The Neuropsychiatric Spectrum of Motivational Disorders
Abstract
Adaptive behavior requires neural systems that mediate the evaluation of stimuli in terms of the well-being of the organism and generate subsequent goal-directed behavior. The authors provide an overview of these systems, with an emphasis on those related to positive motivation/approach. The authors outline the contributions of various disciplines to the current understanding of these systems and discuss their dysfunction in the context of multiple neuropsychiatric disorders in terms of deficits, dysregulation, excess, and related syndromes. Illustrative examples are provided, with an emphasis on functional neuroimaging studies. This approach can provide a foundation for conceptualization, diagnosis, and targeted neuromodulatory therapeutics of neuropsychiatric disorders.
(Reprinted with permission from The Journal of Neuropsychiatry and Clinical Neurosciences 2015; 27:7-18)
Human adaptive behavior requires neural systems that mediate the evaluation of stimuli in terms of the well-being of the organism and the subsequent generation of goal-directed behavior. In this article, we provide an overview of these systems, with an emphasis on those related to positive motivation/approach and review current knowledge on motivational processing and behavior, including relevant contributions from a variety of disciplines allied with the cognitive/affective neurosciences. We illustrate, with an emphasis on systems level function, how the various phenomena and terms encountered in clinical neuropsychiatry may be broadly viewed in light of this knowledge and discuss dysfunction of motivational systems in the setting of multiple neuropsychiatric disorders speaking broadly in terms of deficits, dysregulation, excess, and related syndromes. Illustrative examples are provided, with an emphasis on functional neuroimaging studies. This approach can provide a foundation for conceptualization, diagnosis, and targeted neuromodulatory therapeutics of neuropsychiatric disorders and is consistent with the National Institute of Mental Health (NIMH) Research Domain Criteria (RDoC) project, an initiative that seeks to develop new ways of classifying psychopathology based on dimensions of observable behavior and neurobiological measures (see http://www.nimh.nih.gov/research-priorities/rdoc/index.shtml). The goal of the initiative is to define basic dimensions of functioning across multiple units of analysis, cutting across disorders as traditionally defined, to translate progress in basic neurobiological and behavioral research to an improved integrative understanding of psychopathology and the development of new and/or optimally matched treatments for mental disorders. The RDoC research framework currently consists of five major domains of functioning with related subconstructs, including a Positive Valence Systems domain with a subconstruct of Approach Motivation, centered on the mesolimbic dopamine pathway, which is the focus of this article. Approach motivation can in turn be divided into several components: wanting (related terms include motivation, anticipation, incentive salience, and appetitive behavior), liking (related terms include pleasure, hedonia, and consummatory behavior), and incentive learning or prediction, with each mediated by differing control systems within nucleus accumbens-ventral pallidal pathways and modulated by differing neurochemical signals, as discussed below.
Neural Systems Underlying Motivation/Emotion
Motivation/emotion can be viewed as an adaptational system, comprised of evaluative and effector/behavioral arms that produces behaviors in response to changing internal and environmental conditions. This system has been shaped by natural selection to promote behaviors that increase the likelihood of genetic propagation via survival, sexual reproduction, and subsequent survival of offspring/relatives. Basic evaluation involves comparison of information, of external or internal origin, with stored information attained through experience or inheritance, to assess its significance for the evolutionary well-being of the organism. Basic behaviors include fight or flight from predators, inhibition of ongoing behavior in response to stimuli associated with punishment or omission of reward, and approach to positive/appetitive stimuli (Figure 1). Those components of the system involved in evaluation and subsequent action related to positive stimuli are commonly termed reward processes and are discussed below within the context of the broader motivational/emotional system.
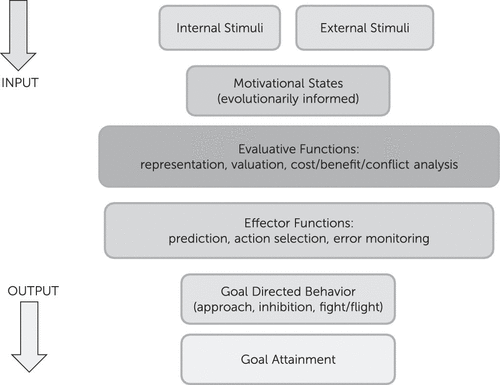
FIGURE 1. Motivation/Emotion can be Viewed as an Adaptational System, Comprised of Evaluative and Effector Arms, that Produces Behaviors in Response to Changing Internal and Environmental Conditions. The System is Modulated by Recurrent Neural Networks.
In humans, neural circuits underlying motivation/emotion form an integrated network that appears to function according to principles of hierarchic organization laid down by Hughlings Jackson in the late 19th century.1 While certain details of his model have not withstood the test of time, this evolutionary scaffolding provides a helpful perspective from which to view the regions/functions involved in motivational processing and behavior. Jackson described the nervous system as an evolutionary accretion of functional levels ranging from the lowest, most organized, and most automatic to the highest, most flexible, and most voluntary. In keeping with Jackson’s model, motivational/emotional processing can be seen to originate at the level of the brainstem and incorporate layers of neural tissue of increasing differentiation and structural complexity during the course of phylogenetic and ontogenetic development.2 As phylogenesis and ontogenesis unfold, the emotional system interacts increasingly with nonaffective cognitive processes, shaping the development of higher order adaptation to a wide range of stimuli and conditions (Figure 2).
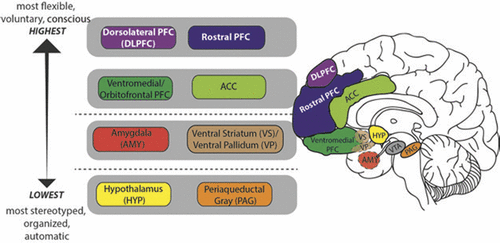
FIGURE 2. In Humans, Neural Circuits Underlying Motivation/Emotion Form a Hierarchically Organized, Integrated Network that Originates at the Level of the Brainstem and Incorporates Layers of Neural Tissue of Increasing Differentiation and Structural Complexity During the Course of Phylogenetic and Ontogenetic Development. Functional Attributes of these Regions are Described Within the Text Under the Heading Neural Systems Underlying Motivation/Emotion. OFC; Orbitofrontal Cortex; ACC: Anterior Cingulate Cortex; PAG: Periaquaductal Gray; VTA: Ventral Tegmental Area.
Key structures at the lower, diencephalic/mesenchephalic level include the hypothalamus and periaquaductal gray (PAG). The hypothalamus, which plays a major role in homeostatic regulation, coordinates somatic responses to changes in the internal milieu, or to stimuli initially processed at higher evaluative levels. It operates via a system of antagonistic excitatory and inhibitory nuclei that mediate production of preprogrammed adaptive behaviors, or fixed-action patterns. The stimulus-response associations mediated by the hypothalamus appear to be genetically determined, and modifiable only by descending inputs from higher levels of the network. The hypothalamus receives input from multiple sources including higher levels of the emotional/motivational network, visceral organs, circulating hormones, neurotransmitters, and osmotic components of the blood stream. Output targets the autonomic nervous system, pituitary gland/endocrine system, and subcortical motor centers that elicit stereotypic movements, and also affects higher level processing via noradrenergic, serotonergic, dopaminergic, and cholinergic projections.
The periaqueductal gray (PAG) is involved in defensive, reproductive and maternal behaviors, as well as arousal, analgesia, thermoregulation, vocalization, micturition, and regulation of REM sleep.3 It receives input from higher levels of the emotional/motivational network including medial prefrontal cortex (PFC), anterior cingulate cortex (ACC), amygdala, and hypothalamus, as well as from nociceptive pathways. Output is to brainstem nuclei that coordinate specific patterns of cardiovascular, respiratory, motor and pain modulation, intralaminar and midline nuclei of the thalamus (relays to PFC), amygdala, hypothalamus, and substantia innominata.
Key structures at the intermediate, limbic level include the amygdala and ventral striatum/pallidum. While hypothalamic evaluative processes involve reference to genetically coded information, those at the level of the amygdala utilize information attained largely through experience, in the form of stimulus-reinforcement associations. Though most strongly associated with evaluation of threat, the amygdala responds to a broad array of significant and potentially significant (i.e., novel) stimuli.4 It receives both interoceptive and exteroceptive input, the latter both directly from modality-specific thalamic relays, and following more complex cortical processing in primary sensory, unimodal and heteromodal association areas, and hippocampus. Output reaches higher and lower levels of the emotional/motivational network, the hippocampus and ventral striatum, sensory association cortex, and numerous effector regions.
The ventral striatum/pallidum, along with extended amygdala, can be viewed as linking motivation to action. While classically studied in the context of drug addiction or reward per se, their role extends to a broad range of positive stimuli/social rewards,5 consistent with the conceptualization of happiness as an approach emotion.6 The key structure is the nucleus accumbens (NAcc), which is comprised of a core and shell—the latter intimately related to the extended amygdala; the former to the dorsal striatum. More anterior regions of the nucleus accumbens show relative selectivity for rewards; more posterior regions, for losses.7
The ventral striatum also receives input from multiple levels of the emotional/motivational network, including hippocampus, basal amygdaloid complex, and limbic prefrontal cortex, and from midline and intralaminar thalamus, median raphe nuclei (serotonergic transmission), and the nucleus of the solitary tract (noradrenergic transmission). Outputs reach the ventral pallidum (to thalamus and frontal cortex), extended amygdale, lateral hypothalamus, basal forebrain cholinergic projection neurons, and dopaminergic neurons in the ventral tegmental area and substantia nigra.
Higher level processing involves paralimbic and prefrontal cortices. Like lower level components of the emotional/motivational network, with which they are interconnected, these regions receive exteroceptive and interoceptive input, and participate in the evaluation of significance and subsequent selection of action. Unlike lower level components, they are able to rapidly readjust behavioral responses to stimuli when their reinforcement value is changed, or when a more complex assessment of the current context suggests the need for modification.8 These higher level regions, which receive top down cognitive and attentional modulation via input from dorsolateral and rostral PFC, are crucial for planning and sustaining goal-directed activity, anticipating consequences of behavior, and acting in accordance with socially determined norms.
While neurophysiologic studies have identified value-related signals in multiple brain regions, a large body of data indicates that orbitofrontal, ventromedial prefrontal, and anterior cingulate cortices play central roles in mediating motivationally guided stimulus evaluation and decision making.9,10 Teasing out the role of specific frontal regions in the complex computations required to guide decision-making and goal-directed behavior is complicated by the fact that multiple processes, such as attention, arousal, motivation, and motor preparation correlate strongly with value, and the precise contributions of various regions is currently a matter of active investigation and hypothesis testing.9–11 However, current evidence supports a number of conclusions, as reviewed by Kennerley and Walton.10 Orbitofrontal cortex plays an important role in determining the current incentive value of a behavioral outcome, potentially influenced by current internal states, and in assigning the value of an outcome to the choice that produced that outcome. Ventromedial prefrontal cortex also plays a role in determining the current incentive value of a behavioral outcome, and in comparing alternative choices. Anterior cingulate cortex may integrate information about a decision’s expected value with information about an action’s value to determine the overall value of each choice alternative, and encodes reward prediction error signals. Lateral prefrontal regions also appear to track history of choices and outcomes, and to encode value information to support allocation of attentional resources or cognitive control toward behaviorally relevant information. In addition to their roles in motivated decision making, orbitofrontal and anterior cingulate cortices appear to mediate pleasure (“liking”).9
Dopaminergic neurons projecting from the ventral tegmental area to ventral striatal and prefrontal regions play a major role in reward processing, particularly the components of wanting and incentive learning or prediction. Rewarding stimuli initially stimulate phasic dopamine release in the nucleus accumbens, reinforcing appetitive behaviors. With repeated reinforcement, or habituation, rewarding objects lose the ability to stimulate dopamine release, while cues predictive of reward availability continue to do so, such that the signal is transferred to the cue. If reward is anticipated but not received, dopaminergic neurons decrease their firing rate. Thus, dopaminergic signals are involved in responding to novel or unanticipated rewards, anticipation or prediction of award, and reward-related learning, including monitoring of prediction errors, independent of reward modality.12 The liking component of reward, in contrast, is most closely associated with opioid and endocannabinoid stimulation of hedonic “hot spots” found most notably in the shell of the nucleus accumbens and ventral pallidum.13–15 There may also be a role for serotonin in modulating reward outcome value.16
Understanding the Motivational/Reward System: Foundations
Contributions to our understanding of the reward system come from a variety of fields. Major examples in the domain of animal studies include Heimer’s identification and mapping of the nucleus accumbens and ventral striatum,17 McGinty’s investigations of molecular and neurochemical aspects of reward,18 Haber’s elucidation of the structure and function of reward pathways,19 Schultz’s work on dopaminergic mechanisms of reward prediction error and their relation to the computational-learning method of temporal difference learning,12 and Gray’s Reinforcement Sensitivity Theory, which postulates a Behavioral Activation System that facilitates reactions to appetitive/rewarding stimuli and regulates approach behavior; a Fight-Flight-Freeze-System that mediates reactions to aversive/punishing stimuli and regulates avoidance behavior, and a Behavioral Inhibition System that mediates conflict within/between the Fight-Flight-Freeze and Behavioral Activation Systems.20 Also crucial are Berridge and Robinson’s parsing of the circuitry and neurochemistry of wanting, liking and learning,13 and development of the incentive sensitization theory of addiction,21 and Rolls’ detailed neurophysiologic mapping of reward, especially in the orbitofrontal cortex,9 and its relation to broader models of emotion, consciousness and computational neuroscience. More recently, a number of investigators have used optogenetic methods to expand our understanding of the distal circuit dynamics, extended inputs and outputs, and causal relationships underlying behavior in the mesolimbic dopamine system.22 Within the human clinical/lesion literature, investigators such as Marin,23 Robinson, Starkstein and Jorge,24 Van Reekum,25 Cummings,26 Stuss,25 and others have enhanced our understanding of apathy/amotivational syndromes, as discussed below.
The field of psychology has provided crucial research on traits related to reward. Major examples include Eysenck’s construct of extraversion27; Watson, Clark, and Tellegen’s positive affectivity28; and Depue and Collins’ agentic and affiliative modes of motivated behavior,29 in which agentic behavior is driven by motivation/pleasure related to social dominance, leadership roles, assertiveness, and a subjective sense of potency in accomplishing goals, and affiliative behavior is driven by motivation/pleasure related to interpersonal warmth/affection (attachment). Behavioral economics/neuroeconomics is an interdisciplinary field that investigates human decision-making. Its origins lie in the application, by Kahneman and Tversky, of cognitive and social psychological approaches to economic decision-making,30 giving rise to the field of behavioral economics; and the further application of cognitive neuroscience/neuroimaging approaches, referred to as neuroeconomics (e.g., Platt and Glimcher31). Increasingly interdisciplinary, this field currently merges perspectives/approaches from cognitive and social psychology, experimental and behavioral economics, neuroscience, neuroimaging, theoretical biology, computer science, and mathematics (computational approaches). Important decision-related constructs from the field include utility, value, probabilistic and temporal uncertainty, risk, loss aversion, intertemporal choice (costs and benefits distributed over time), temporal discounting, and social decision making/game theory.
Translational/human studies, often involving concepts derived from the literatures described above, have also significantly enhanced our understanding of the motivational/reward system. The following examples provide a sense of their scope and intricacy. Using a monetary incentive delay task, Knutson et al.32 demonstrated that anticipation of reward (versus nonreward) activated foci in the ventral striatum, whereas reward outcome was associated with activation in ventromedial prefrontal cortex. Peters and Buchel33 observed correlations between the value of delayed rewards and activation in fronto-polar, lateral parietal, and posterior cingulate cortices; probabilistic rewards and activation in superior parietal and middle occipital regions; and domain-general coding of subjective value regardless of reward type in ventral striatum and orbitofrontal cortex. Similarly, Chib et al.34 reported a correlation between activity in a specific region of ventromedial prefrontal cortex and subjects’ valuations of multiple categories of goods, suggesting an encoding of “common currency” that allows for shared valuation of different categories. Focusing on subjective pleasantness (“liking”) of food, Kringelbach et al.35 found a correlation between decrease in subjective pleasantness and activation of orbitofrontal cortex when food is eaten to satiety, whereas Hare et al.36 reported a correlation between activity in ventromedial prefrontal cortex and goal values in dieters engaged in real decisions about food consumption, regardless of amount of self-control. Investigating trait differences in tendencies to approach/avoidance, Pizzagalli et al.6 showed an association between electroencephalographic activity in left dorsolateral and orbitolateral prefrontal cortex and increased bias to respond to reward-related cues, while Baik et al.37 demonstrated a correlation between extraversion and dopaminergic receptor availability in the striatum using 18F-fallypride positron emission tomography.
Motivational/Reward System Dysfunction
Symptoms related to dysfunction of the motivational/reward system are manifest in a broad array of neuropsychiatric contexts/etiologies that involve pathology of the neural substrates and processes described above. For heuristic purposes, we discuss them here in terms of the broader categories of deficits, dysregulation, excess, and related symptoms, and provide illustrative examples with an emphasis on functional neuroimaging studies.
Motivational Deficits
A variety of overlapping terms have been used to describe deficits in motivational/reward system function, though usage is often imprecise and without universal agreement. Relevant terms include anhedonia, apathy, akinetic mutism, abulia, avolition, psychomotor retardation or slowing, and anergia. These and related constucts are described and discussed below.
Anhedonia.
Anhedonia refers to a lack of interest/pleasure in normally rewarding activities. While the construct was originally viewed as pertaining to pleasure/liking, converging evidence implicates deficits in wanting and/or other aspects of reward processing in the generation of symptoms generally described by this term. Anhedonia is most strongly associated with pathology in ventral striatal and ventromedial prefrontal regions, and encountered most often in the context of depression and schizophrenia.
In terms of depression, it has been well established in rodents that chronic stress can cause decreased responsiveness to rewards, and decreases in striatal dopamine that are reversible with antidepressant medication.38 Abnormalities of motivational/reward processing are also well documented in humans with major depressive disorder and may be a trait marker of vulnerability. Initial functional neuroimaging studies by Keedwell et al.39 and Epstein et al.40 demonstrated that severity of anhedonia, but not depression, was positively and negatively correlated, respectively, with ventromedial prefrontal cortex and amygdala/ventral striatal activity in response to happy stimuli; and that depressed subjects showed significantly less activation than healthy controls to positive stimuli in bilateral ventral striatal regions including ventral head of caudate, putamen and nucleus accumbens, correlating with decreases decreased interest/pleasure in, and performance of activities. Similarly, Pizzagalli et al.41 demonstrated that subjects with depression showed weaker responses to gains in a monetary incentive delay task in the left nucleus accumbens and bilateral caudate, and that both anhedonia and depression severity were associated with reduced caudate volume bilaterally.
In keeping with these findings, Schlaepfer et al.42 reported on three patients treated with deep brain stimulation for refractory depression, using electrodes implanted bilaterally in the nucleus accumbens. Within 1 minute of stimulation onset, two of the three patients manifested an increase in positively motivated behavior, with one spontaneously reporting a plan to visit the local cathedral; the other, to resume bowling, which had been a favorite pastime 12 years previously. Of interest, neither patient reported any subjective changes in feeling state, demonstrating that anhedonia can be dissociated from depressed mood. Dichter et al.43 examined changes in brain activity in depressed subjects during a reward-related (wheel of fortune) task both before and after participation in Behavioral Activation Therapy, a treatment designed to increase engagement with rewarding stimuli and reduce avoidance behaviors. Changes were seen in paracingulate gyrus during reward selection, right (dorsal) caudate during reward anticipation, and paracingulate and orbitofrontal cortex during reward feedback.
More recently McCabe et al.44 examined neural responses to pleasant and aversive sights and tastes in young people with a parental history of depression versus controls with no such family history. Those with a parental history of depression showed decreases in response to rewarding stimuli in orbitofrontal cortex, decreases to both rewarding and aversive stimuli in anterior cingulate cortex, and increases to aversive stimuli in lateral orbitofrontal cortex and insula. In another developmental study, Goff et al.45 observed a failure to develop typical increases in nucleus accumbens reactivity during adolescence in subjects exposed to early life stress, with lower reactivity of nucleus accumbens correlated with higher depression scores. Using optogenetic methods to induce enhanced phasic firing in ventral tegmental area (VTA) dopamine neurons of mice undergoing a subthreshold social-defeat paradigm, Chaudhury et al.46 demonstrated rapid induction of susceptibility to social-stress-induced behavioral abnormalities (social avoidance and decreased sucrose preference) that serve as a validated model of depression. Of note, susceptibility was induced by activation of ventral tegmental neurons projecting to the nucleus accumbens, but not to medial prefrontal cortex, while conversely, optogenetic inhibition of the nucleus accumbens projection induced resilience, and inhibition of the medial prefrontal projection promoted susceptibility.
Deficits in motivational processing are also seen in patients with schizophrenia, including those in the unmedicated state, and are often exacerbated by antidopaminergic medications. These deficits are associated with a range of overlapping symptoms/terminology including anhedonia, apathy, avolition, negative symptoms and psychomotor poverty.47,48 In a study by Juckel et al.,49 unmedicated subjects with schizophrenia displayed reduced ventral striatal activation during presentation of reward-indicating cues, with left ventral striatal activation inversely correlated with negative symptoms. Similarly, Morris et al.50 demonstrated decreased L ventral striatal responses to unexpected outcomes, and increased R ventral striatal responses to expected rewards, in subjects with schizophrenia. Dowd and Barch51 also examined brain activation in schizophrenic subjects associated with reward prediction and receipt, using noninstrumental tasks to control for abnormalities in response selection and execution. In this context, responses to reward anticipation and receipt were largely similar between groups. However, within-group analyses revealed an inverse relationship between physical anhedonia and ventral striatal activity during reward anticipation restricted to the group with schizophrenia, whereas an inverse relationship between physical anhedonia and ventromedial prefrontal activity during reward anticipation was seen in both groups.
Apathy.
Apathy refers to a lack of motivation to engage in thought, feeling and action. It is encountered in a variety of settings and is usually related to pathology of frontal-subcortical circuits, particularly those involving medial frontal/anterior cingulate cortex.26 Etiologies include insults such as stroke, traumatic brain injury, tumor, and anoxia; basil ganglia disease such as Parkinson’s, progressive supranuclear palsy, corticobasal degeneration, Huntington’s, and occasionally Wilson’s: other neurodegenerative conditions such as Alzheimer’s, frontotemporal degeneration, amyotrophic lateral sclerosis and lewy body disease; white matter disorders such as multiple sclerosis and small vessel disease; infections such as human immunodeficiency virus and progressive multifocal leukoencephalopathy; medications such as antipsychotics and selective serotonin reuptake inhibitors; and primary psychiatric disorders such as schizophrenia and depression. Illustrative studies include the use of diffusion tensor imaging by Oto et al.52 to examine the association between apathy and white matter integrity in patients with Alzheimer’s Disease, revealing negative correlations between a measure of apathy and fractional anisotropy values in right anterior cingulate, right thalamus, and bilateral parietal regions; and the use of voxel-based lesion-symptom mapping by Knutson et al.53 in veterans with traumatic brain injury, which demonstrated an association between apathy and damage to limbic and cortical areas of the left hemisphere including anterior cingulate, inferior, middle, and superior frontal regions, insula and supplementary motor area.
Akinetic mutism.
Akinetic mutism refers to a severe form of apathy. Classically seen in patients with lesions of bilateral anterior cingulate cortex, it has also been observed with pathology involving other frontal regions, basal ganglia, mesencephalon, and thalamus.26 Patients with akinetic mutism manifest severely reduced motor output including gestures, facial expression and speech, in the context of preserved arousal and visual tracking of environmental stimuli.54,55
Abulia.
Abulia refers to a lack of self-generated thought, feeling, and action, and is most often encountered in patients with lesions of bilateral subcortical structures, particularly the caudate and globus pallidus, as seen in carbon monoxide poisoning.56,57 Though similar in presentation to patients with akinetic mutism if left to their own devices, individuals with abulia will act at the prompting of others, demonstrating that this syndrome does not truly represent a disorder of motivation, but of self-generation of behavior.58
Avolition.
The term avolition refers to a construct similar to that described by abulia and is most often used in reference to patients with schizophrenia, an illness that involves deficits both in the reward system, and in neural/mental representations of the self. While often referring to deficits of voluntary behavior, there is no universally agreed upon concept of volition in philosophy or neuroscience, and discussions of volition often become entangled in confusion or controversy surrounding concepts of free will/determinism and consciousness.59 While neuroscientific investigations of the construct have approached it from a variety of perspectives, including those of intention, decision-making, initiation, executive control, and feeling of agency, the majority have implicated medial and dorsolateral prefrontal cortex, temporoparietal junction, precuneus, and insula in volitional behavior.60,61 Although a number of studies have pointed to an association between activation of temporoparietal junction and the feeling of agency, more recent investigations suggest that the insula may be more closely associated with self-agency, while temporoparietal regions may play a role in the identification of experiences of nonagency in the setting of incongruent feedback, as part of a more general mismatch detection mechanism.61,62
Psychomotor retardation/slowing.
Psychomotor retardation or slowing refers to a slowing of thought and action. This is a heterogeneous construct involving multiple contributing mechanisms, and is encountered in a variety of neuropsychiatric settings involving pathology of basal ganglia, white matter, and prefrontal cortex. Psychomotor retardation in the contexts of depression and schizophrenia has been associated with structural and functional abnormalities of the striatum, white matter, dorsolateral prefrontal cortex, left prefrontal cortex, angular gyrus, and anterior cingulate gyrus.63,47
Anergia.
Anergia, which refers to a lack of energy, can be seen in depression, schizophrenia, and other disorders involving frontosubcortical pathology. Anergia may be viewed in terms of effort-related aspects of motivated behavior, and the weighing of response costs in decision making.64 Dopamine’s role in the regulation of effort-related functions is primarily one of helping the organism overcome such costs. Most empirical data on this aspect of motivated behavior have been derived from animal studies, but translational work has recently begun, using the Effort Expenditure for Rewards Task.65 Utilizing this task, investigators demonstrated that amphetamine enhanced willingness to exert effort in healthy human subjects, particularly when reward probability was lower, and that depressed patients were less willing than controls to expend effort for rewards, with duration of current episode a significant negative predictor of task performance.66,67 In addition, using positron emission tomography in healthy subjects, they revealed that individual differences in dopamine responsivity in the left striatum and ventromedial prefrontal cortex were correlated with a willingness to expend greater effort for larger rewards, particularly when probability of reward receipt was low, and that variability in dopamine responses in the bilateral insula was negatively correlated with willingness to expend effort for rewards, consistent with evidence implicating this region in the processing of response costs.68
Social/affiliative amotivation.
The hypothesis that deficits of social motivation are central to autism spectrum disorders has recently gained renewed interest.69 Both intervention research and behavioral investigations suggest that individuals with autism spectrum disorders manifest low responsiveness to social rewards such as facial expression (smile), spoken language (praise), and gestures (thumbs-up).69 Electrophysiologic and imaging studies in this population to date suggest alterations in various aspects of reward processing, though findings are somewhat mixed as to the relative specificity of these abnormalities to social versus other forms of reward.70–72
Motivational Dysregulation
Addiction.
Disruption of the motivational/reward system lies at the core of addictive disorders, which involve intensive craving, seeking, and consuming/performing of a given substance/activity despite associated negative consequences. As reviewed bv Volkow et al.,73 addictive substances exert reinforcing effects via abrupt, transient increases in extracellular dopamine in the nucleus accumbens that mimic, either directly or indirectly, phasic dopaminergic cell firing. Repeated exposure to the drug causes changes in dopaminergic function that affect additional brain circuits modulated by dopamine, leading to changes in motivation/drive, inhibitory control/executive function, and memory/conditioning that develop over time. The subsequent hypodopaminergic state results in decreased sensitivity to natural rewards, or anhedonia, and perpetuation of drug use as a compensatory mechanism. Subjects with addiction generally display hyperactivation of reward regions in response to addiction-related cues, and hypoactivation to other forms of reward, likely representing disrupted feedback regulation of the reward circuit by prefrontal regions. Lowered dopamine receptor levels in anterior cingulate cortex are associated with increases in impulsivity; lowered dopamine receptor levels in orbitofrontal cortex, with increases in compulsivity. Impulsivity is associated with increased vulnerability to initiation of drug abuse; compulsivity with continued use, over which one has diminished control. Elevated impulsivity is also seen in those at risk of addiction, prior to the onset of drug use. Some examples of individual studies that have contributed to this understanding of reward processing in addiction are as follows.
Volkow et al.74 demonstrated that detoxified alcoholics display an attenuation of methylphenidate-induced ventral striatal increases in dopamine and lack the negative association between these increases and orbitofrontal cortex metabolism seen in healthy controls, consistent with the hypothesis that orbitofrontal cortex modulates the value of rewards by regulating the magnitude of dopaminergic increases in ventral striatum, and that disruption of this regulation may underlie decreased sensitivity to rewards in addicted subjects. Asensio et al.75 demonstrated hypo-activation of ventral striatum, including nucleus accumbens, as well as dorsal striatum, thalamus, parietal, and dorso-medial prefrontal cortex in cocaine-dependent subjects in response to pleasant stimuli. Using a seed-based analysis to examine resting state functional connectivity in subjects with and without heroin addiction, Ma et al.76 observed increased functional connectivity between nucleus accumbens and ventral/rostral anterior cingulate cortex, and nucleus accumbens and orbitofrontal cortex; along with decreased functional connectivity between prefrontal cortex and orbitofrontal cortex, and nucleus accumbens and orbitofrontal cortex, in addicted subjects. At the molecular level, repeated exposure of rats to drugs of abuse has been shown to induce striatal ΔFosB, a gene transcription factor implicated in many of the neural adaptations associated with drug addiction. Damez-Werno et al.77 demonstrated increased inducibility of Fosb in the nucleus accumbens of rats with a history of prior chronic cocaine administration (followed by extended withdrawal) after repeated cocaine reexposure, revealing a mechanism for epigenetic priming of drug response.
Similar mechanisms to those at play in substance abuse are seen also in behavioral addictions such as pathological gambling, sex addiction, gaming addiction, and some food-related pathologies. For example, in subjects with online gaming addiction, Ko et al.78 observed increased activation in right orbitofrontal cortex, right nucleus accumbens, bilateral anterior cingulate and medial prefrontal cortex, right dorsolateral prefrontal cortex, and right caudate while viewing gaming-related stimuli. Activation was positively correlated with self-reported gaming urge, and with recall of gaming experience provoked by gaming stimuli.
Impulsive-compulsive spectrum disorders in Parkinson’s disease.
The pathophysiology of Parkinson’s Disease, and its treatment with dopaminergic agents, is associated with a spectrum of behavioral, affective, and motor phenomena related to alterations in both dorsal and ventral striatal dopaminergic activity. As reviewed by Voon et al.,79 these range from apathy, depression and decreased motor activity in the hypodopaminergic state, to impulse control disorders, compulsive medication use, punding, and dyskinesias in the hyperdopaminergic state. Impulse control disorders associated with Parkinson’s disease include pathological gambling and shopping, hypersexuality and binge eating. Dopamine dysregulation syndrome, also known as hedonistic homeostatic dysregulation, can develop in the context of prolonged exposure to dopamine replacement therapy, and may also be seen in the setting of deep brain stimulation for Parkinson’s Disease. It usually involves craving for, and compulsive use of dopamine replacement therapy, with the taking of doses beyond that required for control of motor symptoms in an effort to avoid off-period dysphoria. On-periods can be associated with euphoria. Individuals with this syndrome meet criteria for substance dependence and addiction. Dopaminergic medications are also associated with punding, a form of compulsive behavior involving repetitive, purposeless, prolonged activities that generally develop from preexisting habits. Dopamine dysregulation syndrome, impulse control disorders and punding may occur in isolation, but often present in the same patient. Evans et al.80 examined the percentage change in 11C-raclopride binding potential after an oral dose of levodopa in Parkinson’s disease patients with and without dopamine dysregulation syndrome in an off drug state. Patients with the syndrome exhibited enhanced levodopa-induced ventral striatal dopamine release that correlated with self-reported compulsive drug “wanting” but not “liking,” and was related to heightened psychomotor activation (punding).
Impulsivity.
Elevated impulsivity is seen across multiple neuropsychiatric disorders, including attention deficit/hyperactivity disorder, traumatic brain injury, mania, frontotemporal degeneration, addictions, antisocial disorders, and borderline personality disorder. While most often conceptualized in terms of decreased inhibitory control, impulsivity is actually a multifaceted construct including aspects related to attention, reflection, reward sensitivity, choice, and response control.81–83 In terms of the reward system, impulsivity is associated most strongly with increased valuation of immediate over delayed rewards, due to either enhanced discounting of delayed rewards, or overvaluation of immediate rewards. For example, in a behavioral study of subjects with damage to orbitofrontal cortex, a lesion associated with increased impulsivity, Sellitto et al.84 found that subjects with orbitofrontal lesions, versus non-frontal-lobe lesioned subjects and healthy controls, showed significantly increased preference for hypothetical small-immediate over larger-delayed rewards, which is to say steeper temporal discounting. Similarly, in a study of children with attention deficit/hyperactivity disorder, combined type, Costa Dias et al.85 observed atypical functional connectivity of the nucleus accumbens, and increased functional connectivity between the nucleus accumbens and prefrontal cortex associated with steeper delayed-reward discounting.
Motivational Excess
Bipolar disorder/mania.
The increase in goal-directed activity and excessive involvement in pleasurable activities associated with mania suggest hyperactivity of approach motivation. As reviewed by Johnson et al.,86 multiple behavioral studies have examined the Behavioral Activation System20 in bipolar subjects in their baseline state, most using Carver & White’s BAS scale.87 Prospective behavioral findings, which do not appear to be artifacts of baseline manic symptoms, indicate that Behavioral Activation System sensitivity is related to the onset of bipolar spectrum disorder, as well as the transition from cyclothymia to bipolar II, the transition from bipolar II to bipolar I, and a more severe course of manic symptoms. Cross-sectional studies show similar, but less consistent results. In terms of specific aspects of reward processing, compared with healthy subjects, subjects with bipolar disorder appear to expend more effort in pursuit of reward, sustain positive affective responses to reward for a longer duration, and continue to choose previously rewarded stimuli no longer associated with reward. They do not appear to tolerate greater risks to gain rewards, experience greater pleasure in response to rewards, or learn stimulus-reward associations more quickly.
Nusslock et al.88 measured resting electroencephalogram (EEG) activity in healthy individuals and subjects with cyclothymia or bipolar II disorder, who were then followed for an average of 4.7 years. They found that elevation in left frontal EEG activity, a neurophysiological index of approach-system sensitivity, predicted a greater likelihood of converting from cyclothymia or bipolar II disorder to bipolar I disorder, and was associated with an earlier age-of-onset of first bipolar spectrum episode. In addition, left frontal activity was significantly elevated in bipolar spectrum individuals who were in a hypomanic episode at the time of EEG recording. These same investigators89 used functional MRI to scan euthymic bipolar and healthy control subjects engaged in a card-guessing paradigm involving anticipation and receipt of monetary reward and loss. Bipolar subjects displayed greater activation in ventral striatum, right orbitofrontal cortex (Brodmann Area 11), and left lateral orbitofrontal cortex (Brodmann Area 47) during anticipation, but not outcome, of reward. Similarly, Linke et al.90 scanned euthymic bipolar subjects, relatives, and healthy nonrelative controls engaged in a probabilistic reversal learning task, and found increased activation in response to reward and reward reversal contingencies in left medial orbitofrontal cortex in bipolar subjects, and right medial orbitofrontal cortex in relatives. In the latter group, amydgalar activation to reward was also increased, while in bipolar subjects, it was significantly negatively correlated with medication. Both patients and relatives displayed increased amygdala activation to reward reversal.
Psychopathy.
There is also evidence of motivational/reward system hyperfunction associated with psychopathy. Buckholtz et al.91 demonstrated that impulsive-antisocial psychopathic traits selectively predicted nucleus accumbens dopamine release, and reward anticipation-related neural activity, in response to pharmacological and monetary reinforcers, respectively. Similarly, Bjork et al.92 observed that Psychopathic Personality Inventory (PPI) scores correlate positively with recruitment of ventral striatum and anterior cingulate cortex during anticipation of instrumental rewards, and with middle frontal cortex recruitment during anticipation of passively received rewards.
Related Phenotypes and Terms
There are a number of syndromes that appear, at first glance, to involve disturbances in approach behavior but result from distinct, though related, mechanisms.
Anxiety.
Unlike depression, anxiety disorders are not associated with deficits in motivational processing; rather, they are associated with enhanced activity of behavioral inhibition and fight/flight/freeze systems that may interfere with approach behavior.93,94
Kluver Bucy syndrome.
While symptoms of the Kluver Bucy syndrome include hyperorality and hypersexuality, these do not reflect enhanced approach per se, but rather a lack of countervailing behavioral inhibition arising from impaired amygdalar function.
Disinhibitory syndromes.
While changes in functioning of the motivational/reward system associated with orbito-frontal lesions were described above, it should be noted that disinhibitory syndromes, such as frontotemporal degeneration, involve impaired top down control of both approach and inhibitory systems, rather than a primary increase in bottom up generation of motivated behavior.
Catatonia.
Catatonia is a complex state that shares some similarities of appearance to disorders of motivation. While the neurobiology of catatonia is not well understood - it has been reported in the setting of diffuse central nervous system/neurochemical dysfunction as well as with lesions in a variety of brain regions - the existing data are more suggestive of enhancement of fear processing than reward-related deficits.95,96
Compulsive behaviors.
Compulsive behavior can appear similar to impulsive behavior, but differs in important aspects. While impulsive behavior is a manifestation of dysregulation of goal-directed action-outcome learning and maladaptive initiation of behavior, compulsive behavior involves dysregulation of stimulus-response habit learning and maladaptive perseveration of behavior.84
Tics.
Tics such as those seen in Tourette’s syndrome reflect a disturbance in the realm of volition, rather than motivation, in that they are experienced as being generated without will or intent. Although their pathophysiology is not fully understood, it appears to involve striatal disinhibition.97,98
Negative symptoms of schizophrenia.
The term negative symptoms refers to a constellation of symptoms seen in individuals with schizophrenia that includes affective flattening, alogia, avolition, apathy, anhedonia, and asociality.99 These symptoms clearly overlap with many of those described above under the rubric of motivational deficits and are, thus, discussed within that context.
Conclusions: Toward an Integrated Model
Motivational processes are mediated by evolutionarily shaped neurobehavioral systems that allow for the evaluation of, and response to, changing internal and external stimuli, states, and situations. Those components of the system involved in evaluation and subsequent action related to positive stimuli are commonly termed reward processes, and discussed in terms of approach motivation. Our knowledge of positive motivational systems is derived from basic neuroscientific studies, animal models (including, more recently, those employing optogenetic methods), translational/human studies, and clinical findings, as well as psychological and neuroeconomic approaches, which together have elucidated underlying systems-level, cellular, and molecular mechanisms. Multiple etiologic and pathophysiologic mechanisms—environmental, genetic, infectious, vascular and degenerative, among others—can interact with and alter motivational systems, with disruption of key subcortical, limbic, paralimbic and neocortical nodes, and/or their connections acting as final common pathways for clinical symptom or syndrome expression. The resulting deficits, excess, and dysregulation of reward-related behavior can occur across the neuropsychiatric spectrum. Neuroimaging techniques have played an important role in elucidating the functional, systems-level alterations in motivational circuits underlying these symptoms and may also serve a clinical function in the future, as our increasing translational understanding of these processes provides a foundation for enhanced clinical diagnostic biomarker and therapeutic target development. The framework provided within this article allows for an informed approach to disturbances in motivational processing and behavior across the spectrum of neuropsychiatric disorders. Extension of this framework to the related, interacting domains of volition and control, briefly touched upon in this article, would allow for a more comprehensive characterization of behavioral symptoms and an integrated multidimensional model of brain/mind disorders (Figure 3).
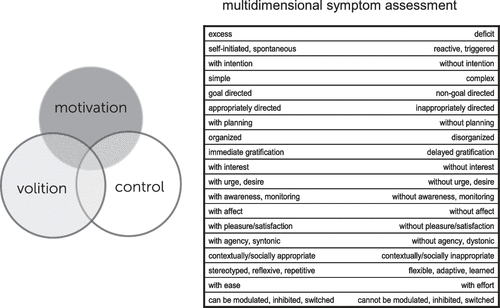
FIGURE 3. The Approach to Disturbances of Motivational Processing/Behavior Presented in this Article may be Extended to the Related, Interacting Domains of Volition and Control, Allowing From a More Comprehensive Characterization of Behavioral Symptoms and an Integrated Multidimensional Model of Brain/Mind Disorders.
1 Taylor J (ed): Selected Writings of John Hughlings Jackson. New York, NY, Basic Books, 1958Google Scholar
2 : Neural mechanisms of emotion. J Consult Clin Psychol 1992; 60:329–338Crossref, Google Scholar
3 : Periaqueductal gray: an interface for behavioral control. Neurology 2012; 78:210–217Crossref, Google Scholar
4 : The amygdala. Curr Biol 2007; 17:R868–R874Crossref, Google Scholar
5 : Functional neuroanatomy of emotion: a meta-analysis of emotion activation studies in PET and fMRI. Neuroimage 2002; 16:331–348Crossref, Google Scholar
6 : Frontal brain asymmetry and reward responsiveness: a source-localization study. Psychol Sci 2005; 16:805–813Crossref, Google Scholar
7 : Differential encoding of losses and gains in the human striatum. J Neurosci 2007; 27:4826–4831Crossref, Google Scholar
8 : The orbitofrontal cortex and reward. Cereb Cortex 2000; 10:284–294Crossref, Google Scholar
9 : Value, pleasure and choice in the ventral prefrontal cortex. Trends Cogn Sci 2011; 15:56–67Crossref, Google Scholar
10 : Decision making and reward in frontal cortex: complementary evidence from neurophysiological and neuropsychological studies. Behav Neurosci 2011; 125:297–317Crossref, Google Scholar
11 : Valuation and decision-making in frontal cortex: one or many serial or parallel systems? Curr Opin Neurobiol 2012; 22:946–955Crossref, Google Scholar
12 : Multiple reward signals in the brain. Nat Rev Neurosci 2000; 1:199–207Crossref, Google Scholar
13 : Dissecting components of reward: ‘liking’, ‘wanting’, and learning. Curr Opin Pharmacol 2009; 9:65–73Crossref, Google Scholar
14 : Towards a functional neuroanatomy of pleasure and happiness. Trends Cogn Sci 2009; 13:479–487Crossref, Google Scholar
15 : Disentangling pleasure from incentive salience and learning signals in brain reward circuitry. Proc Natl Acad Sci USA 2011; 108:E255–E264Crossref, Google Scholar
16 : Serotonin selectively modulates reward value in human decision-making. J Neurosci 2012; 32:5833–5842Crossref, Google Scholar
17 : The concepts of the ventral striatopallidal system and extended amygdala. Ann N Y Acad Sci 1999; 877:1–32Crossref, Google Scholar
18 : Regulation of psychostimulant-induced signaling and gene expression in the striatum. J Neurochem 2008; 104:1440–1449Crossref, Google Scholar
19 : The primate basal ganglia: parallel and integrative networks. J Chem Neuroanat 2003; 26:317–330Crossref, Google Scholar
20 : Reinforcement sensitivity theory and personality. Neurosci Biobehav Rev 2004; 28:317–332Crossref, Google Scholar
21 : The neural basis of drug craving: an incentive-sensitization theory of addiction. Brain Res Brain Res Rev 1993; 18:247–291Crossref, Google Scholar
22 : Optogenetic dissection of neural circuits underlying emotional valence and motivated behaviors. Brain Res 2013; 1511:73–92Crossref, Google Scholar
23 : Apathy: a neuropsychiatric syndrome. J Neuropsychiatry Clin Neurosci 1991; 3:243–254Crossref, Google Scholar
24 : A prospective longitudinal study of apathy in Alzheimer’s disease. J Neurol Neurosurg Psychiatry 2006; 77:8–11Crossref, Google Scholar
25 : Apathy: why care? J Neuropsychiatry Clin Neurosci 2005; 17:7–19Crossref, Google Scholar
26 : Frontal-subcortical circuitry and behavior. Dialogues Clin Neurosci 2007; 9:141–151Google Scholar
27 : The inheritance of extraversion-introversion. Acta Psychol (Amst) 1956; 12:95–110Crossref, Google Scholar
28 : Development and validation of brief measures of positive and negative affect: the PANAS scales. J Pers Soc Psychol 1988; 54:1063–1070Crossref, Google Scholar
29 : Neurobiology of the structure of personality: dopamine, facilitation of incentive motivation, and extraversion. Behav Brain Sci 1999; 22:491–517, discussion 518–569Crossref, Google Scholar
30 : The framing of decisions and the psychology of choice. Science 1981; 211:453–458Crossref, Google Scholar
31 : Neural correlates of decision variables in parietal cortex. Nature 1999; 400:233–238Crossref, Google Scholar
32 : Dissociation of reward anticipation and outcome with event-related fMRI. Neuroreport 2001; 12:3683–3687Crossref, Google Scholar
33 : Overlapping and distinct neural systems code for subjective value during intertemporal and risky decision making. J Neurosci 2009; 29:15727–15734Crossref, Google Scholar
34 : Evidence for a common representation of decision values for dissimilar goods in human ventromedial prefrontal cortex. J Neurosci 2009; 29:12315–12320Crossref, Google Scholar
35 : Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex 2003; 13:1064–1071Crossref, Google Scholar
36 : Self-control in decision-making involves modulation of the vmPFC valuation system. Science 2009; 324:646–648Crossref, Google Scholar
37 : Extraversion and striatal dopaminergic receptor availability in young adults: an [18F]fallypride PET study. Neuroreport 2012; 23:251–254. Available at
38 : Mesolimbic dopamine neurons in the brain reward circuit mediate susceptibility to social defeat and antidepressant action. J Neurosci 2010; 30:16453–16458Crossref, Google Scholar
39 : The neural correlates of anhedonia in major depressive disorder. Biol Psychiatry 2005; 58:843–853Crossref, Google Scholar
40 : Lack of ventral striatal response to positive stimuli in depressed versus normal subjects. Am J Psychiatry 2006; 163:1784–1790Link, Google Scholar
41 : Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am J Psychiatry 2009; 166:702–710Crossref, Google Scholar
42 : Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology 2008; 33:368–377Crossref, Google Scholar
43 : The effects of psychotherapy on neural responses to rewards in major depression. Biol Psychiatry 2009; 66:886–897Crossref, Google Scholar
44 : Neural processing of reward and punishment in young people at increased familial risk of depression. Biol Psychiatry 2012; 72:588–594Crossref, Google Scholar
45 : Reduced nucleus accumbens reactivity and adolescent depression following early-life stress. Neuroscience 2013; 249:129–138Crossref, Google Scholar
46 : Rapid regulation of depression-related behaviours by control of midbrain dopamine neurons. Nature 2013; 493:532–536Crossref, Google Scholar
47 : Dorsolateral prefrontal cortex dysfunction in the major psychoses; symptom or disease specificity? J Neurol Neurosurg Psychiatry 1993; 56:1290–1294Crossref, Google Scholar
48 : Patterns of cerebral blood flow in schizophrenia. Br J Psychiatry 1992; 160:179–186Crossref, Google Scholar
49 : Dysfunction of ventral striatal reward prediction in schizophrenia. Neuroimage 2006; 29:409–416Crossref, Google Scholar
50 : Disambiguating ventral striatum fMRI-related BOLD signal during reward prediction in schizophrenia. Mol Psychiatry 2012; 17:235, 280–289Crossref, Google Scholar
51 : Pavlovian reward prediction and receipt in schizophrenia: relationship to anhedonia. PLoS ONE 2012; 7:e35622Crossref, Google Scholar
52 : Relationship between apathy and diffusion tensor imaging metrics of the brain in Alzheimer’s disease. Int J Geriatr Psychiatry 2012; 27:722–726Crossref, Google Scholar
53 : Neural correlates of apathy revealed by lesion mapping in participants with traumatic brain injuries. Human Brain Mapping 2014; 35:943–953Crossref, Google Scholar
54 : Akinetic mutism following stroke. J Clin Neurosci 2004; 11:25–30Crossref, Google Scholar
55 : Frontal lobe and cingulate cortical metabolic dysfunction in acquired akinetic mutism: a PET study of the interval form of carbon monoxide poisoning. Brain Inj 2004; 18:615–625Crossref, Google Scholar
56 : The behavioural and motor consequences of focal lesions of the basal ganglia in man. Brain 1994; 117:859–876Crossref, Google Scholar
57 : Caudate infarcts. Arch Neurol 1990; 47:133–143Crossref, Google Scholar
58 : Textbook of clinical neuropsychiatry CRC Press, London, 2008Google Scholar
59 : On subjective back-referral and how long it takes to become conscious of a stimulus: a reinterpretation of Libet’s data. Conscious Cogn 2002; 11:144–161Crossref, Google Scholar
60 : How does neuroscience affect our conception of volition? Annu Rev Neurosci 2010; 33:109–130Crossref, Google Scholar
61 : Different brain structures related to self- and external-agency attribution: a brief review and meta-analysis. Brain Struct Funct 2011; 216:151–157Crossref, Google Scholar
62 : Disrupting the experience of control in the human brain: pre-supplementary motor area contributes to the sense of agency. Proc Biol Sci 2010; 277:2503–2509Crossref, Google Scholar
63 : Psychomotor retardation in depression: biological underpinnings, measurement, and treatment. Prog Neuropsychopharmacol Biol Psychiatry 2011; 35:395–409Crossref, Google Scholar
64 : The mysterious motivational functions of mesolimbic dopamine. Neuron 2012; 76:470–485Crossref, Google Scholar
65 : Worth the ‘EEfRT’? The effort expenditure for rewards task as an objective measure of motivation and anhedonia. PLoS ONE 2009; 4:e6598Crossref, Google Scholar
66 : Effort-based decision-making in major depressive disorder: a translational model of motivational anhedonia. J Abnorm Psychol 2012; 121:553–558Crossref, Google Scholar
67 : Amping up effort: effects of d-amphetamine on human effort-based decision-making. J Neurosci 2011; 31:16597–16602Crossref, Google Scholar
68 : Dopaminergic mechanisms of individual differences in human effort-based decision-making. J Neurosci 2012; 32:6170–6176Crossref, Google Scholar
69 : Social‘wanting’dysfunction in autism: neurobiological underpinnings and treatment implications. J Neurodevelopmental Disord 2012; 4:1–20Crossref, Google Scholar
70 : Reward circuitry function in autism spectrum disorders. Soc Cogn Affect Neurosci 2012; 7:160–172Crossref, Google Scholar
71 : Reward circuitry function in autism during face anticipation and outcomes. J Autism Dev Disord 2012; 42:147–160Crossref, Google Scholar
72 : Reward system dysfunction in autism spectrum disorders. Soc Cogn Affect Neurosci 2013; 8:565–572Crossref, Google Scholar
73 : Addiction: decreased reward sensitivity and increased expectation sensitivity conspire to overwhelm the brain’s control circuit. Bioessays 2010; 32:748–755Crossref, Google Scholar
74 : Profound decreases in dopamine release in striatum in detoxified alcoholics: possible orbitofrontal involvement. J Neurosci 2007; 27:12700–12706Crossref, Google Scholar
75 : Altered neural response of the appetitive emotional system in cocaine addiction: an fMRI Study. Addict Biol 2010; 15:504–516Crossref, Google Scholar
76 : Addiction related alteration in resting-state brain connectivity. Neuroimage 2010; 49:738–744Crossref, Google Scholar
77 : Drug experience epigenetically primes Fosb gene inducibility in rat nucleus accumbens. J Neurosci 2012; 32:10267–10272Crossref, Google Scholar
78 : Brain activities associated with gaming urge of online gaming addiction. J Psychiatr Res 2009; 43:739–747Crossref, Google Scholar
79 : Impulse control disorders in Parkinson’s disease: recent advances. Curr Opin Neurol 2011; 24:324–330Crossref, Google Scholar
80 : Compulsive drug use linked to sensitized ventral striatal dopamine transmission. Ann Neurol 2006; 59:852–858Crossref, Google Scholar
81 : Are executive function and impulsivity antipodes? A conceptual reconstruction with special reference to addiction. Psychopharmacology (Berl) 2012; 221:361–387Crossref, Google Scholar
82 : Varieties of impulsivity. Psychopharmacology (Berl) 1999; 146:348–361Crossref, Google Scholar
83 : Neurocognitive endophenotypes of impulsivity and compulsivity: towards dimensional psychiatry. Trends Cogn Sci 2012; 16:81–91Crossref, Google Scholar
84 : Myopic discounting of future rewards after medial orbitofrontal damage in humans. J Neurosci 2010; 30:16429–16436Crossref, Google Scholar
85 : Reward circuit connectivity relates to delay discounting in children with attention-deficit/hyperactivity disorder. Eur Neuropsychopharmacol 2013; 23:33–45Crossref, Google Scholar
86 : The behavioral activation system and mania. Annu Rev Clin Psychol 2012; 8:243–267Crossref, Google Scholar
87 : Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS Scales. J Pers Soc Psychol 1994; 67:319–333Crossref, Google Scholar
88 . Elevated left mid-frontal cortical activity prospectively predicts conversion to bipolar I disorder. 2012; 3:592–601.Google Scholar
89 : Waiting to win: elevated striatal and orbitofrontal cortical activity during reward anticipation in euthymic bipolar disorder adults. Bipolar Disord 2012; 14:249–260Crossref, Google Scholar
90 : Increased medial orbito-frontal and amygdala activation: evidence for a systems-level endophenotype of bipolar I disorder. Am J Psychiatry 2012; 169:316–325Crossref, Google Scholar
91 : Mesolimbic dopamine reward system hypersensitivity in individuals with psychopathic traits. Nat Neurosci 2010; 13:419–421Crossref, Google Scholar
92 : Psychopathic tendencies and mesolimbic recruitment by cues for instrumental and passively obtained rewards. Biol Psychol 2012; 89:408–415Crossref, Google Scholar
93 : A psychophysiological investigation of threat and reward sensitivity in individuals with panic disorder and/or major depressive disorder. J Abnorm Psychol 2013; 122:322–338Crossref, Google Scholar
94 : Testing a tripartite model: I. Evaluating the convergent and discriminant validity of anxiety and depression symptom scales. J Abnorm Psychol 1995; 104:3–14Crossref, Google Scholar
95 : Catatonia: clinical aspects and neurobiological correlates. J Neuropsychiatry Clin Neurosci 2009; 21:371–380Crossref, Google Scholar
96 : Lorazepam modulates orbito-frontal signal changes during emotional processing in catatonia. Hum Psychopharmacol 2010; 25:55–62Crossref, Google Scholar
97 : Tic disorders: what happens in the basal ganglia? Neuroscientist 2013; 19:101–108Crossref, Google Scholar
98 : Tourette syndrome: evolving concepts. Mov Disord 2011; 26:1149–1156Crossref, Google Scholar
99 : Negative symptoms in schizophrenia. Definition and reliability. Arch Gen Psychiatry 1982; 39:784–788Crossref, Google Scholar



