Collaborative Care Models for Comorbid Medical and Behavioral Health Conditions
Abstract
The integration of behavioral health and primary care has become an increasingly important topic, with health care reform placing new emphasis on providing care that meets the spirit of the “Triple Aim”: better clinical outcomes, cost containment, and enhancing the patient experience of care. Models of integration range from the identification and treatment of mental disorders in primary care settings to addressing chronic medical conditions in patients with serious mental illness in public mental health settings. In addition, high utilizers of healthcare resources frequently have comorbid behavioral health conditions and present opportunities for proactive interventions in the other medical settings. Over the past decade, new approaches have been developed to provide solutions in these areas. This paper will discuss these emerging models and new roles for psychiatrists.
Clinical Context
A series of converging factors are leading to increased interest in integrated care. With the passage of the Affordable Care Act, attention is being focused on improving health outcomes and containing costs. The impact of mental and substance use disorders—hereafter referred to as behavioral health—on reaching these goals is being appreciated in new ways. Across the range of healthcare settings, opportunities to implement outcome-changing interventions are underway and being tested.
One in five U.S. citizens has a diagnosable mental disorder (1), with only 40% receiving any treatment for their condition. Of those who do receive care, only half of them see a behavioral health specialist (2), with the majority being treated in physical health settings by primary and specialty medical care clinicians or alternative medicine settings or social service agencies. In the primary and specialty medical care outpatient setting, patients with behavioral disorders are often not recognized or engaged in effectively delivered treatment, resulting in a mere 13% of patients receiving minimally effective treatment (3). In the inpatient medical setting, payment shortfalls and challenges in billing for psychiatric care make it difficult to access behavioral health services either in emergency rooms or on general medical and surgical units. The impact of untreated mental illness on total healthcare costs is significant (4) and can lead to decreased adherence to recommended medical/surgical treatments and lack of follow-up for care. These poor outcomes, coupled with the increase cost in overall health care associated with untreated behavioral illness, have led to the need for better treatment options in primary care and other medical settings. In addition, the lack of a sufficient number of psychiatrists to meet treatment demands necessitates developing models that leverage these limited resources more effectively.
Patients with serious mental illness have a life span shortened by as much as 25 years (5), with 60% of this attributable to untreated chronic medical conditions, leading to increased cardiovascular morbidity in particular (6). As seen in Figure 1, there is a high rate of physical illness in patients with behavioral illnesses (7). Further, the second-generation antipsychotics have a well-known contribution to increased cardiovascular risk (8). A number of other factors also contribute to this mortality, including failure to recognize medical and psychiatric disorders, lack of access to care, poorer quality of care, and inability to afford medical care. A look at baseline data prior to initiating the CATIE study showed a high prevalence of “current” untreated illness, including 30% of patients with diabetes, 66% with hypertension, and 88% with hyperlipidemia (9) with these disorders not receiving care.
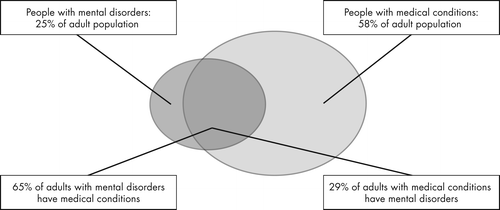
Reproduced with permission of the Robert Wood Johnson Foundation, Princeton, N.J.
In addition, high utilizers of health care resources, i.e., the 5%−10% of patients consuming 50%−70% of the health care dollars, as a group are being targeted for interventions (10). An estimated 70% of these have comorbid behavioral health problems (11). These high utilizers can be found in the population of patients with serious mental illness in public behavioral health settings as well as in general primary care, emergency rooms, and inpatient medical settings. They present an opportunity for collaborative medical and behavioral health interventions designed to improve outcomes and reduce costs.
Outpatient Treatment Strategies and Evidence
Terminology
The terms “collaborative” and “integrated” care are often used interchangeably, although in the former it is implied that independently operating medical and behavioral health groups support care coordination. A recently published lexicon defines integrated care as “the care that results from a practice team of primary care and behavioral health clinicians, working together with patients and families, using a systematic and cost-effective approach to provide patient-centered care for a defined population.” (12). Over the past decade, collaborative care models have often been considered to fall into two camps determined by the primary location of care. These describe common methods for service delivery when payment for medical and behavioral health services are separate. In the first camp, efforts are focused on “integrating” detection and treatment of behavioral health issues into the primary care setting. In the second, the goal is to improve health outcomes in patients seen in the behavioral health sector by addressing comorbid chronic medical problems through the introduction of medical professionals in the behavioral health setting, so called “reverse integration.” Each will be addressed with the understanding that some core principles apply to both and to integrated care in general. For the purposes of this article integrated care and collaborative care will be used interchangeably.
A third model, which is less common than the first two due to the misperception that medical and behavioral health patients fall into two distinct categories (i.e., those with primary medical problems need treatment only in the primary care sector and those with primary behavioral health problems need treatment only in the behavioral health sector), is one in which medical and behavioral health professionals participate in and are accountable for total health of the population they serve regardless of setting. In this model, behavioral health and medical care are considered core components of total health and are part of a single network of clinicians. In this model all practitioners, regardless of background, would be considered full team members. This comes close to being realized in level three patient-centered medical homes but remains a challenge to develop, since independent medical and behavioral health payers continue to drive separate delivery of medical and behavioral health services by maintaining segregated medical and behavioral health provider networks and payment systems that do not incentivize this care.
Integrating Behavioral Health into Primary Care
A robust evidence base for collaboration in primary care settings provides a framework on which to build an effective integrated delivery system. The preponderance of evidence comes from the Improving Mood Across Collaborative Treatment (IMPACT) trial and its many replications throughout the past decade. These studies have shown improved outcomes in depression treatment, lower overall healthcare costs, and patient satisfaction with the care received (13). Over 70 randomized trials in diverse populations conducted in primary care, mostly for the treatment of depression, have proven its effectiveness (14, 15). Another model that adapts a similar design, known as TEAMCare, includes targeted treatment of other chronic medical conditions in addition to depression. This trial demonstrated these interventions can lead to improvement in both medical and behavioral health indicators, such as HbA1c levels, blood pressure control, cholesterol levels, and Patient Health Questionnaire-9 (PHQ-9) scores in addition to saving on costs of outpatient healthcare (16). Both the IMPACT and TEAMCare models provide systematic approaches to care that meet the goals of the Triple Aim (17) and position primary care clinics to be accountable and reimbursed for the quality of care. In the most recent meta-analysis, Woltmann and colleagues (18) demonstrated that using collaborative chronic care models can improve outcomes in the treatment of various mental and physical health outcomes (see Figure 1 of the Woltmann et al. article, which is reprinted in this issue of Focus [p. 557]).
Central to the design of the collaborative care studies that form the evidence base is the Wagner Chronic Care Model (19). It emphasizes care delivery redesign through utilization of proactive care teams and “activated” patients. In May 2011, a summit of leaders met at the Advancing Integrated Mental Health Solutions (AIMS) Center at the University of Washington in Seattle. A set of Core Principles of Effective Collaborative Care (http://uwaims.org/overview-principles.html) were established and can serve as a useful tool for understanding what elements are considered crucial to effective interventions. They help distinguish collaborative care integration from other forms of care. The 5 Core Principles are as follows:
| 1. | |||||
| 2. | Care is population-based. Care is provided for defined populations of patients who are routinely screened and tracked in data bases known as registries. This information is reviewed on a regular basis to determine if improvement is occurring and to follow-up with patents to ensure no one “falls through the cracks.” Data can be utilized to define priorities and adjust treatment strategies. | ||||
| 3. | Care is evidence-based. Treatments are selected on the basis of existing proof for their efficacy in addressing the underlying condition. This can range from the selection of brief interventions (such as motivational interviewing) to pharmacotherapies. | ||||
| 4. | Care is measurement-based. Care is tracked and monitored for progress utilizing rating scales and laboratory values to determine if preset goals are being met. This approach, often called “treat-to-target,” allows for timely adjustments of care determined through caseload review to reach set goals. | ||||
| 5. | Care is accountable for outcomes. The collaborative care team takes responsibility for the outcomes and costs of the care provided. In some instances outcomes can be utilized in pay for performance arrangements. | ||||
Utilizing these core principles, collaborative teams provide a system of care that begins with screening and patient identification through use of tools, such as the PHQ-9 for depression, moves on to patient engagement, and then provision of evidence-based brief interventions and pharmacotherapies. Registries allow tracking and adjustments in treatment as needed. Team members utilize aggregate data to improve the overall care. Treatment in integrated settings follows a system of “stepped care” (20) to allow effective treatment to be provided with the least amount of intervention necessary at that level. The person gets the care they need without over utilizing resources, leading to improved outcomes that are cost efficient.
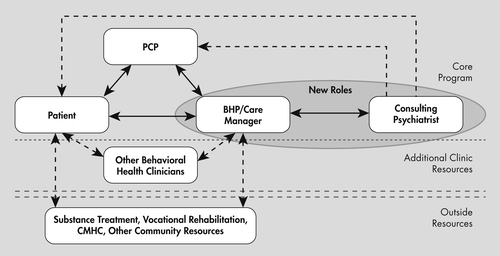
Copyright (c) 2011 University of Washington. Developed by Jurgen Unutzer, MD, MPH, MA, and colleagues. Used with permission.
Unfortunately, the most common form of integrated care in primary and specialty medical settings in the United States today, i.e., the addition of a nondoctoral psychologist or social worker into the medical clinic setting, does not provide these Core Principles. Not surprisingly, meta-analysis of clinical approaches in the United Kingdom, nearly identical to these now being introduced in the United States, have demonstrated that long-term outcomes and cost savings cannot be expected (22). Thus, it is important that the Core Principles are present so that outcome improving and cost savings, i.e., value-added care, can be expected as collaborative services are introduced and delivered.
Integrating Primary Care into Behavioral Health Settings
Determining best practices to promote evidence-based improvement in the health status of persons seen in public behavioral health settings, many of whom have serious mental illness, is a major ongoing area of research, lagging behind its better known counterpart in the primary care setting. Consequently, a robust evidence base for this practice has not been established. However, emerging examples of approaches exist that mirror the collaborative care models described above. These add care managers and specialist consultation to the healthcare team in integrated behavioral health settings. The Patient Care Access, Referral and Evaluation (PCARE) study (23) was one of the first studies published that showed if enhanced efforts by care managers to connect behavioral health patients to offsite primary care services was provided, measures of chronic medical illness could be improved. These efforts included serving as advocates and a communication link between the patients with serious mental illnesses and the primary care providers, providing education on health-related issues and support to dealing with the multiple system barriers that make this process even more difficult for this population.
Two new models are currently being tested nationally. The first is the SAMHSA/HRSA Primary and Behavioral Health Care Initiative (PBHCI) grant program, initiated in 2009 and financed by the 2010 Affordable Care Act to provide 4 years of funding to 93 sites across the country (24). Grants are being used to add primary care providers, care managers, and wellness coaches to create an integrated team to improve the health status of patients seen in behavioral health settings (Figure 3). The addition of care managers is a crucial component to this model where they function to track patients, monitor adherence to treatment, facilitate referrals to medical care, and alert the team to problems. The first study describing program characteristics and early implementation experiences has recently been published (25). While official clinical data from some of the early cohorts is not currently available, there are initial indications that measures such as blood pressure, HbA1c, and cholesterol are showing improvement (26). The addition of primary care staff into the community mental health center (CMHC) environment provides an opportunity for partnership and collaboration with the psychiatric providers, who have struggled with adequately screening and referring patients to other medical services for many years (27).
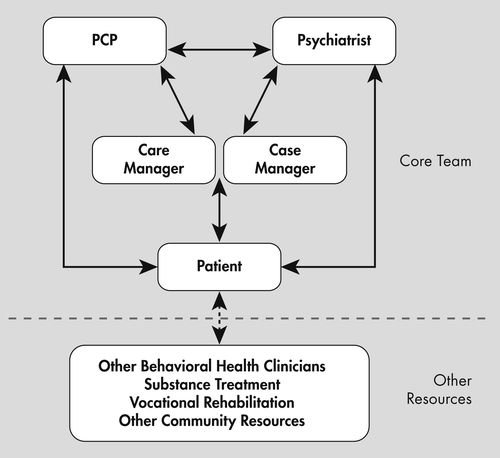
Copyright (c) 2011 University of Washington. Developed by Jurgen Unutzer, MD, MPH, MA, and colleagues. Used with permission.
A second model being tested is the State Option to Provide Health Homes for Medicaid Beneficiaries with Chronic Illnesses (also funded through the 2010 ACA) initiated in 2011. This allows states to amend their Medicaid plan (often called State Plan Amendments or SPAs) and to receive additional funding for services provided to promote health. Patients with behavioral health conditions are qualified to receive services, and for the first time CMHCs can be the location of the health home where the care is provided (28).
Unlike the PBHCI sites, the health homes do not have to provide primary care services directly and instead must provide six additional health home services including comprehensive care management, care coordination, comprehensive transitional care, individual and family support, community linkages, and use of health information technology to link services (29). Eight states have implemented this model to date with Missouri having the most experience one year into implementation. A unique feature of the Missouri model is the addition of a primary care provider “consultant” to assist the CMHC care team with using aggregate data to identify problems and fine-tune medical and behavioral health interventions. This provides a general medical partner to the behavioral health staff for improving total treatment.
No published data exists on the outcomes of SPAs but early information from the Missouri model, which includes a medical care component (30), is showing reductions in emergency room and hospital visits and fewer 30-day readmissions. From provision of onsite primary clinics to the establishment of behavioral health homes, models are being tested with the promise of both improved outcomes and cost containment for these patients who are often high utilizers of healthcare resources.
Merged Clinics With Fully Integrated Medical and behavioral health Care
A final and less common model combines treatment for the total health of a given population into one organizational structure, designed with a diverse team of behavioral health and medical staff working in a unified manner to provide the type of care needed at any visit. These clinics operate under one board of directors with the mission to provide comprehensive collaborative care. Funding limitations, dual licensure requirements, and difficulty with electronic medical records are obstacles that must be overcome in these models. Several of these clinics currently exist nationally and provide a rich environment for the practicing psychiatrist to appreciate fully integrated treatment. Cherokee Health Systems in Tennessee (www.cherokeehealth.com) and Axis Health System in Colorado (www.axishealthsystem.org) are two examples of this model.
Addressing the Needs of Inpatient High Utilizers of Healthcare Resources
Interventions for medically complex patients who often have a behavioral health diagnosis and are high utilizers of healthcare are being identified. The term “hot spotting,” introduced in the 2011 New Yorker article by Atul Gawande, M.D. (31), has become a popular expression for this cohort of patients and entails finding high-cost patients and providing additional services to meet their needs and bring down the costs associated with their care. Jeff Brenner, M.D., from the Camden Coalition, the physician profiled by Gawande, describes a further subgroup of this set of medically complex and expensive patients as the “extreme bucket”, whose difficult to treat behavioral health needs pose an opportunity for intervention for consultant psychiatrists in inpatient settings.
Taking a proactive rather than reactive approach to case finding in hospital settings is an identified shift necessary in the way care is provided (32). One study utilized proactive consult liaison services to identify medically complex patients on general medical units with comorbid behavioral health issues and demonstrated earlier intervention could reduce length of stays (33). Another study demonstrated the utility of delirium prevention interventions in reducing length of stay in intensive care units (34). Utilizing medical complexity tools during the initial nursing assessment helps identify and prioritize which patients need immediate referral and help match the intervention to the level of need (35). Lastly, the idea of a “Complexity Intervention Unit” in inpatient settings has been proposed as an ideal location to take care of this group of patients (32).
Questions and Controversy
“I’m concerned about liability and don’t want to provide ‘curbside’ consults because I have not personally examined the patients I would be offering consultation for in the primary care setting.”
The issue of liability in integrated settings can be considered along two lines. The first is whether or not a doctor-patient relationship was established. Legally, this is usually determined by whether or not there was direct evaluation of a patient (in person or by televideo) and subsequent documentation of findings. Indirect or “curbside” consultations do not establish a doctor-patient relationship so there is presumed minimal liability (36). An additional area of consultation in collaborative settings in primary care is between the consulting psychiatrist and the behavioral health provider. Liability in this situation can depend on the role of the psychiatrist, which can range from consultative to collaborative to supervisory (37). There is increased liability if you are the supervisor of the behavioral health provider and are ultimately responsible for the care they provide. Finally, the primary care provider should remain in charge of the overall care provided, deciding if they wish to implement the consultant psychiatrist’s recommendations and prescribe all medications suggested. Figure 4 shows the nature of the liability and methods to reduce risk.
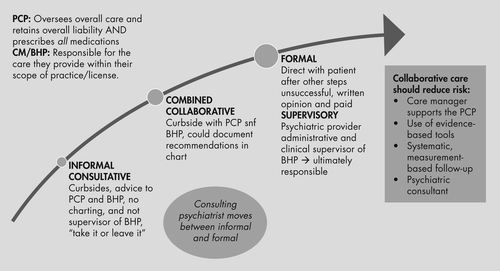
Copyright (c) 2011 University of Washington. Developed by Jurgen Unutzer, MD, MPH, MA, and colleagues. Used with permission.
Recommendations
| 1. | Psychiatrists will benefit from fully understanding new models across a range of opportunities in collaborative care and additional training is encouraged. A course sponsored through the AIMS center is available at national meetings several times a year and participation is encouraged (http://uwaims.org). In addition, several texts will be published in 2014, offering added educational options. | ||||
| 2. | Updating the knowledge base and skill set in the treatment of common medical conditions will enhance work in collaborative settings. This will help in the primary care setting while working with primary care provider colleagues. It will also help in understanding and possibly treating some common medical conditions in patients with serious mental illness who may lack access to primary care. | ||||
| 3. | Learn how to use rating scales to track progress and adjust treatment when goals are not being met. This is an essential element in integrated settings. Utilizing measurement-based care will be important as healthcare reform unfolds in the coming years. | ||||
| 4. | Seek additional training in leadership skills and team building to prepare for new and important psychiatric roles. Consider how psychiatrists will fit into the reformed health care arena and begin to forge relationships with medical colleagues in primary care and other medical settings. Psychiatry is the one specialty where a “physician” is uniquely trained in both general medical and primary behavioral health causes for disturbances of emotions, cognitions, and behaviors. Leadership opportunities to demonstrate skills in this area will become increasingly available as healthcare systems transitions to integrated care. | ||||
1
2 : Twelve-month use of mental health services in the United States: results from the National Comorbidity Survey Replication. Arch Gen Psychiatry 2005; 62:629–640Crossref, Google Scholar
3 : Adequacy of treatment for serious mental illness in the United States. Am J Public Health 2002; 92:92–98Crossref, Google Scholar
4 Melek S, Norris D, Paulus J: Economic impact of integrated medical-behavioral health: implications for psychiatry, Milliman American Psychiatric Association Report, Feb 2013Google Scholar
5 Colton CW, Manderscheid RW: Congruencies in increased mortality rates, years of potential life lost, and causes of death among public mental health clients in eight states. Prev Chronic Dis [serial online] 2006 April [date cited]. Available from: URL: http://www.cdc.gov/pcd/issues/2006/apr/05_0180.htmGoogle Scholar
6 Parks J, Svendsen D, Singer P, Foti ME: (2006). Morbidity and mortality in people with serious mental illness. Alexandria, VA: National Association of State Mental Health Program Directors. Retrieved from www.nasmhpd.org/general_files/publications/med_directors_pubs/Mortality%20and%20Morbidity%20Final%20Report%208.18.08.pdf.Google Scholar
7 Druss BG, Walker R: Mental Disorders and Medical Comorbidity by E:(http://www.rwjf.org/pr/product.jsp?id=71883), Original data from National Comorbidity Survey Replication, 2001-2003Google Scholar
8 : The metabolic effects of atypical antipsychotics. Can J Psychiatry 2006; 51:480–491Crossref, Google Scholar
9 : Low rates of treatment for hypertension, dyslipidemia and diabetes in schizophrenia: data from the CATIE schizophrenia trial sample at baseline. Schizophr Res 2006; 86:15–22Crossref, Google Scholar
10 : Prescription drugs and the changing concentration of health care expenditures. Health Aff (Millwood) 2007; 26:249–257Crossref, Google Scholar
11 Personal communication, Roger Kathol, Cartesian Solutions data baseGoogle Scholar
12 Peek CJ and the National Integration Academy Council: Lexicon for behavioral health and primary care integration: concepts and definitions developed by expert consensus. AHRQ Publication No.13-IP001-EF. Rockville, MD: Agency for Healthcare Research and Quality. 2013. Available at: http://integrationacademy.ahrq.gov/sites/default/files/Lexicon.pdf.Google Scholar
13
14 ;
15 : Collaborative care for depression: a cumulative meta-analysis and review of longer-term outcomes. Arch Intern Med 2006; 166:2314–2321Crossref, Google Scholar
16 : Collaborative care for patients with depression and chronic illnesses. N Engl J Med 2010; 363:2611–2620Crossref, Google Scholar
17 : The triple aim: care, health, and cost. Health Aff (Millwood) 2008; 27:759–769Crossref, Google Scholar
18 : Comparative effectiveness of collaborative chronic care models for mental health conditions across primary, specialty, and behavioral health care settings: systematic review and meta-analysis. Am J Psychiatry 2012; 169:790–804Crossref, Google Scholar
19 : Organizing care for patients with chronic illness. Milbank Q 1996; 74:511–544Crossref, Google Scholar
20 : Individualized stepped care of chronic illness. West J Med 2000; 172:133–137Crossref, Google Scholar
21 : Consultation psychiatry in the medical home and accountable care organizations: achieving the triple aim. Gen Hosp Psychiatry 2011; 33:305–310Crossref, Google Scholar
22 : Counselling for mental health and psychosocial problems in primary care. Cochrane Database Syst Rev 2011; (9, Issue 9)CD001025. Available at doi: 10.1002/14651858.CD001025.pub3Google Scholar
23 : A randomized trial of medical care management for community mental health settings: the Primary Care Access, Referral, and Evaluation (PCARE) study. Am J Psychiatry 2010; 167:151–159Crossref, Google Scholar
24 Request for Applications (RFA) No. SM-09-011: SAMHSA, 2009Google Scholar
25 : Integrating primary care into community behavioral health settings: programs and early implementation experiences. Psychiatr Serv 2013; 64:660–665Link, Google Scholar
26 Personal communication, Center for Integrated Health Solutions (CIHS) 2013.Google Scholar
27 : Medical services for clients in community mental health centers: results from a national survey. Psychiatr Serv 2008; 59:917–920Crossref, Google Scholar
28 Centers for Medicare & Medicaid Services: Letter to state Medicaid directors and state health officials. Nov 16, 2010 www.cms.gov/smdl/downloads/SMD10024.pdf.Google Scholar
29 Alexander L, Druss BG: Behavioral health homes for people with mental health & substance abuse conditions: the core clinical features. Washington, DC, SAMHSA-HRSA Center for Integrated Health Solutions, US Department of Health and Human Services, May 2012.Google Scholar
30 Parks J, Floyd T, Graham D: Medicaid Health Home Implementation in Missouri: A Year Later (webinar). June 27, 2013Google Scholar
31 Gawande A: The Hot Spotters. The New Yorker, Jan 2011.Google Scholar
32 : Psychiatrists for medically complex patients: bringing value at the physical health and mental health/substance-use disorder interface. Psychosomatics 2009; 50:93–107Crossref, Google Scholar
33 : Proactive psychiatric consultation services reduce length of stay for admissions to an inpatient medical team. Psychosomatics 2011; 52:513–520Crossref, Google Scholar
34 : Prevention of delirium in hospitalized older patients: risk factors and targeted intervention strategies. Ann Med 2000; 32:257–263Crossref, Google Scholar
35 : A method to provide integrated care for complex medically ill patients: the INTERMED. Nurs Health Sci 2007; 9:150–157Crossref, Google Scholar
36 : Malpractice liability for informal consultations. Fam Med 2003; 35:476–481Google Scholar
37 : Guidelines for prescribing psychiatrists in consultative, collaborative, and supervisory relationships. Psychiatr Serv 1998; 49:1197–1202Crossref, Google Scholar



