurosci 24:429–458, 2001Crossref, Google Scholar
Wu MF, John J, Maidment N, et al: Hypocretin release in normal and narcoleptic dogs after food and sleep deprivation, eating, and movement. Am J Physiol Regul Integr Comp Physiol 283:R1079–R1086, 2002Crossref, Google Scholar
Yamanaka A, Tsujino N, Funahashi H, et al: Orexins activate histaminergic neurons via the orexin 2 receptor. Biochem Biophys Res Commun 290:1237–1245, 2002Crossref, Google Scholar
Yoshida Y, Fujiki N, Nakajima T, et al: Fluctuation of extracellular hypocretin-1 (orexin A) levels in the rat in relation to the light-dark cycle and sleep-wake activities. Eur J Neurosci 14:1075–1081, 2001Crossref, Google Scholar
Zeitzer JM, Buckmaster CL, Parker KJ, et al: Circadian and homeostatic regulation of hypocretin in a primate model: implications for the consolidation of wakefulness. J Neurosci 23:3555–3560, 2003Crossref, Google Scholar



