Vascular Dementia
Abstract
Vascular dementia is one of the most common causes of dementia after Alzheimer’s disease, causing around 15% of cases. However, unlike Alzheimer’s disease, there are no licensed treatments for vascular dementia. Progress in the specialty has been difficult because of uncertainties over disease classification and diagnostic criteria, controversy over the exact nature of the relation between cerebrovascular pathology and cognitive impairment, and the paucity of identifiable tractable treatment targets. Although there is an established relation between vascular and degenerative Alzheimer’s pathology, the mechanistic link between the two has not yet been identified. This Series paper critiques some of the key areas and controversies, summarises treatment trials so far, and makes suggestions for what progress is needed to advance our understanding of pathogenesis and thus maximise opportunities for the search for new and effective management approaches.
(Reprinted with permission from Lancet 2015; 386:1698-706)
Introduction
Vascular dementia is a very frequent form of dementia and, although much progress has been made over the past decade, several controversies remain to be addressed. In this Series paper, we outline key areas that remain to be clarified, summarise the status of treatment trials, and make suggestions for future research.
Use of the term vascular dementia is controversial. Is dementia an appropriate term, or should vascular cognitive impairment be preferred? Is a dimensional (continuous decline) or categorical (dementia vs no dementia) approach most appropriate for classification? How should we begin to understand the relation between cerebrovascular disease and cognitive impairment, and vascular and degenerative pathology? To understand some of these dilemmas, it is important to place controversies in their historical context. Up until the late 1960s, senile dementia, as it was known, was thought to be attributable to cerebral arteriosclerosis. This vascular aetiology was challenged by the studies of Blessed, Tomlinson, and Roth,1 which established Alzheimer’s disease, rather than vascular pathology, as the main cause of dementia in late life. Subsequently, it was thought that cerebrovascular disease only caused dementia when there were many large cortical infarcts. The multi-infarct dementia approach2 was very influential and subsequent classification systems for vascular dementia, including the international classification of disease (ICD)-10 and diagnostic and statistical manual of mental disorders (DSM)-IV, were largely based on this notion.3,4 Subsequently, however, it became clear that multi-infarct dementia was just one of many possible causes of vascular dementia, and pathological studies from large cohorts showed that subcortical vascular disease, rather than large cortical infarcts, accounted for most cases of vascular dementia.5 This conclusion resulted in competing sets of proposed new criteria for vascular dementia6,7 and specific criteria for some subgroups, such as subcortical ischaemic vascular dementia (which mostly included individuals/patients with what was known as Binswanger’s disease).8
One challenge in validating proposed ideas is the absence of a clear consensus on pathological criteria for vascular dementia. Studies that have attempted pathological valdation show that the different sets of criteria can indeed identify cases of vascular dementia with reasonable accuracy, with the National Institute of Neurological Disorders and Stroke and the Association Internationale pour la Recherche et l’Enseignement en Neurosciences (NINDS-AIREN) criteria arguably the most specific but least sensitive, and the DSM and Alzheimer’s Disease Diagnostic and Treatment Centers (ADDTC) criteria more sensitive but less specific.9 Because of their high specificity, the NINDS-AIREN criteria have been used in most relevant studies thus far.
We searched MEDLINE and Embase until Dec 31, 2014, using the search terms “vascular dementia” (both as a single term and as “vascular” AND “dementia”) and “vascular cognitive impairment”. Publications were selected mostly from the past 5 years, but did not exclude frequently referenced and highly regarded older publications. We searched the reference lists of articles identified by this search strategy and selected those we judged relevant. Review articles and book chapters are cited to provide readers with additional detail.
Although modern criteria allowed new multi-site therapeutic studies to be done, at the same time the use/value of the term vascular dementia was questioned,10 largely because definitions of dementia were based on the concept of Alzheimer’s dementia, and thus included not only the need for more than one cognitive deficit, but for memory to be one of the domains affected. Although highly appropriate for Alzheimer’s disease, memory is affected to various extents in vascular dementia, so a core criterion of memory disturbance is not necessarily appropriate. Because of this limitation, and the increasing recognition that cerebrovascular disease often occurred with other pathological changes to cause cognitive impairment, a broader term of vascular cognitive impairment was introduced, and preferred by many authors.10–12 Vascular cognitive impairment recognises the heterogeneous nature of the contribution of vascular pathology to dementia, and many different subtypes (table 1). However, there are no clear diagnostic criteria for vascular cognitive impairment and it remains a term highlighting the range of pathology, rather than a clearly validated diagnostic entity. Classification systems such as DSM-513 have removed the necessity for memory impairment as one of the criteria for dementia, or as DSM-5 now defines it, major neurocognitive disorder.
| Imaging and pathological changes | |
|---|---|
| Multi-infarct dementia (cortical vascular dementia) | Multiple cortical infarcts |
| Small vessel dementia (subcortical vascular dementia) | Lacunes, extensive white matter lesions; pathologically, infarcts, demyelination, and gliosis |
| Strategic infarct dementia | Infarct in strategic location (eg, thalamus) |
| Hypoperfusion dementia | Watershed infarcts, white matter lesions; pathologically, incomplete infarcts in white matter |
| Haemorrhagic dementia | Haemorrhagic changes, may be associated with amyloid angiopathy |
| Hereditary vascular dementia (CADASIL) | Multiple lacunes and white matter lesions, temporal lobe white matter affected |
| Alzheimer’s disease with cardiovascular disease | Combination of vascular changes and atrophy, especially medial temporal lobe; pathologically, mixture of vascular and degenerative (plaque and tangle) pathology |
TABLE 1. Subtypes of Vascular Dementia
The changes in the nosology of vascular dementia over the past 25 years have reflected new knowledge and progress, but have made harmonised research in the area difficult. Debates over classification and nosology will almost certainly continue until distinct and tractable pathophysiological mechanisms that underpin vascular dementia can be shown. In the meantime, there is consensus for a standardised approach to assessment of patients with vascular cognitive impairment in relation to studies,11 to avoid imposition of a-priori concepts of categories that might not reflect reality. Similar attempts have been made to standardise and operationalise newly proposed sets of diagnostic criteria to provide a common nomenclature for vascular cognitive disorders.14
Epidemiology and Risk Factors for Vascular Dementia
Most epidemiological work has used the standard and narrow definition of vascular dementia; this is important because any broader definitions, for example allowing dementia to be diagnosed in the absence of a memory impairment, or use of the wider term vascular cognitive impairment, would obviously affect estimates of prevalence and incidence. Studies of vascular dementia show it is the second most common cause of dementia after Alzheimer’s disease. Rates rise with age, with risk of vascular dementia roughly doubling every 5·3 years, an exponential rise slightly less pronounced than that of Alzheimer’s disease, which doubles every 4·5 years.15 In addition, dementia develops in around 15–30% of subjects 3 months after a stroke.16 Post-stroke dementia is often viewed as a subtype of dementia in its own right, because the pathophysiology of this disorder is unclear. However, post-stroke dementia is heterogeneous in nature and will include the unmasking of already present cognitive impairment or dementia, the emergence of vascular dementia after recurrent infarct, and the fact that having a stroke places people at high risk of dementia in the long term, with around 20–25% of subjects developing a delayed dementia.16 The close interaction between vascular and Alzheimer’s pathology has prompted a search for whether the pathophysiology of such delayed dementia is attributable to vascular disease, degenerative pathology, or a combination of the two. Although some studies have suggested that Alzheimer’s disease might be more common in people who have had a stroke, a long-term autopsy follow-up study17 of stroke survivors aged over 75 years, a group at high risk of Alzheimer pathology, noted that vascular but not degenerative dementia was the cause of the dementia in over 75% of cases.
Risk factors for dementia after stroke include increasing age, low education, female sex, vascular risk factors, stroke location, presence of strokes, and both global and medial temporal atrophy on structural imaging.16 Similar risk factors have been identified for vascular dementia in the absence of stroke, most especially advancing age and vascular risk.18 A meta-analysis19 showed that late life depression was a risk factor for vascular dementia, as it is for Alzheimer’s disease; this is a relevant finding because late life depression is associated with several vascular abnormalities, shown on brain imaging20,21 and pathology,22,23 and vascular mechanisms provide a plausible mechanistic link between depression and vascular dementia. Vascular risk factors have also emerged as major risk factors for Alzheimer’s disease. In addition to age, risk factors for Alzheimer’s disease include hypertension, smoking, possession of APOE e4 allele, ischaemic heart disease, atrial fibrillation, raised cholesterol and homocysteine concentrations, diabetes, and obesity.24 Many of these risk factors have the strongest association with Alzheimer’s disease when present in mid-life, and the association changes with age. For example, before the onset of dementia, blood pressure, cholesterol, and weight tend to fall, meaning that proximal risk to Alzheimer’s disease is less clear, and in cross-sectional studies there is often no relation, or even an inverse association.24 The presence of common vascular risk factors between Alzheimer’s disease and vascular dementia is both relevant and important to the known interaction between Alzheimer’s and vascular pathology. Several studies have shown that for a similar burden of Alzheimer’s pathology, clinical expression of dementia is greater when there is comorbid vascular disease.25,26
Clinical Features
Cognitive changes in vascular dementia are much more variable than in other disorders such as Alzheimer’s disease, and are highly dependent on the particular neural substrates affected by the vascular pathology. Because subcortical vascular pathology is frequently present, interrupting frontostriatal circuits, predominant deficits in attention, information processing, and executive function are seen.10,27 Standard screening tests for dementia, such as the mini-mental state examination which was devised to detect Alzheimer’s disease,28 might prove less sensitive to impairments, especially in these characteristic deficits. Other tests that highlight attention and executive function, such as the Montreal cognitive assessment scale29 or the vascular dementia assessment scale (VADAS-cog),30 are more likely to pick up deficits in this population. Other functions such as memory, language, and praxis are much more variably affected in vascular dementia. As with other dementias, non-cognitive features are frequent and can be particularly distressing both for the patient and their family. Community studies have shown a substantial overlap in neuropsychiatric features between Alzheimer’s disease and vascular dementia, with a very high burden of all symptoms in both subtypes,31 although some symptoms, particularly depression and apathy, are particularly prominent in those with vascular dementia, and other features such as delusions and hallucinations are less frequent.31,32 As would be expected from the heterogeneous nature of the disorder, outcome is variable, although average rates of cognitive decline are similar in vascular dementia and Alzheimer’s disease; however mortality, largely because of cardiovascular and cerebrovascular causes, is higher in vascular dementia with mean survival of 3–5 years.33
Brain Imaging
Accurate diagnosis of vascular dementia is known to need the presence of sufficient cerebrovascular disease on brain imaging to plausibly account for the degree of cognitive impairment recorded clinically.6 CT is sufficient to show established infarcts and extensive white matter lesions, although MRI is highly preferable to show more precisely the degree, location, and extent of cerebrovascular disease. The absence of an obvious relation between brain vascular disease and dementia is exemplified by a study comparing the imaging criteria for vascular dementia from NINDS-AIREN between post-stroke patients with and without dementia, which reported no significant differences.34 However, several studies have suggested that many lacunes, strategic infarcts, substantial burden (often defined as >25%) of white matter lesions, or combinations thereof are consistent with vascular dementia16,35 (figure 1). White matter lesions, which often indicate subcortical vascular disease, might be particularly important here. Some caveats are needed, because white matter lesions can indicate other non-ischaemic causes, but in the context of people aged over 75 years are most probably vascular in origin, and prospective studies show that even if they are not initially associated with cognitive and functional impairment, white matter lesions are strong predictors of both over the next 3 years.36 Imaging studies have shown that atrophy, both generalised and hippocampal, is at least as strongly associated with dementia as the extent of vascular pathology.37 Whether this association suggests that atrophy is a common pathway secondary to vascular disease, or the full extent of vascular and degenerative changes, is unclear, although the finding that hippocampal atrophy during life can be associated with vascular dementia or hippocampal sclerosis at autopsy17,21,38 suggests the contribution of vascular disease to atrophy can often be underestimated. In terms of assessing the contribution of Alzheimer’s pathology, the availability of in-vivo imaging and cerebrospinal fluid (CSF) markers of both amyloid and tau promise to make a substantial contribution.39,40 Biomarkers of vascular dementia, apart from imaging changes, are less well developed than for Alzheimer’s disease but candidates have been proposed, including albumen, metalloproteinases, and inflammatory markers, but need further validation.41
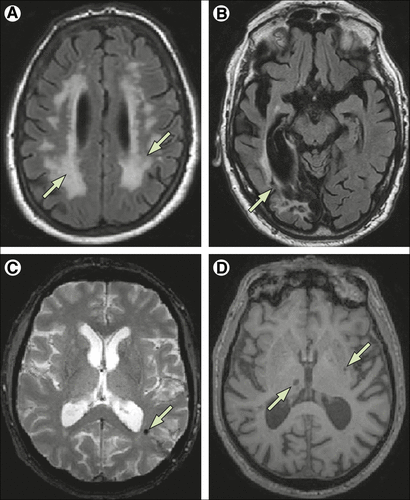
FIGURE 1. Vascular Imaging Changes on MRI. (A) Arrows Indicate Extensive (>25%) White Matter Lesions (Fluid Attenuation Inversion Recovery Image). (B) Arrow Indicates Large Cortical Infarction (Fluid Attenuation Inversion Recovery Image). (C) Arrow Indicates Microbleed (T2*-Weighted Image). (D) Arrows Indicate Multiple Lacunar Infarcts (T1-Weighted Image).
Genetics
Most genetic research in dementia has been on Alzheimer’s disease and investigations in vascular dementia have mainly been on rare familial syndromes, especially cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), related to a frameshift mutation in the notch gene on chromosome 19.42 These rare syndromes might provide important insights into the mechanisms underlying the development of vascular brain ischaemia, but the relevance of such disorders to the genetics of most late onset vascular dementia is unclear. Only one genome-wide association study (GWAS) has been reported.43 Only one gene (rs12007229) on the X chromosome was identified, and although the association was repeated in replication analyses, the odds ratio fell from 3·7 to 1·5, making it possible this was a chance finding, a possibility perhaps increased by the uncertainty about the biological importance of this locus. A systematic review44 of all reported association studies in the broader construct of vascular cognitive impairment included a meta-analysis of six polymorphisms with the strongest associations (APOE, ACT, ACE, MTHFR, PON1, and PSEN-1 genes) but only APOE e4 (OR 1·82, p<0·001) and MTHFR rs1801133 (OR 1·32, p=0·013) remained significant. The association with APOE e4 was reported in an independent meta-analysis45 and this gene is strongly associated with Alzheimer’s disease and cardiovascular disease,46 making this a plausible association, albeit one not likely to help identify mechanisms or treatments specific to vascular dementia. The association with Alzheimer’s disease might be because of diagnostic difficulties in accurately identifying and distinguishing these dementia subtypes, or it could point to such shared pathological mechanisms, a possibility increased by reports of common associations of genes between neurodegenerative disorders. Similarly MTHFR polymorphisms, especially C677T, have been identified in previous meta-analyses47 and MTHFR is a vascular gene related to homocysteine metabolism, suggesting it might be a genuine association, and vascular dementia has been associated with high homocysteine concentration.48 However, effects are slight and again it might not be specific for vascular dementia.49 In summary, there are few genetic studies in vascular dementia and only one GWAS analysis, and in other related disorders further investigation, including repeated GWAS studies, has nullified such modest evidence as is currently available in vascular dementia.50
Neuropathological Features
Although it seems obvious that cerebrovascular disease causes pathological damage and impairs cognition, finding the exact contribution of cerebrovascular pathology to cognitive decline and dementia is exceedingly difficult. This difficulty shows the inherent heterogeneous nature of vascular pathology, in which large vessel atherosclerosis and small vessel arteriosclerosis (and other vascular diseases—eg, cerebral amyloid angiopathy) can lead to cortical and subcortical infarcts, subinfarct ischaemic lesions (microinfarcts in grey matter and white matter lesions), and large and small cerebral haemorrhages (microbleeds).51 All these pathological changes can occur throughout the brain and can contribute to vascular dementia.52Figures 1 and 2 give examples of some of these lesions. In autopsy studies, it is difficult to relate cognitive impairments in life to post-mortem pathology, even when using data from prospective studies. Alzheimer’s disease has a reasonably well defined and predictable pattern of disease progression, but this is not the case for cerebrovascular disease, and there remains no agreed pathological scheme for staging or diagnosing vascular dementia. Different studies therefore use different criteria to report whether individuals have autopsy evidence of substantial cerebrovascular disease.52 Abnormalities in vascular brain pathology are almost universal in people aged over 75 years53 and are thought to contribute to cognitive impairments in mild cognitive impairment and more severe dementia.54 Small vessel disease, seen on MRI neuroimaging as white matter hyperintense lesions, seems to account for most of this contribution in milder cases.55 But a large burden of vascular disease pathology is needed to produce dementia in the absence of Alzheimer’s disease or other degenerative pathology, and so the prevalence of pure vascular dementia seems much lower than previously accepted, accounting for perhaps only about 10% of cases,56 most with large infarcts.51 The burden of cerebrovascular disease increases with age and with the severity of cognitive impairment, as is likewise the case for the major neurodegenerative diseases.53 Hence, with ageing and greater dementia severity, the presence of various cerebral pathologies escalates and the proportion of pure disease cases decreases. In the oldest old (people aged over 80 years), mixed dementia is the norm not the exception. When clinically assessing the contribution of cerebrovascular disease to dementia, the absence of large infarcts on imaging or a clear relation of such lesions to the onset or progression of cognitive impairments suggest that it is wisest to regard any vascular pathology as making a contribution to overall impairment rather than being the principal cause (see figure 2).
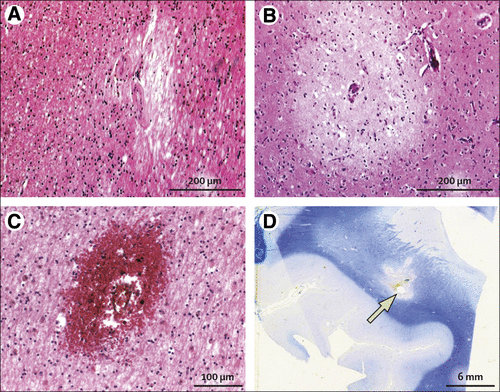
FIGURE 2. Pathological Vascular Changes Associated With Vascular Dementia. (A) Microinfarct in Cingulate White Matter (100 μm, Haematoxylin and Eosin Stain). (B) Microinfarct in Frontal Cortex (100 μm, Haematoxylin and Eosin Stain). (C) Microbleed in White Matter From Occipital Lobe (100 μm, Haematoxylin and Eosin Stain). (D) Arrow Indicates Lacunar Infarct in Frontal White Matter With Associated Pallor (100 μm, Luxol Fast Blue Stain).
Management of Vascular Dementia
General management principles of dementia, which include ensuring a timely diagnosis, assessing and treating comorbidities, providing information and support for the patient with dementia and their carers, and maximising independence, apply equally well to vascular dementia as to Alzheimer’s disease. However, progress towards finding effective treatments for vascular dementia has proved even more elusive than for Alzheimer’s disease. The best studied treatments are cholinesterase inhibitors and memantine, both of which are licensed and well established drugs for Alzheimer’s disease, albeit with modest effectiveness. The rationale for trial of these drugs in vascular dementia was largely based on suggestive evidence showing neuropathological and neurochemical overlap between the two disorders, in particular the suggestion of cholinergic deficit in vascular dementia.57 However, this notion has been challenged by subsequent neurochemical analysis, which suggested that the cholinergic system might be intact in pure vascular dementia, but affected to the same extent as in Alzheimer’s disease in cases of mixed dementia.58 An early study of galantamine in a group of patients with both pure and mixed vascular dementia showed possible benefit.59 However, several subsequent large and well conducted randomised controlled 6-month trials of galantamine, donepezil, and rivastigmine either in NINDS-AIREN probable, or probable and possible vascular dementia, have shown mixed results60–64 (table 2).
| Significant benefit for cognition | Significant global benefits | Significant benefits for activities of daily living | Significant benefits for neuropsychiatric symptoms | |
|---|---|---|---|---|
| Galantamine (Gal-INT-06) (n=121 with NINDS-AIREN probable vascular dementia), Erkinjuntti et al59 | No (p=0⋅06) –1⋅8 points on ADAS-cog | No | No | No |
| Galantamine (Gal-INT-26) (n=788 with NINDS-AIREN probable vascular dementia), 26 weeks Auchus et al60 | Yes (p<0⋅001) –1⋅9 points on ADAS-cog | No | No | No |
| Donepezil (307) (n=603 with NINDS-AIREN probable or possible vascular dementia), 24 weeks Black et al61 | Yes (p<0⋅001) –2⋅24 points on ADAS-cog | No | Yes | NA |
| Donepezil (308) (n=616 with NINDS-AIREN probable or possible vascular dementia), Wilkinson et al62 | Yes (p<0⋅001) –2⋅09 points on ADAS-cog | Yes | No | NA |
| Donepezil (319) (n=974 with NINDS-AIREN probable or possible vascular dementia), 24 weeks Roman et al63 | Yes (p<0⋅001) –0⋅91 points on VADAS-cog | No | No | NA |
| Rivastigmine (VantageE) (n=710 with NINDS-AIREN probable vascular dementia), 24 weeks Ballard et al64 | Yes (p=0⋅028) –1⋅3 points on VADAS-cog | No | No | No |
TABLE 2. Significant Benefits of Drugs Reported from Randomised Controlled Trials of Cholinesterase Inhibitors in Vascular Dementia
Although most have shown a small but significant benefit of cholinesterase inhibitors on cognition, the magnitude of this effect has been slight (about 2 points on the VADAS-cog scale, roughly half the improvement seen in the Alzheimer’s studies) and benefits on global functioning, activities of daily living, and behaviour have not been consistently reported. Combined with concerns over diagnostic validity and possible side-effects, this small effect has led both regulatory bodies and guideline groups to conclude that cholinesterase inhibitors and memantine should not be used in patients with vascular dementia.65
Two well conducted randomised trials66,67 were done with 6 months of memantine, an NMDA antagonist, in vascular dementia. Similar to the studies of cholinesterase inhibitors, both reported a significant but small effect on cognition that did not seem to generalise as there was no effect on global outcome measures. Meta-analyses of the studies have supported these conclusions.68 Unfortunately, there have been few other promising avenues. A study of nimodipine in subcortical vascular dementia69 missed its primary outcome measures, but did find some secondary improvements in some outcome scales and in terms of memory, prompting other studies of calcium channel blockers (eg, AFFECT; EudraCT Number: 2014-000926-39). There have been some positive trials of cerebrolysin,70 a putative neurotrophic neuropeptide derived from pigs’ brains needing daily infusion. A Cochrane review of six studies71 concluded that there was evidence of a positive effect of cerebrolysin on cognition and global outcome in vascular disease, but that wider use was not recommended because of the scarcity and short duration of trials, heterogeneity between trials, and limited follow-up.71
Some primary prevention studies have been done,72–76 although it should be noted that cognition was not a primary outcome in these studies. In terms of reducing cholesterol, the PROSPER study72 randomly assigned 6000 people to pravastatin or placebo and reported no differences in cognitive outcome between groups after 6 years. Similarly, the Heart Protection Study73 randomly assigned 20 537 subjects aged 40–80 years with vascular disease or diabetes to simvastatin or placebo, but reported rates of cognitive impairment 5 years later were almost identical between the two groups, at around 24%, with dementia developing in 0·3% of each group.73 These studies provide no evidence that primary prevention by lowering cholesterol prevents vascular dementia, but can be criticised for the insensitivity of outcome measures and inclusion of relatively well people with low rates of cognitive decline. Studies of blood pressure reduction are more promising, but remain equivocal. One difficulty is that such studies have often been stopped prematurely because endpoints based on cardiovascular and cerebrovascular outcomes have been reached before there has been adequate ascertainment of dementia outcomes to draw clear conclusions. The HYVET study74 seemed to show a trend towards antihypertensive treatment reducing dementia incidence, although this was non-significant, and the Syst-Eur study75 recorded a significant effect of nitrendipine in reducing dementia compared with placebo, although most of the dementias prevented were actually Alzheimer’s disease rather than vascular dementia. However, reviews and meta-analyses74,76 of these studies have generally shown that antihypertensive treatment can prevent vascular dementia by preventing stroke; however, apart from this, any effect in reducing dementia incidence is borderline, but in the right direction, and needs to be substantiated in larger, longer-term studies. The SPS3 trial,77 in a two-by-two factorial design, compared intensive lowering of blood pressure versus usual targets and dual antiplatelet treatment versus single aspirin in 3200 subjects (mean age 63 years) who had a lacunar infarct. The main outcome, the cognitive assessment and screen instrument, at a median of 3 years showed no significant effect of either blood pressure reduction (which was a mean of 11 mm Hg between the groups) or of dual antiplatelet therapy over the control groups. With regard to antiplatelet drugs, the results of SPS3 are very much in accord with other studies that suggested no clear evidence of benefit in preventing cognitive impairment or dementia, and the one trial of aspirin in established vascular dementia,78 although positive, has been criticised because of methodological limitations including a very small sample size, very high dropout rate, and inadequate randomisation.76 Results of single pharmacological strategies such as antiplatelet agents, reducing blood pressure, or use of statins to prevent or treat vascular dementia do not provide support for these interventions, although these remain important treatments for the vascular dementia risk factors themselves. By contrast, preventive studies are taking a more multidimensional approach—for example the FINGER study,79 which combined vascular risk reduction, nutritional advice, cognitive training, and exercise in high risk individuals, has reported promising results with reduced cognitive decline in the active group at 2 years. Consistent with this are findings from epidemiological cohort studies that suggest that overall dementia prevalence might actually be decreasing.80 Although the reasons for this decrease are not entirely clear, reduction in vascular risk is a plausible explanation.
Mild Cognitive Impairment Caused by Cerebrovascular Disease
Mild cognitive impairment caused by cerebrovascular disease has been much less comprehensively studied than the syndrome of mild cognitive impairment caused by Alzheimer’s disease, which is largely defined clinically on the basis of an amnestic deficit in the absence of dementia, although diagnostic criteria have been proposed.81 Far from being a benign disease, the few longitudinal studies of vascular mild cognitive impairment have reported rates of progression to dementia of similar magnitude to mild cognitive impairment caused by Alzheimer’s disease.82 The presentation of early vascular disease is much more heterogeneous because subtle cerebrovascular disease is common with ageing—for example, in imaging samples of representative samples of patients aged over 65 years, at least 30% have silent infarcts on brain imaging,83 and up to 90% have varying degrees of white matter lesions.84 The clinical significance of silent white matter lesions is uncertain, although a large pan-European study (the Leukoaraiosis And DISability [LADIS] study)36 reported that in a population of people aged over 65 years without disabilities, the presence at baseline of severe confluent white matter lesions conveyed a particularly adverse outcome in terms of a high rate of progression to disability over a 3-year period (rates around three times higher than those with only mild white matter disease). The study strongly implied the adverse nature of substantial white matter pathology, even if not accompanied by symptoms and disability, and points the need to investigate potential therapeutic approaches that could have a substantial effect in reducing future disability.
Conclusions and Future Directions
Although there has been much progress in defining and understanding the relation between cerebrovascular disease and cognitive impairment and dementia, some uncertainties remain. Clinical diagnostic criteria are sufficiently robust to be useful for clinical trials, but need further refinement and validation. For example, the development and validation of a range of biomarkers for neurodegenerative Alzheimer’s pathology, including amyloid PET imaging, CSF markers of tau and amyloid, and in-vivo tau imaging, now offer unparalleled opportunities for in-vivo stratification of individuals with pure dementia from those with mixed dementia for future naturalistic and therapeutic studies. Such work will be important to answer the key question as to whether and in what circumstances it is relevant to separate out neurodegenerative and vascular entities and so, in combination with pathological investigation, provide important validation of our clinical concepts of vascular dementia. In these studies we endorse proposals for harmonisation of data capture, avoiding narrow a-priori assumptions that could hamper progress. Some studies, clinical trials in particular, might need to focus on specific subgroups to ensure a more homogeneous population to study. One thing that has been learned from the trials of cholinesterase inhibitors and memantine in vascular dementia is that translation of Alzheimer’s treatments to vascular dementia on the basis of shared neurochemical mechanisms might not be appropriate. Little research exists on vascular dementia and its mechanisms compared with other dementias, and there is a clear need for further pathophysiological studies to investigate mechanisms that predispose or accelerate cognitive impairment. Large scale genetic studies, which have been so informative in many other disorders, are in their infancy for vascular dementia and could add to our understanding of the shared risk with Alzheimer’s disease. Potential treatments are in clinical trials, including calcium channel blockers, and other agents targeting endothelial function or the renin angiotensin system are putative candidates for further clinical trials.
Management of vascular dementia should focus on identifying and managing comorbidities, ensuring that vascular risk factors are optimally managed, ensuring appropriate recognition and management of non-cognitive symptoms, and appropriate psychosocial and other support to optimise quality of life for patients and carers. Cholinesterase inhibitors do not seem to confer benefit in pure vascular dementia, but at least one good randomised controlled trial suggests they are beneficial in cases of mixed Alzheimer’s disease and vascular dementia.59
1 . The association between quantitative measures of dementia and of senile change in the cerebral grey matter of elderly subjects. Br J Psychiatry 1968; 114: 797–811.Crossref, Google Scholar
2 . Multi-infarct dementia. A cause of mental deterioration in the elderly. Lancet 1974; 2: 207–10.Crossref, Google Scholar
3
4
5 . Subcortical ischaemic vascular dementia. Lancet Neurol 2002; 1: 426–36.Crossref, Google Scholar
6 . Vascular dementia: diagnostic criteria for research studies. Report of the NINDS-AIREN International Workshop. Neurology 1993; 43: 250–60.Crossref, Google Scholar
7 . Criteria for the diagnosis of ischemic vascular dementia proposed by the State of California Alzheimer’s Disease Diagnostic and Treatment Centers. Neurology 1992; 42: 473–80.Crossref, Google Scholar
8 . Research criteria for subcortical vascular dementia in clinical trials. J Neural Transm Suppl 2000; 59: 23–30.Google Scholar
9 . Clinicopathological validation study of four sets of clinical criteria for vascular dementia. Am J Psychiatry 2002; 159: 82–87.Crossref, Google Scholar
10 . Vascular cognitive impairment. Lancet Neurol 2003; 2: 89–98.Crossref, Google Scholar
11 . National Institute of Neurological Disorders and Stroke-Canadian Stroke Network vascular cognitive impairment harmonization standards. Stroke 2006; 37: 2220–41.Crossref, Google Scholar
12 . Vascular cognitive impairment. Curr Atheroscler Rep 2013; 15: 330.Crossref, Google Scholar
13 . Mild cognitive impairment should be considered for DSM-V. J Geriatr Psychiatry Neurol 2006; 19: 147–54.Crossref, Google Scholar
14 , and the International Society for Vascular Behavioral and Cognitive Disorders. Diagnostic criteria for vascular cognitive disorders: a VASCOG statement. Alzheimer Dis Assoc Disord 2014; 28: 206–18.Crossref, Google Scholar
15 . The incidence of dementia: a meta-analysis. Neurology 1998; 51: 728–33.Crossref, Google Scholar
16 . Prevalence, incidence, and factors associated with pre-stroke and post-stroke dementia: a systematic review and meta-analysis. Lancet Neurol 2009; 8: 1006–18.Crossref, Google Scholar
17 . Long term incidence of dementia, predictors of mortality and pathological diagnosis in older stroke survivors. Brain 2011; 134: 3716–27.Crossref, Google Scholar
18 . Vascular aspects of cognitive impairment and dementia. J Cereb Blood Flow Metab 2013; 33: 1696–706.Crossref, Google Scholar
19 . Late-life depression and risk of vascular dementia and Alzheimer’s disease: systematic review and meta-analysis of community-based cohort studies. Br J Psychiatry 2013; 202: 329–35.Crossref, Google Scholar
20 , and the LADIS Group. White matter changes and late-life depressive symptoms: longitudinal study. Br J Psychiatry 2007; 191: 212–17.Crossref, Google Scholar
21 , and the LADIS group. Relationship between progression of brain white matter changes and late-life depression: 3-year results from the LADIS study. Br J Psychiatry 2012; 201: 40–45.Crossref, Google Scholar
22 . Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch Gen Psychiatry 2002; 59: 785–92.Crossref, Google Scholar
23 . Depression and vascular disease: what is the relationship? J Affect Disord 2004; 79: 81–95.Crossref, Google Scholar
24 . Cardiovascular risk factors and future risk of Alzheimer’s disease. BMC Med 2014; 12: 130.Crossref, Google Scholar
25 . Brain infarction and the clinical expression of Alzheimer disease. The Nun Study. JAMA 1997; 277: 813–17.Crossref, Google Scholar
26 . Challenges of multimorbidity of the aging brain: a critical update. J Neural Transm 2015; 122: 505–21.Crossref, Google Scholar
27 . Gray matter atrophy in patients with ischemic stroke with cognitive impairment. Stroke 2008; 39: 785–93.Crossref, Google Scholar
28 . “Mini-mental state”. A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res 1975; 12: 189–98.Crossref, Google Scholar
29 . Test accuracy of cognitive screening tests for diagnosis of dementia and multidomain cognitive impairment in stroke. Stroke 2014; 45: 3008–18.Crossref, Google Scholar
30 , and the LADIS Study Group. Comparison of the Alzheimer’s Disease Assessment Scale Cognitive Subscale and the Vascular Dementia Assessment Scale in differentiating elderly individuals with different degrees of white matter changes. The LADIS Study. Dement Geriatr Cogn Disord 2007; 24: 73–81.Crossref, Google Scholar
31 . Prevalence of neuropsychiatric symptoms in dementia and mild cognitive impairment: results from the cardiovascular health study. JAMA 2002; 288: 1475–83.Crossref, Google Scholar
32 . Behavioural and psychological symptoms in poststroke vascular cognitive impairment. Behav Neurol 2014; 2014: 430128.Crossref, Google Scholar
33 . The natural history of dementia. Psychogeriatrics 2014; 14: 196–201.Crossref, Google Scholar
34 . NINDS AIREN neuroimaging criteria do not distinguish stroke patients with and without dementia. Neurology 2004; 63: 983–88.Crossref, Google Scholar
35 . Subcortical vascular dementia: integrating neuropsychological and neuroradiologic data. Neurology 2005; 65: 376–82.Crossref, Google Scholar
36 , and the LADIS Study Group. Changes in white matter as determinant of global functional decline in older independent outpatients: three year follow-up of LADIS (leukoaraiosis and disability) study cohort. BMJ 2009; 339: b2477.Crossref, Google Scholar
37 . Hippocampal and cortical atrophy predict dementia in subcortical ischemic vascular disease. Neurology 2000; 55: 1626–35.Crossref, Google Scholar
38 . MRI shows more severe hippocampal atrophy and shape deformation in hippocampal sclerosis than in Alzheimer’s disease. Int J Alzheimers Dis 2011; 2011: 483972.Google Scholar
39 . Clinical amyloid imaging in Alzheimer’s disease. Lancet Neurol 2011; 10: 667–70.Crossref, Google Scholar
40 . CSF biomarkers and incipient Alzheimer disease in patients with mild cognitive impairment. JAMA 2009; 302: 385–93.Crossref, Google Scholar
41 . Biomarkers in vascular dementia: A recent update. Biomarkers Genomic Med 2014; 7: 43–56.Crossref, Google Scholar
42 . Cadasil. Lancet Neurol 2009; 8: 643–53.Crossref, Google Scholar
43 . Genome-wide association study of vascular dementia. Stroke 2012; 43: 315–19.Crossref, Google Scholar
44 . Using Alzgene-like approaches to investigate susceptibility genes for vascular cognitive impairment. J Alzheimers Dis 2013; 34: 145–54.Crossref, Google Scholar
45 . Association between apolipoprotein E gene polymorphism and the risk of vascular dementia: a meta-analysis. Neurosci Lett 2012; 514: 6–11.Crossref, Google Scholar
46 . The pathobiology of vascular dementia. Neuron 2013; 80: 844–66.Crossref, Google Scholar
47 . The MTHFR C677T polymorphism contributes to an increased risk for vascular dementia: a meta-analysis. J Neurol Sci 2010; 294: 74–80.Crossref, Google Scholar
48 . Is high homocysteine level a risk factor for cognitive decline in elderly? A systematic review, meta-analysis, and meta-regression. Am J Geriatr Psychiatry 2011; 19: 607–17.Crossref, Google Scholar
49 . Is hyperhomocysteinemia an Alzheimer’s disease (AD) risk factor, an AD marker, or neither? Trends Pharmacol Sci 2011; 32: 562–71.Crossref, Google Scholar
50 . The genetics of major depression. Neuron 2014; 81: 484–503.Crossref, Google Scholar
51 . Vascular dementia: different forms of vessel disorders contribute to the development of dementia in the elderly brain. Exp Gerontol 2012; 47: 816–24.Crossref, Google Scholar
52 . Toward a pathological definition of vascular dementia. J Neurol Sci 2010; 299: 136–38.Crossref, Google Scholar
53 . Staging and natural history of cerebrovascular pathology in dementia. Neurology 2012; 78: 1043–50.Crossref, Google Scholar
54 . Alzheimer and vascular neuropathological changes associated with different cognitive States in a non-demented sample. J Alzheimers Dis 2012; 29: 309–18.Crossref, Google Scholar
55 . White matter hyperintensities and cognition: testing the reserve hypothesis. Neurobiol Aging 2011; 32: 1588–98.Crossref, Google Scholar
56 . Is there pure vascular dementia in old age? J Neurol Sci 2010; 299: 150–54.Crossref, Google Scholar
57 . The cholinergic approach for the treatment of vascular dementia: evidence from pre-clinical and clinical studies. Clin Exp Hypertens 2002; 24: 697–713.Crossref, Google Scholar
58 . Absence of cholinergic deficits in “pure” vascular dementia. Neurology 2005; 64: 132–33.Crossref, Google Scholar
59 . Efficacy of galantamine in probable vascular dementia and Alzheimer’s disease combined with cerebrovascular disease: a randomised trial. Lancet 2002; 359: 1283–90.Crossref, Google Scholar
60 . Galantamine treatment of vascular dementia: a randomized trial. Neurology 2007; 69: 448–58.Crossref, Google Scholar
61 , and the Donepezil 307 Vascular Dementia Study Group. Efficacy and tolerability of donepezil in vascular dementia: positive results of a 24-week, multicenter, international, randomized, placebo-controlled clinical trial. Stroke 2003; 34: 2323–30.Crossref, Google Scholar
62 , and the Donepezil 308 Study Group. Donepezil in vascular dementia: a randomized, placebo-controlled study. Neurology 2003; 61: 479–86.Crossref, Google Scholar
63 . Randomized, placebo-controlled, clinical trial of donepezil in vascular dementia: differential effects by hippocampal size. Stroke 2010; 41: 1213–21.Crossref, Google Scholar
64 . Efficacy, safety and tolerability of rivastigmine capsules in patients with probable vascular dementia: the VantagE study. Curr Med Res Opin 2008; 24: 2561–74.Crossref, Google Scholar
65
66 , and the MMM 500 group. A double-blind, placebo-controlled multicentre study of memantine in mild to moderate vascular dementia (MMM500). Int Clin Psychopharmacol 2002; 17: 297–305.Crossref, Google Scholar
67 . Efficacy and safety of memantine in patients with mild to moderate vascular dementia: a randomized, placebo-controlled trial (MMM 300). Stroke 2002; 33: 1834–39.Crossref, Google Scholar
68 . Efficacy and adverse effects of cholinesterase inhibitors and memantine in vascular dementia: a meta-analysis of randomised controlled trials. Lancet Neurol 2007; 6: 782–92.Crossref, Google Scholar
69 . Impact of age-related cerebral white matter changes on the transition to disability—the LADIS study: rationale, design and methodology. Neuroepidemiology 2005; 24: 5l–62.Crossref, Google Scholar
70 . Cerebrolysin in vascular dementia: improvement of clinical outcome in a randomized, double-blind, placebo-controlled multicenter trial. J Stroke Cerebrovasc Dis 2010; 20: 310–18.Crossref, Google Scholar
71 . Cerebrolysin for vascular dementia. Cochrane Database Syst Rev 2013; 1: CD008900.Crossref, Google Scholar
72 , and the PROSPER study group. PROspective Study of Pravastatin in the Elderly at Risk. Pravastatin in elderly individuals at risk of vascular disease (PROSPER): a randomised controlled trial. Lancet 2002; 360: 1623–30.Crossref, Google Scholar
73 . Statin use and the risk of incident dementia: the Cardiovascular Health Study. Arch Neurol 2005; 62: 1047–51.Crossref, Google Scholar
74 , and the HYVET investigators. Incident dementia and blood pressure lowering in the Hypertension in the Very Elderly Trial cognitive function assessment (HYVET-COG): a double-blind, placebo controlled trial. Lancet Neurol 2008; 7: 683–89.Crossref, Google Scholar
75 . Prevention of dementia in randomised double-blind placebo-controlled Systolic Hypertension in Europe (Syst-Eur) trial. Lancet 1998; 352: 1347–51.Crossref, Google Scholar
76 . Blood pressure lowering in patients without prior cerebrovascular disease for prevention of cognitive impairment and dementia. Cochrane Database Syst Rev 2009; 4: CD004034.Crossref, Google Scholar
77 , and the SPS3 Investigators. Effects of long-term blood pressure lowering and dual antiplatelet treatment on cognitive function in patients with recent lacunar stroke: a secondary analysis from the SPS3 randomised trial. Lancet Neurol 2014; 13: 1177–85.Crossref, Google Scholar
78 . Randomized clinical trial of daily aspirin therapy in multi-infarct dementia. A pilot study. J Am Geriatr Soc 1989; 37: 549–55.Crossref, Google Scholar
79 . A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015; 385: 2255–63.Crossref, Google Scholar
80 , and the Medical Research Council Cognitive Function and Ageing Collaboration. A two-decade comparison of prevalence of dementia in individuals aged 65 years and older from three geographical areas of England: results of the Cognitive Function and Ageing Study I and II. Lancet 2013; 382: 1405–12.Google Scholar
81 , and the American Heart Association Stroke Council, Council on Epidemiology and Prevention, Council on Cardiovascular Nursing, Council on Cardiovascular Radiology and Intervention, and Council on Cardiovascular Surgery and Anesthesia. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the american heart association/american stroke association. Stroke 2011; 42: 2672–713.Crossref, Google Scholar
82 . Progression of impairment in patients with vascular cognitive impairment without dementia. Neurology 2001; 57: 714–16.Crossref, Google Scholar
83 . Lacunar infarcts defined by magnetic resonance imaging of 3660 elderly people: the Cardiovascular Health Study. Arch Neurol 1998; 55: 1217–25.Crossref, Google Scholar
84 . Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry 2001; 70: 9–14.Crossref, Google Scholar



