Immune System Contributions to the Pathophysiology of Depression
Abstract
Major depression is a devastating disorder that represents a major public health concern. Of special relevance is the high percentage of patients whose depression does not respond to or who are unable to tolerate conventional antidepressant medications, which primarily target monoamine neurotransmission. Recent data indicate that the immune system may play a role in the pathophysiology of depression, representing a novel pathway for therapeutic development. Patients with major depression have been found to exhibit evidence of an activated innate immune response as reflected by increased biomarkers of inflammation, including innate immune cytokines, acute-phase proteins, chemokines, and adhesion molecules. In addition, administration of innate immune cytokines to laboratory animals and humans has been shown to induce behavioral changes that significantly overlap with the symptom criteria of major depression. Treatment of patients with inflammatory disorders using anticytokine therapies has also been found to reduce depressive symptoms. Interestingly, psychosocial stress, a well-known precipitant of depressive disorders, has been shown to activate the innate immune response. Finally, innate immune cytokines have been shown to influence virtually every pathophysiological domain relevant to depression including monoamine neurotransmission, neuroendocrine function, synaptic plasticity, and regional brain metabolism. Of note, a response to conventional antidepressant medications is associated with a decrease in inflammatory biomarkers, whereas patients with treatment-resistant depression are more likely to exhibit evidence of increased inflammation. Taken together, these data provide the foundation for considering an activated innate immune response as a potential target for further study and therapeutic development in mood disorders, especially in the context of treatment resistance.
Major depression has become a health crisis of epidemic proportions in the modern world. The prevalence of major depression has risen over the last several generations in every country examined (1), and age of symptom onset has decreased (2). One in six individuals in the United States will experience an episode of major depression in his or her lifetime (3), and the risk of subsequent episodes rises dramatically once a person has been depressed (4). Between 10% and 15% of severely depressed people eventually commit suicide (5), and studies indicate that depression significantly increases all-cause mortality (6) and predicts the later development of a number of medical conditions, including cardiac and cerebrovascular disease (7, 8), hypertension (9, 10), diabetes (11, 12), obesity and the metabolic syndrome (13, 14), and cancer (15).
Unfortunately, most patients with depression do not experience a complete resolution of symptoms with conventional antidepressant treatment, and 10%–20% of patients have depression that is refractory to all currently available modalities, including electroconvulsive therapy (16). In addition to efficacy issues, many patients are unable to tolerate the side effects associated with antidepressants or electroconvulsive therapy. The risks of not responding to (or tolerating) treatment have been highlighted by recent studies documenting the fact that a partial—but incomplete—response is associated with an increased risk of full symptomatic relapse (even when the patient is receiving therapy) and worse long-term disease course, as well as significantly impaired quality of life (17, 18). Treatment resistance also results in a six times increase in direct health care costs (19). These factors highlight the tremendous need to identify novel treatment strategies for depression, especially for depressed patients whose depression is unresponsive to treatment or who are intolerant of conventional therapies.
THE IMMUNE SYSTEM AND DEPRESSION
One consideration that has received increasing attention is the possibility that the immune system may contribute to the pathophysiology of depressive disorders and may thus represent a heretofore unrecognized and novel target for future research and therapeutic exploration. Although early studies focused largely on acquired immune responses in patients with depression (e.g., T- and B-cell functions, which were generally found to be suppressed), more recent research suggests that a significant percentage of depressed patients may experience activation of the innate immune response (2021–22).
In contrast to the acquired immune response (Table 1), which develops slowly (i.e., over days) and is highly specific in its recognition of pathogens, the innate immune system provides a rapid, front-line defense against a variety of pathogens and damaged or dead cells, using relatively crude (nonspecific) pattern recognition receptors referred to as Toll-like receptors to initiate and mobilize the response to infection and/or tissue damage and destruction (23). Toll-like receptors in turn are linked to fundamental inflammatory signaling pathways including nuclear factor-κB (NF-κB) and mitogen-activated protein kinases (MAPKs), which when activated stimulate the production of the innate immune cytokines interferon (IFN)-α, interleukin (IL)-1, IL-6, and tumor necrosis factor-α (TNF-α); chemokines; adhesion molecules; and other inflammatory mediators including the prostaglandins, histamine, and reactive oxygen and nitrogen species (23, 24). These molecules then orchestrate the local immune response by recruiting and activating relevant immune cells, which leads to the swelling (tumor), redness (rubor), heat (calor), and pain (dolor) that constitute the clinical characteristics of inflammation. Innate immune cytokines also enter the peripheral blood and stimulate local nerve fibers (see below) to mobilize a systemic response to infection and tissue trauma that includes activation of the acute-phase response in the liver, which involves the production of acute-phase proteins such as C-reactive protein (CRP), and a central nervous system (CNS) response, which involves fever, fatigue, reduced environmental exploration, anorexia, and altered sleep. This CNS response, which has been referred to as “sickness behavior,” is believed to represent a reorganization of behavioral priorities to conserve and divert essential energy resources to pathogen elimination, tissue repair processes, and protection from future injury or attack (20, 22, 25).
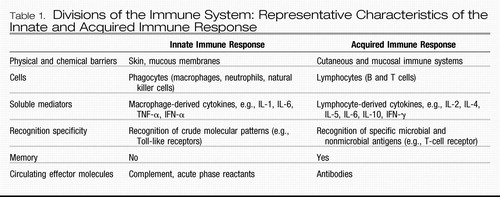 |
Table 1. Divisions of the Immune System: Representative Characteristics of the Innate and Acquired Immune Response
A major breakthrough in terms of the recognition of the potential contribution of the immune system to depression has been the demonstration that all of the cardinal features of inflammation are apparent in patients with major depression (Figure 1). Patients with major depression have been found to exhibit significant elevations of innate immune cytokines and their soluble receptors in both peripheral blood and cerebrospinal fluid (CSF) and have exhibited elevations in acute-phase proteins, chemokines, and adhesion molecules as well as inflammatory mediators such as the prostaglandins. Given the number and variety of studies done in this area, a meta-analysis has been conducted, and the data indicate that of these markers of inflammation, elevations in IL-6 and CRP are some the most reliable (26). Because of the relationship between body mass index and CRP and IL-6 (adipocytes are capable of producing IL-6 as are macrophages within fatty tissues) (27), elevations of these markers in patients with obesity should be interpreted with caution. In addition to mean increases in inflammatory biomarkers in depressed patients versus control subjects, correlations between depressive symptom severity and increases in measures of peripheral inflammation have been observed in multiple studies (20, 28–31). Although the linkage of inflammatory markers with specific behavioral profiles is still under investigation, it should be noted that fatigue, loss of energy, and psychomotor retardation are some of the most common symptoms after cytokine administration (32, 33).
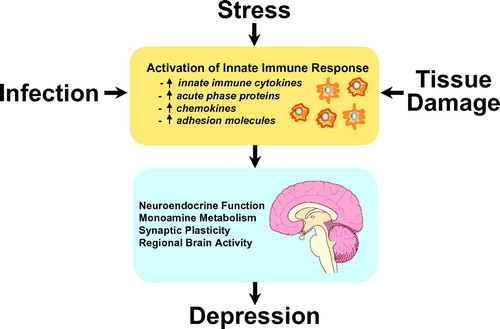
Figure 1. Activation of Innate Immune Responses and Depression
Note: There are many factors that can lead to activation of the innate immune response, which is characterized by increases in a variety of immune mediators that can be measured in the peripheral blood. These immune mediators can then interact with pathways known to be involved in the pathophysiology of depression.
Another major body of evidence supporting an immune system contribution to the development of depression is the profound behavioral disturbances that occur in patients treated with the innate immune cytokine, IFN-α. IFN-α has both antiviral and antiproliferative activities and is therefore used to treat both infectious diseases and cancer (34). Although an effective therapy, IFN-α is notorious for causing a variety of behavioral alterations including symptoms sufficient to meet criteria for major depression in up to 50% of subjects, depending on the dose (34, 35). Treatment of patients before and during IFN-α therapy with antidepressants has been shown to markedly reduce the incidence of depression (35–37), supporting the notion that cytokine-induced depression not only shares behavioral similarities with major depression in otherwise healthy individuals but also shares pharmacological response characteristics. Of note, rhesus monkeys administered IFN-α also exhibit depressive-like huddling behavior that was initially described in monkeys administered the monoamine-depleting drug, reserpine (38).
A final consideration regarding the role of the immune system in depression is the high rate of depression in medically ill patients with disorders that involve the immune system including infectious diseases, cancer, and autoimmune disorders (39). Rates of depression are on average 5–10 times higher in these diseases (40), and studies have shown a relationship between inflammatory markers and depression and other behavioral alterations in these disorders (41–45). These data indicate that there appears to be a specific relationship between inflammation and behavioral symptoms as opposed to a more nonspecific relationship between being ill and emotional distress. Further supporting the specificity of the cytokine-depression link in those who are medically ill is that patients with autoimmune disorders treated with cytokine antagonists have exhibited significant improvement in depressive symptoms (see below) (46, 47). In addition, there is increasing recognition that inflammation may play a prominent role in a number of common disorders including cardiovascular disease, diabetes, and metabolic syndrome and cancer—all disorders with increased rates of depression (48–50). Taken together these data suggest that inflammation may be a shared pathology between these diseases and depression.
IMMUNOLOGICAL MECHANISMS OF BEHAVIORAL CHANGE
Consistent with the notion that innate immune cytokines may be associated with the development of depression are data that these cytokines can influence virtually every pathophysiological domain relevant to depression including neurotransmitter metabolism, neuroendocrine function, synaptic plasticity, and regional brain activity (Figure 2).
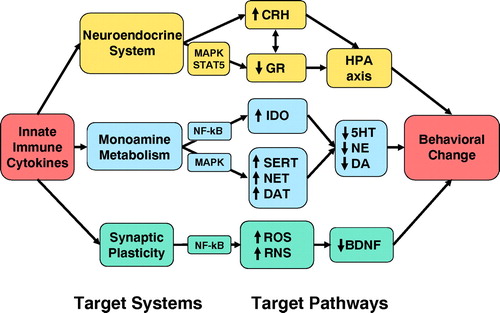
Figure 2. Mechanisms by which Innate Immune Cytokines Can Contribute to Depression
Note: Through influences on the neuroendocrine system, monoamine metabolism, and synaptic plasticity, innate immune cytokines and their signaling pathways (e.g., NF-κB and MAPK) can influence molecules that are believed to play a role in depression including CRH, the GR, serotonin (5HT), norepinephrine (NE), dopamine (DA), and BDNF. Important intermediaries in the effects of cytokines on these target pathways include enzymes that influence monoamine synthesis and release such as indoleamine 2,3-dioxygenase (IDO), monoamine transporters [such as the serotonin transporter (SERT), norepinephrine transporter (NET), and dopamine transporter (DAT)] and reactive oxygen species (ROS) and nitrogen species (RNS).
COMMUNICATION WITH THE BRAIN
As part of the consideration of the impact of peripherally elaborated cytokines on the brain and behavior, a great deal of attention has been paid to the pathways by which cytokines signal the brain. In general, four routes have been described including 1) passage through leaky regions of the blood-brain barrier (51), 2) active transport through saturable transport molecules (52), 3) activation of endothelial cells (as well as other cells lining the cerebral vasculature), which then release inflammatory mediators (53, 54), and 4) binding to cytokine receptors in association with afferent nerve fibers (e.g., vagus nerve), which in turn relay cytokine signals to relevant brain nuclei (55). Once cytokine signals access the brain, activation of relevant inflammatory signaling molecules (e.g., NF-κB) in appropriate cells, including astrocytes and microglia, leads to the release of cytokines within the brain parenchyma (22).
NEUROTRANSMITTER METABOLISM
Administration of cytokines to laboratory animals and humans has been shown to alter the metabolism of the monoamines, serotonin, norepinephrine, and dopamine, in both the peripheral blood and in brain regions relevant to mood regulation (56–59). Much attention regarding the impact of cytokines on monoamine metabolism has been focused on serotonin. Indeed, data indicate that cytokine activation of the enzyme, indoleamine 2,3-dioxygenase, leads to the breakdown of tryptophan (22, 59), the primary precursor of serotonin, into the metabolites, kynurenine and quinolinic acid, which have neurotoxic properties (60). Studies in patients undergoing treatment with IFN-α have shown correlations between decreases in peripheral blood tryptophan and increases in depression as well as increases in peripheral blood kynurenine in patients who develop symptoms of major depression (59, 61). Activation of the cytokine signaling pathway p38 MAPK may also contribute to alterations in serotonin. For example, activation of p38 MAPK by IL-1β or TNF-α has been shown to upregulate the expression and activity of the serotonin transporter (62). Relevant in this regard, increased phosphorylated (activated) p38 MAPK in peripheral blood mononuclear cells of monkeys subjected to early life abuse and neglect were associated with significant decreases in CSF concentrations of the serotonin metabolite, 5-hydroxyindoleacetic acid (63). Thus, through activation of both indoleamine 2,3-dioxygenase and p38 MAPK, cytokines may impose a double hit on serotonin availability by influencing both serotonin synthesis and availability in the synapse.
In addition to effects on serotonergic neurotransmission, the effects of cytokines on dopamine metabolism have also received attention. For example, IFN-α-treated rhesus monkeys that displayed depressive-like behavior exhibited significantly greater decreases in CSF concentrations of the dopamine metabolite, homovanillic acid, than monkeys that did not exhibit such behavior (38). Relevant to cytokine-induced activation of indoleamine 2,3-dioxygenase, data indicate that intrastriatal administration of kynurenic acid, a breakdown product of kynurenine, dramatically reduces extracellular dopamine in the rat striatum (64). Of note, cytokine induction of nitric oxide also has been shown to inhibit the activity of tyrosine hydroxylase (the rate-limiting enzyme in the synthesis of dopamine) through effects on the tyrosine hydroxylase coenzyme, tetrahydrobiopterin (65).
HYPOTHALAMIC-PITUITARY-ADRENAL AXIS
Innate immune cytokines are potent activators of the hypothalamic-pituitary-adrenal (HPA) axis and the release of corticotropin-releasing hormone (CRH) (66, 67). Indeed, the HPA axis response to the first injection of IFN-α was found to be significantly greater in patients who developed depression during IFN-α therapy than in those who did not become depressed (67), indicating that sensitivity of CRH pathways may represent a vulnerability factor to cytokine-induced behavioral disturbances. Given the role of CRH in depression (68), cytokine-induced activation of CRH in the brain may be a major pathway by which cytokines influence behavior. Another pathway by which cytokines may influence the HPA axis is through their effects on the glucocorticoid receptor (GR). For example, activation of cytokine signaling pathways such as p38 MAPK has been shown to disrupt translocation of the GR from cytoplasm to nucleus and thereby reduce GR functional capacity (69). Moreover, cytokines have been shown to increase the expression of the inert β-isoform of the GR, which serves to divert glucocorticoids from the active α-isoform (70). Reduced GR function (as manifested typically by an abnormal dexamethasone suppression test and/or a dexamethasone-CRH test) is a hallmark of depression and may contribute to impaired regulation of CRH, which is under negative regulation by glucocorticoids (71, 72). Decreased GR function may also contribute to increased inflammation, given the well-known role of glucocorticoids in suppressing inflammatory responses through inhibition of NF-κB signaling (73).
SYNAPTIC PLASTICITY
Data indicate the importance of growth factors including brain-derived neurotrophic factor (BDNF) and synaptic plasticity in the vulnerability to and development as well as treatment of depression (74). For example, physical and psychological stressors suppress neurogenesis in the hippocampus, promote apoptotic cell death, and reduce density of synaptic connectivity between nerve cells (75). Increasing evidence suggests that inflammatory signaling pathways within the CNS may play a role in these detrimental stress-induced processes. For example, social isolation stress in rodents has been shown to suppress hippocampal neurogenesis and reduce hippocampal BDNF levels, with both effects being reversed by CNS administration of an antagonist to IL-1 (IL-1 receptor antagonist) before exposure to stressors (76). In addition, induction of NF-κB in the brain by cytokines such as IL-1 may contribute to alterations in neuronal growth and survival through the induction of reactive oxygen and nitrogen species such as nitric oxide, which has been shown to decrease the production of BDNF and to reduce neuronal survival of cells in the hippocampus (77, 78).
REGIONAL BRAIN ACTIVITY
Results from studies using positron emission tomography and functional magnetic resonance imaging provide further evidence that peripheral cytokine activity can induce centrally mediated behavioral changes. For example, during a functional magnetic resonance imaging task of visuospatial attention, patients given IFN-α exhibited significantly greater activation of the dorsal anterior cingulate cortex compared with control subjects (79). Interestingly, increased dorsal anterior cingulate cortex activity during cognitive tasks has also been demonstrated in patients vulnerable to mood disorders, such as those with high trait anxiety, neuroticism, or obsessive-compulsive disorder (80, 81). IFN-α has also been shown to lead to changes in frontal cortex and basal ganglia metabolic activity (as measured by positron emission tomography) (82, 83). Basal ganglia changes resemble those seen in Parkinson's disease and correlated with fatigue (83).
STRESS, DEPRESSION, AND THE INNATE IMMUNE RESPONSE
One of the most profound discoveries that has linked depression and the immune system is the finding that psychosocial stress, a well-known precipitant of mood disorders, can activate the innate immune response. For example, in a study of normal volunteers subjected to the Trier Social Stress Test (a public speaking task followed by mental arithmetic), examination of peripheral blood inflammatory markers revealed significant increases in NF-κB DNA binding within minutes after stressor cessation (84). These data complement results from a host of studies demonstrating that a variety of both acute and chronic emotional and physical stressors are associated with increases in inflammatory markers including innate immune cytokines and their soluble receptors as well as acute-phase proteins (85–87). Interestingly, the innate immune response to stress appears to be exaggerated in depressed patients exposed to early life stress (ELS). Indeed, depressed male patients with increased ELS exhibited significantly greater peripheral blood IL-6 responses and increased NF-κB DNA binding in response to the Trier Social Stress Test than nondepressed control subjects (88). This relationship between ELS and increased inflammation has also been observed in a large population-based study wherein individuals exposed to increasing levels of ELS were found to exhibit increasing levels of CRP in adulthood (87). Given the relationship between stress, depression, and inflammation, these data raise the question as to whether inflammation plays a role in the link between stress, depression, and disease, especially given the recent recognition that inflammation may represent a common mechanism for a number of illnesses including cardiovascular disease, diabetes, and cancer (48–50, 89, 90). As noted above, the effects of stress on relevant growth factors through its effects on the immune system may also play a role in neurodegenerative disorders (in addition to depression) (91).
Regarding the mechanism(s) by which stress influences the innate immune response, emerging data from humans and laboratory animals indicate that the sympathetic and parasympathetic nervous systems may be involved. Antagonism of both α- and β-adrenergic receptors has been shown to abrogate the effects of stress on the induction of innate immune cytokines in both the peripheral blood and brain of laboratory animals (92). Interestingly, increased peripheral blood IL-6 concentrations due to the stress of increased altitude were eliminated by administration of the α-adrenergic agent, prazosin (93). It should be noted, however, that β-agonists are known to have potent anti-inflammatory effects (94, 95), and therefore the role of catecholamines in the regulation of the innate immune response is in need of further study. Indeed, there is some suggestion that stress or inflammation-related induction of α1-adrenergic receptors (in combination with the effects of stress or inflammation on the β-receptor) may be an important component in determining the net effect of catecholamines on the inflammatory response (96). Finally, there has been recent interest in the role of parasympathetic pathways in inhibiting inflammation. For example, stimulation of the vagus nerve (and parasympathetic outflow pathways) has been shown to inhibit endotoxin induction of TNF-α and the signs of sepsis (97). These effects appear to be mediated by the release of acetylcholine, which through binding to the α7 subunit of the nicotinic acetylcholine receptor can inhibit NF-κB. Nevertheless, given the rich interconnection between sympathetic and parasympathetic nervous systems including shared mediators, the exact mechanism by which the autonomic nervous system modulates the inflammatory response is an area that warrants further study.
THERAPEUTIC IMPLICATIONS
Given the potential role of the immune system in the development of depression, there has been mounting interest in targeting the innate immune response as a novel therapeutic strategy to treat depression. Of relevance in this regard, successful antidepressant treatment of major depression with selective serotonin reuptake inhibitors or tricyclic antidepressants has been associated with reduced circulating cytokine concentrations, including TNF-α (98, 99) and IL-6 (100) Recent observations suggest that bupropion, a marketed antidepressant, can also reduce circulating concentrations of TNF-α in mice and in subjects with inflammatory disorders including Crohn's disease (101, 102). In vitro studies also indicate that a number of antidepressants can suppress the release of inflammatory cytokines while increasing the release of cytokines that inhibit inflammation such as IL-10 (103). Importantly, patients with increased plasma concentrations of innate immune cytokines are less likely to respond to currently available therapies (100, 104–107) and, conversely, patients with treatment-resistant depression have been shown to be especially likely to demonstrate evidence of an activated innate immune response, including elevated plasma concentrations of innate immune cytokines (100, 104–108). These findings suggest that pharmacological strategies aimed to downregulate inflammatory signaling pathways may have unique antidepressant efficacy and may be especially relevant in patients with treatment-resistant depression with increased inflammation.
Preliminary data suggest that targeting the innate immune response may be a viable antidepressant strategy. For example, increased response rates and improvement of depressive symptoms in patients with major depression was reported in an add-on study using the anti-inflammatory agent, celecoxib, a cyclooxygenase-2 inhibitor, in combination with reboxetine (109). In addition, in patients with psoriasis, the anti-TNF-α agent, etanercept, was found to reduce symptoms of depression (determined by the Hamilton Depression Rating Scale and the Beck Depression Inventory) independently from the clinical improvement of the primary disorder (46). Animal data are also consistent with the notion that immune targeted therapies may have antidepressant potential. Indeed, mice who have had the gene for the TNF-α receptor “knocked out” have been found to exhibit an antidepressant phenotype and are resistant to the anxiety-inducing effects of viral infection (110, 111).
Taken together, the data suggest that interventions that block inflammation may hold therapeutic promise especially in depressed individuals with increased inflammation. Given established cutoff values for increased inflammation as defined by the American Heart Association (i.e., CRP >3 ng/liter) (48), such patients may be readily identified for further study and exploration of novel treatment approaches targeting cytokines or their signaling pathways.
1 Anonymous: The changing rate of major depression: cross-national comparisons. Cross-National Collaborative Group. JAMA 1992; 268: 3098– 3105Crossref, Google Scholar
2 Chengappa KN, Kupfer DJ, Frank E, Houck PR, Grochocinski VJ, Cluss PA, Stapf DA: Relationship of birth cohort and early age at onset of illness in a bipolar disorder case registry. Am J Psychiatry 2003; 160: 1636– 1642Crossref, Google Scholar
3 Kessler RC, Berglund P, Demler O, Jin R, Koretz D, Merikangas KR, Rush AJ, Walters EE, Wang PS, National Comorbidity Survey Replication: The epidemiology of major depressive disorder: results from the National Comorbidity Survey Replication (NCS-R) [see comment]. JAMA 2003; 289: 3095– 3105Crossref, Google Scholar
4 Frank E, Kupfer DJ, Wagner EF, McEachran AB, Cornes C: Efficacy of interpersonal psychotherapy as a maintenance treatment of recurrent depression: contributing factors [erratum appears in Arch Gen Psychiatry 1992; 49:401]. Arch Gen Psychiatry 1991; 48: 1053– 1059Crossref, Google Scholar
5 Sudak HS. Suicide, in Kaplan & Sadock's Comprehensive Textbook of Psychiatry, 8th ed. Edited by Sadock BJ, Sadock VA. New York, Lippincott, Williams & Wilkins, 2005, pp 2442– 2453Google Scholar
6 Wulsin LR, Vaillant GE, Wells VE: A systematic review of the mortality of depression [see comment]. Psychosom Med 1999; 61: 6– 17Crossref, Google Scholar
7 Joynt KE, Whellan DJ, O'Connor CM: Depression and cardiovascular disease: mechanisms of interaction. Biol Psychiatry 2003; 54: 248– 261Crossref, Google Scholar
8 Thomas AJ, Kalaria RN, O'Brien JT: Depression and vascular disease: what is the relationship? J Affect Disord 2004; 79: 81– 95Crossref, Google Scholar
9 Davidson K, Jonas BS, Dixon KE, Markovitz JH: Do depression symptoms predict early hypertension incidence in young adults in the CARDIA study? Coronary Artery Risk Development in Young Adults. Arch Intern Med 2000; 160: 1495– 1500Crossref, Google Scholar
10 Jonas BS, Lando JF: Negative affect as a prospective risk factor for hypertension. Psychosom Med 2000; 62: 188– 196Crossref, Google Scholar
11 Eaton WW, Armenian H, Gallo J, Pratt L, Ford DE: Depression and risk for onset of type II diabetes: a prospective population-based study [see comment]. Diabetes Care 1996; 19: 1097– 1102Crossref, Google Scholar
12 Kawakami N, Takatsuka N, Shimizu H, Ishibashi H: Depressive symptoms and occurrence of type 2 diabetes among Japanese men [see comment]. Diabetes Care 1999; 22: 1071– 1076Crossref, Google Scholar
13 Pine DS, Goldstein RB, Wolk S, Weissman MM: The association between childhood depression and adulthood body mass index. Pediatrics 2001; 107: 1049– 1056Crossref, Google Scholar
14 Raikkonen K, Matthews KA, Kuller LH: The relationship between psychological risk attributes and the metabolic syndrome in healthy women: antecedent or consequence? Metab Clin Exp 2002; 51: 1573– 1577Crossref, Google Scholar
15 Spiegel D, Giese-Davis J: Depression and cancer: mechanisms and disease progression [see comment]. Biol Psychiatry 2003; 54: 269– 282Crossref, Google Scholar
16 Greden JF. The burden of disease for treatment-resistant depression. J Clin Psychiatry 2001; 62( suppl 16): 26– 31.Google Scholar
17 Judd LL, Paulus MJ, Schettler PJ, Akiskal HS, Endicott J, Leon AC, Maser JD, Mueller T, Solomon DA, Keller MB: Does incomplete recovery from first lifetime major depressive episode herald a chronic course of illness? Am J Psychiatry 2000; 157: 1501– 1504Crossref, Google Scholar
18 Simon GE: Long-term prognosis of depression in primary care. Bull World Health Org 2000; 78: 439– 445Google Scholar
19 Crown WH, Finkelstein S, Berndt ER, Ling D, Poret AW, Rush AJ, Russell JM: The impact of treatment-resistant depression on health care utilization and costs. J Clin Psychiatry 2002; 63: 963– 971Crossref, Google Scholar
20 Raison CL, Capuron L, Miller AH: Cytokines sing the blues: inflammation and the pathogenesis of major depression. Trend Immunol 2006; 27: 24– 31Crossref, Google Scholar
21 Kling MA, Alesci S, Csako G, Costello R, Luckenbaugh DA, Bonne O, Duncko R, Drevets WC, Manji HK, Charney DS, Gold PW: Sustained low-grade pro-inflammatory state in unmedicated, remitted women with major depressive disorder as evidenced by elevated serum levels of the acute phase proteins C-reactive protein and serum amyloid A. Biol Psychiatry 2007; 62: 309– 313Crossref, Google Scholar
22 Dantzer R, O'Connor JC, Freund GG, Johnson RW, Kelley KW: From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci 2008; 9: 46– 56Crossref, Google Scholar
23 Abbas AK, Lichtman AH. Cellular and Molecular Immunology, 5th ed. Philadelphia, WB Saunders 2003Google Scholar
24 Sweeney SE, Firestein GS: Primer: signal transduction in rheumatic disease—a clinician's guide. Nat Clin Pract Rheum 2007; 3: 651– 660Crossref, Google Scholar
25 Maier SF, Watkins LR: Cytokines for psychologists: implications of bidirectional immune-to-brain communication for understanding behavior, mood, and cognition. Psychol Rev 1998; 105: 83– 107Crossref, Google Scholar
26 Zorilla E, Luborsky L, McKay J, Roesnthal R, Houldin A, Tax A, McCorkle R, Seligman DA, Schmidt K: The relationship of depression and stressors to immunological assays: a meta-analytic review. Brain Behav Immun 2001; 15: 199– 226Crossref, Google Scholar
27 Vgontzas AN, Bixler EO, Chrousos GP: Obesity-related sleepiness and fatigue: the role of the stress system and cytokines. Ann NY Acad Sci 2006; 1083: 329– 344Crossref, Google Scholar
28 Alesci S, Martinez PE, Kelkar S, Ilias I, Ronsaville DS, Listwak SJ, Ayala AR, Licinio J, Gold HK, Kling MA, Chrousos GP, Gold PW: Major depression is associated with significant diurnal elevations in plasma interleukin-6 levels, a shift of its circadian rhythm, and loss of physiological complexity in its secretion: clinical implications. J Clin Endocrinol Metab 2005; 90: 2522– 2530Crossref, Google Scholar
29 Kop WJ, Gottdiener JS, Tangen CM, Fried LP, McBurnie MA, Walston J, Newman A, Hirsch C, Tracy RP: Inflammation and coagulation factors in persons > 65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol 2002; 89: 419– 424Crossref, Google Scholar
30 Miller GE, Freedland KE, Duntley S, Carney RM: Relation of depressive symptoms to C-reactive protein and pathogen burden (cytomegalovirus, herpes simplex virus, Epstein-Barr virus) in patients with earlier acute coronary syndromes. Am J Cardiol 2005; 95: 317– 321Crossref, Google Scholar
31 Suarez EC: C-reactive protein is associated with psychological risk factors of cardiovascular disease in apparently healthy adults. Psychosom Med 2004; 66: 684– 691Crossref, Google Scholar
32 Capuron L, Gumnick JF, Musselman DL, Lawson DH, Reemsnyder A, Nemeroff CB, Miller AH: Neurobehavioral effects of interferon-α in cancer patients: phenomenology and paroxetine responsiveness of symptom dimensions. Neuropsychopharmacology 2002; 26: 643– 652Crossref, Google Scholar
33 Wichers MC, Koek GH, Robaeys G, Praamstra AJ, Maes M: Early increase in vegetative symptoms predicts IFN-α-induced cognitive-depressive changes. Psychol Med 2005; 35: 433– 441Crossref, Google Scholar
34 Raison CL, Demetrashvili M, Capuron L, Miller AH: Neuropsychiatric side effects of interferon-α: recognition and management. CNS Drugs 2005; 19: 1– 19Crossref, Google Scholar
35 Musselman DL, Lawson DH, Gumnick JF, Manatunga AK, Penna S, Goodkin RS, Greiner K, Nemeroff CB, Miller AH: Paroxetine for the prevention of depression induced by high-dose interferon alfa [see comments.]. N Engl J Med 2001; 344: 961– 966Crossref, Google Scholar
36 Raison CL, Woolwine BJ, Demetrashvili MF, Borisov AS, Weinreib R, Staab JP, Zajecka JM, Bruno CJ, Henderson MA, Reinus JF, Evans DL, Asnis GM, Miller AH: Paroxetine for prevention of depressive symptoms induced by interferon-α and ribavirin for hepatitis C. Aliment Pharmacol Ther 2007; 25: 1163– 1174Crossref, Google Scholar
37 Schaefer M, Schwaiger M, Garkisch AS, Pich M, Hinzpeter A, Uebelhack R, Heinz A, van Boemmel F, Berg T: Prevention of interferon-α associated depression in psychiatric risk patients with chronic hepatitis C [see comment]. J Hepatol 2005; 42: 793– 798Crossref, Google Scholar
38 Felger JC, Alagbe O, Hu F, Mook D, Freeman AA, Sanchez MM, Kalin NH, Ratti E, Nemeroff CB, Miller AH: Effects of interferon-α on rhesus monkeys: a non-human primate model of cytokine-induced depression. Biol Psychiatry 2007; 62: 1324– 1333Crossref, Google Scholar
39 Evans DL, Charney DS, Lewis L, Golden RN, Gorman JM, Krishnan KR, Nemeroff CB, Bremner JD, Carney RM, Coyne JC, Delong MR, Frasure-Smith N, Glassman AH, Gold PW, Grant I, Gwyther L, Ironson G, Johnson RL, Kanner AM, Katon WJ, Kaufmann PG, Keefe FJ, Ketter T, Laughren TP, Leserman J, Lyketsos CG, McDonald WM, McEwen BS, Miller AH, Musselman DL, O'Connor C, Petitto JM, Pollock BG, Robinson RG, Roose SP, Rowland J, Sheline Y, Sheps DS, Simon G, Spiegel D, Stunkard A, Sunderland T, Tibbits PJ, Valvo WJ: Mood disorders in the medically ill: scientific review and recommendations. Biol Psychiatry 2005; 58: 175– 189Crossref, Google Scholar
40 Evans DL, Staab JP, Petitto JM, Morrison MF, Szuba MP, Ward HE, Wingate B, Luber MP, O'Reardon JP: Depression in the medical setting: biopsychological interactions and treatment considerations. J Clin Psychiatry 1999; 60( Suppl 4): 40– 55; discussion 56Google Scholar
41 Musselman DL, Miller AH, Porter MR, Manatunga A, Gao F, Penna S, Pearce BD, Landry J, Glover S, McDaniel JS, Nemeroff CB: Higher than normal plasma interleukin-6 concentrations in cancer patients with depression: preliminary findings. Am J Psychiatry 2001; 158: 1252– 1257Crossref, Google Scholar
42 Bower JE, Ganz PA, Aziz N, Fahey JL: Fatigue and proinflammatory cytokine activity in breast cancer survivors. Psychosom Med 2002; 64: 604– 611Crossref, Google Scholar
43 Kahl KG, Kruse N, Faller H, Weiss H, Rieckmann P: Expression of tumor necrosis factor-α and interferon-γ mRNA in blood cells correlates with depression scores during an acute attack in patients with multiple sclerosis. Psychoneuroendocrinology 2002; 27: 671– 681Crossref, Google Scholar
44 Parissis JT, Adamopoulos S, Rigas A, Kostakis G, Karatzas D, Venetsanou K, Kremastinos DT: Comparison of circulating proinflammatory cytokines and soluble apoptosis mediators in patients with chronic heart failure with versus without symptoms of depression. Am J Cardiol 2004; 94: 1326– 1328Crossref, Google Scholar
45 Owen BM, Eccleston D, Ferrier IN, Young AH: Raised levels of plasma interleukin-1β in major and postviral depression. Acta Psychiatr Scand 2001; 103: 226– 228Crossref, Google Scholar
46 Tyring S, Gottlieb A, Papp K, Gordon K, Leonardi C, Wang A, Lalla D, Woolley M, Jahreis A, Zitnik R, Cella D, Krishnan R: Etanercept and clinical outcomes, fatigue, and depression in psoriasis: double-blind placebo-controlled randomised phase III trial. Lancet 2006; 367: 29– 35Crossref, Google Scholar
47 Persoons P, Vermeire S, Demyttenaere K, Fischler B, Vandenberghe J, Van Oudenhove L, Pierik M, Hlavaty T, Van Assche G, Noman M, Rutgeerts P: The impact of major depressive disorder on the short- and long-term outcome of Crohn's disease treatment with infliximab. Aliment Pharmacol Ther 2005; 22: 101– 110Crossref, Google Scholar
48 Willerson JT, Ridker PM: Inflammation as a cardiovascular risk factor. Circulation 2004; 109: II2– II10Crossref, Google Scholar
49 Pradhan AD, Manson JE, Rifai N, Buring JE, Ridker PM: C-reactive protein, interleukin 6, and risk of developing type 2 diabetes mellitus [see comment]. JAMA 2001; 286: 327– 334Crossref, Google Scholar
50 Aggarwal BB, Shishodia S, Sandur SK, Pandey MK, Sethi G: Inflammation and cancer: how hot is the link? Biochem Pharmacol 2006; 72: 1605– 1621Crossref, Google Scholar
51 Vitkovic L, Konsman JP, Bockaert J, Dantzer R, Homburger V, Jacque C: Cytokine signals propagate through the brain [erratum appears in Mol Psychiatry 2001; 6:249]. Mol Psychiatry 2000; 5: 604– 615Crossref, Google Scholar
52 Banks WA: The blood-brain barrier in psychoneuroimmunology. Neurol Clin 2006; 24: 413– 419Crossref, Google Scholar
53 Quan N, Stern EL, Whiteside MB, Herkenham M: Induction of pro-inflammatory cytokine mRNAs in the brain after peripheral injection of subseptic doses of lipopolysaccharide in the rat. J Neuroimmunol 1999; 93: 72– 80Crossref, Google Scholar
54 Rivest S, Lacroix S, Vallieres L, Nadeau S, Zhang J, Laflamme N: How the blood talks to the brain parenchyma and the paraventricular nucleus of the hypothalamus during systemic inflammatory and infectious stimuli. Proc Soc Exp Biol Med 2000; 223: 22– 38Crossref, Google Scholar
55 Maier SF, Goehler LE, Fleshner M, Watkins LR: The role of the vagus nerve in cytokine-to-brain communication. Ann NY Acad Sci 1998; 840: 289– 300Crossref, Google Scholar
56 Dunn AJ, Swiergiel AH, de Beaurepaire R: Cytokines as mediators of depression: what can we learn from animal studies? Neurosci Biobehav Rev 2005; 29: 891– 909Crossref, Google Scholar
57 Shuto H, Kataoka Y, Horikawa T, Fujihara N, Oishi R: Repeated interferon-α administration inhibits dopaminergic neural activity in the mouse brain. Brain Res 1997; 747: 348– 351Crossref, Google Scholar
58 Rivier C, Vale W, Brown M: In the rat, interleukin-1α and -β stimulate adrenocorticotropin and catecholamine release. Endocrinology 1989; 125: 3096– 3102Crossref, Google Scholar
59 Capuron L, Neurauter G, Musselman DL, Lawson DH, Nemeroff CB, Fuchs D, Miller AH: Interferon-α-induced changes in tryptophan metabolism: relationship to depression and paroxetine treatment. Biol Psychiatry 2003; 54: 906– 914Crossref, Google Scholar
60 Wichers MC, Maes M: The role of indoleamine 2,3-dioxygenase (IDO) in the pathophysiology of interferon-α-induced depression. J Psychiatry Neurosci 2004; 29: 11– 17Google Scholar
61 Bonaccorso S, Marino V, Puzella A, Pasquini M, Biondi M, Artini M, Almerighi C, Verkerk R, Meltzer H, Maes M: Increased depressive ratings in patients with hepatitis C receiving interferon-α-based immunotherapy are related to interferon-α-induced changes in the serotonergic system. J Clin Psychopharmacol 2002; 22: 86– 90Crossref, Google Scholar
62 Zhu CB, Blakely RD, Hewlett WA: The proinflammatory cytokines interleukin-1β and tumor necrosis factor-α activate serotonin transporters. Neuropsychopharmacology 2006; 31: 2121– 2131Crossref, Google Scholar
63 Sanchez MM, Alagbe O, Felger JC, Zhang J, Graff AE, Grand AP, Maestripieri D, Miller AH: Activated p38: MAPK is associated with decreased CSF 5-HIAA and increased maternal rejection during infancy in rhesus monkeys. Mol Psychiatry 2007; 12: 895– 897Crossref, Google Scholar
64 Wu HQ, Rassoulpour A, Schwarcz R: Kynurenic acid leads, dopamine follows: a new case of volume transmission in the brain? J Neural Transm 2007; 114: 33– 41Crossref, Google Scholar
65 Kitagami T, Yamada K, Miura H, Hashimoto R, Nabeshima T, Ohta T: Mechanism of systemically injected interferon-α impeding monoamine biosynthesis in rats: role of nitric oxide as a signal crossing the blood-brain barrier. Brain Res 2003; 978: 104– 114Crossref, Google Scholar
66 Sapolsky R, Rivier C, Yamamoto G, Plotsky P, Vale W: Interleukin-1 stimulates the secretion of hypothalamic corticotropin-releasing factor. Science 1987; 238: 522– 524Crossref, Google Scholar
67 Capuron L, Raison CL, Musselman DL, Lawson DH, Nemeroff CB, Miller AH: Association of exaggerated HPA axis response to the initial injection of interferon-α with development of depression during interferon-α therapy. Am J Psychiatry 2003; 160: 1342– 1345Crossref, Google Scholar
68 Owens MJ, Nemeroff CB: Physiology and pharmacology of corticotropin-releasing factor. Pharmacol Rev 1991; 43: 425– 473Google Scholar
69 Pace TW, Hu F, Miller AH: Cytokine-effects on glucocorticoid receptor function: relevance to glucocorticoid resistance and the pathophysiology and treatment of major depression. Brain Behav Immun 2007; 21: 9– 19Crossref, Google Scholar
70 Webster JC, Oakley RH, Jewell CM, Cidlowski JA: Proinflammatory cytokines regulate human glucocorticoid receptor gene expression and lead to the accumulation of the dominant negative beta isoform: a mechanism for the generation of glucocorticoid resistance. Proc Natl Acad Sci USA 2001; 98: 6865– 6870Crossref, Google Scholar
71 Holsboer F: The corticosteroid hypothesis of depression. Neuropsychopharmacology 2000; 23: 477– 501Crossref, Google Scholar
72 Raison CL, Miller AH: When not enough is too much: the role of insufficient glucocorticoid signaling in the pathophysiology of stress-related disorders. Am J Psychiatry 2003; 160: 1554– 1565Crossref, Google Scholar
73 Rhen T, Cidlowski JA: Antiinflammatory action of glucocorticoids—new mechanisms for old drugs. N Engl J Med 2005; 353: 1711– 1723Crossref, Google Scholar
74 Duman RS: Depression: a case of neuronal life and death? Biol Psychiatry 2004; 56: 140– 145Crossref, Google Scholar
75 Schmidt HD, Duman RS: The role of neurotrophic factors in adult hippocampal neurogenesis, antidepressant treatments and animal models of depressive-like behavior. Behav Pharmacol 2007; 18: 391– 418Crossref, Google Scholar
76 Barrientos RM, Sprunger DB, Campeau S, Higgins EA, Watkins LR, Rudy JW, Maier SF: Brain-derived neurotrophic factor mRNA downregulation produced by social isolation is blocked by intrahippocampal interleukin-1 receptor antagonist. Neuroscience 2003; 121: 847– 853Crossref, Google Scholar
77 Canossa M, Giordano E, Cappello S, Guarnieri C, Ferri S: Nitric oxide down-regulates brain-derived neurotrophic factor secretion in cultured hippocampal neurons. Proc Natl Acad Sci USA 2002; 99: 3282– 3287Crossref, Google Scholar
78 Fritzen S, Schmitt A, Koth K, Sommer C, Lesch KP, Reif A: Neuronal nitric oxide synthase (NOS-I) knockout increases the survival rate of neural cells in the hippocampus independently of BDNF. Mol Cell Neurosci 2007; 35: 261– 271Crossref, Google Scholar
79 Capuron L, Pagnoni G, Demetrashvili M, Woolwine BJ, Nemeroff CB, Berns GS, Miller AH: Anterior cingulate activation and error processing during interferon-α treatment. Biol Psychiatry 2005; 58: 190– 196Crossref, Google Scholar
80 Paulus MP, Feinstein JS, Simmons A, Stein MB: Anterior cingulate activation in high trait anxious subjects is related to altered error processing during decision making. Biol Psychiatry 2004; 55: 1179– 1187Crossref, Google Scholar
81 Ursu S, Stenger VA, Shear MK, Jones MR, Carter CS: Overactive action monitoring in obsessive-compulsive disorder: evidence from functional magnetic resonance imaging. Psychol Sci 2003; 14: 347– 353Crossref, Google Scholar
82 Juengling FD, Ebert D, Gut O, Engelbrecht MA, Rasenack J, Nitzsche EU, Bauer J, Lieb K: Prefrontal cortical hypometabolism during low-dose interferon α treatment. Psychopharmacology (Berl) 2000; 152: 383– 389Crossref, Google Scholar
83 Capuron L, Pagnoni G, Demetrashvili MF, Lawson DH, Fornwalt FB, Woolwine BJ, Berns GS, Nemeroff CB, Miller AH: Basal ganglia hypermetabolism and symptoms of fatigue during interferon-α therapy. Neuropsychopharmacology 2007; 32: 2384– 2392Crossref, Google Scholar
84 Bierhaus A, Wolf J, Andrassy M, Rohleder N, Humpert PM, Petrov D, Ferstl R, von Eynatten M, Wendt T, Rudofsky G, Joswig M, Morcos M, Schwaninger M, McEwen B, Kirschbaum C, Nawroth PP: A mechanism converting psychosocial stress into mononuclear cell activation. Proc Natl Acad Sci USA 2003; 100: 1920– 1925Crossref, Google Scholar
85 Steptoe A, Hamer M, Chida Y: The effect of acute psychological stress on circulating inflammatory factors in humans: a review and meta-analysis. Brain Behav Immun 2007; 21: 901– 912Crossref, Google Scholar
86 Kiecolt-Glaser JK, Preacher KJ, MacCallum RC, Atkinson C, Malarkey WB, Glaser R: Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci USA 2003; 100: 9090– 9095Crossref, Google Scholar
87 Danese A, Pariante CM, Caspi A, Taylor A, Poulton R: Childhood maltreatment predicts adult inflammation in a life-course study. Proc Natl Acad Sci USA 2007; 104: 1319– 1324Crossref, Google Scholar
88 Pace TW, Mletzko TC, Alagbe O, Musselman DL, Nemeroff CB, Miller AH, Heim CM: Increased stress-induced inflammatory responses in male patients with major depression and increased early life stress. Am J Psychiatry 2006; 163: 1630– 1633Crossref, Google Scholar
89 Bierhaus A, Humpert PM, Nawroth PP: NF-κB as a molecular link between psychosocial stress and organ dysfunction. Pediatr Nephrol 2004; 19: 1189– 1191Crossref, Google Scholar
90 Hiratsuka S, Watanabe A, Aburatani H, Maru Y: Tumour-mediated upregulation of chemoattractants and recruitment of myeloid cells predetermines lung metastasis [see comment]. Nat Cell Biol 2006; 8: 1369– 1375Crossref, Google Scholar
91 Perry VH, Cunningham C, Holmes C: Systemic infections and inflammation affect chronic neurodegeneration. Nat Rev Immun 2007; 7: 161– 167Crossref, Google Scholar
92 Johnson JD, Campisi J, Sharkey CM, Kennedy SL, Nickerson M, Greenwood BN, Fleshner M: Catecholamines mediate stress-induced increases in peripheral and central inflammatory cytokines. Neuroscience 2005; 135: 1295– 1307Crossref, Google Scholar
93 Mazzeo RS, Donovan D, Fleshner M, Butterfield GE, Zamudio S, Wolfel EE, Moore LG: Interleukin-6 response to exercise and high-altitude exposure: influence of α-adrenergic blockade. J Appl Physiol 2001; 91: 2143– 2149Crossref, Google Scholar
94 Palmas W, Ma S, Psaty B, Goff DC Jr, Darwin C, Barr RG: Antihypertensive medications and C-reactive protein in the multi-ethnic study of atherosclerosis. Am J Hypertens 2007; 20: 233– 241Crossref, Google Scholar
95 Nagatomo Y, Yoshikawa T, Kohno T, Yoshizawa A, Anzai T, Meguro T, Satoh T, Ogawa S: Effects of β-blocker therapy on high sensitivity C-reactive protein, oxidative stress, and cardiac function in patients with congestive heart failure. J Card Fail 2007; 13: 365– 371Crossref, Google Scholar
96 Heijnen CJ, Rouppe van der Voort C, Wulffraat N, van der Net J, Kuis W, Kavelaars A: Functional α1-adrenergic receptors on leukocytes of patients with polyarticular juvenile rheumatoid arthritis. J Neuroimmunol 1996; 71: 223– 226Crossref, Google Scholar
97 Pavlov VA, Tracey KJ: The cholinergic anti-inflammatory pathway. Brain Behav Immun 2005; 19: 493– 499Crossref, Google Scholar
98 Tuglu C, Kara SH, Caliyurt O, Vardar E, Abay E: Increased serum tumor necrosis factor-α levels and treatment response in major depressive disorder. Psychopharmacology (Berl) 2003; 170: 429– 433Crossref, Google Scholar
99 Narita K, Murata T, Takahashi T, Kosaka H, Omata N, Wada Y: Plasma levels of adiponectin and tumor necrosis factor-α in patients with remitted major depression receiving long-term maintenance antidepressant therapy. Progr Neuropsychopharmacol Biol Psychiatry 2006; 30: 1159– 1162Crossref, Google Scholar
100 Lanquillon S, Krieg JC, Bening-Abu-Shach U, Vedder H: Cytokine production and treatment response in major depressive disorder. Neuropsychopharmacology 2000; 22: 370– 379Crossref, Google Scholar
101 Brustolim D, Ribeiro-dos-Santos R, Kast RE, Altschuler EL, Soares MB: A new chapter opens in anti-inflammatory treatments: the antidepressant bupropion lowers production of tumor necrosis factor-α and interferon-γ in mice. Int Immunopharmacol 2006; 6: 903– 907Crossref, Google Scholar
102 Kast RE, Altschuler EL: Anti-apoptosis function of TNF-α in chronic lymphocytic leukemia: lessons from Crohn's disease and the therapeutic potential of bupropion to lower TNF-α. Arch Immunol Ther Exp 2005; 53: 143– 147Google Scholar
103 Maes M, Song C, Lin AH, Bonaccorso S, Kenis G, De Jongh R, Bosmans E, Scharpe S: Negative immunoregulatory effects of antidepressants: inhibition of interferon-γ and stimulation of interleukin-10 secretion. Neuropsychopharmacology 1999; 20: 370– 379Crossref, Google Scholar
104 Benedetti F, Lucca A, Brambilla F, Colombo C, Smeraldi E: Interleukin-6 serum levels correlate with response to antidepressant sleep deprivation and sleep phase advance. Progr Neuropsychopharmacol Biol Psychiatry 2002; 26: 1167– 1170Crossref, Google Scholar
105 Mikova O, Yakimova R, Bosmans E, Kenis G, Maes M: Increased serum tumor necrosis factor α concentrations in major depression and multiple sclerosis. Eur Neuropsychopharmacol 2001; 11: 203– 208Crossref, Google Scholar
106 Lee SK, Lee HS, Lee TB, Kim DH, Koo JR, Kim YK, Son BK: The effects of antidepressant treatment on serum cytokines and nutritional status in hemodialysis patients. J Korean Med Sci 2004; 19: 384– 389Crossref, Google Scholar
107 Sluzewska A, Sobieska M, Rybakowski JK: Changes in acute-phase proteins during lithium potentiation of antidepressants in refractory depression. Neuropsychobiology 1997; 35: 123– 127Crossref, Google Scholar
108 Maes M, Delange J, Ranjan R, Meltzer HY, Desnyder R, Cooremans W, Scharpe S: Acute phase proteins in schizophrenia, mania and major depression: modulation by psychotropic drugs. Psychiatry Res 1997; 66: 1– 11Crossref, Google Scholar
109 Muller N, Schwarz MJ, Dehning S, Douhe A, Cerovecki A, Goldstein-Muller B, Spellmann I, Hetzel G, Maino K, Kleindienst N, Moller HJ, Arolt V, Riedel M: The cyclooxygenase-2 inhibitor celecoxib has therapeutic effects in major depression: results of a double-blind, randomized, placebo controlled, add-on pilot study to reboxetine. Mol Psychiatry 2006; 11: 680– 684Crossref, Google Scholar
110 Simen BB, Duman CH, Simen AA, Duman RS: TNFα signaling in depression and anxiety: behavioral consequences of individual receptor targeting. Biol Psychiatry 2006; 59: 775– 785Crossref, Google Scholar
111 Silverman MN, Macdougall MG, Hu F, Pace TW, Raison CL, Miller AH: Endogenous glucocorticoids protect against TNF-α-induced increases in anxiety-like behavior in virally infected mice. Mol Psychiatry 2007; 12: 408– 417Crossref, Google Scholar



