Brighten the Future: Photobiomodulation and Optogenetics
Abstract
Safe, noninvasive, and effective treatments for brain conditions are everyone’s dream. Low-level light therapy (LLLT) based on the photobiomodulation (PBM) phenomenon has recently been adopted in practice, with solid scientific evidence. Optogenetics provides high spatiotemporal resolution to precisely switch on and off a particular circuitry in the brain. However, there are currently no human trials of optogenetics on the human brain. These two approaches—PBM and optogenetics—are promising photonic treatments that target the brain using completely different technologies. PBM is based on the mitochondrial reaction to the photons for up- or downregulation on the cytochrome c oxidase synthase in cellular respiration. It is safe, noninvasive, and good for long-term treatments, with wide applications using light wavelengths ranging from 650 nm to ≈1,100 nm, the red to near-infrared range. Optogenetics is based on the expression of engineered opsins on targeted tissues through viral vectors. The opsins are engineered to be sensors, actuators, or switches and could be precisely controlled by light wavelength ranging from 450 nm to ≈650 nm, the visible light range. The penetration of visible light is limited, and thus the photons cannot be applied directly outside the head without surgical means to create a physical window. PBM using near-infrared light could reach deeper tissues for light directly applied outside the head. Detailed scientific foundations and the state of the art for both technologies are reviewed. Ongoing developments are discussed to provide insight for future research and applications.
The deterioration of brain function during the aging or healing process from trauma is the key factor to quality of life. Can brain function be enhanced? Or can brain deterioration due to aging be delayed? That has been a heavily studied topic in the areas of physical medicine and rehabilitation (1).
Photobiomodulation and Low-Level Light Therapy
Introduction
Among the noninvasive treatments, low-level light therapy (LLLT), with the fundamental phenomenon called photobiomodulation (PBM), has increasingly become a mainstream modality (1). Although at first LLLT was mainly used for wound healing and pain relief, the medical applications of LLLT have broadened to include diseases such as strokes, myocardial infarctions, and degenerative or traumatic brain disorders (2–8). In addition, upconversion nano-particles that are excited at near infrared (NIR) and emit blue light have been fabricated and demonstrated to trigger neural activations in the deep brain through optogenetics (9).
LLLT uses low-powered laser light in the range of 1–1,000 mW, at wavelengths ranging from 632 nm to 1,064 nm, to stimulate a biological response (1). LLLT is different from other external stimuli, such as heat, sound, or vibration, as LLLT acts through inducing a photochemical reaction within the cell, a process referred to as biostimulation or PBM (1). There are many different mechanisms of the photon to affect the bio tissue or the metabolic system. For the LLLT on the brain, its basic mechanism is to excite the cytochrome c oxidase (CCO), which is a mitochondrial respiratory enzyme. The CCO is a photoacceptor that could be excited at 620 nm to 1,100 nm (10–14). The benefits of PBM could be observed through hemodynamics. Specifically, the increase of oxygenated hemoglobin concentration, the decrease of deoxygenated hemoglobin concentration, and the improvement of cerebral oxygenation (i.e., the difference between the oxygenated hemoglobin and the deoxygenated hemoglobin concentrations) (3, 6).
The first controlled study demonstrating the beneficial effects of LLLT on cognitive and emotional functions in humans showed that LLLT could be used as a noninvasive and efficacious approach to increase brain function (15). In another study, the LLLT’s relation to electroencephalography (EEG) was showcased, along with its enhancement of cognitive function in elderly people (10). More and more experiments with promising results (2–8) have indicated that LLLT, using NIR light, is an effective and noninvasive approach to enhance brain function.
Mechanism
PBM involves the absorption of photons and the subsequent modulation of metabolic processes in cells, including neurons (16, 17). Generally, PBM is the interaction of the photons/light with biological tissues, particularly the interaction with mitochondria. These interactions could be classified into at least three categories: the CCO regulation, nitric oxide synthase, and the shaping of membrane cristae. These interactions are not completely independent. Instead, they affect each other closely, making PBM a very sophisticated phenomenon. A simplified flow chart of the PBM mechanism can be found in the article by Wang et al. (18, Figure 6, part a).
For red to NIR light, the major intracellular molecule-absorbing photons is CCO (19), a mitochondrial respiratory enzyme that can be upregulated in vitro and in vivo (3, 11, 20, 21). Upregulation of CCO serves to convert high-energy photons into a source for ATP-based metabolic energy production in the brain (22). PBM of neural functions has been successfully demonstrated at 633-nm to 1,070-nm wavelengths (2, 23, 24).
Hormetic Effect
LLLT does not induce classical linear dose-response pharmacological effects. LLLT effects are characterized by inverted U-shaped dose-response curves, as shown in Figure 1, in which linear responses may be seen only at very low doses. Whereas linear effects may be negligible, maximal stimulatory effects are typically observed at intermediate doses. However, the linear relationship does not hold at high doses, because inhibitory effects are observed instead. In fact, the inhibitory effects of very high LLLT doses might be worse than control conditions (e.g., tissue destruction). A key observation concerning the modulatory effects of light in tissues is that maximal responses at intermediate doses tend to represent less than twofold increases in biological variables relative to baseline conditions. Yet these effects have been shown to have major relevance, especially when energy metabolism is involved in nervous tissue. Thus, hormesis is an essential concept for the development of neurotherapeutic applications of LLLT (25).
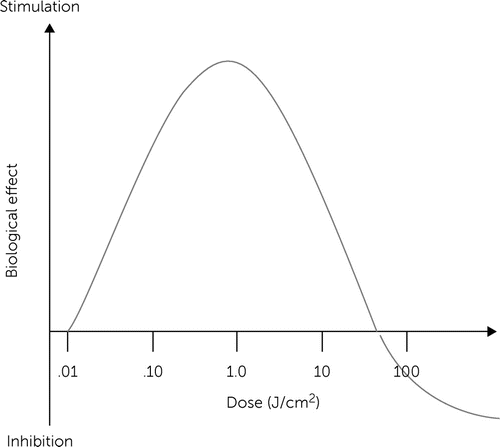
FIGURE 1. Hormetic effects of low-level light therapya
a Source: Rojas and Gonzalez-Lima (25). Reprinted with permission.
Clinical Trials
Naeser and collaborators (26) pioneered using transcranial PBM with light-emitting diode (LED) arrays of 633 nm and 870 nm. Studies showed improvement of cognitive function in patients with mild traumatic brain injury. In addition, 1,064-nm transcranial NIR laser stimulation (TILS) has been proven effective in increasing cognitive and emotional functions in controlled studies using PBM aimed at the right prefrontal cortex (4, 5, 7, 15, 27). In particular, TILS of the human prefrontal cortex with a wavelength of 1,064 nm increases the levels of oxidized CCO, the conformation of the enzyme that has the highest activity, which leads to improved cerebral oxygenation (6, 18).
This photonics bioenergetics in vivo mechanism is important for cognitive brain function because nerve cells are critically dependent on oxidative energy metabolism (8, 23, 25). The TILS may also improve cognitive performance in middle-aged and older adults at risk of cognitive decline (10). This risk may be associated with aging-related subjective memory complaints, cardiovascular disease, or brain trauma. For example, cerebrovascular disease poses a severe threat to public health and is expected to become more widespread as the worldwide population ages. Specifically, atherosclerosis of the carotid artery is a strong predictor of cognitive decline (28). The carotid artery intima-media thickness (IMT) is also recognized as a risk factor for brain damage in asymptomatic patients (29). Middle-aged and older adults reporting memory complaints, showing early signs of carotid atherosclerosis (increased IMT values), or with a history of brain trauma are prime candidates for early interventions aimed to minimize or prevent cognitive decline.
One potential noninvasive intervention may be increasing cerebral oxygenation by upregulating mitochondrial respiration using TILS (18, 30). In one study, TILS was administered using the same 1,064-nm laser and stimulation parameters as in the previous cognitive studies with young adults (4, 7, 15, 27) but with repeated weekly sessions for 5 weeks (10). The laser was aimed at the forehead using an internal red diode aiming light. Because the 1,064-nm laser is invisible, the beam area provided visual confirmation to facilitate precise tissue targeting. During laser operation, participants were instructed to keep their eyes closed, and the experimenters and participants both wore dark safety glasses to block the specific infrared wavelength from reaching the eyes (10). The goal was to investigate the neurocognitive effects of TILS on the prefrontal cortex’s ability to retain attention and memory in a middle-aged and older population, using IMT values measured by carotid ultrasound, and to evaluate the potential neural mechanisms mediating the cognitive effects using exploratory brain studies of both EEG and functional magnetic resonance imaging. The increased resting-state EEG alpha, beta, and gamma power, by an increment of prefrontal blood oxygen level after irradiation with 1,064 nm and 816 J (3.4 W, 13.6 cm2) was first demonstrated in the study by Vargas et al. (10). That study established the fundamental link between PBM and EEG.
Excitatory and Inhibitory Regulation
The modulation phenomenon is not limited to upregulation. Sanderson et al. (12) found that scanning the NIR spectrum between 700 nm and 1,000 nm revealed two NIR wavelengths (750 nm and 950 nm) that reduced the activity of isolated CCO. The CCO-inhibitory NIR was adopted as a potential form of therapy for cerebral reperfusion injury using a rat model of global brain ischemia. Untreated animals demonstrated an 86% loss of neurons in the CA1 hippocampus postreperfusion, whereas inhibitory NIR groups were robustly protected, with neuronal loss ranging from 11% to 35%. Moreover, neurologic function, assessed by radial arm maze performance, was preserved at control levels in rats treated with a combination of both CCO-inhibitory NIR wavelengths. The data from this study suggest that CCO-inhibitory NIR may be a viable nonpharmacologic and noninvasive therapy for the treatment of cerebral reperfusion injury (12).
Photon Propagation in the Brain
Because brain tissue, such as gray matter, is below the scalp and the skull, among the cerebrospinal fluid (CSF), and within the gyri folding—the tissues that increase surface area for the billions of neurons and connections—the brain model is a fundamentally multilayer structure with various optical parameters. Therefore, in the past decades, researchers have faced the challenge of finding the proper wavelength, the power, and the exposure time necessary to achieve consistent results. In one study, the 810-nm wavelength was considered to deliver the most energy to target tissues (31). In others, 1,064 nm was used to show enhanced cognitive function in various human trials (10, 15).
In several studies, there has been a continuous argument regarding the best settings of the wavelengths, power, and exposure time (10, 16, 17, 31). Several computational model and simulation results have been presented. However, all these results are incomplete because the three-dimensional (3D) volume head models are inaccurate or the optical parameters settings are biased to a certain range. Therefore, the concluded best setting of LLLT has varied quite substantially. In one study, researchers examined a wide range of wavelengths and considered the optical parameters for all the boundary conditions (i.e., upper bound and lower bound) at wavelengths ranging from 650 nm to 1,064 nm (13). The researchers built the 3D volume head model for each participant in order to study the variation among them. Results indicated that the customized setting for each person is very important to achieve the optimized result. That finding also explains why there is no conclusive answer for the best settings for every individual, simply because there is no optimal setting for all the conditions.
The delivery of photon energy to the target tissues depends on the multilayered anatomical structure with various optical parameter combinations (13). A 3D volume computational model is built on the magnetic resonance imaging of each participant to encompass variations in the thickness and shape of each tissue layer and gyri. The upper and lower bounds of the optical parameters, such as absorption and scattering coefficients at various wavelengths, are considered for each layer per person. Monte Carlo simulations are performed with all possible combinations of the optical parameters for wavelengths ranging from 650 nm to 1,064 nm. Furthermore, different geometrical patterns of radiation sources, such as collimated beams and cone beams with different spatial and angular distributions, are implemented to find different profiles of photon energy delivery for LLLT on the prefrontal cortex.
The biphasic hormetic dose-response, where bioenergetics are stimulated at a low dose and inhibited at a high dose, is well observed in all photon stimulations. The amount of photon energy delivered to the brain is affected by the wavelength, as well as by the multilayered head structure, with variations of optical parameters. A real 3D volume head model was built for each participant in this study, and the boundary conditions of each optical parameter in each layer were considered (13). The Monte Carlo simulation with wavelengths ranging from 650 nm to 1,064 nm was implemented to investigate the energy delivered to the brain under different radiation profiles. Generally, 1,064-nm photons penetrated deeper than 810-nm photons, except for scalp absorption at the lower bound due to low melanin content. Collimated-beam radiation was better than diverging-beam due to more uniform intensity distribution at the surface of the scalp. Further research to optimize LLLT dosage for each individual is imperative because of the high interperson variability in structure and optical parameters.
Fundamental Cell Studies
PBM is a fundamental phenomenon and can be observed in all cells with mitochondria (i.e., the eukaryotic cell). Recently, researchers focused on the effects of acute infrared treatment on mitochondria respiration in human cell lines under different wavelengths and intensities (Wei A, Huang CH, Huang LD, unpublished study). The oxygen consumption rate of suspended cultured cells was measured using a Clarke-type electrode under different light wavelengths and intensities that ranged from NIR to short-wavelength infrared. In the wavelength ranges, 810 nm and 1,050 nm showed upregulation of mitochondrial respiration, and 750 nm and 940 nm showed downregulation. The same trend was observed in a parallel CCO activity, where a colorimetric assay measured the absorption of cytochrome c at 550 nm. Among five tested cell lines with different human organ origins (AC16, PANC-1, HEK293, HepG2, and IMR32 cell lines), the HepG2 cell line showed the greatest activity increase under low-energy light treatment of 1,050 nm (1.07≈3.06 J/cm2). The AC16 cells maintained the best TMRM fluorescence intensity among these five cell lines (Figure 2).
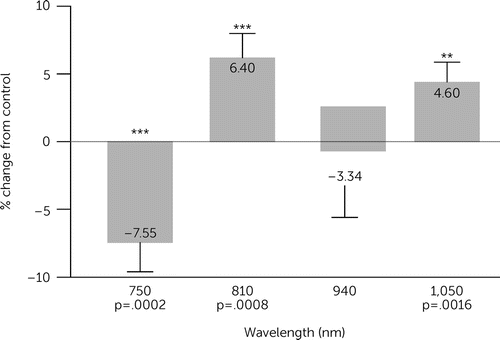
FIGURE 2. Effects of acute infrared treatment on mitochondria respiration in human cell lines under different wavelengths and intensitiesa
a 810-nm and 1,050-nm wavelength showed upregulation of mitochondrial respiration, and 750 nm and 940 nm showed downregulation. Image courtesy of Prof. Anchi Wei, National Taiwan University, Taipei.
Further Applications
The mitochondria are a fundamental subcellular organelle that could be modulated by the photons. Therefore, PBM covers very wide applications, from musculoskeletal pain and inflammation management (32) to treatments of Alzheimer diseases (33), Parkinson’s diseases (34), brain traumas (1), cognitive functions impairment (10, 35), depression (36), bipolar conditions (37), and general longevity wellness. The first controlled study demonstrated that TILS improved human cognitive and emotional brain function (10).
EEG measures voltage fluctuations resulting from ionic current within the neurons of the brain. Because ATP is the major energy unit consumed by the ion pump and gate, PBM will theoretically affect EEG. The first study to establish the relationship between PBM and EEG demonstrated that PBM could increase resting-state alpha, beta, and gamma power by an increment of prefrontal blood oxygen level after irradiation with 1,064 nm and 816 J (3.4 W, 13.6 cm2) (10). Since then, many studies have been conducted to further investigate the modulation caused by PBM on electrophysiology—specifically, neural oscillations in the human brain.
A recent randomized, sham-controlled study tested the effects of PBM caused by laser stimulation on CCO oxidation and hemoglobin oxygenation in the prefrontal cortex of 68 healthy younger and older adults, ages 18–85 (35). To quantize the cerebrovascular changes, a broadband NIR spectroscopy was used for noninvasive monitoring of the oxidized CCO and hemoglobin oxygenation before, during, and after the 1,064-nm wavelength laser (IR-A laser; area, 13.6 cm2; power density, 250 mW/cm2) or the sham stimulation of the right anterior prefrontal cortex (Brodmann area 10). Compared with sham control, there was a significant laser-induced increase in oxidized CCO during laser stimulation, followed by a significant poststimulation increase in oxygenated hemoglobin and a decrease in deoxygenated hemoglobin. Furthermore, there was a greater laser-induced effect on CCO with increasing age, whereas laser-induced effects on cerebral hemodynamics decreased with increasing age. No adverse laser effects were found.
A proof-of-concept study examined whether TILS improves cognition in older euthymic bipolar patients who exhibit greater cognitive decline than is expected for age-matched controls (37). The researchers measured cognitive flexibility, verbal fluency, working memory, sustained attention, and impulsivity with tasks that have been shown to differentiate between healthy older adults and older adults with bipolar disorder. The results showed TILS-induced improvements in cognitive performance on the tasks that measure cognitive flexibility and impulsivity after five weekly sessions of TILS. This suggests that TILS is both safe and effective in helping alleviate the accelerated cognitive decline present in older patients with bipolar disorder.
Summary and Future Directions
LLLT based on PBM has been shown to be a very safe, noninvasive treatment for many conditions that are not limited to the brain. In the COVID-19 era, “brain fog” conditions have been reported globally without any effective medicine. LLLT has been adopted by some practitioners, and very positive results have been recorded.
The power densities and the light sources are two major factors in safety. The power density adopted in LLLT is relatively low, ranging from tens mW/cm2 up to hundreds mW/cm2. For the brain, 250 mW/cm2 is the highest setting in the research literature. For musculoskeletal pain and inflammation management, the power density setting usually is higher, such as 600 mW/cm2. The light from the LED diverges naturally, so it is considered relatively safe. On the other hand, the laser beam will be more dangerous to the eyes due to the refocus of the beam on the retina through the lens system of the eye. Generally, the laser equipment for the LLLT applications will be Class II medical devices, which need to go through the clinical trials or a 510K procedure in the Food and Drug Administration (FDA), whereas the LED devices could be considered as “low risk devices” under FDA “general wellness policy” when not claiming to “treat” or “restore” a function impaired due to a “disease” or “condition.” Instead, promoting relaxation and managing stress is the right way to adopt LED devices to enhance the cognitive functions of the brain.
Transcranial magnetic stimulation (TMS) is a noninvasive procedure that uses magnetic fields to stimulate nerve cells in the brain to improve symptoms of depression. TMS is typically used when other depression treatments have not been effective. However, results are usually inconsistent, except in the group with better cognitive function. The pretreatment of participants on PBM before TMS is an approach worthy of investigation. In the integrated treatment, the participants will do the PBM treatment right before the following TMS procedure. Further fundamental research is needed to fully reveal the potential of the PBM phenomenon. Integration of the stem cell or exosome therapies with PBM are ongoing topics.
Even though PBM is a fundamental phenomenon on the subcellular level, the response time is usually slow, and there is a delay between the stimulation and activation of the cell. Although the spatiotemporal resolution is low, the effective results and safety due to the noninvasive approach are a large advantage. On the other hand, the optogenetics focus on resolving the spatiotemporal resolution trades off somewhat with safety and invasiveness. The science and utilization of optogenetic technologies are very promising.
Optogenetics
Introduction
“Optogenetics” was introduced in 2006 by Deisseroth et al. (38) and broadly refers to an elegant approach that utilizes genetic engineering and optical technology to control and monitor biological functions of isolated, or in situ, cells, tissues, organs, or organisms, modified to express photosensitive proteins (38–41). The photons (light) could be any wavelength as long as there is a matching photosensitive protein working as an optical sensor or actuator to read out the fluorescent changes in biological activities or to allow light to manipulate the cellular biological functions. The goal is for photons to interact with a specific cell type, tissue, or organ of interest together, while the system would control the radiation of the photons and monitor the biological functions with high spatiotemporal resolutions.
In 1979, Francis Crick conceived the idea of using light to provide a rapid spatiotemporal control for targeting specific neurons. Although he lacked a method to apply photosensitive proteins in neuroscience at the time, the microbiologists had already known the existence of photosensitive proteins that regulate ion flow across the plasma membrane in some microorganisms. The breakthrough in this field was in 2003 by Nagel et al. (42), who demonstrated the feasibility of expressing microbial opsins, a light-sensitive ion channel protein, in nonexcitable mammalian cells and enabling fast, light-induced cell depolarization by tens of mV. Similarly, another pioneering study by Boyden et al. (43) demonstrated the efficacy of light in modulating the electrical excitability of neurons with high spatial and temporal resolution upon expression of microbial opsins in mammalian neurons.
Afterward, the biomedical applications of optogenetics evolved from precise and rapid control of individual cells in a vertebrate brain for deciphering the neural circuitry underlying behavior and diseases, to replacing approaches that were not precise enough to target specific neuron populations, highly invasive, or too slow in kinetics (43–46). Precise spatiotemporal control of individual cells is the key feature of optogenetics. This relies on techniques that develop the photosensitive proteins and use of genetic engineering to express these photosensitive proteins in the target cells, tissues, and organs (47).
Photoreceptor Proteins
Rhodopsin.
Photoreceptor proteins are actually very common in all organisms. From archaebacteria to humans, the protein called rhodopsin provides the capability to sense and to respond to light. Rhodopsin was discovered by Franz Christian Boll and generally consists of two components, opsin and retinal. Opsin is a G protein coupled receptor that is embedded in the lipid bilayer of cell membranes using seven protein transmembrane domains and passes through the cell membrane seven times (48). The retinal is a photosensitive chromophore. Based on the primary sequence and mode of action, opsins are categorized as microbial (type I) opsins and animal (type II) opsins. Despite the striking similarities between the type I and type II rhodopsins, the two families are distinguishable by sequence, structure, and mechanism. Type I is found in microbes, such as archaea, eubacteria, fungi, and algae, while type II is found in animals and humans (45). Until recently, type II rhodopsins were thought to be exclusively eumetazoan, although they have now also been found in fungal genomes (49, 50).
Due to the fast response and relative ease of genetic engineering on a single component protein, the microbial opsins (type I) are adopted more commonly than the animal opsins (type II) in optogenetics (47, 50).
Light-driven ion pumps, such as bacteriorhodopsins and halorhodopsins, ion channels, such as channelrhodopsins (ChRs), and sensors, such as sensory rhodopsin I and II, are all microbial opsins (type I) (51). Upon light stimulation, these opsins mediate transmembrane ionic currents and elicit specific biological responses, such as phototaxis and photophobia, by coupling with specific transducers (42, 52).
Animal opsins (type II) indirectly mediate transmembrane ionic potential by coupling with G-protein–mediated transduction pathways and are primarily involved in dim light vision and circadian clock (52, 53). However, they are less used in optogenetics.
These microbial opsins can be functionally divided into excitatory opsins and inhibitory opsins. The most commonly used excitatory opsin is channelrhodopsin (ChR2), a cation channel that opens in the presence of blue light (≈470 nm) to depolarize neurons (42). In contrast, inhibitory opsins, such as the chloride pump halorhodopsin (eNpHR) and the proton pump archaerhodospin (Arch), mediate hyperpolarizing currents that impede action potentials upon yellow light illumination (≈580 nm) (54, 55).
ChR, Blue-Light Sensitive (≈470-nm Wavelengths).
ChR was first discovered in unicellular green algae Chlamydomonas reinhardtii, where it provides the algal eyespot the ability to sense and respond to light (42, 52, 56). It has two forms, ChR1 and ChR2. In 2003, Nagel et al. (42) first cloned channelopsin-2 (Chop2) in Xenopus oocytes and in the presence of all-trans retinal to examine their functional characteristics and determine if functional rhodopsin (having covalently linked retinal, ChR2) can be obtained. Because the ChR2 has a faster response time and Chop2 exhibits the capability to link with an endogenous all-trans retinal and form functional ChR2 in the mammalian system (57), it has remained a prototypical and very commonly used optogenetic tool (39, 45) that is mostly activated by blue light (460 nm to ≈480 nm wavelengths).
ChR2 was originally demonstrated to drive spike trains ranging from 5 Hz to 30 Hz (43). However, many neurons are capable of firing far beyond 30 Hz (58). High-speed opsins are indispensable to investigate fast-spiking neuron types, such as Pvalb interneurons (59, 60); hence, oChIEF was developed in 2009 (57), ChETA developed in 2010 (61), and Chronos developed in 2014 (62). These ChR2 variants can reliably evoke ultra-fast spiking in neurons, up to 100 Hz (63).
Halorhodopsin, Yellow-Light Sensitive (≈580-nm Wavelengths).
Halobacteria utilizes halorhodopsin (HR) for light-induced, inward-chloride transport across the outer membrane (64). Particularly, the HR from an archaeon, Natronomonas pharaonis (NpHR), is one of the most commonly used optogenetic proteins for generating hyperpolarizing—i.e., inhibitory currents—in mammalian cells (46, 65). It has been demonstrated to rapidly and reversibly silence targeted neurons using yellow-light pulses (560±27.5 nm, 10 mW/mm2) (66). The hippocampal neurons, expressing NpHR, elicited outward currents with fast kinetics (<20 ms) when voltage-clamped and produced rapid membrane hyperpolarization (≈100 ms) when current-clamped. Thus, in target regions with heterologous expression of NpHR and with the application of defined light stimulus, specific populations of neurons can be reversibly and reliably silenced with high spatiotemporal resolution. The mechanisms have been further explored, which has resulted in enhanced versions of NpHR pumps (eNpHR) to improve the ability to render mammalian cells electrically inexcitable and for safe and improved membrane trafficking (46, 67). The applications of eNpHR go beyond neuroscience and into cardiac optogenetics (68). The first reported use of eNpHR in zebrafish hearts caused hyperpolarization of myocardial cells and stopped heart contractions. Since then, several investigators have reported the use of eNpHR and other inhibitory optogenetic tools, such as Arch, for inhibition of cardiomyocytes activity (47, 69–71).
Optogenetic Sensors
To monitor the environment or the properties of the cells or tissue, the optogenetic sensors could be adopted. For example, the electrical and biochemical parameters, such as membrane voltage, calcium and chloride concentrations, pH, neurotransmitter release, etc., could be observed through optogenetic sensors (72, 73). Generally, this function is achieved by combining the sensing component with a single fluorescent protein or pair of fluorescent proteins (41). Thus, changes of the conformation of the sensing component caused by changes in the environment, such as voltage, pH, calcium, or any other physiological parameter, will alter the influence and optical properties of the linked fluorescent proteins, such as changes in their brightness or Förster resonance energy transfer (41). Particularly, the great progress in genetically encoded calcium indicators has made them available with a wide spectral sensitivity, binding affinity, and subcellular localization capability, which have allowed subtle monitoring of calcium changes both in in vivo and in situ settings—not attainable with conventional optical dyes (41, 74). If measurement of the voltage is needed, then genetically encoded voltage indicators (GEVIs) could be adopted. Based on the bacterial rhodopsin or voltage-sensing domain (VSD) of voltage-sensitive proteins, the GEVIs are also gaining greater attention in the field (41). VSD-based sensors evolved with the discovery of VSD linked to phosphatase in the sea squirt Ciona intestinalis and with the first construction and efficient plasma membrane localization of the voltage-sensitive fluorescent probe in mammalian cells by Dimitrov et al. (75–78).
New Variants of Opsins
Faster response time and enrichment of functionalities are the main driving forces to introduce new variants of opsins. Interestingly, fast response time achieves faster kinetics, and the extremely slow response time achieves more functionality, like a bistable switch.
Since 2010, the ChR2 has been modified to achieve faster kinetics. In addition to faster response time for higher temporal resolution, several functions have been added to the ChR2 variants, such as step function opsin (SFO) (79) and stabilized step function opsin (SSFO) (80), which have been engineered to produce very slow kinetics. Furthermore, the bistable switch could be established by using blue light to produce prolonged depolarization and by using amber light to rapidly terminate the depolarization. These opsins have mutations at the C128 position in ChR2 that substantially extend the period of activation (63). These functionalities enable many unique sets of experimental advantages.
Although SFO can support stable depolarization for minutes (79), SSFO-mediated depolarization is stable over the 30-minute time scale (80). This unique property makes SSFO useful for in vivo investigations, because complex animal behaviors typically occur on the time scale of minutes.
An example of using these functional ChRs is to utilize SSFO as a bistable switch that is paired with two-photon calcium imaging. It solves the issue of visible light from optogenetic stimulation interfering with GCaMP-based calcium imaging.
Makino and Komiyama (81) used a virus carrying a Cre-dependent SSFO gene to conditionally express SSFO in Sst inhibitory neurons of Sst-Cre mice and expressed GCaMP6f in pyramidal neurons. Thus the researchers used blue light to turn on the Sst interneuron activity and used amber light to turn it off, while performing calcium imaging of mostly pyramidal neurons. The use of SSFO in this study enabled cell-type-specific optogenetic manipulation throughout the entire course of a complex behavioral task with simultaneous calcium imaging. The flexibility of optogenetics enables control within animals and within sessions.
The level of control could be extended to using light to initiate intracellular signaling pathways (82) by using the opto-XR family of opsins. This family of opsins was engineered by replacing the intracellular loops of rhodopsin with those from specific G-protein–coupled receptors. One example is opto-α1-adrenergic receptor (AR), which, when photostimulated, led to a significant increase in IP3 signaling in HEK cells. When this chimeric opsin-receptor was expressed in the nucleus accumbens of mice and photoactivated during a place preference task, mice spent significantly more time in the conditioned area in the subsequent session than did control mice without the optical stimulation, indicating that opto-α1-AR can alter cell signaling in vivo and affect animal behavior (82). This experiment demonstrated the applicability of optogenetics beyond ionic manipulations and examined the effects of metabotropic cell signaling on behavior.
Issues With Optogenetics and Human Trials
The wavelengths adopted in optogenetics are almost in visible light (450 nm to ≈650 nm), blue to red light. For example, ChRs are mostly in blue light (≈470 nm), and the HRs are in yellow light (≈580 nm). The photons in these ranges cannot penetrate through the skin, bone, and other tissues, particularly the head. The head has the scalp, skull, dura mater, and CSF before the photons could reach the gray matter (i.e., the neuron) and the white matter. The optical window for the light to penetrate these multilayer tissues is around 650 nm to ≈1,350 nm, which is in the NIR range. This is why surgery to open the skull in rats is needed in in vivo research.
The expression of the opsin is another important factor in optogenetics. These engineered opsins are not originally in the target tissues. The delivery methods are through viruses and are considerably invasive. Any clinical application of optogenetics requires the use of a viral vector to transduce the target tissue with the chosen opsin. Introducing vectors to the brain requires a neurosurgical procedure, necessitating the use of a vector that ensures long-term protein expression within neuronal cells from a single transduction. This is one of the major factors in determining why no clinical trial on the human brain has been done yet. Nevertheless, a human trial using optogenetics to help a blind patient achieve partial recovery of the visual function has been done recently (83, 84).
To overcome the delivery of visible light into deep tissues, there are proposals suggesting the adoption of upconversion nano-particles that are excited at NIR (700 nm to ≈1,100 nm) and emit blue light and that have been fabricated and demonstrated to trigger neural activations in the deep brain through optogenetics (9). The NIR light is noninvasive and could penetrate deeper into the brain from outside the scalp. The nano-particles upconvert the NIR light to visible light (450 nm to ≈650 nm) to activate the opsins. However, the efficiency of the upconversion and the dosage needed to effectively enable the optogenetics is still under investigation.
Conclusions
Two major noninvasive photonic treatments for brain conditions and relevant research have been reviewed and compared. PBM is adopted mostly in the red or NIR band (650 nm to ≈1,100 nm). Optogenetics adopts mostly on the visible light spectrum (450 nm to ≈650 nm). PBM is a fundamental and natural phenomenon of the cell. Mitochondria respond to the acceptable photons with up- or downregulation. Optogenetics relies on rhodopsins to respond to the matched light and turn on or off the designed actuators or sensors. These engineered rhodopsins are not within the original target tissues, and thus genetic engineering approaches need to be adopted to express these photosensitive proteins in the target cells, tissues, and organs. However, the spatiotemporal resolution is extremely excellent.
LLLT using PBM has been adopted in many practical applications and treatments by practitioners with positive results and no adverse side effects recorded. Optogenetics has no human clinical trials on the brain yet, but a human trial that helped a blind patient achieve partial recovery of visual function has been reported.
PBM research continues on a deeper understanding of the mechanism within the mitochondria machinery, which will be based on quantum mechanical properties. In addition, many applications and reports of clinical applications and integrations are widely spreading out. Optogenetics will continue to explore the safe deployment of engineered opsins into target tissues and how to effectively deliver the required photon to activate these opsins.
1 : Role of low-level laser therapy in neurorehabilitation. PM R 2010; 2(suppl 2):S292–S305.. doi: 10.1016/j.pmrj.2010.10.013Crossref, Google Scholar
2 : Neurological and psychological applications of transcranial lasers and LEDs. Biochem Pharmacol 2013; 86:447–457. doi: 10.1016/j.bcp.2013.06.012Crossref, Google Scholar
3 : Interplay between up-regulation of cytochrome-c-oxidase and hemoglobin oxygenation induced by near-infrared laser. Sci Rep 2016; 6:30540.. doi: 10.1038/srep30540Crossref, Google Scholar
4 : Transcranial laser stimulation as neuroenhancement for attention bias modification in adults with elevated depression symptoms. Brain Stimul 2016; 9:780–787.. doi: 10.1016/j.brs.2016.05.009Crossref, Google Scholar
5 : Cognitive enhancement by transcranial laser stimulation and acute aerobic exercise. Lasers Med Sci 2016; 31:1151–1160.. doi: 10.1007/s10103-016-1962-3Crossref, Google Scholar
6 : Transcranial laser stimulation improves human cerebral oxygenation. Lasers Surg Med 2016; 48:343–349.. doi: 10.1002/lsm.22471Crossref, Google Scholar
7 : Improving executive function using transcranial infrared laser stimulation. J Neuropsychol 2017; 11:14–25.. doi: 10.1111/jnp.12074Crossref, Google Scholar
8 : Augmentation of cognitive brain functions with transcranial lasers. Front Syst Neurosci 2014; 8:36. 10.3389/fnsys.2014.00036Crossref, Google Scholar
9 : Near-infrared deep brain stimulation via upconversion nanoparticle-mediated optogenetics. Science 2018; 359:679–684. 10.1126/science.aaq1144Crossref, Google Scholar
10 : Beneficial neurocognitive effects of transcranial laser in older adults. Lasers Med Sci 2017; 32:1153–1162. doi: 10.1007/s10103-017-2221-yCrossref, Google Scholar
11 : Photobiomodulation directly benefits primary neurons functionally inactivated by toxins: role of cytochrome c oxidase. J Biol Chem 2005; 280:4761–4771.. doi: 10.1074/jbc.M409650200Crossref, Google Scholar
12 : Inhibitory modulation of cytochrome c oxidase activity with specific near-infrared light wavelengths attenuates brain ischemia/reperfusion injury. Sci Rep 2018; 8:3481.. doi: 10.1038/s41598-018-21869-xCrossref, Google Scholar
13 : Simulation study on the optimization of photon energy delivered to the prefrontal cortex in low-level-light therapy using red to near-infrared light. IEEE J Sel Top Quantum Electron 2021; 27(4):1–10.. doi: 10.1109/JSTQE.2021.3051671Google Scholar
14 : From Jöbsis to the present day: a review of clinical near-infrared spectroscopy measurements of cerebral cytochrome-c-oxidase. J Biomed Opt 2016; 21:091307. 10.1117/1.JBO.21.9.091307Crossref, Google Scholar
15 : Transcranial infrared laser stimulation produces beneficial cognitive and emotional effects in humans. Neuroscience 2013; 230:13–23.. doi: 10.1016/j.neuroscience.2012.11.016Crossref, Google Scholar
16 : In vitro and in vivo optimization of infrared laser treatment for injured peripheral nerves. Lasers Surg Med 2014; 46:34–45.. doi: 10.1002/lsm.22212Crossref, Google Scholar
17 : Low-level light/laser therapy versus photobiomodulation therapy. Photomed Laser Surg 2015; 33:183–184.. doi: 10.1089/pho.2015.9848Crossref, Google Scholar
18 : Up-regulation of cerebral cytochrome-c-oxidase and hemodynamics by transcranial infrared laser stimulation: a broadband near-infrared spectroscopy study. J Cereb Blood Flow Metab 2017; 37:3789–3802Crossref, Google Scholar
19 : Absorption measurements of a cell monolayer relevant to phototherapy: reduction of cytochrome c oxidase under near IR radiation. J Photochem Photobiol B 2005; 81:98–106.. doi: 10.1016/j.jphotobiol.2005.07.002Crossref, Google Scholar
20 : Neuroprotective effects of near-infrared light in an in vivo model of mitochondrial optic neuropathy. J Neurosci 2008; 28:13511–13521.. doi: 10.1523/JNEUROSCI.3457-08.2008Crossref, Google Scholar
21 : Low-level light therapy improves cortical metabolic capacity and memory retention. J Alzheimers Dis 2012; 32:741–752.. doi: 10.3233/JAD-2012-120817Crossref, Google Scholar
22 : Effects of near-infra-red laser irradiation on adenosine triphosphate and adenosine diphosphate contents of rat brain tissue. Neurosci Lett 2002; 323:207–210.. doi: 10.1016/s0304-3940(02)00159-3Crossref, Google Scholar
23 :
24 : Shining light on the head: photobiomodulation for brain disorders. BBA Clin 2016; 6:113–124Crossref, Google Scholar
25 : Low-level light therapy of the eye and brain. Eye Brain 2011; 3:49–67Google Scholar
26 : Transcranial, red/near-infrared light-emitting diode therapy to improve cognition in chronic traumatic brain injury. Photomed Laser Surg 2016; 34:610–626Crossref, Google Scholar
27 : Transcranial infrared laser stimulation improves rule-based, but not information-integration, category learning in humans. Neurobiol Learn Mem 2017; 139:69–75Crossref, Google Scholar
28 : Verbal working memory and atherosclerosis in patients with cardiovascular disease: an fMRI study. J Neuroimaging 2007; 17:227–233. doi: 10.1111/j.1552-6569.2007.00110.xCrossref, Google Scholar
29 : Screening for subclinical coronary artery disease measuring carotid intima media thickness. Am J Cardiol 2009; 104:1383–1388. doi: 10.1016/j.amjcard.2009.07.005Crossref, Google Scholar
30 : Mitochondrial respiration as a target for neuroprotection and cognitive enhancement. Biochem Pharmacol 2014; 88:584–593Crossref, Google Scholar
31 : Selective photobiomodulation for emotion regulation: model-based dosimetry study. Neurophotonics 2019; 6:015004. doi: 10.1117/1.NPh.6.1.015004Crossref, Google Scholar
32 : Low level laser therapy for osteoarthritis and rheumatoid arthritis: a metaanalysis. J Rheumatol 2000; 27:1961–1969Google Scholar
33 : Photobiomodulation for Alzheimer’s disease: has the light dawned? Photonics 2019; 6:77. doi: 10.3390/photonics6030077Crossref, Google Scholar
34 : Trials begin for a new weapon against Parkinson’s: light. Science 2020; 369:1415–1416. doi: 10.1126/science.369.6510.1415Crossref, Google Scholar
35 : Transcranial laser stimulation: mitochondrial and cerebrovascular effects in younger and older healthy adults. Brain Stimul 2021; 14:440–449. doi: 10.1016/j.brs.2021.02.011Crossref, Google Scholar
36 : Psychological benefits 2 and 4 weeks after a single treatment with near infrared light to the forehead: a pilot study of 10 patients with major depression and anxiety. Behav Brain Funct 2009; 5:46. doi: 10.1186/1744-9081-5-46Crossref, Google Scholar
37 : Transcranial infrared laser stimulation improves cognition in older bipolar patients: proof of concept study. J Geriatr Psychiatry Neurol (Epub ahead of print, Feb 2, 2021). doi: 10.1177/0891988720988906Crossref, Google Scholar
38 : Next-generation optical technologies for illuminating genetically targeted brain circuits. J Neurosci 2006; 26:10380–10386. doi: 10.1523/JNEUROSCI.3863-06.2006Crossref, Google Scholar
39 : Cardiac optogenetics. Am J Physiol Heart Circ Physiol 2013; 304:H1179–H1191. doi: 10.1152/ajpheart.00432.2012Crossref, Google Scholar
40 : Cardiac optogenetics. Annu Int Conf IEEE Eng Med Biol Soc 2012; 2012:1386–1389. doi: 10.1109/EMBC.2012.6346197Google Scholar
41 : Cardiac optogenetics: using light to monitor cardiac physiology. Basic Res Cardiol 2017; 112:56. doi: 10.1007/s00395-017-0645-yCrossref, Google Scholar
42 : Channelrhodopsin-2, a directly light-gated cation-selective membrane channel. Proc Natl Acad Sci USA 2003; 100:13940–13945. doi: 10.1073/pnas.1936192100Crossref, Google Scholar
43 : Millisecond-timescale, genetically targeted optical control of neural activity. Nat Neurosci 2005; 8:1263–1268. doi: 10.1038/nn1525Crossref, Google Scholar
44 : Optogenetic interrogation of neural circuits: technology for probing mammalian brain structures. Nat Protoc 2010; 5:439–456. doi: 10.1038/nprot.2009.226Crossref, Google Scholar
45 : Molecular tools and approaches for optogenetics. Biol Psychiatry 2012; 71:1033–1038. doi: 10.1016/j.biopsych.2012.02.019Crossref, Google Scholar
46 : Making sense of optogenetics. Int J Neuropsychopharmacol 2015; 18:pyv079. doi: 10.1093/ijnp/pyv079Crossref, Google Scholar
47 : Optogenetics: background, methodological advances and potential applications for cardiovascular research and medicine. Front Bioeng Biotechnol 2020; 7:466. doi: 10.3389/fbioe.2019.00466Crossref, Google Scholar
48 : Crystal structure of rhodopsin: a G protein-coupled receptor. Science 2000; 289:739–745. doi: 10.1126/science.289.5480.739Crossref, Google Scholar
49 : An empirical test of convergent evolution in rhodopsins. Mol Biol Evol 2014; 31:85–95. doi: 10.1093/molbev/mst171Crossref, Google Scholar
50 : Plant and fungal photopigments. WIREs Membr Transp Signal. 2012; 1:411–432Crossref, Google Scholar
51 : Microbial rhodopsins: diversity, mechanisms, and optogenetic applications. Annu Rev Biochem 2017; 86:845–872. doi: 10.1146/annurev-biochem-101910-144233Crossref, Google Scholar
52 : Microbial and animal rhodopsins: structures, functions, and molecular mechanisms. Chem Rev 2014; 114:126–163. doi: 10.1021/cr4003769Crossref, Google Scholar
53 : Crystal structure of the channelrhodopsin light-gated cation channel. Nature 2012; 482:369–374. 10.1038/nature10870Crossref, Google Scholar
54 : Multimodal fast optical interrogation of neural circuitry. Nature 2007; 446:633–639. doi: 10.1038/nature05744Crossref, Google Scholar
55 : High-performance genetically targetable optical neural silencing by light-driven proton pumps. Nature 2010; 463:98–102. doi: 10.1038/nature08652Crossref, Google Scholar
56 : Channelrhodopsin-1: a light-gated proton channel in green algae. Science 2002; 296:2395–2398. doi: 10.1126/science.1072068Crossref, Google Scholar
57 : Characterization of engineered channelrhodopsin variants with improved properties and kinetics. Biophys J 2009; 96:1803–1814. doi: 10.1016/j.bpj.2008.11.034Crossref, Google Scholar
58 : Neural activity in barrel cortex underlying vibrissa-based object localization in mice. Neuron 2010; 67:1048–1061. doi: 10.1016/j.neuron.2010.08.026Crossref, Google Scholar
59 : Gamma oscillations in the entorhinal cortex of the freely behaving rat. J Neurosci 1998; 18:388–398. doi: 10.1523/JNEUROSCI.18-01-00388.1998Crossref, Google Scholar
60 : Spike timing of distinct types of GABAergic interneuron during hippocampal gamma oscillations in vitro. J Neurosci 2004; 24:9127–9137. doi: 10.1523/JNEUROSCI.2113-04.2004Crossref, Google Scholar
61 : Ultrafast optogenetic control. Nat Neurosci 2010; 13:387–392. doi: 10.1038/nn.2495Crossref, Google Scholar
62 : Independent optical excitation of distinct neural populations. Nat Methods 2014; 11:338–346. doi: 10.1038/nmeth.2836Crossref, Google Scholar
63 : Light up the brain: the application of optogenetics in cell-type specific dissection of mouse brain circuits. Front Neural Circuits 2020; 14:18. doi: 10.3389/fncir.2020.00018Crossref, Google Scholar
64 : Halorhodopsin: a light-driven chloride ion pump. Annu Rev Biophys Biophys Chem 1986; 15:11–28. doi: 10.1146/annurev.bb.15.060186.000303Crossref, Google Scholar
65 : Silencing neurons: tools, applications, and experimental constraints. Neuron 2017; 95:504–529. doi: 10.1016/j.neuron.2017.06.050Crossref, Google Scholar
66 : Multiple-color optical activation, silencing, and desynchronization of neural activity, with single-spike temporal resolution. PLoS One 2007; 2:e299. doi: 10.1371/journal.pone.0000299Crossref, Google Scholar
67 : eNpHR: a Natronomonas halorhodopsin enhanced for optogenetic applications. Brain Cell Biol 2008; 36:129–139. doi: 10.1007/s11068-008-9027-6Crossref, Google Scholar
68 : Optogenetic control of cardiac function. Science 2010; 330:971–974. doi: 10.1126/science.1195929Crossref, Google Scholar
69 : Cardiac optogenetics; in 2012 International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, 2012. doi: 10.1109/EMBC.2012.6346197Google Scholar
70 : Modulation of cardiac tissue electrophysiological properties with light-sensitive proteins. Cardiovasc Res 2014; 102:176–187. doi: 10.1093/cvr/cvu037Crossref, Google Scholar
71 : Optical mapping of optogenetically shaped cardiac action potentials. Sci Rep 2014; 4:6125. doi: 10.1038/srep06125Crossref, Google Scholar
72 :
73 : Cardiac optogenetics: a novel approach to cardiovascular disease therapy. Europace 2018; 20:1741–1749. doi: 10.1093/europace/eux345Google Scholar
74 : Optogenetic sensors and effectors: CHROMus—the Cornell Heart Lung Blood Institute Resource for Optogenetic Mouse Signaling. Front Physiol 2014; 5:428. doi: 10.3389/fphys.2014.00428Crossref, Google Scholar
75 : Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature 2005; 435:1239–1243. doi: 10.1038/nature03650Crossref, Google Scholar
76 : Engineering and characterization of an enhanced fluorescent protein voltage sensor. PLoS One 2007; 2:e440. doi: 10.1371/journal.pone.0000440Crossref, Google Scholar
77 : Engineering of a genetically encodable fluorescent voltage sensor exploiting fast Ci-VSP voltage-sensing movements. PLoS One 2008; 3:e2514. doi: 10.1371/journal.pone.0002514Crossref, Google Scholar
78 : Effect of voltage sensitive fluorescent proteins on neuronal excitability. Biophys J 2009; 96:3959–3976. doi: 10.1016/j.bpj.2009.02.046Crossref, Google Scholar
79 : Bi-stable neural state switches. Nat Neurosci 2009; 12:229–234. doi: 10.1038/nn.2247Crossref, Google Scholar
80 : Neocortical excitation/inhibition balance in information processing and social dysfunction. Nature 2011; 477:171–178. doi: 10.1038/nature10360Crossref, Google Scholar
81 : Learning enhances the relative impact of top-down processing in the visual cortex. Nat Neurosci 2015; 18:1116–1122. doi: 10.1038/nn.4061Crossref, Google Scholar
82 : Temporally precise in vivo control of intracellular signalling. Nature 2009; 458:1025–1029. doi: 10.1038/nature07926Crossref, Google Scholar
83 : Partial recovery of visual function in a blind patient after optogenetic therapy. Nat Med 2021; 27:1223–1229. doi: 10.1038/s41591-021-01351-4Crossref, Google Scholar
84 : Taking optogenetics into the human brain: opportunities and challenges in clinical trial design. Open Access J Clin Trials 2020; 2020:33–41. doi: 10.2147/OAJCT.S259702Crossref, Google Scholar



