Attention Deficit Hyperactivity Disorder Through The Life Cycle
Abstract
It is estimated that 5%–8% of school-aged children and 4% of adults in the United States suffer from some form of attention deficit disorder and that the incidence of the disorder is increasing in the population. Although it is the most widely studied behavior disorder of childhood, its etiology remains unclear, its outcome is variable, and its treatment is both complex and moderately successful. Advances in neuroscience have provided new insights into the pathophysiology of ADHD, pointing to key neural circuits involved in attention, behavioral control, learning, and reward maintenance that appear to be underperforming in patients with the disorder. Moreover, the etiology of this heterogeneous disorder points to the key role of genetic x environment interactions during prenatal and perinatal periods. Clinical assessment of ADHD requires a comprehensive approach, including gathering a detailed developmental history, obtaining data from scales and structured interviews, screening for comorbid conditions, and obtaining psychological testing where indicated. The mainstay of treatment is a multimodal approach combining medications and psychosocial interventions that are tailored to the patient and family’s priorities. Optimal clinical care necessitates ongoing monitoring of treatment response and adverse reactions in a longitudinal framework that is commonly used in managing chronic disorders.
Definition
The most widely used definition of attention deficit hyperactivity disorder is provided by the American Psychiatric Association’s Diagnostic and Statistical Manual, Fourth Edition (DSM-IV) (1), which outlines two major dimensions for the disorder (see Table 1). Several changes being considered for DSM-5 include raising the age at onset to 12 years old, adding new criteria for impulsivity (e.g. “acting without thinking,” “impatience,” “difficulty resisting temptations or opportunities”), revising the number of criteria for adolescents and adults (e.g., 2-3 from each of the three categories) and removing the exclusion for autism spectrum disorders. While the core 18 criteria will be unchanged, descriptions will be added to contextualize them for different age groups. There are also likely to be changes in the subtypes of ADHD emphasizing the presenting pattern of symptoms (2).
| A. Either (1) or (2) |
| 1. six (or more) of the following symptoms of inattention have persisted for at least 6 months to a degree that is maladaptive and inconsistent with developmental level: |
| Inattention |
| a. often fails to give close attention to details or make careless mistakes in schoolwork, work or other activities |
| b. often has difficulty sustaining attention in tasks or play activities |
| c. often does not seem to listen when spoken to directly |
| d. often does not follow through on instructions and fails to finish schoolwork, chores, or duties in the workplace (not due to oppositional behavior or failure to understand instructions) |
| e. often has difficulty organizing tasks and activities |
| f. often avoids, dislikes, or is reluctant to engage in tasks that require sustained mental effort (such as schoolwork or homework) |
| g. often loses things necessary for tasks or activities (e.g. toys, school assignments, pencils, books, or tools) |
| h. is often easily distracted by extraneous stimuli |
| i. is often forgetful in daily activities |
| 2. six (or more) of the following symptoms of hyperactivity-impulsivity have persisted for at least 6 months to a degree that is maladaptive and inconsistent with development level |
| Hyperactivity |
| a. often fidgets with hands or feet or squirms in seat |
| b. often leaves seat in classroom or in other situations in which remaining seated is expected |
| c. often runs about or climbs excessively in situations in which it is inappropriate (in adolescents or adults, may be limited to subjective feelings of restlessness |
| d. often has difficulty playing or engaging in leisure activities quietly |
| e. is often “on the go” or often acts as if “driven by a motor” |
| f. often talks excessively |
| Impulsivity |
| g. often blurts out answers before questions have been completed |
| h. often has difficulty awaiting turn |
| i. often interrupts or intrudes on others (e.g. butts into conversations or games) |
| B. Some hyperactive-impulsive or inattentive symptoms that caused impairment were present before age 7 years |
| C. Some impairment from the symptoms is present in two or more settings (e.g. at school [or work] and at home) |
| D. There must be clear evidence of clinically significant impairment in social, academic, or occupational functioning |
| E. Symptoms do not occur exclusively during the course of a psychotic disorder and are not better accounted for by another mental disorder |
| F. Types of ADHD |
| 1. Combined - if both criteria A1 and A2 are met for past 6 months |
| 2. Predominantly Inattentive Type - if only Criterion A1 is met for past 6 months |
| 3. Predominantly Hyperactive-Impulsive Type - if only criterion A2 is met for past 6 months |
Epidemiology and natural history
It is now known that ADHD is a common neuropsychiatric disorder associated with a wide range of functional impairments throughout the lifespan (3, 4). Between 5%–8% of school-aged children (5) and 4% of adults (6) suffer from some form of attention deficit disorder depending upon the method of assessment. There are marked cross-national differences in prevalence rates due to variations in the criteria used to make the diagnosis (7). A survey by Kessler et al. (8) revealed that 36.3% of children meeting the criteria for ADHD continued to meet full diagnostic criteria for the disorder as adults and about two thirds of all children with ADHD continue to have residual symptoms into adolescence and adulthood (9).Inattention is a prominent feature in more than 90% of adults (10). ADHD is associated with impairments in educational, occupational, neuropsychological, and social functioning in adults (11). Adults with ADHD tend to have more frequent job changes and work difficulties; lower socioeconomic status; higher rates of marital problems and divorce; and more speeding violations, driver’s license suspensions, and automobile accidents than those without ADHD. Therefore, the successful diagnosis and management of the disorder in adults is of great importance.
Etiology
ADHD is a heterogeneous neurobehavioral disorder with multiple likely causes. Approximately 65%–75% of cases are thought to be due to genetics, with another 10%–15% caused by CNS insults from prenatal, perinatal, and postnatal sources (12, 13). Prenatal causes include maternal cigarette smoking (which increases the odds by 2.5 times), maternal alcohol drinking (2.5 odds ratio), premature birth (with an incidence of 45% when intracerebral hemorrhage occurs), maternal respiratory infections, maternal anxiety, and high maternal phenylalanine levels. Perinatal asphyxia or anoxia also increases risk of ADHD. Contrary to popular myth, cocaine or crack exposure does not add risk when other variables are controlled. Postnatal factors associated with ADHD include head trauma, brain hypoxia, CNS tumors, CNS infection, febrile seizures, lead poisoning, pediatric acute lymphoblastic leukemia (ALL), streptococcal infection, and elevated phenylalanine levels.
There is a large and growing body of literature on the genetic basis of ADHD dating back over 30 years. Early family, twin, and adoption studies have converged on a mean heritability of 0.75, which places ADHD just below autistic-like traits (0.82-0.87) and schizophrenia (0.80-0.85). Recent family-based and case-control studies of candidate genes have shown a statistically significant correlation between ADHD and variants of seven genes: serotonin HTR1B receptor, serotonin transporter, synaptosomal-associated protein 25 (SNAP 25), dopamine β-hydroxylase, dopamine transporter, dopamine D5 receptor, and the dopamine D4 receptor. One particular common variant of the dopamine D4 receptor (7-repeat) has been highly studied and found to increase risk for ADHD when coupled with both dopamine transporter (SLC6A3 10 repeat) and maternal exposure to smoking (14). The presence of each of these along with maternal smoking increases the risk of ADHD by 2.5 – 3.0 times. The presence of both of them along with smoking increases the risk to 9 times (15, 16).
Neuroanatomy / pathophysiology
Studies over the past several decades suggest that ADHD results from disruptions in several key neural circuits involving the frontal lobe, anterior and medial to the precentral motor cortex. The motor circuit includes a subcortical feedback loop from the motor and somatosensory areas of the cortex, through restricted portions of the basal ganglia and thalamus, and back to the premotor cortex, supplementary motor area, and motor cortex. In addition, the locus ceruleus appears to play a major role in the initiation and maintenance of attention, particularly in response to novel stimuli. Two major subsystems are involved in attention regulation: a posterior system which disengages from current environmental stimuli in order to orient to new stimuli, and an anterior system which works to integrate the various executive functions of the frontal lobe. Imbalances in these pathways have also been implicated in ADHD.
Pliszka et al. (17) proposed an interesting model in which ADHD is hypothesized to be caused by imbalances in catecholamine functioning throughout several brain regions. The central norepinephrine system (via the locus coeruleus) may be hypoactive, causing insufficient response of the posterior attention system to novel stimuli. The dopaminergically mediated anterior attention system (governing executive function) may also be underactive, leading to poor planning, faulty working memory, lack of attention to details, and inefficient problem-solving. The peripheral epinephrine system is also hypothesized to play a role in mediating the individual’s response to psychostimulant medication. The beauty of this multistage model is that it integrates neurochemistry, neuroanatomy and neurophysiology. It also helps to explain why neurotransmitter studies have failed to show a specific deficiency pattern in patients with ADHD.
Several reviews of the neuroanatomy of ADHD (18–21) suggest three related circuits that may be dysregulated in this disorder. The fronto-striatalcircuit is associated with deficits in response suppression, freedom from distraction, working memory, organization, and planning. The fronto-limbiccircuit is associated with symptoms of emotional dyscontrol, motivation deficits, hyperactivity-impulsivity, and proneness to aggression. And the fronto-striatal-cerebellarcircuit is associated with motor coordination deficits, and problems with the timing and timeliness of behavior (see Figure 1).
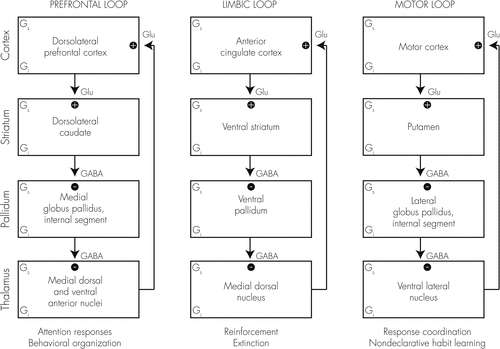
Neuropsychological deficits seen in ADHD suggest the involvement of the prefrontal cortex (especially in the right hemisphere) where classical studies of patients with damage to this area show patterns of loss of working memory, forgetfulness, increased susceptibility to interference, distractibility, poor concentration, impulsivity, and poor organization. This has led to a growing recognition of ADHD as a developmental disorder of executive functioning (EF) (22, 23) although there is as yet no consensus on this point (24). EFs are a wide range of central control processes previously referred to as “frontal lobe functions” that connect, prioritize, and integrate cognitive functions on a moment-by-moment basis. Brain structures and interconnections subserving EF are immature at birth and undergo continuous development into early adulthood. This maturation process depends upon mylenization, synaptic pruning, elaboration of DA & NE systems, and other developmental processes. Thus, EF can become impaired developmentally, traumatically, and/or secondary to disease processes.
Structural and functional neuroimaging studies over the past two decades have shown smaller, less active, and less developed brain regions in three areas: the orbital prefrontal cortex (primarily right side), the basal ganglia (mainly the striatum and globus pallidus) and the cerebellum (central vermis area, more on right side). In addition, the anterior cingulate gyrus has been shown to be under-activated in ADHD patients who are performing an error detection task. The size of the attentional network is correlated with degree of ADHD symptoms, particularly inhibition. Interestingly, there appears to be a 3 year lag in brain development with patients achieving typical brain volumes by age 16. Moreover, these results are not due to taking stimulant medication (15).
The neurochemical evidence for ADHD is contradictory at best. Urine, serum, and cerebrospinal fluid metabolites of serotonin, norepinephrine, and dopamine are not consistently different in ADHD patients as compared with matched controls. Dopamine beta hydroxylase, monoamine oxidase, and catechol-O-methyl transferase are also similar in these two groups. There is some evidence for a decreased turnover of dopamine (DA) and for a supersensitivity to released DA in ADHD patients. More recently, researchers have demonstrated a reduction of DA synaptic markers in the DA reward pathways (mesoaccumbens) of ADHD individuals, which may explicate the chronically diminished motivational state that many patients experience (25). Pharmacologic studies with dopamine agonists, however, fail to demonstrate a primary deficiency of dopamine. The most significant pharmacologic effects on ADHD symptoms have been found with stimulants such as methylphenidate and dextroamphetamine, both of which work on catecholamine metabolism, lending strong support to some role for both norepinephrine and dopamine in this disorder.
Differential diagnosis
ADHD is associated with a variety of other psychiatric problems, and numerous psychiatric conditions can present as attention difficulties. Comorbidity has become an important area of research in recent years, as studies reveal that high percentages of children and adolescents with ADHD experience other disturbances such as oppositional defiant behavior, conduct disorder, and aggressive behaviors; mood disorders (particularly depression and bipolar affective disorder); anxiety disorders; learning disabilities and language disorders; and, among adolescents and young adults, substance abuse and personality disorders. Special patient populations have high rates of ADHD including those with Tourette syndrome, obsessive-compulsive disorder, autistic spectrum disorders, fetal alcohol syndrome, and posttraumatic stress disorder (3, 26) [See Table 2]. In adults, comorbid conditions are also the rule rather than the exception, with even higher rates of alcohol and substance abuse and dependence, mood disorders and anxiety disorders reported (6).
| Oppositional Defiant Disorder |
| Conduct Disorder |
| Anxiety Disorders |
| Mood Disorders |
| Bipolar Disorder |
| Major Depression |
| Dysthymia |
| Learning Disorders |
| Language Disorders |
| Tourette Syndrome |
| Obsessive Compulsive Disorder |
| Autistic Spectrum Disorders |
| Fetal Alcohol Syndrome |
| Sleep Disorders |
| Substance Use Disorders |
| Post-Traumatic Stress Disorder |
Seizure disorders, including petit mal (absence) or partial complex seizures may be mistaken for ADHD. Sensory deficiencies, particularly deafness and partial hearing impairment, can also mimic ADHD. Approximately 40%–50% of ADHD children suffer from a learning disability of sufficient magnitude that school performance is negatively affected. In adults, a number of medication conditions can either cause or worsen ADHD symptoms including head injury (TBI), sleep apnea, COPD, anoxic encephalopathy, dementia, delirium, hypothyroidism, hyperthyroidism, renal insufficiency, hepatic insufficiency, vitamin deficient states, and medication-induced cognitive impairments (see Table 3).
| Head injury (TBI) |
| Sleep apnea |
| COPD |
| Anoxic encephalopathy |
| Dementia |
| Delirium |
| Hypothyroidism |
| Hyperthyroidism |
| Renal insufficiency |
| Hepatic insufficiency |
| Vitamin deficient states |
| Medication induced |
Assessment
Children and adolescents
The diagnosis of ADHD is made by a comprehensive clinical evaluation that should include interviews of parent(s) and patient, and the gathering of collateral information from teachers and school records. Assessment begins with a careful description of the child or adolescent’s problem behaviors. When interviewing parents, it is important that they give examples of situations in which the child or teen is having difficulty. Terms like “hyperactive, disruptive, and impulsive” should be defined as precisely as possible. When parents report that the patient won’t sit still, won’t pay attention, and won’t follow instructions, it is helpful to find out when they first became aware of these difficulties. It also helps to clarify if the problems occur both at home and at school. Parents should describe their strategies for handling these behaviors, and share their insights into what works and what doesn’t. Additionally, the clinician should review all 18 symptoms of ADHD and ask the parent to describe the presence or absence of these symptoms, give examples, and clarify their onset and course.
In addition to the cardinal signs of inattention, impulsivity, and hyperactivity, the clinician should inquire about the degree of oppositional behavior, aggressiveness, moodiness, and temper outbursts that the child or teen is manifesting. Whenever possible, parents should be observed interacting with their child. The clinician should note how the child addresses the parents, and whether s/he listens to their instructions and commands. If the patient begins to misbehave in the office, this is an opportunity to learn how parents handle challenging behaviors.
After the problem description, a comprehensive history should be obtained including pregnancy; perinatal period; medical history; developmental milestones; speech and language function; sleep pattern; presence of pica, enuresis or encopresis; early temperament; and diet and medications. Social and family history should include an inquiry into the presence of ADHD, learning disabilities, and other developmental/or psychiatric disturbances in the parents or siblings. Finally, the patient’s school history should be traced, especially regarding behavior and achievement in the early grades. Copies of old report cards and of teachers’ descriptions of the child are extremely valuable. It is also important to speak directly to the current teacher to learn about the patient’s typical behavior in class, and to understand how the teacher views and handles the child or teen.
Parent and teacher rating scales are extremely useful as adjuncts to the diagnostic interview. There are numerous instruments available (27); however, no single scale is perfect, nor can any scale “make” a diagnosis of ADHD. Scales offer a relatively quick measure of the youth’s behaviors as compared with those of age- and sex-matched peers. They can also be used to measure change in targeted areas following the initiation of treatment (3).
Minor congenital anomalies, general neurologic status, speech and language functioning, and overall mental status are important to evaluate. Signs of fetal alcohol effects should be noted, and the presence of unusual physical stigmata is an indication to order chromosome analysis. The patient’s speech and receptive language abilities are important to screen insofar as communication disorders and learning disabilities can be present in children and adolescents with ADHD. In addition to hyperactivity, inattentiveness, and impulsivity, the mental status examination should note signs of affective disturbance (i.e., anxiety, depression, irritability), autism and other developmental disorders, and general intellectual functioning. Standard grade-level screening tests (e.g., Slosson Oral Reading Test) and perceptual motor tasks (including drawings and the Bender-Gestalt) can provide additional information regarding the patient’s cognitive abilities. These steps can help the clinician assess the child as well as help to rule in or rule out co-occurring conditions or conditions that may mask themselves as ADHD.
For the most part, medical laboratory tests are of little value in making the diagnosis of ADHD. Since patients with mildly elevated lead levels (i.e., 10 μg/dl) may show signs of ADHD, plasma lead level, free erythrocyte protoporphyrin, and a complete blood count should be obtained at the initial visit in order to rule out lead poisoning and iron deficiency anemia. If there is suspicion of absence seizures or other neuropathology, an EEG is indicated. Evidence of altered metabolism (e.g., elevated resting heart rate, palpitations, tremors, agitation) suggests that a thyroid panel and a urine screen for VMA should be obtained.
Psychoeducational testing of intellectual ability and of scholastic achievement can pinpoint cognitive difficulties which may be interfering with the patient’s school performance. Speech and language assessment is indicated for patients who seem to have communication problems. And while computerized measures of attention and impulsivity are not required to make a diagnosis, they may be helpful in quantifying the symptom profile and in evaluating the effects of intervention.
Adults
Diagnosing ADHD in adults necessitates a complete evaluation, focusing on the core ADHD symptoms starting in childhood (28) especially as they may have affected academic achievement, social relationships, and self management skills. It is important to elicit details of early school experiences and to track these throughout the patient’s academic career. It can be useful to explore the degree of assistance and support provided by key adults (e.g., parents, teachers), the patient’s changing symptom patterns and responses to these, and the impact of these on academic achievement, occupational choices, job performance, intimacy, and self-esteem. Rating scales such as the Wender Utah Rating Scale (29), the Brown Attention-Deficit Disorder Scale for Adults (30), the Conners’ Adult ADHD Rating Scale (CAARS) (31), and the Adult Self-Report Scale (32) are extremely important in the assessment of past and current ADHD symptoms in adults with suspected ADHD (33)[See (34) for an excellent comparative review]. Measures of intellectual ability and academic skills may be helpful in gauging the likely success of occupational aspirations and job performance. Object measures of attention and impulsivity may help to clarify the patient’s symptom severity but are not required for general clinical practice.
Given the very high rate of comorbidities seen in this patient group, a thorough medical history is of critical importance in determining whether there are any medical conditions that could confound the diagnosis of ADHD (27). Equally important, a detailed history of drug and alcohol use and of other psychiatric disorders should be taken. Throughout the entire procedure, collateral observational information should be obtained from spouse, significant other, parent, or friend. This is important to verify the patient’s story (hence reducing the chance of missing factitious disorder) and to corroborate and/or add important clinical data that the patient may have missed or forgotten.
Treatment
Children and adolescents
Practice guidelines for the treatment of ADHD have been developed by a number of working groups, including the American Academy of Child and Adolescent Psychiatry (27), the American Academy of Pediatrics (35), the British Association for Psychopharmacology (36), the Canadian ADHD Resource Alliance (37), the European Network for Hyperkinetic Disorders (38), the National Institute for Health and Clinical Excellence (39), and the National Institutes of Health (40). All of these practice parameters stress the importance of a multimodal treatment approach to patients with ADHD.
Multimodal treatment of children and adolescents with ADHD includes careful treatment planning, psychoeducation, medication, behavior management, school-based interventions, family therapy, and social competence training. While the precise combination of these interventions will vary depending upon the needs of the patient and family, it is clear that no single treatment approach is sufficient, and that in order to be effective, treatment must extend over long periods of time
Treatment planning needs to incorporate patient/family priorities and available resources and identify potential obstacles to successful treatment adherence. In addition, systems issues must be taken into consideration so that professional helpers can form constructive relationships with patient/family, school personnel, key community supports, and with one another in order to maximize chances for successful treatment.
The goal of psychoeducation and counseling is to help parents and youth cope better with the consequences of having ADHD. Patients/families need reliable information and supportive guidance when confronted with the diagnosis of ADHD. It is important that the clinician offer the patient and family sufficient time to discuss their concerns and answer their questions. Factual information should be provided in a comprehensible fashion so as to clarify any misunderstandings or confusion about the disorder. The clinician should emphasize the child’s positive traits and the parents’ strengths in order to alleviate feelings of guilt, confusion, and anger. While parents are likely to be relieved to hear that their child’s problems are not the result of inadequate parenting, they are also likely to experience grief reactions as they learn more about the implications of the diagnosis. While children may be pleased to hear their problems are not their fault, they are also likely to feel ashamed and resentful about having “something wrong” with them, and they may resist taking their medication or participating in behavioral treatment. It is important for the physician to monitor the emotional reactions of parents and children, and to be supportive of their efforts to pursue treatment. There are numerous references written for parents that are very helpful in explaining the diagnosis and treatment of ADHD, which are available on websites sponsored by the Centers for Disease Control, National Institute of Mental Health, and CHADD (Children and Adults with Attention Deficit/Hyperactivity Disorder. These include resources like the Parents’ Medication Guide for ADHD, coauthored by the APA and the AACAP [see website listings in Reference section]. Support groups for parents of children with ADHD have proliferated in recent years. These groups hold meetings, sponsor lectures, publish newsletters, and offer emotional assistance to families. Clinicians should be familiar with local chapters of CHADD and similar organizations and should have their contact information readily available for patients and families.
Pharmacotherapy
At present, there are numerous FDA-approved medical treatments for ADHD (Table 4). While medications have been shown to provide short-term benefits for children and adolescents with ADHD, longitudinal studies in-dicate that pharmacotherapy is only one aspect of treatment and that without behavioral inter-ventions, the child’s difficulties at home and at school are likely to persist. The decision to use medication is mediated by several factors, including the child’s age, severity and profile of the child’s symptoms, comorbidity, and parental attitudes. Children under 5 years of age are less likely to respond to medications and are at greater risk of having adverse side effects, suggesting that behav-ioral interventions are an important first step with this age group (41). School-aged children with moderate to severe symptoms of inattention and distractibility (with or without impulsivity and hyperactivity) are extremely likely to benefit from medication. Children with mild symptoms are also likely to benefit, although it is usually preferable to initiate behavioral treatment prior to starting medication with this group. The presence of other disturbances such as tics, anxiety, aggression, or depression tends to influence the choice of phar-macologic agent. Finally, parental attitudes are extremely important to consider when recom-mending medication. Most parents are ambivalent about starting their child on medication, so it is best to give them ample time to consider the decision carefully. The following guidelines are suggested when instituting a medication regimen:
| Medication | Duration of effect |
|---|---|
| Methylphenidate-based formulations | |
| Concerta (methylphenidate) | ∼12 hours |
| Ritalin (methylphenidate) | 3-4 hours |
| Metadate CD (methylphenidate) | 8-10 hours |
| Ritalin LA (methylphenidate) | ∼8 hours |
| Focalin (XR) (dexmethylphenidate) | 3-4 (8-10) hours |
| Daytrana (methylphenidate) | ∼12 hours (worn for 9) |
| Amphetamine-based treatments | |
| Adderall XR (mixed amphetamine salts) | ∼12 hours |
| Adderall (mixed amphetamine salts) | 4-6 hours |
| Dexedrine Spansule (dextroamphetamine) | 6-8 hours |
| Vyvanse (lisdexamfetamine) | ∼12 hours |
| Nonstimulants | |
| Strattera (atomoxetine) | Up to 24 hours |
| Intuniv (guanfacine) | Up to 24 hours |
| Kapvay (clonidine) | Up to 24 hours |
| 1. | Specify the target behaviors that the medications are intended to ameliorate. Where possible, measure the behaviors; otherwise, use parent and teacher rating scales. | ||||
| 2. | Obtain baseline laboratory measures such as CBC, serum electrolytes, and liver function tests. | ||||
| 3. | Begin with low doses, increase gradually, and aim for the lowest effective dose possible. | ||||
| 4. | Follow side effects closely, and discontinue the medication if no positive effects are seen or if side effects become severe. | ||||
| 5. | Discuss the child reactions to and parents’ feelings about the medication. Give support and encouragement if initial results are not as good as expected. | ||||
| 6. | Document beneficial and adverse effects on a regular basis. | ||||
Algorithms have been developed to guide the selection of medication regimens according to best available evidence (42), most of which suggest that clinicians initiate treatment with a stimulant medication and only if there is insufficient response should alternative medications be introduced (Figure 2).
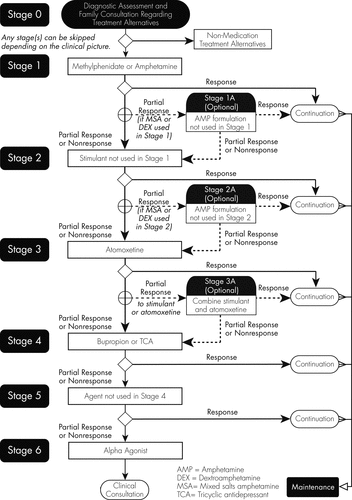
Reprinted with permission from: The Texas Children's Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. Pliszka SR, Crismon ML, Hughes CW, Corners CK, Emslie GJ, Jensen PS, McCracken JT, Swanson JM, Lopez M; Texas Consensus Conference Panel on Pharmacotherapy of Childhood Attention Deficit Hyperactivity Disorder. J Am Acad Child Adolesc Psychiatry. 2006 Jun;45(6):642-57.
Psychostimulants
Psychostimulants have direct and indirect agonist effects on ∂-adrenergic and β-adrenergic receptors as well as on dopaminergic receptors via three mechanisms: release of stored catecholamines (dopamine and norepinephrine); agonist effects on postsynaptic receptors; and inhibition of presynaptic reuptake of released catecholamine (note: methylphenidate works primarily via mechanism 3, whereas amphetamine works via all three mechanisms [see Figure 3]).
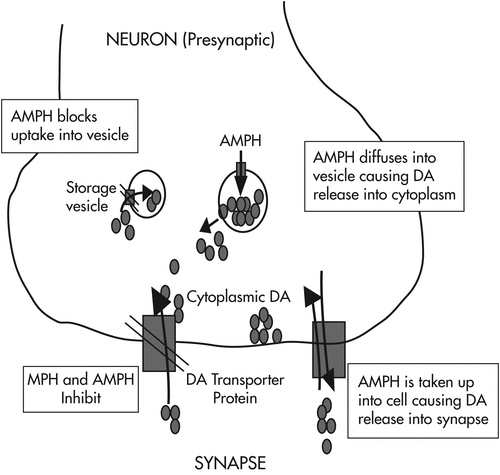
Over 80% of patients with ADHD demonstrate a positive response to psychostimulants, although it is impossible to predict in advance which medication will produce the best results for any given patient. Clinical effects include reduced hyperactivity and impulsivity, improved attention and concentration, improved fine motor skills (including handwriting), and improved social interactions (e.g., reduced oppositional behaviors). Behavioral measures of cognitive performance with psychostimulants demonstrate improvements in attention span, impulse control, short-term memory, and problem solving. These results are seen in both patients with ADHD and controls, so a positive response cannot be used to diagnose ADHD. In general, while cognition of ADHD patients can be optimized by the medication, this improvement can be eradicated with improper dosing.
Up to 5% of patients will experience adverse effects serious enough to warrant discontinuation of the medication. Adverse effects include appetite suppression, gastrointestinal discomfort, sleep disturbance, increased heart rate and blood pressure (clinically insignificant), tics, and minor physical complaints (e.g., headaches, stomach-aches). Irritability, dysphoria, heightened anxiety, lack of spontaneity and over-sedation may be seen, but these are often due to overmedication. It appears that a subgroup of patients who respond with intense mood lability and dysphoria may be expressing early signs of a mood disorder rather than ADHD. This should prompt a change in medication and close monitoring (43, 44).
Extremely serious side effects such as delusions, paranoia, and frank psychosis are rare but can be seen with over-dosage and abuse of the medication. Concerns have been raised about cardiovascular side effects of stimulants, although recent reports seem to indicate that there are few, if any, serious adverse cardiac events due to stimulant use (45, 46). Current recommendations include careful screening for cardiac symptoms in the patient (e.g., dyspnea, palpitations, and exercise intolerance), for abnormal physical findings, and for family history of premature deaths or significant morbidity from heart disease.
Clinical and biochemical predictors of nonresponse or adverse effects have not yet been identified, although there is some evidence of diminished efficacy of stimulants in ADHD children with symptoms of anxiety (42). On the other hand, it appears that stimulants are helpful in reducing the aggressive behavior of conduct disordered ADHD children. There are also studies suggesting that stimulant treatment in childhood and adolescence reduces the risk of later alcohol or substance abuse.
Discussions with parents and teachers are helpful to identify any incipient problems with stimulant medication. It is also important to decide upon the type and frequency of medication use. For example, most long-acting medications are sufficient to improve concentration during the school day, but an additional dose of a short-acting preparation is often prescribed to help the child complete homework assignments in the late afternoon and early evening. Weekend dosing assists ADHD children with participation in team sports, social events, church activities, and instructional programs. It also enables them to complete homework assignments and study for examinations. For teenagers who drive, weekend dosing is essential to minimize the risk of hazardous driving and accidents.
After several weeks on a steady dose and schedule, it may be necessary to increase the dose slightly. The goal of treatment should be symptom remission, which often necessitates steadily titrating the dose upward until either symptoms improve or significant adverse effects emerge. If there is no clear positive response to the initial stimulant chosen, or if side effects emerge that might interfere with adherence as the dose is increased, it is advisable to introduce other stimulant preparations before additional pharmaceutical agents are introduced. Using parent- and teacher-report rating scales to track symptom response to medication can be very helpful. Above all, it is important to continuously monitor progress toward target goals and to adjust the medication regimen accordingly.
For the most part, children can be maintained on the same dose for up to 6 to 12 months. It is important to monitor clinical and adverse effects on a regular basis. Height, weight and blood pressure measurements and a brief physical examination should be conducted approximately every 2 months if things are going smoothly. If the child begins losing weight, the dose and schedule should be revised in order to optimize appetite around mealtimes. Growth delay, although rarely seen with current dosage recommendations, is an indication for stopping the medication for several weeks to allow “catch up” growth to take place.
One of the most common problems seen with stimulants is referred to as “rebound.” This is generally seen in the late afternoon as the medication wears off. Typically, the child becomes restless, hyperactive, inattentive, irritable, and prone to temper tantrums and emotional outbursts. It is often helpful to add an additional slightly lower dose when the child returns from school. Parents should be advised to allow the child to do something enjoyable and to avoid making too many demands on the child during this time. If the rebound period becomes extremely difficult for the child and family to handle, it may be necessary to switch medications or introduce longer acting preparations.
Atomoxetine
Atomoxetine is the first nonstimulant medication to be FDA approved for the treatment of ADHD. A norepinephrine (NE) reuptake inhibitor, it has been shown to have a moderate effect size (0.4 – 0.6) in numerous studies involving children, adolescents and adults with ADHD (47, 48). The advantages of atomoxetine are its milder side effects profile (compared with stimulants), and the absence of potential for abuse or misuse. Increased CNS NE levels resulting from this medication are associated with downstream increases in dopamine (DA) levels in the frontal cortex without concomitant changes in levels found in the nucleus accumbens or the basal ganglia (49, 50). Recent studies suggest that atomoxetine may be helpful in patients with comorbid anxiety (51), and/or tic disorders (52). An open trial of atomoxetine in children and adolescents with dyslexia showed improved reading task performance (53). Side effects include: dizziness, high blood pressure, headache, irritability, nervousness, abdominal pain, nausea, vomiting, loss of appetite, weight loss, dry mouth, constipation, urinary hesitancy, decreased sexual desire and a very slight chance of reversible hepatic insufficiency.
Alpha-adrenergic agents (Clonidine, Guanfacine)
Alpha2 adrenergic agonists were first introduced as antihypertensive agents over 40 years ago but were supplanted by other medications (e.g., calcium channel blockers) with fewer side effects. Their effects on the CNS include modulation of the tonic and phasic activity of the locus coeruleus (major source of NE in the brain) and enhanced adrenergic activity in the prefrontal cortex. Their effects on NE neurotransmission occur via up-regulation of intracellular cyclic adenosine monophosphate (cAMP) which, in turn, improves signal conduction along the axon (54, 55). Clonidine works on alpha2A, 2B, and 2C re-ceptors (which are located throughout the CNS, including the brain stem), leading to its producing more hypotensive and sedative effects than guanfacine, which is selective for the alpha2A receptors. Extended release clonidine has been found to help ADHD symptoms either alone (56) or in conjunction with psychostimulants (57). Clinical trials of extended-release guanfacine have shown it to be effective as monotherapy for ADHD in children and adolescents (58–60). Recent studies have shown guanfacine can be combined with stimulants to produce enhanced clinical effects in children and adolescents with suboptimal response to stimulants alone (61, 62).
Clinical experience with these agents suggests that they are especially useful in reducing irritability, overarousal, emotional lability, and explosiveness in patients with ADHD. They are also helpful in controlling tics and managing high blood pressure. The most common side effects seen with alpha2 adrenergic agonists are sedation, fatigue, dizziness, syncope, cardiac rhythm disturbances, dry mouth, indigestion, nausea, nightmares, insomnia, anxiety, dysphoria, and depression. In addition, hypertensive crises can be induced by sudden discontinuation of these medications.
Psychosocial Interventions
Whereas youth with ADHD have trouble controlling their impulses, focusing their attention and following rules, parents need to learn basic methods of managing these challenging behaviors. Using techniques such as positive reinforcement, rewards, response cost, pun-ishments, contracts, token economies, extinction procedures, environmental manipulation and stimulus controls, parents can be taught to exert a positive influence on behavior.
The first step in developing a behavior management program is to specify which behaviors are acceptable and which ones need to be modified. It is important that parents learn to focus more of their attention on the child’s positive behaviors, and to “catch them being good” as often as possible. By shifting more energy and attention to the child’s “good” behaviors, parents and teachers will inevitably spend less time harping on the “bad.” Next, parents choose a specific behavior (or behavior sequence) that they would like to change. They should describe the behavior in ways they can observe and measure. It is generally best for parents to begin by focusing on a relatively simple behavior which is easy for the child to perform and for the parents to observe and quantify. Behaviors like getting ready in the morning, doing homework, putting toys away, completing chores, temper tantrums, and getting ready for bed are good for starters. It is also important for them to consider factors that prevent the child from successfully completing tasks.
Once a target behavior is chosen, parents will need to identify ways to increase the child’s motivation to cooperate. Rewards should be given for successful efforts, and penalties should be given for overt resistance or major oppositional behavior. An accounting system must be set up to keep track of the child’s performance and to distribute the rewards and penalties in an impartial fashion. It is important that parents not get angry or upset with the child when they are administering a penalty. After parents have decided upon the rewards and penalties, it is advisable for them to draw up a contract. The contract should include the date on which the agreement begins, the specific behaviors that are being targeted for change, the types of rewards and penalties that will be used to enforce the contract, the accounting system that will be used to keep track of rewards and penalties, the time and frequency of rewards, the start and duration of penalties, and the schedule for reviewing the contract. The contract should be written in a language that the child can understand, and should be posted in a prominent place in the home. Once it has been discussed and reviewed, the contract should be signed by everyone who will be involved in its enforcement (including other adults).
After the contract becomes operational, its efficacy will need to be closely monitored, and its terms will need to be refined and corrected in order to ensure that it is helping the child to achieve desired behavior changes. The child should succeed at the desired behaviors 80% of the time. If s/he is succeeding more often, the task should be made more difficult; if less, it should be made easier.
It should always be kept in mind that the purpose of any behavior management system is to help the child learn to follow rules and to complete important tasks. This is a major challenge for most children with ADHD, and parents will need to work very diligently to keep a behavior management program running. Although it takes a great deal of patience, resourcefulness and perseverance, parents can look forward to modest rewards for the child and the family. If the program succeeds in improving the child’s ability to care for him/herself and in increasing his/her self-control, it will have the added benefits of reducing stress and improving the emotional climate of the family.
Classroom behavioral interventions have been developed to improve both classroom deportment and academic performance. The best studied have been daily report cards and contingency management programs with effect sizes in the range of 1.44 (63). Classroom academic interventions seem to have moderate beneficial effects, although these are not as well studied and sample sizes are small. The most common interventions include task and instructional modification, homework assistance, peer tutoring, computer-assisted instruction, and strategy training (for an excellent review, see (64)). More recent innovations include family-school interventions that promote close collaboration between parents and teachers in order to achieve improved academic performance and classroom behavior (65).
Recently, computer-assisted programs have been introduced to enhance cognitive functioning in children and adolescents with ADHD. The best studied of these, CogMed, is a software program developed by Klingberg and colleagues at the Karolinska Institute. It entails web-based training on a series of rotating exercises designed to enhance visuospatial and verbal working memory, five times per week for 30-40 minutes. Initial results of randomized controlled trials show modest improvements in these domains (66, 67).
Treatment considerations for adults with ADHD
Treatment guidelines for adults with ADHD are still evolving, but there is a growing consensus that multimodal approaches similar to those used with children and adolescents are the most likely to address the multiplicity of issues that these patients present to clinicians.
Pharmacotherapy
The most important step before starting pharmacotherapy for ADHD adults is thorough education. To begin with, the objectives for using medications need to be clarified by delineating the target symptoms that are the focus of treatment (e.g., inattention, distractibility, restlessness, and impulsivity). Next, a method for measuring and keeping track of symptom improvement needs to be agreed upon. It is best to have the patient keep a log that is user friendly and likely to be implemented (Table 5). Once a medication is selected, both written and oral information is provided to the patient, and specific plans are discussed for starting the medication. Typically this means starting at a relatively low dose, observing initial clinical effects, noting side effects, and setting a schedule for increasing the dosage to appropriate levels. Frequent follow-up visits are scheduled at first to ensure that the patient has ample opportunity to discuss the positive and negative effects observed and to address any questions or concerns. Further adjustments to the regimen are made in accordance with patient reports, although it is a good idea to get input from significant others in the patient’s life.
| Medication response form | Patient name | |||||
|---|---|---|---|---|---|---|
| Medication | Dose, schedule | |||||
| Instructions to patient: Rate concentration, task completion, and mood on a scale of 1 to 10, | ||||||
| where 1 = poor, 5 = average, and 10 = excellent. Write comments in the appropriate column. | ||||||
| Day | Time | Dose | Concentration | Task Completion | Mood | Comments |
Pharmacotherapy for ADHD in adults is not as well-studied as in children and adolescents, but the American Academy of Child and Adolescent Psychiatry guidelines and others (4, 68) endorse the use of both stimulant and nonstimulant medications depending upon patient variables. Initial treatment choice should consider the patient’s clinical profile (including risk factors and comorbid conditions), treatment goals, past history of medication use, preferences for medication effects and dosing patterns (once-daily versus multiple times), and potential side effects. For relatively uncomplicated patients, stimulants or atomoxetine are first-line agents.
Stimulants work quickly and are generally effective (80%–90% response rate, effect size = 0.9) and well-tolerated, although long-term adverse effects have not been studied. While short-acting stimulants are the most widely prescribed medications, there is some evidence that longer-acting preparations are better tolerated, have less abuse potential, and are easier for patients to remember to take consistently. It is important to monitor blood pressure and heart rate at each visit, and to inquire about treatment emergent cardiovascular symptoms.
Atomoxetine is FDA-approved for ADHD in adults (69–71).It has a long duration of action (half-life>12 hours) but works with gradual onset (4 to 6 weeks), so that positive effects emerge over a longer time period than with stimulants. The response rate to atomoxetine is 60% and the effect size is 0.4 (moderate). It is most helpful with patients who do not tolerate stimulants, who are highly anxious, or who express a preference for a medication that works “around the clock.” It has also shown to be effective in ADHD patients with comorbid social anxiety disorder (72). The most common side effects from atomoxetine are nausea, GI upset, headache, sedation, fatigue, reduced sexual drive, and difficulty with urination. Mild increases in heart rate and blood pressure have also been reported, but rarely are these significant enough to require discontinuation.
There is some evidence that the alpha2 agonists can improve symptoms in adults with ADHD. A double-blind placebo-controlled study comparing guanfacine to dextroamphetamine in adults with ADHD found that each were comparable in their clinical effects as well as their impact on neuropsychological measures (73).
Bupropion is a medication with proven efficacy for treatment of depression, smoking cessation, and adult ADHD (74, 75). It is a potent DA reuptake inhibitor with less potent inhibition of NE reuptake. The regular-release preparation has a half-life of approximately 14 hours, while the extended-release form has a half-life of 24 hours. Although bupropion is activating (even stimulating for some patients) and occasionally worsens anxiety symptoms, it produces less sexual dysfunction than other antidepressants. The most significant side effect of bupropion is an increased incidence of seizures, reported in 0.4% of patients taking the immediate-release preparation. Seizures remit with discontinuation of the medication. Other adverse effects include dry mouth, constipation, nausea, vomiting, anorexia, weight loss, headache, dizziness, fainting spells, insomnia, tremor, restlessness, excitability, mood swings, and irritability.
Combining SSRIs with stimulants can be used safely for adults with ADHD and comorbid anxiety or depression. The more sedating agents (e.g., paroxetine or sertraline) might be preferable when patients report difficulty with insomnia or overactivation, and the less sedating compounds (e.g., fluoxetine or citalopram) when they complain of being too tired or underactive. Since there is no significant interaction between stimulants and SSRIs, both types of medications can be prescribed at usual dosage strengths.
Psychosocial Interventions
There is a growing body of research indicating that psychosocial treatments are effective for adult patients with ADHD, even those that are already taking medications. In contrast to the large literature on children and adolescents, there are about 20 published psychosocial treatment studies to date using a variety of manualized approaches to improving symptoms and functioning in this population (76) (See Table 6). Several investigators have adapted CBT either for individual patients (77–82) or using a group approach (83–87). While the emphasis of each approach is somewhat different, they share certain important features: psychoeducation about ADHD and its impact on daily functioning, redesigning the environment to improve task completion and reduce distractions, modifying distorted beliefs, practical problem-solving skills, and affect regulation. What is impressive about these interventions is that they all seem to work equally well with patients who are medicated or nonmedicated.
| Ratey et al. (1992) – “treatment failures” |
| Wiggins et al. (1999) – educational gp (TFA) |
| Wilens et al. (1999) – chart review CBT/meds |
| Hesslinger et al. (2002) – DBT group |
| Philipsen et al (2007) – DBT-group, multisite replication |
| Stevenson et al. (2002, 2003) – CRP group treatment & self-directed CRP |
| Safren et al. (2005, 2010) – CBT/meds v. meds only; CBT v. relaxation |
| Weiss et al. (2006) – PST & meds |
| Rostain & Ramsay (2006) – CBT & meds |
| Ramsay & Rostain (2011) – CBT only |
| Solanto et al. (2008, 2010) – CBT gp focused on EFs |
| Virta et al. (2008, 2010); Salakari et al. (2010) – CBT gp rehabilitation/ 6 mo. fu |
| Bramham et al. (2009) – CBT group |
Outcomes
While it was once believed that hyperactive children “outgrew” their condition, evidence is accumulating that ADHD persists into adolescence and adulthood. It appears that over 50% of children with ADHD will continue to exhibit some symptoms of the disorder (e.g., inattention, impulsivity) into later life. Associated difficulties for adolescents include school failure, aggression, antisocial behavior, poor social skills, emotional immaturity, low self-esteem and interpersonal conflicts. Adults have an increased incidence of anxiety, low self-esteem, personality disorders (especially antisocial personality disorder), alcohol and substance abuse, interpersonal difficulties, and occupational changes. For instance, Kessler et al. (6) found that 4.4% of adults surveyed in the National Comorbidity Survey Replication study met criteria for ADHD, and a substantial proportion of these individuals also met criteria for other psychiatric disorders. Negative outcomes are associated with factors such as aggression and antisocial behavior, severity of hyperactivity and impulsivity, lower intelligence, lower socioeconomic status, and the presence of additional individual and family psychopathology (e.g., alcohol or substance abuse).
Although follow-up studies raise serious concerns about the long-term outcomes of ADHD children, it should be kept in mind that individual outcomes cannot be predicted from group-derived statistics. Given that ADHD is a heterogeneous condition, and that the poorest outcomes are associated with aggression and antisocial behavior rather than with the core symptoms of ADHD, it is best to regard the prognosis as uncertain and mediated by factors other than ADHD. Professionals and parents need to take a “long view” of ADHD as a chronic condition in order to avoid a sense of failure or frustration if the course of treatment is long and arduous. There are no simple solutions to this complex biopsychosocial disorder.
Future directions
In the future, clinicians will undoubtedly use genetic testing to aid in the diagnosis of ADHD, as well as to assist in selecting appropriate treatment modalities. The role of gene × environment interactions in the unfolding of ADHD and related disorders will be much better understood allowing for preventive interventions to be targeted at highest risk individuals. Pharmacogenomics holds the promise of better prediction of treatment response (both pharmacologic and psychosocial). It is also likely that new medications targeting different genotypes will be available in the near future, as will agents that work at the “epigenetic” level, modulating the gene products that fundamentally influence key neurodevelopmental processes seen in ADHD. Finally, advances in computer technology, particularly portable devices such as smart phones and microcomputers, will offer patients new tools for improving executive functioning and self management, and will enable clinicians to obtain better measures of patient functioning and adaptation as well as symptom severity.
Websites
Patient/Parent Information about ADHD
1 : Diagnostic and Statistical Manual of Mental Disorders, 4th ed. Washington, DC, APA, 1994Google Scholar
2 : Do the diagnostic criteria for ADHD need to change? Comments on the preliminary proposals of the DSM-5 ADHD and Disruptive Behavior Disorders Committee. Eur Child Adolesc Psychiatry 2011; 20:75–81Crossref, Google Scholar
3 Barkley RA (ed): Attention Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment, 3rd ed. New York, Guilford Press, 2006Google Scholar
4 : Understanding attention-deficit/hyperactivity disorder from childhood to adulthood. Postgrad Med 2010; 122:97–109Crossref, Google Scholar
5 : Prevalence, recognition, and treatment of attention-deficit/hyperactivity disorder in a national sample of US children. Arch Pediatr Adolesc Med 2007; 161:857–864Crossref, Google Scholar
6 : The prevalence and correlates of adult ADHD in the United States: results from the National Comorbidity Survey Replication. Am J Psychiatry 2006; 163:716–723Link, Google Scholar
7 : The worldwide prevalence of ADHD: is it an American condition? World Psychiatry 2003; 2:104–113Google Scholar
8 : The prevalence and effects of adult attention deficit/hyperactivity disorder on work performance in a nationally representative sample of workers. J Occup Environ Med 2005; 47:565–572Crossref, Google Scholar
9 : The age-dependent decline of attention deficit hyperactivity disorder: a meta-analysis of follow-up studies. Psychol Med 2006; 36:159–165Crossref, Google Scholar
10 : ADHD in adults: a review of the literature. J Atten Disord 2008; 11:628–641Crossref, Google Scholar
11 : ADHD in Adults: What the Science Says. New York, Guilford Press, 2008Google Scholar
12 : What Causes ADHD? New York, Guilford Press, 2006Google Scholar
13 : The impact of perinatal stress on the functional maturation of prefronto-cortical synaptic circuits: implications for the pathophysiology of ADHD? Prog Brain Res 2011; 189:155–169Crossref, Google Scholar
14 : Molecular genetics of attention-deficit/hyperactivity disorder. Biol Psychiatry 2005; 57:1313–1323Crossref, Google Scholar
15 : Longitudinal mapping of cortical thickness and clinical outcome in children and adolescents with attention-deficit/hyperactivity disorder. Arch Gen Psychiatry 2006; 63:540–549Crossref, Google Scholar
16 : Prenatal smoking exposure and dopaminergic genotypes interact to cause a severe ADHD subtype. Biol Psychiatry 2007; 61:1320–1328Crossref, Google Scholar
17 : Neuroimaging of inhibitory control areas in children with attention deficit hyperactivity disorder who were treatment naive or in long-term treatment. Am J Psychiatry 2006b; 163:1052–1060Crossref, Google Scholar
18 : Attention-deficit/hyperactivity disorder: a selective overview. Biol Psychiatry 2005; 57:1215–1220Crossref, Google Scholar
19 : A dynamic developmental theory of attention-deficit/hyperactivity disorder (ADHD) predominantly hyperactive/impulsive and combined subtypes. Behav Brain Sci 2005; 28:397–419, discussion 419–468Crossref, Google Scholar
20 : The neurobiology of impulsivity and self-regulatory control in children with attention-deficit/hyperactivity disorder, in Neurobiology of Mental Illness, 3rd ed. Edited by Charney DSNestler EJ. New York, NY, Oxford University Press, 2009, pp 1129–1152Google Scholar
21 : Towards conceptualizing a neural systems-based anatomy of attention-deficit/hyperactivity disorder. Dev Neurosci 2009; 31:36–49Crossref, Google Scholar
22 : ADHD Comorbidities: Handbook for ADHD Complications in Children and Adults. Washington, DC, APPI, 2005Google Scholar
23 : Differential diagnosis of adults with ADHD: the role of executive function and self-regulation. J Clin Psychiatry 2010; 71:e17Crossref, Google Scholar
24 : Characterizing cognition in ADHD: beyond executive dysfunction. Trends Cogn Sci 2006; 10:117–123Crossref, Google Scholar
25 : Motivation deficit in ADHD is associated with dysfunction of the dopamine reward pathway. JAMA 2010; 302:1084–1091Crossref, Google Scholar
26 Brown TE (ed): Attention Deficit Disorder: The Unfocused Mind in Children and Adults. New Haven, CT, Yale University Press, 2009Google Scholar
27 : AACAP Work Group on Quality Issues: Practice parameter for the assessment and treatment of children and adolescents with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2007; 46:894–921Crossref, Google Scholar
28 : American Academy of Child and Adolescent Psychiatry: Practice parameter for the use of stimulant medications in the treatment of children, adolescents, and adults. J Am Acad Child Adolesc Psychiatry 2002; 41(Suppl):26S–49SCrossref, Google Scholar
29 : Attention deficit disorder (‘minimal brain dysfunction’) in adults. A replication study of diagnosis and drug treatment. Arch Gen Psychiatry 1981; 38:449–456Crossref, Google Scholar
30 : Brown Attention Deficit Disorder Scales: Manual. San Antonio, TX, Pearson Education, Inc., 1996Google Scholar
31 : CAARS Adult ADHD Rating Scales. New York, Multi Health Systems, 1998Google Scholar
32 Adler LA, Kessler RC, Spencer T: (2004) Adult ADHD Self-Report Scale-v 1.1 (ASRS-v1.1) symptom checklist. New York, NY: 2004. Available at: http://www.med.nyu.edu/psych/assets/adhdscreen18.pdf.Google Scholar
33 : Diagnostic approaches to adult attention-deficit/hyperactivity disorder. Prim Psychiatry 2004; 11:49–53Google Scholar
34 : Psychopathological rating scales for diagnostic use in adults with attention-deficit/hyperactivity disorder (ADHD). Eur Arch Psychiatry Clin Neurosci 2006; 256(Suppl 1):i3–i11Crossref, Google Scholar
35 American Academy of Pediatrics: ADHD: Clinical Practice Guideline for the Diagnosis, Evaluation, and Treatment of Attention-Deficit/Hyperactivity Disorder in Children and Adolescents. Pediatrics 2011; http://pediatrics.aappublications.org/content/early/2011/10/14/peds.2011-2654Google Scholar
36 : British Association for Psychopharmacology: Evidence-based guidelines for management of attention-deficit/hyperactivity disorder in adolescents in transition to adult services and in adults: recommendations from the British Association for Psychopharmacology. J Psychopharmacol 2007; 21:10–41Crossref, Google Scholar
37 Canadian Attention Deficit Hyperactivity Disorder Resource Alliance (CADDRA) Canadian ADHD Practice Guidelines, 1st ed. Toronto, ON, CADDRA Guidelines Committee, 2006Google Scholar
38 : European clinical guidelines for hyperkinetic disorder — first upgrade. Eur Child Adolesc Psychiatry 2004; 13(Suppl 1):I7–I30Crossref, Google Scholar
39 National Institute for Health and Clinical Excellence: Attention deficit hyperactivity disorder. September 2008. Clinical guideline 72. Available at http://guidance.nice.org.uk/CG72Google Scholar
40 : Diagnosis and treatment of attention deficit hyperactivity disorder (ADHD). NIH Consens Statement 1998; 16:1–37Google Scholar
41 : Efficacy and safety of immediate-release methylphenidate treatment for preschoolers with ADHD. J Am Acad Child Adolesc Psychiatry 2006; 45:1284–1293Crossref, Google Scholar
42 : Texas Consensus Conference Panel on Pharmacotherapy of Childhood Attention Deficit Hyperactivity Disorder: The Texas Children’s Medication Algorithm Project: revision of the algorithm for pharmacotherapy of attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2006a; 45:642–657Crossref, Google Scholar
43 : Methylphenidate-induced mania in a prepubertal child. J Clin Psychiatry 1986; 47:566–567Google Scholar
44 : Stimulants, in Attention Deficit Hyperactivity Disorder: A Handbook for Diagnosis and Treatment, 3rd ed. Edited by Barkley RA. New York, Guilford Press, 2006, pp 634Google Scholar
45 : ADHD drugs and serious cardiovascular events in children and young adults. N Engl J Med 2011; 365:1896–1904Crossref, Google Scholar
46 : ADHD medications and risk of serious cardiovascular events in young and middle-aged adults. JAMA 2011; 306:2673–2683Crossref, Google Scholar
47 : Once-daily atomoxetine treatment for children and adolescents with attention deficit hyperactivity disorder: a randomized, placebo-controlled study. Am J Psychiatry 2002; 159:1896–1901Crossref, Google Scholar
48 : Once-daily atomoxetine treatment for children with attention-deficit/hyperactivity disorder, including an assessment of evening and morning behavior: a double-blind, placebo-controlled trial. Pediatrics 2004; 114:e1–e8Crossref, Google Scholar
49 : Atomoxetine increases extracellular levels of norepinephrine and dopamine in prefrontal cortex of rat: a potential mechanism for efficacy in attention deficit/hyperactivity disorder. Neuropsychopharmacology 2002; 27:699–711Crossref, Google Scholar
50 : Effect of the attention deficit/hyperactivity disorder drug atomoxetine on extracellular concentrations of norepinephrine and dopamine in several brain regions of the rat. Neuropharmacology 2006; 50:755–760Crossref, Google Scholar
51 : Atomoxetine treatment for pediatric patients with attention-deficit/hyperactivity disorder with comorbid anxiety disorder. J Am Acad Child Adolesc Psychiatry 2007; 46:1119–1127Crossref, Google Scholar
52 : Atomoxetine treatment of ADHD in children with comorbid Tourette syndrome. J Atten Disord 2008; 11:470–481Crossref, Google Scholar
53 : Atomoxetine for the treatment of Attention-Deficit/Hyperactivity Disorder (ADHD) in children with ADHD and dyslexia. Child Adolesc Psychiatry Ment Health 2009; 3:40Crossref, Google Scholar
54 : Toward a new understanding of attention-deficit hyperactivity disorder pathophysiology: an important role for prefrontal cortex dysfunction. CNS Drugs 2009; 23(Suppl 1):33–41Crossref, Google Scholar
55 : alpha2-Adrenergic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: emerging concepts from new data. J Child Adolesc Psychopharmacol 2007; 17:393–406Crossref, Google Scholar
56 . Clonidine extended-release tablets for pediatric patients with attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry. 2011;50(2):171–179.Crossref, Google Scholar
57 . Clonidine extended-release tablets as add-on therapy to psychostimulants in children and adolescents with ADHD. Pediatrics. 2011;127(6):1406–1413.Crossref, Google Scholar
58 : Long-term, open-label extension study of guanfacine extended release in children and adolescents with ADHD. CNS Spectr 2008; 13:1047–1055Crossref, Google Scholar
59 : SPD503 STUDY GROUP: Guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder: a placebo-controlled trial. J Am Acad Child Adolesc Psychiatry 2009a; 48:155–165Crossref, Google Scholar
60 : Long-term safety and efficacy of guanfacine extended release in children and adolescents with attention-deficit/hyperactivity disorder. J Child Adolesc Psychopharmacol 2009b; 19:215–226Crossref, Google Scholar
61 : Guanfacine extended release as adjunctive therapy to psychostimulants in children and adolescents with attention-deficit/hyperactivity disorder. Adv Ther 2012; 29:385–400Crossref, Google Scholar
62 Wilens TE, Bukstein O, Brams M, Cutler AJ, Childress A, Rugino T, Lyne A, Grannis K, Youcha S: A controlled trial of extended-release guanfacine and psychostimulants for attention-deficit/hyperactivity disorder. J Am Acad Child Adolesc Psychiatry 2012; 51:74–85, e2Google Scholar
63 : Evidence-based psychosocial treatments for children and adolescents with attention-deficit/hyperactivity disorder. Clin Psychol Rev 2006; 26:486–502Crossref, Google Scholar
64 : Strategies for implementing evidence-based psychosocial interventions for children with attention-deficit/hyperactivity disorder. Child Adolesc Psychiatr Clin N Am 2012; 21:145–159 [x]Crossref, Google Scholar
65 Power TJ, Mautone JA, Soffer SL, Clarke AT, Marshall SA, Sharman J, Blum NJ, Glanzman M, Elia J, Jawad AF: A family-school intervention for children with ADHD: results of a randomized clinical trial. J Consult Clin Psychol 2012; 80:611–623 10.1037/a0028188 Epub 2012 Apr 16.Google Scholar
66 : Computerized training of working memory in children with ADHD—a randomized, controlled trial. J Am Acad Child Adolesc Psychiatry 2005; 44:177–186Crossref, Google Scholar
67 : Adaptive training leads to sustained enhancement of poor working memory in children. Dev Sci 2009; 12:F9–F15Crossref, Google Scholar
68 : Attention-deficit/hyperactivity disorder in adults: evidence-based recommendations for management. Postgrad Med 2008; 120:27–38Crossref, Google Scholar
69 : Atomoxetine in adults with ADHD: two randomized, placebo-controlled studies. Biol Psychiatry 2003; 53:112–120Crossref, Google Scholar
70 : Atomoxetine: a review of its use in adults with attention deficit hyperactivity disorder. Drugs 2004; 64:205–222Crossref, Google Scholar
71 : Emotional dysregulation in adult ADHD and response to atomoxetine. Biol Psychiatry 2005; 58:125–131Crossref, Google Scholar
72 : Atomoxetine treatment in adults with attention-deficit/hyperactivity disorder and comorbid social anxiety disorder. Depress Anxiety 2009; 26:212–221Crossref, Google Scholar
73 : Comparing guanfacine and dextroamphetamine for the treatment of adult attention-deficit/hyperactivity disorder. J Clin Psychopharmacol 2001; 21:223–228Crossref, Google Scholar
74 : A controlled clinical trial of bupropion for attention deficit hyperactivity disorder in adults. Am J Psychiatry 2001; 158:282–288Crossref, Google Scholar
75 : Bupropion XL in adults with attention-deficit/hyperactivity disorder: a randomized, placebo-controlled study. Biol Psychiatry 2005; 57:793–801Crossref, Google Scholar
76 : Nonmedication Treatments for Adult ADHD: Evaluating Impact on Daily Functioning and Well-Being. Washington, DC, American Psychological Association, 2012Google Scholar
77 : Cognitive-behavioral therapy for ADHD in medication-treated adults with continued symptoms. Behav Res Ther 2005a; 43:831–842Crossref, Google Scholar
78 : Mastering Your Adult ADHD: A Cognitive-Behavioral Treatment Program Therapist Guide (Treatments That Work). New York, Oxford University Press, 2005bCrossref, Google Scholar
79 : Cognitive behavioral therapy vs relaxation with educational support for medication-treated adults with ADHD and persistent symptoms: a randomized controlled trial. JAMA 2010; 304:875–880Crossref, Google Scholar
80 : A combined treatment approach for adults with ADHD—results of an open study of 43 patients. J Atten Disord 2006; 10:150–159Crossref, Google Scholar
81 Ramsay JR, Rostain AL: Cognitive Behavioral Therapy for Adult ADHD: An Integrative Psychosocial and Medical Approach. New York, Routledge, Taylor & Francis Group, 2008Google Scholar
82 : CBT without medications for adult ADHD: an open pilot study of five patients. J Cogn Psychother 2012;26 (in press)Google Scholar
83 : Structured group psychotherapy in adults with attention deficit hyperactivity disorder: results of an open multicentre study. J Nerv Ment Dis 2007; 195:1013–1019Crossref, Google Scholar
84 : Evaluation of the efficacy and effectiveness of a structured disorder tailored psychotherapy in ADHD in adults: study protocol of a randomized controlled multicentre trial. Atten Defic Hyperact Disord 2010; 2:203–212Crossref, Google Scholar
85 : Development of a new psychosocial treatment for adult ADHD. J Atten Disord 2008; 11:728–736Crossref, Google Scholar
86 : Efficacy of meta-cognitive therapy for adult ADHD. Am J Psychiatry 2010; 167:958–968Crossref, Google Scholar
87 : Cognitive Behavior Therapy for Adult ADHD: Targeting Executive Dysfunction. New York, Guilford Press, 2011Google Scholar



