Evaluation and Management of Insomnia in the Psychiatric Setting
Abstract
Insomnia is experienced by nearly one-third of the population over the course of a year. It affects many dimensions of daily human function. Although its pathophysiology is poorly understood, it is felt to be the final product of excessive arousal in multiple neurophysiological and psychological systems. Because it can coexist with a wide variety of medical and psychiatric disorders, the first task of the clinician in the management of this condition is to engage in a comprehensive evaluation to identify comorbid disorders. Once these are identified, specific treatment can be conducted with confidence. A variety of cognitive/behavioral and pharmacological management techniques are also available for primary insomnia.
Sleep accounts for one-third of human life. It is a highly valued state, as is evident in the magnitude of complaints expressed regarding difficulties with sleep. Sleep is also a powerful drive; human beings cannot remain awake voluntarily for longer than 2–3 days (1). All organisms sleep for hours every day, despite the potential consequences that such a state creates, namely vulnerability to predators and the inability to feed and mate. It is highly likely, therefore, that sleep serves important adaptive functions, yet their nature is not understood.
NORMAL SLEEP NEUROPHYSIOLOGY
Human sleep is believed to be regulated by three basic neural processes: a homeostatic process, whose magnitude depends on the amount of prior sleep and wakefulness, a circadian process, which is governed by an endogenous circadian pacemaker generating near 24-hour cycles of behavior; and an arousal network, which promotes wakefulness and opposes the drive for sleep (Figure 1) (2). It is hypothesized that the interaction between homeostatic, circadian, and arousal systems is responsible for helping humans to maintain wakefulness during the day and consolidated sleep at night.
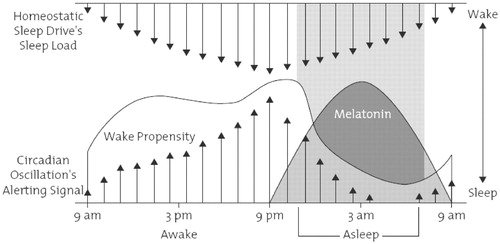
(From Richardson GS: The human circadian system in normal and disordered sleep. The Journal of Clinical Psychiatry 2005; 66(Suppl 9):3–9. © 2005. Physicians Postgraduate Press. Reprinted by permission.)
The homeostatic factor represents the drive for sleep and increases with increasing duration of prior wakefulness, as evidenced by the common experience of increasing daytime sleepiness after nights of sleep restriction. Under normal conditions, this drive builds up during wakefulness and reaches its peak in the evening. It declines in strength during sleep, reaching a nadir upon awakening in the morning. The strength of this drive is an important determinant of the propensity to fall and to stay asleep at night. A variety of substances have been hypothesized to underlie the homeostatic sleep drive, including neuromodulators such as adenosine. Adenosine levels accumulate in the brain during wakefulness periods, eventually activating sleep-promoting neurons and inhibiting neurons promoting wakefulness (3).
Opposing the homeostatic drive is the circadian sleep/wake process, presumed to be regulated by a pacemaker, with an intrinsic periodicity of slightly more than 24 hours (Figure 1). The “master biological clock,” which regulates sleep-wake and all other circadian rhythms, resides in the suprachiasmatic nuclei (SCN) of the hypothalamus, which are bilaterally paired and located slightly above the optic chiasm in the anterior hypothalamus. Other circadian rhythms regulated by the SCN include the release of cortisol, thyroid-stimulating hormone, and melatonin. During normal wakefulness, the circadian process promotes wakefulness by transmitting stimulatory signals throughout the CNS from the SCN. During the wake period, these alerting signals are dominant, reaching a peak about 2–3 hours before one's habitual bedtime and serving to offset the homeostatic drive for sleep that accumulates during waking hours. During the evening, this alerting signal is diminished, in part, via increased melatonin levels, eventually allowing sleep to occur.
Circadian clocks are normally synchronized to environmental cues by a process called entrainment. In most mammals and human beings, the light-dark cycle is the most potent entraining stimulus. In the absence of environmental cues, the endogenous rhythmicity of the circadian pacemaker persists independently of the light-dark cycle (4). The genes of the SCN cells, through transcription-translation, are responsible for maintaining the 24-hour clock (5).
The arousal system, which encompasses the stress hormones and the autonomic nervous system, opposes the sleep drive and fosters alertness. One of the most important networks for wakefulness is the reticular activating system, which sends projections from the brainstem to multiple areas of the forebrain. Included in this arousal system are the laterodorsal tegmental and pedunculopontine tegmental nuclei whose cholinergic fibers interconnect with key forebrain targets such as the thalamus, an area critical to regulating cortical activity. Other important arousal centers include aminergic nuclei such as the tuberomammillary nucleus (histamine), the raphe nuclei (serotonin), and the locus coeruleus (norepinephrine) that diffusely innervate the forebrain, regulating cortical and hypothalamic function (6).
INSOMNIA
Insomnia is the complaint of an inability to fall or stay asleep or unrefreshing sleep. It represents the second most commonly expressed complaint (after pain) in clinical settings (7).
Prevalence
Of the general adult population, 35% experience insomnia during the course of 1 year (8). Half experience the problem as severe, and 20.1% of adults are dissatisfied with their sleep or take medication for sleeping difficulties (9).
Studies on the prevalence of insomnia in adults have yielded discrepant results, largely owing to differences in defining insomnia. In a recent study (10) insomnia was divided into four categories: 1) difficulty in initiating and maintaining sleep or nonrestorative sleep; 2) insomnia symptoms accompanied by daytime consequences; 3) dissatisfaction with sleep quality or quantity; and 4) insomnia diagnosis based on definitions established by DSM-IV-TR or International Classification of Sleep Disorders (ICSD) (11). The first category revealed a prevalence of 30%–48%. This dropped to 16%–12% when frequency modifiers were added to symptoms such as the presence of symptoms on at least 3 nights a week or “often” or “always.” When severity criteria were added to insomnia symptoms, the prevalence of insomnia ranged from 10% to 28%. The prevalence of insomnia based on insomnia symptoms with daytime consequences (category 2) was approximately 10%. The prevalence of insomnia based on dissatisfaction with sleep quality and quantity (category 3) was 8%–18% with a higher prevalence being consistently being reported in females. The prevalence of insomnia based on DSM-IV-TR classification varied from 4.4% to 6.4%. Primary insomnia was the most frequent diagnosis, its prevalence ranging between 2% and 4%.
There are fewer studies in the pediatric population, but they show a similar prevalence. One of the earliest studies in preadolescent children showed that 14% of an outpatient pediatric population between the ages of 6 and 12 had insomnia with a mean duration of 5 years (12).
Insomnia is more common in women by a factor of 1.5:1 (13). In women, its prevalence peaks during pregnancy and in the peri- and postmenopausal years. It is also more common in adolescents (ages 11–14) than in younger girls (30.4% versus 16.8%) (14). Its prevalence also increases with advancing age and it affects more than one-third of the population aged 65 and older (15). The risk of insomnia is based on levels of inactivity, dissatisfaction with social life, and the presence of organic and mental disorders, without which the risk of insomnia in healthy seniors is similar to that of younger individuals.
Insomnia is more common in shift workers than in individuals working fixed schedules (20.1% versus 12.0%) (16), and its prevalence increases in proportion to the number of shifts worked (17). Working the night or third shift may not only acutely cause insomnia but may also have a persistent deleterious effect on sleep quality even after the individual has reverted to working a day or evening shift (18).
Unemployment, lower socioeconomic status (15, 16), marital status (being divorced, widowed, or single) (10), poor mental and physical health (19), noisy environments (20), medical problems (depressive disorders, anxiety disorders, substance abuse, schizophrenia, congestive heart failure, obstructive airway disease and other respiratory illnesses, back and hip problems, and prostate problems) are also associated with an increased prevalence of insomnia (21–25).
Seasonal differences have been reported in patients with chronic insomnia. In Norway a survey done among a representative sample of 14,667 adults living in the municipality of Tromsø, north of the Arctic Circle, revealed increased incidence of complaints of insomnia during the dark period of the year (26).
Impact
Insomnia is associated with decreased quality of life (directly related to the severity of insomnia), greater use of health care resources, functional decrements (27–29), and cognitive deficits (30, 31). A fundamental methodological issue in many studies examining the impairments associated with insomnia has been the inclusion of insomniacs with a wide variety of comorbid conditions. Nevertheless, a limited number of recent studies revealed psychomotor performance deficits in primary insomniacs when responding to challenging reaction time tasks.
One of two studies conducted over the course of 6 years revealed an increased mortality rate and the other revealed no increase in insomniacs (32, 33). A more recent study in older adults with a mean follow-up of 12.8 years revealed that participants who had baseline sleep latencies of greater than 30 minutes had a 2.14 times greater mortality risk and those with sleep efficiency <80% had a 1.93 times greater risk of death (34). Mechanisms that may underlie an association between insomnia and mortality are unclear, yet a study showing an association between difficulties falling asleep and mortality due to coronary artery disease in men (35) may provide some clues into this question. The existence and continuation of insomnia also confer a risk for the future occurrence of new psychiatric disorders (see below).
Insomnia causes a significant burden for the health care system (36) and also for employers of insomniacs in both direct and indirect expenses including medical expenses, ramifications of accidents, and reduced productivity due to absenteeism and decreased work efficiency (29). Insomnia costs the American public $92.5–$107.5 billion annually (37).
PATHOPHYSIOLOGY
The pathophysiology of primary insomnia is poorly understood. Nevertheless, a variety of etiological models have been advanced. Each of these is, in turn, linked to a set of therapeutic options, which are discussed in the section on treatment modalities.
Neurophysiologic models
Studies in insomnia reveal evidence of hyperarousal, an overly active arousal system. This is evident not only in the environment and timing of sleep but also during the day, as insomniacs display enhanced alertness or a decreased ability to fall asleep during the daytime nap opportunities of the multiple sleep latency test compared with normal sleepers (38). Hyperarousal is also evident in the following areas in patients with primary insomnia:
| 1. | Sleep electroencephalography: Tests have shown reduced low-frequency delta power (typical of deep non-REM sleep) across the night, particularly in the first part of the night, and increased high-frequency beta power across the entire night compared with those in healthy control subjects (39). | ||||
| 2. | Neuroendocrine axis: Increased cortisol and ACTH levels have been found before and during sleep, particularly during the first half of sleep (40). | ||||
| 3. | Metabolic brain activity: Single-photon emission computed tomography and positron emission tomography scans reveal increased global glucose metabolic rates during both wakefulness and sleep compared with those in healthy control subjects, and the usual sleep-related decline in metabolism in brainstem arousal centers is attenuated (41). | ||||
| 4. | Physiological and metabolic systems: An increase in heart rate, a decrease in heartbeat-to-beat variability (42), and an increase in whole-body metabolic rate (43) during sleep have been noted in patients with insomnia compared with control subjects. | ||||
Other neurophysiologic models indicate the importance of the circadian system in the genesis of insomnia, pointing to various circadian rhythm disorders such as delayed sleep phase syndrome and advanced sleep phase syndrome, which are associated with difficulty in falling asleep and early awakening, respectively. In these disorders, the circadian system is disturbed, causing sleep/wakefulness, melatonin, cortisol, and presumably other endogenous rhythms to be either delayed or advanced in relation to the environmental light/dark cycle. Recent findings of a genetic basis for these disorders in at least some individuals [a mutation in a human clock gene (Per2) was shown to produce advanced sleep phase syndrome, and a functional polymorphism in Per3 is associated with delayed sleep phase syndrome] further support genetic models of insomnia (44).
Genetic models
One study noted that the correlation between insomnia was greater with monozygotic than in dizygotic twins (0.47 versus 0.15) (45). Other studies have shown similar genetic mutations and genotypes in a rare form of insomnia, fatal familial insomnia (46).
Psychological models
Although a variety of psychoanalytic models have been proposed throughout the past century, the one most widely recognized is that of Sigmund Freud. This construct proposed, in essence, that insomnia represents a failure of dream work (47). Dreams, according to Freud, are created by a combination of daily events (“residue”) and conflictual, anxiety-producing, unconscious wishes. These are, in turn, transformed into dreams through a series of mental processes, including displacement, condensation, symbolic representation, and secondary elaboration, which are collectively subsumed under the rubric of “dream work.” Freud further hypothesized that successful dream work is necessary for the preservation of sleep. However, in insomnia, “evil, repudiated wishes become active precisely at night and disturb us during sleep” (48). Dream work is unsuccessful because of the intensity of the anxiety caused by the underlying conflict, resulting in the awakening of the dreamer and the complaint of insomnia.
Cognitive/behavioral constructs in the genesis of insomnia rely on the importance of sleep-related thoughts and associated behaviors. Cognitive and emotional arousal due to traumatic events and stressors can precipitate insomnia in most individuals. However, insomniacs are theorized to have a predisposition for an exaggerated reaction to such stressors, requiring less activation to achieve high levels of arousal, which, in turn, lead to disturbed sleep. This predisposition is supported by studies indicating that insomniacs report difficulty relaxing, feeling tense and anxious, being overly preoccupied with a myriad of thoughts, being worried, and being depressed more frequently than control subjects (49). Other predisposing factors, such as distorted beliefs about sleep itself, including the belief that poor sleep is inevitable, that the lack of a full night of sleep will inevitably lead to disastrous health consequences, and that a minimum of 8 hours of sleep per day is critical to maintain health, can induce further emotional and cognitive arousal. Such catastrophizing is encouraged by many nights of poor sleep, which can foment cognitive rumination and worry about not falling asleep and about the potential for disastrous next-day consequences of sleeplessness. Therefore, such a cycle of apprehension and worry can perpetuate insomnia and make future sleep even less likely. Finally, insomniacs are prone to excessive cognitive monitoring at bedtime, which can further perpetuate insomnia; insomniacs carefully monitor mental and body sensations as well as external cues such as the bedroom clock and environmental noises.
Arousal at bedtime can also be a learned response. The repeated experience of poor sleep promotes an association between sleeplessness and both presleep activities and the sleep setting. Once these connections are established, the bedtime rituals and environment become contextual cues for arousal rather than for sleep (59). The interaction between predisposing, precipitating, and perpetuating factors in the genesis and progression of insomnia are depicted in Figure 2.
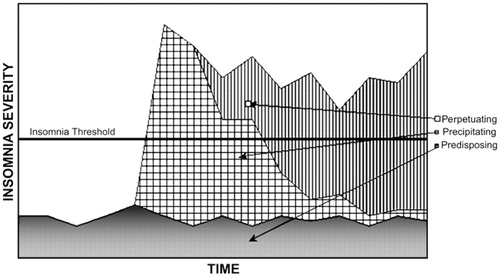
(From Yang CM, Spielman AJ, Glovinsky P: Nonpharmacologic strategies in the management of insomnia. Psychiatr. Clin. North Am 2006; 29:895–919. Reprinted by permission.)
CLASSIFICATION AND DIFFERENTIAL DIAGNOSIS
The three major classification systems used by clinicians and researchers for insomnia are the DSM-IV-TR, the International Classification of Sleep Disorders, 2nd edition (ICSD-2) and the ICD-10.
The DSM-IV-TR organizes sleep disorders into four major sections according to presumed etiology (primary, related to another mental disorder, due to a general medical condition, and substance-induced). According to the DSM-IV-TR, the essential feature of primary insomnia is difficulty initiating or maintaining sleep or of nonrestorative sleep (i.e., sleep that is restless, light, or of poor quality), which lasts for at least 1 month and causes clinically significant distress or impairment in social, occupational, or other important areas of functioning.
The ICSD-2, which was developed by sleep specialists, organizes diagnoses according to the presumed pathophysiology underlying the sleep disturbance, including 11 diagnoses related to insomnia. The ICD-10 criteria differ from those of the DSM-IV-TR and ICSD-2 in that they include, for insomnia, specific frequency criteria, i.e., the sleep disturbance must occur at least 3 nights a week. The systems also differ in terms of requirements for symptom severity (DSM-IV-TR specifies that symptoms must cause significant distress or impairment) and duration (DSM-IV-TR specifies at least 4 weeks).
From a clinical standpoint, insomnia can be classified into the following categories.
Primary insomnia
In primary insomnia, no coexisting disorder has been identified (51). Primary insomnia can also be referred to as an insomnia syndrome, presumed to be an independent disorder, which does not preclude the possibility that other medical, psychiatric, or sleep disorders can coexist with primary insomnia. However, they are not thought to contribute to the insomnia complaint.
Comorbid insomnia
This category includes insomnia disorders that are comorbid with other medical and psychiatric conditions and other sleep disorders. It represents the vast majority of insomnia. Historically, this has also been referred to as “secondary insomnia.” However, a recent National Institutes of Health State of the Science Conference statement recommended the use of term “comorbid insomnia,” noting that the limited understanding of mechanistic pathways in insomnia precludes drawing firm conclusions about the nature of the associations between insomnia and comorbid conditions or the direction of causality.
Insomnia can be associated with a host of medical, psychiatric, and sleep disorders. Some of these are listed in Table 1 (52). Table 2 lists some of the commonly encountered comorbid insomnia disorders and their defining symptoms (53). A variety of medications can also cause the complaint of insomnia, including anticholinergic agents, antihypertensives, antineoplastics, CNS stimulants, hormones, antidepressants, and antipsychotics as well as withdrawal from sedating agents (52). Anxiety and mood disorders represent some of the most commonly encountered disorders in psychiatric practice and are highly associated with insomnia.
 |
Table 1. Conditions Associated with Insomnia
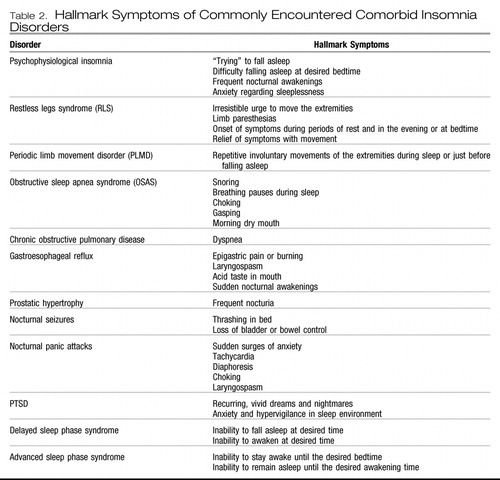 |
Table 2.
Mood disorders
There is a strong association between insomnia and mood disorders. The complaint of insomnia is voiced by most patients with major depression. Sleep patterns include difficulty falling asleep, frequent nocturnal awakenings, early morning awakening, nonrestorative sleep, decreased total sleep, and disturbing dreams. Daytime fatigue is also common (54). Insomnia is also a common complaint in bipolar patients in the depressed phase, yet hypersomnia is also a frequent complaint, with extended nocturnal sleep periods, difficulty awakening, and excessive daytime sleepiness (55). Hypersomnia is also common in seasonal affective disorder during episodes of winter depression. During manic periods, however, patients usually report significantly reduced amounts of total sleep, often with a subjective sense of a decreased need for sleep.
Conversely, 14%–20% of individuals with significant complaints of insomnia show evidence of major depression, whereas rates of depression are less than 1% in those without sleep complaints (8, 56). Although clinical wisdom suggests that insomnia is a consequence of mood disorders, longitudinal studies indicate that insomnia is more likely to emerge before rather than after the onset of the acute phase of a mood disorder (57). Indeed, the complaint of insomnia also confers an increased risk for the development of new psychiatric disorders over the course of the ensuing year, a risk that diminishes if the insomnia resolves (56). Other studies have noted an enhanced risk for mood disorders for a median of 34 years after the complaint of insomnia (57). Thus, insomnia in early adulthood was associated with an increased risk of depression some 17–30 years later. Other studies have shown that the occurrence and persistence of insomnia confers an enhanced risk for the future occurrence of anxiety and substance use disorders (36, 59). From a clinical standpoint, these data suggest that the presence of chronic insomnia should alert the clinician to the possibility of the future emergence of a mood, anxiety, or substance use disorder. In addition, the lack of sufficient response to the treatment of presumed primary insomnia should alert the clinician to the possibility of an underlying, disguised, mood, substance use, or anxiety disorder that may warrant independent management.
Polysomnographic studies have revealed a constellation of findings during major depressive episodes (54, 59). These are listed in Table 3.
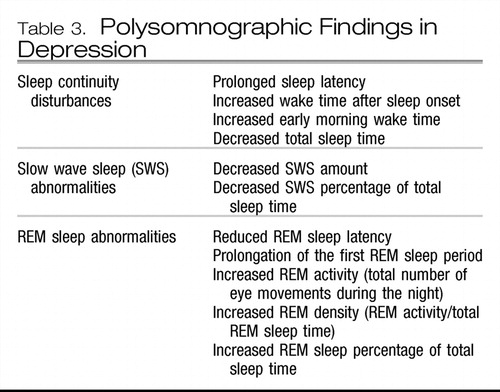 |
Table 3.
The clinical relevance of these findings is not straightforward. They have, however, been used historically in research settings to establish a diagnosis of depression during the acute phase of a depressive episode, to identify individuals in the population who may be vulnerable for the future development of depression, to predict relapse, and to predict response to treatment. They have also been used to support various neurophysiological formulations regarding the pathophysiology of depression (61).
The treatment of disturbed sleep in mood disorders should start with the management of the underlying mood disorder. The short-term subjective effects of some of the more commonly used pharmacological agents are listed in Table 4 (61). It should be noted that antidepressant medications are not approved by the U.S. Food and Drug Administration (FDA) for the treatment of insomnia.
 |
Table 4. Primary Effect of Pharmacological Agents for Mood Disorders
Nonpharmacological treatments directed primarily at the mood disorder can also diminish sleep disturbances, yet their effects may be more modest than those of antidepressants. Clinical wisdom suggests that effective management of the underlying mood disorder should provide relief from related sleep disturbances. However, pharmacological treatment studies indicate that less than 20% of full responders to antidepressant treatment are free of all major depressive disorder symptoms (62) and nearly half of responders have persistent sleep disturbances. Therefore, it is often necessary to supplement antidepressants with sleep-specific treatments such as hypnotic medications and insomnia-specific cognitive behavior therapy, described in greater detail below.
Anxiety disorders
Insomnia and anxiety are closely related in a variety of ways. These symptoms coexist frequently in the clinical practice of medicine and may even involve common etiologies. Both can be symptomatic manifestations of other comorbid conditions. In addition, both can represent distinct primary conditions or syndromes in their own right. Anxiety disorders represent the most frequent psychiatric disorder in individuals with insomnia (16). As noted above, insomnia often precedes the onset of an anxiety disorder. However, unlike the situation in mood disorders, insomnia is more likely to emerge at the same time (>38%) or after (>34%) the onset of the anxiety disorder (57).
The comorbidity of generalized anxiety disorder (GAD) and insomnia is more frequent than that for all other anxiety disorders (63). Objective studies in GAD reveal a longer sleep latency and an increase in the frequency of awakenings compared with those in control subjects. However, unlike in patients with major depression, patients with GAD appear to have normal REM latency (64).
Insomnia is one of the core symptoms of PTSD and is thought to be a reflection of increased psychological and physiologic arousal (DSM-IV-TR) associated with the original experience of the traumatic event or its reexperiencing. Another core symptom of PTSD is distressing dreams and nightmares, reflections of the reexperiencing of the traumatic event. As with insomnia, their presence early in the aftermath of trauma is a strong predictor of the later development of PTSD itself (65, 66). Nightmares may incorporate elements of or the entirety of the actual traumatic event and its affective correlates. However, the latent content of the dream (i.e., the traumatic event and its psychic correlates) may be disguised so that dreams may be affectively charged yet lack the factual recollection of the actual event or may not be remembered at all. Evidence is emerging to suggest that in the aftermath of trauma, the latter experience carries a more positive prognosis. Mellman et al. (67) reported that within 6 weeks of traumatic events reports of dreams rated as “highly similar” to the traumatic experience and as distressing were associated with concurrent and subsequent PTSD severity. On the other hand, lack of dream recall or dreams that did not depict actual memories of the traumatic event were more characteristic of the group of individuals exposed to trauma who did not subsequently develop PTSD, although their dreams did contain some threatening scenarios. The authors theorized that dreams with highly replicative content represent a failure of adaptive emotional memory processing that is a normal function of REM sleep and dreaming.
Polysomnographic studies in PTSD have not revealed consistent findings in sleep initiation and maintenance, possibly because of the diversity in the nature of the populations examined and in the temporal relationship between the development of PTSD symptoms and the traumatic event itself. Some studies have shown an enhancement of phasic activity and eye movement density during REM sleep in those with PTSD as well as reports of nightmares and awakenings from REM sleep even early in the development of the disorder (68).
Insomnia is not one of the diagnostic symptoms of panic disorder. However, insomnia, characterized by both impaired sleep initiation and maintenance, is more commonly reported in those with panic disorder than in normal individuals (69). Insomnia can be related to episodes of panic, which can arise during sleep and result in the complaint of insomnia. The first awareness of panic in most people with this disorder is a somatic symptom such as racing heart or shortness of breath (70), which results in sudden awakening with a feeling of physical intensity. Taylor et al. (71) found that major spontaneous panic attacks clustered during the sleep period between 1:30 and 3:30 a.m. Contrary to popular belief, they generally do not occur in REM sleep. They have been observed to occur in slow-wave sleep and during the transition from stage 2 to slow-wave sleep (66). Dreams are not commonly reported preceding panic episodes. Not all patients with panic disorder experience panic episodes during sleep.
Insomnia can also arise from recurrent sleep panic attacks, which, in turn, can create a state of anticipatory anxiety and apprehension over the prospect of yet another night of sleeplessness followed by another day of fatigue. A vicious cycle can then ensue in which excessive focus on sleep and trying to sleep causes greater tension and even greater impairment in sleep initiation and maintenance, thereby resulting in a second comorbid condition, i.e., psychophysiologic insomnia (see above).
Nonpharmacologic treatments for anxiety disorders include cognitive behavior therapy (CBT) and exposure therapy alone, in combination, or combined with relaxation training. These therapies share some similarities with the nonpharmacologic treatments for insomnia, described in greater detail below. Preliminary studies indicate that when used for the management of anxiety disorders, these therapies can also ameliorate insomnia. GAD-specific CBT can ameliorate insomnia when used for the treatment of GAD (72), and imagery rehearsal for the nightmares of patients with PTSD can improve sleep, presumably as a secondary consequence of diminished nightmares (73).
The benzodiazepine receptor agonists (GABAA receptor agonists) are effective for both anxiety and insomnia symptoms in patients with comorbid insomnia and anxiety disorders, although individual agents may not have an FDA-approved indication for both conditions. Antidepressants can also ameliorate insomnia in various anxiety disorders, as noted above, and, if insomnia persists after the management of the underlying anxiety disorder, the addition of hypnotic agents can be helpful.
EVALUATION
The primary goal of the evaluation process is to identify comorbid disorders. Once all of the related disorders have been established, treatment can be conducted with greater accuracy and confidence. Because insomnia can be related to a host of medical, psychiatric, neurological, and other disorders, we recommend that the psychiatrist conduct a comprehensive evaluation (53, 74).
History
Essential elements of the history are outlined in Table 5. The hallmark symptoms of the various disorders described above should be asked for (Table 2). Collateral information from bed partners can be useful in obtaining information that the patient may not be aware of, such as snoring; breathing pauses during sleep, limb movements, and the extent and frequency of naps. The history should also include the past medical history, history of medications and substance use, family history [certain sleep disorders, such as restless leg syndrome and obstructive sleep apnea syndrome (OSAS), can have a familial basis and the genetic bases of insomnia and certain circadian rhythm disorders are discussed above], and social and occupational history for entities that can contribute to irregular sleep/wake habits.
 |
Table 5. Essential Elements of the Sleep/Wake History in the Evaluation of Insomnia
A physical examination is essential; for example, a neck circumference of 16 in. or greater in women and 17 in. or greater in men, is associated with an increased risk for sleep-related breathing disorders. Useful scales and inventories include the Insomnia Severity Index, the Fatigue Severity Scale, the Epworth Sleepiness Scale, sleep diaries, and the Mallampati Airway Classification (53). General serum laboratory tests including thyroid function studies can be considered if data are not current. Polysomnography or a referral to a sleep medicine specialist should be considered if the evaluation raises the suspicion of OSAS or periodic limb movement disorder, if the patient reports violent or potentially injurious nocturnal behaviors, for the diagnosis of paroxysmal arousals or other sleep disruptions thought to be seizure related, if the office-based evaluation is not fruitful, or if the patient does not respond appropriately to treatment of the presumed disorder.
MANAGEMENT
cbt
Sleep hygiene education.
Many versions of sleep hygiene instructions exist. Most sleep hygiene instructions recommend limiting the use of alcohol, tobacco, and caffeine, avoiding napping, maintaining a regular sleep/wake routine, reducing time in bed, increasing exposure to light during the daytime, participating in exercise activities in the morning or in the afternoon, and ensuring that sleep environment is dark, cool, and quiet. There is insufficient evidence to recommend sleep hygiene instructions as a stand-alone therapeutic modality, yet they are a necessary component of any CBT modality (75).
Stimulus control therapy.
Stimulus control therapy is based on the premise that insomnia is caused by the conditioned arousal that occurs in response to the stimulus of repeated wakefulness and the bedroom environment. The assumption is that spending time awake in bed while eating, talking, watching TV, or worrying strengthens the association between the bedroom cues and sleeplessness, perpetuating insomnia. The goal of stimulus control therapy is to break the association between bedroom cues and conditioned arousal that occurs with repeated wakefulness, to imprint bed as a sleep stimulus, and to associate the bedroom environment with falling asleep (Table 6) (49).
 |
Table 6.
Sleep restriction therapy.
Sleep restriction therapy is a behavioral approach that is based on systematic curtailment of time in bed to further consolidate sleep, here modified from the original approach proposed by Dr. Arthur Spielman. At the beginning of therapy, the patient is asked to keep a sleep log for 1–2 weeks, on whose basis the average nightly sleep time is calculated. The patient is then asked to delay bedtime and curtail time in bed, so that he or she spends no more time in bed than the average sleep duration during the previous 1–2 weeks, never being less than 5 hours, and to avoid napping. The sleep efficiency is then calculated, which is the total sleep time divided by the time spent in bed expressed as a percentage, every 1–2 weeks, and the time in bed is increased by 15–30 minutes if sleep efficiency is greater that 90% (85% in the elderly) or decreased by 15–30 minutes if sleep efficiency is less than 90% (85% in the elderly). The goal of treatment is to increase the homeostatic sleep drive, which leads to consolidation of sleep and an improvement in sleep depth (49).
Cognitive therapy.
Cognitive therapy attempts to address the dysfunctional cognitions, catastrophization, and preoccupation with sleeplessness that accompany insomnia and ultimately contribute to poor sleep. In the course of the therapy, insomniacs' dysfunctional beliefs and attitudes toward sleep need to be challenged, their unrealistic expectations about sleep need to be corrected, and their perceptions of the consequences of insomnia need to be reappraised.
Relaxation therapy.
Relaxation therapy was developed around the theory that hyperarousal leads to insomnia. The goal of the relaxation therapy is not to induce sleep; the goal is to reduce arousal and induce a relaxed state (relaxation), thus allowing sleep to occur. Progressive muscle relaxation, autogenic training, guided imagery, and biofeedback are among the many techniques that have been used to reduce arousal and induce relaxation. Before applying relaxation therapy at bedtime, the patients are trained and instructed to practice relaxation techniques during the day.
Paradoxical intention.
Paradoxical intention is an effective treatment modality for patients with sleep onset insomnia. The paradox is that patients are not asked to try to fall asleep; instead, they are instructed to try to stay awake for as long as possible. It has been hypothesized that paradoxical intention works by redefining the task and removing the performance anxiety some insomniacs feel when facing the task of falling asleep (76).
CBT has been shown to be effective for patients who have both primary and secondary insomnia. We do not have evidence to be able to predict which specific type of psychotherapy should be prescribed for a given patient. Therefore, it is recommended that a combination of therapeutic approaches be used as deemed clinically appropriate for the individual patient. The efficacy of CBT for insomnia is similar to the efficacy of hypnotic medications.
Pharmacological agents
Historical overview.
Sedating substances, such as alcohol and extract of opium, have been available for centuries, and alcohol is commonly used for sleep induction to this day. However, both are now considered to be undesirable choices because alcohol can lead to sleep fragmentation and can also worsen the severity of sleep-related breathing disorders such as OSAS; the opiates can lead to central sleep apnea. Chloral hydrate (introduced in 1869) and barbital (introduced in 1903) and phenobarbital (introduced in 1912) were originally popular remedies for insomnia but are no longer used extensively because of their narrow therapeutic index and potential for lethality in an overdose.
The benzodiazepines were introduced in 1960s and are now known to be safer than the barbiturates. Nonbenzodiazepine benzodiazepine receptor agonists became available in 1980s. Ramelteon, a melatonin receptor agonist, was recently introduced and is regarded as not having a potential for abuse. In recent years there have also been fewer precautions regarding the use of hypnotics on a longer term basis; before 2005, medications were labeled for short-term treatment of insomnia yet, since that time, owing primarily to data showing lack of tolerance after longer term usage, these qualifiers were dropped for newly introduced hypnotics (77).
FDA-approved hypnotic medications.
Currently, FDA-approved medications for insomnia include benzodiazepines and nonbenzodiazepine agonists at the benzodiazepine receptor site on the GABAA receptor complex as well as the recently approved melatonin receptor agonist ramelteon. All of these agents are indicated for sleep onset insomnia; some of these medications are also indicated for sleep maintenance insomnia. These are summarized in Tables 7 and 8. The most relevant clinical distinction between these agents lies in their elimination half-lives. Agents with longer half-lives may have a longer duration of action and are beneficial for sleep maintenance, yet may promote residual sedation during the day, at the time when the patient is expected to be awake. On the other hand, agents that have a short half-life are better suited for sleep initiation than maintenance. Some of them, such as zaleplon, have also been evaluated for administration in the middle of the night after nocturnal awakenings (78), a practice that should not be used unless the patient has the opportunity to be in bed for at least 4 hours after administration, although no hypnotic agents are FDA-approved for this specific use.
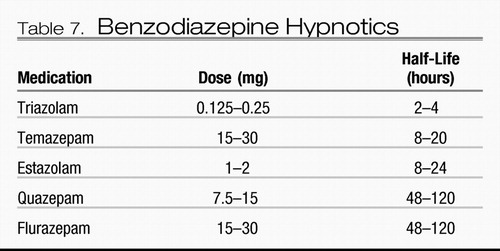 |
Table 7. Benzodiazepine Hypnotics
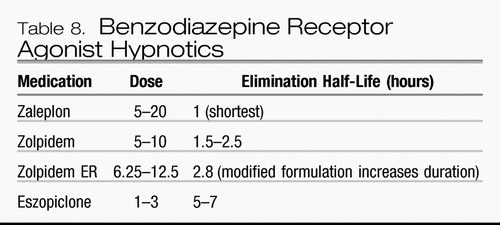 |
Table 8. Benzodiazepine Receptor Agonist Hypnotics
Adverse effects of hypnotics include ataxia, somnolence, impairment of cognitive function, confusion, and fatigue. The discontinuation of these drugs may lead to rebound insomnia, thought to be more likely after the discontinuation of shorter-acting hypnotics, especially if higher doses of the drug were used for longer periods of time (77).
Ramelteon is the only Drug Enforcement Administration nonscheduled hypnotic medication. It is approved by the FDA for insomnia characterized by difficulty with sleep onset. The recommended dose is 8 mg. Ramelteon does not worsen respiratory measures in sleep apnea syndrome or chronic obstructive pulmonary disease in patients with mild to moderate disease. It is not recommended for patients with severe liver impairment or for patients taking fluvoxamine. Adverse effects of ramelteon include dizziness, somnolence, nausea, headaches, and fatigue (79).
Hypnotics should be used at reduced dosages in elderly individuals, and, whenever possible, those with the shortest half-lives should be considered. The benzodiazepine receptor agonist hypnotics should also not be offered to patients with a history of drug and alcohol dependence without close monitoring. The addition of alcohol and other sedating agents may lead to potentiation of sedative effects and a decreased margin of safety.
Over-the-counter agents.
These products that are marketed for insomnia contain the antihistamines diphenhydramine or doxylamine. Although the evidence supporting their efficacy in insomnia is scant, antihistamines may produce mild to moderate sedation and may improve sleep for some individuals. However, tolerance to their sedating effects can develop rapidly (80). They are also associated with morning grogginess, daytime sleepiness possibly leading to impairment of driving ability, delirium, urinary retention, constipation, dry mouth, blurry vision, and psychomotor impairment (81).
Although their use is not regulated by the FDA, dietary supplements and herbal remedies are also used extensively, owing to a variety of factors, including their widespread availability, lack of prescription requirements, relatively low cost, and the widespread belief that they are safe and have a relatively low abuse risk. These include, among others, valerian, kava-kava (Piper methysticum), melatonin, chamomilla, passiflora, avena sativa, and humulus lupulus. Most of these have not been well studied for safety and efficacy; melatonin, which has received the widest evaluations, may be effective in the treatment of delayed sleep phase syndrome but does not appear to be consistently effective in treating most primary or secondary sleep disorders with short-term use (82).
Medications not FDA-approved for insomnia.
At appropriate doses, sedating antidepressants have demonstrated efficacy for mood and anxiety disorders. However, in populations with insomnia, they are typically used at low doses and are considered to be subtherapeutic for the treatment of depression or anxiety disorders. The latter use has received little scientific attention. Trazodone is a heterocyclic antidepressant and has an elimination half-life of 5–12 hours. It has received little scientific attention as a sleep aid in primary insomnia (83). Its hypnotic properties appear to be complicated by the rapid development of tolerance. Doxepin is a tricyclic antidepressant. At doses of 25–50 mg, it has demonstrated an improvement in total sleep time but not in sleep latency. Withdrawal insomnia was evident after abrupt discontinuation (28). Mirtazapine is a newer antidepressant with an elimination half-life of 20–40 hours. It has not been examined in patients with primary insomnia.
Therefore, despite the potential advantages of the sedating antidepressants, a major disadvantage is the paucity of available data regarding their effects on sleep and wakefulness in insomnia. A recent National Institutes of Health state-of-the-science conference concluded that “evidence supports the efficacy of cognitive behavior therapy and benzodiazepine receptor agonists in the treatment of this disorder. Very little evidence supports the efficacy of other treatments, despite their widespread use” (51). Their use is also complicated by daytime sedation and cognitive impairment, anticholinergic effects, weight gain, and drug-drug interactions. The tricyclic antidepressants are also potentially fatal in an overdose.
Insomnia comorbid with psychiatric disorders.
Monotherapy with sedating antidepressants or sedating antipsychotics is a useful choice for the treatment of psychiatric conditions complicated by insomnia. However, as noted above, this approach may not result in resolution of insomnia. Therefore, the addition of a hypnotic agent may be warranted (84). The relative advantages and disadvantages of initial monotherapy and polytherapy have not been adequately explored.
1 Roth T, Roehrs T: Sleep organization and regulation. Neurology 2000; 54( 5 Suppl 1): S2– S7Crossref, Google Scholar
2 Richardson GS: The human circadian system in normal and disordered sleep. J. Clin.Psychiatry 2005; 66( Suppl 9): 3– 9, quiz 42–43Google Scholar
3 Mignot E, Taheri S, Nishino S: Sleeping with the hypothalamus: emerging therapeutic targets for sleep disorders. Nat Neurosci 2002; 5( Suppl): 1071– 1075Crossref, Google Scholar
4 Hattar S, Liao HW, Takao M, Berson DM, Yau KW: Melanopsin-containing retinal ganglion cells: architecture, projections, and intrinsic photosensitivity. Science 2002; 295: 1065– 1070Crossref, Google Scholar
5 Pace-Schott EF, Hobson JA: The neurobiology of sleep: genetics, cellular physiology and subcortical networks. Nat Rev Neurosci 2002; 3: 591– 605Crossref, Google Scholar
6 Saper CB, Chou TC, Scammell TE: The sleep switch: hypothalamic control of sleep and wakefulness. Trends Neurosci 2001; 24: 726– 731Crossref, Google Scholar
7 Mahowald MW, Kader G, Schenck CH: Clinical categories of sleep disorders. I. Continuum 1997; 3: 35– 65Google Scholar
8 Mellinger GD, Balter MB, Uhlenhuth EH: Insomnia and its treatment: prevalence and correlates. Arch Gen Psychiatry 1985; 42: 225– 232Crossref, Google Scholar
9 Ohayon M: Epidemiological study on insomnia in the general population. Sleep 1996; 19( 3 Suppl): S7– S15Crossref, Google Scholar
10 Ohayon MM: Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev 2002; 6: 97– 111Crossref, Google Scholar
11 International Classification of Sleep Disorders, 2nd ed. Westchester, Ill., American Academy of Sleep Medicine, 2005Google Scholar
12 Dixon KN, Monroe LJ, Jakim S: Insomniac children. Sleep 1981; 4: 313– 318Crossref, Google Scholar
13 Sutton DA, Moldofsky H, Badley EM: Insomnia and health problems in Canadians. Sleep 2001; 24: 665– 670Crossref, Google Scholar
14 Camhi SL, Morgan WJ, Pernisco N, Quan SF: Factors affecting sleep disturbances in children and adolescents. Sleep Med 2000; 1: 117– 123Crossref, Google Scholar
15 Ohayon MM, Zulley J, Guilleminault C, Smirne S, Priest RG: How age and daytime activities are related to insomnia in the general population: consequences for older people. J Am Geriatr Soc 2001; 49: 360– 366Crossref, Google Scholar
16 Ohayon MM, Lemoine P, Arnaud-Briant V, Dreyfus M: Prevalence and consequences of sleep disorders in a shift worker population. J Psychosom Res 2002; 53: 577– 583Crossref, Google Scholar
17 Harma M, Tenkanen L, Sjoblom T, Alikoski T, Heinsalmi P: Combined effects of shift work and life-style on the prevalence of insomnia, sleep deprivation and daytime sleepiness. Scand J Work Environ Health 1998; 24: 300– 307Crossref, Google Scholar
18 Dumont M, Montplaisir J, Infante-Rivard C: Sleep quality of former night-shift workers. Int J Occup Environ Health 1997; 3( Suppl. 2): S10– S14Google Scholar
19 Li RH, Wing YK, Ho SC, Fong SY: Gender differences in insomnia—a study in the Hong Kong Chinese population. J Psychosom Res 2002; 53: 601– 609Crossref, Google Scholar
20 Kageyama T, Kabuto M, Nitta H, Kurokawa Y, Taira K, Suzuki S, Takemoto, K: A population study on risk factors for insomnia among adult Japanese women: a possible effect of road traffic volume. Sleep 1997; 20: 963– 971Google Scholar
21 Ishigooka J, Suzuki M, Isawa S, Muraoka H, Murasaki M, Okawa M: Epidemiological study on sleep habits and insomnia of new outpatients visiting general hospitals in Japan. Psychiatry Clin Neurosci 1999; 53: 515– 522Crossref, Google Scholar
22 Morgan K, Clarke D: Risk factors for late-life insomnia in a representative general practice sample. Br J Gen Pract 1997; 47: 166– 169Google Scholar
23 Costa e Silva JA, Chase M, Sartorius N, Roth T: Special report from a symposium held by the World Health Organization and the World Federation of Sleep Research Societies: an overview of insomnias and related disorders—recognition, epidemiology, and rational management. Sleep 1996; 19: 412– 416Crossref, Google Scholar
24 Dodge R, Cline MG, Quan SF: The natural history of insomnia and its relationship to respiratory symptoms. Arch Intern Med 1995; 155: 1797– 1800Crossref, Google Scholar
25 Katz DA, McHorney CA: Clinical correlates of insomnia in patients with chronic illness. Arch Intern Med 1998; 158: 1099– 1107Crossref, Google Scholar
26 Pallesen S, Nordhus IH, Nielsen GH, Havik OE, Kvale G, Johnsen BH, Skjøtskift S: Prevalence of insomnia in the adult Norwegian population. Sleep 2001; 24: 771– 779Google Scholar
27 Chevalier H, Los F, Boichut D, Bianchi M, Nutt DJ, Hajak G, Hetta J, Hoffmann G, Crowe C: Evaluation of severe insomnia in the general population: results of a European multinational survey. J Psychopharmacol 1999; 13( 4 Suppl 1): S21– S24Crossref, Google Scholar
28 Hajak G, Rodenbeck A, Voderholzer U, Riemann D, Cohrs S, Hohagen F, Berger M, Rüther E: Doxepin in the treatment of primary insomnia: a placebo-controlled, double-blind, polysomnographic study. J Clin Psychiatry 2001; 62: 453– 463Crossref, Google Scholar
29 Leger D, Scheuermaier K, Philip P, Paillard M, Guilleminault C: SF-36: evaluation of quality of life in severe and mild insomniacs compared with good sleepers. Psychosom Med 2001; 63: 49– 55Crossref, Google Scholar
30 Hauri PJ: Cognitive deficits in insomnia patients. Acta Neurol Belg 1997; 97: 113– 117Google Scholar
31 Espie CA, Inglis SJ, Harvey L, Tessier S: Insomniacs' attributions: psychometric properties of the Dysfunctional Beliefs and Attitudes about Sleep Scale and the Sleep Disturbance Questionnaire. J Psychosom Res 2000; 48: 141– 148Crossref, Google Scholar
32 Kripke DF, Garfinkel L, Wingard DL, Klauber MR, Marler MR: Mortality associated with sleep duration and insomnia. Arch Gen Psychiatry 2002; 59: 131– 136Crossref, Google Scholar
33 Rumble R, Morgan K: Hypnotics, sleep, and mortality in elderly people. J Am Geriatr Soc 1992; 4: 787– 791Google Scholar
34 Dew MA, Hoch CC, Buysse DJ, Monk TH, Begley AE, Houck PR, Hall M, Kupfer DJ, Reynolds CF 3rd: Healthy older adults' sleep predicts all-cause mortality at 4 to 19 years of follow-up. Psychosom Med 2003; 65: 63– 73Crossref, Google Scholar
35 Mallon L, Broman JE, Hetta J: Sleep complaints predict coronary artery disease mortality in males: a 12-year follow-up study of a middle-aged Swedish population. J Intern Med 2002; 251: 207– 216Crossref, Google Scholar
36 Weissman MM, Greenwald S, Nino-Murcia G, Dement WC: The morbidity of insomnia uncomplicated by psychiatric disorders. Gen Hosp Psychiatry 1997; 19: 245– 250Crossref, Google Scholar
37 Stoller MK: Economic effects of insomnia. Clin Ther 1994; 16: 873– 897, discussion 854Google Scholar
38 Edinger JD, Means MK, Carney CE, Krystal AD: Psychomotor performance deficits and their relation to prior nights' sleep among individuals with primary insomnia. Sleep 2008; 31: 599– 607Crossref, Google Scholar
39 Merica H, Blois R, Gaillard JM: Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci 1998; 10: 1826– 1834Crossref, Google Scholar
40 Vgontzas AN, Bixler EO, Lin HM, Prolo P, Mastorakos G, Vela-Bueno A, Kales A, Chrousos GP: Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab 2001; 86: 3787– 3794Crossref, Google Scholar
41 Nofzinger EA, Buysse DJ, Germain A, Price JC, Miewald JM, Kupfer DJ: Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry 2004; 161: 2126– 2128Crossref, Google Scholar
42 Bonnet MH, Arand DL: Heart rate variability in insomniacs and matched normal sleepers. Psychosom Med 1998; 60: 610– 615Crossref, Google Scholar
43 Bonnet MH, Arand DL: Physiological activation in patients with sleep state misperception. Psychosom Med 1997; 59: 533– 540Crossref, Google Scholar
44 Hamet P, Tremblay J: Genetics of the sleep-wake cycle and its disorders. Metabolism 2006; 55( 10 Suppl 2): S7– S12Crossref, Google Scholar
45 Watson NF, Goldberg J, Arguelles L, Buchwald D: Genetic and environmental influences on insomnia, daytime sleepiness, and obesity in twins. Sleep 2006; 29: 645– 649Crossref, Google Scholar
46 Tabernero C, Polo JM, Sevillano MD, Munoz R, Berciano J, Cabello A, Cabello A, Báez B, Ricoy JR, Carpizo R, Figols J, Cuadrado N, Claveria LE: Fatal familial insomnia: clinical, neuropathological, and genetic description of a Spanish family. J Neurol Neurosurg Psychiatry 2000; 68: 774– 777Crossref, Google Scholar
47 Freud S: The Interpretation of Dreams, 2nd ed. London, Hogarth Press, 1955Google Scholar
48 Freud S. Dreams, in Complete Psychological Works, standard ed, London, Hogarth Press, 1986Google Scholar
49 Kales JD, Kales A, Bixler EO, Soldatos CR, Cadieux RJ, Kashurba GJ, Vela-Bueno A: Biopsychobehavioral correlates of insomnia. V: Clinical characteristics and behavioral correlates. Am J Psychiatry 1984; 141: 1371– 1376Crossref, Google Scholar
50 Yang CM, Spielman AJ, Glovinsky P: Nonpharmacologic strategies in the management of insomnia. Psychiatr Clin North Am 2006; 29: 895– 919; abstract viiiCrossref, Google Scholar
51 National Institutes of Health: National Institutes of Health State of the Science Conference statement on Manifestations and Management of Chronic Insomnia in Adults, June 13–15, 2005. Sleep 2005; 28: 1049– 1057Crossref, Google Scholar
52 Doghramji K, Doghramji P: Clinical Management of Insomnia. 1st ed. West Islip, N.Y., Professional Communications, Inc., 2006Google Scholar
53 Doghramji K, Choufani D: Taking a sleep history, in Foundations of Psychiatric Sleep Medicine. Edited by Winkelman J, Plante D. Cambridge, Mass., Cambridge University Press, in pressGoogle Scholar
54 Reynolds CF 3rd, Kupfer DJ: Sleep research in affective illness: state of the art circa 1987. Sleep 1987; 10: 199– 215Crossref, Google Scholar
55 Detre T, Himmelhoch J, Swartzburg M, Anderson CM, Byck R, Kupfer DJ: Hypersomnia and manic-depressive disease. Am J Psychiatry 1972; 128: 1303– 1305Crossref, Google Scholar
56 Ford DE, Kamerow DB: Epidemiologic study of sleep disturbances and psychiatric disorders: an opportunity for prevention? JAMA 1989; 262: 1479– 1484Crossref, Google Scholar
57 Ohayon MM, Roth T: Place of chronic insomnia in the course of depressive and anxiety disorders. J Psychiatr Res 2003; 37: 9– 15Crossref, Google Scholar
58 Chang PP, Ford DE, Mead LA, Cooper-Patrick L, Klag MJ: Insomnia in young men and subsequent depression. The Johns Hopkins Precursors Study. Am J Epidemiol 1997; 146: 105– 114Crossref, Google Scholar
59 Breslau N, Roth T, Rosenthal L, Andreski P: Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry 1996; 39: 411– 418Crossref, Google Scholar
60 Benca RM, Obermeyer WH, Thisted RA, Gillin JC: Sleep and psychiatric disorders: a meta-analysis. Arch Gen Psychiatry 1992; 49: 651– 670Crossref, Google Scholar
61 Benca RM: Mood disorders, in Principles and Practice of Sleep Medicine, 4th ed. Edited by Kryger MH, Roth T, Dement WC. New York, Elsevier, 2005, pp 1311– 1326Crossref, Google Scholar
62 Nierenberg AA, Keefe BR, Leslie VC, Alpert JE, Pava JA, Worthington JJ 3rd, Rosenbaum JF, Fava M: Residual symptoms in depressed patients who respond acutely to fluoxetine. J Clin Psychiatry 1999; 60: 221– 225Crossref, Google Scholar
63 Ohayon MM, Caulet M, Lemoine P: Comorbidity of mental and insomnia disorders in the general population. Compr Psychiatry 1998; 39: 185– 197Crossref, Google Scholar
64 Saletu-Zyhlarz G, Saletu B, Anderer P, Brandstatter N, Frey R, Gruber G, Klösch G, Mandl M, Grünberger J, Linzmayer L: Nonorganic insomnia in generalized anxiety disorder. 1. Controlled studies on sleep, awakening and daytime vigilance utilizing polysomnography and EEG mapping. Neuropsychobiology 1997; 36: 117– 129Crossref, Google Scholar
65 Mellman TA, David D, Kulick-Bell R, Hebding J, Nolan B: Sleep disturbance and its relationship to psychiatric morbidity after Hurricane Andrew. Am J Psychiatry 1995; 152: 1659– 1663Crossref, Google Scholar
66 Mellman TA, Uhde TW: Electroencephalographic sleep in panic disorder: a focus on sleep-related panic attacks. Arch Gen Psychiatry 1989; 46: 178– 184Crossref, Google Scholar
67 Mellman TA, David D, Bustamante V, Torres J, Fins A: Dreams in the acute aftermath of trauma and their relationship to PTSD. J Trauma Stress 2001; 14: 241– 247Crossref, Google Scholar
68 Harvey AG, Jones C, Schmidt DA: Sleep and posttraumatic stress disorder: a review. Clin Psychol Rev 2003; 23: 377– 407Crossref, Google Scholar
69 Stein MB, Chartier M, Walker JR: Sleep in nondepressed patients with panic disorder: I. Systematic assessment of subjective sleep quality and sleep disturbance. Sleep 1993; 16: 724– 726Crossref, Google Scholar
70 Shapiro CM, Sloan EP: Nocturnal panic—an underrecognized entity. J Psychosom Res 1998; 44: 21– 23Crossref, Google Scholar
71 Taylor CB, Sheikh J, Agras WS, Roth WT, Margraf J, Ehlers A, Maddock RJ, Gossard D: Ambulatory heart rate changes in patients with panic attacks. Am J Psychiatry 1986; 143: 478– 482Crossref, Google Scholar
72 Belanger L, Morin CM, Langlois F, Ladouceur R: Insomnia and generalized anxiety disorder: effects of cognitive behavior therapy for gad on insomnia symptoms. J Anxiety Disord 2004; 18: 561– 571Crossref, Google Scholar
73 Krakow B, Hollifield M, Johnston L, Koss M, Schrader R, Warner TD, Tandberg D, Lauriello J, McBride L, Cutchen L, Cheng D, Emmons S, Germain A, Melendrez D, Sandoval D, Prince H: Imagery rehearsal therapy for chronic nightmares in sexual assault survivors with posttraumatic stress disorder: a randomized controlled trial. JAMA 2001; 286: 537– 545Crossref, Google Scholar
74 Sateia MJ, Doghramji K, Hauri PJ, Morin CM: Evaluation of chronic insomnia: an American Academy of Sleep Medicine review. Sleep 2000; 23: 243– 308Google Scholar
75 Chesson AL Jr, Anderson WM, Littner M, Davila D, Hartse K, Johnson S, Hartse K, Johnson S, Wise M, Rafecas J: Practice parameters for the nonpharmacologic treatment of chronic insomnia: an American Academy of Sleep Medicine report. Standards of Practice Committee of the American Academy of Sleep Medicine. Sleep 1999; 22: 1128– 1133Crossref, Google Scholar
76 Ascher LM, Efran JS: Use of paradoxical intention in a behavioral program for sleep onset insomnia. J Consult Clin Psychol 1978; 46: 547– 550Crossref, Google Scholar
77 Curry DT, Eisenstein RD, Walsh JK: Pharmacologic management of insomnia: past, present, and future. Psychiatr Clin North Am 2006; 29: 871– 893; abstract vii–viiiCrossref, Google Scholar
78 Zammit GK, Corser B, Doghramji K, Fry JM, James S, Krystal A, Mangano RM: Sleep and residual sedation after administration of zaleplon, zolpidem, and placebo during experimental middle-of-the-night awakening. J Clin Sleep Med 2006; 2: 417– 423Google Scholar
79 Zammit G, Erman M, Wang-Weigand S, Sainati S, Zhang J, Roth T: Evaluation of the efficacy and safety of ramelteon in subjects with chronic insomnia. J Clin Sleep Med 2007; 3: 495– 504Google Scholar
80 Richardson GS, Roehrs TA, Rosenthal L, Koshorek G, Roth T: Tolerance to daytime sedative effects of H1 antihistamines. J Clin Psychopharmacol 2002; 22: 511– 515Crossref, Google Scholar
81 Basu R, Dodge H, Stoehr GP, Ganguli M: Sedative-hypnotic use of diphenhydramine in a rural, older adult, community-based cohort: effects on cognition. Am J Geriatr Psychiatry 2003; 11: 205– 213Crossref, Google Scholar
82 Buscemi N, Vandermeer B, Pandya R, Hooton N, Tjosvold L, Hartling L, Baker G, Vohra S, Klassen T: Melatonin for treatment of sleep disorders. Evid Rep Technol Assess (Summ) 2004; 108: 1– 7Google Scholar
83 James SP, Mendelson WB: The use of trazodone as a hypnotic: a critical review. J Clin Psychiatry 2004; 65: 752– 755Crossref, Google Scholar
84 Fava M, McCall WV, Krystal A, Wessel T, Rubens R, Caron J, Amato D, Roth T: Eszopiclone co-administered with fluoxetine in patients with insomnia coexisting with major depressive disorder. Biol Psychiatry 2006; 59: 1052– 1060Crossref, Google Scholar



