Cognitive Dysfunction in Major Depressive Disorder: Assessment, Impact, and Management
Abstract
Cognitive dysfunction is increasingly being recognized as an important clinical dimension in major depressive disorder. This review summarizes the existing data on the epidemiology, assessment, and treatment of cognitive dysfunction among nonelderly adults with the disorder. Overall, cognitive dysfunction is prevalent, persists through periods of symptom remission, and may be independently associated with functional outcomes. However, although the evidence increasingly suggests that clinicians should be heedful of their patients’ cognitive functioning, there is as yet no consensus on how best to monitor cognition clinically. In addition, although most studies have reported improved cognition with antidepressant medications, psychotherapy, and neuromodulation, the clinical significance of these improvements is unclear, and high-level evidence to guide decision making is limited. Nonetheless, given the important functional implications, clinicians should assess and monitor cognition and optimize both medication and psychological treatments to mitigate cognitive dysfunction among patients with major depressive disorder.
Definition
Cognition, the process of acquiring knowledge and understanding, is reciprocally connected to emotions (1). It has long been recognized that depression, conceptualized as an emotional disorder, has a cognitive component; decreased concentration and focus are listed as core symptoms of major depressive disorder (2). It is also well established that major depressive disorder may result in pronounced cognitive dysfunction among elderly adults (3, 4). However, a large body of research now shows that, even among younger age groups, depression affects cognition in multiple domains. This review focuses on the epidemiology, clinical manifestations, and management of cognitive dysfunction among nonelderly adults with major depressive disorder, which may have pathophysiology and treatment implications distinct from those of late-life depression (4–6).
Cognition is a multifaceted construct with a number of constituent domains. In studies involving psychiatric patients, these domains are generally classified as psychomotor speed, attention, working memory, declarative memory (verbal and visual), and executive functioning (including planning, decision making, response inhibition, and cognitive flexibility) (7–10) (Table 1). The delineation of these domains can vary between studies; for example, cognitive control may be included as a component of executive functioning or defined as a separate category (11).
| Domain | Description | Representative Tests |
|---|---|---|
| Attention | Encompasses ability to focus, maintain, and shift attention | Continuous Performance Test–Identical Pairs |
| Processing speed | Ability to rapidly perceive or respond to stimuli | Trail Making Test Part A; Digit Symbol Substitution Test; Category Fluency: Animal Naming and Phonetic Fluency |
| Working memory | Temporary storage and manipulation of information | Spatial Span subtest (WMS-III); Letter-number sequencing (WMS-III); Digit Span Forward and Backward (WAIS-R); Spatial Working Memory (CANTAB); n-Back Test |
| Verbal learning and memory | Encompasses learning, encoding, retention, and retrieval of verbal information | California Verbal Learning Test–Revised; Hopkins Verbal Learning Test–Revised; Rey Auditory Verbal Learning Test |
| Visual learning and memory | Ability to retain and retrieve visual images | Brief Visuospatial Memory Test–Revised; Rey-Osterrieth Complex Figure Test; Visual Memory Index; Pattern Recognition Memory (CANTAB); Spatial Recognition Memory (CANTAB); Delayed Matching to Sample (CANTAB); Paired Associates Learning (CANTAB); |
| Executive functioning | Ability to plan, problem solve, and inhibit affectively charged or inappropriate responses | Wisconsin Card Sorting Test; Trail-Making Test Part B; Stroop Color-Word Interference Test; Tower of London (CANTAB); Controlled Oral Word Association Test; Stockings of Cambridge (CANTAB); Intra/Extradimensional Shift Test (CANTAB) |
Table 1. Commonly Assessed Cognitive Domains and Representative Neuropsychological Tests in Major Depressive Disorder Studiesa
Functioning within these cognitive domains is assessed through subjective or objective measures. Subjective assessments rely on patients’ own evaluations of their cognitive capacity and are measured with patient-rated scales. Objective assessments are based on standard neuropsychological tests (Table 1). However, there can be overlap among and disagreement around which cognitive functions a test is measuring, and in reality most tests require the integration of multiple domains (11). For example, the Trail Making Test B and Stroop task can be conceptualized as tests of executive functioning, processing speed, or both (12). Subjective cognitive complaints and objective performance are poorly correlated and thus provide complementary, rather than overlapping, information regarding a patient’s cognitive status (see Assessment and Differential Diagnosis section) (13).
Specifically relevant to depression is the classification of cognitive dysfunction as hot or cold. “Hot cognition” refers to affective biases toward negative stimuli and self-evaluation that can have an impact on working memory and attention; “cold cognition” refers to deficits in executive functioning, attention, and memory that are independent of emotion (2, 14–16). Both types of dysfunction appear to be present in major depressive disorder, and each may affect either subjective or objective cognitive performance (12).
Epidemiology and Natural History
Prevalence of Cognitive Dysfunction in Depression
Data regarding the prevalence of cognitive dysfunction in major depressive disorder have been heterogeneous, perhaps because of a lack of uniformity among the patient groups examined (i.e., symptom severity, depressive subtype, comorbid conditions) and cognitive tests used (14). However, the growing consensus is that objective cognitive impairment is present in acute episodes of major depressive disorder and persists during periods of remission (17). Although elderly patients have more severe deficits, nonelderly adults also display objective decrements in executive functioning, attention, and processing speed in acute depression and small to moderate deficits in these cognitive domains when euthymic (18–23). In addition, a large subset of adult patients may meet criteria—for example, scoring less than two standard deviations below a normative age- and gender-matched mean—for cognitive impairment. In one study of 285 patients with major depressive disorder, 38% were impaired in one cognitive domain and 20% in two or more domains on the basis of these criteria (24).
Subjective cognitive complaints are also highly prevalent in major depressive disorder. Nearly 90% of acutely depressed patients have self-reported difficulties with concentration and focus (25, 26), and while in remission patients experience ongoing problems with poor focus and concentration 44% of the time (27).
Impact of Cognitive Functioning on Clinical and Functional Outcomes
Although the literature investigating the relationship between cognition and psychosocial outcomes is limited by small studies and lack of prospective designs, evidence that cognitive dysfunction is associated with poorer occupational and psychosocial functioning is growing (8, 14, 28, 29). Subjective decrements in concentration and attention, and embarrassment related to these perceived deficits, is a major mediator of perceived decreased role functioning among patients with depression (28). Objective cognitive measures were associated with overall levels of functioning among patients six months after hospital discharge, and objective visuospatial, language, and total cognitive performance was lower among patients in remission who remained unemployed than among those who were able to resume work (30, 31). Although further research is needed to examine the mediating effects of cognition on functioning, existing data have suggested that targeting cognitive dysfunction can improve overall outcomes among patients recovering from depression.
The presence of cognitive dysfunction may also be negatively associated with treatment response and represent a risk factor for relapse (14, 32, 33). A meta-analysis of 17 studies found that baseline performance on certain tests of executive function, processing speed, and verbal memory significantly differentiated between patients who did and did not respond to antidepressants, although the authors noted that their results were limited by the heterogeneity between studies (34). Cognitive, and particularly executive, functioning may also significantly relate to suicide risk. Executive dysfunction was found among depressed patients with high-lethality suicide attempts but not among depressed patients with no history of suicidality (35, 36); similarly, patients with suicidal ideation have impaired executive functioning particularly with regard to decision making, problem solving, and mental flexibility (37–39).
Course of Cognitive Dysfunction in Depression
Cognitive dysfunction may precede the onset of a depressive episode and represent a premorbid trait marker for major depressive disorder. Baseline episodic memory performance among a large community-based cohort was associated with higher risk of developing depressive symptoms, as were lower executive functioning and attention among 234 high-risk subjects followed over 9 years (33, 40, 41). Numerous studies have also detected cognitive deficits among patients experiencing their first episode of depression, suggesting that this may be a target for intervention early in the disease course. A meta-analysis of 15 studies of first-episode patients found deficits in psychomotor speed, attention, visual memory, attentional switching, verbal fluency, and cognitive flexibility, with small to moderate effect sizes (42).
Although cognitive dysfunction is present at, or possibly prior to, illness onset, it is unclear whether cognition worsens with an increased number of episodes or illness duration. Many studies have not found a correlation between illness duration and cognition, and in the above-mentioned meta-analysis of euthymic patients, these variables were not associated with cognitive performance (19, 43–45). Conversely, a large study of 1,140 patients with major depressive disorder found a significant negative correlation between performance of attentional and executive functioning and number of previous episodes. Similarly, episodic memory performance among 8,229 outpatient treatment responders was negatively correlated with previous episodes and illness duration (46, 47). Thus, although the longitudinal course of cognition in depression still requires further study, it is plausible that preventing recurrence could potentially ameliorate cognitive deterioration.
Moderators of Cognitive Dysfunction in Depression
The clinical setting and patient characteristics can affect the magnitude and prevalence of cognitive impairment. For example, younger, medically healthy patients with depression treated in an outpatient setting—such as those typically seen in primary care—may have only mild or minimal cognitive dysfunction (48, 49). Conversely, hospitalized patients with severe symptoms or psychotic or melancholic features will exhibit more severe deficits (42, 50–55). Comorbid anxiety may additionally impair executive functioning, memory, and psychomotor speed, although comorbid anxiety appears to have a less significant impact on cognition of younger outpatients with mild to moderate symptoms (56–58). The impact of other comorbid psychiatric conditions has not been well studied.
Comorbid medical conditions can also exert a significant effect on cognition. Metabolic disturbances such as obesity and diabetes contribute to neurocognitive decline in major depressive disorder (59, 60). Similarly, sleep disturbance can have significant adverse consequences for cognition (61).
Biopsychosocial Underpinnings
Neurobiological
Cognition requires the parallel and integrated functioning of circuits involving the prefrontal cortex, parietal cortex, basal ganglia, thalamus, hippocampus, and amygdala (1). Changes in some of these regions have been identified in depression and linked to cognitive functioning. One of the most consistently implicated regions is the hippocampus, a key structure mediating learning and declarative memory. Reduction in hippocampal volumes has consistently been observed among patients with depression (62–64), and studies have found an inverse correlation between hippocampal volume and declarative memory and functional outcomes (65). The hippocampus is also a hub for the integration of other emotional and cognitive functions, and hippocampal dysfunction has been linked to poorer executive functioning in depression (1, 66–69).
Patients with depression also appear to have deficits in the functional connectivity and activation of networks involved in cognitive control—the ability to use working memory, cognitive flexibility, planning, and problem solving to achieve internal behavioral goals (70). Cognitive control requires the coordinated activity of brain networks involved in detecting and processing relevant stimuli, providing top-down inhibitory control and self-referential processing (see Figure 1) (71–75). Dysfunctional connectivity between these networks results in increased negative, self-focused rumination and decreased engagement of brain regions required to complete the task at hand (76–79).
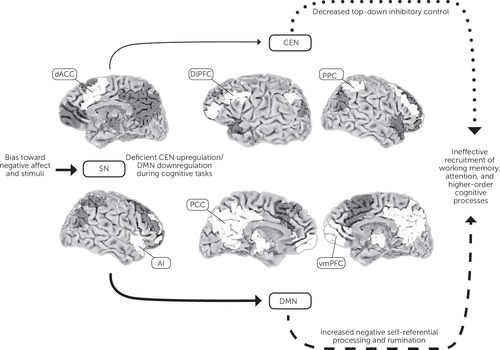
Figure 1. Proposed Network Model of Cognitive Dysfunction in Major Depressive Disordera
a Abnormal recruitment and connectivity of the SN (consisting of dACC and AI), CEN (consisting of dlPFC and PPC), and DMN (consisting of PCC and vmPFC) contribute to hot (emotion dependent, indicated with dashed arrow) and cold (emotion independent, indicated with dotted arrow) cognitive dysfunction in major depressive disorder. The SN, responsible for detecting and filtering relevant information, is disproportionately affected by negative stimuli and emotional perception. The SN in major depressive disorder is also less efficient in mediating the anticorrelated activation of the CEN and DMN during cognitive tasks. Subsequent DMN overactivity results in increased negative self-referential processing and rumination that distracts from task-relevant stimuli, and the underactive CEN is less able to provide top-down inhibitory control. The net result is less efficient recruitment of networks involved in attention, working memory, and higher-order processes necessary for successful completion of cognitive tasks. SN, salience network; dACC, dorsal anterior cingulate cortex; AI, anterior insula; CEN, central executive network; dlPFC, dorsolateral prefrontal cortex; PPC, posterior parietal cortex; DMN, default mode network; PCC, posterior cingulate cortex; vmPFC, ventromedial prefrontal cortex. Adapted from Hamilton et al. (77) and Menon (74).
Stress and the Hypothalamic-Pituitary-Adrenal Axis
Activation of the hypothalamic-pituitary-adrenal (HPA) axis past a certain optimal level has a deleterious effect on cognition in general and memory encoding in particular (1). Stress in early life has been linked to increased vulnerability to affective disorders and cognitive deficits in adulthood (80). In addition, increased levels of cortisol appear to result in the preferential encoding of negative rather than neutral or positive memories (80–82), and chronic stress and hypercortisolemia cause hippocampal atrophy and also inhibit hippocampal neurogenesis (83).
HPA hyperactivity and hypercortisolemia among patients with major depressive disorder may contribute to cognitive dysfunction (84). Most studies have found an association between basal cortisol levels and cognition in major depressive disorder (85), and a higher level of HPA activity among patients whose depression had remitted has been linked with poorer cognition when euthymic (80, 86, 87). Psychosocial stress, which is a common antecedent of depressive episodes, may therefore play a role in perpetuating cognitive dysfunction, and depressed patients who experienced early childhood stress appear to manifest greater cognitive deficits (1, 80). In addition, HPA axis dysregulation may mediate the link between obesity and metabolic disturbances and cognitive deficits in major depressive disorder (59).
Monoamine Modulation
Disturbances in norepinephrine, dopamine, and serotonin activity have been linked to cognitive dysfunction (15, 88, 89). The serotonergic system modulates learning, memory, and cognitive flexibility (90). Acute tryptophan depletion, an experimental modality that reduces central serotonergic activity and putatively models serotonergic functioning in depression, impairs episodic memory consolidation among both healthy and clinical populations (7). Similarly, sensitive regulation of norepinephrine and dopamine activity in the prefrontal cortex is necessary for attention, working memory, and executive functioning. Decreased activity of these neurotransmitters may underlie some of the cognitive deficits seen in major depressive disorder (91–95). Because most pharmacological treatments for depression aim to improve monoamine functioning in some regard, they may also help to enhance cognition.
Cognitive Schemas and Negative Affective Bias
Cognitive schemas directing attention and memory to negative themes have been conceptualized as a core feature of major depressive disorder (81, 96). This negative affective bias is believed to contribute to hot cognitive dysfunction because self-deprecatory ruminations interfere with memory among patients with major depressive disorder (81). Targeting these cognitive distortions and improving conscious emotional regulation may therefore have positive effects on cognition.
Assessment and Differential Diagnosis
Given the persistence of cognitive dysfunction in major depressive disorder during both active and remitted states, and its relationship to functional outcomes, serial assessment of cognition is important even in periods of euthymia (97). However, there are no guidelines addressing how such clinical monitoring should be undertaken (32, 98). Efforts to develop a standardized cognitive battery to be used in clinical trials with patients with schizophrenia have identified five criteria for an appropriate clinical test, including test–retest reliability, utility as a repeated measure, potential response to pharmacological agents, practicality for clinical use, and relationship to functional outcome (10). Currently, the MATRICS Consensus Cognitive Battery (MCCB) for schizophrenia, which consists of 10 tests that putatively meet these criteria, has been validated for use in clinical trials (99). Although efforts are underway to adapt and validate this battery for affective disorders, there is currently no consensus on what constitutes an appropriate cognitive assessment in major depressive disorder (98, 100).
Although the MATRICS criteria provide a useful guide for selection of clinical cognitive tests, such objective tests can still be difficult to administer clinically. The MCCB, for example, requires at least 65 minutes to complete, as well as some degree of training to administer and interpret (99). Briefer and more clinician-friendly objective batteries are in the process of being validated for depression (such as the THINC-it screening tool; www.THINCcognition.com), but the options are currently limited in terms of objective tests that are feasible for clinical use. Common bedside screening tests such as the Montreal Cognitive Assessment (101) have not been evaluated in clinical studies of cognitive dysfunction among adults with depression; the Mini-Mental State Examination has only been studied with elderly patients (102, 103). Even among an elderly population, extreme ceiling effects may preclude the Mini-Mental State Examination from capturing the full breadth of cognitive deficits found in major depressive disorder.
Evaluation of subjective cognitive functioning may be more feasible in busy clinical settings. However, commonly used depression symptom rating scales such as the Montgomery-Åsberg Depression Rating Scale, Beck Depression Inventory, Patient Health Questionnaire, and Quick Inventory of Depressive Symptomatology include only single items related to patients’ perceived difficulties with concentration or indecisiveness (104–107) and may not provide adequate assessment of all relevant cognitive domains. There are many specific cognitive assessment scales, but most have been developed to detect dementia, and few have been studied with depressed patients. Table 2 summarizes the three self-report scales that have been psychometrically validated for major depressive disorder (108–110).
| Study | Scale | No. of Items | Validation Sample |
|---|---|---|---|
| Iverson and Lam (108) | British Columbia Cognitive Complaints Inventory | 6 | Major depressive disorder, N=62; healthy, N=112 |
| Fava et al. (109) | Cognitive and Physical Functioning Questionnaire | 7 | Major depressive disorder, N=150 and N=50; generalized anxiety disorder, N=381 |
| Lam et al. (110) | Perceived Deficits Questionnaire–Depression | 20 (also 5-item version) | Major depressive disorder, N=400; healthy, N=400 |
Table 2. Self-Report Cognitive Assessments That Have Been Psychometrically Validated in Major Depressive Disorder
It should be noted that the high degree of discordance between subjective cognitive scales and objective performance necessitates the systematic assessment of both (8, 14). Objective deficits tend to be more independent from symptom severity than subjective complaints and thus require continued assessment through active treatment and remission (111–113). Subjective cognitive complaints, however, provide a better understanding of patients’ perceived capacities and skills; for example, a negatively distorted self-perception can contribute to hot cognitive dysfunction and may be a therapeutic target in and of itself. Evaluation of subjective complaints may also detect a decline from a patient’s individual baseline that objective testing may not capture (11).
Patients also require assessment for other conditions that may have a negative impact on cognition (see Epidemiology and Natural History section). These conditions include comorbid physical conditions, such as sleep, cardiovascular, and metabolic disorders; comorbid psychiatric conditions such as anxiety, attention-deficit hyperactivity disorder (ADHD), and substance use; and medication side effects (e.g., side effects resulting from anticholinergic or benzodiazepine use) (8, 16, 17, 59, 60).
Treatment and Outcomes
There are currently no approved treatments specifically for cognitive dysfunction in major depressive disorder (103), but numerous studies have examined the effect of standard treatments for depression—such as pharmacotherapy, psychotherapy, and neuromodulation—on cognition.
Antidepressant Monotherapy
There is limited good-quality evidence regarding the effects of antidepressants on cognition among adults because a substantial portion of the literature consists of small, open-label studies (Table 3) (114–129). Results are heterogeneous as a result of differences in patient characteristics as well as variability in the cognitive measures used (103, 130). The magnitude of improvement is also questionable; although the majority of studies have reported statistically significant improvements in cognition with antidepressant treatment, a pooled analysis showed the effect sizes were small (103). A meta-analysis exclusive to randomized controlled trials (RCTs; encompassing nine RCTs including both elderly and nonelderly adults) found statistically significant but small improvements in delayed recall (d=.24) and psychomotor speed (d=.16) across all age groups (130), but most of the effect was attributed to studies of vortioxetine (see below). Separate analysis of early-onset patients was possible only for psychomotor speed and showed an overall moderate benefit (130).
| Medication | Studies | Summary of Evidenceb | Description of Studies |
|---|---|---|---|
| SSRI | |||
| Paroxetine | Ferguson et al. (114), Gorlyn et al. (115), Deuschle et al. (116), Nickel et al. (117) | 0*/+/+/+ | DBRCT (N=23, 20–40 mg/day); did not separate from placebo. Three trials found improvement compared with baseline (Ns=30, 24, and 44) |
| Escitalopram | Herrera-Guzmán et al. (118, 119), Soczynska et al. (120) | +/+ | Improvements in objective cognition compared with baseline performance and untreated controls (Ns=36 and 19). |
| Sertraline | Constant et al. (121) | + | Improvements in attention, executive function, and psychomotor speed (N=20) |
| Fluoxetine | Chang et al. (122), Richardson et al. (123), Levkovitz et al. (124) | +/+/+ | Three trials showing improvement in objective cognition compared with baseline (Ns=73, 18, and 8) |
| SNRI | |||
| Duloxetine | Herrera-Guzmán et al. (118, 119), Mahableshwarkar et al. (125) | 0*/+ | Improved memory, attention, and executive function compared with baseline in one sample (N=37). DBRCT (N=176, 60 mg/day) showed improved subjective cognition but no difference in processing speed and functional capacity compared with placebo. |
| Venlafaxine | Chang et al. (122) | + | Improved attention and executive function compared with baseline (N=72) |
| SSRI/SNRI | Nagane et al. (126) | − | Remitted patients receiving unspecified SSRI or SNRI antidepressants showed decreased visual memory compared with nonmedicated controls (N=21) |
| NDRI: buproprion | Gorlyn et al. (115), Soczynska et al. (120), Herrera-Guzmán et al. (127) | +/+/+ | Improved verbal and nonverbal memory compared with baseline in two studies (Ns=17 and 27) and improved visual memory and processing speed (N=20) |
| NaSSA: mirtazapine | Borkowska et al. (128) | + | Improvements in working memory, processing speed, and executive function after six months of treatment compared with baseline (N=71) |
| NRI: reboxetine | Ferguson et al. (114) | +* | DBRCT (N=25, 8–10 mg/day) demonstrated improved attention and processing speed compared with placebo |
| Multimodal vortioxetine | Mahableshwarkar et al. (125), McIntyre et al. (129) | +*/+* | DBRCT (N=168, 10–20 mg/day) showed improved processing speed and functional capacity compared with placebo; DBRCT of 10 mg/day (N=195) and 20 mg/day (N=207) found improvements in subjective and objective cognition compared with placebo at both doses |
Table 3. Evidence for Treatment of Cognitive Dysfunction With Newer-Generation Antidepressant Medications for Nonelderly Adults With Major Depressive Disordera
With two large RCTs with nonelderly adults, vortioxetine has been the most studied antidepressant and has the strongest evidence for efficacy in improving cognition. Approved by the Food and Drug Administration for the treatment of depression in 2013, vortioxetine is a multimodal agent that acts as a serotonin (5-HT) reuptake inhibitor; an agonist at 5-HT1A receptors; a partial agonist at 5-HT1B receptors; and an antagonist at 5-HT1D, 5-HT3, and 5-HT7 receptors (131). In preclinical studies, vortioxetine has also exerted effects on a number of different neurotransmitters, such as histamine, dopamine, glutamate, and acetylcholine (129, 132). Compared with placebo, vortioxetine resulted in significant improvements on measures of attention, executive functioning, learning, and episodic memory among both adult and elderly populations (125, 129, 133). It has also demonstrated superiority compared with placebo in improving executive functioning as well as functional capacity, as determined by a performance-based cognitive skills assessment, whereas the comparator, duloxetine, did not (125). In this study, path analysis indicated that 75% of vortioxetine’s effect on cognition was independent of improvement in depressive symptoms, as opposed to approximately 50% for duloxetine.
Although vortioxetine showed differential cognitive effects from a comparator, overall few studies have compared different agents (8). Tricyclic antidepressants (TCAs) are less effective in improving cognition compared with newer antidepressants such as sertraline, fluoxetine, and venlafaxine, possibly because of their deleterious anticholinergic effects (130, 134). It has also been postulated that drugs targeting multiple neurotransmitters (i.e., serotonin–norepinephrine reuptake inhibitors [SNRIs]) may be more effective than those modulating a single neurotransmitter (i.e., selective serotonin reuptake inhibitors [SSRIs]) (14, 17, 118). A comparison of 73 patients treated with 24 weeks of escitalopram or duloxetine found that although both groups improved in measures of processing speed and episodic and working memory, the duloxetine group experienced greater improvement in the latter two domains (118). This increased benefit of duloxetine over escitalopram persisted after patients achieved remission and had been withdrawn from medications for six months (135). In contrast, two RCTs comparing the norepinephrine–dopamine reuptake inhibitor bupropion with the SSRIs escitalopram and paroxetine found similar improvements in verbal and working memory (115, 120).
It should be noted that ongoing antidepressant treatment may also adversely affect cognition (136). A comparison of nonmedicated and medicated patients with remitted depression demonstrated that, although both groups displayed lower verbal memory performance than healthy controls, those continuing on medications (SSRI, SNRI, or TCA) showed additional deficits in visual memory and executive functioning (126). Another study found that, compared with baseline, patients with remitted depression experienced ongoing cognitive improvement six months after discontinuing escitalopram or duloxetine, although it should be noted that these patients had minimal residual depressive symptoms at the time of medication cessation (135). Rapid discontinuation of SSRIs may also temporarily worsen psychomotor and subjective cognitive functioning, particularly with shorter acting agents such as paroxetine (137).
In summary, evidence has suggested that antidepressants may improve cognition, but the conclusions that can be drawn are limited by small sample sizes, relatively few randomized placebo-controlled designs, variable results, and lack of comparator studies. Vortioxetine has the most consistent evidence for improvement of cognitive dysfunction in major depressive disorder, but the clinical significance of these effects, and how they may translate into improved functional outcomes, still needs to be clarified.
Adjunctive Pharmacotherapy
There are few studies of the cognitive effects of augmentation agents commonly used with adults. A small open-label study of aripiprazole augmentation among 13 patients who had partially responded to first-line antidepressants found objective improvements in executive functioning, working memory, and psychosocial functioning after six weeks (138). A placebo-controlled double-blind trial of 25 patients receiving augmentation therapy with olanzapine examined cognitive measures as a secondary outcome and found no significant differences between groups (139). Lisdexamfetamine may improve executive functioning in combination with antidepressants among adults with mild depression (140). Older stimulants such as methylphenidate and dextroamphetamine can improve cognition and depressive symptoms among patients with comorbid ADHD, traumatic brain injury, and medical illness, but evidence for their use with healthy younger adults is currently limited (141, 142). Healthy subjects taking modafinil have shown improvement in cognition, and in one study nonelderly patients with residual depressive symptoms improved their performance on the Stroop interference test when modafinil was added as an adjunctive agent (143, 144).
The efficacy of S-adenosylmethionine (SAMe) in the treatment of depression has been demonstrated in numerous RCTs (145), and it may be a useful adjunct to SSRI treatment for nonresponders (146, 147). Thus far, one trial has found adjunctive SAMe treatment to improve subjective reports of cognition and memory for people with depression (148). Omega-3 fatty acids may be another nutraceutical with efficacy as an adjunctive treatment in depression (145). However, one RCT found no effect of omega-3 supplementation on cognitive function with subjects with depression, and omega-3 supplementation with healthy volunteers did not have an effect on cognitive domains commonly affected in depression (149, 150).
Neuromodulation
Electroconvulsive therapy (ECT) is one of the most efficacious acute treatments for depression, but its use is limited largely because of concerns about possible detrimental cognitive effects, such as confusion and anterograde and retrograde amnesia (151, 152). Although patients may report long-term subjective memory deficits after ECT, most studies that have examined objective neuropsychological performance have found post-ECT cognitive impairment to be transient (152). A meta-analysis of these studies found that, although patients had significant decreases in verbal episodic memory, executive functioning, and verbal memory within three days of ECT completion, all measures either returned to baseline or improved after 15 days (153).
Treatment factors have been identified that may attenuate the acute cognitive effects, such as appropriate dose titration, ultrabrief versus brief pulse, and less frequent (i.e., two vs. three per week) treatments (151, 152). Bilateral electrode placement may also result in greater cognitive impairment than unilateral placement, although some have found this detrimental effect again to be transient (151, 154–156). Concomitant pharmacotherapy may also alter the cognitive effects of ECT; a large multicenter RCT found that patients receiving nortriptyline experienced fewer ECT-related cognitive side effects than those on placebo, whereas those on venlafaxine demonstrated a trend toward worsened anterograde and retrograde memory (156).
Aside from ECT, repetitive transcranial magnetic stimulation (rTMS) has been the most studied neuromodulation treatment in terms of cognitive effects. An approved therapy for treatment-resistant depression, rTMS uses a pulsed magnetic field to generate electrical activity in the underlying brain region, most commonly the left dorsolateral prefrontal cortex (157, 158). There have thus far been 10 double-blind, sham-controlled, or crossover placebo RCTs, with seven of these reporting an association between rTMS treatment and improved cognition. Although ECT is generally regarded as being more effective than rTMS, two studies randomizing patients to either ECT or rTMS did not find any significant differences in changes in depressive symptoms or cognition between the two modalities (159).
Psychotherapy
Few studies have examined the effects of psychotherapy on cognition in major depressive disorder (8), but overall they have reported a beneficial effect. Patients with recurrent depression who had received combined psychoeducation and cognitive-behavioral therapy reported fewer subjective cognitive symptoms than those who had received psychoeducation alone (160). Combination long-term psychodynamic therapy and fluoxetine appeared to result in greater improvement in certain cognitive domains than fluoxetine alone (161). Similarly, patients receiving combination psychotherapy and medication management for depression improved on measures of short-term memory and attention after eight weeks of treatment compared with patients receiving either treatment alone (162). The cognitive effects of mindfulness practices, which have been gaining popularity in the treatment of depression, have also been examined. In healthy populations, mindfulness meditation appears to improve sustained and selective attention and may improve memory and negative attentional bias in depression (163).
Cognitive remediation approaches are used extensively with people with schizophrenia as a means to specifically target cognitive dysfunction (164). Cognitive remediation involves patients practicing computerized tasks related to planning, working memory, and attention, usually at a frequency of one to two hours a week for several weeks (164). Studies of cognitive remediation in depression have found significant improvements in learning, verbal memory, and attention among both acutely depressed patients and those whose depression was in remission (165). Studies thus far have been small, however, and there is currently no easily accessible, standardized cognitive remediation protocol for patients with depression.
Other psychosocial interventions targeting lifestyle changes have also been examined. In particular, aerobic exercise was found to improve depressive symptoms and cognition among depressed inpatients (166). The largest study to date has been Treatment With Exercise Augmentation for Depression (“TREAD”), which randomly assigned 39 patients in partial remission to receive either low-dose or high-dose aerobic exercise. Although both groups improved in psychomotor processing, attention, and visual memory, high-dose patients had additional improvements in executive functioning and working memory. The authors concluded that among patients with residual depressive symptoms, there may be a dose-dependent beneficial effect of exercise on cognition (167).
Conclusions and Future Directions
Cognitive dysfunction is prevalent in depression, often persists in remission, and is linked to clinical and functional outcomes. However, despite growing recognition of its clinical importance, a lack of consensus regarding clinical monitoring strategies remains a barrier to clinicians. Although clinically accessible cognitive batteries are under development, clinicians can still make efforts to routinely assess patients’ subjective cognitive functioning using validated scales.
Overall, the data suggest that most of the standard treatments for depression result in improved cognition. However, the evidence continues to be limited by few studies and small sample sizes. In addition, the magnitude of these therapies’ effects on cognition, and the degree to which they translate into functional outcomes, remains to be determined. The vortioxetine studies, however, have suggested that there may be differential effects of specific antidepressants (and, likely, psychological treatments) on cognitive dysfunction.
As awareness regarding cognitive dysfunction in depression grows, so too is the interest in developing newer treatments that specifically address these deficits. There have thus far been promising developments in biological therapies targeting the neurotransmitter systems involved in cognitive processes and psychosocial interventions such as cognitive remediation specifically aimed at improving cognitive skills. These treatments require further study and long-term follow-up to definitively ascertain their effects on cognition.
Until such time as these specific therapies have been validated, there is a general consensus that clinicians should pursue a multimodal approach in trying to improve patients’ cognitive outcomes. Such an approach would address basic lifestyle factors such as exercise, diet, and sleep, in addition to psychotherapeutic and pharmacological treatments for depression (14). The combination of medication (especially medication that has been shown to have an effect on cognition) and psychotherapy may be particularly beneficial in targeting both the maladaptive top-down, higher-level cognitive processes and the monoamine deficiencies in bottom-up circuits believed to contribute to cognitive dysfunction (15). Combination therapy may also be useful in modulating the negative attentional bias that may underlie hot cognitive dysfunction. A cognitive neuropsychological model proposes that antidepressants work by shifting attentional bias from negative to positive stimuli; further psychological treatments are then primed to consolidate this shift (82, 168, 169). Because the factors mediating cognition and cognitive dysfunction are numerous and complex, the treatment of such deficits will likely need to be similarly multifaceted.
1 : Cognitive dysfunction in psychiatric disorders: characteristics, causes and the quest for improved therapy. Nat Rev Drug Discov 2012; 11:141–168Crossref, Google Scholar
2 : Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA, American Psychiatric Publishing, 2013Crossref, Google Scholar
3 : The cognitive neuropsychology of depression in the elderly. Psychol Med 2007; 37:1693–1702Crossref, Google Scholar
4 : The vascular depression hypothesis: mechanisms linking vascular disease with depression. Mol Psychiatry 2013; 18:963–974Crossref, Google Scholar
5 : White matter hyperintensities, cortisol levels, brain atrophy and continuing cognitive deficits in late-life depression. Br J Psychiatry 2010; 196:143–149Crossref, Google Scholar
6 : MRI and neuropsychological differences in early- and late-life-onset geriatric depression. Neurology 1996; 46:1567–1574Crossref, Google Scholar
7 : Effects of acute tryptophan depletion on memory, attention and executive functions: a systematic review. Neurosci Biobehav Rev 2009; 33:926–952Crossref, Google Scholar
8 : Cognitive dysfunction in major depressive disorder: effects on psychosocial functioning and implications for treatment. Can J Psychiatry 2014; 59:649–654Crossref, Google Scholar
9 : Executive functions. Annu Rev Psychol 2013; 64:135–168Crossref, Google Scholar
10 : Approaching a consensus cognitive battery for clinical trials in schizophrenia: the NIMH-MATRICS conference to select cognitive domains and test criteria. Biol Psychiatry 2004; 56:301–307Crossref, Google Scholar
11 : Cognitive symptoms in patients with major depressive disorder and their implications for clinical practice. J Clin Psychiatry 2014; 75:8–14Crossref, Google Scholar
12 : Domains of cognitive impairment in bipolar disorder: commentary on “The International Society for Bipolar Disorders–Battery for Assessment of Neurocognition (ISBD-BANC).” Bipolar Disord 2011; 13:217–218Crossref, Google Scholar
13 : Is there an association between subjective and objective measures of cognitive function in patients with affective disorders? Nord J Psychiatry 2012; 66:248–253Crossref, Google Scholar
14 : Cognitive dysfunction in major depressive disorder: a state-of-the-art clinical review. CNS Neurol Disord Drug Targets 2014; 13:1804–1818Crossref, Google Scholar
15 : Hot and cold cognition in depression. CNS Spectr 2013; 18:139–149Crossref, Google Scholar
16 : Cognitive dysfunction in depression: neurocircuitry and new therapeutic strategies. Neurobiol Learn Mem 2011; 96:553–563Crossref, Google Scholar
17 : Cognitive deficits and functional outcomes in major depressive disorder: determinants, substrates, and treatment interventions. Depress Anxiety 2013; 30:515–527Crossref, Google Scholar
18 : Cognitive impairment in depression: a systematic review and meta-analysis. Psychol Med 2014; 44:2029–2040.Crossref, Google Scholar
19 : Cognitive impairment in euthymic major depressive disorder: a meta-analysis. Psychol Med 2013; 43:2017–2026Crossref, Google Scholar
20 : Neuropsychological functioning in adolescents and young adults with major depressive disorder—a review. Psychiatry Res 2014; 218:261–271Crossref, Google Scholar
21 : Domain-specific impairment in cognitive control among remitted youth with a history of major depression. Early Interv Psychiatry (Epub July 14, 2015). Available at doi: 10.1111/eip.12253Google Scholar
22 : A review on cognitive impairments in depressive and anxiety disorders with a focus on young adults. J Affect Disord 2008; 106:1–27Crossref, Google Scholar
23 : Cognitive impairment in the remitted state of unipolar depressive disorder: a systematic review. J Affect Disord 2011; 134:20–31Crossref, Google Scholar
24 : The frequency of cognitive impairment in patients with anxiety, depression, and bipolar disorder: an unaccounted source of variance in clinical trials. J Clin Psychiatry 2008; 69:1122–1130Crossref, Google Scholar
25 : Presenting characteristics of depressed outpatients as a function of recurrence: preliminary findings from the STAR*D clinical trial. J Psychiatr Res 2006; 40:59–69Crossref, Google Scholar
26 : Major depression symptoms in primary care and psychiatric care settings: a cross-sectional analysis. Ann Fam Med 2007; 5:126–134Crossref, Google Scholar
27 : Presence of individual (residual) symptoms during depressive episodes and periods of remission: a 3-year prospective study. Psychol Med 2011; 41:1165–1174Crossref, Google Scholar
28 : mediators of the association between depression and role functioning. Acta Psychiatr Scand 2008; 118:451–458Crossref, Google Scholar
29 : The relationship between neurocognitive and psychosocial functioning in major depressive disorder: a systematic review. J Clin Psychiatry 2014; 75:1359–1370Crossref, Google Scholar
30 : Neurocognitive deficits and disability in major depressive disorder. Psychiatry Res 2006; 145:39–48Crossref, Google Scholar
31 : The role of cognitive impairment in general functioning in major depression. Psychiatry Res 2010; 176:183–189Crossref, Google Scholar
32 : Cognitive dysfunction in unipolar depression: implications for treatment. J Affect Disord 2014; 152-154:19–27Crossref, Google Scholar
33 : Cognition as predictor of current and follow-up depressive symptoms in the general population. Acta Psychiatr Scand 2009; 120:45–52Crossref, Google Scholar
34 : The depression-executive dysfunction (DED) syndrome and response to antidepressants: a meta-analytic review. Int J Geriatr Psychiatry 2010; 25:933–944Crossref, Google Scholar
35 : Neuropsychological function and suicidal behavior: attention control, memory and executive dysfunction in suicide attempt. Psychol Med 2013; 43:539–551Crossref, Google Scholar
36 : Neuropsychological dysfunction in depressed suicide attempters. Am J Psychiatry 2001; 158:735–741Crossref, Google Scholar
37 : Executive functioning in depressed patients with suicidal ideation. Acta Psychiatr Scand 2005; 112:294–301Crossref, Google Scholar
38 : Executive performance of depressed suicide attempters: the role of suicidal ideation. Eur Arch Psychiatry Clin Neurosci 2008; 258:414–421Crossref, Google Scholar
39 : Cognitive predictors of suicide risk among hospitalized psychiatric patients: a prospective study. Death Stud 1993; 17:103–124Crossref, Google Scholar
40 : Low episodic memory performance as a premorbid marker of depression: evidence from a 3-year follow-up. Acta Psychiatr Scand 2007; 115:458–465Crossref, Google Scholar
41 : Impairment of executive function and attention predicts onset of affective disorder in healthy high-risk twins. J Clin Psychiatry 2013; 74:e747–e753Crossref, Google Scholar
42 : A meta-analysis of cognitive deficits in first-episode major depressive disorder. J Affect Disord 2012; 140:113–124Crossref, Google Scholar
43 : Effects of recurrent major depressive disorder on behavior and cognitive function in female depressed patients. Psychiatry Res 2004; 125:73–79Crossref, Google Scholar
44 : Cognitive functioning in euthymic recurrently depressed patients: relationship with future relapses and prior course of disease. J Affect Disord 2012; 141:300–307Crossref, Google Scholar
45 : Neuropsychological function in subjects with psychotic and affective disorders: relationship to diagnostic category and duration of illness. Eur Psychiatry 2000; 15:236–243Crossref, Google Scholar
46 : Psychomotor retardation is a scar of past depressive episodes, revealed by simple cognitive tests. Eur Neuropsychopharmacol 2014; 24:1630–1640Crossref, Google Scholar
47 : Toxic effects of depression on brain function: impairment of delayed recall and the cumulative length of depressive disorder in a large sample of depressed outpatients. Am J Psychiatry 2008; 165:731–739Crossref, Google Scholar
48 : Cognitive functioning in a population-based sample of young adults with a history of non-psychotic unipolar depressive disorders without psychiatric comorbidity. J Affect Disord 2008; 110:36–45Crossref, Google Scholar
49 : Cognitive disturbance in outpatient depressed younger adults: evidence of modest impairment. Biol Psychiatry 2001; 50:35–43Crossref, Google Scholar
50 : Neuropsychological deficits in psychotic versus nonpsychotic unipolar depression. Neuropsychology 1999; 13:69–75Crossref, Google Scholar
51 : Neuropsychological deficits in psychotic versus nonpsychotic major depression and no mental illness. Am J Psychiatry 2000; 157:1095–1100Crossref, Google Scholar
52 : Depressive symptom profiles and severity patterns in outpatients with psychotic vs nonpsychotic major depression. Compr Psychiatry 2008; 49:421–429Crossref, Google Scholar
53 : Neuropsychological dysfunction in antipsychotic-naive first-episode unipolar psychotic depression. Am J Psychiatry 2004; 161:996–1003Crossref, Google Scholar
54 : A meta-analysis of depression severity and cognitive function. J Affect Disord 2009; 119:1–8Crossref, Google Scholar
55 : A longitudinal study of cognitive function in melancholic and non-melancholic subtypes of major depressive disorder. J Affect Disord 2010; 123:150–157Crossref, Google Scholar
56 : Comorbid anxiety corresponds with neuropsychological dysfunction in unipolar depression. Cogn Neuropsychiatry 2007; 12:437–456Crossref, Google Scholar
57 : Comorbid anxiety disorder in late life depression: association with memory decline over four years. Int J Geriatr Psychiatry 2005; 20:848–854Crossref, Google Scholar
58 : The effect of psychiatric co-morbidity on cognitive functioning in a population-based sample of depressed young adults. Psychol Med 2010; 40:29–39Crossref, Google Scholar
59 : Towards a “metabolic” subtype of major depressive disorder: shared pathophysiological mechanisms may contribute to cognitive dysfunction. CNS Neurol Disord Drug Targets 2014; 13:1693–1707Crossref, Google Scholar
60 : Should depressive syndromes be reclassified as “metabolic syndrome type II”? Ann Clin Psychiatry 2007; 19:257–264Crossref, Google Scholar
61 : Neurocognitive consequences of sleep deprivation. Semin Neurol 2009; 29:320–339Crossref, Google Scholar
62 : Hippocampal volume and depression: a meta-analysis of MRI studies. Am J Psychiatry 2004; 161:1957–1966Crossref, Google Scholar
63 : A meta-analysis examining clinical predictors of hippocampal volume in patients with major depressive disorder. J Psychiatry Neurosci 2009; 34:41–54Google Scholar
64 : Lower hippocampal volume in patients suffering from depression: a meta-analysis. Am J Psychiatry 2004; 161:598–607Crossref, Google Scholar
65 : Deficits in hippocampal and anterior cingulate functioning during verbal declarative memory encoding in midlife major depression. Am J Psychiatry 2004; 161:637–645Crossref, Google Scholar
66 : Dysfunctional hippocampal activity affects emotion and cognition in mood disorders. Brain Res 2012; 1476:58–70Crossref, Google Scholar
67 : The hippocampus in major depression: evidence for the convergence of the bench and bedside in psychiatric research? Mol Psychiatry 2011; 16:252–264Crossref, Google Scholar
68 : Gray matter reduction associated with psychopathology and cognitive dysfunction in unipolar depression: a voxel-based morphometry study. J Affect Disord 2008; 109:107–116Crossref, Google Scholar
69 : Reduced hippocampal volume correlates with executive dysfunctioning in major depression. J Psychiatry Neurosci 2006; 31:316–323Google Scholar
70 : The variable nature of cognitive control: a dual mechanisms framework. Trends Cogn Sci 2012; 16:106–113Crossref, Google Scholar
71 : Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 2007; 27:2349–2356Crossref, Google Scholar
72 : A critical role for the right fronto-insular cortex in switching between central-executive and default-mode networks. Proc Natl Acad Sci USA 2008; 105:12569–12574Crossref, Google Scholar
73 : A neurobiological approach to the cognitive deficits of psychiatric disorders. Dialogues Clin Neurosci 2013; 15:419–429Google Scholar
74 : Large-scale brain networks and psychopathology: a unifying triple network model. Trends Cogn Sci 2011; 15:483–506Crossref, Google Scholar
75 : Functional connectivity in the resting brain: a network analysis of the default mode hypothesis. Proc Natl Acad Sci USA 2003; 100:253–258Crossref, Google Scholar
76 : The role of default network deactivation in cognition and disease. Trends Cogn Sci 2012; 16:584–592Crossref, Google Scholar
77 : Default-mode and task-positive network activity in major depressive disorder: implications for adaptive and maladaptive rumination. Biol Psychiatry 2011; 70:327–333Crossref, Google Scholar
78 : The default mode network and self-referential processes in depression. Proc Natl Acad Sci USA 2009; 106:1942–1947Crossref, Google Scholar
79 : Resting-state functional connectivity in major depressive disorder: A review. Neurosci Biobehav Rev 2015; 56:330–344Crossref, Google Scholar
80 : Effects of stress throughout the lifespan on the brain, behaviour and cognition. Nat Rev Neurosci 2009; 10:434–445Crossref, Google Scholar
81 : Cognition and depression: current status and future directions. Annu Rev Clin Psychol 2010; 6:285–312Crossref, Google Scholar
82 : Antidepressant drug action: a neuropsychological perspective. Depress Anxiety 2010; 27:231–233Crossref, Google Scholar
83 : Effects of adverse experiences for brain structure and function. Biol Psychiatry 2000; 48:721–731Crossref, Google Scholar
84 : Major depressive disorder and hypothalamic-pituitary-adrenal axis activity: results from a large cohort study. Arch Gen Psychiatry 2009; 66:617–626Crossref, Google Scholar
85 : Cognitive impairment in major depression: association with salivary cortisol. Biol Psychiatry 2009; 66:879–885Crossref, Google Scholar
86 : Associations between cognitive performance and cortisol reaction to the DEX/CRH test in patients recovered from depression. Psychoneuroendocrinology 2013; 38:447–454Crossref, Google Scholar
87 : Stress hormones and human memory function across the lifespan. Psychoneuroendocrinology 2005; 30:225–242Crossref, Google Scholar
88 : Depression: the case for a monoamine deficiency. J Clin Psychiatry 2000; 61(Suppl 6):7–11Crossref, Google Scholar
89 : History and evolution of the monoamine hypothesis of depression. J Clin Psychiatry 2000; 61(Suppl 6):4–6Crossref, Google Scholar
90 : Serotonin and prefrontal cortex function: neurons, networks, and circuits. Mol Neurobiol 2011; 44:449–464Crossref, Google Scholar
91 : Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry 2005; 57:1377–1384Crossref, Google Scholar
92 : Adrenergic pharmacology and cognition: focus on the prefrontal cortex. Pharmacol Ther 2007; 113:523–536Crossref, Google Scholar
93 : Molecular modulation of prefrontal cortex: rational development of treatments for psychiatric disorders. Behav Neurosci 2011; 125:282–296Crossref, Google Scholar
94 : Catecholamine influences on dorsolateral prefrontal cortical networks. Biol Psychiatry 2011; 69:e89–e99Crossref, Google Scholar
95 : Catecholamine and second messenger influences on prefrontal cortical networks of “representational knowledge”: a rational bridge between genetics and the symptoms of mental illness. Cereb Cortex 2007; 17(Suppl 1):i6–i15Crossref, Google Scholar
96 : The evolution of the cognitive model of depression and its neurobiological correlates. Am J Psychiatry 2008; 165:969–977Crossref, Google Scholar
97 : Using measurement strategies to identify and monitor residual symptoms. J Clin Psychiatry 2013; 74(Suppl 2):14–18Crossref, Google Scholar
98 : The International Society for Bipolar Disorders–Battery for Assessment of Neurocognition (ISBD-BANC). Bipolar Disord 2010; 12:351–363Crossref, Google Scholar
99 : The MATRICS Consensus Cognitive Battery, part 1: test selection, reliability, and validity. Am J Psychiatry 2008; 165:203–213Crossref, Google Scholar
100 : Systematic review of appropriate cognitive assessment instruments used in clinical trials of schizophrenia, major depressive disorder and bipolar disorder. Psychiatry Res 2014; 216:291–302Crossref, Google Scholar
101 : The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc 2005; 53:695–699Crossref, Google Scholar
102 : The MMSE is not an adequate screening cognitive instrument in studies of late-life depression. J Psychiatr Res 2009; 43:464–470Crossref, Google Scholar
103 : Cognitive effects of pharmacotherapy for major depressive disorder: a systematic review. J Clin Psychiatry 2014; 75:864–876Crossref, Google Scholar
104 : A new depression scale designed to be sensitive to change. Br J Psychiatry 1979; 134:382–389Crossref, Google Scholar
105 : An inventory for measuring depression. Arch Gen Psychiatry 1961; 4:561–571Crossref, Google Scholar
106 : The PHQ-9: validity of a brief depression severity measure. J Gen Intern Med 2001; 16:606–613Crossref, Google Scholar
107 : The 16-Item Quick Inventory of Depressive Symptomatology (QIDS), Clinician Rating (QIDS-C), and Self-Report (QIDS-SR): a psychometric evaluation in patients with chronic major depression. Biol Psychiatry 2003; 54:573–583Crossref, Google Scholar
108 : Rapid screening for perceived cognitive impairment in major depressive disorder. Ann Clin Psychiatry 2013; 25:135–140Google Scholar
109 : Reliability and validity of the Massachusetts General Hospital Cognitive and Physical Functioning Questionnaire. Psychother Psychosom 2009; 78:91–97Crossref, Google Scholar
110 : Psychometric validation of Perceived Deficits Questionnaire–Depression (PDQ-D) in patients with major depressive disorder (MDD). Value Health 2013; 16:A330Crossref, Google Scholar
111 : The relation between objective and subjective impairment in cognitive function among multiple sclerosis patients—the role of depression. Mult Scler 2001; 7:131–135Google Scholar
112 : Association between severity of depression and self-perceived cognitive difficulties among full-time employees. Prim Care Companion CNS Disord 2013; 15.Google Scholar
113 : Effects of depressed mood on objective and subjective measures of attention. J Neuropsychiatry Clin Neurosci 2003; 15:98–104Crossref, Google Scholar
114 : Reboxetine versus paroxetine versus placebo: effects on cognitive functioning in depressed patients. Int Clin Psychopharmacol 2003; 18:9–14Crossref, Google Scholar
115 : Treatment-related improvement in neuropsychological functioning in suicidal depressed patients: paroxetine vs. bupropion. Psychiatry Res 2015; 225:407–412Crossref, Google Scholar
116 : Impaired declarative memory in depressed patients is slow to recover: clinical experience. Pharmacopsychiatry 2004; 37:147–151Crossref, Google Scholar
117 : Clinical and neurobiological effects of tianeptine and paroxetine in major depression. J Clin Psychopharmacol 2003; 23:155–168Crossref, Google Scholar
118 : Effects of selective serotonin reuptake and dual serotonergic-noradrenergic reuptake treatments on memory and mental processing speed in patients with major depressive disorder. J Psychiatr Res 2009; 43:855–863Crossref, Google Scholar
119 : Effects of selective serotonin reuptake and dual serotonergic-noradrenergic reuptake treatments on attention and executive functions in patients with major depressive disorder. Psychiatry Res 2010; 177:323–329Crossref, Google Scholar
120 : The effect of bupropion XL and escitalopram on memory and functional outcomes in adults with major depressive disorder: results from a randomized controlled trial. Psychiatry Res 2014; 220:245–250Crossref, Google Scholar
121 : Effects of sertraline on depressive symptoms and attentional and executive functions in major depression. Depress Anxiety 2005; 21:78–89Crossref, Google Scholar
122 : Treatment response and cognitive impairment in major depression: association with C-reactive protein. Brain Behav Immun 2012; 26:90–95Crossref, Google Scholar
123 : Verbal learning by major depressive disorder patients during treatment with fluoxetine or amitriptyline. Int Clin Psychopharmacol 1994; 9:35–40Crossref, Google Scholar
124 : The SSRIs drug fluoxetine, but not the noradrenergic tricyclic drug desipramine, improves memory performance during acute major depression. Brain Res Bull 2002; 58:345–350Crossref, Google Scholar
125 : A randomized, placebo-controlled, active-reference, double-blind, flexible-dose study of the efficacy of vortioxetine on cognitive function in major depressive disorder. Neuropsychopharmacology 2015; 40:2025–2037Crossref, Google Scholar
126 : Comparative study of cognitive impairment between medicated and medication-free patients with remitted major depression: class-specific influence by tricyclic antidepressants and newer antidepressants. Psychiatry Res 2014; 218:101–105Crossref, Google Scholar
127 : Cognitive predictors of treatment response to bupropion and cognitive effects of bupropion in patients with major depressive disorder. Psychiatry Res 2008; 160:72–82Crossref, Google Scholar
128 : Enhancing effect of mirtazapine on cognitive functions associated with prefrontal cortex in patients with recurrent depression. Neuropsychopharmacol Hung 2007; 9:131–136Google Scholar
129 : A randomized, double-blind, placebo-controlled study of vortioxetine on cognitive function in depressed adults. Int J Neuropsychopharmacol 2014; 17:1557–1567Crossref, Google Scholar
130 : The cognitive effects of antidepressants in major depressive disorder: a systematic review and meta-analysis of randomized clinical trials. Int J Neuropsychopharmacol [Epub July 25, 2015]. Available at doi: 10.1093/ijnp/pyv082Google Scholar
131 : An overview of vortioxetine. J Clin Psychiatry 2014; 75:1411–1418Crossref, Google Scholar
132 : Discovery of 1-[2-(2,4-dimethylphenylsulfanyl)phenyl]piperazine (Lu AA21004): a novel multimodal compound for the treatment of major depressive disorder. J Med Chem 2011; 54:3206–3221Crossref, Google Scholar
133 : A randomized, double-blind, placebo-controlled, duloxetine-referenced, fixed-dose study comparing the efficacy and safety of Lu AA21004 in elderly patients with major depressive disorder. Int Clin Psychopharmacol 2012; 27:215–223Crossref, Google Scholar
134 : Pharmacological and non-pharmacological interventions to improve cognitive dysfunction and functional ability in clinical depression—a systematic review. Psychiatry Res 2014; 219:25–50Crossref, Google Scholar
135 : Major depressive disorder in recovery and neuropsychological functioning: effects of selective serotonin reuptake inhibitor and dual inhibitor depression treatments on residual cognitive deficits in patients with major depressive disorder in recovery. J Affect Disord 2010; 123:341–350Crossref, Google Scholar
136 : On “cognitive dysfunction” as a novel target for antidepressant treatment. Hum Psychopharmacol 2013; 28:535–537Crossref, Google Scholar
137 : Abrupt and brief discontinuation of antidepressant treatment: effects on cognitive function and psychomotor performance. Int Clin Psychopharmacol 2000; 15:305–318Crossref, Google Scholar
138 : Cognitive and psychosocial improvements following aripiprazole augmentation of SSRI antidepressant therapy in treatment refractory depression: a pilot study. Open J Depress. 2013; 2:45–53Crossref, Google Scholar
139 : Sleep architecture and cognitive changes in olanzapine-treated patients with depression: a double blind randomized placebo controlled trial. BMC Psychiatry 2014; 14:202Crossref, Google Scholar
140 : Lisdexamfetamine dimesylate augmentation in adults with persistent executive dysfunction after partial or full remission of major depressive disorder. Neuropsychopharmacology 2014; 39:1388–1398Crossref, Google Scholar
141 : Depression and cognitive complaints following mild traumatic brain injury. Am J Psychiatry 2009; 166:653–661Crossref, Google Scholar
142 : Using psychostimulants to treat depression in the medically ill. Prim Care Companion J Clin Psychiatry 2004; 6:44–46Crossref, Google Scholar
143 : Modafinil for cognitive neuroenhancement in healthy non-sleep-deprived subjects: a systematic review. Eur Neuropsychopharmacol 2015; 25:1865–1881Crossref, Google Scholar
144 : A prospective trial of modafinil as an adjunctive treatment of major depression. J Clin Psychopharmacol 2004; 24:87–90Crossref, Google Scholar
145 : Are nutritional supplements ready for prime time? J Clin Psychiatry 2008; 69:1497–1498Crossref, Google Scholar
146 : S-adenosyl methionine (SAMe) augmentation of serotonin reuptake inhibitors for antidepressant nonresponders with major depressive disorder: a double-blind, randomized clinical trial. Am J Psychiatry 2010; 167:942–948Crossref, Google Scholar
147 : S-adenosyl-L-methionine (SAMe) as an adjunct for resistant major depressive disorder: an open trial following partial or nonresponse to selective serotonin reuptake inhibitors or venlafaxine. J Clin Psychopharmacol 2004; 24:661–664Crossref, Google Scholar
148 : Effects of S-adenosylmethionine augmentation of serotonin-reuptake inhibitor antidepressants on cognitive symptoms of major depressive disorder. J Affect Disord 2012; 136:1174–1178Crossref, Google Scholar
149 : No effect of n-3 long-chain polyunsaturated fatty acid (EPA and DHA) supplementation on depressed mood and cognitive function: a randomised controlled trial. Br J Nutr 2008; 99:421–431Crossref, Google Scholar
150 : Omega-3 fatty acids (fish-oil) and depression-related cognition in healthy volunteers. J Psychopharmacol 2009; 23:831–840Crossref, Google Scholar
151 : Efficacy and safety of electroconvulsive therapy in depressive disorders: a systematic review and meta-analysis. Lancet 2003; 361:799–808Crossref, Google Scholar
152 : Neuropsychologic effects of neuromodulation techniques for treatment-resistant depression: a review. Brain Stimulat 2011; 4:17–27Crossref, Google Scholar
153 : Objective cognitive performance associated with electroconvulsive therapy for depression: a systematic review and meta-analysis. Biol Psychiatry 2010; 68:568–577Crossref, Google Scholar
154 : Randomized comparison of ultra-brief bifrontal and unilateral electroconvulsive therapy for major depression: cognitive side-effects. J Affect Disord 2010; 122:60–67Crossref, Google Scholar
155 : Clinical efficacy and cognitive side effects of bifrontal versus right unilateral electroconvulsive therapy (ECT): a short-term randomised controlled trial in pharmaco-resistant major depression. J Affect Disord 2007; 101:149–157Crossref, Google Scholar
156 : Effect of concomitant pharmacotherapy on electroconvulsive therapy outcomes: short-term efficacy and adverse effects. Arch Gen Psychiatry 2009; 66:729–737Crossref, Google Scholar
157 : The expanding evidence base for rTMS treatment of depression. Curr Opin Psychiatry 2013; 26:13–18Crossref, Google Scholar
158 : Daily left prefrontal transcranial magnetic stimulation therapy for major depressive disorder: a sham-controlled randomized trial. Arch Gen Psychiatry 2010; 67:507–516Crossref, Google Scholar
159 : The effects of repetitive transcranial magnetic stimulation on cognitive performance in treatment-resistant depression: a systematic review. Neuropsychobiology 2015; 71:125–139Crossref, Google Scholar
160 : Cognitive-behavioural therapy v. usual care in recurrent depression. Br J Psychiatry 2008; 193:505–506Crossref, Google Scholar
161 : Neurocognitive changes in depressed patients in psychodynamic psychotherapy, therapy with fluoxetine and combination therapy. J Affect Disord 2013; 151:1066–1075Crossref, Google Scholar
162 : Impact of psychotherapy and antidepressive treatment on cognitive functions in patients treated for depression. Psychiatr Danub 2012; 24(Suppl 1):S130–S134Google Scholar
163 : Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clin Psychol Rev 2011; 31:449–464Crossref, Google Scholar
164 : Cognitive remediation as a treatment for major depression: A rationale, review of evidence and recommendations for future research. Aust N Z J Psychiatry 2013; 47:1165–1175Crossref, Google Scholar
165 : Cognitive training in affective disorders improves memory: a preliminary study using the NEAR approach. J Affect Disord 2010; 121:258–262Crossref, Google Scholar
166 : Effects of aerobic exercise on cognitive performance and individual psychopathology in depressive and schizophrenia patients. Eur Arch Psychiatry Clin Neurosci 2014; 264:589–604Crossref, Google Scholar
167 : Dose-dependent changes in cognitive function with exercise augmentation for major depression: results from the TREAD study. Eur Neuropsychopharmacol 2015; 25:248–256Crossref, Google Scholar
168 : Why do antidepressants take so long to work? A cognitive neuropsychological model of antidepressant drug action. Br J Psychiatry 2009; 195:102–108Crossref, Google Scholar
169 : Cognitive mechanisms of treatment in depression. Neuropsychopharmacology 2012; 37:117–136Crossref, Google Scholar



