Neuropsychological Correlates of Behavioral Symptoms in Alzheimer’s Disease, Frontal Variant of Frontotemporal, Subcortical Vascular, and Lewy Body Dementias: A Comparative Study
Abstract
The aim of this study was to investigate the neuropsychological correlates of behavioral and psychological symptoms (BPSD) in patients affected by various forms of dementia, namely Alzheimer’s disease (AD), frontal-variant frontotemporal dementia (fvFTD), Lewy body dementia (LBD), and subcortical ischemic vascular dementia (SIVD). 21 fvFTD, 21 LBD, 22 AD, and 22 SIVD patients matched for dementia severity received a battery of neuropsychological tests and the Neuropsychiatry Inventory (NPI). The possible association between performance on neuropsychological tests and severity of BPSD was assessed by correlational analysis and multivariate regression. BPSD were present in 99% of patients. Most behavioral symptoms were not related to a particular dementia group or to a specific cognitive deficit. Euphoria and disinhibition were predicted by fvFTD diagnosis. Hallucinations correlated with the severity of visuospatial deficits in the whole sample of patients and were predicted by LBD diagnosis. Apathy, which was found in all dementia groups, correlated with executive functions and was predicted by both reduced set-shifting aptitude and fvFTD diagnosis. The results confirm the high prevalence of BPSD in the mild to moderate stages of dementia and show that most BPSD are equally distributed across dementia groups. Most of the cognitive and behavioral symptoms are independent dimensions of the dementia syndromes. Nevertheless, hallucinations in LBD and euphoria and disinhibition in fvFTD are related to the structural brain alterations that are responsible for cognitive decline in these dementia groups. Finally, apathy arises from damage in the frontal cortical areas that are also involved in executive functions.
(Reprinted with permission from Journal of Alzheimerʼs Disease 2014; 39:669–677)
Introduction
Cognitive and behavioral and psychological symptoms (BPSD) are an integral part of dementia syndromes [1, 2]. Nevertheless, the interrelations among these two dimensions are complex and difficult to perceive. Studies that investigated the relationship between cognitive and behavioral aspects in dementia, considered as overall dimensions, obtained contrasting results [3–6]. Casanova et al. [7] suggested that some BPSD are the expression of regional rather than diffuse brain pathology. Nevertheless, investigations that focused on more specific relationships between particular BPSD and selective cognitive deficits also obtained contrasting results [8–14], making it difficult to compare because of the variability of patients included (both as for etiology and severity of dementia) [15], the type of BPSD and cognitive functions investigated, the instruments used [8–13], and the kind of the analyses performed [2]. In sum, data present in the literature show a complex interplay between cognitive and behavioral disorders, probably reflecting different types of relationship among these two dimensions according to individual BPSD. A specific problem concerns the investigation of specific etiological groups of dementia patients. Indeed, the simultaneous presence of specific behavioral problems and cognitive deficits in these patients might be evidence a statistical association revealing a common neural substrate underlying the two kinds of disorders or, alternatively, it might be an epiphenomenon. For example, the simultaneous presence of hallucinations and visuospatial deficits in patients with Lewy body dementia (LBD) could reflect a common basic mechanism, expression of the involvement of a common neural substrate; alternatively, it could reflect the large involvement of posterior cerebral areas in these patients, without an effective identity of the neural networks at the base of these two behavioral and cognitive deficits.
Here we investigated the relationship between BPSD and cognitive functions in relatively large groups of patients affected by different forms of dementia, namely Alzheimer’s disease (AD), frontal variant of frontotemporal dementia (fvFTD), subcortical ischemic vascular dementia (SIVD), and LBD, matched for disease severity. We submitted the four dementia groups to a detailed neuropsychological and behavioral assessment, and then we performed a correlational analysis between the cognitive and behavioral scores obtained by the whole group of patients in order to reveal specific relationships among these two dimensions. Furthermore, since in the whole sample of dementia patients a significant correlation between a specific cognitive and behavioral disorder could be determined by the high prevalence of the two deficits in a particular group of patients (thus revealing a strong association in that dementia group but not in others), we also performed a series of regression analyses to determine whether the performance on cognitive tasks was able to predict the severity of each BPSD over and above the dementia group membership. In this regard, in these analyses, both cognitive scores and the type of dementia were introduced as possible predictors of the behavioral disorders. In fact, we reasoned that if specific cognitive and behavioral abnormalities are functionally and anatomically related, the association between a particular BPSD and the related cognitive deficit should emerge in all patients suffering from that specific BPSD, irrespective of the dementia type.
Methods
Subjects.
All patients who participated in this study were consecutive outpatients referred by their primary care physician to the Brain Aging and Alzheimer’s disease unit of the IRCCS Santa Lucia Foundation in Rome. Internationally accepted clinical criteria were adopted to diagnose each form of dementia [16–19]. To be included in the study, patients had to undergo complete clinical, neuropsychological, behavioral, and functional evaluation as part of the diagnostic process scheduled in the outpatient service as well as a CT or MR examination. Only patients who were not under anticholinesterase or neuroleptic therapy and had mild to moderate levels of dementia (Clinical Dementia Rating (CDR) total score <3) were considered.
Twenty-two patients (10 males, 12 females) met the clinical criteria for AD, 21 (11 males, 10 females) were diagnosed as fvFTD, 21 (13 males, 8 females) met the criteria for the clinical diagnosis of probable LBD, and 22 patients (18 males, 4 females) were diagnosed as SIVD.
The groups differed for age (F(3,82) = 3.76, p = 0.01) and years of education (F(3,82) = 2.75, p = 0.04). fvFTD patients were younger than both LBD (p = 0.005) and SIVD patients (p = 0.01), and AD patients were younger than LBD patients (p = 0.03). LBD patients were less educated than both fvFTD (p = 0.01) and AD (p = 0.04) patients. Groups had comparable dementia severity, as shown by the CDR [20] mean-boxes scores (F(3,82) = 2.0) (Table 1).
| Group | Age | Education | CDR |
|---|---|---|---|
| AD | 66.8 (3.5) | 10.5 (5.4) | 0.5 (0.4) |
| fvFTD | 64.7 (11.5) | 11.2 (3.4) | 0.7 (0.6) |
| SIVD | 72.1 (10.9)1 | 8.8 (4.3) | 0.9 (0.6) |
| LBD | 72.6 (6.4)2 | 7.8 (3.6)2 | 0.8 (0.6) |
TABLE 1. Mean and Standard Deviation (SD) of Age, Education, and Mean-Boxes Clinical Dementia Rating (CDR) Scores of the Four Experimental Groups
Standard protocol approvals, registrations, and patient consents.
The Joint Ethics Committee of the IRCCS Foundation Santa Lucia approved the experimental protocol. All patients gave written informed consent for participation in the study.
Neuropsychological and behavioral evaluation.
The neuropsychological battery included tests for the assessment of the following cognitive areas: declarative memory (15-word list delayed recall [21]; Prose delayed recall [22]), visuo-spatial abilities (Copy of drawings [21]), logical reasoning (Raven’s Coloured Progressive Matrices [21]), and executive functions (Phonemic verbal fluency (PVF) [21]; Modified Card Sorting Test (MCST) - Number of Criteria achieved [23]); general cognitive efficiency (Mini-Mental State Examination (MMSE) [24]).
Frequency of occurrence and severity of BPSD were evaluated using the Neuropsychiatry Inventory ten-items (NPI) assessing delusions, hallucinations, agitation/aggression, depression/dysphoria, anxiety, euphoria, apathy, disinhibition, irritability/lability, and aberrant motor behavior [25].
Data analysis.
ANOVAs co-varied for age, education, and MMSE scores were performed to compare scores obtained by patients in each dementia group on the neuropsychological and behavioral evaluation. Post-hoc comparisons were performed using LSD. χ2 tests were used to compare occurrence frequency of the individual BPSD. Partial correlation analyses were performed to investigate the relationship between cognitive and behavioral scores obtained by the whole group of patients, controlled for age and education. Finally, ten linear regression analyses (one for each NPI item) were performed to determine whether performance on cognitive tasks was able to predict the severity of each BPSD over and above the dementia type. In these analyses, the score in each behavioral domain was the dependent variable and age, education, dementia group membership (a dummy variable for each of the four groups, with 1 encoding the patients in a specific dementia group and 0 all others) and the six neuropsychological scores were the independent variables. In all analyses, significance level was set at p ≤ 0.003 according to Bonferroni’s correction.
Results
Neuropsychological evaluation.
Table 2 reports the mean scores obtained by each dementia group on the cognitive tests. ANOVAs showed that groups performed different on Prose delayed recall (Prose F(3,82) = 6.18, p = 0.001) and Copy of Drawings (F(3,82) = 14.18, p < 0.001), but no difference emerged on the Word-list delayed recall (F(3,82) = 4.51), PVF (F(3,82) = 2.96), Progressive Matrices (F(3,82) = 1.34), MCST criteria (F(3,82) = 1.86), and the MMSE (F(3,82) = 3.83). Post-hoc comparisons revealed that on the Prose task, AD patients obtained lower scores than fvFTD (p < 0.001) and marginally lower than SIVD (p = 0.004) groups; on the Copy of Drawings, the LBD group performed worse than all other groups (p < 0.001 in all comparisons) and the SIVD group performed worse than the fvFTD group (p < 0.001). No other difference was detected.
| Word list delayed recall | Prose delayed recall | Progressive matrices | Copy of drawings | Phonemic Verbal Fluency | MCST criteria achieved | MMSE | |
|---|---|---|---|---|---|---|---|
| AD | 1.59 (1.4) | 1.16 (1.8)1 | 20.97 (7.4) | 7.95 (2.2) | 20.72 (10.7) | 2.38 (1.6) | 23.2 (1.9) |
| fvFTD | 3.68 (2.2) | 3.78 (2.3) | 21.89 (7.5) | 9.00 (1.3) | 17.61 (10.7) | 1.90 (1.2) | 23.4 (2.8) |
| SIVD | 2.18 (1.5) | 3.04 (1.9) | 18.29 (5.8) | 6.70 (2.1)2 | 12.40 (5.7) | 1.22 (1.2) | 22.7 (2.7) |
| LBD | 2.85 (2.3) | 2.28 (1.9) | 14.69 (7.4) | 4.47 (2.6)3 | 12.70 (7.0) | 1.20 (1.2) | 20.8 (3.4) |
TABLE 2. Mean Scores and (SD) Obtained by Alzheimer’s Disease (AD), Frontal-Variant Frontotemporal Dementia (fvFTD), Subcortical Ischemic Vascular Dementia (SIVD), and Lewy Body Dementia (LBD) Groups on Each Test of the Neuropsychological Battery
Behavioral evaluation.
Table 3 shows the frequency of occurrence of behavioral changes as measured by NPI in the four dementia groups. The most frequent symptom in the whole sample was apathy, followed by depression, irritability, and agitation. All behavioral changes were equally distributed among the four dementia groups, except for hallucinations ( =30.41, p < 0.001), euphoria (
=30.41, p < 0.001), euphoria ( = 18.52, p < 0.001), and disinhibition (
= 18.52, p < 0.001), and disinhibition ( =25.1, p < 0.001). Indeed, hallucinations were more frequent in the LDB group compared to all other groups (p < 0.001 in all comparisons), and euphoria and disinhibition were more frequent in the fvFTD group (euphoria: p = 0.008 in the comparison with the AD group and p < 0.001 in all the other comparisons; disinhibition: p consistently <0.001).
=25.1, p < 0.001). Indeed, hallucinations were more frequent in the LDB group compared to all other groups (p < 0.001 in all comparisons), and euphoria and disinhibition were more frequent in the fvFTD group (euphoria: p = 0.008 in the comparison with the AD group and p < 0.001 in all the other comparisons; disinhibition: p consistently <0.001).
| BPSD | AD | vfFTD | SIVD | LBD | All groups |
|---|---|---|---|---|---|
| Delusions | 9.1 | 28.6 | 22.7 | 42.9 | 25.6 |
| Hallucinations | 4.5 | 4.8 | 4.5 | 57.1 | 17.4 |
| Agitation | 45.5 | 61.9 | 45.5 | 47.6 | 50.0 |
| Depression | 50.0 | 52.4 | 63.6 | 71.4 | 59.3 |
| Anxiety | 36.4 | 42.9 | 40.9 | 52.4 | 43.0 |
| Euphoria | 18.2 | 57.1 | 9.1 | 9.5 | 23.3 |
| Apathy | 54.5 | 90.5 | 86.4 | 61.9 | 73.3 |
| Disinhibition | 22.7 | 81.0 | 27.3 | 14.3 | 36.0 |
| Irritability | 50.0 | 66.7 | 50.0 | 47.6 | 53.5 |
| Aberrant motor behavior | 18.2 | 52.4 | 27.3 | 47.6 | 36.0 |
| NPI tot | 95.5 | 100.0 | 100.0 | 100.0 | 98.8 |
TABLE 3. Frequency of Occurrence of Symptoms (% of Affected Patients) for Each Dementia Group and the Whole Sample of Patients
Scores obtained by dementia groups on each NPI subscale are reported in Fig. 1. The NPI total-score was significantly different among groups (F(3,82) = 6.14, p = 0.001), due to the higher NPI score obtained by fvFTD compared with AD patients (p < 0.001); no other difference was detected. Significant differences among groups emerged for the hallucinations (F(3,82) = 12.45, p < 0.001), apathy (F(3,82) = 6.42, p = 0.001), and dishinibition (F(3,82) = 16.29, p < 0.001) subscales. LBD patients obtained hallucination scores higher than all other groups (p consistently <0.001), fvFTD patients had disinhibition scores higher than all others groups (p < 0.001 in all comparisons), and the apathy score was higher in the fvFTD group than in the AD one (p < 0.001). No other significant difference was detected.
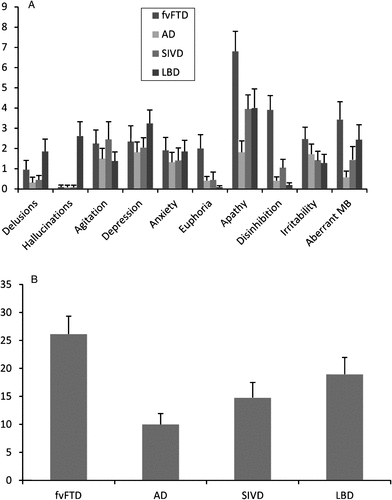
FIGURE 1. Neuropsychiatry Inventory (NPI) Scores Obtained by the Dementia Groups. A) Mean Scores (and Standard Errors) Obtained by the Four Dementia Groups on Each NPI Subscale. B) Mean Scores (and Standard Errors) Obtained by the Four Dementia Groups on the NPI Total Score. AD, Alzheimer’s Disease; fvFTD, Frontal-Variant Frontotemporal Dementia; SIVD, Subcortical Ischemic Vascular Dementia; LBD, Lewy Body Dementia; Aberrant MB, Aberrant Motor Behavior.
Correlations.
The correlational analysis between cognitive and behavioral scores obtained by the whole sample of patients revealed that patients who obtained the lower MCST criteria score had the higher apathy score (Pearson’s r –0.343, p = 0.003) and patients who performed poorly on the Copy of Drawings test had the higher hallucination scores (Pearson’s r –0.356, p = 0.002). No other correlation was significant.
Regression analyses.
Results of linear regression analysis showed that hallucinations were predicted by LBD group-membership (beta = 0.555, p < 0.001), euphoria and disinhibition by fvFTD group-membership (beta = 0.35, p = 0.002; beta = 0.625, p < 0.001, respectively), and apathy by fvFTD group-membership and performance scores on the MCST (beta = 0.403, p < 0.001; beta= –0.359, p = 0.001, respectively). No variable entered in the equations to predict the other BPSD.
Discussion
We investigated behavioral changes in groups of patients affected by different forms of dementia and correlated them with deficits of specific cognitive areas. We first analyzed the distribution of different BPSD in groups of patients affected by AD, fvFTD, SIVD, and LBD in the mild to moderate stage of dementia. Then, we performed correlational analyses between the cognitive and behavioral scores in the whole group of patients in order to reveal specific relationships among these two dimensions. Finally, we made a series of regression analyses to verify whether performance on cognitive tasks was able to predict the severity of each BPSD over and above the dementia group membership.
In agreement with previous reports of the high incidence of BPSD in dementia patients [1, 15], 99% of the patients in this study showed some behavioral symptom, even if mild. Comparisons of the occurrence frequency and severity level among the dementia groups revealed great similarity for most of the BPSD investigated. Depression, anxiety, irritability, and aberrant motor behavior were no more present in a particular group of dementia patients than in the others. Even delusions, usually reported as characteristic of patients with LBD, did not significantly differ among the four dementia groups. This unexpected finding may be due to the fact that the NPI subscale includes different kinds of delusions, such as well-structured and paranoid delusions, which are prevalent in the LBD pathology [26] and less defined ones, such as delusions of theft or infidelity, most common in AD patients [27]. According to the notion that behavioral changes are the core symptom of fvFTD, patients in this group had the highest NPI total score. In line with previous studies, fvFTD patients showed higher frequency and/or severity than the other groups of euphoria (more frequent in this group than all other groups), disinhibition (which occurred with greater frequency and severity in the fvFTD patients than in all others), and apathy (more severe in the fvFTD group than in the AD one) [28]. As expected, hallucinations were more frequent and severe in the LBD group than in all other ones, since hallucinations are a core feature used to diagnose this type of dementia [18].
The main aim of this study was to investigate the relationship between BPSD and cognitive deficits. Correlational analyses were conducted on the whole sample of patients. Results showed a significant correlation between the MCST criteria and apathy scores and between the Copy of Drawings and hallucination. Linear regression analyses performed to determine whether performance on cognitive tasks can predict severity of BPSD over and above the dementia type, showed that most BPSD (i.e., delusions, depression, agitation, anxiety, irritability, and aberrant motor behavior) were not predicted by performance on any cognitive task and did not show any association with a particular dementia group. Differently, hallucinations were predicted by the LBD group membership and euphoria and disinhibition by fvFTD diagnosis. Finally, apathy was predicted both by the number of criteria achieved on the MCST and by fvFTD group membership.
In sum, the results of the present study seem to confirm previous evidence of a lack of association between most of the cognitive and behavioral disorders in dementia patients. Indeed, the correlational analysis and the multiple regression approach evidenced three patterns of results which are in keeping with this general conclusion. The first pattern concerned most of neurobehavioral symptoms, which were not more prevalent in any of the four dementia groups and did not correlate with performance on any of the neuropsychological tests. The lack of any clear indication of a neuropathological substrate for these neurobehavioral symptoms may be interpreted in the view that most BPSD derive from a complex interplay between biological and psycho-social factors [2], which renders them somewhat unspecific and common to the different etiological dementia groups.
A second group of behavioral symptoms is represented by euphoria and disinhibition, which were independent of any of the neuropsychological functions assessed but were closely associated with fvFTD. This suggests that these BPSD are associated with frontal cerebral areas. Nevertheless, we found no correlation between patients’ scores on the euphoria and disinhibition NPI subscales and performance scores on any cognitive tasks, even those that tapped cognitive functions known to be subsumed by frontal regions such as PVF and MCST. These results are in line with previous studies documenting the independence of these behavioral changes from the level of executive function abilities in fvFTD patients [29]. Indeed, euphoria and disinhibition have been related to reduced social competence in fvFTD patients which, in turn, are related to the cognitive abilities involved in the “theory of mind” competences, greatly impaired in fvFTD patients [29, 30]. They are linked to orbitofrontal and ventromedial frontal area degeneration and largely independent of executive function abilities such as those measured by the MCST or PVF, which are mainly related to dorsolateral frontal structures [29, 30].
A third pattern regarding hallucinations emerged, with hallucinations being more represented in LBD group with respect to the others and significantly correlated with performance on the visuo-spatial task. When submitted to regression analysis, however, the relationship with the neuropsychological scores did not survive and only LBD group membership predicted occurrence and severity of hallucinations. The most cautious interpretation of these data is that there is an overlap between the neuropathological changes characteristic of LBD and those which underlie hallucinations. Conversely, the relationship with the deficit of visuo-spatial competences was only an epiphenomenon, resulting from the high prevalence of both the neurobehavioral and the neuropsychological disorder in the same dementia group. Indeed, in the present study, hallucinations were almost always present only in the patients with LBD and these patients also showed the most severe visuo-spatial deficits. Alternatively, the association between hallucinations and visuo-spatial deficits is really due to an association between the cerebral areas responsible for both the behavioral and cognitive deficits but this association is not complete in that it does not cover the entire spectrum of neuropathological changes involved in the altered behavioral manifestation. Neuroimaging studies have shown that in LBD patients complex visual hallucinations are related to hypometabolism in the extrastriate visual areas in the occipital lobes and the parietal association areas [26, 31], thus suggesting the involvement of the same cerebral regions in the genesis of both visual hallucinations and visuospatial deficits [32]. However, it has been proposed that in LBD hallucinations are also associated with high Lewy-body densities in the amygdala and parahippocampal formation [33] and to decreased cholinergic activity in the same areas [34]. Therefore, in the present study, the correlation between hallucinations and visuo-spatial deficits found in the whole sample of patients may really reflect involvement of cerebral areas involved in the genesis of both neuropsychological and behavioral symptoms. However, as LBD membership is the unique predictor of hallucinations in regression analysis, it may indicate the existence of additional factors (primarily in LBD) not related to visuo-spatial functions.
However, at variance with the general conclusion of independence between neurobehavioral disorder and neuropsychological deficits in dementia, we determined a final pattern of results in which both group membership and performance on a specific cognitive task contributed to the prediction of a neurobehavioral disorder in the regression model. In this case, it should be concluded that the neuropathological changes underlying the behavioral symptom are part of the cortical areas specifically damaged in a particular dementia group. However, these cortical areas also represent the ones whose damage is responsible for a specific cognitive deficit. The only symptom which fitted this kind of relationship was apathy. Indeed, apathy was predicted by fvFTD membership and by the number of criteria achieved on the MCST.
Apathy is a complex phenomenon characterized by a reduction of voluntary, goal-directed behaviors. In agreement with previous investigations, in this study apathy was the BPSD most represented in all dementia groups, with up to 90% occurrence in the fvFTD group [29, 35, 36]. Apathy can be divided into emotional-affective, cognitive, and auto-activation components [37]. Emotional-affective component refers to the inability to associate affective and emotional signals with ongoing and forthcoming behaviors and has been related to lesions in the orbital and medial-prefrontal cortex [11]. Conversely, cognitive apathy is due to impairment of the abilities needed to elaborate a plan of action, that is, working memory, rule-finding, and set-shifting, specifically assessed by cognitive tasks such as the MCST. Cognitive apathy has been related to damage including the dorsolateral-prefrontal cortex and the dorsal portions of the basal ganglia [37]. Finally, auto-activation deficits, which are responsible for difficulties in activating thoughts or initiating motor programs, have been related to lesions involving the basal ganglia and/or deep frontal white matter connecting basal ganglia structures to prefrontal regions [37]. The complex nature of apathy may well explain the present results. Indeed, the executive functions (as assessed by MCST) were diffusely impaired in all dementia groups due to direct involvement of the dorsolateral-prefrontal cortex in fvFTD patients [29] and to a lesser extent in AD patients [38] and to the disruption of the frontal-subcortical circuits in LBD [32] and SIVD [39]. In patients with fvFTD, correlations have been reported between apathy and performance on tests of planning and goal-directed behavior as well as severity of dorsolateral-prefrontal cortex atrophy [40]. A similar relationship between apathy and executive functions has been reported in AD patients [10, 12]. Thus, the ability of the MCST to predict apathy severity is due to the cognitive (executive) component of this behavioral symptom, which is disturbed in all dementia groups. However, it has been proposed that apathy affects all domains (cognitive, emotional, and auto-activation) in fvFTD patients due to involvement of the orbital, medial, or dorsolateral-prefrontal cortex and their connections with the basal ganglia in this form of dementia [11]. As the NPI assesses apathy as a whole, without distinguishing among different components, and as the cognitive tests used in the present study are sensitive only to dorsolateral-prefrontal dysfunction, it is probable that the link between emotional and/or auto-activation apathy components and the orbital and medial-prefrontal cortex and their connections with the basal ganglia (which are primarily disturbed in fvFTD patients) could be captured in the regression analysis only by vfFTD membership.
In sum, the results of the present investigation permit us to conclude that a clear relationship between specific cognitive and behavioral symptoms of dementia syndromes probably subsumed by the identity of the same anatomical areas in their manifestation, over and above the dementia type, can be evidenced only between apathy and executive dysfunctions. In other cases, however, a similar relation between BPSD and cognitive deficit probably failed to be revealed due to a low sensitivity of NPI (as for particular aspects of the apathy syndrome) and/or the inadequacy of the neuropsychological tasks (as for the investigation of the theory of mind abilities).
Conclusions
To our knowledge, this is the first study that has simultaneously investigated the relationship between a large spectrum of BPSD and different cognitive areas in four different dementia groups comparable for dementia severity. Findings confirm the high prevalence of BPSD in the mild to moderate stages of dementia, irrespective of the underlying pathology, and show that most behavioral changes are equally distributed in the four dementia groups. The study also confirms that most of the cognitive and behavioral symptoms are independent dimensions of the dementia syndromes [6]. Nevertheless, some behavioral disorders seem related to the same structural brain alterations responsible for cognitive decline in specific dementia types, such as hallucinations in LBD or euphoria and disinhibition in fvFTD. Finally, apathy symptoms, which are significantly represented in all dementia groups, arise from damage in frontal cortical areas also involved in cognitive abilities, such as set-shifting aptitude [7].
[1] (1996) Behavioral and psychological signs and symptoms of dementia: A consensus statement on current knowledge and implications for research and treatment. Int Psychogeriatr 8(Suppl 3), 497–500.Google Scholar
[2] (2005) Grouping for behavioral and psychological symptoms in dementia: Clinical and biological aspects. Consensus paper of the European Alzheimer disease consortium. Eur Psychiatry 20, 490–496.Crossref, Google Scholar
[3] (2003) Psychiatric symptoms vary with the severity of dementia in probable Alzheimer’s disease. J Neuropsychiatry Clin Neurosci 15, 346–353.Crossref, Google Scholar
[4] (2010) Behavioral and psychological symptoms associated with dementia subtype and severity. Int Psychogeriatr 22, 300–305.Crossref, Google Scholar
[5] ,
[6] (2004) Cognition and behaviour are independent and heterogeneous dimensions in Alzheimer’s disease. J Neurol 251, 688–695.Crossref, Google Scholar
[7] (2011) Clinicopathological correlates of behavioral and psychological symptoms of dementia. Acta Neuropathol 122, 117–135.Crossref, Google Scholar
[8] (1998) Executive dysfunction in Alzheimer’s disease: Association with neuropsychiatric symptoms and functional impairment. J Neuropsychiatry Clin Neurosci 10, 426–432.Crossref, Google Scholar
[9] (2002) Apathy and executive function in Alzheimer’s disease. J Int Neuropsychol Soc 8, 373–381.Crossref, Google Scholar
[10] (1999) Neuropsychological correlates of apathy and depression in patients with dementia. Neurology 52, 1403–1407.Crossref, Google Scholar
[11] (2012) Apathy in frontotemporal dementia: Behavioral and neuroimaging correlates. Behav Neurol 25, 127–136.Crossref, Google Scholar
[12] (2006) Behavioural and neuropsychological correlates of frontal lobe features in dementia. Psychol Med 36, 1173–1182.Crossref, Google Scholar
[13] (2004) Alzheimer disease with psychosis: Excess cognitive impairment is restricted to the misidentification subtype. Am J Geriatr Psychiatry 12, 449–456.Crossref, Google Scholar
[14] (2000) Neurobehaviors and psychotic symptoms in Alzheimer’s disease. J Int Neuropsychol Soc 6, 815–820.Crossref, Google Scholar
[15] (2012) Systematic reviews on behavioural and psychological symptoms in the older or demented population. Alzheimers Res Ther 4, 28.Crossref, Google Scholar
[16] (2011) The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimers Dement 7, 263–269.Crossref, Google Scholar
[17] (1998) Frontotemporal lobe degeneration. A consensus on clinical diagnostic criteria. Neurology 51, 1546–1554.Crossref, Google Scholar
[18] ;
[19] (2002) Subcortical ischaemic vascular dementia. Lancet Neurol 1, 426–436.Crossref, Google Scholar
[20] (1982) A new clinical scale for the staging of dementia. Br J Psychiatry 140, 566–572.Crossref, Google Scholar
[21] (1996) The Mental Deterioration Battery: Normative data, diagnostic reliability and qualitative analyses of cognitive impairment. The Group for the Standardization of the Mental Deterioration Battery. Eur Neurol 36, 378–384.Crossref, Google Scholar
[22] (2002) Standardizzazione di due test di memoria per uso clinico: Breve Racconto e Figura di Rey. Nuova Riv Neurol 12, 1–13.Google Scholar
[23] (2002) La valutazione delle funzioni esecutive nella pratica neuropsicologica; dal Modified Card Sorting Test al Modified Card Sorting Test-Roma Version. Dati di standardizzazione. Nuova Riv Neurol 12, 13–24.Google Scholar
[24] . (1993) Il Mini-Mental State Examination: Studio normativo di un campione random della popolazione italiana. Dev Neuropsychol 9, 77–85.Crossref, Google Scholar
[25] (1994) The Neuropsychiatry Inventory: Comprehensive assessment of psychopathology in dementia. Neurology 44, 2308–2314.Crossref, Google Scholar
[26] (2007) Classification of psychotic symptoms in dementia with Lewy bodies. Am J Geriatr Psychiatry 15, 961–967.Crossref, Google Scholar
[27] (2007) National Institute of Mental Health Clinical Antipsychotic Trials of Intervention Effectiveness-Alzheimer’s Disease (CATIE-AD): Baseline characteristics. Curr Alzheimer Res 4, 325–335.Crossref, Google Scholar
[28] (2001) Behaviour in frontotemporal dementia, Alzheimer’s disease and vascular dementia. Acta Neurol Scand 103, 367–378.Crossref, Google Scholar
[29] (1999) Specific cognitive deficits in mild frontal variant frontotemporal dementia. Brain 122, 1469–1493.Crossref, Google Scholar
[30] (1999) Theory of mind in patients with frontal variant frontotemporal dementia and Alzheimer’s disease: Theoretical and practical implications. Brain 125, 752–764.Crossref, Google Scholar
[31] (2008) Cerebral metabolic dysfunction in patients with dementia with Lewy bodies and visual hallucinations. Dement Geriatr Cogn Disord 25, 531–538.Crossref, Google Scholar
[32] (2001) Perception, attention, and working memory are disproportionately impaired in dementia with Lewy bodies compared with Alzheimer’s disease. J Neurol Neurosurg Psychiatry 70, 157–164.Crossref, Google Scholar
[33] (1999) Clinical and quantitative pathologic correlates of dementia with Lewy bodies. Neurology 53, 1284–1291.Crossref, Google Scholar
[34] . (1991) Topography, extent, and clinical relevance of neurochemical deficits in dementia of Lewy body type, Parkinson’s disease, and Alzheimer’s disease. Ann N Y Acad Sci 640, 197–202.Crossref, Google Scholar
[35] (1999) The neuropsychiatry of subcortical ischemic brain disease. Curr Psychiatry Rep 1, 69–77.Crossref, Google Scholar
[36] (2001) Apathy in Alzheimer’s disease. J Am Geriatr Soc 49, 1700–1707.Crossref, Google Scholar
[37] (2006) Apathy and the functional anatomy of the prefrontal cortex-basal ganglia circuits. Cereb Cortex 16, 916–928.Crossref, Google Scholar
[38] (2010) Executive deficits and regional brain metabolism in Alzheimer’s disease. Int J Geriatr Psychiatry 25, 1150–1158.Crossref, Google Scholar
[39] ;
[40] (2013) Sensitivity and specificity of ventromedial prefrontal cortex tests in behavioral variant frontotemporal dementia. Alzheimers Dement 9(Suppl), S84–S94.Crossref, Google Scholar



