Vaccines in the Treatment of Substance Abuse
Substances of abuse exert their initial central effects by stimulation of reward and reinforcement pathways in the brain. Typically, this requires rapid entrance into the circulation, crossing the blood-brain barrier, and activation of one or more neurotransmitter systems (1). It is important to recognize that the addictive liability of a drug is, in part, related to the speed with which it is able to traverse the blood-brain barrier as well as its ability to stimulate certain (i.e., dopaminergic) neurotransmitter systems. Volkow et al. (2) demonstrated that route of administration determines intensity of subjective euphoria, because those substances of abuse that are delivered to the brain more rapidly are associated with increased levels of “high” experienced by the user, independent of levels of brain receptor occupancy or transporter blockade (2). Consequently, slowing the entry of drugs of abuse into the brain may be as effective as reducing the amount of substance in the brain, because it would allow for concurrent reductions in (behaviorally) reinforcing properties (1).
Conceptually, immunotherapy for SUDs involves both active and passive immunization. With active immunization, vaccines are administered to an individual, who subsequently mounts an immunological response against the agent. An immunological memory, in which reexposure to the vaccine (through booster injection) results in amplified response of the immune system, is also created. Once the immune system has been primed by administration of the vaccine, upon reintroduction of the substance of abuse into the system, the body will then produce an IgG-mediated antibody response and clear the toxin while it is in the periphery, thereby reducing the action of the drug in the CNS.
Drugs of abuse, because of their small size (molecular mass <1000 Da), do not typically result in the activation of the immune cascade. Thus, it is necessary to attach the drug hapten to a foreign carrier protein with immunogenicity, such as inactivated cholera vaccine or bovine albumin. Structurally, then, substance abuse vaccines are composed of the chemical structure of the drug along with another agent that is present in widely used (and available) vaccines.
The extent of the response to the vaccine can be measured by 1) receptor binding affinity and 2) concentration of antibody produced (3). Receptor binding affinity references the quality of the antibody produced, whereas the amount/concentration related to quantity. It is necessary to have a certain threshold amount of antibody produced, and to have a clinical benefit, these antibodies must have an affinity for the drug sufficient to bind the drug and reduce the amount of free drug available in the circulation. Under ideal circumstances, drug is bound by antibodies, and the resulting immune complexes are cleared before the central activation by the substance. Drugs of abuse readily cross the blood-brain barrier, whereas antibodies, which are comparatively larger molecules (molecular mass ∼150,000 Da), are unable to do so. This is an important factor, because it means that antibodies bound to substances of abuse cannot act centrally or stimulate target neurotransmitter systems. However, it is also important to note that simply slowing the progression of these substances across the blood-brain barrier can result in decreased activation of the CNS and limit the reward experienced by the user. Thus, even antibodies with weak affinity for the drug may be helpful, because they would transiently bind the substance and impede the rapid stimulation of the reward system.
Passive immunization involves the administration of exogenously produced antibodies to specific substances of abuse. Two general classes of antibodies exist, polyclonal and monoclonal. After immunization of an animal (i.e., rat, mouse, or goat), polyclonal antibodies are generated by multiple cell lines within the animal. The IgG molecules created as a result of the immune response are collected from the animal serum. Of note, polyclonal antibodies are used primarily within the context of animal studies, and there are no current approved uses in humans. Monoclonal antibodies originate from a single cell line, typically a hybrid of human and mouse (i.e., hybridoma). These cell lines are created within the laboratory and exposed to an antigen so that they might express IgG antibodies, which are then harvested for treatment. At present, monoclonal antibodies are being used for treatment of a wide variety of illnesses including malignancies and infectious diseases.
CURRENT STATUS OF IMMUNIZATION STRATEGIES
At present, clinical trials for both nicotine and cocaine vaccines are being conducted. Preclinical development of vaccines for methamphetamine, morphine, and phencyclidine has also been a focus. The status of each specific drug vaccine is discussed in Table 1.
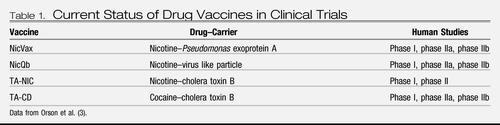 |
Table 1. Current Status of Drug Vaccines in Clinical Trials
Nicotine
Although the rates of cigarette smoking in the United States have declined over the past several years, nicotine dependence continues to represent a significant cause of morbidity and mortality. The health consequences of smoking have been well defined, and greater than 400,000 people die annually from illness related to smoking (4, 5). Despite high reported rates of desire to quit smoking and numerous quit attempts by those who smoke, the majority of persons who use nicotine remain dependent (6). Smoking cessation programs and widely available pharmacotherapeutic treatment options offer some benefit; however, the ultimate success rate for quitting remains low (7).
There are three nicotine conjugate vaccines currently undergoing clinical trials: NicVax, NicQb, and TA-NIC. NicVax, a nicotine hapten conjugated to Pseudomonas exoprotein A, is undergoing its second of two phase III clinical trials (8). In previous studies, it was found to produce antibody titers in a linear dose-response fashion sufficient to reduce smoking among participants. Interestingly, there was no observed increase in compensatory smoking to overcome the effect of the vaccine. Further, in those receiving the highest dose (200 μg), significantly greater 30-day abstinence rates were observed (9).
NicVax demonstrated a favorable safety profile (9). Local reactions to the vaccine, ache and tenderness being most commonly reported, were mild to moderate in severity in almost all events (99.5%). Systemic reactions most commonly reported include malaise, headache, and myalgia. Of importance, there were no significant differences between experimental and placebo groups in terms of reported systemic adverse events. Of the 68 subjects enrolled, 56 successfully completed the study and received all scheduled vaccinations. Dropout was due to consent withdrawal (six participants), noncompliance with protocol (one participant), loss to follow-up (three participants), and development of medical problems (two participants). Of note, the two medically related, serious adverse events were unrelated to the study drug.
NicQb, composed of a nicotine hapten similar to that used in NicVax conjugated with a virus-like particle formed by bacteriophage Qb, is currently being tested in phase IIB/III clinical trials (10). In previous trials, NicQb demonstrated an ability to produce a nicotine-specific antibody response as well as a favorable safety profile (11). During phase I study of NicQb, recipients of the vaccine demonstrated an immunologic response with development of nicotine-specific IgM antibodies at day 7 and nicotine-specific IgG antibodies at day 14 (11). Adverse events were characterized as either mild (93%) or moderate (7%), with participants most commonly reporting an injection-site reaction. During the phase II trial of NicQb, most adverse events were deemed mild to moderate in scale (95.7%). Pain at the injection site was the most commonly reported local reaction, with relatively little reporting of swelling, erythema, and edema. The most prominent systemic adverse event was characterized as “flu-like symptoms,” reported by 69.4% of vaccine recipients compared with 12.5% of the placebo group. In general, the development of these symptoms occurred within 2–12 hours after receipt of the vaccine and resolved within 24 hours. Other commonly reported adverse events in the vaccine group compared with the placebo group included pyrexia (42% versus 8%), headache (40% versus 27%), nasopharyngitis (32% versus 26%), rigors (13.5% versus 0%), and myalgia (13.5% versus 5%). Nine serious adverse events were reported; however, only one event was deemed to be treatment-related (e.g., flu-like symptoms associated with chest pain).
TA-NIC, a nicotine hapten conjugated with inactivated cholera toxin B, also demonstrated encouraging results. Subjects received four doses of the vaccine over the first 8 weeks of the trial and an additional booster injection at week 32. At the 12-month follow-up, cessation rates were significantly higher among those receiving the highest dose of the vaccine comparison with rates in the placebo group (38% versus 8%) (12). Of importance, during a double-blind placebo-controlled dose-escalation study of TA-NIC, no serious safety issues were identified (13).
Taken together, these vaccines represent a powerfully important clinical advance in the treatment of nicotine dependence, particularly because high antibody titers resulting from their use correlate with increased rates of smoking cessation (3).
Cocaine
Cocaine abuse and dependence have consistently been demonstrated to be a source of significant morbidity and mortality worldwide and a continuing public health concern. The medical, psychological, and social consequences of cocaine abuse have been extensively studied in an effort to characterize its impact on the public. Chronic drug use has been demonstrated to be associated with increased utilization of health care services (14). In the United States, cocaine use is associated with increased numbers of emergency room visits (14, 15) as well as hospital admissions (16). Cocaine, because of its direct toxicity, has been associated with medical complications affecting numerous organ systems including cardiovascular (17), neurological (18), renal (19), respiratory (20), and immunological (21). At present, there are no U.S. Food and Drug Administration (FDA) approved pharmacotherapeutic options for treatment of this debilitating illness, and, unfortunately, behavioral therapies have demonstrated low success rates (22).
The cocaine vaccine, TA-CD, is a cocaine hapten conjugated to inactivated cholera toxin B and is currently undergoing large-scale, multisite, phase IIb clinical testing. In the phase I trial to assess clinical safety and immunogenicity (N=34), the vaccine demonstrated an ability to elicit an immunological response in subjects (23). In those persons with an antibody response, there were noted reductions in the pleasurable effects from smoked cocaine (24, 25). Almost all recipients (33 of 34) reported local pain/tenderness after administration of the injection with no difference between groups (23). Treatment-related systemic adverse events occurring in all experimental groups and independent of vaccine dosage included tachycardia, elevated temperature (greatest mean increase of 1.9°F observed in the high-dose vaccine group), and hypertension.
In the phase IIa trial, the vaccine was well tolerated at two dose levels (100 μg×4 injections or 400 μg×5 injections), and subjects (N=18) developed cocaine-specific antibodies that persisted for at least 6 months (26). Of note, those receiving the higher dose of vaccine demonstrated a significantly higher mean antibody titer and were more likely to maintain abstinence compared with the low-dose group (24, 26). Sixteen of 18 subjects (89%) completed the vaccination series (24). There were no hospitalizations, deaths, or serious adverse events. No group differences were found for the most commonly reported adverse events (e.g., rhinitis, pharyngolaryngeal pain, nasal congestion, nausea, and vomiting) with the exception of headache, which was noted more frequently in the 400-μg group (nine of nine subjects) than in the 2000-μg group (one of seven subjects). The two subjects leaving the study received only one vaccination and did not complete the series; however, neither participant reported any adverse effects before discontinuation.
In the initial phase IIb trial of TA-CD in methadone-maintained individuals (N=115), the vaccine was found to produce substantial antibody response in one-third of the recipients. This high-antibody group demonstrated a higher percentage of cocaine-free urine samples (27). The safety profile of the vaccine also remained favorable. There were no differences between groups in regard to treatment-emergent adverse events or subject dropout (27). Of 115 subjects randomly assigned to receive either vaccine or placebo, 94 (82%) completed the study. Reasons cited for withdrawal from the study included incarceration and loss of housing due to cocaine use. All severe adverse effects were deemed to be unrelated to the vaccine but include various substance abuse-related consequences (i.e., cocaine-induced paranoia, alcoholic pancreatitis, and cellulitis from drug injection). Common adverse events reported among vaccine recipients compared with those receiving placebo included hot flashes (19% versus 12%), hyperhidrosis (15% versus 10%), nausea (14% versus 2%), and feeling cold (12% versus 7%).
Taken together, these findings suggest that not only are recipients of the vaccine able to mount an immunological response to the vaccine but also the antibodies created are sufficient to block the rewarding effects of cocaine that may lead to reduction in use of the substance. As with nicotine, the effects of the vaccine can be overcome by increasing the amount of cocaine used. Thus, it remains important to consider use of the vaccine in those persons who are motivated to abstain from drug use.
Phencyclidine
Although traditionally thought to be uncommon, phencyclidine (PCP) abuse represents a potential area of growing concern in the field of addiction. PCP, an arylcyclohexylamine, acts as an antagonist of the N-methyl-d-aspartate (NMDA) receptor. It has become increasingly more popular among select groups of substance abusers, as cigarettes or marijuana sticks are often laced with this drug (28). Although use of PCP in comparison with other substances like nicotine and cocaine is quite low, the medical and psychiatric impact of acute toxicity is considerable, and thus continued consideration should be given to this drug. Current treatment options for PCP abuse are limited to behavioral interventions, because there are no FDA-approved pharmacotherapy options available.
Interestingly, PCP was the first drug of abuse to be examined in studies of passive immunity. Purified goat anti-PCP polyclonal antibodies were found to “substantially alter” the pharmacokinetics of PCP in dogs, resulting in redistribution of PCP from the systemic circulation and organ systems and back into plasma (29). In rat studies, passively administered monoclonal antibodies were found to result in similar findings, with reduction of PCP levels in brain as well as other organs (i.e., lung, heart, and testis), while renal elimination was increased (30, 31). In additional animal studies, monoclonal anti-PCP antibodies have demonstrated the ability to block locomotor activity and ataxia associated with PCP use and reverse acute toxicity from high-dose PCP administration (30, 31, 32).
The production of high-affinity monoclonal antibodies with affinity for PCP that can be safely used in humans has been a growing focus of research. The murine anti-PCP monoclonal antibody, mAb6B5, demonstrated long-term reductions in PCP brain concentration and locomotor activity. A murine/human chimera of mAb6B5 was also tested and found to have similar effects (33). Chimeric monoclonal antibodies represent another important option for treatment of SUDs, particularly because medications of this kind have already been approved by the FDA for treatment of other disorders, as in the case of rituximab treatment of lymphoma (34)
Methamphetamine
Methamphetamine (METH) use in the United States peaked in 2005, with an estimated 1.3 million users; however, more recent estimates suggest there are slightly more than 500,000 current users of METH (35). Despite this decrease in the overall number of users, the devastating impact to the individual as well as to the community cannot be underestimated. METH exerts its mechanism of action through the indirect agonism of monoamine transporters, as well as the vesicular monoamine transporter-2 (VMAT-2). Acute administration of METH results in the reversal of function of the VMAT-2, which effectively causes the release of monoamines from storage vesicles into the cytosol of neurons. In addition, METH reverses the function of the monoamine transporters, causing the release of dopamine, norepinephrine, and serotonin into the synaptic cleft. As a result, these monoamines are available to stimulate postsynaptic receptors (36). METH is highly addictive, due in large part to its active metabolite (amphetamine) and long half-life, which result in sustained action within the CNS. Currently, there are no medications approved for its treatment, and a vaccine that could address not only METH, but also demonstrate cross-reactivity for amphetamine, would be a formidable agent in the treatment of this particularly destructive SUD.
The preclinical development of METH immunotherapy is an area of active research and potential future targets of treatment include METH-induced acute overdose, relapse prevention, and use in at-risk populations using the drug but not yet dependent (37). Passive immunotherapy with monoclonal antibodies, which has been demonstrated in several animal studies, is likely to be beneficial in those with drug overdose. Monoclonal antibodies have demonstrated safety when administered to mice and an ability to block the hemodynamic and locomotor alterations typically induced by METH (38). Monoclonal antibodies have also been found to reduce brain concentrations of METH, shifting the drug into the circulation as evidenced by increasing serum concentration (39). Furthermore, METH self-administration in rats was reduced in those receiving monoclonal antibodies, possibly suggesting a use for passive immunotherapy in relapse prevention as well (40). In addition, the development of active vaccination is currently ongoing, with testing of several combinations of METH haptens and carrier protein conjugates (37).
Heroin and morphine
In animal studies in the 1970s, administration of a morphine hapten conjugated with 6-hemisuccinyl-bovine serum albumin was found to result in the creation of anti-morphine antibodies, which reduced the concentration of the drug in plasma (in rats) and decreased self-administration of heroin (in rhesus monkey) (41, 42). However, the successful pharmacological management of opiate dependence with methadone and/or buprenorphine and its integration into the fabric of addiction treatment led to a slowing in the effort to create a vaccine for the treatment of heroin addiction. Years later, despite the availability of pharmacotherapy, opiate dependence remains a significant cause of morbidity and mortality in those with the illness. Increased rates of HIV and hepatitis C transmission, criminal involvement, and the general burden to governmental programs contribute to its societal consequences (43). Given the ongoing impact of opiate dependence, current consideration of immunotherapeutic approaches is warranted, because both active and passive immunization could be helpful in treatment of opiate overdose and relapse prevention.
In a recent study conducted in rats, a morphine/heroin vaccine created by conjugation of haptenized morphine to tetanus toxoid demonstrated significant immunogenicity with robust antibody production (44). Because the antibodies are unable to cross the blood-brain barrier, there is little chance for psychoactive effects or an impact on the endogenous opioidergic system. In this case, the creation of high-affinity antibodies with cross-reactivity for both morphine and heroin is also particularly important, because it will allow binding of both heroin and its metabolite (morphine). Ultimately, this novel vaccine may represent an exciting option for the treatment of opioid dependence.
CONCLUSION
Approaching substance abuse treatment with the principles of immunology in mind has allowed introduction of several novel pharmacological agents and treatment strategies. With immunotherapy, the ultimate goal is to block or reduce uptake of drug into the CNS and diminish its effects. Through both passive and active immunization strategies, the transit of drugs across the blood-brain barrier is substantially slowed and activation of reward and reinforcement is attenuated. The decrease in subjective euphoria experienced with use of the drug as well as the reduction in drug reinforcement or craving can greatly assist those individuals who are attempting to establish abstinence and/or maintain sobriety.
The efficacy of substance abuse vaccines can be determined by their ability to elicit production of antibodies in sufficient quantity and with adequate binding capacity for specific drug molecules. Animal and human studies of nicotine and cocaine vaccines show great promise; in addition, recent advances with creation of methamphetamine and heroin/morphine vaccines are intriguing. Animal studies of phencyclidine and methamphetamine monoclonal antibodies represent exciting areas of future discovery, because these agents have shown efficacy in reduction of both brain levels of the drug and toxic effects of these substances. Ultimately, these treatment strategies should complement behavioral therapy for substance use disorders to optimize management of these complex illnesses.
1 Kosten T, Owens SM: Immunotherapy for the treatment of drug abuse. Pharmacol Ther 2005; 108:76–85Crossref, Google Scholar
2 Volkow ND, Wang GJ, Fischman MW, Foltin R, Fowler JS, Franceschi D, Franceschi M, Logan, Gatley SJ, Wong C, Ding YS, Hitzemann R, Pappas N: Effects of route of administration on cocaine induced dopamine transporter blockade in the human brain. Life Sci 2000; 67:1507–1515Crossref, Google Scholar
3 Orson FM, Kinsey BM, Singh RA, Wu Y, Gardner T, Kosten TR: Substance abuse vaccines. Ann NY Acad Sci 2008; 1141:257–269Crossref, Google Scholar
4 Rehm J, Taylor B, Room R: Global burden of disease from alcohol, illicit drugs and tobacco. Drug Alcohol Rev 2006; 25:503–513Crossref, Google Scholar
5 Danaei G, Ding EL, Mozaffarian D, Taylor B, Rehm J, Murray CJ, Ezzati M: The preventable causes of death in the United States: comparative risk assessment of dietary, lifestyle, and metabolic risk factors. PLoS Med 2009; 6:e1000058Crossref, Google Scholar
6 Centers for Disease Control and Prevention: Cigarette smoking among adults—United States, 2000. Morb Mortal Wkly Rep 2002; 51:642–645. http://www.cdc.gov/mmwr/preview/mmwrhtml/mm5129a3.htmGoogle Scholar
7 Fiore MC, Bailey WC, Cohen SJ, Dorfman, SF, Goldstein MG, Gritz, ER, Heyman, RB, Jaen, CR, Kottke, TE, Lando HA, Mecklenburg, RE, Mullen PD, Nett LM, Robinson L, Stitzer ML, Tommasello TC, Villejo L, Wewers ME: Treating Tobacco Use and Dependence. Quick Reference Guide for Clinicians. Rockville, MD, US Department of Health and Human Services. Public Health Service, 2000.Google Scholar
8 Riedmann EM: Pivotal phase III study initiated. Hum Vaccin 2010; 6:430–435Google Scholar
9 Hatsukami DK, Rennard S, Jorenby D, Fiore M, Koopmeiners J, de Vos A, Horwith F, Pentel PR: Safety and immunogenicity of a nicotine conjugate vaccine in current smokers. Clin Pharmacol Ther 2005; 78:456–467Crossref, Google Scholar
10 Heading CE: Drug evaluation: CYT-002-NicQb, a therapeutic vaccine for the treatment of nicotine addiction. Curr Opin Investig Drugs 2007; 8:71–77Google Scholar
11 Maurer P, Jennings GT, Willers J, Rohner F, Lindman Y, Roubicek K, Renner WA, Muller P, Bachmann MF: A therapeutic vaccine for nicotine dependence: preclinical efficacy, and phase I safety and immunogenicity. Eur J Immun 2005; 35:2031–2040Crossref, Google Scholar
12 LeSage MG, Keyler DE, Pentel PR: Current status of immunologic approaches to treating tobacco dependence: vaccines and nicotine-specific antibodies. AAPS J 2006; 8:E65–E75Crossref, Google Scholar
13 St Clair Roberts J, Dobson J, Wood D, Settles M: Safety and immunogenicity of a human nicotine conjugate vaccine. Drug Alcohol Depend. 2002; 66:S148Google Scholar
14 French MT, McGeary KA, Chitwood DD, McCoy CB: Chronic illicit drug use, health services utilization and the cost of medical care. Soc Sci Med 2000; 50:1703–1713Crossref, Google Scholar
15 Vitale S, van de Mheen D: Illicit drug use and injuries: a review of emergency room studies. Drug Alcohol Depend 2006; 82:1–9Crossref, Google Scholar
16 Cherpitel: 2003;Google Scholar
17 Pozner CN, Levine M, Zane R: The cardiovascular effects of cocaine. J Emer Med 2005; 29:173–178Crossref, Google Scholar
18 Devlin RJ, Henry JA: Major consequences of illicit drug consumption. Crit Care 2008; 12:202–209Crossref, Google Scholar
19 Crowe AV, Howse M, Bell GM, Henry JA: Substance abuse and the kidney. Q J Med 2000; 93:147–152Crossref, Google Scholar
20 Perper JA, Van Thiel DH: Respiratory complications of cocaine abuse. Recent Dev Alcohol 1992; 10:363–377Crossref, Google Scholar
21 Cook JA, Burke-Miller JK, Cohen MH, Cook RL, Vlahov D, Wilson TE, Golub ET, Schwartz RM, Howard AA, Ponath C, Plankey MW, Grey DD: Crack cocaine, disease progression, and mortality in a multi-center cohort of HIV-1 positive women. AIDS 2008; 22:1355–1363Crossref, Google Scholar
22 Simpson DD, Joe GW, Fletcher BW, Hubbard RL, Anglin MD: A national evaluation of treatment outcomes for cocaine dependence. Arch Gen Psychiatry 1999; 56:507–514Crossref, Google Scholar
23 Kosten TR, Rosen M, Bond J, Settles M, St. Clair Roberts J, Shields J, Jack L, Fox B: Human therapeutic cocaine vaccine: safety and immunogenicity. Vaccine 2002; 20:1196–1204Crossref, Google Scholar
24 Martell BA, Mitchell E, Poling J, Gonsai K, Kosten TR: Vaccine pharmacotherapy for the treatment of cocaine dependence. Biol Psychiatry 2005; 58:158–164Crossref, Google Scholar
25 Haney M, Kosten TR: Therapeutic vaccines for substance dependence. Expert Rev Vaccines 2004; 3:11–18Crossref, Google Scholar
26 Orson FM, Kinsey BM, Singh RA, Wu Y, Kosten TR: Vaccines for cocaine abuse. Hum Vaccin 2009; 5:194–199Crossref, Google Scholar
27 Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, Kosten TR: Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double blind, placebo-controlled efficacy trial. Arch Gen Psychiatry 2009; 66:1116–1123Crossref, Google Scholar
28 Peters RJ, Williams M, Ross MW, Atkinson J, McCurdy SA: The use of fry (embalming fluid and PCP-laced cigarettes or marijuana sticks) among crack cocaine smokers. J Drug Educ 2008; 38:285–295Crossref, Google Scholar
29 Owens SM, Mayersohn M: Phencyclidine-specific Fab fragments alter phencyclidine disposition in dogs. Drug Metab Dispos 1986; 14:52–58Google Scholar
30 Valentine JL, Mayersohn M, Wessinger WD, Arnold LW, Owens SM: Antiphencyclidine monoclonal Fab fragments reverse phencyclidine-induced behavioral effects and ataxia in rats. J Pharmacol Exp Ther 1996; 278:709–716Google Scholar
31 Hardin JS, Wessinger WD, Proksch JW, Owens SM: Pharmacodynamics of a monoclonal antiphencyclidine Fab with broad selectivity for phencyclidine-like drugs. J Pharmacol Exp Ther 1998; 285:1113–1122Google Scholar
32 Valentine JL, Owens SM: Antiphencyclidine monoclonal antibody therapy significantly changes phencyclidine concentrations in brain and other tissues in rats. J Pharmacol Exp Ther 1996; 278:717–724Google Scholar
33 Lacy HM, Gunnell MG, Laurenzana EM, Owens SM: Engineering and characterization of a mouse/human chimeric anti-phencyclidine monoclonal antibody. Int Immunopharmacol 2008; 8:1–11Crossref, Google Scholar
34 Keating GM: Rituximab: a review of its use in chronic lymphocytic leukaemia, low-grade or follicular lymphoma and diffuse large B-cell lymphoma. Drugs 2010; 70:1445–1476Crossref, Google Scholar
35 Substance Abuse and Mental Health Services Administration: Results from the 2009 National Survey on Drug Use and Health: Volume I. Summary of National Findings. NSDUH Series H-38A, HHS Publication No. SMA 10-4856Findings. Rockville, MD, Office of Applied Studies, 2010Google Scholar
36 Cruikshank CC, Dyer KR: A review of the clinical pharmacology of methamphetamine. Addiction 2009; 104:1085–1099Crossref, Google Scholar
37 Gentry WB, Laurenzana EM, Williams DK, West JR, Berg RJ, Terlea T, Owens SM: Safety and efficacy of an anti-(+)-methamphetamine monoclonal antibody in the protection against cardiovascular and central nervous system effects of (+)-methamphetamine in rats. Int Immunopharmacol 2006; 6:968–977Crossref, Google Scholar
38 Gentry WB, Ruedi-Bettschen D, Owens SM: Development of active and passive human vaccines to treat methamphetamine addiction. Hum Vaccin 2009; 5:206–213Crossref, Google Scholar
39 Byrnes-Blake KA, Laurenzana EM, Landes RD, Gentry WB, Owens SM: Monoclonal IgG affinity and treatment time alters antagonism of (+)-methamphetamine effects in rats. Eur J Pharmacol 2005; 521:86–94Crossref, Google Scholar
40 McMillan DE, Hardwick WC, Li M, Gunnell MG, Carroll FI, Abraham P, Owens SM: Effects of murine-derived anti-methamphetamine monoclonal antibodies on (+)-methamphetamine self-administration in the rat. J Pharmacol Exp Ther 2004; 309:1248–1255Crossref, Google Scholar
41 Berkowitz B, Spector S: Evidence for active immunity to morphine in mice. Science 1972; 178:1290–1292Crossref, Google Scholar
42 Bonese KF, Wainer BH, Fitch FW, Rothberg RM, Schuster CR: Changes in heroin self-administration by a rhesus monkey after morphine immunization. Nature 1974; 252:708–710Crossref, Google Scholar
43 Anton B, Salazar A, Flores A, Matus M, Marin R, Hernandez JA, Leff P: Vaccines against morphine/heroin and its use as effective medication for preventing relapse to opiate addictive behaviors. Hum Vaccin 2009; 5:214–229Crossref, Google Scholar
44 Anton B, Leff P: A novel bivalent morphine/heroin vaccine that prevents relapse to heroin addiction in rodents. Vaccine 2006; 24:3232–3240Crossref, Google Scholar
45 Rose JE: Disrupting nicotine reinforcement: from cigarette to brain. Ann NY Acad Sci 2008; 1141:233–256Crossref, Google Scholar



