Posttraumatic Stress Disorder: A State-of-the-Science Review
Abstract
This article reviews the state-of-the-art research in posttraumatic stress disorder (PTSD) from several perspectives: (1) Sex differences: PTSD is more frequent among women, who tend to have different types of precipitating traumas and higher rates of comorbid panic disorder and agoraphobia than do men. (2) Risk and resilience: The presence of Group C symptoms after exposure to a disaster or act of terrorism may predict the development of PTSD as well as comorbid diagnoses. (3) Impact of trauma in early life: Persistent increases in CRF concentration are associated with early life trauma and PTSD, and may be reversed with paroxetine treatment. (4) Imaging studies: Intriguing findings in treated and untreated depressed patients may serve as a paradigm of failed brain adaptation to chronic emotional stress and anxiety disorders. (5) Neural circuits and memory: Hippocampal volume appears to be selectively decreased and hippocampal function impaired among PTSD patients. (6) Cognitive behavioral approaches: Prolonged exposure therapy, a readily disseminated treatment modality, is effective in modifying the negative cognitions that are frequent among PTSD patients. In the future, it would be useful to assess the validity of the PTSD construct, elucidate genetic and experiential contributing factors (and their complex interrelationships), clarify the mechanisms of action for different treatments used in PTSD, discover ways to predict which treatments (or treatment combinations) will be successful for a given individual, develop an operational definition of remission in PTSD, and explore ways to disseminate effective evidence-based treatments for this condition.
(Reprinted with permission from Journal of Psychiatric Research 2006; 40:1–21)
1. INTRODUCTION
It has long been known that people sometimes develop maladaptive symptoms after exposure to extreme stress. Jacob Mendez Da Costa, an eminent Philadelphia physician, described an eponymous condition resembling posttraumatic stress disorder (PTSD) among veterans of the American Civil War (Vaisrub, 1975). The relatively high prevalence of this condition among veterans of the Vietnam War (Card, 1987; Long et al., 1996; Beals et al., 2002) was one important impetus for the burgeoning of PTSD research over the last several decades. The diagnosis of PTSD was first included in the third edition of the Diagnostic and Statistical Manual of Mental Disorders (DSM) in 1980; (APA, 1980), since then, considerable research effort has been directed towards the etiology, phenomenology, clinical and neuro-biological characteristics, and treatment of PTSD and related and common comorbid disorders.
In this article, we review the state of the art in PTSD from six different perspectives: (1) sex differences in trauma and PTSD; (2) risk and resilience factors in the mass traumas of disasters and terrorism; (3) the impact of early life trauma and its relationship to psychiatric sequelae including PTSD; (4) imaging studies of depression which can serve as a paradigm of failed adaptation to chronic emotional stress; (5) alterations in neural circuits and memory in PTSD; and (6) cognitive therapy approaches to the treatment of PTSD. We conclude with suggestions for key directions in future PTSD research.
2. SEX, TRAUMA, AND PTSD: WHAT ARE THE DIFFERENCES?
While much of the early research on PTSD involved men with combat-related disorders, population studies have revealed that PTSD is more prevalent in women. In the National Comorbidity Survey, Kessler et al. (1995) found an overall lifetime prevalence of PTSD of 7.8%, but women were over twice as likely as men to have suffered from the condition (10.4% vs 5.0%; p < 0.05). Community surveys consistently reveal elevated rates of PTSD among women [e.g., a Swedish study by Frans et al. (2005)]. Why are most PTSD sufferers female?
Combat exposure accounts for a large proportion of the PTSD seen among men in the United States: the population-attributable fraction is reported to be 27.8% of 12-month PTSD (Prigerson et al., 2002). The main burden of PTSD in the United States, however, stems not from war or terrorism but from far more common events such as criminal victimization, motor vehicle accidents, and childhood maltreatment (physical, sexual, and emotional) (Kessler et al., 1995; Stein, 2002; Kessler, 2000). Men and women differ in the types of trauma most frequently encountered; molestation and sexual abuse are more frequent in women, while fights, accidents, and threats involving a weapon (and combat) are more frequent in men. Despite this, even when subjected to the same type of trauma as men, women still have approximately twice the risk of developing PTSD symptoms, and their symptoms are more likely to persist than symptoms among men (Fig. 1) (Kessler et al., 1995; Kessler, 2000; Breslau et al., 1997; Breslau and Davis, 1992; Van Loey et al., 2003; Holbrook et al., 2002; Stein et al., 2000; DeLisi et al., 2003). However, it should be noted that not all studies find this increased susceptibility in women. In a nested case-control analysis of a population-based survey of 30,000 Gulf War era veterans, exposure to either of two kinds of severe trauma—sexual assault and combat—was associated with comparable risk for PTSD in men and women (Kang et al., 2005). Furthermore, there is preliminary evidence from studies of recent US veterans of war in Iraq and Afghanistan that men and women are at equal risk for PTSD symptoms (Kang et al., 2005). It may be that gender differences are negligible under circumstances of extreme trauma exposure: this possibility would be compatible with findings from a population-based twin study in which there was an effect of gender on major depression in the presence of lower, but not higher, levels of psychosocial stress (Kendler et al., 2004). The implications of this observation for mental health prevention efforts remain to be determined.
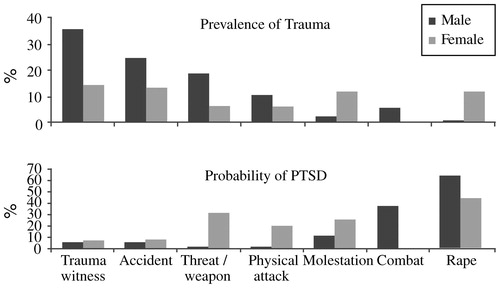
Figure 1. Prevalence of Trauma and Probability of PTSD
In one of the very few prospective studies of PTSD, Perkonigg et al. (2000) followed a German sample of 3021 individuals aged 14–24 and found that predictors of trauma exposure included a pre-existing anxiety disorder (odds ratio (OR) 1.3–3.0) or substance use disorder (OR ∼ 3–7); once trauma had occurred, the strongest predictors of PTSD symptoms were female sex (OR ∼ 2–3) and assault or sexual trauma (OR ∼ 2–4).
Many studies have now demonstrated that female gender is a strong risk factor for the development of PTSD (Stein, 2002). Though this gender difference is fairly clear from an epidemiologic perspective, the mechanisms for this disparity are uncertain; they may involve both differences in types of trauma exposure and differences in response to trauma. Conceivably, there may be a biological basis to women's apparent vulnerable to certain kinds of traumatic stressors. Barr et al. (2004) have shown that the serotonin transporter promoter polymorphism (5-HTTLPR) modulates the effect of early adversity in female—but not male—macaque monkeys. They speculate that women with the short (“s”) allele may be more susceptible to the effects of early adversity, a mechanism that explains the increased risk among women for certain stress-related syndromes such as PTSD This work remains to be replicated and extended to humans, but it does pose a testable hypothesis for gender differences in PTSD.
Regarding explanations that focus on differences in type of exposure, violence at the hands of an intimate partner is an epidemic problem that predominantly affects women; it contributes significantly to the burden of PTSD in the female population. Plichta and Falik (2001) used a nationally representative sample of 2850 American women from the Commonwealth Fund's 1998 Survey of Women's Health to estimate that over 40% of women had experienced some form of violence; 34.6% had experienced intimate partner violence, and 8% had been physically abused by their partners within the previous 12 months. Victims of intimate partner violence often develop psychiatric disorders. In this context, Bennice et al. (2003) suggested that sexual violence was a greater risk factor than physical violence for the development of PTSD symptoms. In a sample of 44 women who had been victims of intimate partner violence within the last 2 years, Stein and Kennedy (2001) found that PTSD had a lifetime prevalence of 50%, and 31.8% of PTSD related to violence by intimate partners. Most of the participants in this study had experienced childhood maltreatment, and it was therefore not possible to explore the impact of this factor on PTSD risk. However, other studies have shown that adults with histories of childhood trauma have increased rates of PTSD. Although childhood sexual trauma has been the most intensively studied in this regard, other types of maltreatment—emotional abuse in particular—may be at least as important in explaining gender differences in PTSD. Studying 8667 adult members of an HMO, Edwards et al. (2003) found that lower scores on the mental health scale of the SF-36 were associated with more categories of abuse (sexual, physical and emotional abuse, and witnessing of maternal battering), and that both an emotionally abusive family environment and the interaction of such an environment with the different types of maltreatment significantly lowered mental health scores. While men reported significantly more physical abuse, women reported a significantly higher prevalence of sexual abuse as well as moderate or severe emotional abuse in the family environment (Fig. 2). Thus, differences in types of trauma exposure—possibly relating to sexual trauma (in childhood and adulthood) and intimate partner violence—may explain some of the gender differences in rates of PTSD.
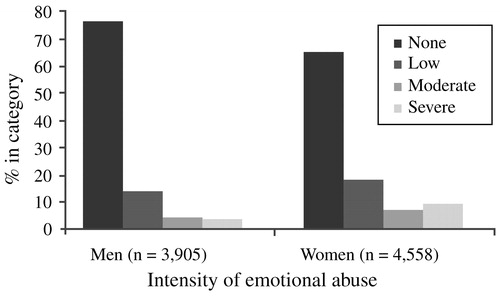
Figure 2. Emotional Abuse and Gender in an HMO Sample of Adults
Psychosocial factors such as social support and stigma are also likely to influence gender differences in PTSD following trauma exposure. Andrews et al. (2003) assessed 118 male and 39 female victims of violent crime for PTSD symptoms as well as for levels of positive support and negative reactions from others. They found that women reported significantly more negative responses from family and friends, and that this factor mediated the relationship between trauma exposure and subsequent PTSD symptoms at 6 months post-exposure. This study serves to remind us that it may be unwarranted to assume that men and women's experience of the same type of trauma will be similar. For example, motor vehicle collisions—even those that are similar in extent of personal injury or vehicular damage—may be experienced very differently by men and women, perhaps in large part because of psychosocial factors (e.g., spousal and/or community support) that mediate the response to trauma. The existence of such factors does not negate the epidemiological fact that women are more at risk for PTSD after many types of trauma exposure, but it may provide another mechanistic explanation for these differences.
The presence of PTSD adversely affects women's health and functioning in many domains (Dobie et al., 2004; Ouimette et al., 2004). Walker et al. found that in a sample of 1225 women belonging to an urban HMO, health care costs were doubled among those with high PTSD scores (≥45 on the PTSD Symptom Check-list) (Walker et al., 2003). Better identification of PTSD in primary care settings might well lead not only to appropriate treatment but also to cost savings.
Do women differ from men in the extent to which they have conditions comorbid with PTSD? In the National Comorbidity Survey, Kessler et al. (1995) found that, compared with men with PTSD, women with PTSD had lower rates of comorbid alcohol abuse or dependence (27.9% vs 51.9%), drug abuse or dependence (26.9% vs 34.5%), but higher rates of panic disorder (12.6% vs 7.3%) and agoraphobia (22.4% vs 16.1%). However, other data suggest that prior substance abuse may increase the risks of trauma exposure and PTSD, and that childhood maltreatment may increase the risk of substance abuse (McCauley et al., 1997). Rates of social phobia, major depression, and dysthymia were similar, but a more recent study by Oquendo et al. (2003) showed that among 156 inpatients with major depression, those with comorbid lifetime PTSD were more likely to have attempted suicide than those without comorbid PTSD (75% vs 54%; p ≤ 0.01), and among the subgroup with both conditions, the risk of a suicide attempt was higher among women than among men.
Finally, it is possible that there may be gender differences in response to drug treatment for PTSD. However, an early study by Brady et al. (2000) showing that men responded poorly to treatment with sertraline was not confirmed by later studies using larger sample sizes.
3. RISK AND RESILIENCE FACTORS AFTER DISASTERS AND TERRORISM
Resilience has been addressed since ancient times by authors as diverse as Confucius (“Our greatest glory is not in never falling, but in rising every time we fall”) and Nietzsche (“That which does not kill us can only make us stronger”). Merriam-Webster's Collegiate Dictionary (M-W Collegiate Dictionary, 1993) defines resilience as: (1) the capability of a strained body to recover its size and shape after deformation and (2) an ability to recover from or adjust easily to misfortune or change. The metaphor of flexibility and elasticity is also apparent in mental health definitions, which involve the capacity to bounce back, withstand hardship and repair oneself (Wolin, 1993), or to master cycles of disruption and reintegration (Flach, 1980). Psychiatric resilience can be defined as resistance to and rapid recovery from psychiatric illness. A wealth of elements that comprise resilience has been proposed in the literature—including active problem-solving, responsibility, self-esteem, independence, well-being, initiative, humor, insight, creativity, and many others. Measuring these concepts and understanding their respective roles presents a formidable challenge.
Disasters tend to be random events that expose unselected populations to trauma. Thus, they offer unique opportunities for researchers interested in studying risk and resilience to disentangle the confounding issue of pre-existing risk for exposure to traumatic events. Within a given community, individuals who are highly exposed to a traumatic experience—who are directly in harm's way—will be distressed and challenged by their experience, but only some of them will develop PTSD. A second group of individuals are indirectly exposed to the trauma—they may have lost jobs or had electrical and water utilities cut off, or sustained minor property damage. Finally, if the traumatic event is massive, many individuals outside the particular community may be remotely affected, as much of the American population was affected after September 11, 2001 (Fig. 3). Each of these groups deserves separate formal intervention. PTSD and other psychiatric disorders are the domain of a medical model that deals with disease and subsumes wellness models that focus on restoring homeostasis. These models need not be in conflict with each other; together they form a comprehensive whole that addresses the effects of disasters, both negative (distress, symptoms, disease) and positive (personal challenge and growth).
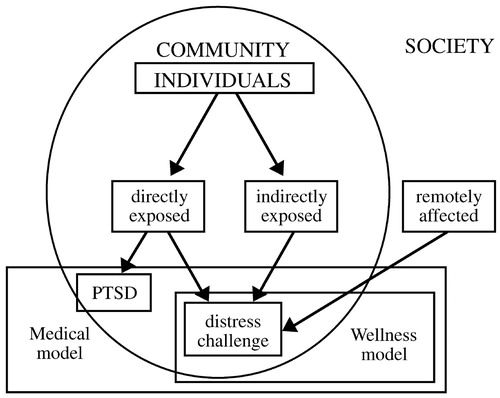
Figure 3. Approaches to the Mental Health Effects of Disasters and Terrorism
Epidemiological studies that attempt to estimate the level of psychopathology in a population after a disaster often neglect important aspects of the PTSD diagnosis. Many popular questionnaires do not determine the sufficiency of exposure to the traumatic event, separate new from pre-existing symptoms, or inquire into the duration of the symptoms or the duration or extent of the resulting disability. Epidemiological estimates of prevalence of PTSD may be inflated as a result.
North et al. (1999) used structured diagnostic interviews to assess 182 adult survivors of the bombing of the Alfred P. Murrah Federal Building in Oklahoma City. Interviewees were selected randomly from the Oklahoma City Health Department's bombing registry. Of these individuals, 87% reported injuries, and 77% had required medical intervention for them. At 6 months after the disaster, even in this highly exposed group, 55% had no psychiatric diagnosis. PTSD was seen in 36% of this population, and 2% of these survivors had PTSD related to traumatic events other than the bombing. Development of PTSD was generally rapid—76% of cases began on the day of the bombing, 94% within the first week, and 98% within the first month. The strongest predictors of PTSD were female sex (45% vs 23%; p = 0.002) and a pre-disaster history of psychiatric illness (45% vs 26%; p = 0.009). Over half of those with PTSD had a comorbid psychiatric diagnosis, usually major depression. The second most common psychiatric diagnosis was major depression, seen in 23% of the sample; 78% of those with prior history of major depression had recurrent or persistent major depression at the time of assessment. All cases of substance abuse seen in this sample had developed prior to the bombing, and no new cases were seen.
Individuals with PTSD reported widespread social and occupational dysfunction. For example, 52% of those with PTSD alone and 87% of those with PTSD and a comorbid diagnosis reported some type of functional interference, compared with 27% of those with a non-PTSD diagnosis and 16% of those with no psychiatric diagnosis.
Over the first 6 months after the disaster, 79% of the study participants met the DSM-III-R criterion for group B symptoms (intrusive memories, dreams or nightmares of the event, flashbacks, and being upset by reminders), and 82% met the criterion for group D symptoms (insomnia, difficulty in concentrating, irritability, hypervigilance, and being jumpy or easily startled). However, only 36% met the criteria for group C symptoms (avoidance, psychogenic amnesia, detachment or estrangement, loss of interest, restricted affect, and a sense of shortened future). Of those who met the group C symptom criteria, 94% had a diagnosis of PTSD. The presence of group C symptoms also predicted other diagnoses, the presence of comorbid conditions, interference with activities, dissatisfaction with work performance, and receiving mental health treatment (Fig. 4).
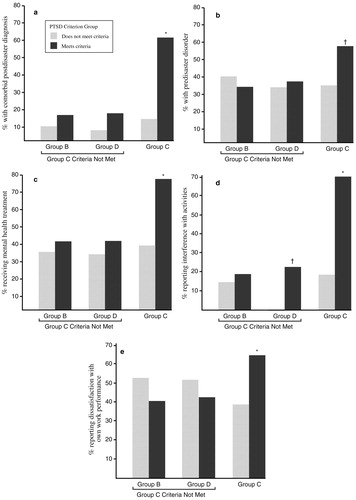
Figure 4. Diagnosis, Treatment and Functional Indicators Associated with PTSD Criterion Groups
The above results suggest that a simplified algorithm could be constructed for mental health assessment and triage in the aftermath of a disaster. The first step would be to assess for PTSD, the most common diagnosis; if the population at risk is too large for full assessment of everyone, this task may be facilitated by screening for group C criteria, which constitute a marker for the likelihood of PTSD. The second step would be to assess for other diagnoses, whose presence may be critical to selection and outcome of treatment. Finally, treatment would be selected based on the diagnosis (Fig. 5).
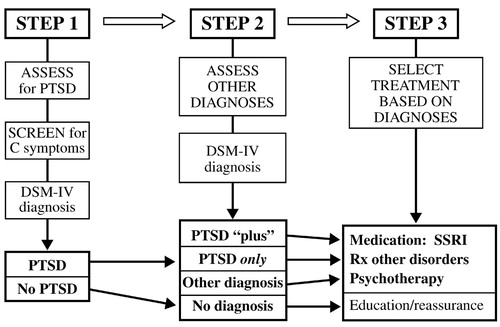
Figure 5. Flow Chart for Mental Health Assessment and Triage After Disasters
A recent analysis of 160 samples of disaster victims (Norris et al., 2002) indicated that impairment and adverse outcomes were associated with female gender, ethnic minorities, youthfulness, prior psychiatric problems, secondary stressors, inadequate psychosocial resources, developing rather than developed countries, severity of exposure, and mass violence (e.g., terrorism, shooting sprees) rather than natural or technological disasters.
What about the resilience of those in the larger community—the intended target of demoralization and intimidation by terrorist groups—who are indirectly affected by disasters? While individuals directly exposed to the September 11 disaster have not yet been studied as were the Oklahoma City survivors, numerous studies of indirectly exposed groups have found that 1–2 months after the attacks, 7.5% of a representative sample of the adult Manhattan population reported posttraumatic symptoms (Galea et al., 2002). A variety of risk factors were identified, including prior stressors, residence near the World Trade Center, loss of possessions or job due to the attacks, the death of a friend or relative during the attack, and low levels of social support. At least two national studies (Schlenger et al., 2002; Schuster et al., 2001) have reported an association between extensive viewing of television coverage and substantial stress reactions, although the causal directionalities of this association have not been elucidated. A Pew poll (Associated Press, 2001) found that a few days after the attacks, 90% of a nationally representative sample found that 7 of 10 reported feeling depressed, half had trouble concentrating, and one third had disturbed sleep. However, a longitudinal study by Silver et al. (2002) found that the prevalence of posttraumatic stress symptoms related to September 11 among the US population outside New York City declined from 17% at 2 months to 5.8% at 6 months. Not surprisingly, coping strategies assessed shortly after the attacks were the strongest predictors of posttraumatic stress symptoms. Avoidance and numbing symptoms, reflecting inability to cope effectively, were associated with several indicators of problem functioning; these findings were consistent with those of a study of a mass shooting episode in which early coping problems were found to predict PTSD 3 years later (North et al., 2001).
How can a community's resilience to disasters be increased? Policies and practices that address this question need to be empirically driven, tailoring the interventions to the needs of the individuals being served, rather than the ideologies of service providers. It is essential to recognize and treat the subset of community members with psychiatric illnesses—facilitated by screening for group C symptoms—and to find ways to assist people who have group B and D symptoms (which are nearly ubiquitous among high-exposure groups) without pathologizing them. Mass cognitive reframing could help to correct widespread cognitive distortions and substitute more rational and adaptive thoughts. In this regard, the potential for positive effects of disasters should not be overlooked. Amazingly, many participants in disaster studies have reported favorable outcomes; a review by McMillen (1999) reported strengthened spiritual or religious beliefs in 15–40% and improvements in interpersonal relationships with family or friends in 10–40%. Finally, a focus on community cohesiveness may also be useful to help people pull together their individual resilience for the good of the greater community.
4. EARLY LIFE TRAUMA AND PTSD
Until the last decade, the hypothesis that early life trauma is associated with an increased risk of adult mood and anxiety disorders was supported largely by anecdotal reports inspired by psychoanalytic concepts of early critical periods of development. Research on the biology of depression and some anxiety disorders has commonly been plagued by the confounding factor of early life stress—the hypercortisolemia and structural brain changes attributed solely to the disorder is likely due, at least in part, to the biological sequelae of early life trauma.
If early trauma is indeed a principal risk factor for later depression and anxiety disorders, one would expect the baseline prevalence of such trauma histories to be very high. However, prevalence data are surprisingly sparse, being derived mainly from small samples or spontaneous reports of trauma from social service departments or hospital emergency rooms. We do know that reported cases constitute a relatively small fraction of all cases, and that although prevalence estimates of childhood abuse and other traumas such as early loss of parents are extremely approximate, they are indeed sufficient to account in part for the high prevalence of depression and anxiety disorders among the general adult population.
In a retrospective study involving 17,337 adult HMO members, Dube et al. (2001) examined the relationship between adverse childhood experiences and later suicide attempts. In this population, whose mean age was 57 years and which was 54% female, the lifetime prevalence of any suicide attempt was 3.8%, but adverse childhood experiences of any type (emotional, physical, or sexual abuse, substance abuse, mental illness or incarceration of a family member, or parental domestic violence, separation or divorce) increased this risk 2- to 5-fold. Moreover, the greater the number of adverse experiences, the greater the risk of suicide attempts; those with seven or more such experiences had an adjusted odds ratio of 31.1 for a suicide attempt compared with those who had none of these adverse experiences. McHolm et al. (2003) found consistent results in a community sample of 347 Canadian women aged 15–64 with major depressive disorder: suicidal ideation among them was most strongly associated with a self-reported history of childhood physical abuse (OR 2.77; 95% CI = 1.26–6.12).
The striking results of a study by McCauley et al. (1997) have also received a great deal of attention, though it has been criticized for the potential confound of retrospective reporting. This cross-sectional survey of 1,931 women from all socioeconomic classes, drawn from four community-based primary care internal medicine practices, found a 22% prevalence of reported childhood or adolescent physical or sexual abuse. Compared with the remainder of the sample, those with childhood, but not adult, abuse histories reported significantly more physical symptoms (mean 6.2 vs 4.0; p < 0.001), as well as significantly higher Symptom Checklist-22 scores for depression, anxiety, somatization, and interpersonal sensitivity, a 4.7-fold higher prevalence of drug abuse, a 2.2-fold higher alcohol abuse, a 3.7-fold higher history of suicide attempts, and a 3.2-fold higher risk of psychiatric admission to hospital.
More recently, Safren et al. (2002) found that patients with panic disorder were significantly more likely to have histories of childhood physical or sexual abuse than those with social phobia, while the prevalence of such histories was intermediate among patients with generalized anxiety disorders; these findings held regardless of the presence or absence of comorbid anxiety or depression. Such results suggest that, consistent with other studies (Engel et al., 1993; Bremner et al., 1993a,b), early abuse is a factor in the later development of anxiety disorders as well as depression.
A suggested model of vulnerability to major depressive episodes, based on both animal and clinical studies, posits that genetic factors, temperament, and trauma early and later in life markedly increase the risk of depression. When superimposed on this background of risk, stressful life events and other recent difficulties trigger a major depressive episode. Many of these effects are mediated by corticotropin-releasing factor (CRF), which plays a key role in modulating the autonomic, immune, and behavioral effects of stress; increases in CRF are associated with increased symptoms of depression and anxiety. Indeed, these findings are consistent with the repeated demonstration that mood and anxiety disorders are frequently comorbid—if not syndromally, then certainly in terms of dimensional measures of symptom severity. In fact, depression and anxiety share common effective treatments, such as SSRIs, dual serotonin-norepinephrine reuptake inhibitors, and certain forms of psychotherapy.
Is this general model consistent with preclinical findings linking early trauma with hypothalamic-pituitary-adrenal (HPA) axis functioning? In a rat model of neglect, rat pups were removed from their mothers for 3 h daily between the ages of 2–14 days, and then returned to their mothers in the animal colony for a week before weaning; this naturalistic stressor is thought to be analogous to neglect in human childhood up to the age of 4–5 years. Pihoker et al. (1993) found that rat pups subjected to this type of maternal deprivation at the age of 10 days had significant reductions in median eminence CRF concentrations after 24 h, interpreted as an increase in hypothalamic CRF release. However, the effect was seen only during a critical time window—maternal deprivation did not reduce CRF concentrations in older, 18-day old rat pups.
The effects of early maternal deprivation appear to be remarkably persistent (Heim et al., 2004). Ladd et al. (1996) found that adult male rats isolated from their mothers for 6 h daily from postnatal days 2–20 (before weaning) showed increases in ACTH concentrations, both basal and induced by a mild foot shock. This effect appeared to be mediated by large increases in CRF concentrations in the median eminence of the hypothalamus, where CRF and mRNA expression were greatly increased compared to unstressed control animals. The increase in CRF mRNA expression was also seen extra-hypothalamically—for example, in the central nucleus of the amygdala and in the parabrachial region of the locus coeruleus. Moreover, direct measurements of CRF concentrations in cerebrospinal fluid (CSF) also demonstrated increases over levels in control animals.
Other intriguing changes in maternally deprived rats have also been noted. For example, Heim et al. (2004) demonstrated that rats with histories of early maternal deprivation displayed anhedonia, as indicated by lack of reinforcement for choosing saccharin-sweetened water over plain water. Neumaier et al. (2002) found increased 5HT1B mRNA expression in the dorsal raphe nucleus; other pilot studies have demonstrated persistent changes including reduced hippocampal neurogenesis (unpublished observations), hippocampal mossy fiber development (Huot et al., 2002) and GABAA receptor binding (Caldji et al., 2000). Gene chips are currently being used to further characterize changes in gene expression in maternally deprived animals.
What about behavioral alterations in maternally deprived rats? A hypersensitive startle to an acoustic stimulus is a well-validated marker of hyperarousal, analogous to the exaggerated startle response seen in human adults with PTSD. Plotsky et al found that maternally deprived animals showed increased acoustic startle responses compared to non-deprived and minimally separated animals (unpublished observations). Maternally deprived animals have also been shown to prefer alcohol-sucrose to water-sucrose solutions (Huot et al., 2001), though total fluid intake was unchanged.
Interestingly, drug treatment of maternally deprived rats with paroxetine (via implantable mini-pump) has been shown to normalize—at least partially—several of the trauma-associated perturbations, both biochemical and behavioral (Plotsky et al., unpublished observations). For example, paroxetine reduced CRF mRNA expression in the paraventricular nucleus, bed nucleus of the stria terminalis, and central nucleus of the amygdala, reduced CRF concentrations in cerebrospinal fluid, normalized pituitary CRF receptor binding, attenuated the exaggerated ACTH response to startle, reversed the blunted ACTH response to exogenous CRF challenge, and decreased peripheral corticosterone levels. In contrast, paroxetine had no significant effects on these parameters in nondeprived rats. Drug treatment also normalized behaviors such as the reduced preference for sucrose and increased preference for ethanol (Huot et al., 2001), as well as anxiety-like behavior. The time course of some of these effects is instructive: 3–4 weeks were required for the full effect of paroxetine on CRF mRNA levels in the paraventricular nucleus. However, CRF mRNA expression returned to its abnormally high level 14 days after discontination of the drug. Pilot studies with reboxetine and mirtazepine have revealed similar effects of these antidepressants (unpublished observations).
Non-human primate models of maternal deprivation have shown a similar pattern of results. Coplan et al. (1996) found that infant bonnet macaques reared by mothers who faced variable and unpredictable foraging conditions had later persistent and significant elevations in CSF concentrations of CRF (Fig. 6), as well as decreases in CSF concentrations of cortisol. The effects were not noted among offspring of mothers who faced high or low foraging demands, likely because these demands were predictable.
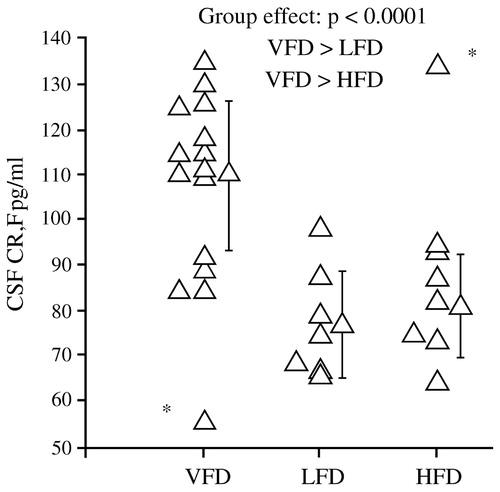
Figure 6. Levels of CSF CRF Among Infant Bonnet Macaques Reared Under Different Foraging Conditions
Can these preclinical findings on HPA axis responses to early life trauma be extended to the clinical arena? We believe that they can, but one must do so cautiously. In a prospective study of 49 women, Heim et al. (2000) scrutinized four groups aged 18–45: those with no early trauma history or current psychiatric illness (controls); those with a trauma history only; those with a current episode of major depression only; and those with both major depression and early life trauma. Approximately 85% of the last group also fulfilled the diagnostic criteria for PTSD, while the rate of PTSD was 36% among those with early trauma without major depression. These women were exposed to a standard laboratory stressor, the Trier Social Stress Test, in which participants are assigned an oral presentation and mental arithmetic tasks. This stressor reproducibly raised plasma cortisol concentrations in all groups including controls, but the increases were much more dramatic among the group with both early life trauma and major depression (Fig. 7). Whether or not they were depressed, women with early life trauma also had a much greater ACTH response to stress than controls or those with major depression only (Fig. 7). In addition, heart rates increased modestly in controls but dramatically in subjects with early life trauma and major depression; the magnitude of the heart rate response was intermediate in the other two groups. These results are consistent with a model in which the stress system is sensitized, and subsequent stresses in adult life lead to markedly hyperactive responses. In a case-control study of 27 medication-free adults with major depression and 25 matched controls, Carpenter et al. (2004) also found that perceived early life stress was a significant predictor of CSF CRF concentrations in both normal volunteers and depressed subjects.
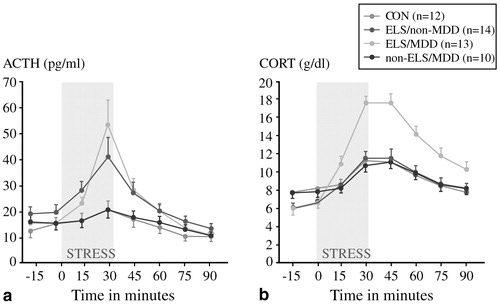
Figure 7. Trier Social Stress Test
Recently, Caspi et al. (2003) reported that individuals either homozygous (s/s) or heterozygous (s/l) for the short arm of the serotonin transporter (SERT) promoter are at considerably greater risk than individuals with the l/l genotype for the development of major depression if exposed to trauma early in life. Moreover, there was a dose-response relationship for risk, such that individuals with the s/s genotype were at greater risk than those with the s/I genotype.
Several clinical studies have linked elevated CSF CRF concentrations not only with depression (Raad-sheer et al., 1994; Banki et al., 1987; Nemeroff, 1989), but also with PTSD. For example, Bremner et al. (1997a,b) studied 11 Vietnam veterans with PTSD and found significant elevations in CSF CRF compared to controls; these elevations were seen in veterans with or without comorbid major depression. Baker et al. (1999) subsequently measured sequentially sampled CSF cortisol concentrations and 24-h urinary free cortisol excretion in 11 combat veterans with PTSD and 12 matched control subjects, and found that CSF CRF concentrations were significantly higher among the combat veterans, though CRF levels were not correlated with PTSD symptomatology. In contrast, 24-h urinary free cortisol excretion was similar in the two groups. Interestingly, however, cortisol excretion was inversely correlated with PTSD symptoms.
Finally, what implications do these results have for treatment selection? Keller et al. (2000) were among the first to compare directly the efficacy of antidepressant (nefazodone) treatment, psychotherapy, and the combination of the two in the treatment of chronic depression. This study involved 681 adults with nonpsychotic major depressive episodes at least two years long (mean duration 7.5–8.0 years) and scores ≥20 on the 24-item Hamilton Rating Scale for Depression (HAM-D). Many also had long-standing dysthymia, anxiety disorders, substance abuse disorders or personality disorders, and had undergone prior treatment with antidepressants, psychotherapy, or both. These patients were randomized to receive either the cognitive-behavioral-analysis system of psychotherapy (CBASP) (McCullough, 1984), delivered in 16–20 sessions over 12 weeks, or nefazodone (mean dose 460–466 mg daily), or both treatments. After 12 weeks, remission rates (HAM-D ≤8) among study completers were 22% in the nefazo-done group, 24% in the CBASP group, and 42% in the combined group (p < 0.001 vs either single modality) (Fig. 8). These findings were consistent with the wide-spread clinical impression that combination treatment is superior to single modalities.
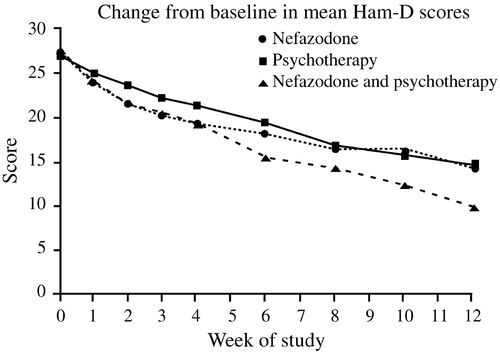
Figure 8. Nefazodone Chronic Depression Study.
A secondary analysis (Nemeroff et al., 2003) of the data set from the foregoing study revealed several novel findings. First, there is an extremely high prevalence of early life trauma in this chronically depressed population. Only about one-third of the patients had no trauma history. Overall, 32–35% had suffered parental loss before age 15 years, 40–48% physical abuse, 15–19% sexual abuse and 7–12% neglect. Patients with histories of trauma had dramatically poorer responses to antidepressant treatment than to psychotherapy or combined treatment. The differences in remission rates were even more striking—32% on antidepressant therapy, 48% on psychotherapy, and approximately 52% on combined therapy. Given that treatment of maternally deprived animals with SSRIs can reverse many of the adverse biochemical and behavioral effects of trauma, it would be desirable to conduct a similar study with an SSRI in humans; a trial involving fluoxetine is currently underway.
5. WHAT CAN IMAGING STUDIES OF DEPRESSION TEACH US ABOUT FAILED ADAPTATION TO CHRONIC EMOTIONAL STRESS AND ANXIETY DISORDERS?
Research on functional brain imaging in depression (and on the biology of depression in general) has “matured” much more than similar research in PTSD. Not only can we learn a great deal from the depression literature that is relevant to PTSD, but it is now well established that these syndromes are frequently comorbid, and that certain antidepressants, the SSRIs in particular, are effective for both conditions.
Depression may be conceptualized as an end-product of failed adaptation to chronic emotional stress. The individual, who begins with a given set of biological risks (e.g., female sex, certain gene polymorphisms, temperament, and reactivity of the HPA axis) is subjected to a variety of exogenous stressors, which act to destabilize the usual homeostatic state of the brain circuits that are involved in mood regulation. The result is a depressive episode, and the goal of treatment is to restabilize the key brain circuits. Defining these brain circuits and their perturbations as precisely as possible by separating their putative components experimentally is a major research challenge.
Do depressed patients differ from healthy individuals in their acute adaptive responses to stress? Several studies have used provoked sadness and neuroimaging studies to determine changes in regional brain activity in response to this sad mood challenge. Normal individuals, depressed patients, and those thought to be at risk for depression may be studied in this way. Mayberg et al. (1999) asked eight healthy right-handed euthymic women to prepare scripts of sad personal experiences and then to allow themselves to feel sadness while undergoing [15O]H2O positron emission tomography (PET). Limbic-paralimbic blood flow was increased, particularly in the subgenual cingulate (Brodmann's area 25) and the anterior insula, while neocortical blood flow (in the right dorsolateral prefrontal and inferior parietal areas) was reciprocally decreased. This pattern was consistent with the well-known clinical observation that intense sadness is often accompanied by cognitive difficulties.
A very different pattern was seen in depressed patients. Liotti et al. (2002a,b) compared regional blood flow changes after the same sad mood challenge among acutely depressed patients (mean HAM-D score 21.3), euthymic patients who were in remission (mean HAM-D score 4.8 without medications), and normal volunteers. Decreases in blood flow in the medial orbitofrontal cortex (area 10/11) and a variety of other areas were virtually identical in both the remitted and symptomatic depressed patients, and differed substantially from control subjects. Remitted patients (but not acutely depressed patients or controls) exhibited decreased blood flow in the pregenual anterior cingulate (area 24a). Conversely, the depressed groups lacked the increase in area 25 and the decrease in right prefrontal cortex that were observed in the controls. More recently, Kruger et al. (2003) studied bipolar patients and found patterns of cerebral blood flow in response to sad mood challenge that were similar whether the patients were acutely depressed or in remission. Taken together, these results suggest that the unique changes seen across patient subtypes, irrespective of clinical state, may be trait markers of a depressive diathesis.
Can depressive illness also be conceived of as the product of more chronic maladaptive changes to emotional triggers? Most depressed participants in neuroimaging studies are recruited well after the onset of symptoms, and may present at various points in the course of the brain's attempts at adaptation. Moreover, patients recruited from tertiary care centers are likely to be different from those recruited by newspaper advertising, and their clinical states can range from complete failure of adaptation to partial adaptation or hypersensitive adaptations. The heterogeneity of neuroimaging results reported in the literature is therefore not surprising. In fact, this baseline variability may be a potential clue to understanding differences in both the clinical and brain response patterns across different treatment modalities (Mayberg, 2003).
In a placebo-controlled, double-blind trial, Mayberg et al. (2000a,b) explored changes in brain glucose metabolism using PET in unipolar depressed male inpatients after 1 week and 6 weeks of treatment with fluoxetine. Patients receiving active fluoxetine had a similar pattern of changes in glucose metabolism after 1 week of treatment: increases in the hippocampus and brainstem and decreases in the posterior cingulate, striatum, and thalamus. By 6 weeks, however, fluoxetine responders could be distinguished from non-responders by decreases in limbic and striatal glucose metabolism (subgenual cingulate, hippocampus, insula, and pallidum) and reciprocal increases in brainstem and dorsal cortical areas (prefrontal, parietal, anterior, and posterior cingulate). In contrast, the patterns seen during the first week persisted in the non-responders. Interestingly, regional changes were not merely corrections of pretreatment abnormalities. Some regions showed changes in the direction of reversing baseline abnormalities, while others were new effects in regions showing apparently normal pretreatment activity. These findings suggest that successful treatment is not a simple matter of absolute changes, but instead entails a more complex adaptation of multiple brain regions to a new setpoint. Conversely, clinical treatment failure may be the result of an inability to institute or maintain these adaptations. The metabolic changes with response were not specific to fluoxetine treatment, because they were also noted among the placebo responders in the study. The time course and localization of these changes were also consistent with the data obtained by several groups on upregulation of the cyclic AMP second messenger system and increased expression of brain-derived neurotrophic factor (BDNF) in association with antidepressant treatment (Vaidya et al., 1997; Duman et al., 1999; Chen et al., 2001). Finally, the changes appear to persist over time; decreased activity in area 25 was seen 18 months later (Mayberg, 2003; Liotti et al., 2000) (Fig. 9—Mayberg et al., 2000a,b; Mayberg et al., 2002; Liotti et al., 2002a,b; Stefurak and Mayberg, 2003; Goldapple et al., 2004a,b).
Kennedy et al. (2001) carried out a similar study of brain glucose metabolism among 13 depressed male patients before and after 6 weeks of therapy with paroxetine 20–40 mg daily, comparing the patterns obtained with those of 24 healthy male volunteers. All patients responded to treatment (HAM-D scores reduced by at least 50%). With successful treatment, glucose metabolism increased in the prefrontal cortex, parietal cortex, and dorsal anterior cingulate, and decreased in left anterior and posterior insula (though it remained higher than in controls) and right hippocampus and parahippocampal regions. Anterior cingulate metabolism, already hyperactive before treatment began, increased even further after treatment. Reanalysis of the data obtained by Kennedy et al showed that the subgenual cingulate (area 25) was also suppressed with paroxetine treatment (Fig. 9).
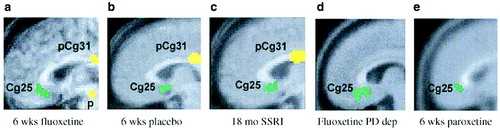
Figure 9. Common Limbic Cortical Changes With Pharmacotherapy.
Changes in subgenual cingulate may be central to recovery from depression due to its very high concentrations of serotonin transporters, a marker of presynaptic serotonin-containing nerve terminal density, and a primary target of SSRIs. This region also has reciprocal cortical and subcortical connections to the hippocampus, hypothalamus, amygdale, brainstem, and frontal cortex—regions involved in regulating circadian and autonomic as well as cognitive functions disturbed during a major depressive episode (Freedman et al., 2000). Modulation of this area therefore has the potential for broad cortical effects (Ongur et al., 1998; Barbas et al., 2003).
It might be tempting to conclude that successful treatment of depression occurs through a prerequisite final pathway—cortical normalization and limbic suppression. This is apparently not the case. Goldapple et al. (2004a,b) recruited 17 depressed patients with no comorbid psychiatric illnesses and studied changes in cerebral metabolism before and after they received 15–20 sessions of cognitive-behavioral therapy (CBT). Fourteen patients completed the study and all had significant clinical improvement (mean posttreatment HAM-D score 6.7). The metabolic changes among these patients were in many regions precisely the opposite of those seen with paroxetine treatment: increases in the hippocampus and dorsal cingulate and decreases in the dorsal, ventral, and medial frontal cortex. Dorsal cingulate and medial frontal regions were unique targets of CBT. Similarly, the subgenual cortex, thalamus, and brainstem were targeted with medication but were unaffected by the CBT (Fig. 10). The decreases in cortical metabolism are consistent with the idea that CBT has the effect of decreasing rumination about emotionally salient aversive events and memories.
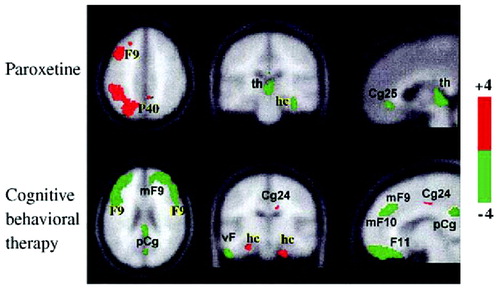
Figure 10. Effects on Cerebral Glucose Metabolic After Treatment With Paroxetine or Cognitive Behavioral Therapy.
These results imply that instead of going through a single final common pathway, different treatment modalities modulate the system in different ways—drug treatment primarily via a subcortical approach (“bottom-up”) and cognitive approaches primarily via a cortical approach (“top-down”; Fig. 11). They also suggest that subtyping depression according to the brain's baseline metabolic state might be an effective method of selecting the best treatment modality for a given individual.
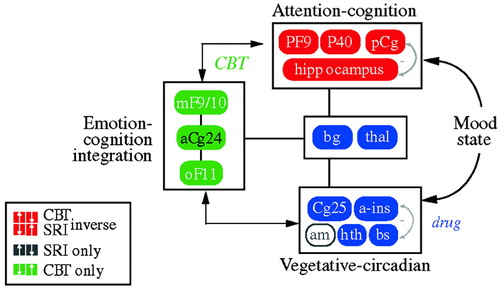
Figure 11. Relationships Among Regions Mediating CBT and Drug Response.
In an attempt to define common denominators of successful treatments, Seminowicz et al. (2004) developed a simplified 7-region model derived from the results of these treatment studies; this was mathematically tested using structural equation modeling. The simplified model examining regional interactions of medial frontal (MF10), orbital frontal (OF11), lateral prefrontal (PF9), subgenual cingulate (Cg25), anterior cingulate (Cg24), hippocampus (hc), and thalamus (thal) activity prior to treatment succeeded in accommodating all patient cohorts tested—a total of 119 patients from two institutions. Patients who went on to respond to CBT showed a mainly cortical pattern of aberrant brain connections at baseline, while drug responders showed a combined limbic-cortical pattern. Patients who were eventual nonresponders to medication showed a third distinct baseline network pattern suggestive of a more complex limbic-subcortical disturbance. The modeling results lay the groundwork for future clinical trials examining treatment response based on brain subtypes.
Finally, can the interactions between Brodmann's area (BA) 25 and frontal cortex be useful in detecting populations who are vulnerable to depression? Keightley et al. (2003) examined activation patterns in response to sad mood challenge among healthy college students with no personal or family history of depression who were selected for resilience (low scores on the dispositional Depression facet of Neuroticism [N3] and high scores on the Positive Emotions face of Extraversion [E6] on the NEO Personality Inventory-Revised) or for the reverse pattern—high N3 scores and low E6 scores. In response to the mood challenge, all subjects had decreases in lateral frontal cortical activation and increases in the activation of BA 25, but the high-N3 subjects had low medial frontal activation that resembled the pattern seen in depressed patients. These data suggest that this paradigm is highly sensitive to risk of depression, a hypothesis that requires testing in other vulnerable subject groups.
The foregoing results illustrate the value of a meticulous dissection of the different neural circuits involved in psychiatric illness and its treatment. While this approach may initially appear to be oversimplified, it has the advantage of allowing the complex mechanisms that underlie vulnerability, treatment, and relapse to be clarified.
6. NEURAL CIRCUITS, MEMORY, AND PTSD
The neural circuitry implicated in PTSD probably involves complex interactions between the thalamus (a gateway for sensory inputs), the hippocampus (which is involved in short-term memory and probably fear of the context of an event), the amygdala (which is involved in conditioned fear responses), the posterior cingulate, parietal and motor cortex (which are involved in visuospatial processing and assessment of threat), and the medial prefrontal cortex, including the anterior cingulate, orbitofrontal, and subcallosal gyrus (which is believed to extinguish more primitive subcortical responses).
The effects of stress on the hippocampus have been recognized since the 1990s, but the effects of stress on neurogenesis are of more recent vintage: Woolley et al. (1990) demonstrated that exposure of adult rats to excess corticosterone (equivalent to cortisol in humans) reduced dendritic branching in specific neuronal populations of the hippocampus, suggesting early stages of degeneration, and Gould et al. (1998) found decreased neurogenesis in socially subordinated non-human primates compared with dominant animals. Moreover, Duman et al. (2001) demonstrated that chronic administration of electroconvulsive therapy or a variety of antidepressants, but not other psychotropic drugs, to adult rodents upregulated hippocampal neurogenesis, Santarelli et al. (2003) found that irradiation of the hippocampus in mice prevented the neurogenic and behavioral effects of fluoxetine or imipramine, suggesting that hippocampal neurogenesis may be one of the mechanisms of action of antidepressants in the treatment of stress-related disorders.
Bremner et al. (1993a,b) used neuropsychological testing (paragraph recall) as a probe of hippocampal function in 26 Vietnam veterans with combat-related PTSD and 15 matched healthy control subjects. Compared to controls, the combat veterans had decreased immediate and delayed recall as well as percent retention, but IQ scores were similar in the two groups. Bremner et al. (1995a,b) also found similar results among adult survivors of severe childhood physical or sexual abuse who presented for psychiatric treatment.
The results of these studies introduced the possibility that stress in humans may also lead to hippocampal damage (Bremner, 1998; Pitman, 2001; Bremner, 2002). The first neuroimaging study in PTSD was carried out using magnetic resonance imaging (MRI) to measure hippocampal volumes in 26 Vietnam combat veterans with PTSD and 22 matched control subjects. Mean right hippocampal volume was 8% smaller in the veterans than in the control subjects (1,184 mm3 vs 1,286 mm3; p < 0.05; Fig. 12), and was correlated with short-term memory deficits on the Wechsler Memory Scale (r = 0.64; p < 0.05). Decreases in right hippocampal volume in the PTSD patients were associated with deficits in short-term memory. Left hippocampal volume was also decreased in the veterans, but the difference did not reach statistical significance (Bremner et al., 1995a,b). Findings of smaller hippocampal volume and/or a reduction in N-acetylaspartate (NAA) in the hippocampus—a marker of neuronal integrity—in adults with chronic, long-standing PTSD have been replicated several times in the published literature (Gurvits et al., 1996; Bremner et al., 1997a,b; Stein et al., 1997a,b; Freeman et al., 1998; Schuff et al., 2001; Bremner et al., 2002; Villareale et al., 2002).
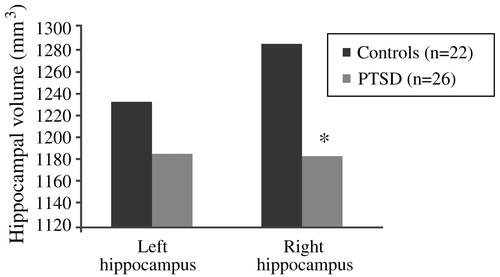
Figure 12. Decreased Right Hippocampal Volume in Combat-Related PTSD
Using [15O]H2O PET, Bremner et al. (1999a,b) measured cerebral blood flow in 22 women with histories of childhood sexual abuse while they listened to scripts that were either neutral or traumatic (personalized accounts of childhood sexual abuse events). Compared with those without PTSD, the traumatic scripts evoked increased blood flow in the posterior cingulate, anterior prefrontal cortex, and motor cortex, but decreased blood flow in the right hippocampus, visual association cortex, and the subcallosal gyrus (area 25) and adjacent anterior cingulate (area 32) of the medial prefrontal cortex. In tandem with this, women with PTSD (but not those without the diagnosis) had increased PTSD symptom scores as they listened to the traumatic scripts. This pattern of activation was similar to that observed in Vietnam veterans who were exposed to slides and sounds evoking combat situations (Bremner et al., 1999a,b). These results are consistent with a general model of cortical failure to suppress exaggerated subcortical reactions to stress.
Are the hippocampal changes seen in PTSD actually a function of the early childhood traumas seen in so many PTSD patients, or are they specific to PTSD itself? In a more recent study, Bremner et al. (2003) studied 33 women with early childhood sexual abuse and PTSD (n = 10), early childhood sexual abuse without PTSD (n = 12) and no abuse or PTSD (n = 11). Hippocampal volumes (as measured with MRI) among the women with abuse histories and PTSD were 16% smaller than among those with abuse histories without PTSD, and 19% smaller than among the normal controls, with the right hippocampal volume somewhat more diminished than the left. The women were also given two tasks: to count the number of times they heard the letter “d” in a paragraph that was read aloud to them (control condition) and to recall as much as possible of a different paragraph they had been read (verbal memory encoding task). In contrast to the women with abuse histories without PTSD, the women with PTSD did not show increased blood flow to the hippocampus during the verbal encoding memory task. Thus, there was a failure of left hippocampal activation with a memory task among women with abuse-related PTSD, which remained significant after controlling for differences in hippocampal volume (measured with MRI in the same subjects) (Fig. 13). Shin et al. (2004) have also demonstrated a failure of hippocampal activation with a declarative memory task in PTSD.
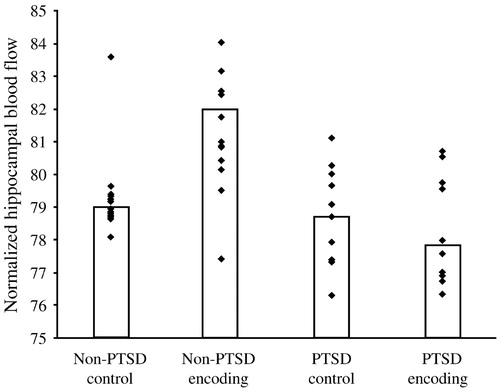
Figure 13. Failure of Hippocampal Activation Encoding in Women with Abuse-related PTSD
However, studies in children with PTSD (De Bellis et al., 1999; Carrion et al., 2001; De Bellis et al., 2001) and in adult subjects with new-onset PTSD (Bonne et al., 2001; Notestine et al., 2002) have not demonstrated a smaller hippocampal volume, suggesting that chronic PTSD is required for the effect. One study of monozygotic twins discordant for exposure to trauma found a correlation between PTSD symptoms and hippocampal volume in the unexposed twin, suggesting a genetic contribution to smaller hippocampal volume (Gilbertson et al., 2002). However, another unpublished twin study of twins discordant for PTSD has shown a pattern of hippocampal volume that is consistent with a combined genetic and environmental effect.
Preclinical studies have suggested that stress-induced lesions of the hippocampus attenuate its normal inhibitory effect on the HPA axis. The cascade of consequences includes enhanced release of CRF from the hypothalamus, a blunted ACTH response to CRF challenge, increased peripheral plasma cortisol concentrations, and—similar to what is observed in depression—resistance to the usual suppressive effects of dexamethasone. However, there are puzzling inconsistencies in findings among patients with PTSD; for example, some studies—but not all—have found decreased urinary cortisol levels (Mason et al., 1986; Yehuda et al., 1995) or “super” suppression of cortisol by low doses of dexamethasone (Yehuda et al., 1993; Stein et al., 1997a,b).
Elzinga et al. (2003) measured cortisol responses to traumatic reminders (via personalized trauma script) in 24 women with abuse histories in the presence or absence of PTSD. Cortisol concentrations in the patients with PTSD were 60% higher during anticipation of the script reading (from 20 min prior to the reading), 122% higher during the reading and 69% higher during the recovery phase (up to 75 min later; Fig. 14). Thus, PTSD appears to involve low baseline cortisol levels, but an exaggerated release of cortisol in response to a stressor.
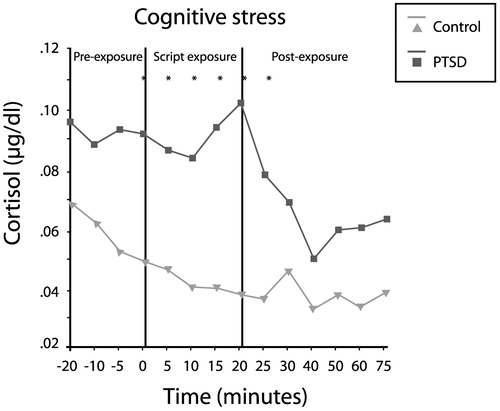
Figure 14. Increased Cortisol Response to Trauma-specific Stress in PTSD.
Paroxetine has been shown to be highly effective in the treatment of PTSD (Marshall et al., 2001; Marshall et al., 1998; Tucker et al., 2001). Vermetten et al. (2003) measured hippocampal volume and verbal declarative memory (a hippocampus-based task) in 28 patients with PTSD before and after 9–12 months of paroxetine treatment. Twenty-three patients completed the study, and 20 had MRI for assessment of hippocampal volume. Paroxetine significantly reduced symptoms in all three domains of the CAPS-2 (Fig. 15(a)), improved verbal declarative memory (Fig. 15(b)) and increased left and right hippocampal volume by 5.6% and 3.7%, respectively (p < 0.05; Fig. 15(c)). The effects of other medications on these parameters in PTSD remain to be explored.
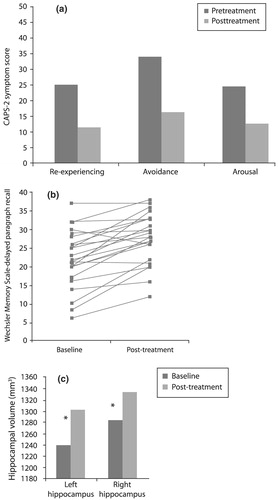
Figure 15. Effects of 9–12 Months of Treatment with 10–40 mg/day Paroxetine in PTSD
7. COGNITIVE BEHAVIORAL APPROACHES TO THE TREATMENT OF PTSD: WHAT WORKS?
Like re-experiencing, hyperarousal and avoidance symptoms, negative cognitions are ubiquitous immediately after an individual suffers a trauma (Foa and Jaycox, 1999). Usually, however, subsequent everyday life experiences gradually correct these negative cognitions, and the traumatized individual has the opportunity to regain a sense of competence and safety in the world. In contrast, those who make extensive use of avoidance and numbing will also avoid the very experiences that could have corrected their cognitive distortions. Thus, these individuals may be at higher risk for the development of PTSD.
Foa and Jaycox (1999) have suggested that two particular groups of erroneous cognitions are associated with the later development of PTSD: that the world is extremely dangerous (for example, that no place is safe and that people are untrustworthy) and that the sufferer is extremely incompetent (e.g., that others would have been able to prevent the trauma somehow, and that the sufferer's PTSD symptoms are a clear sign of weakness). Foa et al. (1999a,b) explored self-blame and negative thoughts about the self and the world among three groups of subjects with PTSD, trauma exposure without PTSD, or no trauma exposure. The group with chronic PTSD had significantly higher levels of these negative cognitions than the other two groups. While this study was not longitudinal and therefore could not address causality, the similarity between the non-traumatized subjects and those who had been traumatized but did not develop PTSD suggests that the negative cognitions may be more important than the experience of trauma per se in driving the development of PTSD.
Although most therapists working with traumatized individuals use psychodynamic or supportive counseling approaches—for which there are no efficacy data—most studies of PTSD treatment outcomes have explored cognitive-behavioral therapies, which fall into three general subtypes. In exposure therapies such as systematic desensitization and flooding, patients confront their fears, object, situation, memories, and images without being as overwhelmed as they had anticipated. These experiences of exposure thus serve to disconfirm and correct cognitive distortions harbored by the patient. The anxiety management component includes a variety of techniques such as relaxation, controlled breathing, and self-distraction (thought stopping); patients carry out exercises designed to improve their anxiety management skills. Finally, cognitive therapy identifies and challenges dysfunctional and erroneous cognitions, aiming to replace them with more functional and realistic cognitions. This procedure can be said to contain elements of exposure in the sense that the patient is exposed to the traumatic memories and thoughts and must process them differently in order to recover.
The clinical practice guidelines developed by the International Society for Traumatic Stress Studies (Foa et al., 2000) suggest that exposure therapy is the most empirically supported for PTSD, but cognitive therapy (e.g., Resick et al., 2002) and interpersonal psychotherapy (Bleiberg and Markowitz, 2005) have also been shown to be effective for this disorder. Keane et al. (1989) conducted the first study of implosion therapy (flooding) combined with relaxation in Vietnam veterans, a difficult population, and obtained a modest improvement in PTSD symptoms compared to wait-listed control subjects. Foa and Rothbaum (1998) developed a prolonged exposure (PE) therapy program consisting primarily of prolonged, repeated exposure to the traumatic memory and repeated in vivo exposure to situations that are being avoided because of trauma-related fear; the treatment also contains elements of breathing retraining and psychoeducation about common reactions to trauma. (For a comparison between the efficacy of imaginal and in vivo exposure, see Bryant et al., 2003.)
Stress inoculation training (SIT) (Meichenbaum, 1974) contains elements of relaxation training, thought stopping, self-guided dialogue, cognitive restructuring and covert modeling, and role play. Foa et al. (1991) compared SIT, PE, supportive counseling and waiting list conditions in 45 rape victims with PSTD, and found that while PTSD symptoms improved in all groups, SIT produced significantly more improvement immediately after treatment (55% symptom reduction, vs 40% for PE, 26% for supportive counseling, and 20% for waiting list controls). However, PE was superior at 3 months post-treatment—PTSD symptom reduction at this point was 60% for this modality compared with 49% for SIT and 36% for supportive counseling.
In a second study, Foa et al. (1999a,b) compared the efficacy of PE, SIT, both treatments combined, and waiting list among 96 female assault victims with chronic PTSD. These patients, whose post-assault intervals averaged 5 years, had a high prevalence of comorbid depression, but any medication regimens were to remain unchanged throughout the course of the study. Treatment consisted of nine sessions over 5 weeks. All three active treatments significantly reduced both PTSD and depressive symptoms to a similar extent; contrary to initial expectations, combined therapy did not improve outcomes over either single modality. In fact, exposure therapy alone yielded an effect size of 1.92, larger than SIT (1.61) or the combination (1.50). The gains were maintained through a 12-month followup period.
Using a slightly different design in which nine sessions of exposure therapy were extended over 9 weeks, optionally augmented by three additional sessions for those patients with a partial treatment response (<70% improvement), Foa et al. (in press) obtained similar results: adding cognitive restructuring to exposure therapy did not produce a significant benefit over that conferred by exposure therapy alone. One possible reason for this result is that exposure therapy alone is successful in modifying negative cognitions.
Similar results in favor of exposure therapy were found by Marks et al. (1998) among 87 male and female subjects who had suffered a variety of traumas. These authors compared the efficacy of 10 sessions of prolonged imaginal and live exposure, cognitive restructuring alone, the combination of the two, or relaxation therapy without prolonged exposure or cognitive restructuring. Both PE and cognitive restructuring were effective, but the combination did not offer increased benefits. In fact, PE produced over 50% improvement in end-state functioning, more than any of the other conditions including combination treatment (Fig. 16).
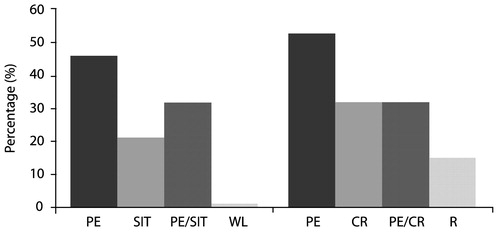
Figure 16. Good End-state Functioning Post-treatment*
Eye movement desensitization and reprocessing (EMDR) (Shapiro, 1995) is a therapy which involves accessing traumatic images and memories, evaluating their aversive qualities, and generating and focusing on alternative cognitive appraisals of these images and memories while performing sets of saccadic eye movements. Comparing EMDR with exposure therapy in female rape victims with PTSD, Rothbaum and Astin (2002) found that the latter produced a numerically but not statistically higher treatment response rate at 6 months follow-up (defined as a CAPS score reduction of ≥50%, a Beck Depression Inventory score <10, and a STAI-S score of <40). A meta-analysis (Davidson and Parker, 2001) encompassing 34 studies of EMDR in a variety of populations, including 20 studies of PTSD or treatment of a trauma memory, concluded that while EMDR is superior to no treatment and to non-exposure therapies, it is no more effective than other exposure therapies, and that the eye movements appear to be unimportant in its overall efficacy.
Does combining medication treatment with exposure therapy enhance treatment outcomes? In a study by Rothabuam et al. (unpubished observation), 64 patients who had been treated with sertraline for 10 weeks were randomized to continue sertraline alone or to receive, in addition, PE for 10 sessions over 5 weeks. In the entire sample, the effect size for the patients who received sertraline treatment alone was 1.7, while that for the combination was a striking 2.8. There was evidence of a floor effect; adding exposure therapy greatly improved treatment outcomes for patients with partial responses to medication, but not for those with excellent responses. However, in a subsample of 45 patients, those who had received PE did not relapse on discontinuation of medication, whereas 30% of those who had not had PE relapsed (Cahill et al., 2003).
The techniques of PE readily lend themselves to dissemination in order to serve a larger population. In a recent study by Foa et al. (in press), Masters-degree level therapists with no expertise in CBT were trained and supervised in PE with and without cognitive restructuring. Training consisted of 5 days for PE and 5 days for cognitive restructuring and 2 h of weekly supervision. These counselors went on to successfully treat patients with long-standing PTSD (mean duration of 9 years), some of whom had been refractory to other treatments. The initial reluctance of the trainees to make patients “suffer” from the exposure techniques was replaced by adoption of the treatment after experiencing its positive results. Prolonged exposure for PTSD has been selected by the Substance Abuse and Mental Health Services Administration (SAMHSA) as a model program for national dissemination.
8. FUTURE DIRECTIONS FOR RESEARCH
What are the key priorities for future research in the PTSD field? In the area of neurobiology, future studies are required to determine brain mechanisms underlying the successful response to treatment. Studies are also needed to examine changes in brain receptor and neuro-transmitter systems in greater detail in PTSD. In the areas of phenomenology and etiology, it is important to assess the validation of the PTSD and acute stress disorder constructs and their distinction from other disorders, as well as to improve our understanding of genetic and other biological contributions to PTSD susceptibility and the complex interrelationships between these factors and life experiences. The mechanisms that underlie success in treatment will continue to be elucidated, with the help of a variety of animal models for PTSD. Increased knowledge of the complex etiology of PTSD would not only help develop new therapeutic targets and treatments with improved short- and long-term efficacy, but also assist in identifying predictors of treatment response: Which patients require psychotherapy, pharmacotherapy, or a combination of modalities? Which drugs and/or types of psychotherapy are most suitable for a given individual? How can evidence-based psychotherapies be made available to a wider population in need? Finally, in analogy with major depressive disorder, PTSD needs an operational definition of remission, since the ultimate goal of treament is not merely reduction of symptoms, but full remission from this devastating condition.
American Psychiatric Association, Committee on Nomenclature and Statistics. Diagnostic and Statistical Manual of Mental Disorders. 3rd ed. Washington (DC): American Psychiatric Association; 1980.Google Scholar
Andrews B, Brewin CR, Rose S. Gender, social support, and PTSD in victims of violent crime. J Trauma Stress 2003; 16: 421– 7.Crossref, Google Scholar
Associated Press. Poll: Americans depressed, sleepless. Associated Press. 9/19/2001. Washington DC, Associated Press. Available from: www.chron.com/cs/CDA/story.hts/special/terror/impact/1055126.Google Scholar
Baker DG, West SA, Nicholson WE, et al. Serial CSF corticotropin-releasing hormone levels and adrenocortical activity in combat veterans with posttraumatic stress disorder. Am J Psychiatry 1999; 156: 585– 8.Google Scholar
Banki CM, Bissette G, Arato M, et al. CSF corticotropin-releasing factor-like immunoreactivity in depression and schizophrenia. Am J Psychiatry 1987; 144: 873– 7.Crossref, Google Scholar
Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Seial pathways from preimate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci 2003; 4: 25.Crossref, Google Scholar
Barr CS, Newman TK, Schwandt M, et al. Sexual dichotomy of an interaction between early adversity and the serotonin transporter gene promoter variant in rhesus macaques. Proc Natl Acad Sci USA 2004; 101: 12358– 63.Crossref, Google Scholar
Beals J, Manson SM, Shore JH, et al. The prevalence of posttraumatic stress disorder among American Indian Vietnam veterans: disparities and context. J Trauma Stress 2002; 15: 89– 97.Crossref, Google Scholar
Bennice JA, Resick PA, Mechanic M, Astin M. The relative effects of intimate partner physical and sexual violence on post-traumatic stress disorder symptomatology. Violence Vict 2003; 18: 87– 94.Crossref, Google Scholar
Bleiberg KL, Markowitz JC. A pilot study of interpersonal psychotherapy for posttraumatic stress disorder. Am J Psychiatry 2005; 162: 181– 3.Crossref, Google Scholar
Bonne O, Brandes D, Gilboa A, et al. Longitudinal MRI study of hippocampal volume in trauma survivors with PTSD. Am J Psychiatry 2001; 158: 1248– 51.Crossref, Google Scholar
Brady K, Pearlstein T, Asnis GM, et al. Efficacy and safety of sertraline treatment of posttraumatic stress disorder: a randomized controlled trial. JAMA 2000; 283: 1837– 44.Crossref, Google Scholar
Bremner JD. Neuroimaging of posttraumatic stress disorder. Psychiatric Ann 1998; 28: 445– 50.Crossref, Google Scholar
Bremner JD. Does stress damage the brain? In: Understanding trauma-related disorders from a mind-body perspective. New York: W.W. Norton; 2002.Google Scholar
Bremner JD, Licinio J, Darnell A, et al. Elevated CSF corticotropin-releasing factor concentrations in posttraumatic stress disorder. Am J Psychiatry 1997a; 154: 624– 9.Crossref, Google Scholar
Bremner JD, Narayan M, Staib LH, et al. Neural correlates of memories of childhood sexual abuse in women with and without posttraumatic stress disorder. Am J Psychiatry 1999a; 156: 1787– 95.Google Scholar
Bremner JD, Randall P, Scott TM, et al. Deficits in short-term memory in adult survivors of childhood abuse. Psychiatry Res 1995a; 59: 97– 107.Crossref, Google Scholar
Bremner JD, Randall PR, Vermetten E, et al. MRI-based measurement of hippocampal volume in posttraumatic stress disorder related to childhood physical and sexual abuse: a preliminary report. Biol Psychiatry 1997b; 41: 23– 32.Crossref, Google Scholar
Bremner JD, Scott TM, Delaney RC, et al. Deficits in short-term memory in posttraumatic stress disorder. Am J Psychiatry 1993a; 150: 1015– 9.Crossref, Google Scholar
Bremner JD, Randall P, Scott TM, et al. MRI-based measurement of hippocampal volume in patients with combat-related posttraumatic stress disorder. Am J Psychiatry 1995b; 152: 973– 81.Crossref, Google Scholar
Bremner JD, Southwick SM, Johnson DR, Yehuda R, Charney DS. Childhood physical abuse and combat-related posttraumatic stress disorder in Vietnam veterans. Am J Psychiatry 1993b; 150: 235– 9.Crossref, Google Scholar
Bremner JD, Staib LH, Kaloupek D, et al. Neural correlates of exposure to traumatic pictures and sound in Vietnam combat veterans with and without posttraumatic stress disorder: a positron emission tomography study. Biol Psychiatry 1999b; 45: 806– 16.Crossref, Google Scholar
Bremner JD, Vythilingam M, Vermetten E, et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am J Psychiatry 2003; 160: 924– 32.Crossref, Google Scholar
Breslau N, Davis GC. Posttraumatic stress disorder in an urban population of young adults: risk factors for chronicity. Am J Psychiatry 1992; 149: 671– 5.Crossref, Google Scholar
Breslau N, Davis GC, Andreski P, et al. Sex differences in posttraumatic stress disorder. Arch Gen Psychiatry 1997; 54: 1044– 8.Crossref, Google Scholar
Bryant RA, Moulds ML, Guthrie RM, et al. Imaginal exposure alone and imaginal exposure with cognitive restructuring in treatment of posttraumatic stress disorder. J Consulting Clin Psychol 2003; 71: 706– 12.Crossref, Google Scholar
Cahill SP, Foa EB, Rothbaum BO, Connor K, Smith R, Davidson JRT. Augmentation of sertraline with prolonged exposure (PE) in the treatment of PTSD: does PE protect against relapse? In: Poster presented at
Caldji C, Francis D, Sharma S, et al. The effects of early rearing environment on the development of GABAA and central benzodiazepine receptor levels and novelty-induced fearfulness in the rat. Neuropsychopharmacology 2000; 22: 219– 29.Crossref, Google Scholar
Card JJ. Epidemiology of PTSD in a national cohort of Vietnam veterans. J Clin Psychol 1987; 43: 6– 17.Crossref, Google Scholar
Carpenter LL, Tyrka AR, McDougle CJ, et al. Cerebrospinal fluid corticotropin-releasing factor and perceived early-life stress in depressed patients and healthy control subjects. Neuropsycho-pharmacology 2004; 29: 777– 84.Crossref, Google Scholar
Carrion VG, Weems CF, Eliez S, et al. Attenuation of frontal asymmetry in pediatric posttraumatic stress disorder. Biol Psychiatry 2001; 50: 943– 51.Crossref, Google Scholar
Caspi A, Sugden K, Moffitt TE, et al. Influence of life stress on depression: moderation by a polymorphism in the 5-HTT gene. Science 2003; 301: 386– 9.Crossref, Google Scholar
Chen B, Dowlatshahi D, MacQueen GM, Wang JF, Young LT. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol Psychiatry 2001; 50: 260– 5.Crossref, Google Scholar
Coplan JD, Andrews MW, Rosenblum LA, et al. Persistent elevations of cerebrospinal fluid concentrations of corticotropin-releasing factor in adult nonhuman primates exposed to early-life stressors: implications for the pathophysiology of mood and anxiety disorders. Proc Natl Acad Sci USA 1996; 93: 1619– 23.Crossref, Google Scholar
Davidson PR, Parker KC. Eye movement desensitization and reprocessing (EMDR): a meta-analysis. J Consult Clin Psychol 2001; 69: 305– 16.Crossref, Google Scholar
De Bellis MD, Hall J, Boring AM, Frustaci K, Moritz G. A pilot longitudinal study of hippocampal volumes in pediatric maltreatment-related posttraumatic stress disorder. Biol Psychiatry 2001; 50: 305– 9.Crossref, Google Scholar
De Bellis MD, Keshavan MS, Clark DB, et al. A.E. Bennett Research Award: Developmental traumatology: Part II. Brain development. Biol Psychiatry 1999; 45: 1271– 84.Crossref, Google Scholar
DeLisi LE, Maurizio A, Yost M, et al. A survey of New Yorkers after the Sept. 11, 2001, terrorist attacks. Am J Psychiatry 2003; 160: 780– 3.Crossref, Google Scholar
Dobie DJ, Kivlahan DR, Maynard C, Bush KR, Davis TM, Bradley KA. Posttraumatic stress disorder in female veterans: association with self-reported health problems and functional impairment. Arch Intern Med 2004; 164: 394– 400.Crossref, Google Scholar
Dube SR, Anda RF, Felitti VJ, et al. Childhood abuse, household dysfunction, and the risk of attempted suicide throughout the life span: findings from the Adverse Childhood Experiences Study. JAMA 2001; 286: 3089– 96.Crossref, Google Scholar
Duman RS, Malberg J, Thome J. Neural plasticity to stress and antidepressant treatment. Biol Psychiatry 1999; 19: 1181– 91.Crossref, Google Scholar
Duman RS, Nakagawa S, Malberg J. Regulation of adult neurogenesis by antidepressant treatment. Neuropsychopharmacology 2001; 25: 836– 44.Crossref, Google Scholar
Edwards VJ, Holden GW, Felitti VJ, Anda RF. Relationship between multiple forms of childhood maltreatment and adult mental health in community respondents: results from the adverse childhood experiences study. Am J Psychiatry 2003; 160: 1453– 60.Crossref, Google Scholar
Elzinga BM, Schmahl CG, Vermetten E, van Dyck R, Bremner JD. Higher cortisol levels following exposure to traumatic reminders in abuse-related PTSD. Neuropsychopharmacology 2003; 28: 1656– 65.Crossref, Google Scholar
Engel CC, Engel AL, Campbell SJ, McFall ME, Russo J, Katon W. Posttraumatic stress disorder symptoms and precombat sexual and physical abuse in Desert Storm veterans. J Nerv Ment Dis 1993; 181: 683– 8.Crossref, Google Scholar
Flach FF. Psychobiologic resilience, psychotherapy, and the creative process. Compr Psychiatry 1980; 21: 510– 8.Crossref, Google Scholar
Foa EB, Hembree EA, Cahill SP, Rauch SA, Riggs DS, Feeny NC, et al. Randomized trail of prolonged exposure for PTSD with and without cognitive restructuring: Outcome at academic and community clinics. J Consult Clin Psychol ( in press).Google Scholar
Foa EB, Jaycox LH. Cognitive-behavioral theory and treatment of post-traumatic stress disorder. In: Spiegel DS, editor. Efficacy and cost-effectiveness of psychotherapy. Washington (DC): American Psychiatric Press; 1999. p. 23– 61.Google Scholar
Foa EB, Rothbaum BO. Treating the trauma of rape. New York (NY): Guilford Press; 1998.Google Scholar
Foa EB, Dancu CV, Hembree EA, Jaycox LH, Meadows EA, Street GP. A comparison of exposure therapy, stress inoculation training, and their combination for reducing posttraumatic stress disorder in female assault victims. J Consult Clin Psychol 1999a; 67: 194– 200.Crossref, Google Scholar
Foa EB, Ehlers A, Clark D, Tolin DF, Orsillo SM. Posttraumatic Cognitions Inventory (PTCI): development and validation. Psychol Assess 1999b; 11: 303– 14.Crossref, Google Scholar
Foa EB, Keane TM, Friedman MJ, editors. Effective treatments for PTSD: practice guidelines from the International Society for Traumatic Stress Studies. New York: Guilford Press; 2000.Google Scholar
Foa EB, Rothbaum BO, Riggs DS, Murdock TB. Treatment of posttraumatic stress disorder in rape victims: a comparison between cognitive-behavioral procedures and counseling. J Consult Clin Psychol 1991; 59: 715– 23.Crossref, Google Scholar
Frans O, Rimmo PA, Aberg L, Fredrikson M. Trauma exposure and post-traumatic stress disorder in the general population. Acta Psychiatr Scand 2005; 111: 291– 9.Crossref, Google Scholar
Freedman LJ, Insel TR, Smith Y. Subcortical projections of area 25 (subgenual cortex) of the macaque monkey. J Comp Neurol 2000; 421: 172– 88.Crossref, Google Scholar
Freeman TW, Cardwell D, Karson CN, Komoroski RA. In vivo proton magnetic resonance spectroscopy of the medial temporal lobes of subjects with combat-related posttraumatic stress disorder. Magn Reson Med 1998; 40: 66– 71.Crossref, Google Scholar
Galea S, Ahern J, Resnick H, et al. Psychological sequelae of the September 11 terrorist attacks in New York City. New Engl J Med 2002; 346: 982– 7.Crossref, Google Scholar
Gilbertson MW, Shenton ME, Ciszewski A, et al. Smaller hippocampal volume predicts pathologic vulnerability to psychological trauma. Nature Neurosci 2002; 5: 1242– 7.Crossref, Google Scholar
Goldapple K, Segal Z, Garson C, et al. Modulation of cortical-limbic pathways in major depression: treatment specific effects of cognitive behavioral therapy. Arch Gen Psychiatry 2004a; 61: 34– 41.Crossref, Google Scholar
Goldapple K, Segal Z, Garson C, et al. Modulation of cortical-limbic pathways in major depression: treatment-specific effects of cognitive behavior therapy. Arch Gen Psychiatry 2004b; 61: 34– 41.Crossref, Google Scholar
Gould E, Tanapat P, McEwen BS, Flugge G, Fuchs E. Proliferation of granule cell precursors in the dentate gyrus of adult monkeys is diminished by stress. Proc Natl Acad Sci USA 1998; 95: 3168– 71.Crossref, Google Scholar
Gurvits TG, Shenton MR, Hokama H, et al. Magnetic resonance imaging study of hippocampal volume in chronic combat-related posttraumatic stress disorder. Biol Psychiatry 1996; 40: 192– 9.Crossref, Google Scholar
Heim C, Newport DJ, Heit S, et al. Pituitary-adrenal and autonomic responses to stress in women after sexual and physical abuse in childhood. JAMA 2000; 284: 592– 7.Crossref, Google Scholar
Heim C, Plotsky PM, Nemeroff CB. Importance of studying the contributions of early adverse experience to neurobiological findings in depression. Neuropsychopharmacology 2004; 29: 641– 8.Crossref, Google Scholar
Holbrook TL, Hoyt DB, Stein MB, Sieber WJ. Gender differences in long-term posttraumatic stress disorder outcomes after major trauma: women are at higher risk of adverse outcomes than men. J Trauma 2002; 53: 882– 8.Crossref, Google Scholar
Huot RL, Plotsky PM, Lenox RH, McNamara RK. Neonatal maternal separation reduces hippocampal mossy fiber density in adult Long Evans rats. Brain Res 2002; 950: 52– 63.Crossref, Google Scholar
Huot RL, Thrivikraman KV, Meaney MJ, Plotsky PM. Development of adult ethanol preference and anxiety as a consequence of neonatal maternal separation in Long Evans rats and reversal with antidepressant treatment. Psychopharmacology (Berl) 2001; 158: 366– 73.Crossref, Google Scholar
Kang H, Dalager N, Mahan C, Ishii E. The role of sexual assault on the risk of PTSD among Gulf War veterans. Ann Epidemiol 2005; 15: 191– 5.Crossref, Google Scholar
Keane TM, Fairbank JA, Caddell JM, et al. Implosive (flooding) therapy reduces symptoms of PTSD in Vietnam combat veterans. Behav Ther 1989; 20: 245– 60.Crossref, Google Scholar
Keightley ML, Seminowicz DA, Bagby RM, et al. Personality influences limbic-cortical interactions during sad mood induction. Neuroimage 2003; 20: 2031– 9.Crossref, Google Scholar
Keller MB, McCullough JP, Klein DN, et al. A comparison of nefazodone, the cognitive-behavioral-analysis system of psychotherapy, and their combination for the treatment of chronic depression. N Engl J Med 2000; 342: 1462– 70.Crossref, Google Scholar
Kendler KS, Kuhn J, Prescott CA. The interrelationship of neuroticism, sex, and stressful life events in the prediction of episodes of major depression. Am J Psychiatry 2004; 161: 631– 6.Crossref, Google Scholar
Kennedy SH, Evans KR, Kruger S, et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry 2001; 158: 899– 905.Crossref, Google Scholar
Kessler RC. Posttraumatic stress disorder: the burden to the individual and to society. J Clin Psychiatry 2000; 61( Suppl 5): 4– 12, discussion: 13–4.Google Scholar
Kessler RC, Sonnega A, Bromet E, et al. Posttraumatic stress disorder 0in the National Comorbidity Survey. Arch Gen Psychiatry 1995; 52: 1048– 60.Crossref, Google Scholar
Kruger S, Seminowicz D, Goldapple K, et al. State and trait influences on mood regulation in bipolar disorder: blood flow differences with an acute mood challenge. Biol Psychiatry 2003; 54: 1274– 83.Crossref, Google Scholar
Ladd CO, Owens MJ, Nemeroff CB. Persistent changes in corticotropin-releasing factor neuronal systems induced by maternal deprivation. Endocrinology 1996; 137: 1212– 8.Crossref, Google Scholar
Liotti M, Mayberg HS, Brannan SK, McGinnis S, Jerabek P, Fox PT. Differential limbic-cortical correlates of sadness and anxiety in healthy subjects: implications for affective disorder. Biol Psychiatry 2000; 48: 30– 42.Crossref, Google Scholar
Liotti M, Mayberg HS, McGinnis S, Brannan S, Jerabek P. Mood challenge in remitted unipolar depression unmasks disease-specific cerebral blood flow abnormalities. Am Psychiatry 2002a; 159: 1830– 40.Crossref, Google Scholar
Liotti M, Mayberg HS, McGinnis S, et al. Unmasking disease-specific cerebral blood flow abnormalities: mood challenge in patients with remitted unipolar depression. Am J Psychiatry 2002b; 159: 1830– 40.Crossref, Google Scholar
Long N, MacDonald C, Chamberlain K. Prevalence of posttraumatic stress disorder, depression and anxiety in a community sample of New Zealand Vietnam War veterans. Aust N Z J Psychiatry 1996; 30: 253– 6.Crossref, Google Scholar
Marks I, Lovell K, Noshirvani H, Livanou M, Thrasher S. Treatment of posttraumatic stress disorder by exposure and/or cognitive restructuring: a controlled study. Arch Gen Psychiatry 1998; 55: 317– 25.Crossref, Google Scholar
Marshall RD, Beebe KL, Oldham M, Zaninelli R. Efficacy and safety of paroxetine treatment for chronic PTSD: a fixed-dose, placebo-controlled study. Am J Psychiatry 2001; 158: 1982– 8.Crossref, Google Scholar
Marshall RD, Schneider FR, Fallon BA, et al. An open trial of paroxetine in patients with noncombat-related, chronic posttraumatic stress disorder. J Clin Psychopharmacol 1998; 18: 10– 8.Crossref, Google Scholar
Mason JW, Giller EL, Kosten TR, Ostroff RB, Podd L. Urinary free-cortisol levels in posttraumatic stress disorder patients. J Nerv Ment Dis 1986; 174: 145– 9.Crossref, Google Scholar
Mayberg HS. Modulating dysfunctional limbic-cortical circuits in depression: towards development of brain-based algorithms for diagnosis and optimised treatment. Br Med Bull 2003; 65: 193– 207.Crossref, Google Scholar
Mayberg HS, Brannan SK, Mahurin RK, McGinnin S. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry 2000a; 48: 830– 43.Crossref, Google Scholar
Mayberg HS, Brannan SK, Tekell JL, et al. Regional metabolic effects of fluoxetine in major depression: serial changes and relationship to clinical response. Biol Psychiatry 2000b; 48: 830– 43.Crossref, Google Scholar
Mayberg HS, Liotti M, Brannan SK, et al. Reciprocal limbic-cortical function and negative mood: converging PET findings in depression and normal sadness. Am J Psychiatry 1999; 156: 675– 82.Google Scholar
Mayberg HS, Silva JA, Brannan SK, et al. The functional neuroanatomy of the placebo effect. Am J Psychiatry 2002; 159: 728– 37.Crossref, Google Scholar
McCauley J, Kern DE, Kolodner K, et al. Clinical characteristics of women with a history of childhood abuse: unhealed wounds. JAMA 1997; 277: 1362– 8.Crossref, Google Scholar
McCullough JP. Cognitive-behavioral analysis system of psychotherapy: an interactional treatment approach for dysthymic disorder. Psychiatry 1984; 47: 234– 50.Crossref, Google Scholar
McHolm AE, MacMillan HL, Jamieson E. The relationship between childhood physical abuse and suicidality among depressed women: results from a community sample. Am J Psychiatry 2003; 160: 933– 8.Crossref, Google Scholar
McMillen JC. Better for it: how people benefit from adversity? Soc Work 1999; 44: 455– 68.Crossref, Google Scholar
Meichenbaum D. Cognitive behavior modification. Morristown (NJ): General Learning Press; 1974.Google Scholar
Merriam-Webster's Collegiate Dictionary, 10th ed. Springfield (MA): Merriam-Webster; 1993.Google Scholar
Nemeroff CB. Clinical significance of psychoneuroendocrinology in psychiatry: focus on the thyroid and adrenal. J Clin Psychiatry 1989; 50( Suppl): 13– 20, discussion: 21–2.Google Scholar
Nemeroff CB, Heim CM, Thase ME, Klein DN, Rush J, Schatzberg AF, et al. Differential responses to psychotherapy versus pharmacotherapy in patients with chronic forms of major depression and childhood trauma. Proc Natl Acad Sci USA 2003; 100: 14293– 6.Crossref, Google Scholar
Neumaier JF, Edwards E, Plotsky PM. 5-HT(1B) mrna regulation in two animal models of altered stress reactivity. Biol Psychiatry 2002; 51: 902– 8.Crossref, Google Scholar
Norris FH, Friedman MJ, Watson PJ, Byrne CM, Diaz E, Kaniasty K. 60,000 disaster victims speak. Part I. An empirical review of the empirical literature, 1981–2001. Psychiatry 2002; 65: 207– 39.Crossref, Google Scholar
North CS, Nixon SJ, Shariat S, et al. Psychiatric disorders among survivors of the Oklahoma City bombing. JAMA 1999; 282: 755– 62.Crossref, Google Scholar
North CS, Spitznagel EL, Smith EM. A prospective study of coping after exposure to a mass murder episode. Ann Clin Psychiatry 2001; 13: 81– 7.Crossref, Google Scholar
Notestine CF, Stein MB, Kennedy CM, Archibald SL, Jernigan TL. Brain morphometry in female victims of intimate partner violence with and without posttraumatic stress disorder. Biol Psychiatry 2002; 51: 1089– 101.Crossref, Google Scholar
Ongur D, An X, Price JL. Prefrontal cortical projections to the hypothalamus in macaque monkeys. J Comp Neurol 1998; 401: 480– 505.Crossref, Google Scholar
Oquendo MA, Friend JM, Halberstam B, et al. Association of comorbid posttraumatic stress disorder and major depression with greater risk for suicidal behavior. Am J Psychiatry 2003; 160: 580– 2.Crossref, Google Scholar
Ouimette P, Cronkite R, Henson BR, Prins A, Gima K, Moos RH. Posttraumatic stress disorder and health status among female and male medical patients. J Traumatic Stress 2004; 17: 1– 9.Crossref, Google Scholar
Perkonigg A, Kessler RC, Storz S, Wittchen H-U. Traumatic events and post-traumatic stress disorder in the community: prevalence, risk factors and comorbidity. Acta Psychiatr Scand 2000; 101: 46– 59.Crossref, Google Scholar
Pihoker C, Owens MJ, Kuhn CM, Schanberg SM, Nemeroff CB. Maternal separation in neonatal rats elicits activation of the hypothalamic-pituitary-adrenocortical axis: a putative role for corticotropin-releasing factor. Psychoneuroendocrinology 1993; 18: 485– 93.Crossref, Google Scholar
Pitman RK. Investigating the pathogenesis of posttraumatic stress disorder with neuroimaging. J Clin Psychiatry 2001; 62: 47– 54.Google Scholar
Plichta SB, Falik M. Prevalence of violence and its implications for women's health. Womens Health Issues 2001; 11: 244– 58.Crossref, Google Scholar
Prigerson HG, Maciejewski PK, Rosenheck RA. Population attributable fractions of psychiatric disorders and behavioral outcomes associated with combat exposure among US men. Am J Public Health 2002; 92: 59– 63.Crossref, Google Scholar
Raadsheer FC, Hoogendijk WJ, Stam FC, et al. Increased numbers of corticotropin-releasing hormone expressing neurons in the hypothalamic paraventricular nucleus of depressed patients. Neuroendocrinology 1994; 60: 436– 44.Crossref, Google Scholar
Resick PA, Nishith P, Weaver TL, Astin MC, Feuer CA. A comparison of cognitive-processing therapy with prolonged exposure and a waiting condition for the treatment of chronic posttraumatic stress disorder in female rape victims. J Consulting Clin Psychol 2002; 70: 867– 79.Crossref, Google Scholar
Rothbaum BO, Astin MC. Prolonged exposure versus EMDR for PTSD rape victims. In: Zoellner LA (Chair), Recent innovations in post traumatic stress disorder treatment. Paper presented at
Safren SA, Gershuny BS, Marzol P, et al. History of childhood abuse in panic disorder, social phobia, and generalized anxiety disorder. J Nerv Ment Dis 2002; 190: 453– 6.Crossref, Google Scholar
Santarelli L, Saxe M, Gross C, et al. Requirement of hippocampal neurogenesis for the behavioral effects of antidepressants. Science 2003; 301: 805– 9.Crossref, Google Scholar
Schlenger WE, Caddell JM, Ebert L, et al. Psychological reactions to terrorist attacks: findings from the National Study of Americans' Reactions to September 11. JAMA 2002; 288: 581– 8.Crossref, Google Scholar
Schuff N, Neylan TC, Lenoci MA, et al. Decreased hippocampal N-acetylaspartate in the absence of atrophy in posttraumatic stress disorder. Biol Psychiatry 2001; 50: 952– 9.Crossref, Google Scholar
Schuster MA, Stein BD, Jaycox L, et al. A national survey of stress reactions after the September 11, 2001, terrorist attacks. New Engl J Med 2001; 345: 1507– 12.Crossref, Google Scholar
Seminowicz DA, Mayberg HS, McIntosh AR, et al. Limbic-frontal circuitry in major depression: a path modeling metanalysis. Neuroimage 2004; 22: 409– 18.Crossref, Google Scholar
Shapiro F. Eye movement desensitization and reprocessing: basic principles, protocols, and procedures. New York (NY): Guilford Press; 1995.Google Scholar
Shin LM, Shin PS, Heckers S, et al. Hippocampal function in posttraumatic stress disorder. Hippocampus 2004; 14: 292– 300.Crossref, Google Scholar
Silver RC, Holman EA, McIntosh DN, et al. Nationwide longitudinal study of psychological responses to September 11. JAMA 2002; 288: 1235– 44.Crossref, Google Scholar
Stefurak T, Mayberg H. Cortico-limbic-striatal dysfunction in depression: converging findings in basal ganglia disease & primary affective disorders. In: Bedard MA, Agid Y, Chouinard, et al., editors. Mental & behavioral dysfunction in movement disorders. Clifton, UK: Humanae Press; 2003. p. 321– 38.Google Scholar
Stein MB. A 46-year-old man with anxiety and nightmares after a motor vehicle collision. JAMA 2002; 288: 1513– 22.Crossref, Google Scholar
Stein MB, Kennedy C. Major depressive and post-traumatic stress disorder comorbidity in female victims of intimate partner violence. J Affect Disord 2001; 66: 133– 8.Crossref, Google Scholar
Stein MB, Walker JR, Forde DR. Gender differences in susceptibility to posttraumatic stress disorder. Behav Res Ther 2000; 38: 619– 28.Crossref, Google Scholar
Stein MB, Koverola C, Hanna C, Torchia MG, McClarty B. Hippocampal volume in women victimized by childhood sexual abuse. Psychol Med 1997a; 27: 951– 9.Crossref, Google Scholar
Stein MB, Yehuda R, Koverola C, Hanna C. Enhanced dexa-methasone suppression of plasma cortisol in adult women traumatized by childhood sexual abuse. Biol Psychiatry 1997b; 42: 680– 6.Crossref, Google Scholar
Tucker P, Zaninelli R, Yehuda R, Ruggiero L, Dillingham K, Pitts CD. Paroxetine in the treatment of chronic posttraumatic stress disorder: results of a placebo-controlled, flexible-dosage trial. J Clin Psychiatry 2001; 62: 860– 8.Crossref, Google Scholar
Unpublished observations.Google Scholar
Vaidya VA, Marek GJ, Aghajanian GK, Duman RS. 5-HT2A receptor-mediated regulation of brain-derived neurotrophic factor mRNA in the hippocampus and the neocortex. J Neurosci 1997; 17: 2785– 95.Crossref, Google Scholar
Vaisrub S. Editorial: Da Costa syndrome revisited. JAMA 1975; 232: 164.Crossref, Google Scholar
Van Loey NE, Maas CJ, Faber AW, Taal LA. Predictors of chronic posttraumatic stress symptoms following burn injury: results of a longitudinal study. J Trauma Stress 2003; 16: 361– 9.Crossref, Google Scholar
Vermetten E, Vythilingam M, Southwick SM, Charney DS, Bremner JD. Long-term treatment with paroxetine increases verbal declarative memory and hippocampal volume in posttraumatic stress disorder. Biol Psychiatry 2003; 54: 693– 702.Crossref, Google Scholar
Villareale G, Hamilton DA, Petropoulos H, et al. Reduced hippocampal volume and total white matter in posttraumatic stress disorder. Biol Psychiatry 2002; 15: 119– 25.Crossref, Google Scholar
Walker EA, Katon W, Russo J, et al. Health care costs associated with posttraumatic stress disorder symptoms in women. Arch Gen Psychiatry 2003; 60: 369– 74.Crossref, Google Scholar
Wolin SJ. The resilient self: how survivors of troubled families rise above adversity? New York: Villard Books; 1993.Google Scholar
Woolley CS, Gould E, McEwen BS. Exposure to excess glucocorticoids alters dendritic morphology of adult hippocampal pyramidal neurons. Brain Res 1990; 531: 225– 31.Crossref, Google Scholar
Yehuda R, Kahana B, Binder-Brynes K, Southwick S, Mason J, Giller E. Low urinary cortisol excretion in Holocaust survivors with posttraumatic stress disorder. Am J Psychiatry 1995; 152: 982– 90.Crossref, Google Scholar
Yehuda R, Southwick SM, Krystal JH, Bremner D, Charney DS, Mason JW. Enhanced suppression of cortisol following dexamethasone administration in posttraumatic stress disorder. Am J Psychiatry 1993; 150: 83– 6.Crossref, Google Scholar



