Testing an APA Practice Guideline: Symptom–Targeted Medication Utilization for Patients With Borderline Personality Disorder
Abstract
Background: The goal of this study was to test whether the use of psychotropic medication treatment for borderline personality disorder (BPD) was consistent with proposals in the recently published American Psychiatric Association’s Practice Guideline for the Treatment of Patients With Borderline Personality Disorder. Method: Medication utilization by patients with BPD was assessed prospectively over a 2-year period prior to the publication of the Guideline. Three BPD symptom clusters—cognitive-perceptual, affective dysregulation, and impulsive-behavioral dyscontrol—along with demographic and functioning variables were used to predict the use of five classes of medication. Results: Symptoms of impulsive-behavioral dyscontrol significantly predicted use of both neuroleptics and anticonvulsants. Cognitive-perceptual symptoms were inversely related to anticonvulsant use. Conclusion: While some pre-Guideline medication practices with BPD patients were consistent with the recommended algorithms, physicians may also have been influenced by a variety of considerations, including level of functional impairment and the presence of comorbid Axis I conditions.
Borderline personality disorder (BPD) is associated with serious impairment in psychosocial functioning (1). Patients with BPD demonstrate significant affective instability, impulsive dyscontrol, cognitive distortions, and interpersonal discord. They also use more mental health services than patients with other personality disorders or those with major depression (2). However, because of a lack of empirical evidence, there has historically been little consensus on the best treatment(s) for this debilitating disorder.
Consequently, the American Psychiatric Association (APA) developed a practice guideline for the treatment of patients with BPD (3). This guideline represents a comprehensive set of evidence-based, “best practice” recommendations for the psychiatric community. Based on a review of the literature, psychotherapy was designated as the primary treatment, with pharmacotherapy recommended as an adjunctive, symptom-targeted component of treatment. Specific algorithms, supported by a growing body of empirical research (e.g., Soloff 1998 [4]), were developed to guide clinicians in the use of psychotropic medications.
These medication algorithms address three clusters of BPD symptoms: cognitive-perceptual symptoms, affective dysregulation, and impulsive-behavioral dyscontrol (3). While all three algorithms have a number of successive decision points, the first and second steps of each will be described here (Figure 1). According to the Guideline, the first-line treatment for cognitive-perceptual symptoms is low-dose neuroleptics; the next step is to increase the dose of the neuroleptic, if needed. For affective dysregulation, the Guideline recommends beginning with an SSRI or related antidepressant, switching to a second SSRI or other antidepressant if the first one tried is not fully effective. The Guideline recommends that impulsive-behavioral dyscontrol symptoms first be addressed with an SSRI, with the next step changing to or adding a low-dose neuroleptic, if necessary.
While these algorithms were developed by experts on the treatment of BPD, psychiatrists and other physicians had obviously been making decisions about medications for the treatment of BPD for some time prior to the publication of the Guideline. In the absence of recommendations such as those given in the Guideline, how closely did established clinical approaches to the use of medication for BPD approximate what has been promoted in the new algorithms? The purpose of the present study was to empirically explore how actual psychotropic medication practices for BPD relate to those that have subsequently been proposed in the APA Practice Guideline. Findings from this study can then be used to establish a baseline for future assessments of the Guideline’s impact.
Method
Treatment-seeking individuals aged 18 to 45 years were recruited from clinical services affiliated with each of the four sites conducting the Collaborative Longitudinal Personality Disorders Study (CLPS). CLPS is a study of the longitudinal course and impact of four representative and contrasting personality disorders (PDs): schizotypal, borderline, avoidant, and obsessive-compulsive. The primary aims and design of the CLPS are described in detail elsewhere (5). Potential participants were prescreened to exclude patients with active psychosis, acute substance intoxication or withdrawal, or a history of schizophrenia, schizoaffective, or schizophreniform disorders. The patients were predominately white (76%), female (64%), and from Hollingshead and Redlich (6) socioeconomic classes II and III (65%). All eligible participants signed written informed consent after the research procedures had been fully explained to them.
The Diagnostic Interview for DSM-IV Personality Disorders (DIPD-IV) (7) was used by trained clinical interviewers to assess the presence of symptoms of BPD at baseline. The DIPD-IV is a semi-structured interview for the diagnosis of all DSM-IV PDs, by which information is elicited to rate the presence or absence of diagnostic criteria for each PD over the preceding 2 years. In addition, patients must confirm that the PD features they report have been characteristic of them for most of their adult lives and are descriptive of their “personality or temperament,” in order to receive a diagnosis. A follow-along version, the DIPD-FAV, was used to track the presence of BPD symptoms on a monthly basis at 6-month, 1-year, and 2-year follow-up assessments. The final sample for these analyses was 165 patients who met criteria for BPD (interrater reliability k=0.68; test-retest k=0.69) (8) at study intake and for whom medication utilization data were available for the subsequent 2 years.
The Longitudinal Interval Follow-up Evaluation Adapted for the Personality Disorders Study (LIFE-PS) (9) was also administered at 6-month, 1-year, and 2- year follow-up assessments to collect prospective data on medications used over a 2-year period (data were collected during the period 1996–1999, prior to the publication of the APA Guideline in 2001). The LIFE-PS provides for a detailed assessment of patient reported use of over 100 varieties of psychotropic medications, with weekly recordings of specific medications used and daily dosages. In the present analyses, relevant medications were aggregated into five groups, selective serotonin reuptake inhibitors (SSRIs), neuroleptics, anticonvulsants, lithium, and clonazepam, all five of which are explicitly mentioned in the Guideline algorithms.
The three symptom clusters—cognitive-perceptual, affective dysregulation, and impulsive-behavioral dyscontrol—were measured using DIPD-IV items. The cognitive-perceptual cluster was represented by a DIPD criterion assessing transient stress-related paranoia or dissociation; the affective dysregulation cluster was comprised of items measuring inappropriate and intense anger, affective instability, and frantic efforts to avoid abandonment; and the impulsive-behavioral dyscontrol cluster was made up of criteria assessing recurrent suicidal or self-mutilating behavior and impulsivity in at least two self-damaging areas. Item selection was informed by a previous comfirmatory factor analysis of BPD criteria by Sanislow et al. (10) using CLPS data.
To be counted in a symptom cluster, the patient had to have met one or more criteria for that cluster at least 50% of the time over the 2-year period. Because the symptom clusters are not independent and patients most often presented with symptoms that met criteria for more than one cluster, we also analyzed the three symptom cluster variables as continuous measures, in addition to separating patients into different cluster groups. Each criterion was rated on the baseline DIPD-IV as absent (0), present but of questionable clinical significance (1), or present and clinically significant (2). Total scores for each cluster for each participant were then computed by adding the pertinent item scores.
All statistical analyses were conducted using SAS Version 8.2 (11). Chi-square comparisons were used to assess differences in the proportion of patients having each symptom cluster and also receiving each class of medication. Five logistic regression analyses were conducted to examine the association of personality and other baseline variables with use of medication over the 2-year follow-up period. Goodness-of-fit for each model was assessed using Hosmer and Lemeshow’s C statistic (12). This was calculated by grouping cases according to deciles of predicted probabilities and then comparing the expected number of events in each group with the number observed. The statistic is well approximated by the chi-square distribution with eight degrees of freedom. Variables entered into each model were the following: the three BPD symptom clusters, age category (below 30 years or 30 years and older), race (minority or Caucasian), gender, socioeconomic status (Hollingshead 5 classes), and Global Assessment of Functioning Scale (GAFS) (13), averaged over the 24 months.
Results
Chi-square analyses (Table 1) showed that use of neuroleptics and use of anticonvulsants were significantly related to the presence of impulsive-behavioral dyscontrol. There were no significant associations, however, between symptom clusters and use of SSRIs, lithium, or clonazepam.
Of the five regression models, three were significant: neuroleptics (p<0.0001), anticonvulsants (p<0.0002), and clonazepam (p<0.0009). However, only two classes of medications, the neuroleptics and anticonvulsants, were related to any of the BPD symptom cluster variables (Table 2). Affective dysregulation did not predict any of the five categories of medication use. Impulsive-behavioral dyscontrol symptoms significantly predicted both neuroleptic (OR=1.53, 95% CI=1.03–2.27) and anticonvulsant (OR=1.57, 95% CI=1.08–2.26) use. Cognitive-perceptual symptoms were inversely related to anticonvulsant use (OR=.60, 95% CI=0.36–0.99). In addition, GAF scores were related to both neuroleptic (OR=0.90, 95% CI=0.86–0.94) and anticonvulsant (OR=0.94, 95% CI= 0.90–0.98) use, with those at lower levels of functioning more likely to receive these medications. Anticonvulsants were also significantly more likely to be taken by women (OR=2.45, 95% CI=1.09–5.50).
Discussion
The APA Practice Guideline for the Treatment of Borderline Personality Disorder recommends SSRIs as first line medications for adjunctive pharmacotherapy when symptoms of affective dysregulation or impulsive-behavioral dyscontrol are prominent. Although borderline patients in our study had significant histories of SSRI use, the prominence of affective dysregulation or impulsive-behavioral dyscontrol symptoms did not predict their utilization. The prescription of SSRIs may have been linked to the frequent presence of comorbid depression in patients with BPD. In fact, over 70% of the patients with BPD in the CLPS sample had a history of major depression (14).
In addition, even though the Guideline recommends neuroleptics as the initial pharmacological approach for cognitive-perceptual symptoms, impulsive-behavioral dyscontrol but not cognitive-perceptual symptoms predicted neuroleptic use. It may be that, in psychiatric practice, careful delineation of distinct symptom patterns has not been typical. Furthermore, the diagnosis of BPD alone may have led physicians to prescribe medications from multiple categories, including SSRIs and neuroleptics.
Symptoms of impulsive-behavioral dyscontrol predicted not only neuroleptic use, but also anticonvulsant use, and both classes of medications are recommended as alternative or augmentation strategies for this symptom cluster. Also, cognitive-perceptual symptoms were not preferentially treated with anticonvulsants, which is consistent with the APA Guideline. Finally, more seriously impaired patients in our study (i.e., patients with lower GAF scores indicating more impairment in functioning) were more likely to receive neuroleptics and anticonvulsants. This practice was not addressed in the Guideline, but it may reflect sound clinical judgment in deciding when to use adjunctive pharmacotherapy in patients with BPD.
It should be noted that the Practice Guideline algorithms have been the subject of some controversy. It has been observed that, while there has been some empirical work on medications for BPD, that body of inquiry has been somewhat sparse and preliminary in nature (15, 16). In addition, the nature of BPD and its response to medications is likely to be different from that of Axis I disorders. It has been argued that none of the medications that are currently suggested for BPD has been shown to be specifically effective over time for a majority of patients (16), which could also be linked to the potentially detrimental practice of polypharmacy observed in BPD (17). Nevertheless, it is hoped that the Guideline will stimulate more empirical clinical trials concerning the psychopharmacological component of treatment for patients with BPD.
Several potential limitations of the current study should be noted. First, we relied upon patient self-reports about medication use. It is possible that certain medications were prescribed but not actually taken by some of the patients. However, it is still of clinical interest to examine which medications BPD patients reported using. Retrospective reports of treatment obtained by interviewers using the LIFE interview have been shown to highly reliable (18). The LIFE has been used to report treatment received by patients with depressive (19) and anxiety (20) disorders in multi-site longitudinal studies similar to the CLPS. Recently, Posternak and Zimmerman demonstrated that, in general, patients were able to accurately recall their antidepressant treatment histories over a 5-year period (21).
A second limitation is that our approximation of the symptom clusters was based on the BPD criteria from the DIPD-IV, which were designed to assess the presence or absence of the disorder itself, and not to elaborate on specific sub-areas of phenomenology. Future prospective studies should more specifically measure the symptom clusters and also examine whether patients with BPD are treated according to standard algorithms, as physicians become more familiar with the Guideline.
Finally, previous experience with psychotropic medications and other co-occurring treatments may also have influenced the treatments selected by physicians in this sample in ways that may not have agreed with the recommendations in the Guideline. Certainly, patients with BPD have extensive experience with a variety of medications and other treatments, as documented by Bender et al. (2). Few patients in this study were treatment naïve.
In conclusion, in this prospective study of reported medication utilization, significant proportions of patients with BPD reported the use of major classes of psychotropic drugs recommended in the APA Practice Guideline for the Treatment of Borderline Personality Disorder.With the exception of symptoms of impulsive-behavioral dyscontrol, however, BPD symptom clusters did not predict medication utilization in line with the algorithms included in the Guideline. This may have been because this study examined medication utilization during the period before the Guideline was published and the Guideline may eventually impact clinical practice. It may also be the case that reported use does not correspond to physician prescribing practices and actual prescribing was more in line with the Guideline recommendations. Given the high frequency with which symptoms of affective dysregulation and impulsive-behavioral dyscontrol occur in patients with BPD, however, it seems more likely that prescribing practices and reported medication utilization are determined by other clinical factors, including prior experience with medications, the relative effectiveness of other treatments, comorbid Axis I disorders, and the severity of functional impairment, in addition to target symptomatology. Several of these hypotheses will be explored with data from a future prospective follow-up, which will examine prescribing practices after the publication of the Guideline.
| Medication Class | Cognitive-perceptual | Affective dysregulation | Impulsive-behavioral |
|---|---|---|---|
| n = 101 | n = 48 | n = 23 | |
| SSRI (N = 138) | 79 (78.2) | 36 (75.0) | 17 (73.9) |
| Neuroleptics (N = 66) | 44 (43.6) | 19 (39.6) | 13 (56.5)* |
| Anticonvulsants (N = 78) | 47 (46.5) | 18 (37.5) | 18 (78.3)** |
| Lithium (N = 34) | 18 (17.8) | 6 (12.5) | 5 (21.7) |
| Clonazepam (N = 53) | 36 (35.6) | 18 (37.5) | 6 (26.1) |
| Neuropleptics | Anticonvulsants | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | B | S.D. | Wald χ2 | OddsRatio | 95%CI | B | S.D. | Waldχ2 | OddsRatio | 95%CI |
| Cognitive-perceptual | −0.19 | 8.96 | 0.52 | 0.82 | 0.49–1.40 | −0.51 | 3.33 | 3.98* | 0.60 | 0.36–0.99 |
| Affective dysregulation | −0.31 | 2.43 | 2.56 | 0.74 | 0.51–1.07 | 0.06 | 2.30 | 0.12 | 1.07 | 0.75–1.52 |
| Impulsive-behavioral | 0.42 | 2.56 | 4.40* | 1.53 | 1.03–2.27 | 0.45 | 2.43 | 5.65* | 1.57 | 1.08–2.26 |
| Age | −0.03 | 0.38 | 1.87 | 0.97 | 0.92–1.02 | 0.01 | 0.26 | 0.25 | 1.01 | 0.97–1.06 |
| Race | −0.76 | 6.14 | 2.57 | 0.47 | 0.18–1.18 | −0.63 | 6.14 | 2.14 | 0.53 | 0.23–1.24 |
| Gender | 0.34 | 5.63 | 0.61 | 1.41 | 0.60–3.34 | 0.89 | 5.25 | 4.68* | 2.45 | 1.09–5.50 |
| SES | −0.25 | 2.43 | 1.76 | 0.78 | 0.54–1.13 | 0.01 | 2.30 | 0.01 | 1.01 | 0.71–1.44 |
| GAFS | −0.10 | 0.26 | 21.05*** | 0.90 | 0.86–0.94 | −0.06 | 0.26 | 10.10** | 0.94 | 0.90–0.98 |
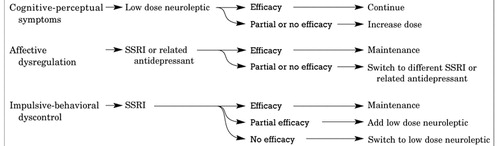
Figure 1. Medication Algorithms for BPD Symptom Clusters: First Two Steps
Source:American Psychiatric Association: Practice Guideline for the Treatment of Patients With Borderline Personality Disorder. Am J Psychiatry 2001; 158(Oct suppl)
1 Skodol AE, Gunderson JG, McGlashan TM, et al. Functional impairment in patients with schizotypal, borderline, avoidant, or obsessive-compulsive personality disorder. Am J Psychiatry 2002;159:276–83Crossref, Google Scholar
2 Bender DS, Dolan RT, Skodol AE, et al. Treatment utilization by patients with personality disorders. Am J Psychiatry 2001;158:295–302Crossref, Google Scholar
3 American Psychiatric Association. Practice guideline for the treatment of patients with borderline personality disorder. Am J Psychiatry 2001;158(Suppl):1–52Crossref, Google Scholar
4 Soloff PH. Symptom-oriented psychopharmacology for personality disorders. Journal of Practical Psychiatry and Behavioral Health 1998;4:3–11Crossref, Google Scholar
5 Gunderson JG, Shea MT, Skodol AE, et al. The Collaborative Longitudinal Personality Disorders Study: Development, aims, design, and sample characteristics. J Personal Disord 2000;14:300–15Crossref, Google Scholar
6 Hollingshead AB, Redlich FC. Schizophrenia and social structure. Am J Psychiatry 1954;110:695–701Crossref, Google Scholar
7 Zanarini MC, Frankenburg FR, Sickel AE, et al. Diagnostic Interview for DSM-IV Personality Disorders. Belmont, MA: McLean Hospital, Laboratory for the Study of Adult Development; 1996Google Scholar
8 Zanarini MC, Skodol AE, Bender D, et al. The Collaborative Longitudinal Personality Disorders Study: Reliability of Axis I and II diagnoses. J Personal Disord 2000;14:291–9Crossref, Google Scholar
9 Keller M, Nielson E. Longitudinal Interval Follow-Up Evaluation Adapted for Personality Disorders Study. Providence, RI: Brown University School of Medicine, Department of Psychiatry and Human Behavior; 1989Google Scholar
10 Sanislow CA, Grilo CM, Morey LC, et al. Confirmatory factor analysis of DSM-IV criteria for borderline personality disorder: Findings from the Collaborative Longitudinal Personality Disorders Study. Am J Psychiatry 2002;159:284–90Crossref, Google Scholar
11 SAS Version 8.2. Cary, NC: SAS Institute; 2001Google Scholar
12 Hosmer DW, Lemeshow S. Assessing the fit of the model. In: Applied logistic regression. New York: John Wiley & Sons; 1989:135–75Google Scholar
13 American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th Edition. Washington, DC: American Psychiatric Association; 1994:32Google Scholar
14 McGlashan TH, Grilo CM, Skodol AE, et al. The Collaborative Longitudinal Personality Disorders Study: Baseline Axis I/II and II/II diagnostic co-occurrence. Acta Psychiatr Scand 2000;102:256–64Crossref, Google Scholar
15 Tyrer P. Practice guideline for the treatment of borderline personality disorder: A bridge too far. J Personal Disord 2002;16:113–8Crossref, Google Scholar
16 Paris J. Commentary on the American Psychiatric Association Guidelines for the Treatment of Borderline Personality Disorder: Evidence-based psychiatry and the quality of evidence. J Personal Disord 2002;16:130–4Crossref, Google Scholar
17 Zanarini MC, Frankenburg FR, Khera GS, et al. Treatment histories of borderline inpatients. Compr Psychiatry 2001;42:144–50Crossref, Google Scholar
18 Keller MB, Lavori PW, McDonald-Scott P, et al. The reliability of retrospective treatment reports. Psychiatry Res 1983;9:81–8Crossref, Google Scholar
19 Keller MB, Klerman GL, Lavori PW, et al. Treatment received by depressed patients. JAMA 1982;248:1848–55Crossref, Google Scholar
20 Goisman RM, Rogers MP, Steketee, GS, et al. Utilization of behavioral methods in a multicenter anxiety disorders study. J Clin Psychiatry 1993;54:213–8Google Scholar
21 Posternak MA, Zimmerman M. How accurate are patients in reporting their antidepressant treatment history? J Affect Disord 2003;75:115–24Crossref, Google Scholar



