Subcallosal Cingulate Gyrus Deep Brain Stimulation for Treatment-Resistant Depression
Abstract
Background:
A preliminary report in six patients suggested that deep brain stimulation (DBS) of the subcallosal cingulate gyrus (SCG) may provide benefit in treatment-resistant depression (TRD). We now report the results of these and an additional 14 patients with extended follow-up.
Methods:
Twenty patients with TRD underwent serial assessments before and after SCG DBS. We determined the percentage of patients who achieved a response (50% or greater reduction in the 17-item Hamilton Rating Scale for Depression [HRSD-17]) or remission (scores of 7 or less) after surgery. We also examined changes in brain metabolism associated with DBS, using positron emission tomography.
Results:
There were both early and progressive benefits to DBS. One month after surgery, 35% of patients met criteria for response with 10% of patients in remission. Six months after surgery, 60% of patients were responders and 35% met criteria for remission, benefits that were largely maintained at 12 months. Deep brain stimulation therapy was associated with specific changes in the metabolic activity localized to cortical and limbic circuits implicated in the pathogenesis of depression. The number of serious adverse effects was small with no patient experiencing permanent deficits.
Conclusions:
This study suggests that DBS is relatively safe and provides significant improvement in patients with TRD. Subcallosal cingulate gyrus DBS likely acts by modulating brain networks whose dysfunction leads to depression. The procedure is well tolerated and benefits are sustained for at least 1 year. A careful double-blind appraisal is required before the procedure can be recommended for use on a wider scale.
(Reprinted with permission from Biological Psychiatry 2008; 64:461–467)
Depression is a common disorder and a leading cause of years lived with disability worldwide (1–3). While treatment is often effective, a substantial subgroup of patients with depression, approximately 10% to 20% of sufferers, continue to be severely disabled despite adequate trials of antidepressant drugs, psychotherapy, and electroconvulsive therapy (ECT) (4). For these patients with treatment-resistant depression (TRD), the illness not only affects quality of life but is also a major cause of suicide and contributes to increased mortality in the context of comorbid disorders including diabetes and cardiovascular disease (5–7). Recent advances in understanding the neurobiology and brain circuitry of depression are driving novel treatment approaches for patients with TRD (8).
Functional imaging has shown that depression is associated with increased activity in the subcallosal cingulate gyrus (SCG), a brain area involved in mood regulation (9–11). That this overactivity is important in the pathophysiology of depression is supported by the finding that disparate interventions including pharmacotherapy, transcranial magnetic stimulation, and electroconvulsive therapy ameliorate the clinical features of depression and alter the activity of the SCG (12, 13). These observations have sparked interest in the possibility of directly modulating the output of the SGC and, consequently, its downstream targets in TRD. Deep brain stimulation (DBS) represents an adjustable and reversible method of focally modulating the activity of dysfunctional brain circuits with electrical stimulation. Leveraging our experience with DBS in treating patients with Parkinson disease (14–16), we implanted DBS electrodes in the SCG in six TRD patients and showed improvements in symptoms of depression were associated with decreased activity in SCG (17).
We now report the clinical effects, safety, and the changes in the activity of brain circuitry in the 6 initial patients and an additional 14 patients with TRD receiving DBS of the SCG for a longer time, 12 months.
METHODS AND MATERIALS
Patients
Twenty patients with TRD received DBS of the SCG between May 2003 and November 2006 (Table 1). Referrals came from hospital and community psychiatrists who were aware of the protocol and were not directly involved in its implementation. All patients met criteria for major depressive disorder (MDD), were in a current major depressive episode (MDE) as determined by the Structured Clinical Interview for DSM-IV Axis I Disorders Patient Edition (SCID-I/P) (18), and had a minimum score of 20 on the 17-item Hamilton Rating Scale for Depression (HRSD-17) (19). Inclusion and exclusion criteria have been previously described (17). Essentially, inclusion criteria required a duration of at least 1 year for the current MDE and treatment resistance as defined as failure to respond to a minimum of four different treatments, including antidepressant pharmacotherapy of sufficient dose and duration, evidence-based psychotherapy, and electroconvulsive therapy. Exclusion criteria included comorbid Axis I psychiatric conditions, a cluster B Axis II diagnosis as determined by the Structured Clinical Interview for DSM-IV Axis II Personality Disorders (SCID-II) (20), suicidal behavior within the past year or a score of 3 or more on the HRSD-17 suicide item, and concurrent neurological or medical conditions that could interfere with the treatment. The study was approved by the Institutional Review Board of the University Health Network, Baycrest Centre, and the Centre for Addiction and Mental Health. Signed informed consent was obtained from each patient.
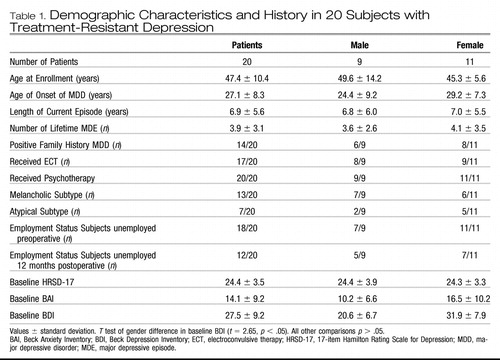 |
Table 1. Demographic Characteristics and History in 20 Subjects with Treatment-Resistant Depression
Assessment
Psychiatric assessments and stimulator adjustments (if warranted) were performed at 1, 2, and 4 weeks after surgery; biweekly for 3 months; and then monthly for up to 12 months. The primary outcome measure was the percentage of patients who achieved a 50% or greater reduction in the severity of depression, as measured by HRSD-17 scores (defined as “response”) with a secondary outcome as those who achieved clinical “remission”(defined as a HRSD-17 score of 7 or less). Other standardized ratings included the Beck Anxiety Inventory (BAI) (21), the Beck Depression Inventory (BDI) (22), and the Clinical Global Impression for Severity (CGI-S) scale. Neuropsychological testing was administered to establish baseline intellectual and cognitive function prior to surgery and to monitor for changes after 3, 6, and 12 months of continuous stimulation. A preliminary report on the neuropsychological battery utilized and results obtained in a subgroup of these patients is described elsewhere (23). Positron emission tomography (PET) scan measures of regional glucose metabolism were used to quantify changes in brain activity with chronic stimulation, expanding on findings reported in the initial cohort of these patients (17).
Surgical procedure
A Leksell stereotactic frame (Elekta, Stockholm, Sweden) was applied using local anesthesia. Magnetic resonance imaging (MRI) was obtained using a 1.5 T scanner (Signa, General Electric, Milwaukee, Wisconsin). Images of 2 mm thickness without overlap were reconstructed in three dimensions using neuronavigation software (Stealth, Medtronic, Minneapolis, Minnesota). The SCG was identified by direct visualization on coronal images and target points spanning the entire vertical height of the gyrus were chosen. Bilateral burr holes were placed 1 cm anterior to the coronal suture and 2 cm from the midline under local anesthesia. Microelectrode recordings were used to identify the upper and lower cortical banks and the intervening white matter of the SCG. Quadripolar DBS electrodes (Medtronic 3387, Medtronic) were implanted with the most distal contact (contact 0 on the right, contact 4 on the left) adjacent to the ventral bank of gray matter, the two central contacts (contacts 1 and 2 right, contacts 5 and 6 left) in white matter, and the uppermost contacts (contact 3 right, contact 7 left) adjacent to the upper bank of gray matter of the SCG gyrus using fluoroscopic guidance and confirmed on postoperative MRI. A dual channel programmable internal pulse generator (Kinetra, Medtronic) was implanted subcutaneously in the infraclavicular area under general anesthesia. Patients were discharged on postoperative days 2 to 5.
Stimulation settings
Blinded stimulation through the implanted DBS electrodes was applied intraoperatively and in the immediate postoperative period. The acute response to stimulation was noted at each contact and each setting with the patient blinded to the stimulator settings or changes. The selection of electrode contacts for the initial settings for chronic stimulation was made on the basis of the observation of acute behavioral effects with stimulation. These included calmness, improved mood, and increased interest and motivation and were achieved using 3 V to 6 V. As part of dose finding, stimulation intensity was increased to look for adverse effects. In two patients, mental slowing occurred at high settings of 8 V to 10 V, particularly at the higher contacts (3 and 7). In patients who showed little or no acute behavioral changes, we started stimulation at contacts 1 and 5 set at 3.5 V, 90 microseconds, and 130 Hz. Adjustments to the neurostimulator at follow-up visits were made if the patient was failing to show improvement (less than a 10% reduction in the HRSD-17 scores) or developed adverse effects, for example increased anxiety. Changes consisted of choosing different contact pairs for stimulation or adjusting the voltage. Patients received continuous monopolar stimulation at settings that ranged from 3.5 V to 5.0 V with the pulse width set at 90 microseconds and the frequency at 130 Hz with the pulse generator case set as the positive terminal. Notably, patients reported inability to discern if the contacts were on or off during these subsequent sessions and remained blinded as to which contact was being stimulated and the parameter settings.
Statistical analysis
A reduction from baseline to end point in total HRSD-17 score of 50% or greater (i.e., responders) was chosen as the primary measure of effectiveness. We also determined the proportion achieving a score of 7 or less at end point on the HRSD-17 (i.e., remitters). We further examined changes in the subcomponents of the HRSD-17 (mood, anxiety, somatic, and sleep) (24). Pairwise comparisons between the results of the baseline evaluation and the results of the follow-up evaluations were made with the Student t test. To evaluate long-term effectiveness, data were examined at 6 and 12 months after surgery. Data from early terminators were analyzed in a last observation carried forward manner.
PET scan acquisition and data analysis
We have previously characterized specific abnormalities in regional cerebral blood flow in patients with TRD (compared with healthy age-matched control subjects), as well as changes with SCG DBS (17). We sought to confirm and expand on these observations in the current study. Positron emission tomography scans were used to establish changes in brain activity after 3 and 6 months of chronic stimulation relative to the presurgical baseline. In the initial report, regional blood flow was measured using 150-water PET (as previously described). Due to technical issues including installation of a new PET scanner and lack of subsequent availability of the blood flow tracer, regional glucose metabolism was measured instead using 18F-fluorodeoxyglucose (18FDG) in the last 11 patients (3 patients were not imaged). The PET protocol and analysis are described in Supplement 1.
RESULTS
Primary outcome measures
The clinical and demographic features of 20 patients with TRD undergoing SCG DBS are shown in Table 1. We treated 9 men and 11 women with SCG DBS. All had failed multiple trials of pharmacotherapy and psychotherapy and all but three (who refused) also received a course of electroconvulsive therapy during the current episode without response. The mean duration of the current major depressive episode was 6.9 years (SD 5.6). One patient who initially was diagnosed with unipolar depression had more accurately, in retrospect, previously undiagnosed bipolar II disorder. All were required to maintain a stable dosing regimen in the 4 weeks prior to surgery.
The mean number of medications at the time of surgery was 4.2 and the median was 4. Eleven patients were receiving two antidepressants from distinct classes augmented with an atypical antipsychotic or lithium and a benzodiazepine. Five patients were receiving one antidepressant combined with a benzodiazepine or antipsychotic agent, two were receiving an antidepressant alone, and two had discontinued all antidepressants but continued to receive a benzodiazepine. So as to not confound the effects of DBS, we attempted not to change medications during the course of the study, and no new antidepressant medications were prescribed in the first 6 months after surgery. The emergence of dose-related, medication-specific adverse effects or clinical improvement led to reductions in the number or dose of medications. Minor medication changes occurred over time as described in Supplement 2.
The mean total HRSD-17 score in the 20 patients was significantly improved at all time points examined 1 month or longer after DBS than at baseline (Figure 1). After 1 week of stimulation, 40% of patients were responders and one patient was in remission. Some of this initial benefit may be related to a microlesion effect as is commonly observed in Parkinson's disease where the mere insertion of a DBS electrode can produce transient clinical benefits. Consistent with this notion, the response rate fell to 30% with one patient in remission 2 weeks after surgery.
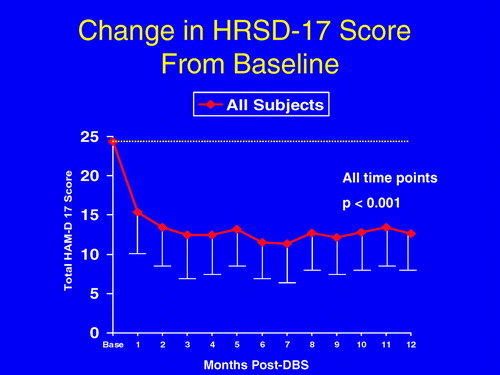
The values at all time points from 1 to 12 months are significantly different compared with baseline p < .001. Error bars indicate standard deviation. DBS, deep brain stimulation; HRSD-17, 17-item Hamilton Rating Scale for Depression; SGC, subcallosal cingulate gyrus; TRD, treatment-resistant depression.
We observed a progressive improvement with chronic DBS. From 2 weeks to 6 months after surgery, an increasing proportion of patients improved, reaching a plateau at 6 months when 60% of patients met response criteria and 35% achieved remission (Figure 2). These benefits were largely maintained for the duration of the study. At 12 months, 55% of patients were responders and 35% achieved or were within 1 point of remission (scoring 8 or less on the HRSD-17 scale). Eight (72.7%) of 11 patients who met criteria for response at 6 months also met criteria for response at 12 months, while 3 (33%) of 9 patients who were not deemed responders at 6 months obtained response status at 12 months (Fisher exact test p = .08). There was no difference in response rates at 12 months post-DBS between those that had previously received ECT and those that had not (9/17 vs. 2/3, Fisher exact test p = .58). There was no significant loss of effect requiring dose adjustments over time, an observation that mimics the relative stability of DBS stimulation parameters in patients with other disorders including Parkinson's disease and dystonia.
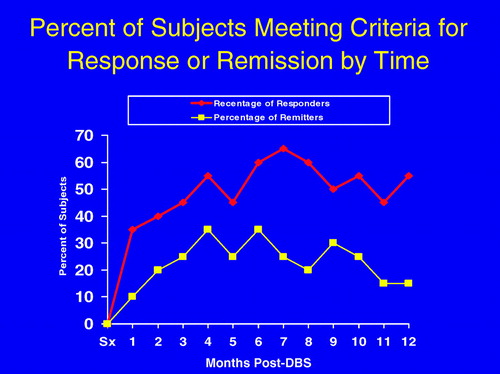
The proportion of patients responding or reaching remission increased over time to plateau from 6 to 12 months. DBS, deep brain stimulation.
Deep brain stimulation was associated with global improvements in depressive symptomatology as measured by the mood, anxiety, somatic, and sleep subcomponents of the HRSD scale (Table 2). Improvements in each of the symptom clusters increased with time after the initiation of stimulation. The maximal improvement in the mood component occurred by 3 months. Longer times were required to reach maximal improvements in anxiety, sleep, and somatic symptoms (Table 2). An analysis of the rates of symptom cluster improvement in responders versus nonresponders at 12 months suggested that responders achieved greater symptom improvement in the mood cluster of symptoms at months 3, 6, and 12 and in the somatic cluster of symptoms at 1 and 12 months compared with nonresponders. There were no baseline differences in total HRSD-17 score or any of the symptom cluster scores between patients who achieved or failed to achieve response status at 12 months. The different responses times of the various depression components suggest that the distinct symptoms of depression may be supported by different neural substrates, a hypothesis further supported by the time course of PET changes described below.
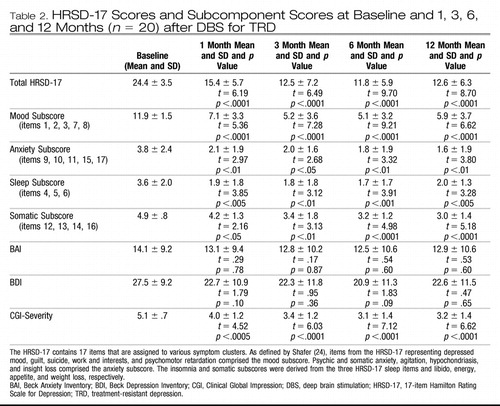 |
Table 2. HRSD-17 Scores and Subcomponent Scores at Baseline and 1, 3, 6, and 12 Months (n = 20) after DBS for TRD
Secondary measures
We sought to validate the improvements in depression as measured with the HRSD-17 ratings obtained by examining the patients with the patient's self-rating of their response to DBS. Self-reported depressive symptoms at 12 months as measured by the BDI demonstrated a robust positive correlation with the physician-rated HRSD-17 at 12 months (r = .73, p < .001), indicating a congruency in the clinical and self-reported impressions of improvement. Additionally, the total BAI score and HRSD-17 scores at 12 months were significantly correlated (r = .58, p < .01). Consistent with these observations, patients improved from a mean baseline Clinical Global ImpressionSeverity rating of 5.1 (markedly to severely ill) to a mean rating of 3.1 at 6 months and 3.2 at 12 months (mildly to moderately ill) (Table 2). Improvements in CGI-S scores were seen at 1 month and remained statistically significant for the entire 12 months of the trial. Further, 6 of 17 patients who were not working due to their illness at enrollment returned to employment after being unemployed for 2 to 7 years, indicating that DBS also led to significant social reintegration.
PET measures of regional glucose metabolism: effects of stimulation
Of the 11 patients undergoing 18FDG PET scans, there were 8 responders and 3 nonresponders. As only two nonresponders completed all three PET scans, results are presented only for the eight responders. The response to treatment with DBS was accompanied by widespread changes in metabolic activity in limbic and cortical areas as measured with 18FDG PET (Figure 3). Areas of significant change included decreases in orbital (Brodmann area [BA] 11), medial frontal cortex (BA 10/9/8), and insula and increases in lateral prefrontal cortex (BA 11/47, BA 46/10/9), parietal (BA 40), anterior midcingulate (BA 24), and posterior cingulate areas (BA 23) (Supplement 3). The medial and orbital frontal decreases were seen by 3 months and were sustained at 6 months (Supplement 3, Figure 3). A different pattern was seen in both ventrolateral and dorsolateral prefrontal, parietal, midcingulate, and posterior cingulate regions, where changes were only significant at 6 months (Supplement 3). The topography of the brain areas affected with DBS is specific and is consistent with the known connectivity of the subcallosal cingulate gyrus. The pattern of metabolic changes seen here generally replicates those seen in the original study of blood flow PET (Figure 3). Taken together, these results indicate that SCG DBS produces striking changes in cognitive and limbic brain areas and they provide a biological basis for the observed improvements in depression in these patients.
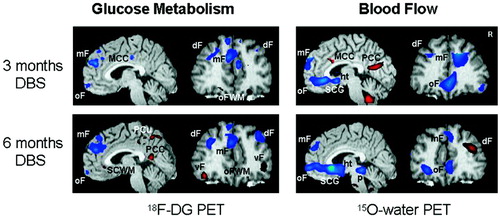
Changes relative to baseline in DBS responders studied at 3 and 6 months of treatment using 18F-fluoro-deoxyglucose PET (FDG, n = 8) or 15O water blood flow PET (n = 3). Increases in activity shown in red; decreases in blue; significance threshold p < .005. Note similar pattern of relative glucose metabolism and blood flow changes in orbital frontal (oF), medial frontal (mF), dorsolateral prefrontal (dF) and posterior cingulate (PCC) with both tracers. Discordance is seen in the subcallosal cingulate gyrus target region (SCG) with focal glucose metabolic increases restricted to the adjacent white matter (SCWM) in the FDG scans and more widespread SCG decreases in the blood flow scans. Abbreviations: 18FDG, 18F-fluoro-deoxyglucose; DBS, deep brain stimulation; dF, dorsal frontal; ht, hypothalamus; mF, medial frontal; MCC, mid cingulate cortex; oF, orbital frontal; ofWM, orbital frontal white matter; p, pons; PCu, precuneus; PCC, posterior cingulate cortex; PET, positron emission tomography; SCG, subcallosal cingulate gyrus; SCWM, subcallosal cingulate white matter; vF, ventral frontal.
Of interest, opposite metabolic changes were present at the target of stimulation, the subcallosal cingulate white matter, and the immediately adjacent gray matter. In contrast to our previous report using blood flow where only decreased SCG activity was observed, we noted focal increases in metabolism in the immediate vicinity of the stimulating electrode with decreases in metabolism in the adjacent caudal subcallosal gray matter consistent with the previous blood flow results (Figure 3, sagittal image, left). These results suggest that there is a direct activation of the white matter at target that can lead to either metabolic activation or inhibition in distinct remote brain areas.
Adverse events
Table 3 shows the adverse events in the 20 patients. Four patients had wound infections. In three cases, this occurred early in the series when electrodes were externalized for several days before the internal pulse generator was inserted. These three patients had their hardware removed. In one patient, the hardware was reimplanted after a 6-month delay with recapture of the clinical benefit. In the two others who did not receive significant benefit, the explanted hardware was not replaced. All patients from patient 6 on had electrode and pulse generator inserted in a single surgery. One patient had superficial scalp cellulitis 2 weeks after surgery that responded to antibiotics. One patient experienced a generalized seizure the evening of surgery. He was treated with phenytoin for 3 months with no further seizures. Four patients reported headache or pain at the site of the pulse generator implant in the immediate postoperative period. The behavioral aspects of this pain included crying and despair. Such responses were unanticipated, as they are rarely seen in other patient populations having similar DBS surgery for other diagnoses. In seven patients, there were no emergent adverse effects. Finally, neuropsychological testing confirmed the absence of any cognitive adverse effects (23). There were no cases of hypomania with stimulation in these patients.
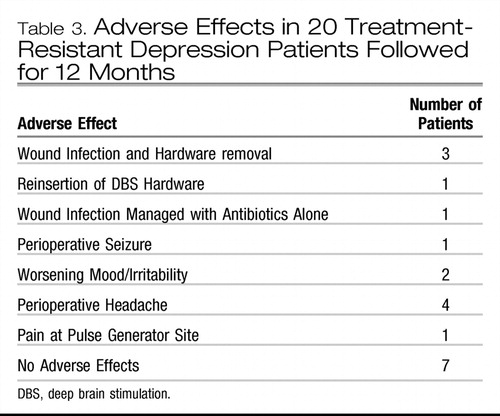 |
Table 3. Adverse Effects in 20 Treatment-Resistant Depression Patients Followed for 12 Months
DISCUSSION
Impact of DBS on TRD
We found that SCG DBS produced robust improvements in depression in patients with TRD. Benefits were seen across multiple domains of depression as reflected by improvements in each of the mood, anxiety, somatic, and sleep subcomponents of the HRSD-17 scale. The maximal benefits were delayed and progressive. They reached a plateau at 6 months and were generally sustained up to last time point measured at 12 months. Patients who showed a benefit at 1 month were likely to maintain response 6 and 12 months later, indicating that the relapse rate was low among responders. Overall, patients were markedly improved with surgery and the benefits were sustained over time, leading in certain cases to reintegration into their family and social activities and a return to their past work activities. These results, particularly in this treatment-resistant patient population, are striking.
Mechanism of action
The SCG is synaptically connected with cortical and subcortical structures implicated in the pathophysiology of major depression including pregenual anterior cingulate, midcingulate, and posterior cingulate; ventromedial and dorsomedial frontal cortex; and the hypothalamus, amygdala, hippocampus, nucleus accumbens, and periaqueductal areas. The SCG is thus in a position to influence neural elements that underlie major aspects of depression including mood state, cognitive processes, sleep and circadian rhythm, appetite, cortisol regulation, pain processing, reward mechanisms, and emotional reactivity. Our PET results show that DBS produces large changes in brain activity that are distributed along these downstream targets of the SCG. Of interest, distinct therapies including antidepressant medications, cognitive behavioral therapy, and electroconvulsive therapy produce similar changes in many of these same brain regions (25). This suggests that SCG DBS together with other therapies that also ameliorate depression exert their effects on a common set of neural substrates. These observations support the idea that the changes in the clinical attributes of depression we have observed with DBS are a consequence of changes in the activity of these brain regions. We cannot be certain, however, to what extent these changes in brain activity are necessary or sufficient to produce the clinical benefit or whether they are merely biological markers of the state of improvement.
Despite the clinical and metabolic changes in brain circuits, we do not know the precise mechanism of action through which stimulation exerts its effects at the cellular level. The proposed physiologic effects of DBS include axonal excitation, depolarization blockade, synaptic release of neurotransmitters, jamming of abnormal neuronal firing, and disruption of pathological neuronal synchrony (26). Both anterograde and retrograde effects may contribute to the observed clinical effects. Our results are consistent with the notion that SCG DBS drives focal activity at the immediate target, which, in turn, leads to inhibition or excitation in adjacent and remote areas to which it is connected. We hypothesize that DBS disrupts pathological activity in the circuits underlying depression and allows more normal cerebral function and behavior.
Responders versus nonresponders
One of the greatest challenges is to understand why some patients with TRD respond to SCG DBS and others do not. The majority of patients in the study receiving DBS met response criteria and most others, indeed all but two patients, showed some drop in HRSD-17 scores; no patient experienced a worsening of symptoms. The reasons for the variability in response across this patient population with TRD are not well understood but likely are related to the heterogeneity of MDD. To date, we have not identified demographic, clinical, or image-based predictors of response. There were too few nonresponders to characterize responder/nonresponder differences in the PET changes, especially since there is a strong partial response in nonresponders. This may change with a larger sample. We also considered the possibility that the response to SCG DBS could be a function of the location of electrode placement and the specificity of the neural structures being stimulated. Our preliminary analysis suggests that there are no significant differences in electrode location within the SCG in responders and nonresponders. We are using diffusion tensor imaging and voxelbased morphometry to study whether neuroanatomic variations including the position of axonal afferents and efferents within the SCG or differences in gray matter density across subjects could contribute to the variability (27).
Limitations
A limitation of this study is the open label assessment of outcomes. While we do not know the relative magnitude of its contribution, there are several observations that argue against a placebo response playing a major role. First, the response was progressive over time. A different pattern with an initial large effect followed by a decay would be more in keeping with a placebo response. Second, we have noted that for each patient, the effects vary with which electrode contacts and electrical stimulation settings are chosen, arguing for the specificity of stimulation. Third, in two patients, one planned and another unplanned, blinded discontinuation of stimulation was associated with a recurrence of depression within 1 week and a recapture of benefit within 1 week of reintroduction of the stimulation. Finally, examination of placebo response rates in patients with chronic and severe depression, particularly those with melancholia, show a reduced propensity for placebo-mediated improvements (28, 29), including a reported placebo response rate to vagus nerve stimulation in a comparably treatment-resistant patient population of 10% at 10 weeks (30). Taken together, the results suggest that the improvements are highly dependent on the delivery of effective stimulation to the SCG.
CONCLUSION
We conclude that SCG DBS improves many of the symptoms of severe depression in patients who have failed to respond to conventional treatments. Improvements are seen within 1 month and last for at least 1 year. The procedure is well tolerated and no patient suffered a permanent serious adverse effect. In contrast to previously utilized ablative neurosurgical procedures for depression, DBS is adjustable and stimulation is reversible. These features increase safety and may offer advantages for both the efficacy of the therapy and its acceptance in the patient, medical, and psychiatric community.
We have shown that DBS applied to the SCG produces striking changes in the metabolic activity of the brain circuits that mediate depression. Our results are consistent with the notion that changes in activity in these selected brain circuits are responsible for the behavioral changes and clinical improvements we have seen in this treatment-resistant population. Deep brain stimulation in treatment-resistant patients requires a dedicated multidisciplinary team of neurosurgeons, psychiatrists, and support staff. While these results, particularly in this difficult-to-treat population, are promising, they represent an initial step and a blinded evaluation of DBS in TRD is required before it could be adopted on a wider scale.
1 The World Health Organization (2004): Annex Table 3: Burden of disease in DALYs by cause, sex, and mortality stratum in WHO regions, estimates for 2002. In: The World Health Report: Changing History. Geneva: World Health Organization.Google Scholar
2 Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE (2005): Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:593–602Crossref, Google Scholar
3 Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE (2005): Prevalence, severity, and comorbidity of 12-month DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry 62:617–627Crossref, Google Scholar
4 Depression Guideline Panel, editor (1993): Depression in Primary Care: Volume 2 Treatment of Major Depression. Rockville, MD: U.S. Department of Health and Human Services, Public Health Service, Agency for Health Care Policy and Research.Google Scholar
5 Conwell Y, Brent D (1995): Suicide and aging. I: Patterns of psychiatric diagnosis. Int Psychogeriatr 7:149–164Crossref, Google Scholar
6 Kochanek KD, Murphy SL, Anderson RN, Scott C (2004): Deaths: Final data for 2002. Natl Vital Stat Rep 53:1–115Google Scholar
7 Weissman MM, Bland RC, Canino GJ, Greenwald S, Hwu HG, Joyce PR, et al. (1999): Prevalence of suicide ideation and suicide attempts in nine countries. Psychol Med 29:9–17Crossref, Google Scholar
8 Dougherty DD, Rauch SL (2007): Somatic therapies for treatment-resistant depression: New neurotherapeutic interventions. Psychiatr Clin North Am 30:31–37Crossref, Google Scholar
9 Mayberg HS, Liotti M, Brannan SK, McGinnis S, Mahurln RK, Jerabek PA, et al. (1999): Reciprocal limbic-cortical function and negative mood: Converging PET findings in depression and normal sadness. Am J Psychiatry 156:675–682Google Scholar
10 Mayberg HS (2003): Modulating dysfunctional limbic-cortical circuits in depression: Towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull 65:193–207Crossref, Google Scholar
11 Seminowicz DA, Mayberg HS, McIntosh AR, Goldapple K, Kennedy S, Segal Z, et al. (2004): Limbic-frontal circuitry in major depression: A path modeling metanalysis. Neuroimage 22:409–418Crossref, Google Scholar
12 Agid Y, Buzsaki G, Diamond DM, Frackowiak R, Giedd J, Girault JA, et al. (2007): How can drug discovery for psychiatric disorders be improved? Nat Rev Drug Discov 6:189–201.Crossref, Google Scholar
13 Ressler KJ, Mayberg HS (2007): Targeting abnormal neural circuits in mood and anxiety disorders: From the laboratory to the clinic. Nat Neurosci 10:1116–1124Crossref, Google Scholar
14 Fine J, Duff J, Chen R, Chir B, Hutchison W, Lozano AM, et al. (2000): Long-term follow-up of unilateral pallidotomy in advanced Parkinson's disease. N Engl J Med 342:1708–1714Crossref, Google Scholar
15 Lang AE, Lozano AM (1998): Parkinson's disease. Second of two parts. N Engl J Med 339:1130–1143Crossref, Google Scholar
16 Lang AE, Lozano AM (1998): Parkinson's disease. First of two parts. N Engl J Med 339:1044–1053Crossref, Google Scholar
17 Mayberg HS, Lozano AM, Voon V, McNeely HE, Seminowicz D, Hamani C, et al. (2005): Deep brain stimulation for treatment-resistant depression. Neuron 45:651–660Crossref, Google Scholar
18 First MB, Spitzer RL, Gibbon M, Williams JBW (2002): Structured Clinical Interview for DSM-IV-TR Axis I Disorders, Research Version, Patient Edition. (SCID-I/P). New York: Biometrics Research, New York State Psychiatric Institute.Google Scholar
19 Hamilton MA (1960): Rating scale for depression. J Neurol Neurosurg Psychiatry 23:56–62Crossref, Google Scholar
20 First MB, Spitzer RL, Gibbon M, Williams JBW (1997): Structured Clinical Interview for DSM-IV Personality Disorders, (SCID-II). Washington, DC: American Psychiatric Press, Inc.Google Scholar
21 Beck AT, Steer RA (1993): Beck Anxiety Inventory Manual. San Antonio, TX: Psychological Corporation.Google Scholar
22 Beck AT, Steer RA, Brown GK (1996): Beck Depression Inventory Manual, 2nd ed. San Antonio, TX: Psychological Corporation.Google Scholar
23 McNeely HE, Mayberg HS, Lozano AM, Kennedy SH (2008): Neuropsychological impact of Cg25 deep brain stimulation for treatment resistant depression: Preliminary results over 12 months. J Nerv Ment Disease 196:405–410Crossref, Google Scholar
24 Shafer AB (2006): Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. J Clin Psychol 62:123–146Crossref, Google Scholar
25 Goldapple K, Segal Z, Garson C, Lau M, Bieling P, Kennedy S, et al. (2004): Modulation of cortical-limbic pathways in major depression: Treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry 61:34–41Crossref, Google Scholar
26 Lozano AM, Dostrovsky J, Chen R, Ashby P (2002): Deep brain stimulation for Parkinson's disease: Disrupting the disruption. Lancet Neurol 1:225–231Crossref, Google Scholar
27 Johansen-Berg H, Gutman DA, Behrens TE, Matthews PM, Rushworth MF, Katz E, et al. (2008): Anatomical connectivity of the subgenual cingulate region targeted with deep brain stimulation for treatment-resistant depression. Cereb Cortex 18:1374–1383Crossref, Google Scholar
28 Brown WA (2007): Treatment response in melancholia. Acta Psychiatr Scand Suppl 433:125–129Crossref, Google Scholar
29 Peselow ED, Sanfilipo MP, Difiglia C, Fieve RR (1992): Melancholic/endogenous depression and response to somatic treatment and placebo. Am J Psychiatry 149:1324–1334Crossref, Google Scholar
30 Rush AJ, Marangell LB, Sackeim HA, George MS, Brannan SK, Davis SM, et al. (2005): Vagus nerve stimulation for treatment-resistant depression: A randomized, controlled acute phase trial. Biol Psychiatry 58:347–354Crossref, Google Scholar



