Neural Mechanisms of Genetic Risk for Impulsivity and Violence in Humans
Abstract
Neurobiological factors contributing to violence in humans remain poorly understood. One approach to this question is examining allelic variation in the X-linked monoamine oxidase A (MAOA) gene, previously associated with impulsive aggression in animals and humans. Here, we have studied the impact of a common functional polymorphism in MAOA on brain structure and function assessed with MRI in a large sample of healthy human volunteers. We show that the low expression variant, associated with increased risk of violent behavior, predicted pronounced limbic volume reductions and hyperresponsive amygdala during emotional arousal, with diminished reactivity of regulatory prefrontal regions, compared with the high expression allele. In men, the low expression allele is also associated with changes in orbitofrontal volume, amygdala and hippocampus hyperreactivity during aversive recall, and impaired cingulate activation during cognitive inhibition. Our data identify differences in limbic circuitry for emotion regulation and cognitive control that may be involved in the association of MAOA with impulsive aggression, suggest neural systems-level effects of X-inactivation in human brain, and point toward potential targets for a biological approach toward violence.
Violent and criminal behavior are likely related to complex environmental and social circumstances, but heritable factors also have been implicated (1, 2). The specific neural mechanisms leading to delinquency and impulsive aggression are poorly understood, although they have been the subject of spirited speculation and debate for literally centuries (2–4). Arguably, the clearest link between genetic variation and aggression exists for monoamine oxidase A (MAO-A, MIM 309850), a key enzyme in the catabolism of monoamines, especially serotonin. The serotonergic system has been implicated in impulsivity and manifest violent behavior in animals and both auto- and heteroaggression in humans (2). MAOA and -B genes, likely derived from the same ancestral gene, are both located on the X chromosome (Xp11.23), comprising 15 exons with identical intron-exon organization (5). MAO-A provides the major enzymatic clearing step for serotonin and norepinephrine during brain development, whereas MAO-B activity increases dramatically after birth (5). Mouse knockouts for MAOA, but not MAOB, have elevated brain levels of serotonin, norepinephrine, and dopamine. They show enhanced amygdala-dependent emotional, but not motor, learning (6), and males exhibit dramatically increased aggressive behavior (7). In humans, a Dutch kindred with a missense mutation in the MAOA gene has been described (8): hemizygous males, representing functional gene knockouts, exhibited a pattern of impulsively violent criminal behavior for generations.
Although functionally disabling variants of the gene are rarities outside of the laboratory setting, a common variable number of tandem repeats polymorphism of the MAOA gene has been described that strongly impacts transcriptional efficiency: enzyme expression is relatively high for carriers of 3.5 or 4 repeats (MAOA-H) and lower for carriers of 2, 3, or 5 repeats (MAOA-L) (9). Although conflicting evidence exists for the association of genotype with trait impulsivity in human cross-sectional studies, a clear and pronounced gene-by-environment interaction was found in a large longitudinal study of children followed for 25 years in which MAOA-L predicted violent offenses in males with adverse early experience (maltreatment) (10). This finding, replicated in the majority of further studies (11–13), but not all (14), suggests a deficiency in the neural systems for emotional regulation and memory as possible substrates for the observed gene-environment interaction, because they are essential for the encoding, retrieval, and extinction of negative emotional information expected to be associated with maltreatment during childhood. This finding agrees with current proposals linking brain structures involved in emotional control, such as amygdala and medial prefrontal and orbitofrontal cortices, to the emergence of violent behavior (3, 4). However, whereas two previous functional MRI (fMRI) studies suggested an effect of MAOA genotype during a cognitive task in small samples (15, 16), no data related to emotion processing or brain structure are available.
In the present study, we examined a large sample of healthy volunteers (Table 1, which is published as supporting information on the PNAS web site) using a multimodal imaging approach that we have shown previously to be sensitive to genetic variation affecting the serotonergic system (17). Because our sample was nonviolent, we are not studying the relationship of MAOA and violence per se, but rather the effects of one specific genetic factor on relevant aspects of brain circuitry without contamination by other interacting genetic and epidemiological risk factors that may be implicated in the emergence of this complex behavior and that could obscure or exaggerate the genetic effect (e.g., drug or alcohol use or maltreatment) (1, 2). Voxel-based morphometry was used to canvass the brain for regional volume changes related to genotype (17), previously seen in genetic variation related to serotonin (17), a major modulator of neurodevelopment (18, 19). Three functional magnetic resonance paradigms were used to assess aspects of emotional and cognitive control, subserved by limbic circuitry and conceptually linked to impulse control. To probe circuits of emotional arousal, we used affectively salient social stimuli (angry and fearful faces) previously shown to reliably activate amygdala (17). To examine the neural circuitry engaged by emotional memory, we used incidental encoding and retrieval of neutral and aversive visual scenes. Finally, because cognitive inhibitory processing has also been implicated as a substrate of impulsivity (4), we studied cognitive inhibitory control using a no-go variant of the “flanker” task (20). We hypothesized that carriers of MAOA-L would exhibit structural and functional changes in brain circuitry subserving these various regulatory functions related to emotion and inhibitory control. Because the behavioral effects of MAOA variation have been consistently more penetrant in males in both animal (7) and human (8) studies, we also expected that some physiological and structural differences would be more pronounced in males than females.
Results
All imaging results are derived from cross-sectional comparison of groups of genotyped healthy volunteers; therefore, reductions or increases are relative to the other genotype group(s) and do not imply that changes happen over time.
Genotype effects on brain structure
Analysis of brain structure revealed that allelic variation in MAOA was associated with pronounced regionally specific changes in gray matter volume (Fig. 1A and Table 2, which is published as supporting information on the PNAS web site); compared with MAOA-H subjects, MAOA-L individuals showed a significant reduction in volume that encompassed virtually the entire cingulate gyrus and bilateral amygdalae, with a maximum in anterior cingulate cortex. In addition, we found significant reductions in insula and hypothalamus. Relative to the volume in MAOA-H subjects, reductions averaged around 8%. Examination of sex-by-genotype interaction maps revealed a sex-specific (males only) increase (of ≈14%) in bilateral lateral orbitofrontal cortex (OFC) [Brodmann’s area (BA) 47] volume in MAOA-L men, relative to MAOA-H men (Fig. 1B), whereas no MAOA genotype-dependent structural changes were present in this region in women. The OFC was the only brain region where an interaction effect was found.
Genotype effects on brain function
Emotional arousal.
Analysis of fMRI data during perceptual matching of angry and fearful faces showed significant activation of amygdala and task-related deactivation of limbic and paralimbic regions implicated in emotion processing, a pattern seen previously by our group (17) and others (21) (Fig. 2 and Table 2). Activity was strongly modulated by genotype: MAOA-L individuals showed significantly increased activity in left amygdala and decreased response of subgenual (BA 25) and supragenual (BA 32) ventral cingulate cortex, left lateral OFC, and left insular cortex, relative to MAOA-H subjects (P < 0.05, corrected for multiple comparisons) (Fig. 2A–D). These effects were present in both sexes (no significant sex-by-genotype interaction). Because both increased and decreased reactivity was seen in these various limbic regions that showed reduced structural volume in MAOA-L individuals, it is unlikely that the fMRI effects are related to smaller volume in these small structures.
Because OFC structure and function were affected by genotype and this prefrontal region has been implicated in amygdala regulation, we examined the effects of genotype and sex on amygdala-orbitofrontal connectivity (Fig. 5, which is published as supporting information on the PNAS web site). OFC was significantly less reactive in carriers of MAOA-L in both sexes, and overall connectivity with amygdala was significantly reduced in men when compared with women.
Emotional memory.
We examined brain activation during emotional memory, i.e., the encoding and retrieval of aversively, compared with neutrally, valenced information. We found a pronounced effect of genotype and sex in left amygdala and hippocampal formation; men, but not women, carrying the low-expression MAOA genotype showed increased reactivity during retrieval of negatively valenced emotional material (Fig. 3 and Table 1). This effect was present for aversive, but not neutral, material and only during retrieval, as confirmed by a significant genotype-by-sex-by-task interaction in a post hoc ANOVA (F(3,258) = 3.0, P = 0.03).
Inhibitory control.
We probed the neural correlates of cognitive inhibitory control using a no-go “flanker task.” In men only, MAOA genotype had a pronounced effect on activation during response inhibition in dorsal anterior cingulate: MAOA-L hemizygotes showed deficient activation (Fig. 4 and Table 2). This finding is compatible with a previous observation in a small group of male subjects (16). Women had no significant effect of genotype, resulting in a significant sex-by-genotype interaction at this locus. Again, this functional change was located within the cingulate region of maximal structural change related to genotype.
Discussion
Our analysis revealed pronounced genotype-related structural and functional changes in corti-colimbic circuits previously linked to affect regulation, emotional memory, and impulsivity. Importantly, because our sample was psychiatrically normal, the variation observed is clearly compatible with normal mental health and does not imply or suggest increased risk for violence in our sample. Rather, our data identify neural mechanisms associated with one specific gene epidemiologically associated with risk for violent and impulsive behavior (10–13). By itself, this gene is likely to contribute only a small amount of risk in interaction with other genetic, epidemiological, and sociobiographical factors. Of the identified functional differences, increased amygdala activation, in particular, is associated with anger and with perception of angry faces (22); amygdala stimulation in animals can induce violent behavior; and amygdala ablation has been reported to reduce impulsive violence in humans (23). Because we used a low-level baseline (fixation) in our imaging tasks to increase reliability of the amygdala response (24), our data probe only the overall reactivity of fear-related circuitry to faces; further work using high-level baselines is necessary to implicate processing of specific facial emotions. It is noteworthy that the most robust structural changes were observed in cingulate, the brain region with the highest density of serotonin receptors within the human cortex (25) and the recipient of dense projections from amygdala (26). Convergent evidence strongly suggests a key regulatory role for cingulate and medial prefrontal cortices in extinguishing amygdala reactivity and in emotional arousal (26): ventral cingulate reactivity predicts amygdala signaling during extinction in humans (21) and lesions of this region markedly impair fear extinction (27). Given evidence that cingulate modulates amygdala activity by inhibition, our finding of reduced cingulate reactivity in MAOA-L subjects provides a potential mechanistic account for the observed increased amygdala activity in this group. In this context, it is worth mentioning that animal studies demonstrate a role for serotonergic neurotransmission in modulating the inhibitory functions of cingulate cortex (28).
Previous work has demonstrated a similar although less statistically robust and more focal effect on amygdala function as a consequence of genetic variation in the serotonin transporter, 5-HTTLPR, (17, 29); there, as here, it was the genetic variant associated with higher synaptic serotonin levels, presumably during neurodevelopment, that was associated with impaired limbic structure, increased amygdala activation, and relatively decreased response of cingulate circuitry regulating amygdala. Serotonin is further implicated by preclinical data showing enduring anxiety in mice with transient transgenic alterations of serotonin signaling shortly after birth or in mice treated perinatally with serotonin reuptake inhibitors (19). These results suggest separate genetic mechanisms apparently converging on the development of limbic circuitry, consistent with evidence that 5-HT impacts on neuronal proliferation, migration, differentiation, and synaptogenesis (18, 19). It is further of relevance to note that abnormal amygdala and orbitofrontal volume, both of which have been linked to deficits in fear perception and social cognition, have been observed in a study of partial deletions on chromosome Xp, including the MAOA locus (30).
One consistent finding in research on MAOA genotype effects on violence and aggression is the more pronounced impact on males. In our analysis of MAOA-dependent structural changes, we found a significant genotype-by-sex interaction in OFC. In human and animal models, OFC activity has been associated with representation of the relative reward value of primary and secondary (learned) reinforcers (31). In particular, OFC and OFC-amygdala interactions are critical for stimulus-reinforcement association learning (31) and have been hypothesized to link sensory representations of stimuli with the social judgements made about them on the basis of their motivational value. Lesions of OFC are associated with disinhibition and antisocial behavior (3, 4), and we have recently obtained imaging results showing reciprocal regulation of amygdala by both OFC and cingulate (32), suggesting that OFC provides a layer of control that may be especially important if cingulate function is suggested to be compromised, as here. Furthermore, we found significantly decreased functional connectivity with amygdala in men, indicating that this regulatory mechanism may be intrinsically weaker in men and that the genotype-dependent variation in OFC structure and function may therefore be more likely to result in insufficient amygdala regulation by this route.
When considering potential neurobiological correlates of aggression, it is important to bear in mind that overt behavior is expressed in a complex interaction of biological, psychological, and social determinants (2). Factor analytic studies of dimensional aspects of human temperament suggest that a distinction can be drawn between so-called impulsive-reactive and instrumental, goal-directed dimensions of aggression (33), although this distinction is not universally accepted. The instrumental factor has been associated with psychopathy, is often accompanied by diminished empathy and remorse, and has been linked to reduced amygdala activation and OFC volume (4). The genetic data presented here, which show the opposite effects associated with the risk allele, suggest that these two dimensions may be genetically dissociable; argue against an association of MAOA genotype with instrumental aggression and for a genetic risk for impulsive violence; and indicate that, whereas both instrumental and impulsive aggression may be present to varying degrees in most violent offenders, the risk imparted by the specific genetic variation studied here contributes to the impulsive dimension of this complex behavior. This finding is in good agreement with the preclinical and human data reviewed above that indicate an association of the serotonergic system with impulsive violence (2) and may clarify findings from epidemiological studies in which outcome measures such as arrests for violent offenses map ambiguously on these two dimensions of violent behavior (10).
A striking finding in the MAOA literature is the mediation of this genotype effect by past environmental adversity, suggesting an impact on brain systems related not only to acute emotional regulation, but also to the processing of emotional experience. Our findings during emotional memory in humans are analogous to enhanced emotional learning observed in MAOA knockout mice (6). If replicated, this finding could suggest that heightened sensitivity to adverse experience may underlie the increased vulnerability of MAOA-L males exposed to abuse during childhood (10–13). As observed previously, abnormal cingulate regulation of amygdala could also contribute to this gene-by-environment interaction by impaired extinction of conditioned fear (17).
Predisposition to impulsive violence by means of abnormal activation and regulation of emotion-related amygdala function might be further enhanced by deficient neural systems for cognitive control (4), especially over inhibition, the capacity to suppress prepotent but inappropriate behavior (20) that might originate from a dysregulated affective response. Although the rostral cingulate is key to the regulation of acute affective arousal and emotional learning, inhibitory control of prepotent cognitive responses is thought to be critically dependent on caudal aspects of anterior cingulate (28, 34). Our study of genetic influences on cognitive impulse control revealed a sex-dependent impairment in precisely this area of cingulate, affecting men only. Our finding of a genotype-by-sex interaction in this region therefore provides a plausible neural mechanism for reduced cognitive inhibitory control in risk allele-carrying males, suggesting synergistic impairment in cognitive and emotional neural regulatory mechanisms that might render MAOA-L men at especially high risk for a neural phenotype that plausibly relates to the slightly greater probability of impulsive violence.
The cellular mechanism for the observed sex-by-genotype interactions is not currently known. Because we found similar effects for amygdala and cingulate volume and activation for both sexes, a simple gene-load-effect mechanism linked to the localization of MAOA on the X chromosome seems unlikely. This conclusion was further substantiated by post hoc analysis of the female data, where the functional response of heterozygotes carrying both a high- and a low-expressing allele was intermediate between female homozygotes, and female homozygotes are similar to male hemizygotes (Fig. 6). This correspondence in functional response indicates similar gene dosage in the homo-/hemizygous groups, providing some in vivo evidence for a physiological consequence of X-inactivation in the human brain. X-inactivation, if sufficiently random across cells, would also predict an intermediate response at the neurobiological systems level in female heterozygotes, as was indeed observed. Of note, estrogens affect transcription of MAOA in brain (35), and sex hormone receptors are prominently expressed in amygdala, cingulate, and OFC (36). It should also be borne in mind that, in addition to direct cellular effects of sex, serotonin-related aggression is a complex behavior that manifests in social contexts that are themselves strongly affected by sex; for example, higher dominance status in primates is associated with MAOA-L and aggression in males (37), but not females.
Because the variation in MAOA related to increased serotonin levels has been predominantly associated with impulsive violence, and the analogous allele of 5-HTLPPR with anxiety and depression, it is instructive to compare similarities and differences in the neural mechanisms associated with these genes (17). Both impact on structure and function of amygdala and perigenual cingulate cortex, indicating a shared mechanism of emotional regulation under serotonergic control and predicting some overlap in clinical association, as is indeed observed (38). However, MAOA showed much more extensive effects in both structure and activation, notably affecting more caudal regions of the cingulate associated with cognitive control, as well as OFC and hippocampus. This finding may reflect the broader metabolic effect of variation in MAO-A, which catabolizes not only serotonin, but also other neurotransmitters, notably norepinephrine (5), which is also implicated in neurodevelopment and emotional experience. We speculate that, whereas our results suggest that a dysregulated and hyperreactive amygdala response contributes to both anxiety and violence (“fight or fright”), the manifestation of violence in behavior may require impairment in additional layers of control, be they emotional (OFC) or cognitive (caudal cingulate). Our genetic results are in good agreement with current hypotheses about the neural substrates of violence (3, 4). In addition, as discussed, an impact on emotional memory may relate to the pronounced gene-environment interaction observed for MAOA.
In summary, we present multimodal imaging data delineating functional and structural differences in a prefrontal-amygdala-hippocampal system for emotional regulation, memory, and cognitive control that suggest neural mechanisms for genetic bias toward impulsive violence. This work implicates neural systems for social adaptation and cognition under partial genetic control and suggests adverse consequences for increased serotonergic tone during brain development in humans.
Methods
Subjects
Subjects were culled from a larger population after careful screening (39) to ensure they were free of any lifetime history of psychiatric or neurological illness, psychiatric treatment, or drug or alcohol abuse (Table 1). Previous results from this ongoing study have been reported in subjects that partially overlap with the groups reported here (17). Only Caucasians of European ancestry were studied to avoid stratification artefacts. All available scans of subjects meeting these criteria were used. Subject demographics are shown in Table 1. Sex distribution differed significantly among genotypes, which did not influence the analyses because the genetic situation mandated that main effects and interactions with sex were explicitly included into the statistical model. A slight age difference in the group studied in fMRI was addressed by adding age as a covariate in all analyses. Subjects gave written informed consent and participated in the study according to the guidelines of the National Institute of Mental Health Institutional Review Board.
DNA collection and statistical analysis
Males are hemizygous carriers of either one MAOA-L or MAOA-H allele, whereas women carry two alleles. Therefore, only women can be heterozygote. For statistical analysis using ANOVA with a fully factorial (genotype by sex) design, the female MAOA-L group included all carriers of three or five repeats, so that two genotype groups were analyzed for each sex. To illustrate the effects of heterozygosity, we provide supplementary plots where female homozygotes for MAOA-L are shown separately from subjects carrying both an MAOA-L and an MAOA-H allele (Fig. 6, which is published as supporting information on the PNAS web site). Genomic control panels were performed to investigate occult genetic stratification between MAOA genotype groups: the sample was genotyped with a panel of 100 unlinked SNP loci (available upon request) to survey for occult genetic stratification and showed no significant differences in frequency overall [omnibus-χ2 (df 200) = 172.5, P = 0.92], and specifically also not for 5-HTTLPR variants of the serotonin transporter. We used standard methods to extract DNA from white blood cells with the Puragen DNA purification kit (Gentra Systems). The 30-bp MAOA variable-number of tandem repeats (vntr) polymorphism was amplified in an Applied Biosytems 9700 thermal cycler by PCR using the primer sequence of Sabol et al. (9). PCRs were performed in a final 25-μl volume containing 100 ng of genomic DNA, 10 pmol of each primer, dNTP mix (200 μmol, Applied Biosystems/Roche), formamide (GIBCO BRL), 0.5 units of Taq polymerase (Applied Biosystems/Roche), GC resolution solution (GC Rich PCR system Cat no. 2140306, Roche) in manufacturer’s (Applied Biosystems/Roche) buffer (10×) with 1.5 mM MgCl2. A 3-μl PCR product was separated on 2% Nusieve 3:1 Agarose gel (Cambrex Bio Science Rockland, Rockland, ME), which contained ethidium bromide (100 μg/200 ml of gel) to visualize the separated bands (Fig. 7, which is published as supporting information on the PNAS web site). Fragment sizes were determined by comparison with molecular length standards. The vntr alleles consist of 3 repeats (209 bp), 3.5 repeats (227 bp), 4 repeats (239 bp), and 5 repeats (269 bp).We did not include in our analysis two individuals who possessed 2 repeats.
Functional imaging tasks
Face matching.
The face matching task is a simple perceptual task previously described to robustly engage the amygdala (32, 40). During two blocks of an emotion task, subjects viewed a trio of faces, selecting one of the two faces (bottom) that was identical to the target face (top). Per block, six images were presented sequentially for 5 s, three of each sex and target affect (angry or afraid) derived from a standard set of pictures of facial affect. Emotion tasks alternated with three blocks of a sensorimotor control task where faces were replaced with simple geometric shapes.
Neutral and aversive encoding and retrieval.
This task is based on previous work on genetic influences on hippocampal function (41) and consisted of the encoding and subsequent retrieval of novel, complex scenes in a blocked paradigm of eight 20-s encoding blocks, followed by eight retrieval blocks in an interleaved design with a passive rest condition. The encoding and retrieval blocks were separated into individual runs, resulting in a total of 34 blocks over the two scan runs. During encoding blocks, subjects viewed six images, presented serially for 3 s each, and determined whether each image represented an “indoor” or “outdoor” scene, responding by button presses with their dominant hand. An equal number of “indoor” and “outdoor” scenes were presented in each encoding block derived from the International Affective Picture System (42). In half of the blocks, neutrally valenced pictures were presented; in the other half, emotionally aversive scenes were shown. During subsequent retrieval blocks, subjects again viewed six images, presented serially in pseudorandom order for 3 s each, and determined whether each scene was “new” or “old.” In each retrieval block, half the scenes were old (i.e., presented during the encoding blocks) and half were new (i.e., not presented during the encoding blocks). Emotional valence was again blocked. Before the beginning of each block, subjects viewed a brief (2-s) instruction: “Indoor or Outdoor?,” “Seen Before?,” or “Rest.”
Flanker task.
Subjects saw an array of five stimuli that included a central target arrow pointing left or right, flanked by two stimuli (arrows, boxes, or Xs) on either side and were instructed to press a button corresponding to the direction of the central target arrow as fast and accurately as possible. Each run included four experimental conditions: one of these, “no-go,” analyzed here, had flanking stimuli that were Xs, which indicated that the subjects had to withhold their response and served to evaluate response inhibition. Each stimulus was presented for 800 ms randomly distributed across the session (43). The randomization of this sequence was specified according to the stochastic design option in spm99. A total of 33 no-go stimuli (of 141) were presented in 10 min, 8 s. During the interstimulus interval (ISI), which varied from 2,200 to 5,200 ms, a fixation crosshair was presented.
Structural image processing
3D structural MRI scans were acquired on a 1.5-Tesla General Electric scanner (Milwaukee, WI) by using a T1-weighted spoiled gradient recalled (SPGR) sequence [repetition time (TR)/echo time (TE)/no. of excitations (NEX) 24/5/1, flip angle 45°, matrix size 256 × 256, field of view (FOV) 24 × 24 cm] with 124 sagittal slices (0.94 × 0.94 × 1.5 mm resolution) and preprocessed as described (17), followed by an optimized voxel-based morphometry (VBM) protocol using customized templates (44, 45). Resulting gray matter images were smoothed with a 10-mm Gaussian kernel before statistics. Analysis was performed on Linux workstation (RedHat Enterprise) by using matlab 6.52sp2 (Math-Works, Natick, MA) within the General Linear Model (46) in spm2 (www.fil.ion.ucl.ac.uk/spm). The specification of a design matrix identical to the one used in this study has been described in detail elsewhere (17). Briefly, effects of MAOA genotype on gray matter volume were examined by using an analysis of covariance model including the following covariates of no interest: total gray matter volume and orthogonalized first-and second-order polynomial expansions of age. Interactions of genotype with sex were explicitly included in the model to probe the hypothesized effects.
Functional image processing
Blood oxygen level-dependent (BOLD) fMRI was performed on a General Electric Signa 3T (Milwaukee, WI) by using gradient echo, echo-planar imaging (EPI) (24 axial slices; 4-mm thickness; 1-mm gap; TR/TE, 2,000/28 ms; FOV, 24 cm; matrix, 64 × 64). Images were processed as described (32, 40, 47) by using spm99 (www.fil.ion.ucl.ac.uk/spm). Briefly, images were realigned to the first image of the scan run, spatially normalized into a standard stereotactic space [Montreal Neurological Institute (MNI) template] by using affine and nonlinear (4 · 5 · 4 basis functions) transformations, smoothed with an 8-mm full-width half-maximum (FWHM) Gaussian filter and ratio normalized to the whole-brain global mean. A statistical image for the contrast of the emotion task versus the sensorimotor control (for face matching) or encoding/retrieval vs. rest (separately for emotionally negative and neutral stimuli) or no-go versus baseline (for the flanker task) was then obtained for each subject and analyzed in a second-level random effects model (ANOVA and one-tailed t test) to identify significant activations within and between genotype groups. Both main effects and interactions with sex were considered in the ANOVA. Age was included as a confounding covariate.
Functional connectivity analyses
Our methods to measure “functional connectivity” have been described (17, 48). This measure examines the covariation across the brain with the activation in a region (volume) of interest, by using the masks specified below. Because it is correlational in nature, functional connectivity should not be assumed to reflect anatomical connections or a causal link (44, 49). After mean and drift correction of the time series, median activity within this region of interest (ROI) was calculated (we prefer median as a robust estimator that coincides with the mean under the assumption of normality) for each scan and then correlated across the brain with all voxel time series, resulting in a map that contained, in each voxel, the correlation coefficient of the time series in this voxel with that of the reference regions. These maps, one per subject, were then analyzed in a random effects model in SPM identical to the one described in the previous paragraph.
Statistical inference
For all imaging methods, the significance threshold was set to P < 0.05, corrected for multiple comparisons within ROI defined by using the Wake Forest University pickatlas (www.fmri.wfubmc.edu) as described (17). For structural neuroimaging, this region included the entire limbic system (cingulate, medial prefrontal, and OFC, and subcortical structures including amygdala). Based on the structural results, hypothesis-driven ROI for analysis of functional data were placed in lateral OFC (BA 47), amygdala, and subgenual (BA 25) anterior cingulate. Additionally, an ROI defining supragenual cingulate (BA 32), derived from a prior study of genetic influences on emotion processing, was used in our analyses (17).
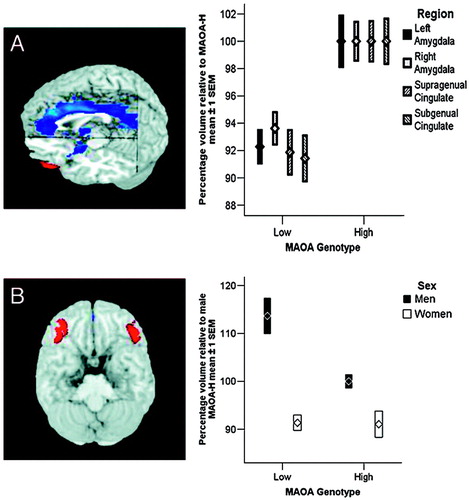
Figure 1. Structural data demonstrate limbic and paralimbic regional volume changes in MAOA-L subjects (n = 97). Plots represent the summed volumes of voxels in pre-defined ROI, normalized to volume measures relative to the MAOA-H group mean (100%). (A) Compared with MAOA-H subjects, MAOA-L individuals exhibit significant volume reductions in bilateral amygdala, supragenual anterior cingulate, and subgenual anterior cingulate cortex. Male and female subjects were combined. (B) Male MAOA-L individuals show increased lateral orbitofrontal volume, bilaterally, relative to MAOA-H subjects. Females show no effect of genotype, resulting in a highly significant sex-by-genotype interaction.
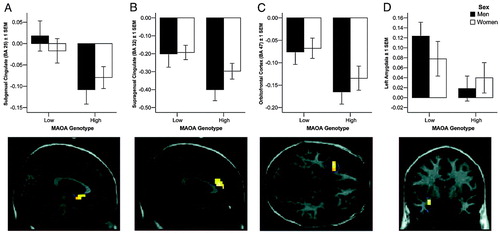
Figure 2. Thresholded (P < 0.05, corrected for multiple comparisons in the ROI) statistical maps and plots of percent blood oxygen level-dependent (BOLD) signal change (mean ± 1 SEM) illustrate differential activation to angry and fearful facial expressions in MAOA-L individuals in several limbic and paralimbic regions (n = 142): subgenual anterior cingulate (BA 25) (A), supragenual anterior cingulate (BA 32) (B), left lateral OFC (BA 47) (C), and left amygdala (D).
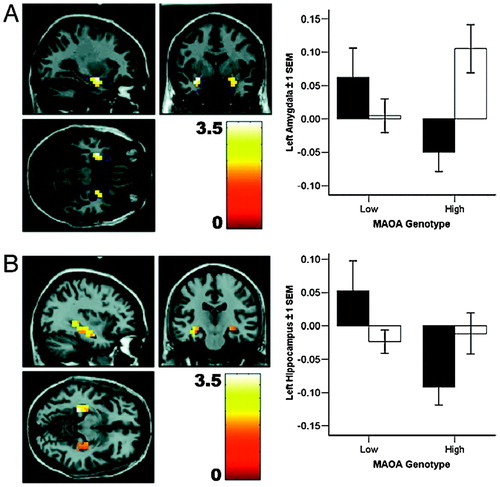
Figure 3. Limbic activation during the retrieval of aversive memories varies according to MAOA genotype (n = 90). (A) Left amygdala response during emotional memory retrieval is higher for male (filled bars) MAOA-L subjects, compared with male MAOA-H individuals. (B) Left hippocampal engagement during emotional memory retrieval is more pronounced for male, but not female (open bars), MAOA-L subjects, relative to MAOA-H individuals (n = 90).
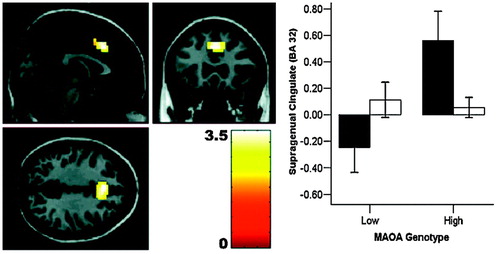
Figure 4. Genotype effect on anterior cingulate activation during response inhibition (no-go flanker task). Anterior cingulate (BA 32) response during response inhibition is higher for male (filled bars) MAOA-H subjects, compared with male MAOA-L individuals, whereas females (open bars) show no significant effect (n = 82).
1 Moffitt, T. E. (2005) Psychol. Bull. 131, 533–554.Crossref, Google Scholar
2 Lesch, K. P. & Merschdorf, U. (2000) Behav. Sci. Law 18, 581–604.Crossref, Google Scholar
3 Davidson, R. J., Putnam, K. M. & Larson, C. L. (2000) Science 289, 591–594.Crossref, Google Scholar
4 Blair, R. J. (2001) J. Neurol. Neurosurg. Psychiatry 71, 727–731.Crossref, Google Scholar
5 Shih, J. C., Chen, K. & Ridd, M. J. (1999) Annu. Rev. Neurosci. 22, 197–217.Crossref, Google Scholar
6 Kim, J. J., Shih, J. C., Chen, K., Chen, L., Bao, S., Maren, S., Anagnostaras, S. G., Fanselow, M. S., De Maeyer, E., Seif, I. & Thompson, R. F. (1997) Proc. Natl. Acad. Sci. USA 94, 5929–5933.Crossref, Google Scholar
7 Cases, O., Seif, I., Grimsby, J., Gaspar, P., Chen, K., Pournin, S., Muller, U., Aguet, M., Babinet, C., Shih, J. C., et al. (1995) Science 268, 1763–1766.Crossref, Google Scholar
8 Brunner, H. G., Nelen, M., Breakefield, X. O., Ropers, H. H. & van Oost, B. A. (1993) Science 262, 578–580.Crossref, Google Scholar
9 Sabol, S. Z., Hu, S. & Hamer, D. (1998) Hum. Genet. 103, 273–279.Crossref, Google Scholar
10 Caspi, A., McClay, J., Moffitt, T. E., Mill, J., Martin, J., Craig, I. W., Taylor, A. & Poulton, R. (2002) Science 297, 851–854.Crossref, Google Scholar
11 Nilsson, K. W., Sjoberg, R. L., Damberg, M., Leppert, J., Ohrvik, J., Alm, P. O., Lindstrom, L. & Oreland, L. (2006) Biol. Psychiatry 59, 121–127.Crossref, Google Scholar
12 Huang, Y. Y., Cate, S. P., Battistuzzi, C., Oquendo, M. A., Brent, D. & Mann, J. J. (2004) Neuropsychopharmacology 29, 1498–1505.Crossref, Google Scholar
13 Foley, D. L., Eaves, L. J., Wormley, B., Silberg, J. L., Maes, H. H., Kuhn, J. & Riley, B. (2004) Arch. Gen. Psychiatry 61, 738–744.Crossref, Google Scholar
14 Haberstick, B. C., Lessem, J. M., Hopfer, C. J., Smolen, A., Ehringer, M. A., Timberlake, D. & Hewitt, J. K. (2005) Am. J. Med. Genet. B Neuropsychiatr. Genet. 135, 59–64.Crossref, Google Scholar
15 Passamonti, L., Fera, F., Magariello, A., Cerasa, A., Gioia, M. C., Muglia, M., Nicoletti, G., Gallo, O., Provinciali, L. & Quattrone, A. (2006) Biol. Psychiatry 59, 334–340.Crossref, Google Scholar
16 Fan, J., Fossella, J., Sommer, T., Wu, Y. & Posner, M. I. (2003) Proc. Natl. Acad. Sci. USA 100, 7406–7411.Crossref, Google Scholar
17 Pezawas, L., Meyer-Lindenberg, A., Drabant, E. M., Verchinski, B. A., Munoz, K. E., Kolachana, B. S., Egan, M. F., Mattay, V. S., Hariri, A. R. & Weinberger, D. R. (2005) Nat. Neurosci. 8, 828–834.Crossref, Google Scholar
18 Buznikov, G. A., Lambert, H. W. & Lauder, J. M. (2001) Cell Tissue Res. 305, 177–186.Crossref, Google Scholar
19 Gross, C. & Hen, R. (2004) Nat. Rev. Neurosci. 5, 545–552.Crossref, Google Scholar
20 Bunge, S. A., Dudukovic, N. M., Thomason, M. E., Vaidya, C. J. & Gabrieli, J. D. (2002) Neuron 33, 301–311.Crossref, Google Scholar
21 Phelps, E. A., Delgado, M. R., Nearing, K. I. & LeDoux, J. E. (2004) Neuron 43, 897–905.Crossref, Google Scholar
22 Murphy, F. C., Nimmo-Smith, I. & Lawrence, A. D. (2003) Cognit. Affect. Behav. Neurosci. 3, 207–233.Crossref, Google Scholar
23 Shaikh, M. B., Steinberg, A. & Siegel, A. (1993) Brain Res. 625, 283–294.Crossref, Google Scholar
24 Johnstone, T., Somerville, L. H., Alexander, A. L., Oakes, T. R., Davidson, R. J., Kalin, N. H. & Whalen, P. J. (2005) NeuroImage 25, 1112–1123.Crossref, Google Scholar
25 Varnas, K., Halldin, C. & Hall, H. (2004) Hum. Brain Mapp. 22, 246–260.Crossref, Google Scholar
26 Paus, T. (2001) Nat. Rev. Neurosci. 2, 417–424.Crossref, Google Scholar
27 Sotres-Bayon, F., Bush, D. E. & LeDoux, J. E. (2004) Learn. Mem. 11, 525–535.Crossref, Google Scholar
28 Carli, M., Baviera, M., Invernizzi, R. W. & Balducci, C. (September 28, 2005) Neuropsychopharmacology, 10.1038/sj.npp.1300893.Google Scholar
29 Canli, T., Omura, K., Haas, B. W., Fallgatter, A., Constable, R. T. & Lesch, K. P. (2005) Proc. Natl. Acad. Sci. USA 102, 12224–12229.Crossref, Google Scholar
30 Good, C. D., Lawrence, K., Thomas, N. S., Price, C. J., Ashburner, J., Friston, K. J., Frackowiak, R. S., Oreland, L. & Skuse, D. H. (2003) Brain 126, 2431–2446.Crossref, Google Scholar
31 Kringelbach, M. L. & Rolls, E. T. (2004) Prog. Neurobiol. 72, 341–372.Crossref, Google Scholar
32 Meyer-Lindenberg, A., Hariri, A. R., Munoz, K. E., Mervis, C. B., Mattay, V. S., Morris, C. A. & Berman, K. F. (2005) Nat. Neurosci. 8, 991–993.Crossref, Google Scholar
33 Vitiello, B. & Stoff, D. M. (1997) J. Am. Acad. Child Adolesc. Psychiatry 36, 307–315.Crossref, Google Scholar
34 Garavan, H., Ross, T. J., Murphy, K., Roche, R. A. & Stein, E. A. (2002) NeuroImage 17, 1820–1829.Crossref, Google Scholar
35 Gundlah, C., Lu, N. Z. & Bethea, C. L. (2002) Psychopharmacology 160, 271–282.Crossref, Google Scholar
36 MacLusky, N. J., Naftolin, F. & Goldman-Rakic, P. S. (1986) Proc. Natl. Acad. Sci. USA 83, 513–516.Crossref, Google Scholar
37 Newman, T. K., Syagailo, Y. V., Barr, C. S., Wendland, J. R., Champoux, M., Graessle, M., Suomi, S. J., Higley, J. D. & Lesch, K. P. (2005) Biol. Psychiatry 57, 167–172.Crossref, Google Scholar
38 Yu, Y. W., Tsai, S. J., Hong, C. J., Chen, T. J., Chen, M. C. & Yang, C. W. (2005) Neuropsychopharmacology 30, 1719–1723.Crossref, Google Scholar
39 Bertolino, A., Callicott, J. H., Mattay, V. S., Weidenhammer, K. M., Rakow, R., Egan, M. F. & Weinberger, D. R. (2001) Biol. Psychiatry 49, 39–46.Crossref, Google Scholar
40 Hariri, A. R., Tessitore, A., Mattay, V. S., Fera, F. & Weinberger, D. R. (2002) NeuroImage 17, 317–323.Crossref, Google Scholar
41 Hariri, A. R., Goldberg, T. E., Mattay, V. S., Kolachana, B. S., Callicott, J. H., Egan, M. F. & Weinberger, D. R. (2003) J. Neurosci. 23, 6690–6694.Crossref, Google Scholar
42 Lang, P. J., Bradley, M. M. & Cuthbert, B. N. (1997) International Affective Picture System (IAPS): Technical Manual and Affective Ratings (NIMH Center for the Study of Emotion and Attention, University of Florida, Gainesville, FL).Google Scholar
43 Friston, K. J., Zarahn, E., Josephs, O., Henson, R. N. & Dale, A. M. (1999) NeuroImage 10, 607–619.Crossref, Google Scholar
44 Good, C. D., Johnsrude, I. S., Ashburner, J., Henson, R. N., Friston, K. J. & Frackowiak, R. S. (2001) NeuroImage 14, 21–36.Crossref, Google Scholar
45 Ashburner, J. & Friston, K. J. (2000) NeuroImage 11, 805–821.Crossref, Google Scholar
46 Wright, I. C., McGuire, P. K., Poline, J. B., Travere, J. M., Murray, R. M., Frith, C. D., Frackowiak, R. S. & Friston, K. J. (1995) NeuroImage 2, 244–252.Crossref, Google Scholar
47 Blasi, G., Mattay, V. S., Bertolino, A., Elvevag, B., Callicott, J. H., Das, S., Kolachana, B. S., Egan, M. F., Goldberg, T. E. & Weinberger, D. R. (2005) J. Neurosci. 25, 5038–5045.Crossref, Google Scholar
48 Meyer-Lindenberg, A., Poline, J. B., Kohn, P. D., Holt, J. L., Egan, M. F., Weinberger, D. R. & Berman, K. F. (2001) Am. J. Psychiatry 158, 1809–1817.Crossref, Google Scholar



