Transcranial Direct Current Stimulation in Psychiatry: What Psychiatrists Need to Know
Abstract
Transcranial direct current stimulation (tDCS) is emerging as a potential treatment for a host of neuropsychiatric disorders. Data appear to indicate that tDCS applied over frontal or prefrontal brain regions may reduce symptoms of major depression, yet results have been mixed. Early studies showed promise, but recent work failed to replicate earlier results. The decision whether to use tDCS is further affected by the complex regulatory environment; no tDCS devices are cleared by the U.S. Food and Drug Administration for clinical use. Older systems have grandfathered regulatory approval for treating mood, anxiety, and insomnia, although they have not demonstrated efficacy in rigorous trials. Furthermore, as the field of noninvasive brain stimulation advances, various side effects and contraindications are increasingly recognized. Over the last few years, research and consumer use of tDCS have outpaced education, thus providing little guidance for clinicians and trainees about how to understand tDCS. Therefore, this focused review includes those items psychiatric clinicians and trainees most need to understand tDCS, including basic electrical and neurophysiological principles, a brief review of efficacy data in major depressive disorder, and suggested guidelines about how to manage patients using tDCS.
Definitions: Basic Information, Device Design, and Nomenclature
The basic concepts underlying transcranial direct current stimulation (tDCS) derive from electrical engineering. Stimulation is based on Ohm’s Law (V=IR), where the interplay between voltage (V), current (I), and resistance (R) forms the basic conceptual framework. tDCS devices deliver a constant and predefined electric current (I; measured in milliampere units and abbreviated mA) through two electrodes that are placed on the head. The amount of current passing between the two electrodes is equal to the electric potential difference, V, divided by the resistance between the electrodes. The device’s wires have a very low resistance, and the main resistance comes from the electrodes and the biological tissue through which the current passes. Ohm’s law states that a higher voltage is required for current to pass through high-resistance spaces, such as tissue. Accordingly, higher resistance during tDCS (leading to higher voltage) can result in scalp discomfort or even skin burns (1) (see the “Risks of tDCS” section for more detail).
A typical tDCS system has a power source connected by two sets of electrodes, the anode (positive, where the electrical current enters the body) and a cathode (negative, where the electrical current leaves the body, also called return electrodes). The arrangement of these electrodes on the scalp or skin (called a montage) roughly defines the area of the brain that receives most of the electrical energy. Figure 1 illustrates an example of tDCS application, including the power source and electrodes.
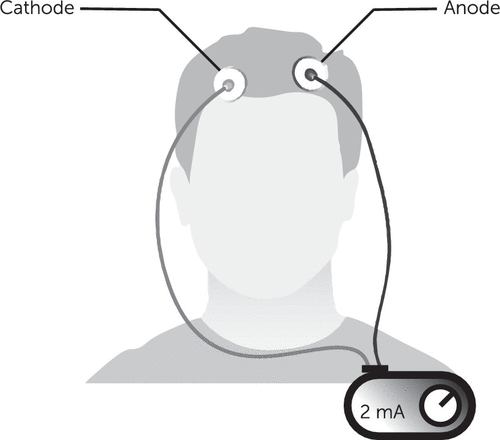
FIGURE 1. Example of transcranial direct current stimulation applicationa
aCurrent is generated from a power source, typically around 2mA; it enters the brain through the anode and then leaves the brain through the cathode. The main resistance in the system is in the tissue between the two electrodes; thus, higher resistance can lead to scalp discomfort or burns if not carefully managed.
Originally, stimulation targeting was done by trial and error; more recently this has been facilitated with software that models the electrical current. Such modeling can be complicated, because electrical current can shunt through the skin or cerebrospinal fluid. More sophisticated electrode montages use multiple electrodes (typically numerous cathodes) to target a particular area of interest. The typical range of current used in clinical tDCS is 0.1 mA to 4.0 mA, with most falling between 1 mA and 2 mA. This current is usually applied for tens of minutes, usually 10–60 minutes.
tDCS Neurobiology and Potential Mechanisms of Action
A constant or direct electrical field delivered by a tDCS device with an intensity that is typically used for treatment interventions (1–2 mA) will produce a shift in neuronal membrane voltage by less than 1 mV. To place this effect in perspective, note that a much larger shift of membrane voltage (10–20 mV of depolarization) would be required to move a resting neuron to the threshold where it fires an action potential. For this reason, tDCS is generally considered subthreshold modulation, such that it can increase or decrease overall excitability of the neural tissue (Figure 2). Such an effect would change the dynamics of cortical networks in a nonlinear fashion.
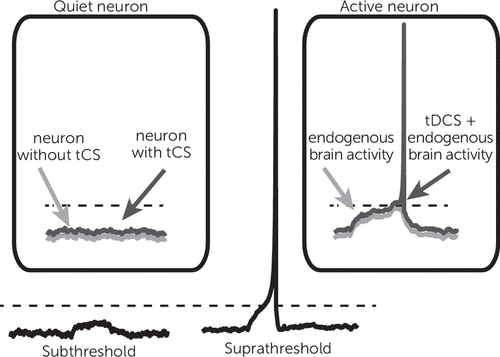
FIGURE 2. Subthreshold versus suprathreshold stimulationa
aSubthreshold membrane fluctuations are not sufficient to generate an action potential (left). However, if intrinsic fluctuations in a neuron’s membrane voltage move it closer to its threshold, application of an inherently subthreshold input, such as transcranial direct current stimulation (tDCS), can trigger an action potential (right). The dashed line indicates the membrane threshold. Adapted from Philip et al. (5) with permission from the American Journal of Psychiatry.
A typical neuron makes synaptic contacts from a very large number of other neurons, and a small fluctuation in excitability can lead to significant change in the behavior of neural networks. Thus, even minute changes in membrane voltage brought about by subthreshold energy neurostimulation can affect population neuronal firing. This also suggests that what the brain is doing while receiving tDCS may be important, because external demands shift internal membrane potential states.
The direction of current flowing in the brain also affects neuronal firing. When the current travels in one direction, the effect is to depolarize the neuronal membrane and enhance the chance of firing, as described above. Current traveling in the opposite direction leads to hyperpolarization, reducing the likelihood of neuronal firing. On the basis of these features, tDCS has been associated with the notion that brain regions under the tDCS anode are excited, whereas neurons under the cathode are inhibited. This, however, seems to be overly simplistic and likely incorrect. The effect of tDCS is complex and depends on many factors, such as location and orientation of cells and associated dendritic trees, and also the rate and nature of the neurons activity (see (2) for an example).
The therapeutic benefit from tDCS arises because its effects extend beyond the stimulation session. As discussed above, stimulation changes how the population of neurons fire, and this change in neural activity during stimulation leads to long-lasting strengthening or weakening of synaptic connections. This phenomenon, referred to as synaptic plasticity, was demonstrated by a comprehensive series of experiments in which the motor cortex activity was assessed after tDCS (3).
Although much of what we know about tDCS comes from studies examining the human motor cortex, it remains unclear whether the same principles apply to brain regions involved in psychiatric disorders (e.g., prefrontal cortex, subcortical areas). Furthermore, it is possible that the organization of neurons into microcircuits differs from one area of the cortex to another, such that different effects are produced when the same type of stimulation is applied. Testing this hypothesis remains an area of active research.
tDCS and the Regulatory Environment
Regulatory concerns are unique for tDCS. Devices used to deliver therapeutic brain stimulation may be “cleared” (for marketing) by the U.S. Food and Drug Administration (FDA) through regulatory processes that are different from the pathway typically required for approval of new drugs. FDA regulation of devices began in 1976 with a classification system based on level of risk associated with using each medical device. Several devices that used low-intensity electrical stimulation, categorized by the FDA as “cranial electrotherapy stimulation” (CES) devices, were introduced to the U.S. market before 1976 and therefore were not subjected to the same FDA regulatory process as devices introduced after this time.
Those original CES devices and subsequently updated versions of them are thus marketed as “FDA cleared,” but they have not passed more recent FDA requirements for clinical testing. Newer models of the pre-1976 CES devices are able to claim technical equivalency with their predicate models to achieve regulatory approval. Methods used to demonstrate efficacy in early CES studies of mood and anxiety symptoms would not be considered rigorous by today’s standards of clinical trial design (4). Further complicating this issue is the fact that no tDCS device currently has FDA clearance for marketing as a medical device. However, as long as the manufacturer does not claim a medical benefit, these devices can be sold directly to consumers.
tDCS in Psychiatry: A Focus on Depression
The studies that have tested tDCS in psychiatric conditions are numerous. These include proof-of-concept studies using small sample sizes. For this article, we focus on the larger studies testing the efficacy of tDCS to reduce symptoms of depression. A more thorough review of literature can be found in the work of Philip et al. (5) and Moffa et al. (6).
To date, three large (N>100) randomized controlled trial (RCT) studies of tDCS for depression have been conducted (Table 1). The first study, by Brunoni et al. (7), combined tDCS with low-dose sertraline (50 mg daily) in a factorial design (i.e., four groups: tDCS+sertraline, tDCS+placebo, sham+sertraline, sham+placebo), which allowed for tests of the main effects of both interventions as well as their interaction. The authors found superior efficacy of tDCS+sertraline and the poorest outcomes in the sham+placebo group.
| Study | N | Anatomical target | Stimulation strength | Session duration and frequency (total sessions, total length) | Main finding |
|---|---|---|---|---|---|
| Brunoni et al. (7) | 120 | L DLPFCa | 2 mA | 30 min/day, 5 times per week for 2 week, then 1 session every other week (12 sessions over 6 weeks) | Sert/activeb tDCSc superior to Sert/sham, PBO/active, and PBO/ sham groups |
| PBO/shamd | 30 | ||||
| Sert/sham | 30 | ||||
| PBO/active | 30 | ||||
| Sert/active | 30 | ||||
| Brunoni et al. (8) | 245 | L DLPFC | 2 mA | 30 min/day, 5 times per week for 3 weeks, then 1 session every week (22 sessions over 10 weeks) | Escite and tDCS both superior to placebo, Escit also superior to tDCS |
| PBO/sham | 60 | ||||
| Escit/sham | 91 | ||||
| PBO/active | 94 | ||||
| Loo et al. (9) | 130 | L DLPFC | 2.5 mA | 30 min/day, 5 times per week (20 sessions over 4 weeks) | Unipolar: more remitted with sham than active; bipolar: no difference between active and sham |
| Unipolar/shamf | 46 | ||||
| Bipolar/shamg | 18 | ||||
| Unipolar/active | 45 | ||||
| Bipolar/active | 21 |
TABLE 1. Large (N > 100) randomized controlled trials of low-intensity electrical stimulation for major depressive disorder
Brunoni et al. (8) conducted a noninferiority study investigating tDCS monotherapy or escitalopram (20 mg daily) versus sham or placebo over 10 weeks. In this study, tDCS was superior to placebo but was not as effective as escitalopram and had more side effects. Most recently, a multisite international study described by Loo et al. (9) tested the use of tDCS versus sham among patients with unipolar and bipolar depression. In that study, active stimulation did not produce outcomes similar to those for sham.
Until the most recent international multisite study, the available RCT evidence supported the use of tDCS delivered to the dorsolateral prefrontal cortex for treating depression. The lack of consistent results across trials, particularly after a larger study, indicates that effects may not be as robust as previously thought; suboptimal parameters or sample characteristics may account for these results. It is possible that cognitive activity of participants during tDCS is a critical element of the treatment intervention that has not been adequately controlled. Questions remain about potential side effects or synergistic therapeutic effects when tDCS is applied in combination with psychotropic medications.
Risks of tDCS
A recent review of tDCS safety found no serious adverse effect or irreversible brain injury when tDCS was applied within the “conventional” parameter range (i.e., ≤40 min, ≤4 mA) (10). The RCTs on the use of tDCS for depression also demonstrated tDCS to have a reasonably safe profile (7–9). In those studies, tDCS was associated with limited side effects, including itching, redness, tingling, headache, and discomfort. In a meta-analysis, all cause discontinuation rates were not statistically different between active tDCS and sham (11).
However, a small number of treatment-emergent mania cases have been reported. To date, there have been at least 12 cases of treatment-emergent mania or hypomania, representing approximately 3% of subjects receiving active tDCS in these trials (12 of 369 subjects) (7, 8, 12–14). Although prior meta-analyses of treatment-emergent mania (15) suggested no relationship between active stimulation and hypomanic or manic symptoms, additional cases have emerged (14), and the majority have occurred with the combination of medication and tDCS.
These results are only true for tDCS as delivered in clinical studies; thus, this claim of safety might not be true for homemade devices or those marketed directly to consumers. Accurately assessing tDCS risks is complicated by the fact that to a large enthusiast and proponent community, the devices are viewed and promoted as a completely safe technology that should be available directly to consumers. The language used to introduce and promote tDCS devices often includes phrases such as “jumper cables for the mind,” “overclock your brain,” or “reach your true potential” (16). Use of this language makes arguing for vigorous clinical studies difficult and trivializes potential risks. Here, we focus on areas that should be considered by clinicians and trainees: risks from the systems or stimulation, and broader risk of interference with standard clinical care.
Device and Stimulation Effects
As described above, tDCS uses very low currents that are orders of magnitude less than the current used in electroconvulsive therapy or repetitive transcranial magnetic stimulation (10). The most common side effects from tDCS are stimulation-site discomfort or itching, redness, tingling, and headache. The most immediate risk arises as a result of high resistance to current from the skin, which causes an increase in delivered voltage. In rare cases, this can lead to skin burns (17). Typical reasons for heightened resistance are poor electrode contact with the skin or use of electrodes made from materials that conduct poorly. This risk is reduced by devices that enforce a maximum upper limit of voltage. Safe tDCS use requires that resistance remains low for the entire stimulation session; this is achieved by use of electrodes with good conductive properties; good contact between skin and electrodes; and integrity of connections among the stimulator, wires, and electrodes. Although skin injury in clinical studies is rare, this risk is by definition greater during unsupervised use.
Technically, whenever the brain is stimulated, there is a risk of seizures. Because of the low current used in tDCS, seizures are unlikely, yet individuals with seizure disorders, those with intracranial metal or other pathology, and those who are excessively using medications or substances that substantially increase cortical excitability are at higher risk. For example, this risk might be substantial among patients engaging in sleep deprivation and illicit stimulant use. Long-lasting mental status changes are also unlikely with short-term tDCS use, although there is potential for more durable effects with repeated administration outside of tested parameters.
Cognitive effects may also be an important risk. For example, in one study testing a commercially available tDCS system marketed to improve performance, stimulation worsened working memory (18). It is possible that stimulation induces a functional “trade-off,” whereby a single cognitive ability improves at the cost of impairing another. Examples of this have been demonstrated previously (19, 20). It is important to note that changing the allocation of cognitive resources might be more consequential for patients who might have reduced cognitive reserves because of their illness state.
Furthermore, as tDCS systems get more sophisticated and home use expands, safety assessments become more complex. To underscore this point, we reviewed several online tDCS forums and found descriptions of unremitting migraines, photophobia, increased anxiety, and mania (e.g., see Reddit’s tDCS subforum, www.reddit.com/r/tDCS/, which has close to 11,000 followers). Although it is impossible to evaluate their veracity, these reports likely represent important safety information. Had they occurred in a clinical trial, researchers would be able to better understand how they happened; through home use, the reports are lost outside of the immediate readership of a particular online post.
Negative Impact on Standard Clinical Care
Another important risk is the potential of tDCS to interfere with safe and successful delivery of standard clinical care. For example, patients may substitute tDCS for evidence-based therapies because of the perceived lack of side effects and ease of access. Furthermore, interactions between tDCS and other interventions (e.g., medications, psychotherapy) remain largely unknown. For example, in many of the early tDCS studies, participants were required to be medication free, yet in later studies using tDCS combined with medication, treatment-emergent hypomanic and manic symptoms emerged (7). Thus, it is easy to imagine a situation in which a patient uses tDCS without telling his or her provider, then develops hypomanic or manic symptoms, and the provider misattributes these symptoms to other factors. Relevant here are studies of tDCS for substance use disorders, which have occasionally described increased in cravings or risk behavior (21–24). Furthermore, there are no large-scale studies of tDCS plus psychotherapy, and it is likely that important results (positive or negative) will emerge from these lines of inquiry.
Recommendations
Because of limited training in noninvasive brain stimulation, the fairly recent emergence of clinical trial data, and atypical regulatory history, most clinicians remain unfamiliar with tDCS. Lacking reliable information and professional guidance, clinicians and trainees may find themselves inclined to avoid the topic and fail to inquire about or discuss the use of tDCS with their patients. Because marketing messages are appealing and devices are inexpensive and abundantly available, patients may seek out tDCS when they are struggling with unresolved symptoms or undesirable side effects from traditional treatments.
However, prescribing or directing patients to use tDCS in place of standard, evidence-based treatments is inappropriate and constitutes an unacceptable standard of care when appropriate steps are not taken. Recommending that otherwise stable patients with residual symptoms withdraw or discontinue ongoing approved therapies may be particularly risky. A patient might misconstrue a clinician’s endorsement of tDCS to mean that the stimulation is proven to be as safe and effective for his or her condition as other recommended therapies.
For today’s clinician, a basic understanding of tDCS and appropriate inquiry are critical for management of patients with mental health disorders. Systematic inquiry about use of nonprescription treatments (e.g., dietary supplements) is already an important part of the initial patient evaluation, and this should be expanded to include questions about tDCS. This is likely particularly important in the treatment of patients who are more likely to engage in tDCS. If patients disclose that they use tDCS, clinicians should seek additional detail, provide relevant information to the extent possible, and document use.
To minimize potential misuse, patients should be discouraged from using do-it-yourself kits. Clinicians who have a strong personal interest in electrical neurostimulation or want to incorporate stimulation into their routine practice can pursue additional training. An increasing number of programs, taught by faculty with knowledge in the area and a track record of training, are available for in-depth instruction on tDCS and related technologies. Patients who wish to pursue tDCS might be directed to receive treatments administered by a licensed health care provider who has appropriate training and experience and routinely offers such treatments under medical supervision in a clinic setting.
Clinicians who prescribe or treat patients using tDCS should evaluate and document their consideration of essential safety elements (e.g., seizure risk, absence of metal in the head and neck). If a clinician chooses to prescribe tDCS, a prescription or order for electrical stimulation treatments should include a description of the device, the duration and frequency of treatments, the placement of electrodes, and the stimulation settings. Informed consent documentation should reflect consideration of risks, benefits, and alternatives.
Education should be provided to patients who use tDCS, including a review of the quality of available evidence for their efficacy and safety concerning diagnoses, symptoms, and conditions that the patient is targeting. Clinicians who monitor patients using tDCS should routinely query them regarding adverse events of the types highlighted above. They should also consider postponing abrupt discontinuation of ongoing medications or initiation of new pharmacotherapy trials while tDCS is concurrently used, to avoid misinterpretation of emergent side effects and to allow systematic observation of potential interactions between medications and stimulation. The interaction between ongoing psychotherapy and tDCS should also be monitored. Best practice standards include regular review of patient experience whenever tDCS is used, including reporting adverse events to the FDA.
Future Directions
In summary, tDCS may have the potential to improve symptoms of depression, although clinical trial outcomes are mixed and further work is needed to evaluate its place in modern clinical care. Although the matter is outside the focus of this review, the efficacy data for tDCS to treat other conditions remain very preliminary and may include potential harms. Like all interventions, tDCS has potential side effects. Because tDCS is increasing in its use and popularity, clinicians and trainees should incorporate inquiry about tDCS and related device use when reviewing their patients’ use of nonstandard treatments and should document accordingly. Providers with an interest in tDCS administration should pursue additional training in this area and develop appropriate medicolegal practices.
It is possible that future tDCS studies will demonstrate efficacy (or harm) for psychiatric disorders; positive results from these studies would have a significant impact on the field, by providing inexpensive and easily deployable technologies to those with psychiatric disorders or those with side effects related to psychiatric medications and other treatments. Despite this promise, however, it is critical that providers, trainees, and patients recognize the limitations of the available information and responsibly consider tDCS within the broader context of available treatments.
1 : Avoiding skin burns with transcranial direct current stimulation: preliminary considerations. Int J Neuropsychopharmacol 2011; 14:425–426Crossref, Google Scholar
2 : Direct current stimulation modulates LTP and LTD: activity dependence and dendritic effects. Brain Stimul 2017; 10:51–58Crossref, Google Scholar
3 : Excitability changes induced in the human motor cortex by weak transcranial direct current stimulation. J Physiol 2000; 527:633–639Crossref, Google Scholar
4 : Meta-analysis of randomized controlled trials of cranial electrostimulation. Efficacy in treating selected psychological and physiological conditions. J Nerv Ment Dis 1995; 183:478–484Crossref, Google Scholar
5 : Low-intensity transcranial current stimulation in psychiatry. Am J Psychiatry 2017; 174:628–639Crossref, Google Scholar
6 : Transcranial direct current stimulation in psychiatric disorders: a comprehensive review. Psychiatr Clin North Am 2018; 41:447–463Crossref, Google Scholar
7 : The sertraline vs electrical current therapy for treating depression clinical study: results from a factorial, randomized, controlled trial. JAMA Psychiatry 2013; 70:383–391Crossref, Google Scholar
8 : Trial of electrical direct-current therapy versus escitalopram for depression. N Engl J Med 2017; 376:2523–2533Crossref, Google Scholar
9 : International randomized-controlled trial of transcranial direct current stimulation in depression. Brain Stimul 2018; 11:125–133Crossref, Google Scholar
10 : Safety of transcranial direct current stimulation: evidence based update 2016. Brain Stimul 2016; 9:641–661Crossref, Google Scholar
11 : Transcranial direct current stimulation for acute major depressive episodes: meta-analysis of individual patient data. Br J Psychiatry 2016; 208:522–531Crossref, Google Scholar
12 : Pilot study of feasibility of the effect of treatment with tDCS in patients suffering from treatment-resistant depression treated with escitalopram. Clin Neurophysiol 2015; 126:1185–1189Crossref, Google Scholar
13 : A double-blind, sham-controlled trial of transcranial direct current stimulation for the treatment of depression. Int J Neuropsychopharmacol 2010; 13:61–69Crossref, Google Scholar
14 : Transcranial direct current stimulation of 20- and 30-minutes combined with sertraline for the treatment of depression. Prog Neuropsychopharmacol Biol Psychiatry 2018; 82:31–38Crossref, Google Scholar
15 : Treatment-emergent mania/hypomania during antidepressant treatment with transcranial direct current stimulation (tDCS): a systematic review and meta-analysis. Brain Stimul 2017; 10:260–262Crossref, Google Scholar
16 : Jumper Cables for the Mind. New York Times Magazine, 2013. Available from: https://archive.nytimes.com/www.nytimes.com/2013/11/03/magazine/jumper-cables-for-the-mind.htmlGoogle Scholar
17 : A review of the efficacy of transcranial magnetic stimulation (TMS) treatment for depression, and current and future strategies to optimize efficacy. J Affect Disord 2005; 88:255–267Crossref, Google Scholar
18 : “Unfocus” on foc.us: commercial tDCS headset impairs working memory. Exp Brain Res 2016; 234:637–643Crossref, Google Scholar
19 : The mental cost of cognitive enhancement. J Neurosci 2013; 33:4482–4486Crossref, Google Scholar
20 : Cognitive enhancement or cognitive cost: trait-specific outcomes of brain stimulation in the case of mathematics anxiety. J Neurosci 2014; 34:16605–16610Crossref, Google Scholar
21 : Modulation of risk-taking in marijuana users by transcranial direct current stimulation (tDCS) of the dorsolateral prefrontal cortex (DLPFC). Drug Alcohol Depend 2010; 112:220–225Crossref, Google Scholar
22 : Behavioral effects of transcranial direct current stimulation (tDCS) induced dorsolateral prefrontal cortex plasticity in alcohol dependence. J Physiol Paris 2013; 107:493–502Crossref, Google Scholar
23 : Modulation of risky choices in recently abstinent dependent cocaine users: a transcranial direct-current stimulation study. Front Hum Neurosci 2014; 8:661Crossref, Google Scholar
24 : State dependent effect of transcranial direct current stimulation (tDCS) on methamphetamine craving. Int J Neuropsychopharmacol 2014; 17:1591–1598Crossref, Google Scholar



