The World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for the Biological Treatment of Bipolar Disorders: Update 2010 on the Treatment of Acute Bipolar Depression
Abstract
Objectives: These guidelines are based on a first edition that was published in 2002, and have been edited and updated with the available scientific evidence until September 2009. Their purpose is to supply a systematic overview of all scientific evidence pertaining to the treatment of acute bipolar depression in adults. Methods: The data used for these guidelines have been extracted from a MEDLINE and EMBASE search, from the clinical trial database clinicaltrials.gov, from recent proceedings of key conferences, and from various national and international treatment guidelines. Their scientific rigor was categorised into six levels of evidence (A–F). As these guidelines are intended for clinical use, the scientific evidence was finally assigned different grades of recommendation to ensure practicability. Results: We identified 10 pharmacological monotherapies or combination treatments with at least limited positive evidence for efficacy in bipolar depression, several of them still experimental and backed up only by a single study. Only one medication was considered to be sufficiently studied to merit full positive evidence. Conclusions: Although major advances have been made since the first edition of this guideline in 2002, there are many areas which still need more intense research to optimize treatment. The majority of treatment recommendations is still based on limited data and leaves considerable areas of uncertainty.
(Reprinted with permission from The World Journal of Biological Psychiatry, 2010; 11:81–109)
Preface and disclosure statement
This practice guideline for the biological, mainly pharmacological treatment of acute bipolar depression was developed by an international Task Force of the World Federation of Societies of Biological Psychiatry (WFSBP) and is part of a series covering the acute treatment of mania, bipolar depression and maintenance treatment of bipolar disorder. The preparation of these guidelines has not been financially supported by any commercial organization.
This guideline has mainly been developed by psychiatrists and psychotherapists who are in active clinical practice. Experts of the task force were selected according to their expertise and with the aim to cover a multitude of different cultures.
In addition, some contributors are primarily involved in research or other academic endeavours. It is possible that through such activities some contributors have received income related to medicines discussed in this guideline.
Some drugs recommended in the present guideline may not be available in all countries, and approved doses may vary.
Introduction
Although mania is considered as the hallmark of bipolar disorder, major depressive episodes and depressive symptoms place an even more significant burden onto bipolar patients (Judd et al. 2002;Goodwin and Jamison 2007). Traditionally, bipolar depression is considered to be more refractory than unipolar depression (Kupfer et al. 2000), with less favourable response to treatments, and the perceived risk of treatment emergent affective switches (TEAS; Tohen et al. 2009). It poses an important challenge for clinicians, since data suggest that bipolar patients once diagnosed spend about three-fold more time being depressed than manic or hypomanic, in addition to a considerable time with subthreshold depression (Kupka et al. 2007). Even subsyndromal depression is characterised by a significant loss of functionality (Altshuler et al. 2006; Marangell et al. 2008) and is associated with an increased risk of relapse into major affective episodes. Thus, patients recovering, but still having residual affective symptoms, experience subsequent major affective episodes more than three times faster than asymptomatic recoverers (Judd et al. 2008). This may add to the utmost importance of full remission as the ultimate treatment goal in bipolar depression, with a full return to normal levels of psychosocial functioning.
Further goals of treatment in bipolar depression are to diminish the risk of suicidal acts and avoid subsequent episodes. Out of all psychiatric disorders, bipolar disorders (both I and II) carry the highest risk of suicide (or suicidal behaviours in its broader sense) (Rihmer 2005).
Diagnosis of bipolar depression
The diagnostic criteria, both in DSM-IV (American Psychiatric Association 1994) and ICD-10 (World Health Organization 1992), for a major depressive episode (MDE) as part of bipolar disorder are not different from those for MDE in unipolar depression. Some symptoms as leaden paralysis, hypersomnia or increased appetite have been reported to be more frequent in bipolar depression (Akiskal et al. 1983; Mitchell and Malhi 2004; Perlis et al. 2006; Goodwin and Jamison 2007). Other variables such as earlier onset of illness or family history of bipolar disorder may point towards an underlying bipolar course (Winokur et al. 1993), and also some biological variables may show subtle differences (Yatham et al. 1997). Looking at differing symptomatology in two large study cohorts of unipolar and bipolar depressed patients, Perlis et al. (2006) identified eight individual symptom items on the Montgomery-Äsberg Depression Rating Scale (MADRS) and the Hamilton Anxiety Rating Scale: inner tension, pessimistic thoughts, suicidal thoughts and fear were more frequent symptoms in bipolar subjects, whereas apparent sadness, reduced sleep and cognitive and several somatic symptoms of anxiety were more frequent in unipolars. A proposed “probabilistic” approach to distinguish between unipolar and bipolar depression in a person with a major depressive episode and no clear prior manic, hypomanic or mixed episode had been put forward by the International Society of Bipolar Disorder Guidelines Taskforce on Bipolar Depression, summarizing the so far available evidence (Table I; Mitchell et al. 2008). However, the presence or absence of any of these characteristics would not contribute to diagnostic certainty in the individual case. In addition, a substantial proportion of patients considered as unipolar depressive for decades eventually experience a hypomanic or mixed episode (Angst 2006).
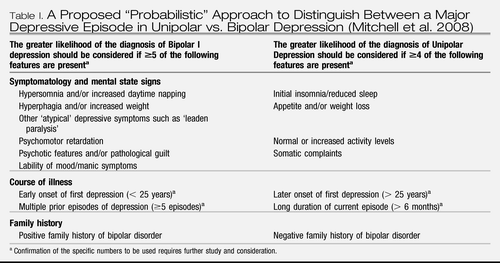 |
Table I. A Proposed “Probabilistic” Approach to Distinguish Between a Major Depressive Episode in Unipolar vs. Bipolar Depression (Mitchell et al. 2008
Careful questioning for past mania and hypomania among those who present with major depressive episode is of utmost importance. While the bipolar nature of major depressive episode is evident for everybody if the patient has had a past manic episode, health professionals are usually less sensitized for detecting past spontaneous hypomania and past “treatment-associated” hypomania. A family history of bipolar disorder, early age of onset (Benazzi and Akiskal 2008) and agitated unipolar major depression (Akiskal et al. 2005) and other soft signs of bipolar spectrum disorder also deserve close attention (Ghaemi et al. 2002).
Patients with those indicators of possible bipolarity are not only particularly vulnerable for affective switches when depressed, but might also be more prone to antidepressant resistance (O'Donovan et al. 2008). In this follow-up study, almost all antidepressant resistant depressives (and those who become suicidal during antidepressant monotherapy) were found among the “pre-bipolar” depressives, as compared to pure unipolar depressives. Similar findings were published by Woo et al. (2008).
Potentially insufficient treatment with antidepressant monotherapy in these cases might result in worsening both the short and long-term outcome including suicidal behaviours as a consequence of worsening of depression (Rihmer and Akiskal 2006). In light of these it is not surprising that particularly juvenile depressives have been found to be vulnerable for “antidepressant-induced” suicidality, since early age of onset is among the best indicators of bipolarity in major depression.
Until recently, it has been widely assumed that evidence from the treatment of unipolar depression can be extrapolated to the bipolar syndrome. This has seemed justified by an acute symptomatology that is virtually undistinguishable. However, since the first edition of this guideline came out in 2002 (Grunze et al. 2002), the available evidence for different medications in bipolar depression has markedly increased, and differences are being proposed. Some caution is needed, since simply to show efficacy in either unipolar or particularly bipolar groups cannot prove specificity. Indeed, unless equal effort is made to study both unipolar and bipolar patient groups, the claim for efficacy in one (and not the other) could be pseudo-specific. Moreover, the most obvious difference between the conditions lies in the potential for TEAS for patients with a bipolar illness history, rather than differential presentation of the depressed state per se.
Methods
The main focus of this guideline is on pharmacological treatments and while best practice regarding other physical treatments and psychotherapy will be summarised briefly, an evidence based review of these modalities is beyond the scope of the present paper. Although the authors are aware that bipolar disorder is a changeable condition which also shows common overlap of the different poles of mood (i.e. mixed mania and mixed depression), the guidelines are initially divided into the classical categories of acute treatments for bipolar depression and mania and prophylaxis. This article will concentrate on the treatment of bipolar depression in adults as there is, despite the clear clinical need (Leverich et al. 2007), unfortunately a paucity of evidence for the treatment in children and adolescents. Due both to the lack of clear-cut and universally accepted diagnostic criteria, and the lack of controlled evidence for treatment, these guidelines will not cover depressive mixed states. There is no clear consensus where the dividing line runs between what some conceptionalize as bipolar mixed depressive state and others as unipolar agitated depression, especially when it comes to the importance of elated mood and motor activity (Maj et al. 2003; Benazzi 2004a,b; Akiskal et al. 2005; Benazzi and Akiskal 2006). However, clinicians should be aware that patients with potentially manic symptoms while depressed constitute a different challenge (Goldberg et al. 2009b), and some medications, e.g., antidepressants, are believed to require caution (Goldberg et al. 2007).
We are also not able to differentiate on an evidence base between the treatment of bipolar depression with or without psychotic symptoms. Unfortunately, there are no controlled studies providing guidance on the drug treatment of bipolar depression with accompanying psychotic symptoms.
The methods of retrieving and reviewing the evidence base and coming up with an recommendation are identical to those described in the WFSBP guideline for acute mania (Grunze et al. 2009). For those readers who are not familiar with the mania guideline, we will summarize the methods in the following paragraphs.
The data used for these guidelines have been extracted from a MEDLINE and EMBASE search, the Science Citation Index at Web of Science (ISI) and a check of the Cochrane library for recent metaanalyses (all until September 2009), and from recent proceedings of key conferences. To ensure comprehensiveness of data, we also consulted various national and international treatment guidelines, consensus statements and comprehensive reviews (Zarin et al. 2002; Licht et al. 2003; Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines Team for Bipolar Disorder 2004; National Collaborating Centre for Mental Health 2006; Yatham et al. 2006; Sartorius et al. 2007; Fountoulakis et al. 2008; Goodwin et al. 2008; Jon et al. 2008; Kasper et al. 2008; Nolen et al. 2008). A few additional trials were found by hand-searching in text books. In addition, www.clinicaltrials.gov was accessed to check for unpublished studies.
The results of metaanalyses have been used as a secondary source of evidence in the absence of conclusive studies or in the case of conflicting evidence. Metaanalyses often compile different drugs into one group, although the individual agents may be quite heterogeneous in their mode of action. In addition, they may have a number of methodological shortcomings, which can make their conclusions less reliable than those of the original studies (Anderson 2000; Bandelow et al. 2008). For bipolar depression, there are few metaanalyses available (e.g., Gijsman et al. 2004) and results and conclusions may be confounded by methodological issues (Fetter and Askland 2005; Ghaemi and Goodwin 2005; Hirschfeld et al. 2005). Metaanalysis may pick up weak signals and magnify them to significance, e.g., in the case of lamotrigine (Geddes et al. 2009); however, statistical significance should not be unthinkingly equated to clinical significance (the latter being also true for individual studies). In general, metaanalyses of negative primary data might identify a small effect size benefit as significant because of the power of Fisherian statistics.
In order to achieve uniform and, in the opinion of this taskforce, appropriate ranking of evidence we adopted the same hierarchy of evidence based rigor and level of recommendation as recently used in other WFSBP guidelines (Bandelow et al. 2008; Grunze et al. 2009) (see Table II). Depending on the number of positive trials and the absence or presence of negative evidence, different categories of evidence for efficacy can be assigned. Ideally, a drug must have shown its efficacy in double-blind placebo-controlled studies in order to be recommended with substantial confidence (categories of evidence (CE) A or B, recommendation grades 1–3); however, as detailed later, these strict criteria may be not suitable in bipolar depression due to a lack of conclusive evidence. A distinction was also made between “lack of evidence” (i.e. studies proving efficacy or non-efficacy do not exist) and “negative evidence” (i.e. the majority of controlled studies shows non-superiority to placebo or inferiority to a comparator drug). When there is lack of evidence, a drug with a potentially positive mechanism of action could still reasonably be tried in a patient unresponsive to standard treatment. Recommendations were then derived from the category of evidence for efficacy (CE) and from additional aspects as safety, tolerability and interaction potential. The grades of recommendation do not fully resemble what is generally understood as “effectiveness.” Clinical effectiveness is composed of efficacy, safety/tolerability and treatment adherence and persistence (Lieberman et al. 2005). As we do not have reliable data on treatment adherence for most of the medications dealt with in this chapter, any statement on clinical effectiveness must be partially based on assumptions.
 |
Table II. Categories of Evidence (CE) and Recommendation Grades (RG)
The recommendation grades (RG) can generally be viewed as steps: Step 1 would be a prescription of a medication with RG 1. When this treatment fails, all other Grade 1 options should ideally be tried first before switching to treatments with RG 2, then 3, 4 and 5. In some cases, e.g., the combination of an RG 1 and an RG 2 option can preferentially be tried instead of combining two RG 1 options, e.g., with some augmentation strategies. In the case of bipolar depression, the primary treatment may still be a medication with a RG as low as 5 as the RG 1 and 2 choices are rather limited and may not suit every patient. In addition, unequal quality of studies may substantially impact on CE and derived RG and even appear contradictory to clinical experience (see paragraph on valproate).
A general problem when reviewing trials is the question of adequate dosing of medication. For several medications, a dose-response relationship is known, especially from studies in unipolar depression. Established drugs which are used as internal comparators in sponsored three-arm studies might be underdosed as it is not in the interest of the sponsor that they came out as superior to the drug under investigation. Using this, although controlled, evidence could induce an unfair bias against established medication, as it might be the case with the two “EMBOLDEN” studies using paroxetine (Young et al. 2008) and lithium (McElroy et al. 2008), respectively, as comparators.
The WFSBP guideline series, including the bipolar guidelines, review acute and long-term treatment issues separately. They do not take into account long-term efficacy when addressing short-term treatment.This approach may be suitable for acute medical conditions, but the WFSBP Bipolar task force still feels uncertain whether an “episode” based approach is really the best way for a disorder which is almost characterised by the chronicity of its symptoms. This dilemma is most obvious in the case of lithium: Acute treatment data are not convincing enough for a higher category of evidence than “D”, however, when long-term considerations, including suicide risk, are taken into account lithium would clearly fall into a higher category (Müller-Oerlinghausen et al. 2006; Young and Newham 2006).
We have not considered the direct or indirect costs of treatments as these vary substantially across different health care systems. Additionally, some of the drugs recommended in this guideline may not (or not yet) have received approval for the treatment of bipolar depression in every country, especially if they have been developed lately. As approval by national regulatory authorities is also dependent on a variety of factors, including the sponsor's commercial interest (or lack thereof) this guideline is exclusively based on the available evidence, not marketing authorisation.
Most RCTs in acute bipolar depression have a duration of 6–8 weeks, and only more recently have double-blind extension periods been added to the protocols. Thus, with the relative paucity of data, the clinically important question of maintenance of effect could not be considered as a core criterion for efficacy, but may become a supportive argument when a choice between similar effective medications has to be made.
Another unsolved issue is the choice of the appropriate rating scale for depression (Möller 2009). Whereas older studies usually applied the Hamilton Rating scale for Depression (HAMD; Hamilton 1967), either in its 17- or 21-item versions, more recent trials choose the Montgomery-Asberg Depression rating scale (MADRS; Montgomery and Asberg 1979). There are subtle differences between these scales, and neither seems to adequately pick up symptoms more frequent in bipolar depression than unipolar depression such as hypersomnia, mood lability and psychomotor disturbances. Other, less frequently used scales in bipolar depression trials include the Inventory of Depressive Symptoms (IDS, (Rush et al. 1986), the self-rated Beck Depression Inventory (BDI; Beck et al. 1961) and the Bech-Rafaelsen Melancholia Scale (MES) (Bech 2002). An issue, which is often not considered is whether the available rating scales fulfil the criteria of unidimensionality by item response theory analysis (Licht et al. 2005). Among the rating scales, the MES is the only scale that has been shown to fulfil such criteria. This heterogeneity of scales for the primary outcomes may have such an impact that it determines whether a study has a positive or failed outcome, e.g., as seen in one study with lamotrigine (Calabrese et al. 1999a). Future studies may, subject to regulatory authorities' acceptance, use more specific scales as the Bipolar Depression Rating Scale (Berk et al. 2007).
The task force is aware of several inherent limitations of these guidelines. When taking negative evidence into consideration, we rely on their publication or their presentation or the willingness of study sponsors to supply this information. Thus, this information may not always be complete and may bias evidence of efficacy in favour of a drug where access to such information is limited. This potential bias has been minimized as much as possible by checking the www.clinicaltrials.gov data base; however, this does not work for older studies conducted prior to the implementation of this website. Another methodological limitation is sponsor bias (Lexchin et al. 2003; Perlis et al. 2005; Heres et al. 2006; Lexchin and Light 2006) inherent in many single studies on which the guidelines are based. Also, all recommendations are formulated by experts who may try their best to be objective but are still subject to their individual pre-determined attitudes and views for or against particular choices. Therefore, no review of evidence and guideline can in itself provide an unchallengeable recommendation but it can direct readers to the original publications and, by this, enhance their own knowledge base and anchor their treatment decisions more securely.
Finally, the value of any guideline is defined by the limitations of evidence. It is a particular additional problem that placebo trials in depression have become harder to conduct, and that those that have been conducted relatively recently tend to have higher placebo response rates. The necessary resources to do them properly can only come from an industry which has had little incentive to study bipolar depression until recently. In addition, one of the most important clinical questions that cannot be sufficiently answered in an evidence-based way is what to do when any first step treatment fails, which happens in a significant number of cases. Some studies, as the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD; Sachs et al. 2003) tried to develop such algorithms, but results are not conclusive and cannot cover the large variety of treatment options (Nierenberg et al. 2006). In particular, there are no systematic studies in bipolar depression that can guide the clinician when to switch medication. In the absence of other, more specific evidence, the task force suggests considering 4-week intervals for the different treatment steps. With the current level of knowledge we can only provide suggestive guidelines and not rigorous algorithms.
Once a draft of this guideline had been prepared by the Secretary and the principal authors it was sent out to the 53 members of the WFSBP Task Force on Treatment Guidelines for Bipolar Disorders for critical review and addition of remarks about specific treatment peculiarities in their respective countries. A second draft, revised according to the respective recommendations, was then distributed for final approval to all task force members and, in addition, to the presidents of the 63 national member societies of the WFSBP.
The acute treatment of bipolar depression
Overview
When initiating treatment for bipolar depression, some general principles apply as outlined in the Canadian Guidelines (Yatham et al. 2006) and its most recent update (Yatham et al. 2009):
| •. | assess safety/functioning | ||||
| •. | establish treatment setting | ||||
| •. | rule out medical causes | ||||
| •. | discontinue caffeine, alcohol and illicit substances | ||||
| •. | consider behavioural strategies/rhythms, psychoeducation | ||||
The Canadian guidelines also recommend as a basic principle to discontinue antidepressants; however, the role of antidepressants in the treatment of bipolar depression remains controversial and will be discussed in more detail in the related chapter. Clinically, the use of antidepressants especially in combination treatment remains common, perhaps reflecting this ongoing controversy, the limited data available (Vieta 2008) and, until recently, the lack of sensible alternatives (Ghaemi et al. 2006a).
Having a thorough review of previous treatment modalities in depression, if there were any, is essential before initiating new treatment. Previous response to a medication appears to be one of the strongest predictor for treatment success. In addition, some medications may be ruled out due to previous non-response or tolerability problems.
The use of lithium rests on old unconvincing trials of small scale and idiosyncratic design (Bhagwagar and Goodwin 2002). The latest controlled evidence in a large cohort study could not show separation of low-serum level lithium from placebo (Young et al. 2008) (CE D, RG 5). Nevertheless, a generally recommended approach in a patient with bipolar depression who is already on treatment with lithium is to increase the dosage to the maximum tolerated level while remaining within the established therapeutic range. This recommendation is indirectly derived from post-hoc analysis of study results (Nemeroff et al. 2001), but mainly based on clinical experience and is in part a variant on “watchful waiting” (CE C3, RG 4). On the other hand, this strategy is to some extend contradicted by a recent analysis suggesting that high lithium serum levels are associated with an increased rate of relapse into bipolar depression (Severus et al. 2009).
Maximizing the benefit from a single medication reduces potential adding up of side effects when medications are combined, and makes it easier to determine the effectiveness of that medication. Only a few studies have examined the role of combination pharmacotherapy when monotherapy is unsuccessful. Based on the results of one such study (van der Loos et al. 2009) lamotrigine might be initiated when lithium optimisation is unsuccessful (CE B, RG 3). Other options with lower grades of evidence include the addition of an atypical antipsychotic to lithium or some augmentation strategies.
The use of anticonvulsants also remains an important option, which will be reviewed in detail below.
Since the previous version of this guideline in 2002, two atypical antipsychotics have emerged as new treatment options in bipolar depression. Based on the findings of large, multicentre, placebo-controlled clinical trials, the initial approach to the pharmacotherapy of bipolar depression (either bipolar I or bipolar II) could be to initiate quetiapine monotherapy (Calabrese et al. 2005; Thase et al. 2006; McElroy et al. 2008; Young et al. 2008) (CE A, RG1) for untreated patients or to add quetiapine to ongoing treatment (CE C1, RG4) (Sokolski and Denson 2003; Suppes et al. 2007) using either the immediate-release or extended-release formulation.
Olanzapine, although mildly effective on its own, is another option-especially when given in combination with fluoxetine (OFC). This combination has been approved and marketed as fixed dose tablets in the US. Its efficacy is supported by one placebo-controlled trial (Tohen et al. 2003) and one head-to-head comparison to lamotrigine (Brown et al. 2006) (CE B, RG 3). However, interpretation of the latter study is difficult as it remains doubtful whether lamotrigine can be considered as a standard comparator for bipolar depression, given its relative small effect size.
Of the different non-medication treatments, ECT is also a reasonable choice (CE C1, RG4) particularly in patients with very severe depression, severe suicide risk, catatonic features, or psychosis (Valenti et al. 2007). ECT may also be used for severe depression during pregnancy. Repetitive transcranial magnetic stimulation (rTMS) (Nahas et al. 2003) and vagus nerve stimulation (VNS) (Goodnick et al. 2001) has, to date, shown only modest benefits (CE F).
Certain psychotherapy modalities may also be helpful as adjuncts to pharmacotherapy (Vieta 2005). Results of the Systematic Treatment Evaluation Program for Bipolar Disorder study indicate that inter-personal and social rhythms therapy, CBT, and family-focused therapy may also speed recovery when added to pharmacotherapy during depressive episodes in patients with either bipolar I or bipolar II disorder (Miklowitz and Otto 2007; Miklowitz et al. 2007) (CE A, RG 1).
In conclusion, there is no choice of first step in treating bipolar depression that shows unequivocal benefits. We are obliged to review the options as just that, without an overwhelming preference for any single treatment based on careful comparisons of head to head efficacy and acceptability.
Antidepressants
Efficacy.
Antidepressants are frequently used in bipolar depression (Simon et al. 2004), at least as part of combination treatment, and the severity of depressive burden is correlated with the use of antidepressants as part of complex combination treatment (Goldberg et al. 2009a). Open studies suggest that what is true about efficacy for acute treatment of unipolar depression seems very likely to be true also for bipolar depression. Some evidence for comparable efficacy of tricyclics in unipolar and bipolar depressed patients is provided by a large retrospective analysis of 2032 inpatients recruited in the years 1980–1992 at the Department of Psychiatry of the University of Munich (Möller et al. 2001). When the routinely recorded clinician rating scales and the length of stay in hospital were compared, no difference could be detected between unipolar and bipolar depressed patients. Other open studies are also in line with similar antidepressant efficacy of antidepressants in unipolar and bipolar depressed patients (for a review, see Grunze (2006).
There is a large body of controlled clinical studies that support the efficacy of the different available antidepressants in treating symptoms of unipolar depression (Sartorius et al. 2007). However, this is unfortunately only true for unipolar depression. Bipolarity has regrettably been an exclusion criterion in most antidepressant trials of the last two decades (Möller et al. 2006).
More recent, some doubts have been raised about the efficacy of antidepressants in milder forms of unipolar depression, as well as in adolescents. The issue of severity is important in establishing or clarifying the size of the effect of antidepressants (Kirsch et al. 2008, see also McAllister-Williams 2008; Möller 2008). The study by Bridge et al. (Bridge et al. 2009) in children reinforces this, as does the metaanalysis of lamotrigine (Geddes et al. 2009) (see chapter on lamotrigine). Unfortunately, the number of study subjects in most trials with antidepressants is too small to allow for separate responder analysis depending on severity of depression.
Overall, the controlled evidence for antidepressant efficacy of antidepressants as a group of medication in bipolar depression is inconclusive (Vieta, 2008). The available evidence is detailed below, and the deduced CE and RG gradings for antidepressants in monotherapy and as part of a combination treatment are given in Table III.
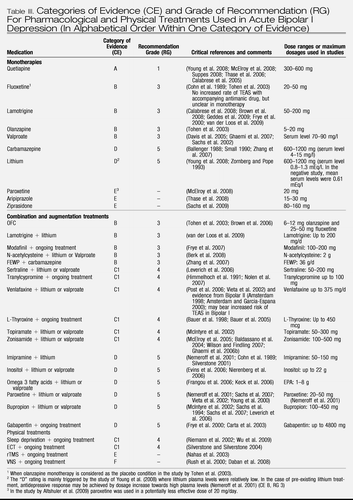 |
Table III. Categories of Evidence (CE) and Grade of Recommendation (RG) For Pharmacological and Physical Treatments Used in Acute Bipolar I Depression (In Alphabetical Order Within One Category of Evidence)
Several small controlled studies support the use of deprenyl (Mendlewicz and Youdim 1980), tranylcypromine (Himmelhoch et al. 1982; Nolen et al. 2007), imipramine and fluoxetine (Cohn et al. 1989). Together with a study examining the effect of olanzapine-fluoxetine combination (OFC) (Tohen et al. 2003), these studies—except the one by Nolen et al. (2007)—have also been subject to a metaanalysis showing beneficial effects of antidepressants as a group in bipolar depression (Gijsman et al. 2004); however, the conclusions of this metaanalysis (which focussed on short-term exposure) have been criticised for not recognizing the perceived long-term harms of antidepressant use (Fetter and Askland 2005; Ghaemi and Goodwin 2005; Hirschfeld et al. 2005). The particular problem has been the inadequate size and small number of monotherapy trials in bipolar depression. Such trials may be negative when considered alone and positive when part of an attempt to synthesize all the available data. It is widely agreed that the evidence is inadequate, and interpretation is accordingly subject to fewer constraints than if the evidence was very clear.
The use of imipramine has not been supported by a failed add-on study to lithium when lithium levels are higher; however, with lower lithium levels imipramine may add some benefit (Nemeroff et al. 2001). Paroxetine has shown no benefit in the treatment of bipolar depression when compared to placebo in one monotherapy study (McElroy et al. 2008), conflicting results in two placebo-controlled add-on studies to lithium (Nemeroff et al. 2001; Sachs et al. 2007), where in one study (Nemeroff et al. 2001) paroxetine was superior to placebo in subjects with lower lithium levels, and potential efficacy in two add-on comparator studies (Young et al. 2000; Vieta et al. 2002). The situation is similar with bupropion: limited evidence exists from small, double-blind comparator studies against desimipramine (Sachs et al. 1994) and topiramate (McIntyre et al. 2002), but in a larger placebo-controlled add-on study to mood stabilizer it could not provide any additional benefit (Sachs et al. 2007). Citalopram appeared effective in a small comparative study (Schaffer et al. 2006), but the choice of the comparator (lamotrigine, see related chapter) makes the study finally inconclusive. One large blinded study did find that a subset of patients benefited from addition of sertraline, bupropion or venlafaxine, but the absence of a placebo comparator means that the extent of benefit cannot be finally estimated (Post et al. 2003; Leverich et al. 2006).
Probably the best positive evidence exists for fluoxetine. Besides the smaller studies mentioned, fluoxetine was also effective in a placebo-controlled study by Cohn et al. (1989). This study alone may not merit a high ranking of fluoxetine monotherapy, as 22 of the 89 patients in this study had concomitant lithium—which, on the other hand, has no signal for efficacy in a recent monotherapy study (McElroy et al. 2008). The strongest evidence comes from another study: fluoxetine add-on to olanzapine was significantly more effective than olanzapine monotherapy and than placebo in a sufficiently powered study (Tohen et al. 2003) maintaining this efficacy without increased rates of treatment emergent affective switches (TEAS) during a 24-week open label extension (Corya et al. 2006). Also, during the acute phase, the risk of TEAS into mania or hypomania was not increased in subjects treated with the combination of fluoxetine and olanzapine as compared to those treated with placebo (Keck et al. 2005).
The two most recent studies, which are also probably those studies with the most elaborate methodology and sufficient number of subjects, did not establish efficacy for antidepressants in bipolar depression. Paroxetine was used as an internal comparator in a study designed to prove the efficacy of quetiapine in bipolar depression. Paroxetine mono-therapy was not superior to placebo after 8 weeks in any depression-related outcome, only in improving symptoms of anxiety (McElroy et al. 2008). One criticism of this study is what is considered as a relatively low dose of paroxetine (20 mg/day), whereas clinically effective doses in unipolar depression are in the range of 30–40 mg/day (Dunner and Dunbar 1992; Möller et al. 1993).
Paroxetine (20–40 mg, mean dose 30 mg) and bupropion (150–300 mg, mean dose 300 mg) were also investigated as adjunctive treatment to mood stabilizer in depressed Bipolar I and II patients. This study was part of the STEP-BD program (Sachs et al. 2003). For the primary outcome, durable recovery as defined as at least eight consecutive weeks of euthymia (with no more than two depressive or two manic symptoms), there was no statistical significant difference between lithium or valproate + placebo and lithium or valproate + antidepressant. Although the chosen outcome criterion may be very meaningful from the clinical perspective, it is unclear how sensitive it may be, and it makes difficult to compare this trial to those with a “classical” outcome, e.g., reduction of a given depression rating scale. Moreover, both the allowed additional use of antipsychotics and psychotherapy which the majority of patients received may have contributed to reduce the ability to detect additional effects of antidepressant therapy. Finally, at randomisation, patients had already been treated within the framework of the STEP-BD for around half a year on average, and presumably a substantial number of patients may have shown non-response to other antidepressants before randomisation. As a matter of fact, an unknown number of patients in this trial still were still using their previous antidepressant during the first 2 weeks of the double-blind phase with an antidepressant or placebo (there was no wash-out). Therefore, the design of the STEP-BD study does not allow a firm conclusion about antidepressants in bipolar depression.
What can we conclude from these latest studies? What we can say with some confidence is that paroxetine (20 mg/day) alone (McElroy et al. 2008) has failed to show efficacy in a controlled bipolar depression trial. Add-on paroxetine (20–40 mg/day) and or bupropion (150–300 mg/day) to mood stabilizers (Sachs et al. 2007) also failed to show effectiveness; however, there are several methodological concerns about this study. But interestingly, paroxetine and bupropion appear in a recent metaanalysis of 12 newer antidepressants in unipolar depression (Cipriani et al. 2009) to belong to the group of weaker antidepressants. In this analysis, it appears that escitalopram, venlafaxine, sertraline and mirtazapine are among those with a relatively stronger action, whereas in another analysis, again venlafaxine and escitalopram, but also clomipramine were judged superior to other antidepressants (Montgomery et al. 2007). Unfortunately, none of them has been tested in bipolar depression in placebo-controlled designs. For two of them, venlafaxine and sertraline, there are less rigorous data in bipolar disorder suggestive of efficacy (Post et al. 2006). But given the large variety of depressive manifestations, it would be somehow naïve to assume that each given antidepressant shows similar efficacy in all conditions (Ayuso-Gutierrez 2005). Therefore, it may be more appropriate in the future not to look at antidepressants as a group but on the individual agents (and their dosing) when making statements on efficacy and TEAS rates in bipolar patients.
Evidence on how to proceed if antidepressant acute treatment is effective is also conflicting. Several observational studies suggesting increased mood instability with long-term antidepressants may be biased by the fact that in clinical settings the more severely ill patients are more likely to be treated with antidepressants (Goldberg et al. 2009a). Open (Altshuler et al. 2003a) and controlled (Altshuler et al. 2009) data of the Stanley Foundation Bipolar Network (SFBN) would favour continuation of antidepressants in selected patients. In both studies, the risk of a depressive relapse appears significantly lower in patients continuing the antidepressant compared to those discontinuing after remission, with no statistically significant difference for breakthrough manic episodes. However, these patients may not be representative in several aspects. A metaanalysis published prior to the controlled study of the SFBN could not establish a benefit from antidepressant continuation (Ghaemi et al. 2008b); however, it was dominated by older studies of the tricyclic impiramine and even short-term data suggest higher risks of switch with tricyclic antidepressants. Looking only into studies which combined imipramine with lithium, no additional risk of TEAS could be observed. In summary, it may again be crucial to look into the individual patient's history to establish whether he or she seems to be at elevated risk of TEAS and whether he or she previously responded well on antidepressants.
Safety, tolerability and practicability.
From the safety and side effect profiles, the newer generation antidepressants are believed to be better tolerated by patients, and are less toxic when taken in overdose (Lader 1996; Barbey and Roose 1998; Frey et al. 2000; Peretti et al. 2000; see also Sartorius et al. 2007). It has to be added, however, that a Cochrane library metaanalysis established only a tendency, but no significant advantage for SSRI compared to TCA when looking at dropout rates in clinical trials in unipolar patients (Barbui et al. 2000). Adherence to treatment is often a highly critical issue, particularly in bipolar patients (Colom et al. 2000), so even a trend of better tolerability might favour the use of the new generation antidepressants unless other effectiveness issues do not contradict it.
A warning concerning the use of antidepressants especially in children and adolescents but also in all age groups has been issued by the FDA (FDA Public Health Advisory 2004). This was due to emerging data suggesting a possible link between suicidality (thinking and behaviour but not completed suicide) and antidepressant use. Both a more thorough view on the available evidence (Moller et al. 2008) and newer, larger population-based studies seem not to support this claim (Simon et al. 2006), and as a matter of fact, suicide rates have increased in adolescents with a drop in antidepressant use (Gibbons et al. 2007). As far as bipolar patients are concerned, data from the large STEP-BD program do not suggest any increased suicidality when treated with antidepressants (Bauer et al., 2006).
Treatment emergent affective switches (TEAS).
Although this chapter gives some general thoughts about the association of antidepressants with TEAS, weighing risks and benefits in the single patient still remains a highly important clinical task. On the one hand, manic episodes can be devastating for the patient and his occupational and family life. On the other hand, insufficient treatment of depression may severely reduce the patients' functional capacities and put them at an increased risk of suicide.
As a matter of fact, the direct transition of depression into hypomania/mania without a symptom-free interval (“switch”) was fundamental for the definition of the “folie à double forme” proposed by Baillarger in 1854 in Paris; the switch was interpreted as a “reaction” to the preceding depression, emphasizing that switching is part of the bipolar course of illness (Pichot 1995) at a time long before the first antidepressant entered the market. Instability of mood is a key feature of bipolar disorder, and any tampering with a scarcely stable system may produce unpredictable effects. For example, there is also some evidence that the withdrawal of antidepressants might also provoke manic episodes (Andrade 2004).
However, more concerns are clearly associated with the introduction of antidepressants into a treatment regimen. A recent systematic review (Visser and Van der Mast 2005) found no strong association between antidepressant use and switch events, and the evidence from observational studies and retrospective self-reports is divergent (Leverich et al. 2006; Carlson et al. 2007; Truman et al. 2007). This may, in part, be due to the fact that there are so far no operationalized criteria for switches, especially the time criterion (how long after beginning/discontinuation of treatment does an affective switch count as treatment emergent?) remains vague and differs between studies. It is just recently that a task force of the ISBD has put forward a suggested definition of switch, irrespectively its relation to treatment, hence it needs validation in prospective trials: a switch (i.e. the appearance of an episode of the opposite pole directly from/after the index episode) would be defined as occurring up to 8 weeks after remission (Tohen et al. 2009). The definition of TEAS itself remains controversial, with many studies requiring high thresholds such as needing to meet full syndromal criteria for mania, and clinically significant but lower thresholds are not explored in many studies.
Two recent reviews have very diligently looked into this and other methodological problems when considering a switch as caused by treatment or as being part of the natural course of the illness (Grunze 2008b; Licht et al. 2008). Similar arguments have also been outlined by Angst and Gamma (2002). Also, only cases with switch events are reported leading to a publication bias. In addition, all studies reporting on switches do not only have a uniform definition of a switch, and also calculate switch rates on an intent-to-treat basis, including non-responders in the analysis. Assuming that antidepressants are efficacious at least in a subgroup of patients, this clearly favours placebo treated patients because only patients who respond can switch, but not those who remain depressed. Finally, placebo treated patients may drop out of trials earlier due to inefficacy and thus have a shorter observational period and smaller chance to develop a switch as part of the natural course of bipolar disorder.
The natural risk of a switch into mania during recovery from a bipolar depression has been estimated to be between 4 and 8% (Bunney et al. 1972; Angst 1985), and mood stabiliser monotherapy show either similar rates of switches or appear to be preventive, especially lithium (Calabrese et al. 1999b). Bipolar I patients appear to be more prone to switch events into manic/hypomanic states than Bipolar II patients (Bond et al. 2008). Monotherapy with some antidepressants, especially tricyclics, without an accompanying mood stabiliser, however, may be associated with an increased rate of TEAS (Lewis and Winokur 1982; Wehr and Goodwin 1987), although the causal relation is impossible to establish in observational studies. When newer antidepressants are used, the switch risk may not be much different from the natural switch risk (Peet 1994). The latest studies (Sachs et al. 2007; McElroy et al. 2008) did not find increased switch rates either with paroxetine monotherapy or paroxetine or bupropion in combination with a mood stabilizer. The switch risk with older TCAs may also be sufficiently controlled with the addition of an antimanic agent (Boerlin et al. 1998), although this cannot totally eliminate the risk of TEAS (Quitkin et al. 1981; Bottlender et al. 1998). However, available data consistently support the low risk of TEAS with the combination of an antidepressant with an antimanic medication (Grunze 2008a). TEAS may occur especially when there are concomitant manic symptoms (Goldberg et al., 2007). Manic symptoms, especially increased motor activity, speech, and language-thought disorder while depressed have been shown to be predictive for an increased risk of TEAS with antidepressants (Frye et al. 2009).
Recommendations.
It is virtually impossible to give a recommendation for antidepressants as a group given the diversity of agents, their dosing, observed outcomes and trial quality. In addition, many data are from combination treatments with antimanic agents, and it is hard to predict the individual contribution of medications and potentiating effects naturally not seen in monotherapy. The main indication for antidepressants in bipolar depression comes from extrapolation of the strong unipolar data, given the absence of proven differences in the underlying biology of bipolar and unipolar depressed states. This may change in the future with emerging data on biological differences, e.g., BDNF serum levels (Fernandes et al. 2009). The task force is aware that the grading of antidepressants as given in Table III is subject to many limitations and thus should be only a preliminary guide for the reader. As to the TEAS into mania with antidepressant use, all the larger studies suggest that this risk is quite modest, at least when combined with a mood-stabilizing medication, and seem to be generally lower in Bipolar II than in Bipolar I patients (Bond et al. 2008). Actually, as reviewed above there is evidence suggesting that when an antidepressant is combined with a mood-stabilizer, there is seemingly no increased risk of TEAS in the sense of full syndromal switches.
Lithium.
Efficacy. There is very limited evidence that lithium may be more effective in bipolar compared to unipolar depression (Goodwin et al. 1972; Baron et al. 1975). Eight of nine small double-blind trials versus placebo suggest that lithium is superior to placebo in treating bipolar depression (Zornberg and Pope 1993). However, most of these trials are methodologically questionable (Grunze 2003) and more recently lithium could not demonstrate clear-cut efficacy in the methodologically most advanced study to date in bipolar depression (Young et al. 2008). In this study, lithium served as an internal comparator in a study investigating the efficacy of quetiapine versus placebo. At study end (week 8) there was only a non-significant trend of separation from placebo for lithium. However, lithium plasma levels in this study were rather low (mean 0.61 mEq/l). In addition, the reported time to onset of antidepressant action of lithium is 6–8 weeks, which is slower than that observed for other antidepressant interventions (Zornberg and Pope 1993). This may also explain the failure of lithium in the Young et al. study, as there was a non-significant tendency for separation of lithium from placebo just towards study end at week 8. It remains speculative whether a significant outcome may have been achieved with higher lithium levels and/or longer study duration.
The strength of the antidepressant effect of lithium monotherapy compared to that of other agents also remains rather unclear. Five rather small double-blind trials have been documented (for a review, see Adli et al. (1998). In particular, we are not aware of published controlled trials in bipolar patients comparing the antidepressant efficacy of lithium with that of antidepressants of the new generation head to head. The previously mentioned study of Young et al. (2008) was not designed and powered to allow comparison for superiority or non-inferiority between quetiapine and lithium, and we are not aware of such a post-hoc analysis.
Lithium is frequently used as an augmentation strategy in refractory unipolar depression (Crossley and Bauer 2007). However, data for lithium augmentation in bipolar depression are scarce and restricted to open studies (Altshuler et al. 2003b). When some antidepressants are combined with lithium, their efficacy may be greater than in mono-therapy (Gyulai et al. 2003; see also following section on valproate).
Safety, tolerability and practicability. Similar to its use in acute mania, the usefulness of lithium in acute bipolar depression may be limited by a slow onset of action and the need for regular plasma level checks to avoid toxicity, as well as by its side effect profile and contraindications (Fountoulakis et al, 2008). Although not absolutely contraindicated, lithium is rarely suitable in certain medical conditions, which therefore should be excluded before treatment initiation, e.g., renal problems or thyroid dysfunction. In these instances, regular medical checkups are mandatory. These limitations have been dealt more extensively in textbooks (Goodwin and Jamison 2007) and reviews (McIntyre et al. 2001). A slower onset of action of lithium, relative to the investigational drug, has been suggested as decisive for inferior outcome in the study by Young et al. (2008)
Lithium has only limited sedating effects, although these may actually be desirable in patients with severe depression and suicidal impulses. Antisuicidal effects of lithium have been pointed out by recent systematic reviews (Baldessarini et al. 2003; Baldessarini et al. 2006; Müller-Oerlinghausen et al. 2006); however, the putative antisuicidal effect of lithium is thought to be not acute but develops over time.
Recommendation. Based on the available studies, lithium monotherapy falls into CE for acute antidepressive efficacy “D”, and the RG is “5”. A positive impression from individually less compelling studies is currently contradicted by a well conducted, negative, large randomized study (Young et al. 2008). If considerations of maintenance treatment or suicidal risks play an additional role at the time of acute treatment initiation, lithium should, however, still be considered as part of a combination or augmentation treatment approach (see also Methods section).
Valproate.
Efficacy. This guideline uses “valproate” as common generic name for the different preparations tested in bipolar disorder, e.g., valproic acid, sodium valproate, divalproate, divalproex sodium, and valpromide. As far as pharmacokinetics and pharmacodynamics are concerned, only valproic acid finally reaches and penetrates the blood-brain barrier. Although tolerability is enhanced with extended release preparations, the difference does not warrant grouping valproic acid derivatives as different medications.
Initially, an open study by Lambert showed a response in only 24% of 103 depressed bipolar patients (Lambert 1984). This 24% response rate is probably not different from an expected placebo response. More recently, however, limited evidence for an acute antidepressant effect of valproate has built up. Three out of four small, but placebo-controlled studies show superiority of valproate over placebo (Davis et al. 2005; Ghaemi et al. 2007; Muzina et al. 2008), the fourth displayed a clear trend, probably missing significance due to lack of power (Sachs et al. 2002). Numbers in these trials were small, the largest one included 54 subjects (Muzina et al. 2008). Unfortunately, only two out of the four studies have been fully published; thus, the methodological accuracy of the unpublished studies (Sachs et al. 2002; Muzina et al. 2008) is difficult to assess.
Some more indirect evidence also comes from a maintenance study comparing valproate, lithium and placebo for 1 year (Bowden et al. 2000). This has been the only maintenance study to date that allowed treatment of breakthrough depression with an antidepressant (either sertraline or paroxetine). Valproate or lithium plus a selective serotonin-reuptake inhibitor (SSRI) provided longer time in study without discontinuation for depression than did placebo plus a SSRI. Fewer patients discontinued prematurely among valproate plus SSRI-treated patients than among placebo-treated patients (Gyulai et al. 2003). These results indirectly suggest that the combination of valproate and an SSRI in acute bipolar depression is more effective than SSRI monotherapy.
Safety, tolerability and practicability. The tolerability of valproate appears fair across trials. Gastointestinal discomfort, sedation and tremor are in most trials more regularly seen with valproate. For rare, but severe complications such as thrombocytopenia, hepatic failure, pancreatitis or hyperammonaemic coma and precaution measures we refer to the pertinent reviews (e.g., Bowden and Singh 2005). When valproate is started during acute bipolar depression, it is mostly not meant as the primary antidepressive agent, but as an augmentation and antimanic cover. This implies that valproate may be continued for quite a considerable time which may require additional precautions, e.g., the use in females of child bearing age (polycystic ovary syndrome (PCOS), teratogenicity). Neurocognitive effects in neonates mean it is now strongly contra-indicated in women of child bearing potential (Meador et al. 2009).
Recommendation. Three out of four small sized, but placebo-controlled studies support antidepressant efficacy of valproate in acute bipolar depression. Thus, the CE is “B” and the RG “3”; however, in special groups as women of child bearing age valproate cannot be recommended due to safety issues.
This graduation of evidence and recommendation grade for valproate strictly follows the pre-setcriteria. The positioning of valproate as an RG “3”, especially when contrasting this to the RG for lithium, has evoked some controversial discussion within the task force. Clinical experience does not seem to reflect a better efficacy of valproate monotherapy than for monotherapy with lithium. As a consequence for this guideline, the task force agreed not to restrict first line treatment to a RG 1–3, but feels that in individual patients also a RG as low as “5” may justify first line use of a medication (e.g., in the case of previous good response in acute and/or long-term treatment) (see Figure 1). The task force also feels that it would be highly desirable to conduct well powered, high quality studies of valproate in bipolar depression in the future to achieve a more reliable ranking of the evidence.
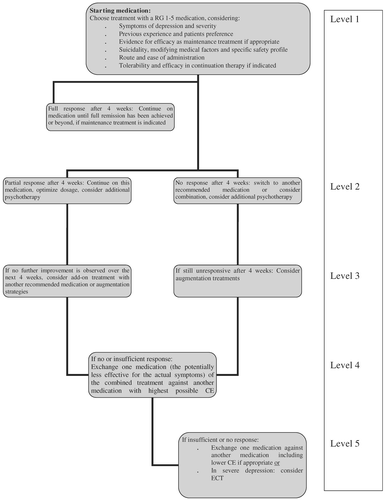
This algorithm applies to bipolar I depression of initially moderate severity, and may vary in mild or severe depression. CE: category of evidence; RG: recommendation grade (see Tables II and III).
Carbamazepine.
Efficacy. Similar to valproate, carbamazepine has been much less studied in the treatment of acute bipolar depression than in mania and prophylaxis (Grunze 2006). The majority of studies are, again, in mixed unipolar and bipolar depressed patients. Some trials suggested moderate efficacy (Ballenger and Post 1980; Neumann et al. 1984; Matkowski and Rybakowski 1992; Dilsaver et al. 1996) including one small placebo-controlled cross-over trial (Ballenger 1988) but others did not replicate this (Small 1990). However, a more recent double-blind, placebo-controlled study showed a significant effect of carbamazepine in a Chinese population at week 12 (endpoint) in the CGI, but not in the HAMD and MADRS (Zhang et al. 2007). When carbamazepine was combined with the herbal remedy “Free and easy wanderer plus” (FEWP) a significant improvement compared to placebo was observed for all three outcomes. Unfortunately, the article does not clarify which of the three scales was chosen as the primary outcome, so the evidence remains inconsistent for carbamazepine monotherapy.
Safety, tolerability and practicability. Common side effects of carbamazepine include oversedation and blurred vision, especially with high dosages and rapid titration. Rare, but potentially severe side effects include allergic reactions, lupus erythematosus, agranulocytosis and hyponatremia. Detailed information on the tolerability and safety profile of carbamazepine is available in recent reviews (Grunze and Walden 2002; Gajwani et al. 2005). In addition, carbamazepine is associated with an increased risk of birth defects (Morrow et al. 2006). The main shortcoming in routine use of carbamazepine, however, is its manifold interactions with other psychotropic medication, including several antipsychotics, antidepressants and anticonvulsants (Spina et al. 1996). If a patient has already received carbamazepine as a prophylactic treatment and has so far responded well to it, continuation of this treatment may be justified. Otherwise, if prophylactic treatment is about to be commenced other treatment options with less interaction potential such as lithium, valproate, lamotrigine or some atypical antipsychotics should be considered.
Recommendation. The evidence base for carbamazepine as monotherapy of acute bipolar depression is not convincing (CE D, RG 5), although it may be helpful to prevent TEAS. Two small trials including a placebo condition gave contradictory results, one placebo-controlled study showed improvement in the CGI at week 12 (endpoint). With complex combination treatment, the RG for carbamazepine may even be lower due to its high interaction potential.
Lamotrigine
Efficacy. Of all anticonvulsants used in bipolar disorder, lamotrigine has the largest portfolio of methodologically well-designed studies in bipolar depression. Numerous early open studies have been conducted (Calabrese et al. 1998) suggesting already that lamotrigine may be more effective in patients with a pre-dominantly depressive polarity (Colom et al. 2006). The first placebo-controlled study was published in 2000 (Frye et al. 2000) showing significant improvement of treatment refractory depression with lamotrigine when compared to placebo or gabapentin. This study applied a cross-over design which raises methodological concerns, and included both unipolar and bipolar patients. Since the mid of the 1990s, five controlled, parallel-group monotherapy studies (Calabrese et al. 2008) and one add-on study to lithium (van der Loos et al. 2009) have looked more systematically at the efficacy of lamotrigine in acute bipolar depression. Results from the first double-blind, placebo-controlled clinical trial (Calabrese et al. 1999a) seemed to confirm its efficacy in bipolar depression at doses of 200 mg daily. However, improvement in the HAMD, which was the primary outcome, was not significant. In the following, there have been four additional negative trials of lamotrigine in bipolar depression. Results of these trials have not been published until recently (Calabrese et al. 2008) which has raised questions as to the extent that publication bias can contribute to widespread use of a medication despite the presence of negative evidence (Ghaemi et al. 2008a). With all monotherapy trials being negative for their primary outcome, several drug licensing authorities did not consider the lamotrigine data strong enough to merit an acute bipolar depression license. A recent individual patient data metanalysis (Geddes et al. 2009) has shed additional light on these studies. Overall, there was a modest, but significant aggregate effect for lamotrigine. However, more importantly, those patients with higher baseline HAM-D scores showed an interaction (P = 0.04) by baseline severity of depression: lamotrigine was superior to placebo in people with HRSD score >24 (RR = 1.47, 95% CI 1.16–1.87, P = 0.001) but not in people with HRSD score < or=24 (RR = 1.07, 95% CI 0.90–1.27, P = 0.445) which might reflect an effect also seen in several antidepressant trials. The result in higher baseline scorers is comparable to that seen with quetiapine. Whether such patients better reflect real world patients is an important question. Lower HRSD scores in these trials were associated with high placebo arm recovery rates.
As noticed previously, these secondary analyses may support the drug and cast at the same time doubt on the subjects included in the monotherapy studies.
This would fit with controlled evidence that lamotrigine may be an effective add-on to lithium in bipolar depressed patients insufficiently responsive to lithium. An investigator initiated, double-blind, placebo-controlled study found a significant improvement in depression related outcomes, including MADRS score reduction and response/remission rates, in patients receiving adjunctive lamotrigine (van der Loos et al. 2009). This augmentation study protocol can be considered as enriched for lithium nonresponse, albeit as a not clinically inappropriate design as patients were required to have continuing depression in the face of lithium use. Furthermore, a small proof-of-concept study in treatment resistant bipolar depression as part of the STEP-BD program randomized 66 patients to lamotrigine, inositol or risperidone added to ongoing lithium or valproate treatment (Nierenberg et al. 2006). No statistically significant difference was found between treatments, but lamotrigine showed numerically clearly higher recovery rates (≥20%) which might, together with the study of Frye et al. (2000) warrant further research of lamotrigine-add on for treatment refractory bipolar depression.
In addition, a large randomized, double-blind, but not placebo-controlled comparison of lamotrigine against combined olanzapine/fluoxetine treatment (Brown et al. 2006) has been conducted. Olanzapine/fluoxetine combination was superior in a number of efficacy related outcomes, including the primary outcome (CGI-S) whereas tolerability was better with lamotrigine.
Unfortunately, at present there are no large controlled monotherapy trials published comparing lamotrigine with a standard antidepressant. Two small randomized studies compared the addition of lamotrigine or an antidepressant to ongoing mood stabilizer treatment. Whereas in one study comparing add-on citalopram and lamotrigine no difference in reducing depressive symptomatology was observed (Schaffer et al. 2006), the other one—adding tranylcypromine or lamotrigine in treatment resistant bipolar depression—observed numerically better outcomes with tranylcypromine; however due to the small number this finding was not significant (Nolen et al. 2007).
Safety, tolerability and practicability. The major concern with lamotrigine is the risk of serious rash, which appears in very rare cases (three per thousand), as opposed to benign rash (10% of patients), which can be prevented by gradually tapering the daily dosage. Cases of severe exfoliative dermatitis and lethal Steven-Johnson syndrome have been described as consequence of an allergic reaction (Bowden et al. 2004). It is recommended that clinicians strictly adhere to the producer's recommended tapering scheme. In patients on concomitant valproate or carbamazepine, the tapering scheme has to be adapted since valproate lowers and carbamazepine increases the metabolism of lamotrigine (Hurley 2002).
Lamotrigine does not appear to possess antimanic properties, since both double-blind clinical trials which focused on this aspect have been negative (Grunze et al. 2009). The rate of TEAS in controlled studies with lamotrigine was not different from placebo, possibly meaning that lamotrigine may not favour switches, but is also not especially protective against treatment emergent mania. Effects against mania were smaller than against depression in the relapse prevention studies (Goodwin et al. 2004).
Recommendation. For lamotrigine monotherapy, the CE would be strictly speaking “E” with negative controlled studies outweighing positive studies.† The effect size of lamotrigine seems to be too small to separate from placebo in five sponsored Phase III studies, and only a metaanalysis of these individual studies with large numbers can detect a small, but significant signal triggered by the more severely ill patients (Geddes et al. 2009). Negative results of the individual trials may be due to patient selection, and if you do not have assay sensitivity in clinical trials the outcome reflects more a property of the patients than the drug. However, the task force takes into account that lamotrigine has shown efficacy in more severely (Geddes et al. 2009) (see above) and treatment refractory depressed patients (Nierenberg et al. 2006; Frye et al. 2000). A CE of “E” would also mean that lamotrigine monotherapy cannot be recommended at all, which is at odds with clinical practice and experience, as well as especially with the positive evidence for adjunctive use together with lithium. This study, together with the metaanalysis of the monotherapy study, might outweigh the negative evidence from the single monotherapy studies. Thus, more members of the task force felt that a CE “B” and a RG of “3” may be more appropriate for lamotrigine monotherapy and especially for combination treatment with lithium.
Olanzapine.
Efficacy. Olanzapine was the first atypical antipsychotic tested in a randomized, controlled 8-week trial (Tohen et al. 2003). Both olanzapine and the fixed combination of olanzapine and fluoxetine (OFC) were superior to placebo treatment in the primary outcome, reduction of the MADRS score, from week 1 onwards. However, OFC was also superior to olanzapine monotherapy from week 4 onwards, and the therapeutic effect size for OFC was twice what it was for olanzapine (0.68 and 0.32). Olanzapine monotherapy separated from placebo in the MADRS total score, but was not superior on the core depressive items, such as reported sadness, apparent sadness, and inability to feel, whereas OFC also significantly improved these items. In a comparator trial, OFC was also superior to lamotrigine in several outcomes (Brown et al. 2006). These results were the basis for approval of a fixed olanzapine/fluoxetine combination preparation for bipolar depression by the FDA, whereas olanzapine monotherapy has no label for bipolar depression.
Safety, tolerability and practicability. The adverse events of greatest concern with olanzapine are related to metabolic issues and weight gain. This topic has already been dealt with in the recent WFSBP mania guideline (Grunze et al. 2009) and will receive more attention in the upcoming maintenance guideline of this series. For an update on this topic, we refer the reader meanwhile to a recent comprehensive review (Kantrowitz and Citrome 2008).
As far as other tolerability issues are concerned, olanzapine was generally well tolerated as an acute treatment. In all controlled trials until 2003, except for one in acute mania, the drop out rates due to adverse events have not been significantly higher than in patients taking placebo (McCormack and Wiseman 2004). Somnolence and dizziness were associated significantly more frequently with olanzapine treatment than with placebo. In the bipolar depression study of Tohen et al. (2003), the Number-needed-to-harm (NNH) was 24 for discontinuation due to sedation (Gao et al. 2008a). EPS, however, were not significantly more frequent when compared to placebo independent from dosage. Anti-cholinergic side effects like dry mouth or constipation occurred in the controlled studies. Olanzapine seems to have a very safe cardiac profile, in none of the olanzapine trials significant QTc prolongations have been observed. However, with intramuscular injections of olanzapine, there is an increased risk of respiratory arrest when patients are on concomitant benzodiazepines.
Side effects seen more frequently with OFC than olanzapine monotherapy were diarrhoea and nausea, otherwise the side effect profile was comparable (Tohen et al. 2003). Side effects significantly more frequent with OFC than with lamotrigine in the head-to head comparison included somnolence, dizziness, sedation, dry mouth, tremor, increased appetite and weight gain, both in short term (Brown et al. 2006) and continuation treatment (Brown et al. 2008).
Concerning TEAS, OFC did not differ from olanzapine or placebo in the placebo-controlled study, or from lamotrigine in the comparator study. Rates of treatment-emergent mania were 6.7% (23/345) for the placebo group, 5.7% (19/335) for the olanzapine group, and 6.4% (5/78) for the olanzapine-fluoxetine group. In the comparator study, rates for TEAs were 4.0% for OFC and 5.2% for lamotrigine.
Recommendation. Both olanzapine and OFC showed efficacy in one double-blind, placebo-controlled trial, corresponding to a CE “B” and RG “3”. However, if a choice has to be made between these two, OFC appears clearly to be the more effective alternative with a more specific action on depressive core items as depicted by single item analysis of the MADRS and a much faster onset of antidepressant action.
Quetiapine.
Efficacy. The record of quetiapine in acute bipolar depression is substantial: five out of five double-blind, placebo-controlled studies in adults showed efficacy for quetiapine 300 or 600 mg/day, four of them using the immediate release formulation (Calabrese et al. 2005; Thase et al. 2006), one the extended release formulation (Suppes 2008). In addition, two of these studies had a comparator arm for assay sensitivity, in one study paroxetine (McElroy et al. 2008), in the other lithium (Young et al. 2008). Effect sizes observed in these studies were moderate to large. Quetiapine was effective both in Bipolar I and II depression (Suppes et al. 2008) with or without rapid cycling (Vieta et al. 2007). Although patients with psychotic symptoms were not excluded, there is no information on the proportion of patients with these symptoms, and whether their outcome differed from the patients without psychotic symptoms.
A feature of quetiapine's pharmacology which might explain its better antidepressant response than that of other atypicals is the noradrenaline reuptake inhibiting properties of its major metabolite norquetiapine (Jensen et al. 2008). There may, however, be a general problem with more sedative medications in placebo-controlled bipolar depression trials which applies not only to quetiapine. They have advantages over non-sedative medications as they might attenuate antidepressant discontinuation syndromes, thereby increasing the effect size in patients previously on anrtidepressant treatment. In addition, it will be more difficult to maintain the blind in studies given their sedative properties.
Safety, tolerability and practicability. The drop-out rates due to side effects were not different from placebo in the quetiapine trials. As expected, somnolence, sedation and dizziness, especially shortly after treatment initiation, were the most frequent side effects. Excesive sedation was also the primary reason for early study discontinuation with a NNH of 7 (Gao et al. 2008a). Other side effects were mostly of anticholinergic nature, such as dry mouth and constipation.
Extrapyramidal side effects were assessed using the Barnes Akathisia and the Simpson Angus Rating Scale for Parkinsonism; no significant differences between quetiapine, placebo or comparator drugs (in two trials) with respect to EPS were observed in single studies. In contrast to schizophrenia and mania trials, however, there was a higher risk of EPS with quetiapine compared with placebo with an NNH of 19 (95% CI −72 to −11) when pooling results of bipolar depression studies (Gao et al. 2008b). With the extended release formulation of quetiapine, EPS were seen in 8.9% of bipolar depressed subjects compared to 3.8 with placebo. This might be suggestive of a higher liability to EPS in depressed patients compared to mania or schizophrenia (Gao et al. 2008b).
Cardiac tolerability was also good, and no significant QTc prolongation was observed when compared to placebo.
The mean weight gain was consistently higher in quetiapine treated patients compared to placebo. Metabolic issues cannot be excluded when quetiapine is taken as long-term medication, but appear not of significance for the short-term use.
In line with quetiapine's antimanic efficacy, TEAS were in all studies numerically lower than with placebo.
Recommendation. Consistent positive results in five placebo-controlled monotherapy trials merit a CE “A” for quetiapine in bipolar depression. There are some concerns about somnolence, weight gain and metabolic issues, but not to a degree that would impact the grade of recommendation for short-term acute treatment. Thus, the corresponding RG is “1.” That said, there is a definite future need for effectiveness trials and naturalistic audit of clinical experience to confirm quetiapine's utility.
Other atypical antipsychotics
Evidence for efficacy of other antipsychotics is currently scarce, but this may change rapidly over the next years. For aripiprazole, two negative controlled studies in bipolar depression have been reported (Thase et al. 2008). Although aripiprazole seemed to enhance improvement from weeks 1–6, it was not better than placebo at week 8 (study end point) (CE “E”). The previously mentioned proof-of-concept study of treatment resistant bipolar depression as part of the STEP-BD program (Nierenberg et al. 2006) found a recovery rate as low as 4.6% for risperidone (one-fifth of what was found for lamotrigine), questioning the efficacy of risperidone in bipolar depression.
Two monotherapy studies and one add-on trial for ziprasidone have been finished recently and the results were negative for monotherapy (Sachs et al. 2009). For other antipsychotics, no data from double-blind, randomized studies exist. Whereas augmentative effects of typical and atypical antipsychotics have been repeatedly described in treatment refractory depression, depressive symptoms in schizophrenia and schizoaffective disorder (Masan 2004), there are no controlled data available in bipolar depression.
Augmenting strategies
Of possible augmentation strategies, i.e. add-on treatments that are not necessarily effective in monotherapy, the combination of modafinil (100–200 mg/ day) with lithium, valproate or ongoing antidepressants was supported by one placebo-controlled trial (Frye et al. 2007) (CE B, RG 3). Interestingly, no increased rate of TEAS was observed despite the supposed dopaminergic effects of modafinil.
Additionally, a fair number of novel add-on treatments have been examined in bipolar depression including inositol (Evins et al. 2006; Nierenberg et al. 2006) (CE D), zonisamide (Baldassano et al. 2004; McElroy et al. 2005; Ghaemi et al. 2006b; Wilson and Findling 2007) (CE C1, RG 4), topiramate (McIntyre et al. 2002) (CE C1, RG 4), omega-3 fatty acids (Frangou et al. 2006; Keck et al. 2006) (CE D, RG 5), FEWP together with carbamazepine (Zhang et al. 2007) (CE B, RG 3) and, more recently, N-acetylcysteine (Berk et al. 2008) (CE B, RG 3). Several open studies suggest antidepressant properties of adjunctive gabapentin (Young et al. 1997; Altshuler et al. 1999; Vieta et al. 2000; Yasmin et al. 2001), but the methodologically best study for gabapentin to date could not find a separation from placebo in treatment-resistant unipolar or bipolar depression. There is some suggestion that adjunctive gabapentin may be effective in patients with alcohol or anxiety comorbidity (Perugi et al. 2002), but in summary the CE for adjunctive gabapentin is “D”, and the RG “5”.
If no sufficient treatment response is observed despite sufficient trials with so-called “mood stabilisers”, some atypical antipsychotics and antidepressants, high dose lthyroxine may also be an augmentative treatment of choice (Bauer et al. 1998; Bauer et al. 2005) (CE C1, RG 4). However, somatic, especially cardiovascular side effects may vary considerably and this strategy should only be applied in treatment-refractory patients and under informed medical surveillance.
Non-pharmacological, biologically based treatments
As a chronobiological intervention strategy, sleep deprivation combined with sleep phase advance protocol is as efficacious in bipolar depression as in unipolar depression (CE C1, RG 4) (Riemann et al. 2002; Wu et al. 2009); however, poorly studied for this indication. When not combined with a mood stabiliser, the risk of TEAS is around 10% (Colombo et al. 1999). It is generally recommended to start the patient on an antimanic medication before sleep deprivation. In summary, sleep deprivation could be considered as an additional therapeutic means to speed up response.
Exercise is of established efficacy in unipolar depression. There is only one non-randomised trial of exercise in bipolar disorder that suggested efficacy (Ng et al. 2007). Exercise nevertheless assists in the management of the metabolic syndrome and as part of activity scheduling and enhancing self-efficacy.
Although controlled data are limited for bipolar depression, the most successful non-pharmacological treatment modality in depression is still electroconvulsive therapy (ECT) (Silverstone and Silverstone 2004; Macedo-Soares et al. 2005) (CE C1, RG 4). Especially in very severe and psychotic depression, or in depression with severe psychomotor retardation, ECT has a major role (Valenti et al. 2007). There is, however, a suggestion of lower ECT efficacy in bipolar than unipolar depression (Hallam et al. 2009). The risk of TEAS with ECT is around 7% (Angst 1985). Protective lithium co-administration may be considered but could increase the risk and duration of a transient post-ECT delirium. However, relapse-preventive medication needs to be initiated once a course of ECT has been finished (Sackeim et al. 2001). The readiness to use ECT is quite different in different countries and mainly reflects public opinion and not its usefulness. Thus, ECT may be used in some countries at an early stage of treatment, whereas in others it is usually only applied in selected, mostly treatment refractory patients.
Transcranial magnetic stimulation (TMS) has undergone extensive evaluation in unipolar depression, but only little is known about its effects in bipolar patients. One small controlled study against sham-TMS could not proof efficacy (Nahas et al. 2003) (CE E). In another study, a non-significant increase of TEAS has been reported for TMS when compared to sham-TMS in bipolar depressed patients (Xia et al. 2008).
Results for vagus nerve stimulation (VNS) have not been reported specifically for bipolar patients. There was a subgroup of bipolar depressed patients included in a larger trial (Rush et al. 2000), but as this trial was negative on its primary outcome for the whole sample, it is highly unlikely that it would work for the subgroup of bipolar depressed patients (Daban et al. 2008) (CE F).
Psychotherapy
Combining pharmacological treatment with psychotherapy, especially those following a standardised procedure or manual, e.g., cognitive-behavioural therapy (CBT; Zaretsky et al. 1999) or interpersonal psychotherapy (IPT; Weissman 1997) is always an option, especially in mildly ill patients. Beneficial effects may include better compliance and adherence to pharmacological treatment as well as avoidance of a stress-inducing lifestyle (Miklowitz et al. 1996).
A RCT over 6 months with CBT in addition to ongoing medication showed lower depression scores and less dysfunctional attitudes in the CBT group (Ball et al. 2006). Results of the Systematic Treatment Evaluation Program for Bipolar Disorder study indicate that interpersonal and social rhythms therapy, CBT, and family-focused therapy may also speed recovery when added to pharmacotherapy during depressive episodes in patients with either bipolar I or bipolar II disorder (Miklowitz and Otto 2007; Miklowitz et al. 2007).
For more detailed information on psychotherapies used in bipolar depression, we refer the reader to comprehensive reviews (Colom and Vieta 2004; Gonzalez-Pinto et al. 2004; Jones 2004; Frank 2007). Psychotherapies, although they may be initiated while acutely depressed, are aiming for intermediate and long-term changes; thus, they cannot be compared to medication or physical treatments which primarily focus on short-term improvement. Therefore, we do not grade their evidence in this chapter,but will pay more attention to them in the upcoming maintenance guidelines.
Specific consideration in bipolar II depression
Bipolar II disorder is sufficiently different from bipolar I to deserve particular attention (Vieta and Suppes 2008). The best available evidence for the acute treatment of bipolar II depression exists for quetiapine which proofed efficacy in a combined analysis of two identically designed randomized, double-blind studies (Suppes et al. 2008) (CE B, RG 3). One study with a mixed Bipolar I and II population (Goldberg et al. 2004) and one pilot study in Bipolar II patients also confirmed efficacy of pramipexole add-on to lithium or valproate in Bipolar II depression (Zarate et al. 2004) (CE B, RG 3). An explorative open study in 19 patients with Bipolar II disorder, depressed phase, demonstrated antidepressant effects of valproate (Winsberg et al. 2001) (CE C1, RG 4).
There is some, but less rigorous evidence for antidepressant monotherapy (e.g., fluoxetine (Amsterdam et al. 1998; Amsterdam and Brunswick 2003), venlafaxine (Amsterdam 1998; Amsterdam and Garcia-Espana 2000) and citalopram (Parker et al. 2006) (all CE1, RG 4). All these studies have also in common that they suggest a low rate of TEAS with antidepressant monotherapy in Bipolar II patients. According to a metaanalysis of available data for antidepressant treatment in Bipolar II patients, the rate of TEAS during acute treatment may be intermediate between Bipolar I and unipolar depression (Bond et al. 2008).
Conclusions
Until recently, treatment of bipolar depression meant either monotherapy with antidepressants, preferably in combination with lithium or an antipsychotic or an anticonvulsant such as valproate or lamotrigine. The evidence for these options was, at its best, moderate, and a relatively slow onset of action is common to all of these. Antidepressants, lithium and lamotrigine have a substantial delay until they show full beneficial action, so additional symptomatic treatment with tranquilizers, e.g., lorazepam, is frequently needed.
Recently, quetiapine and OFC have broadened our treatment options, and both showed moderate to high effect sizes in controlled studies, together with an early separation from placebo, which may hint to a more rapid onset of action. Given the inherent danger of suicide in acute bipolar depression, this can be considered as a major step forward.
For many treatment options, the evidence is not straight forward but generated and extrapolated from several trials, most of them inconclusive by themselves. However, with reasonable caution, we can summarize some key findings:
| •. | There is clear evidence of the efficacy of quetiapine monotherapy at 300 mg/day for the treatment of both Bipolar I and II depression, although there are both short-term tolerability issues and long-term safety issues which should be considered by the clinician and patient. | ||||
| •. | There is strong evidence for the efficacy of olanzapine/fluoxetine combination. There are tolerability and safety issues with regard to this treatment as well which the clinician and patient must deal with. | ||||
| •. | There is also fair evidence for the efficacy of fluoxetine and to some degree also for other antidepressants when used in combination with an antimanic agent, e.g., tranylcypromine, bupropion, sertraline, venlafaxine and imipramine. The issue of TEAS seems to be under control with the combined use of an antimanic agent, at least with SSRIs. | ||||
| •. | Lamotrigine monotherapy in more severely depressed patients has been shown in a post-hoc pooled analysis to have efficacy. Lamotrigine as add-on to lithium in non- or partially responding patients should be considered. | ||||
| •. | Although the evidence is not as good, add-on modafinil and add-on pramipexole (the latter in Bipolar II patients) should be considered. | ||||
Of the non-pharmacological treatments of bipolar depression, adjunctive psychological therapies such as CBT, IPT and social rhythm therapy can add to improved outcomes, although their main evidence base is in the prophylaxis of new episodes. As far as physical treatments are concerned, results for rTMS or VNS in bipolar depression are so far not encouraging or non-existing. ECT remains an effective option especially in treatment resistant bipolar depression.
The task force agrees that, although major advances have been made since the first edition of this guideline (Grunze et al. 2002), there are many areas which still need more intense research to optimize treatment as also outlined in Kasper et al. (2008): Bipolar II depression, psychotic bipolar depression, treatment of children, adolescents and the elderly with bipolar depression, treatment of patients with comorbid conditions, treatment of patients with suicidal risk, treatment of mixed depression, treatment of patients not responding to first or second step treatments and finally comparative studies between the different treatment options and identification of patient subgroups who may do best on a given medication or combination of medications. It is also mandatory to spend more thoughts on trial methodology given the continuous rise in placebo-response rates and, consecutively, failed studies. Clearly defined diagnostic groups and study entry criteria, together with careful site selection, are essential to achieve the best available evidence.
Statement of interests of principal authors
Heinz Grunze received grants/research support, consulting fees and honoraria within the last 3 years from Astra Zeneca, Bial, BMS, Cephalon, Eli Lilly, Glaxo-Smith Kline, Janssen-Cilag, Organon, Pfizer Inc, Sanofi-Aventis, Servier, UBC and UCB Belgium.
Eduard Vieta received grants/research support, consulting fees and honoraria within the last 3 years from Almirall, AstraZeneca, Bial, Bristol-Myers Squibb, Eli Lilly, Forest Research Institute, Glaxo-Smith-Kline, Janssen-Cilag, Jazz, Lundbeck, Merck Sharp and Dohme, Novartis, Organon, Otsuka, Pfizer Inc, Sanofi-Aventis, Servier, Shering-Plough, UBC, and Wyeth.
Guy Goodwin received grants/research support, consulting fees and honoraria within the last 3 years from AstraZeneca, Bristol-Myers Squibb, Eisai, Eli Lilly, Janssen Cilag, Lundbeck, P1 Vital, Sanofi-Aventis, Servier and Wyeth.
Charles Bowden received grants/research support, consulting fees and honoraria within the last 3 years from Abbott Laboratories, Astra Zeneca, Bristol-Myers Squibb, GlaxoSmithKline, Janssen, JDS Inc., Lilly Research, National Institute of Mental Health, Pfizer, R.W. Johnson Pharmaceutical Institute, Sanofi-Aventis, Repligen and the Stanley Medical Research Foundation.
Rasmus W. Licht received research grants, consulting fees and honoraria within the last 3 years from Astra-Zeneca, Bristol-Myers Squibb, Eli Lilly, Glaxo-SmithKline, Janssen Cilag and Sanofi-Aventis.
Hans-Jürgen Möller received grant/research support, consulting fees and honoraria within the last 3 years from AstraZeneca, Bristol-Myers Squibb, Eisai, Eli Lilly, GlaxoSmithKline, Janssen Cilag, Lundbeck, Merck, Novartis, Organon, Pfizer, Sanofi-Aventis, Sepracor, Servier and Wyeth.
Siegfried Kasper received grants/research support, consulting fees and honoraria within the last 3 years from AstraZeneca, Bristol-Myers Squibb, CSC, Eli Lilly, GlaxoSmithKline, Janssen Pharmaceutica, Lundbeck, MSD, Novartis, Organon, Pierre Fabre, Pfizer, Schwabe, Sepracor, Servier and Wyeth.
Adli M, Bschor T, Canata B, Döpfmer S, Bauer M. 1998. Lithium in der Behandlung der akuten Depression. Fortschr Neurol Psychiatr 66:435–441.Crossref, Google Scholar
Akiskal HS, Benazzi F, Perugi G, Rihmer Z. 2005. Agitated “unipolar” depression re-conceptualized as a depressive mixed state: implications for the antidepressant-suicide controversy. J Affect Disord 85:245–258.Crossref, Google Scholar
Akiskal HS, Walker P, Puzantian VR, King D, Rosenthal TL, Dranon M. 1983. Bipolar outcome in the course of depressive illness. Phenomenologic, familial, and pharmacologic predictors. J Affect Disord 5:115–128.Crossref, Google Scholar
Altshuler LL, Keck PE, McElroy SL, Suppes T, Brown ES, Denicoff K, et al. 1999. Gabapentin in the acute treatment of refractory bipolar disorder. Bipolar Disord 1:61–65.Crossref, Google Scholar
Altshuler L, Suppes T, Black D, Nolen WA, Keck PE, Frye MA, et al. 2003a. Impact of antidepressant discontinuation after acute bipolar depression remission on rates of depressive relapse at 1-year follow-up. Am J Psychiatry 160:1252–1262.Crossref, Google Scholar
Altshuler LL, Frye MA, Gitlin MJ. 2003b. Acceleration and augmentation strategies for treating bipolar depression. Biol Psychiatry 53:691–700.Crossref, Google Scholar
Altshuler LL, Post RM, Black DO, Keck PE, Nolen WA, Frye MA, et al. 2006. Subsyndromal depressive symptoms are associated with functional impairment in patients with bipolar disorder: results of a large, multisite study. J Clin Psychiatry 67:1551–1560.Crossref, Google Scholar
Altshuler, LL, Post, RM, Hellemann G, Leverich GS, Nolen WA, Frye MA, et al. 2009. Impact of antidepressant continuation after acute positive or partial treatment response for bipolar depression: a blinded, randomized study. J Clin Psychiatry, 70:450–457.Google Scholar
American Psychiatric Association. 1994. Diagnostic and statistical manual of mental disorders. 4th revised ed. Washington DC: American Psychiatric Press.Google Scholar
Amsterdam J. 1998. Efficacy and safety of venlafaxine in the treatment of bipolar II major depressive episode. J Clin Psychopharmacol 18:414–417.Crossref, Google Scholar
Amsterdam JD, Brunswick DJ. 2003. Antidepressant monotherapy for bipolar type II major depression. Bipolar Disord 5:388–395.Crossref, Google Scholar
Amsterdam JD, Garcia-Espana F. 2000. Venlafaxine monotherapy in women with bipolar II and unipolar major depression. J Affect Disord 59:225–229.Crossref, Google Scholar
Amsterdam JD, Garcia-Espana F, Fawcett J, Quitkin FM, Reimherr FW, Rosenbaum JF, et al. 1998. Efficacy and safety of fluoxetine in treating bipolar II major depressive episode. J Clin Psychopharmacol 18:435–440.Crossref, Google Scholar
Anderson J. 2000. On variability of effects for a metaanalysis of rheumatoid arthritis radiographic progression. J Rheumatol 27:540–542.Google Scholar
Andrade C. 2004. Antidepressant-withdrawal mania:a critical review and synthesis of the literature. J Clin Psychiatry 65:987–993.Crossref, Google Scholar
Angst J. 1985. Switch from depression to mania - a record study over decades between 1920 and 1982. Psychopathology 18:140–154.Crossref, Google Scholar
Angst J. 2006. Do many patients with depression suffer from bipolar disorder? Can J Psychiatry 51:3–5.Crossref, Google Scholar
Angst J, Gamma A. 2002. A new bipolar spectrum concept: a brief review. Bipolar disorders 4 (Suppl 1):11–14.Crossref, Google Scholar
Ayuso-Gutierrez JL. 2005. Depressive subtypes and efficacy of antidepressive pharmacotherapy. World J Biol Psychiatry 6(Suppl 2):31–37.Crossref, Google Scholar
Baldassano CF, Ghaemi SN, Chang A, Lyman A, Lipari M. 2004. Acute treatment of bipolar depression with adjunctive zonisamide: a retrospective chart review. Bipolar Disord 6:432–434.Crossref, Google Scholar
Baldessarini RJ, Tondo L, Hennen J. 2003. Lithium treatment and suicide risk in major affective disorders: update and new findings. J Clin Psychiatry 64(Suppl 5):44–52.Google Scholar
Baldessarini RJ, Tondo L, Davis P, Pompili M, Goodwin FK, Hennen J. 2006. Decreased risk of suicides and atempts during long-term lithium treatment: A meta-analytic review. Bipolar Disord 8:625–639.Crossref, Google Scholar
Ball JR, Mitchell PB, Corry JC, Skillecorn A, Smith M, Malhi GS. 2006. A randomized controlled trial of cognitive therapy for bipolar disorder: focus on long-term change. J Clin Psychiatry 67:277–286.Crossref, Google Scholar
Ballenger JC. 1988. The clinical use of carbamazepine in affective disorder. J Clin Psychiatry 49(Suppl 4):13–19.Google Scholar
Ballenger JC, Post RM. 1980. Carbamazepine in manic-depressive illness: a new treatment. Am J Psychiatry 137:782–790.Crossref, Google Scholar
Bandelow B, Zohar J, Hollander E, Kasper S, Moller HJ, Zohar J, et al. 2008. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for the pharmacological treatment of anxiety, obsessive-compulsive and post-traumatic stress disorders - first revision. World J Biol Psychiatry 9:248–312.Crossref, Google Scholar
Barbey JT, Roose SP. 1998. SSRI safety in overdose. J Clin Psychiatry 59(Suppl 15):42–48.Google Scholar
Barbui C, Hotopf M, Freemantle N, Boynton J, Churchill R, Eccles MP, et al. 2000. Selective serotonin reuptake inhibitors versus tricyclic and heterocyclic antidepressants: comparison of drug adherence. Cochrane Database Syst Rev CD002791.Google Scholar
Baron M, Gershon ES, Rudy V, Jonas WZ, Buchsbaum M. 1975. Lithium carbonate response in depression. Prediction by unipolar/bipolar illness, average-evoked response, catechol-O-methyl transferase, and family history. Arch Gen Psychiatry 32:1107–1111.Crossref, Google Scholar
Bauer M, Hellweg R, Graf KJ, Baumgartner A. 1998. Treatment of refractory depression with high-dose thyroxine. Neuropsy-chopharmacology 18:444–455.Crossref, Google Scholar
Bauer M, London ED, Rasgon N, Berman SM, Frye MA, Altshuler LL, et al. 2005. Supraphysiological doses of levothyroxine alter regional cerebral metabolism and improve mood in bipolar depression. Mol Psychiatry 10:456–469.Crossref, Google Scholar
Bech P. 2002. The Bech-Rafaelsen Melancholia Scale (MES) in clinical trials of therapies in depressive disorders: a 20-year review of its use as outcome measure. Acta Psychiatr Scand 106:252–264.Crossref, Google Scholar
Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. 1961. An inventory for measuring depression. Arch Gen Psychiatry 4:561–571.Crossref, Google Scholar
Benazzi F. 2004a. Agitated depression: a valid depression subtype? Prog Neuropsychopharmacol Biol Psychiatry 28:1279–1285.Crossref, Google Scholar
Benazzi F. 2004b. Mixed states in bipolar II disorder: should full hypomania always be required? Psychiatry Res 127:247–257.Crossref, Google Scholar
Benazzi F, Akiskal HS. 2006. Psychometric delineation of the most discriminant symptoms of depressive mixed states. Psychiatry Res 141:81–88.Crossref, Google Scholar
Benazzi F, Akiskal HS. 2008. How best to identify a bipolar-related subtype among major depressive patients without spontaneous hypomania: Superiority of age at onset criterion over recurrence and polarity? J Affect Disord 107:77–88.Crossref, Google Scholar
Berk M, Malhi GS, Cahill C, Carman AC, Hadzi-Pavlovic D, Hawkins MT, et al. 2007. The Bipolar Depression Rating Scale (BDRS): its development, validation and utility. Bipolar Disord 9:571–579.Crossref, Google Scholar
Berk M, Copolov DL, Dean O, Lu K, Jeavons S, Schapkaitz I,et al. 2008. N-Acetyl cysteine for depressive symptoms in bipolar disorder- a double-blind randomized placebo-controlled trial. Biol Psychiatry 64:468–475.Crossref, Google Scholar
Bhagwagar Z, Goodwin GM. 2002. The role of lithium in the treatment of bipolar depression. Clin Neurosci Res 2: 222–227.Crossref, Google Scholar
Boerlin HL, Gitlin MJ, Zoellner LA, Hammen CL. 1998. Bipolar depression and antidepressant-induced mania: a naturalistic study. J Clin Psychiatry 59:374–379.Crossref, Google Scholar
Bond DJ, Noronha MM, Kauer-Sant'Anna M, Lam RW, Yatham LN. 2008. Antidepressant-associated mood elevations in bipolar II disorder compared with bipolar I disorder and major depressive disorder: A systematic review and meta-analysis. J Clin Psychiatry 69:1589–1601.Crossref, Google Scholar
Bottlender R, Rudolf D, Strauβ A, Möller H-J. 1998. Antidepressant-associated maniform states in acute treatment of patients with bipolar I depression. Eur Arch Psychiatry Clin Neurosci 248:296–300.Crossref, Google Scholar
Bowden CL, Singh V. 2005. Valproate in bipolar disorder: 2000 onwards. Acta Psychiatr Scand Suppl 13–20.Google Scholar
Bowden CL, Calabrese JR, McElroy SL, Gyulai L, Wassef A, Petty F, et al. 2000. A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Divalproex Maintenance Study Group. Arch Gen Psychiatry 57:481–489.Crossref, Google Scholar
Bowden CL, Asnis GM, Ginsberg LD, Bentley B, Leadbetter R, White R. 2004. Safety and tolerability of lamotrigine for bipolar disorder. Drug Saf 27:173–184.Crossref, Google Scholar
Bridge JA, Birmaher B, Iyengar S, Barbe RP, Brent DA. 2009. Placebo response in randomized controlled trials of antidepressants for pediatric major depressive disorder. Am J Psychiatry 166:42–49.Crossref, Google Scholar
Brown E, Dunner DL, McElroy SL, Keck PE, Adams DH,Degenhardt E, et al. 2009. Olanzapine/fluoxetine combination vs. lamotrigine in the 6-month treatment of bipolar I depression. Int J Neuropsychopharmacol 12(6):773–782.Crossref, Google Scholar
Brown EB, McElroy SL, Keck PE, Deldar A, Adams DH, Tohen M, Williamson DJ. 2006. A 7-week, randomized, double-blind trial of olanzapine/fluoxetine combination versus lamotrigine in the treatment of bipolar I depression. J Clin Psychiatry 67:1025–1033.Crossref, Google Scholar
Bunney WE, Murphy DL, Goodwin FK, Borge GF. 1972. The ‘switch process’ in manic-depressive illness. I. A systematic study of sequential behavioral changes. Arch Gen Psychiatry 27:295–302.Crossref, Google Scholar
Calabrese JR, Rapport DJ, Shelton MD, Kimmel SE. 1998. Clinical studies on the use of lamotrigine in bipolar disorder. Neuropsychobiology 38:185–191.Crossref, Google Scholar
Calabrese JR, Bowden CL, Sachs GS, Ascher JA, Monaghan E, Rudd G, for the Lamictal 602 Study Group. 1999a. A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. J Clin Psychiatry 60:79–88.Crossref, Google Scholar
Calabrese JR, Rapport DJ, Kimmel SE, Shelton MD. 1999b. Controlled trials in bipolar I depression: focus on switch rates and efficacy. Eur Neuropsychopharmacol 9(Suppl 4):109–112.Crossref, Google Scholar
Calabrese JR, Keck PE, Macfadden W, Minkwitz M, Ketter TA,Weisler RH, et al. 2005. A randomized, double-blind, placebo-controlled trial of quetiapine in the treatment of Bipolar I or II depression. Am J Psychiatry 162:1351–1360.Crossref, Google Scholar
Calabrese JR, Huffman RF, White RL, Edwards S, Thompson TR,Ascher JA, et al. 2008. Lamotrigine in the acute treatment of bipolar depression: results of five double-blind, placebo-controlled clinical trials. Bipolar Disord 10:323–333.Crossref, Google Scholar
Carlson GA, Finch SJ, Fochtmann LJ, Ye Q, Wang Q, Naz B, Bromet EJ. 2007. Antidepressant-associated switches from depression to mania in severe bipolar disorder. Bipolar Disord 9:851–859.Crossref, Google Scholar
Carta MG, Hardoy MC, Hardoy MJ, Grunze H, Carpiniello B. 2003. The clinical use of gabapentin in bipolar spectrum disorders. J Affect Disord 75:83–91.Crossref, Google Scholar
Cipriani A, Furukawa TA, Salanti G, Geddes JR, Higgins JP, Churchill R, et al. 2009. Comparative efficacy and acceptability of 12 new-generation antidepressants: a multiple-treatments meta-analysis. Lancet 373:746–758.Crossref, Google Scholar
Cohn JB, Collins G, Ashbrook E, Wernicke JF. 1989. A comparison of fluoxetine imipramine and placebo in patients with bipolar depressive disorder. Int Clin Psychopharmacol 4:313–322.Crossref, Google Scholar
Colom F, Vieta E. 2004. A perspective on the use of psychoeducation, cognitive-behavioral therapy and interpersonal therapy for bipolar patients. Bipolar Disord 6:480–486.Crossref, Google Scholar
Colom F, Vieta E, Martinez-Aran A, Reinares M, Benabarre A,Gasto C. 2000. Clinical factors associated with treatment non-compliance in euthymic bipolar patients. J Clin Psychiatry 61:549–555.Crossref, Google Scholar
Colom F, Vieta E, Daban C, Pacchiarotti I, Sanchez-Moreno J. 2006. Clinical and therapeutic implications of predominant polarity in bipolar disorder. J Affect Disord 93:13–17.Crossref, Google Scholar
Colombo C, Benedetti F, Barbini B, Campori E, Smeraldi E. 1999. Rate of switch from depression into mania after therapeutic sleep deprivation in bipolar depression. Psychiatry Res 86:267–270.Crossref, Google Scholar
Corya SA, Perlis RH, Keck PE, Lin DY, Case MG, Williamson DJ, Tohen MF. 2006. A 24-week open-label extension study of olanzapine-fluoxetine combination and olanzapine mono-therapy in the treatment of bipolar depression. J Clin Psychiatry 67:798–806.Crossref, Google Scholar
Crossley NA, Bauer M. 2007. Acceleration and augmentation of antidepressants with lithium for depressive disorders: two meta-analyses of randomized, placebo-controlled trials. J Clin Psychiatry 68:935–940.Crossref, Google Scholar
Daban C, Martinez-Aran A, Cruz N, Vieta E. 2008. Safety and efficacy of Vagus Nerve Stimulation in treatment-resistant depression. A systematic review. J Affect Disord 110:1–15.Crossref, Google Scholar
Davis LL, Bartolucci A, Petty F. 2005. Divalproex in the treatment of bipolar depression: a placebo-controlled study. J Affect Disord 85:259–266.Crossref, Google Scholar
Dilsaver SC, Swann SC, Chen YW, Shoaib A, Joe B, Krajewski KJ, et al. 1996. Treatment of bipolar depression with carbamazepine: results of an open study. Biol Psychiatry 40:935–937.Crossref, Google Scholar
Dunner DL, Dunbar GC. 1992. Optimal dose regimen for paroxetine. J Clin Psychiatry 53(Suppl):21–26.Google Scholar
Evins EA, Demopulos C, Yovel I, Culhane M, Ogutha J,Grandin LD, et al. 2006. Inositol augmentation of lithium or valproate for bipolar depression. Bipolar Disord 8:168–174.Crossref, Google Scholar
FDA Public Health Advisory. 2004. Suicidality in children and adolescents being treated with antidepressant medications. Electronic Citation US Food and Drug Administration.Google Scholar
Fernandes BS, Gama CS, Kauer-Sant'Anna M, Lobato MI,Belmonte-de-Abreu P, & Kapczinski F. 2009. Serum brain-derived neurotrophic factor in bipolar and unipolar depression: A potential adjunctive tool for differential diagnosis. J Psychiatr Res 43:1200–1204.Crossref, Google Scholar
Fetter JC, Askland KD. 2005. Antidepressants for bipolar depression. Am J Psychiatry 162:1546–1548.Crossref, Google Scholar
Fountoulakis KN, Grunze H, Panagiotidis P, Kaprinis G. 2008.Treatment of bipolar depression: An update. J Affect Disord 109:21–34.Crossref, Google Scholar
Frangou S, Lewis M, McCrone P. 2006. Efficacy of ethyl-eicosapentaenoic acid in bipolar depression: randomised double-blind placebo-controlled study. Br J Psychiatry 188:46–50.Crossref, Google Scholar
Frank E. 2007. Interpersonal and social rhythm therapy: a means of improving depression and preventing relapse in bipolar disorder. J Clin Psychol 63:463–473.Crossref, Google Scholar
Frey R, Schreinzer D, Stimpfl T, Vycudilik W, Berzlanovich A, Kasper S. 2000. Suicide by antidepressant intoxication at autopsy in Vienna between 1991–1997: the favourable consequences of the increasing use of SSRIs. Eur Neuropsychopharmacol 10:133–142.Crossref, Google Scholar
Frye M, Ketter T, Kimbrell TA, Dunn R, Speer A, Osuch E, et al. 2000. A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol 20:607–614.Crossref, Google Scholar
Frye MA, Grunze H, Suppes T, McElroy SL, Keck PE, Walden J,et al. 2007. A placebo-controlled evaluation of adjunctive modafinil in the treatment of bipolar depression. Am J Psychiatry 164:1242–1249.Crossref, Google Scholar
Frye MA, Helleman G, McElroy SL, Altshuler LL, Black DO,Keck PE, et al. 2009. Correlates of treatment-emergent mania associated with antidepressant treatment in bipolar depression. Am J Psychiatry 166:164–172.Crossref, Google Scholar
Gajwani P, Forsthoff A, Muzina D, Amann B, Gao K, Elhaj O,et al. 2005. Antiepileptic drugs in mood-disordered patients. Epilepsia 46(Suppl 4):38–44.Google Scholar
Gao K, Ganocy SJ, Gajwani P, Mühlbauer HD, Kemp DE, Calabrese JR. 2008a. A review of sensitivity and tolerability of antipsychotics in patients with bipolar disorder or schizophrenia: focus on somnolence. J Clin Psychiatry 69:302–309.Crossref, Google Scholar
Gao K, Kemp DE, Ganocy SJ, Gajwani P, Xia G, Calabrese JR. 2008b. Antipsychotic-induced extrapyramidal side effects in bipolar disorder and schizophrenia: a systematic review. J Clin Psychopharmacol 28:203–209.Crossref, Google Scholar
Geddes JR, Calabrese JR, Goodwin GM. 2009. Lamotrigine for treatment of bipolar depression: independent meta-analysis and meta-regression of individual patient data from five randomised trials. Br J Psychiatry 194:4–9.Crossref, Google Scholar
Ghaemi SN, Goodwin FK. 2005. Antidepressants for bipolar depression. Am J Psychiatry 162:1545–1546.Crossref, Google Scholar
Ghaemi SN, Ko JK, Goodwin FK. 2002. “Cade's Disease” and beyond: Misdiagnosis, antidepressant use, and proposed definition for bipolar spectrum disorder. Can J Psychiatry 47: 125–134.Crossref, Google Scholar
Ghaemi SN, Hsu DJ, Thase ME, Wisniewski SR, Nierenberg AA, Miyahara S, Sachs G. 2006a. Pharmacological treatment patterns at study entry for the first 500 STEP-BD participants. Psychiatr Serv 57:660–665.Crossref, Google Scholar
Ghaemi SN, Zablotsky B, Filkowski MM, Dunn RT, Pardo TB, Isenstein E, Baldassano CF. 2006b. An open prospective study of zonisamide in acute bipolar depression. J Clin Psychopharmacol 26:385–388.Crossref, Google Scholar
Ghaemi SN, Gilmer WS, Goldberg JF, Zablotsky B, Kemp DE, Kelley ME, et al. 2007. Divalproex in the treatment of acute bipolar depression: a preliminary double-blind, randomized, placebo-controlled pilot study. J Clin Psychiatry 68:1840–1844.Crossref, Google Scholar
Ghaemi NS, Shirzadi AA, Filkowski M. 2008a. Publication bias and the pharmaceutical industry: the case of lamotrigine in bipolar disorder. Medscape J Med 10:211.Google Scholar
Ghaemi SN, Wingo AP, Filkowski MA, Baldessarini RJ. 2008b. Long-term antidepressant treatment in bipolar disorder: meta-analyses of benefits and risks. Acta Psychiatr Scand 118: 347–356.Crossref, Google Scholar
Gibbons RD, Brown CH, Hur K, Marcus SM, Bhaumik DK, Erkens JA, Herings RM, Mann JJ. 2007. Early evidence on the effects of regulators' suicidality warnings on SSRI prescriptions and suicide in children and adolescents. Am J Psychiatry 164:1356–1363.Crossref, Google Scholar
Gijsman HJ, Geddes JR, Rendell JM, Nolen WA, Goodwin GM. 2004. Antidepressants for bipolar depression: a systematic review of randomized, controlled trials. Am J Psychiatry 161:1537–1547.Crossref, Google Scholar
Goldberg JF, Burdick KE, Endick CJ. 2004. Preliminary randomized, double-blind, placebo-controlled trial of pramipexole added to mood stabilizers for treatment-resistant bipolar depression. Am J Psychiatry 161:564–566.Crossref, Google Scholar
Goldberg JF, Perlis RH, Ghaemi SN, Calabrese JR, Bowden CL,Wisniewski S, et al. 2007. Adjunctive antidepressant use and symptomatic recovery among bipolar depressed patients with concomitant manic symptoms: findings from the STEP-BD. Am J Psychiatry 164:1348–1355.Crossref, Google Scholar
Goldberg JF, Brooks JO III, Kurita K, Hoblyn JC, Ghaemi SN, Perlis RH, et al. 2009a. Depressive illness burden associated with complex polypharmacy in patients with bipolar disorder: findings from the STEP-BD. J Clin Psychiatry 70:155–162.Crossref, Google Scholar
Goldberg JF, Perlis RH, Bowden CL, Thase ME, Miklowitz DJ, Marangell LB, Calabrese JR, et al. 2009b. Manic symptoms during depressive episodes in 1,380 patients with bipolar disorder: findings from the STEP-BD. Am J Psychiatry 166:173–181.Crossref, Google Scholar
Gonzalez-Pinto A, Gonzalez C, Enjuto S, Fernandez dC, Lopez P, Palomo J, et al. 2004. Psychoeducation and cognitive-behavioral therapy in bipolar disorder: an update. Acta Psychiatr Scand 109:83–90.Crossref, Google Scholar
Goodnick PJ, Rush AJ, George MS, Marangell LB, Sackeim HA. 2001. Vagus nerve stimulation in depression. Expert Opin Pharmacother 2:1061–1063.Crossref, Google Scholar
Goodwin FK, Jamison KR. 2007. Manic-depressive illness. 2nd ed. New York: Oxford University Press.Google Scholar
Goodwin FK, Murphy DL, Dunner DL, Bunney WE. 1972.Lithium response in unipolar versus bipolar depression. Am J Psychiatry 129:44–47.Crossref, Google Scholar
Goodwin GM, Bowden CL, Calabrese JR, Grunze H, Kasper S, White R, et al. 2004. A pooled analysis of 2 placebo-controlled 18-month trials of lamotrigine and lithium maintenance in bipolar I disorder. J Clin Psychiatry 65:432–441.Crossref, Google Scholar
Goodwin GM, Anderson I, Arango C, Bowden CL, Henry C,Mitchell PB, et al. 2008. ECNP consensus meeting. Bipolar Depression. Nice, March 2007. Eur Neuropsychopharmacol 18:535–549.Crossref, Google Scholar
Grunze H. 2003. Lithium in the acute treatment of bipolar disorders-a stocktaking. Eur Arch Psychiatry Clin Neurosci 253:115–119.Crossref, Google Scholar
Grunze H. 2006. Carbamazepine, other anticonvulsants and augmenting agents. In: Akiskal HS, Tohen M, editors. Bipolar psychopharmacotherapy: Caring for the patient. London: John Wiley & Sons. p 63–84.Google Scholar
Grunze H, Walden J. 2002. Relevance of new and newly rediscovered anticonvulsants for atypical forms of bipolar disorder. J Affect Disord 72(Suppl 1):15–21.Crossref, Google Scholar
Grunze H, Kasper S, Goodwin G, Bowden CL, Baldwin D, Licht RW, Vieta E, Möller H-J, WFSBP Task Force on Treatment Guidelines for Bipolar Disorders. 2002b. The World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for the Biological Treatment of Bipolar Disorders. Part I: Treatment of Bipolar Depression. World J Biol Psychiatry 3: 115–124.Crossref, Google Scholar
Grunze H, Vieta E, Goodwin GM, Bowden C, Licht RW, Moller HJ, et al. 2009. The World Federation of Societies of Biological Psychiatry (WFSBP) Guidelines for the Biological Treatment of Bipolar Disorders: Update 2009 on the Treatment of Acute Mania. World J Biol Psychiatry 10:85–116.Crossref, Google Scholar
Grunze HC. 2008a. Switching, induction of rapid cycling, and increased suicidality with antidepressants in bipolar patients: fact or overinterpretation? CNS Spectr 13:790–795.Crossref, Google Scholar
Grunze HC. 2008b, Switching, induction of rapid cycling, and increased suicidality with antidepressants in bipolar patients: fact or overinterpretation? CNS Spectr 13:790–795.Crossref, Google Scholar
Gyulai L, Bowden CL, McElroy SL, Calabrese JR, Petty F, Swann AC, et al. 2003. Maintenance efficacy of divalproex in the prevention of bipolar depression. Neuropsychopharmacology 28:1374–1382.Crossref, Google Scholar
Hallam KT, Smith DI, Berk M. 2009. Differences between subjective and objective assessments of the utility of Electrocon-vulsive therapy in patients with bipolar and unipolar depression. J Affect Disord 112:212–218.Crossref, Google Scholar
Hamilton M. 1967. Development of a rating scale for primary depressive illness. Br J Soc Clin Psychol 6:278–296.Crossref, Google Scholar
Heres S, Davis J, Maino K, Jetzinger E, Kissling W, Leucht S. 2006. Why olanzapine beats risperidone, risperidone beats quetiapine, and quetiapine beats olanzapine: an exploratory analysis of head-to-head comparison studies of second-generation antipsychotics. Am J Psychiatry 163:185–194.Crossref, Google Scholar
Himmelhoch JM, Fuchs CZ, Symons BJ. 1982. A double-blind study of tranylcypromine treatment of major anergic depression. J Nerv Ment Dis 170:628–634.Crossref, Google Scholar
Himmelhoch JM, Thase ME, Mallinger AG, Houck P. 1991.Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry 148:910–916.Crossref, Google Scholar
Hirschfeld RM, Fochtmann LJ, McIntyre JS. 2005. Antidepressants for bipolar depression. Am J Psychiatry 162:1546–1547.Crossref, Google Scholar
Hurley SC. 2002. Lamotrigine update and its use in mood disorders. Ann Pharmacother 36:860–873.Crossref, Google Scholar
Jensen NH, Rodriguiz RM, Caron MG, Wetsel WC, Rothman RB,Roth BL. 2008. N-Desalkylquetiapine, a potent norepinephrine reuptake inhibitor and partial 5-HT1A agonist, as a putative mediator of quetiapine's antidepressant activity. Neuropsy-chopharmacology 33:2303–2312.Crossref, Google Scholar
Jon DI, Bahk WM, Yoon BH, Shin YC, Cho HS, Lee E, et al. 2008. Revised Korean medication algorithm for bipolar disorder. World J Biol Psychiatry 10:1–10.Google Scholar
Jones S. 2004. Psychotherapy of bipolar disorder: a review. J Affect Disord 80:101–114.Crossref, Google Scholar
Judd LL, Akiskal HS, Schettler PJ, Endicott J, Maser J, Solomon DA, et al. 2002. The long-term natural history of the weekly symptomatic status of bipolar I disorder. Arch Gen Psychiatry 59:530–537.Crossref, Google Scholar
Judd LL, Schettler PJ, Akiskal HS, Coryell W, Leon AC, Maser JD, Solomon DA. 2008. Residual symptom recovery from major affective episodes in bipolar disorders and rapid episode relapse/recurrence. Arch Gen Psychiatry 65: 386–394.Crossref, Google Scholar
Kantrowitz JT, Citrome L. 2008. Olanzapine: review of safety 2008. Expert Opin Drug Saf 7:761–769.Crossref, Google Scholar
Kasper S, Calabrese JR, Johnson G, Tajima O, Vieta E, Viguera AC, et al. 2008. International Consensus Group on the evidence-based pharmacologic treatment of bipolar I and II depression. J Clin Psychiatry 69:1632–1646.Crossref, Google Scholar
Keck PE, Corya SA, Altshuler LL, Ketter TA, McElroy SL, Case M, et al. 2005. Analyses of treatment-emergent mania with olanzapine/fluoxetine combination in the treatment of bipolar depression. J Clin Psychiatry 66:611–616.Crossref, Google Scholar
Keck PE, Mintz J, McElroy SL, Freeman MP, Suppes T,Frye MA, et al. 2006. Double-blind, randomized, placebo-controlled trials of ethyl-eicosapentanoate in the treatment of bipolar depression and rapid cycling bipolar disorder. Biol Psychiatry 60:1020–1022.Crossref, Google Scholar
Kirsch I, Deacon BJ, Huedo-Medina TB, Scoboria A, Moore TJ,Johnson BT. 2008. Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration. PLoS Med 5:e45.Crossref, Google Scholar
Kupfer DJ, Frank E, Grochocinski VJ, Luther JF, Houck PR,Swartz HA, Mallinger AG. 2000. Stabilization in the treatment of mania, depression and mixed states. Acta Neuropsychiatr 12:3–114.Crossref, Google Scholar
Kupka RW, Altshuler LL, Nolen WA, Suppes T, Luckenbaugh DA, Leverich GS, et al. 2007. Three times more days depressed than manic or hypomanic in both bipolar I and bipolar II disorder. Bipolar Disord 9:531–535.Crossref, Google Scholar
Lader MH. 1996. Tolerability and safety: essentials in antidepressant pharmacotherapy. J Clin Psychiatry 57(Suppl 2):39–44.Google Scholar
Lambert PA. 1984. Acute and prophylactic therapies of patients with affective disorders using valpromide (dipropylacetamide). In: Emrich HM, Okuma T, Müller AA, editors. Anticonvulsants in affective disorders. Amsterdam, Oxford, Princeton: Elsevier Science Publishers. p 33–44.Google Scholar
Leverich GS, Altshuler LL, Frye MA, Suppes T, McElroy SL,Keck PE, J, et al. 2006. Risk of switch in mood polarity to hypomania or mania in patients with bipolar depression during acute and continuation trials of venlafaxine, sertraline, and bupropion as adjuncts to mood stabilizers. Am J Psychiatry 163:232–239.Crossref, Google Scholar
Leverich GS, Post RM, Keck PE, Altshuler LL, Frye MA, Kupka RW, et al. 2007. The poor prognosis of childhood-onset bipolar disorder. J Pediatr 150:485–490.Crossref, Google Scholar
Lewis JL, Winokur G. 1982. The induction of mania. A natural history study with controls. Arch Gen Psychiatry 39:303–306.Crossref, Google Scholar
Lexchin J, Light DW. 2006. Commercial influence and the content of medical journals. Br Med J 332:1444–1447.Crossref, Google Scholar
Lexchin J, Bero LA, Djulbegovic B, Clark O. 2003. Pharmaceutical industry sponsorship and research outcome and quality: systematic review. Br Med J 326:1167–1170.Crossref, Google Scholar
Licht RW, Vestergaard P, Kessing LV, Larsen JK, Thomsen PH. 2003. Psychopharmacological treatment with lithium and antiepileptic drugs: suggested guidelines from the Danish Psychiatric Association and the Child and Adolescent Psychiatric Association in Denmark. Acta Psychiatr Scand Suppl 1–22.Google Scholar
Licht RW, Qvitzau S, Allerup P, Bech P. 2005. Validation of the Bech-Rafaelsen Melancholia Scale and the Hamilton Depression Scale in patients with major depression; is the total score a valid measure of illness severity? Acta Psychiatr Scand 111: 144–149.Crossref, Google Scholar
Licht RW, Gijsman H, Nolen WA, Angst J. 2008. Are antidepressants safe in the treatment of bipolar depression? A critical evaluation of their potential risk to induce switch into mania or cycle acceleration. Acta Psychiatr Scand 118:337–346.Crossref, Google Scholar
Lieberman JA, Stroup TS, McEvoy JP, Swartz MS, Rosenheck RA, Perkins DO, et al. 2005. Effectiveness of antipsychoticdrugs in patients with chronic schizophrenia. New Engl J Med 353:1209–1223.Crossref, Google Scholar
Macedo-Soares MB, Moreno RA, Rigonatti SP, Lafer B. 2005. Efficacy of electroconvulsive therapy in treatment-resistant bipolar disorder: a case series. J ECT 21:31–34.Crossref, Google Scholar
Maj M, Pirozzi R, Magliano L, Bartoli L. 2003. Agitated depression in bipolar I disorder: prevalence, phenomenology, and outcome. Am J Psychiatry 160:2134–2140.Crossref, Google Scholar
Marangell LB, Dennehy EB, Miyahara S, Wisniewski SR, Bauer MS, Rapaport MH, et al. 2009. The functional impact of sub-syndromal depressive symptoms in bipolar disorder: Data from STEP-BD. J Affect Disord 114:58–67.Crossref, Google Scholar
Masan PS. 2004. Atypical antipsychotics in the treatment of affective symptoms: a review. Ann Clin Psychiatry 16:3–13.Crossref, Google Scholar
Matkowski K, Rybakowski J. 1992. Karbamazepina w leczeniu chorob depresyjnych. Psychiatr Pol 26:251–258.Google Scholar
McAllister-Williams RH. 2008. Do antidepressants work? A commentary on “Initial severity and antidepressant benefits: a meta-analysis of data submitted to the Food and Drug Administration” by Kirsch et al. Evid Based Ment Health 11:66–68.Crossref, Google Scholar
McCormack PL, Wiseman LR. 2004. Olanzapine: a review of its use in the management of bipolar I disorder. Drugs 64: 2709–2726.Crossref, Google Scholar
McElroy SL, Suppes T, Keck PE, Black D, Frye MA, Altshuler LL, et al. 2005. Open-label adjunctive zonisamide in the treatment of bipolar disorders: a prospective trial. J Clin Psychiatry 66:617–624.Crossref, Google Scholar
McElroy SL, Young AH, Carlsson A, Olausson B, Nordenhem A,Paulsson B, Brecher M. 2008. A double-blind, placebo-controlled study with acute and continuation phase of quetiapine and paroxetine in adults with bipolar deprsssion (EMBOLDEN II). Bipolar Disord 10(Suppl 1):59.Google Scholar
McIntyre RS, Mancini DA, Parikh S, Kennedy SH. 2001. Lithium revisited. Can J Psychiatry 46:322–327.Crossref, Google Scholar
McIntyre RS, Mancini DA, McCann S, Srinivasan J, Sagman D, Kennedy SH. 2002. Topiramate versus bupropion SR when added to mood stabilizer therapy for the depressive phase of bipolar disorder: a preliminary single-blind study. Bipolar Disord 4:207–213.Crossref, Google Scholar
Meador KJ, Baker GA, Browning N, Clayton-Smith J, CombsCantrell DT, Cohen M, et al. 2009. Cognitive function at 3 years of age after fetal exposure to antiepileptic drugs. New Engl J Med 360:1597–1605.Crossref, Google Scholar
Mendlewicz J, Youdim MB. 1980. Antidepressant potentiation of 5-hydroxytryptophan by L-deprenil in affective illness. J Affect Disord 2:137–146.Crossref, Google Scholar
Miklowitz DJ, Otto MW. 2007. Psychosocial interventions for bipolar disorder: a review of literature and introduction of the systematic treatment enhancement program. Psychopharmacol Bull 40:116–131.Google Scholar
Miklowitz DJ, Frank E, George EL. 1996. New psychosocial treatments for the outpatient management of bipolar disorder. Psychopharmacol Bull 32:613–621.Google Scholar
Miklowitz DJ, Otto MW, Frank E, Reilly-Harrington NA, Kogan JN, Sachs GS, Thase ME, Calabrese JR, Marangell LB, Ostacher MJ, Patel J, Thomas MR, Araga M, Gonzalez JM, Wisniewski SR. 2007. Intensive psychosocial intervention enhances functioning in patients with bipolar depression: results from a 9-month randomized controlled trial. Am J Psychiatry 164:1340–1347.Crossref, Google Scholar
Mitchell PB, Malhi GS. 2004. Bipolar depression: phenomenological overview and clinical characteristics. Bipolar Disord 6:530–539.Crossref, Google Scholar
Mitchell PB, Goodwin GM, Johnson GF, Hirschfeld RM. 2008. Diagnostic guidelines for bipolar depression: a probabilistic approach. Bipolar Disord 10:144–152.Crossref, Google Scholar
Möller HJ. 2008. Isn't the efficacy of antidepressants clinically relevant? A critical comment on the results of the metaanalysis by Kirsch et al. 2008. Eur Arch Psychiatry Clin Neurosci 258:451–455.Crossref, Google Scholar
Möller HJ. 2009. Standardised rating scales in Psychiatry: Methodological basis, their possibilities and limitations and descriptions of important rating scales. World J Biol Psychiatry 10:6–26.Crossref, Google Scholar
Möller HJ, Berzewski H, Eckmann F, Gonzalves N, Kissling W, Knorr W, et al. 1993. Double-blind multicenter study of paroxetine and amitriptyline in depressed inpatients. Pharmacopsychiatry 26:75–78.Crossref, Google Scholar
Möller HJ, Bottlender R, Grunze H, Strauss A, Wittmann J. 2001. Are antidepressants less effective in the acute treatment of bipolar I compared to unipolar depression? J Affect Disord 67:141–146.Crossref, Google Scholar
Möller HJ, Grunze H, Broich K. 2006. Do recent efficacy data on the drug treatment of acute bipolar depression support the position that drugs other than antidepressants are the treatment of choice? A conceptual review. Eur Arch Psychiatry Clin Neurosci 256:1–16.Crossref, Google Scholar
Moller HJ, Baldwin DS, Goodwin G, Kasper S, Okasha A, Stein DJ, Tandon R, Versiani M. 2008. Do SSRIs or antidepressants in general increase suicidality? WPA Section on Pharmacopsychiatry: consensus statement. Eur Arch Psychiatry Clin Neurosci 258 Suppl 3:3–23.Google Scholar
Montgomery SA, Asberg M. 1979. A new depression scale designed to be sensitive to change. Br J Psychiatry 134:382–389.Crossref, Google Scholar
Montgomery SA, Baldwin DS, Blier P, Fineberg NA, Kasper S, Lader M, Lam RW, Lepine JP, Moller HJ, Nutt DJ, Rouillon F, Schatzberg AF, Thase ME. 2007. Which antidepressants have demonstrated superior efficacy? A review of the evidence. Int Clin Psychopharmacol 22:323–329.Crossref, Google Scholar
Morrow J, Russell A, Guthrie E, Parsons L, Robertson I, Waddell R, Irwin B, McGivern RC, Morrison PJ, Craig J. 2006. Malformation risks of antiepileptic drugs in pregnancy: a prospective study from the UK Epilepsy and Pregnancy Register. J Neurol Neurosurg Psychiatry 77:193–198.Crossref, Google Scholar
Müller-Oerlinghausen B, Ahrens B, Felber W. 2006. The suicide-preventive and mortality reducing effect of lithium. In: Bauer M, Grof P, Müller-Oerlinghausen B (eds) Lithium in Neuropsychiatry: The Comprehensive Guide. Informa Healthcare, Oxfordshire, UK: pp 179–192.Google Scholar
Muzina DJ, Ganocy SJ, Khalife S, and Gao K. A double-blind, placebo-controlled study of divalproex extended-release in newly-diagnosed mood stabilizer naive patients with acute bipolar depression.
Nahas Z, Kozel FA, Li X, Anderson B, George MS. 2003. Left prefrontal transcranial magnetic stimulation (TMS) treatment of depression in bipolar affective disorder: a pilot study of acute safety and efficacy. Bipolar Disord 5:40–47.Crossref, Google Scholar
National Collaborating Centre for Mental Health (2006) Bipolar disorder. The management of bipolar disorder in adults, children and adolescents, in primary and secondary care. CG38: NICE Guideline. Electronic Citation National Institute for Health and Clinical Excellence.Google Scholar
Nemeroff CB, Evans DL, Gyulai L, Sachs GS, Bowden CL, Gergel IP, Oakes R, Pitts CD. 2001. Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression. Am J Psychiatry 158:906–912.Crossref, Google Scholar
Neumann J, Seidel K, Wunderlich H-P. 1984. Comparative studies of the effect of carbamazepine and trimipramine in depression. In: Emrich HM, Okuma T, Müller AA (eds) Anticonvulsants in affective disorders. Elsevier Science, Amsterdam: pp 160–166.Google Scholar
Ng F, Dodd S, Berk M. 2007. The effects of physical activity in the acute treatment of bipolar disorder: a pilot study. J Affect Disord 101:259–262.Crossref, Google Scholar
Nierenberg AA, Ostacher MJ, Calabrese JR, Ketter TA, Marangell LB, Miklowitz DJ, Miyahara S, Bauer MS, Thase ME, Wisniewski SR, Sachs GS. 2006. Treatment-resistant bipolar depression: a STEP-BD equipoise randomized effectiveness trial of antidepressant augmentation with lamotrigine, inositol, or risperidone. Am J Psychiatry 163:210–216.Crossref, Google Scholar
Nolen WA, Kupka RW, Hellemann G, Frye MA, Altshuler LL, Leverich GS, Suppes T, Keck PE, Jr., McElroy S, Grunze H, Mintz J, Post RM. 2007. Tranylcypromine vs. lamotrigine in the treatment of refractory bipolar depression: a failed but clinically useful study. Acta Psychiatr Scand 115: 360–365.Crossref, Google Scholar
Nolen WA, Kupka RW, Schulte PFJ, Knoppert-van der Klein EAM, Honig A, Reichart CG, Goossens PJJ, Daemen P, Ravelli DP. 2008. Richtlijn bipolaire stoornissen. 2 ed. De Tijdstrom uitgerverij BV, Utrecht.Google Scholar
O'Donovan C, Gamham JC, Hajek T, Alda M. 2008. Antidepressant monotherapy in pre-bipolar depression: Predictive value and inherent risk. J Affect Disord 107:293–298.Crossref, Google Scholar
Parker G, Tully L, Olley A, Hadzi-Pavlovic D. 2006. SSRIs as mood stabilizers for Bipolar II Disorder? A proof of concept study. J Affect Disord 92:205–214.Crossref, Google Scholar
Peretti S, Judge R, Hindmarch I. 2000. Safety and tolerability considerations: tricyclic antidepressants vs. selective serotonin reuptake inhibitors. Acta Psychiatr Scand Suppl 403:17–25.Google Scholar
Perlis RH, Perlis CS, Wu Y, Hwang C, Joseph M, Nierenberg AA. 2005. Industry sponsorship and financial conflict of interest in the reporting of clinical trials in psychiatry. Am J Psychiatry 162:1957–1960.Crossref, Google Scholar
Perlis RH, Brown E, Baker RW, Nierenberg AA. 2006. Clinical features of bipolar depression versus major depressive disorder in large multicenter trials. Am J Psychiatry 163:225–231.Crossref, Google Scholar
Perugi G, Toni C, Frare F, Ruffolo G, Moretti L, Torti C, Akiskal HS. 2002. Effectiveness of adjunctive gabapentin in resistant bipolar disorder: is it due to anxious-alcohol abuse comorbidity? J Clin Psychopharmacol 22:584–591.Crossref, Google Scholar
Pichot P. 1995. The birth of the bipolar disorder. Eur Psychiatry 1–10.Google Scholar
Post RM, Leverich GS, Altshuler LL, Frye MA, Suppes TM, Keck PE, et al. 2003. An overview of recent findings of the Stanley Foundation Bipolar Network (Part I). Bipolar Disord 5:310–319.Crossref, Google Scholar
Post RM, Altshuler LL, Leverich GS, Frye MA, Nolen WA,Kupka RW, et al. 2006. Mood switch in bipolar depression: comparison of adjunctive venlafaxine, bupropion and sertraline. Br J Psychiatry 189:124–131.Crossref, Google Scholar
Quitkin FM, Kane J, Rifkin A, Ramos-Lorenzi JR, Nayak DV.1981. Prophylactic lithium carbonate with and without imipramine for bipolar 1 patients. A double-blind study. Arch Gen Psychiatry 38:902–907.Crossref, Google Scholar
Riemann D, Voderholzer U, Berger M. 2002. Sleep and sleep-wake manipulations in bipolar depression. Neuropsychobiology 45(Suppl 1):13–19.Crossref, Google Scholar
Rihmer Z. 2005. Prediction and prevention of suicide in bipolar disorders. Clin Neuropsychiatry 2:48–54.Google Scholar
Rihmer Z, Akiskal H. 2006. Do antidepressants t(h)reat(en) depressives? Toward a clinically judicious formulation of the antidepressant-suicidality FDA advisory in light of declining national suicide statistics from many countries. J Affect Disord 94:3–13.Crossref, Google Scholar
Royal Australian and New Zealand College of Psychiatrists Clinical Practice Guidelines Team for Bipolar Disorder. 2004. Australian and New Zealand clinical practice guidelines for the treatment of bipolar disorder. Aust NZ J Psychiatry 38: 280–305.Crossref, Google Scholar
Rush AJ, Giles DE, Schlesser MA, Fulton CL, Weissenburger J, Burns C. 1986. The Inventory for Depressive Symptomatology (IDS): preliminary findings. Psychiatry Res 18:65–87.Crossref, Google Scholar
Rush AJ, George MS, Sackeim HA, Marangell LB, Husain MM, Giller C, et al. 2000. Vagus nerve stimulation (VNS) for treatment-resistant depressions: a multicenter study. Biol Psychiatry 47:276–286.Crossref, Google Scholar
Sachs G, Collins MA, Altshuler L, Ketter T, Suppes T, Rasgon N, et al. 2002. Divalproex sodium versus placebo for the treatment of bipolar depression. APA 2002 Syllabus & Proceedings Summary.Google Scholar
Sachs G, Lombardo I, Yang R, Kremer C, Pappadopulos E. 2009. Learnings from the ziprasidone bipolar depression program.
Sachs GS, Lafer B, Stoll AL, Banov M, Thibault AB, Tohen M, Rosenbaum JF. 1994. A double-blind trial of bupropion versus desipramine for bipolar depression. J Clin Psychiatry 55: 391–393.Google Scholar
Sachs GS, Thase ME, Otto MW, Bauer M, Miklowitz D, Wisniewski SR, et al. 2003. Rationale, design, and methods of the systematic treatment enhancement program for bipolar disorder (STEP-BD). Biol Psychiatry 53:1028–1042.Crossref, Google Scholar
Sachs GS, Nierenberg AA, Calabrese JR, Marangell LB, Wisniewski SR, Gyulai L, et al. 2007. Effectiveness of adjunctive antidepressant treatment for bipolar depression. New Engl J Med 356:1711–1722.Crossref, Google Scholar
Sackeim HA, Haskett RF, Mulsant BH, Thase ME, Mann JJ, Pettinati HM, Greenberg RM, et al. 2001. Continuation pharmacotherapy in the prevention of relapse following electroconvulsive therapy: a randomized controlled trial. J Am Med Assoc 285:1299–1307.Crossref, Google Scholar
Sartorius N, Baghai TC, Baldwin DS, Barrett B, Brand U, Fleischhacker W, et al. 2007. Antidepressant medications and other treatments of depressive disorders: a CINP Task Force report based on a review of evidence. Int J Neuropsychopharmacol 10(Suppl 1):1–207.Google Scholar
Schaffer A, Zuker P, Levitt A. 2006. Randomized, double-blind pilot trial comparing lamotrigine versus citalopram for the treatment of bipolar depression. J Affect Disord 96: 95–99.Crossref, Google Scholar
Severus WE, Kleindienst N, Evoniuk G, Bowden C, Moller HJ, Bohus M, et al. 2009. Is the polarity of relapse/recurrence in bipolar-I disorder patients related to serum lithium levels? Results from an empirical study. J Affect Disord 115: 466–470.Crossref, Google Scholar
Silverstone T. 2001. Moclobemide vs. imipramine in bipolar depression: a multicentre double-blind clinical trial. Acta Psychiatr Scand 104:104–109.Crossref, Google Scholar
Silverstone PH, Silverstone T. 2004. A review of acute treatments for bipolar depression. Int Clin Psychopharmacol 19:113–124.Crossref, Google Scholar
Simon GE, Savarino J, Operskalski B, Wang PS. 2006. Suicide risk during antidepressant treatment. American Journal of Psychiatry 163:41–47.Crossref, Google Scholar
Simon NM, Otto MW, Weiss RD, Bauer MS, Miyahara S, Wisniewski SR, et al. 2004. Pharmacotherapy for bipolar disorder and comorbid conditions: baseline data from STEP-BD. J Clin Psychopharmacol 24:512–520.Crossref, Google Scholar
Small JG. 1990. Anticonvulsants in affective disorders. Psychopharmacol Bull 26:25–36.Google Scholar
Sokolski KN, Denson TF. 2003. Adjunctive quetiapine in bipolar patients partially responsive to lithium or valproate. Prog Neuropsychopharmacol Biol Psychiatry 27:863–866.Crossref, Google Scholar
Spina E, Pisani F, Perucca E. 1996. Clinically significant pharmacokinetic drug interactions with carbamazepine. An update. Clin Pharmacokinet 31:198–214.Crossref, Google Scholar
Suppes, T. 2008. Effectiveness of the new extended release formulation of quetiapine as monotherapy for the treatment of acute bipolar depression (trial D144CC00002).
Suppes T, Kelly DI, Keck PE, McElroy SL, Altshuler LL, Mintz J, et al. 2007. Quetiapine for the continuation treatment of bipolar depression: naturalistic prospective case series from the Stanley Bipolar Treatment Network. Int Clin Psychopharmacol 22:376–381.Crossref, Google Scholar
Suppes T, Hirschfeld RM, Vieta E, Raines S, Paulsson B. 2008. Quetiapine for the treatment of bipolar II depression: Analysis of data from two randomized, double-blind, placebo-controlled studies. World J Biol Psychiatry 9:198–211.Crossref, Google Scholar
Thase ME, Macfadden W, Weisler RH, Chang W, Paulsson B, Khan A, Calabrese JR. 2006. Efficacy of quetiapine monotherapy in bipolar I and II depression: a double-blind, placebo-controlled study (the BOLDER II study). J Clin Psychopharmacol 26:600–609.Crossref, Google Scholar
Thase ME, Jonas A, Khan A, Bowden CL, Wu X, McQuade RD, et al. 2008. Aripiprazole monotherapy in nonpsychotic bipolar I depression: results of 2 randomized, placebo-controlled studies. J Clin Psychopharmacol 28:13–20.Crossref, Google Scholar
Tohen M, Vieta E, Calabrese J, Ketter TA, Sachs G, Bowden C, et al. 2003. Efficacy of olanzapine and olanzapine-fluoxetine combination in the treatment of bipolar I depression. Arch Gen Psychiatry 60:1079–1088.Crossref, Google Scholar
Tohen M, Frank E, Bowden CL, Colom F, Ghaemi SN, Yatham LN, et al. 2009. The International Society for Bipolar Disorders (ISBD) Task Force report on the nomenclature of course and outcome in bipolar disorders. Bipolar Disord 11:453–473.Crossref, Google Scholar
Truman CJ, Goldberg JF, Ghaemi SN, Baldassano CF, Wisniewski SR, Dennehy EB, et al. 2007. Self-reported history of manic/hypomanic switch associated with antidepressant use: data from the Systematic Treatment Enhancement Program for Bipolar Disorder (STEP-BD). J Clin Psychiatry 68:1472–1479.Crossref, Google Scholar
Valenti M, Benabarre A, Bernardo M, Garcia-Amador M, Amann B, Vieta E. 2007. La terapia electroconsultiva en el tratamiento de la depresion bipolar. Actas Esp Psiquiatr 35:199–207.Google Scholar
van der Loos ML, Mulder PG, Hartong EG, Blom MB, Vergouwen AC, de Keyzer HJ, et al. 2009. Efficacy and safety of lamotrigine as add-on treatment to lithium in bipolar depression: a multicenter, double-blind, placebo-controlled trial. J Clin Psychiatry 70:223–231.Crossref, Google Scholar
Vieta E. 2005. The package of care for patients with bipolar depression. J Clin Psychiatry 66(Suppl 5):34–39.Crossref, Google Scholar
Vieta E. 2008. Antidepressants in bipolar depression. Acta Psychiatr Scand 118:335–336.Crossref, Google Scholar
Vieta E, Suppes T. 2008. Bipolar II disorder: arguments for and against a distinct diagnostic entity. Bipolar Disord 10:163–178.Crossref, Google Scholar
Vieta E, Martinez-Aran A, Nieto E, Colom F, Reinares M, Benabarre A, Gasto C. 2000. Adjunctive gabapentin treatment of bipolar disorder. Eur Psychiatry 15:433–437.Crossref, Google Scholar
Vieta E, Martinez-Aran A, Goikolea JM, Torrent C, Colom F, Benabarre A, Reinares M. 2002. A randomized trial comparing paroxetine and venlafaxine in the treatment of bipolar depressed patients taking mood stabilizers. J Clin Psychiatry 63:508–512.Crossref, Google Scholar
Vieta E, Calabrese JR, Goikolea JM, Raines S, Macfadden W. 2007. Quetiapine monotherapy in the treatment of patients with bipolar I or II depression and a rapid-cycling disease course: a randomized, double-blind, placebo-controlled study. Bipolar Disord 9:413–425.Crossref, Google Scholar
Visser HM, Van der Mast RC. 2005. Bipolar disorder, antidepressants and induction of hypomania or mania. A systematic review. World J Biol Psychiatry 6:231–241.Crossref, Google Scholar
Wehr TA, Goodwin FK. 1987. Can antidepressants cause mania and worsen the course of affective illness? Am J Psychiatry 144:1403–1411.Crossref, Google Scholar
Weissman MM. 1997. Interpersonal psychotherapy: current status. Keio J Med 46:105–110.Crossref, Google Scholar
Wilson MS, Findling RL. 2007. Zonisamide for bipolar depression. Expert Opin Pharmacother 8:111–113.Crossref, Google Scholar
Winokur G, Coryell W, Endicott J, Akiskal H. 1993. Further distinctions between manic-depressive illness (bipolar disorder) and primary depressive disorder (unipolar depression). Am J Psychiatry 150:1176–1181.Crossref, Google Scholar
Winsberg ME, DeGolia SG, Strong CM, Ketter TA. 2001. Divalproex therapy in medication-naive and mood-stabilizer-naive bipolar II depression. J Affect Disord 67:207–212.Crossref, Google Scholar
Woo YS, Chae JH, Jun TY, Kim KS, Bahk WM. 2008. The bipolar diathesis of treatment-resistant major depressive disorder. Int J Psychiaty Clin Practice 12:142–146.Crossref, Google Scholar
World Health Organization. 1992. The ICD-10 Classification of Mental and behavioural Disorders. Clinical Descriptions and Diagnostic Guidelines. Geneva: WHO.Google Scholar
Wu JC, Kelsoe JR, Schachat C, Bunney BG, Demodena A, Golshan S, et al. 2009. Rapid and sustained antidepressant response with sleep deprivation and chronotherapy in bipolar disorder. Biol Psychiatry 66:298–301.Crossref, Google Scholar
Xia G, Gajwani P, Muzina DJ, Kemp DE, Gao K, Ganocy SJ, Calabrese JR. 2008. Treatment-emergent mania in unipolar and bipolar depression: focus on repetitive transcranial magnetic stimulation. Int J Neuropsychopharmacol 11: 119–130.Crossref, Google Scholar
Yasmin S, Carpenter LL, Leon Z, Siniscalchi JM, Price LH. 2001. Adjunctive gabapentin in treatment-resistant depression: a retrospective chart review. J Affect Disord 63:243–247.Crossref, Google Scholar
Yatham LN, Srisurapanont M, Zis AP, Kusumakar V. 1997. Comparative studies of the biological distinction between unipolar and bipolar depressions. Life Sci 61:1445–1455.Crossref, Google Scholar
Yatham LN, Kennedy SH, O'Donovan C, Parikh SV, Macqueen G, McIntyre RS, et al. 2006. Canadian Network for Mood and Anxiety Treatments (CANMAT) guidelines for the management of patients with bipolar disorder: update 2007. Bipolar Disord 8:721–739.Crossref, Google Scholar
Young AH, Newham JI. 2006. Lithium in maintenance therapy for bipolar disorder. J Psychopharmacol 20:17–22.Crossref, Google Scholar
Young LT, Robb JC, Patelis S, I, MacDonald C, Joffe RT. 1997. Acute treatment of bipolar depression with gabapentin. Biol Psychiatry 42:851–853.Crossref, Google Scholar
Young LT, Joffe RT, Robb JC, MacQueen GM, Marriott M, Patelis-Siotis I. 2000. Double-blind comparison of addition of a second mood stabilizer versus an antidepressant to an initial mood stabilizer for treatment of patients with bipolar depression. Am J Psychiatry 157:124–126.Crossref, Google Scholar
Young AH, Carlsson A, Olausson B, Paulsson B, Brecher M. 2008. A double-blind, placebo-controlled study with acute and continuation phase of quetiapine and lithium in adults with bipolar depression (EmboldenI). Bipolar Disord 10(Suppl 1):451.Crossref, Google Scholar
Zarate CA, Payne JL, Singh J, Quiroz JA, Luckenbaugh DA, Denicoff KD, et al. 2004. Pramipexole for bipolar II depression: a placebo-controlled proof of concept study. Biol Psychiatry 56:54–60.Crossref, Google Scholar
Zaretsky AE, Segal ZV, Gemar M. 1999. Cognitive therapy for bipolar depression: a pilot study. Can J Psychiatry 44: 491–494.Crossref, Google Scholar
Zarin D, Pincus HA, McIntyre JS. 2002. APA Practice Guideline For The Treatment Of Patients With Bipolar Disorder. Electronic Citation http://www.psych.org/clin_res/pg_bipolar.cfmGoogle Scholar
Zhang ZJ, Kang WH, Tan QR, Li Q, Gao CG, Zhang FG, et al. 2007. Adjunctive herbal medicine with carbamazepine for bipolar disorders: A double-blind, randomized, placebo-controlled study. J Psychiatr Res 41:360–369.Crossref, Google Scholar
Zornberg GL, Pope HG. 1993. Treatment of depression in bipolar disorder: new directions for research. J Clin Psychopharmacol 13:397–408.Crossref, Google Scholar



