Insomnia
Insomnia is one of the most prevalent health complaints in the general population, in medical practice, and in psychiatric practice. For many years, clinicians have been encouraged to think of insomnia as a symptom rather than a disorder, hoping to treat insomnia by finding and treating the underlying “cause.”
Recent evidence has called this practice into question. Although insomnia is very commonly associated with other psychiatric and mental disorders, its onset, response to treatment, and long-term course are often independent of comorbid conditions. Furthermore, insomnia is independently associated with substantial morbidity, functional impairment, and health care costs. Finally, psychological, behavioral, and pharmacologic treatments for “primary” insomnia are also efficacious in “secondary” insomnias, potentially improving the comorbid conditions as well. Therefore, insomnia is increasingly viewed not simply as a symptom, but as a syndrome or disorder that frequently co-occurs with other medical and psychiatric conditions and that merits independent treatment.
Definitions
Insomnia symptoms refer to complaints of difficulty falling asleep, frequent or prolonged awakenings, inadequate sleep quality, or short overall sleep duration in an individual who has adequate time available for sleep. Insomnia is not defined by sleep laboratory measures or a specific sleep duration. Because insomnia occurs only when there is adequate opportunity for sleep, it should be distinguished from sleep deprivation, in which the individual has relatively normal sleep ability but has inadequate opportunity for sleep.
An insomnia disorder is a syndrome consisting of the insomnia complaint together with significant impairment or distress and the exclusion of other causes. The most common daytime impairments associated with insomnia include complaints of mood disturbances, impaired cognitive function, and daytime fatigue (Moul et al. 2002). Typical mood symptoms are irritability, mild dysphoria, and difficulty tolerating stress. Cognitive complaints include difficulties in concentrating, completing tasks, and performing complex, abstract, or creative tasks. Fatigue is a common complaint in individuals with insomnia disorders, but actual daytime sleepiness is less common. In fact, many individuals with insomnia are unable to sleep during the day even though they feel tired.
Epidemiology and consequences
The epidemiology of insomnia is complicated by several factors, including the distinction of symptom from disorder. First, because epidemiologic studies of sleep disorders rely on self-reports, biases may occur because of inexact wording of questions, length or form of interview, subjects’ avoidance or exaggeration of symptoms, measurement of confounding comorbidities, concurrent medication use, and memory difficulties (Moul et al. 2004). In addition, deciding when a complaint is clinically significant is a challenge for epidemiological and clinical studies. Finally, the validity of epidemiological estimates of specific insomnia disorders may be limited by the low degree of interrater reliability of clinical diagnoses (Buysse et al. 1994). In-depth reviews of insomnia epidemiology are available elsewhere (Ohayon 2002), but major findings are reviewed below.
The one-year prevalence of insomnia symptoms in the United States and other Western nations is approximately 30%–40% in the general population and up to 66% in primary care and psychiatric settings. The prevalence of primary insomnia as a specific disorder is in the range of 5%–10% of the general population. Aside from estimating the prevalence of nighttime symptoms or syndromal insomnias, investigators continue to expand the study of daytime symptoms in primary insomnia, among them poor concentration, fatigue, dysphoria, sleep dissatisfaction, and mental inefficiency (Moul et al. 2002).
Risk factors for insomnia can be categorized into predisposing, initiating, and maintaining factors (Spielman et al. 1987). Established vulnerability factors include advancing age, female sex, being divorced or separated, unemployment, and comorbid medical and psychiatric illness. Genetic factors, although inadequately studied, represent another possible risk. Factors that commonly initiate or maintain insomnia include psychosocial stresses such as moves, relationship difficulties, occupational and financial problems, and caregiving responsibilities (Kappler and Hohagen 2003). An additional maintaining factor that forms the basis for many therapeutic interventions is the adoption of counterproductive sleep habits (described later in this chapter). It remains difficult to estimate the exact roles of different risk factors in the initiation and maintenance of insomnia in specific populations.
The natural history of insomnia, determined from population-based studies, has not been well described. However, follow-up studies of clinical patients and population samples indicate that insomnia often persists for years and may become more severe with time (Mendelson 1995; Vollrath et al. 1989), although remission has been described in one study of older adults (Foley et al. 1999). The longitudinal epidemiology of insomnia is complicated by normal aging, which leads to lighter and more fragmented sleep. Studies of older adults again suggest that insomnia is strongly related to medical and psychiatric conditions and that it decreases when these problems are adequately treated (Katz and McHorney 1998).
The consequences of insomnia can be substantial. Several studies have identified insomnia as a risk factor for the later development of depressive, anxiety, and substance use disorders (Breslau et al. 1996; Chang et al. 1997; reviewed in Riemann and Voderholzer 2003). In patients with major depressive disorder (MDD), insomnia is associated with worse treatment outcomes (Buysse et al. 1999), suicidal ideation (Agargun et al. 1997), symptom persistence (Moos and Cronkite 1999), and recurrence of MDD (Perlis et al. 1997a; Reynolds et al. 1997). Insomnia is also associated with persistence and relapse in alcohol dependence (Brower et al. 2001). Insomnia is associated with increased medical care utilization, more absenteeism from work (Simon and Von Korff 1997), and increased direct and indirect medical costs (Walsh and Engelhardt 1999). Insomnia may be associated with increased motor vehicle and other accidents (Powell et al. 2002), as well as increased incidence of falls in the elderly (Brassington et al. 2000).Various studies have also substantiated reduced quality of life in individuals with insomnia (Hatoum et al. 1998; Léger et al. 2001) that is similar in magnitude to that seen with chronic conditions such as congestive heart failure and MDD (Katz and McHorney 2002). Quality of life is associated with the severity of the insomnia complaint, and in individuals with medical disorders, quality of life has an effect independent of the comorbid condition (Karlsen et al. 1999; McCall et al. 2000). Quality of life problems among individuals with insomnia include role impairments across a broad set of domains such as job performance, social life, and family life (Léger et al. 2002).
Pathophysiology and etiology
Physiological models
Insomnia is often considered to be a disorder of increased arousal. Arousal may refer to many different phenomena, but a working definition of arousal is the individual’s state of central nervous system (CNS) activity and reactivity, ranging from sleep at one end of the spectrum to wakefulness with excitement or panic at the other (Coull 1998). Arousal is related to the function of wake-promoting structures in the ascending reticular activating system, hypothalamus, and basal forebrain, which interact with sleep-promoting brain centers in the anterior hypothalamus and thalamus (see Chapter 1, “Introduction”). Hyperarousal is a state characterized by a high level of alertness that may be present tonically or in response to specific situations, such as the sleep environment.
Psychophysiological and metabolic evidence for hyperarousal in insomnia patients includes findings of increased body temperature and galvanic skin response near sleep onset and increased heart rate and decreased heart period variability during sleep (reviewed in Bonnet and Arand 1997a). Whole-body metabolic rate measured by volume of oxygen consumption per unit of time (VO2) is higher during sleep in patients with insomnia compared with healthy sleepers (Bonnet and Arand 1995, 1997b), again supporting the general concept of hyperarousal.
Electrophysiological evidence for hyperarousal comes from studies showing elevated high-frequency electroencephalographic (EEG) activity (beta activity) during non–rapid eye movement (NREM) sleep in insomnia patients (Krystal et al. 2002; Merica et al. 1998; Perlis et al. 2001). Beta activity is normally associated with mental activity during wakefulness. Reduced homeostatic sleep drive in insomnia patients, indicated by reduced delta EEG activity, has been found in some studies as well (Merica and Gaillard 1992).
Neuroendocrine evidence for hyperarousal includes increased cortisol and adrenocorticotropic hormone levels before and during sleep, particularly during the first half of sleep, in insomnia patients (Rodenbeck et al. 2002; Vgontzas et al. 2001). Decreased melatonin levels have been found less consistently (Attenburrow et al. 1996; Hajak et al. 1995).
Finally, hyperarousal is suggested by functional neuroanatomic studies of arousal, as indicated by patterns of regional brain metabolic activity during NREM sleep during single-photon emission computed tomography (SPECT) and positron emission tomography (PET) scans. In the first PET study of primary insomnia, patients had increased global glucose metabolic rates during both wakefulness and sleep compared with healthy control subjects, and the usual sleep-related decline in metabolism in brainstem arousal centers was attenuated (Nofzinger et al. 2004) (Figure 1, part A). During wakefulness, primary insomnia patients have reduced dorsolateral prefrontal cortical activity. This constellation of findings suggests hyperarousal during NREM sleep and frontal hypoarousal during wakefulness, which may correspond to patients’ sleep- and wake-related complaints (Figure 1, part B). In patients with insomnia in the context of major depression, EEG beta activity was positively associated with metabolic activity in the orbitofrontal cortex and with complaints of poor sleep quality (Nofzinger et al. 2000), further substantiating the hyperarousal hypothesis. On the other hand, a study using [99mTc]-HMPAO SPECT in primary insomnia patients (M.T. Smith et al. 2002a) found hypoperfusion across eight preselected regions of interest (including frontal medial, occipital, and parietal cortex) during NREM sleep, with the most prominent effects in the basal ganglia. Thus, imaging studies have begun to show functional neuroanatomic changes during NREM sleep associated with primary and secondary insomnia, although the precise nature of these changes awaits further confirmation.
Cognitive-behavioral models
All cognitive-behavioral models of insomnia outline the relationships between sleep-related thoughts, behaviors, and perceived arousal, their antecedents, and their consequences. However, the core dysfunction underlying insomnia differs across models. In the behavioral model of Spielman et al. (1987) (Figure 2), individual predisposing factors (e.g., heightened physiological or cognitive arousal) interact with external precipitating factors (e.g., life stressors) to produce insomnia. Perpetuating factors (e.g., maladaptive coping strategies such as spending more time in bed) maintain and reinforce insomnia even after the original precipitants recede.
In Morin’s (1993) model, cognitive hyper-arousal, indicated by sleep-focused, ruminative thoughts, particularly around bedtime, is the central component. Cognitive arousal increases physiological arousal. With repetition, this pairing facilitates conditioning between temporal (e.g., bedtime routines) and environmental (e.g., bed, bedroom) cues and sleeplessness. Resulting sleep disturbance and ruminations ultimately lead to daytime consequences such as mood disturbances and fatigue. Over time, these experiences can alter one’s beliefs regarding the ability to sleep and the consequences of sleep difficulties and can lead to the development of maladaptive strategies aimed at maximizing sleep (e.g., spending more time in bed) that further reinforce sleep disturbances and cognitive hyperarousal.
Perlis and colleagues (1997b) proposed a neurocognitive model of insomnia to explain discrepancies between objective polysomnographic findings and subjective reports of poor sleep quality and sleep misperception. Specifically, this model emphasizes brain cortical arousal as a central component and suggests that both physiological and cognitive arousal arise from increased cortical arousal at or around the sleep onset period, as measured by beta EEG activity. The authors posited that this arousal can be conditioned and that it further contributes to the insomnia experience.
Another cognitive model, by Harvey (2002), emphasizes the role of attention biases in the maintenance of insomnia. The model proposes that cognitive strategies (rather than behavioral strategies) employed by people with insomnia are maladaptive and maintain sleep disturbances. Excessive negative cognitive activity leads to increased physiological hyperarousal, which in turn leads to selective attention to and monitoring of autonomic symptoms and environmental cues indicative of sleeplessness. Such selective attention distorts perceptions regarding sleep deficits and adverse daytime effects. Individuals develop maladaptive safety behaviors, such as thought suppression and emotional inhibition, to avoid sleep loss. However, these behaviors further reinforce the negative cognitive activity, physiological arousal, and erroneous beliefs regarding sleep and daytime deficits seen in insomnia.
Clinical assessment and diagnosis
History
The assessment of patients with insomnia rests on a detailed clinical history. As with any clinical disorder, the history for insomnia should focus on specific symptoms, chronology, exacerbating and alleviating factors, and response to previous treatments. However, some aspects of the insomnia history differ from those of other disorders and deserve special emphasis.
Other assessment tools
Although the clinical history is the key to making an insomnia diagnosis, several other tools may aid this process.
Sleep-wake diaries covering 1 or 2 weeks, in which patients record their actual sleep hours and sleep experiences, can be invaluable. Diaries are useful for establishing patterns of sleep, as well as for indicating the day-to-day variability in sleep hours and sleep problems. An example of a sleep diary for insomnia patients is shown in Figure 1–4 in Chapter 1.
Actigraphy is an objective means of assessing rest-activity patterns by use of a motion-sensitive device worn on the nondominant wrist. Commercially available software provides descriptive statistics and graphical displays of rest-activity patterns. Validation studies have shown a strong correlation between actigraphy patterns and sleep as monitored by polysomnography (PSG), although actigraphy tends to overestimate the actual amount of sleep (Sadeh and Acebo 2002). Like the sleep diary, actigraphy can be useful for examining temporal patterns, variability, and responses to treatment.
Polysomnography, or a sleep study, is the gold standard for quantifying sleep and sleep disturbances. PSG is not routinely recommended for the evaluation of chronic insomnia (Sateia et al. 2000) because in most cases, PSG simply confirms the patient’s subjective report without indicating a cause for awakenings. However, PSG may be useful in specific situations involving patients with symptoms of sleep apnea (see Chapter 3), periodic limb movements (Chapter 5), or parasomnias (Chapter 6). Patients who have atypical complaints or a history of poor response to usually efficacious treatments may be candidates for polysomnography.
Differential diagnosis
Many classifications have been proposed for insomnia, some based on symptoms, others on duration, and still others on presumed etiology.
| • | Symptom-based classifications (i.e., sleep-onset, sleep maintenance, or mixed-type insomnia) are of limited value because the specific type of sleep complaint often varies within an individual over time (Hohagen et al. 1994a) and a majority of patients actually complain of more than one type of sleep disturbance. | ||||
| • | Duration-based classifications (e.g., acute, short-term, and chronic insomnia) are also of limited value because of the high rate of chronicity or recurrence in insomnia symptoms. Duration-based classification is most useful in that specific causes of insomnia may be associated with their duration. For instance, transient and short-term insomnias are often related to specific psychosocial or environmental stresses, whereas chronic insomnia is more often related to intrinsic sleep disorders or primary insomnia. | ||||
| • | Etiology-based classifications are the most useful for categorizing insomnia. Specific classification systems include the International Statistical Classification of Diseases and Related Disorders, 9th Revision (ICD-9) and 10th Revision (ICD-10; World Health Organization 1992), the Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision (DSM-IV-TR; American Psychiatric Association 2000), and the International Classification of Sleep Disorders, Revised (ICSD; American Sleep Disorders Association 1997) and 2nd Edition (ICSD-2; American Academy of Sleep Disorders, in press). | ||||
In general, the ICD has the broadest, least well described categories, DSM-IV-TR has somewhat more specific categories, and ICSD has the most specific of all. However, each basically describes three major categories of insomnia:
| 1. | Insomnia secondary to other conditions. This includes insomnias associated with medical disorders, insomnia related to mental disorders, and insomnia related to the acute effects of a substance or withdrawal from a substance/medication. This is the largest single group of chronic insomnia diagnoses seen in epidemiological studies and in clinical samples (Buysse et al. 1994; Ohayon 1997). | ||||
| 2. | Insomnia as a symptom of other specific sleep disorders. This group includes the insomnia seen in restless legs syndrome, some cases of obstructive sleep apnea syndrome, and some cases of parasomnias. Insomnia, particularly difficulty in falling asleep, is a very frequent symptom of restless legs syndrome. Insomnia is somewhat less common in obstructive sleep apnea syndrome, although older adults and those with more central sleep apneas may have this presentation. | ||||
| 3. | Primary insomnia. This category refers to disorders in which insomnia is the primary symptom and no other disorder is a possible cause. DSM-IV-TR includes a single category for primary insomnia. ICSD subdivides it into a number of categories, such as psychophysiological insomnia, idiopathic insomnia, and paradoxical insomnia. | ||||
Behavioral treatment
Behavioral treatments aim to reduce sleep latency and improve sleep consolidation by changing behaviors and habits that interfere with sleep. A cognitive component is sometimes included to address distorted or maladaptive beliefs related to sleep. Behavioral treatments of insomnia can be administered in an individual or a group format.
Types of treatments
Cognitive-behavioral interventions for insomnia are described in this section and are listed in Table 3.
Stimulus control therapy is based on principles of operant learning (Bootzin and Nicassio 1978) and aims to reinforce associations between sleepiness, sleep, and the sleep environment. The patient is instructed to use the bed and bedroom only for sleep and sex, and to go to the bedroom only when feeling sleepy. If awake for long periods of time in bed (e.g., 20 minutes or longer), the individual is instructed to get out of bed and leave the bedroom until feeling sleepy again.
Sleep restriction therapy involves restricting the time spent in bed by setting strict bedtime and rising schedules that closely match the number of hours of actual sleep reported by the patient (Spielman et al. 1987). The aim is to increase sleep efficiency, or the ratio of total time spent asleep to total time spent in bed. Initially, sleep restriction is often associated with slight to moderate sleep deprivation, which increases sleepiness and enhances the ability to fall asleep and to maintain sleep. Sleep restriction may lead to increased daytime sleepiness, and patients should be cautioned about operating machinery or performing duties that require high levels of alertness.
Relaxation techniques aim to reduce muscular tension and cognitive arousal, which are incompatible with sleep (Jacobson 1974; Woolfolk and McNulty 1983). Several specific relaxation techniques have been evaluated for insomnia. Autogenic training involves focusing on physiological sensations, such as heat, to induce relaxation via modulation of the autonomic nervous system. Biofeedback relies on a similar principle, but the process of reducing autonomic arousal is aided by the use of visual or auditory feedback to monitor biological signals such as heart rate or skin temperature to indicate arousal levels to the patient, who can then voluntarily reduce physiological arousal through active concentration. Acupuncture is distinct from conventional relaxation and other behavioral techniques, but preliminary evidence suggests that it too may be efficacious for insomnia (see Sok et al. 2003 for meta-analysis).
Cognitive interventions involve the identification, challenge, and replacement of initial erroneous beliefs or fears regarding loss of sleep (Gross and Borkovec 1982). Cognitive distortions increase arousal and tension, further preventing sleep. Challenging the erroneous beliefs and fears can be done through education and discussion within a psychotherapeutic context. Replacing the irrational beliefs and fears requires behavioral changes to test the new beliefs and cognitive strategies to face resurgence of distorted thoughts about sleep. Cognitive therapy is most often combined with more behaviorally based interventions (Morin 1993). Paradoxical intention is another example of a cognitive technique used for insomnia. The rationale is that actively trying to maintain wakefulness will reduce performance anxiety related to falling asleep and thus will facilitate sleep onset. Data indicate that paradoxical intention may provide some benefit, but responses are highly variable (Morin et al. 1999).
Sleep hygiene refers to a set instructions aimed at reinforcing sleep-promoting behaviors and reducing the frequency of behaviors that may interfere with sleep (Hauri 1991). Exercising, relaxing evening routines, avoiding stimulants and naps, and limiting alcohol intake are examples of behaviors that may enhance sleep quality. Although sleep hygiene alone has limited efficacy to reduce insomnia (Lacks and Morin 1992), it is often combined with other behavioral interventions.
Efficacy of behavioral treatments
Behavioral interventions for insomnia significantly reduce sleep onset latency, reduce wake time after sleep onset, and improve total sleep time. Two meta-analyses support the efficacy of behavioral or cognitive-behavioral treatments of chronic insomnia (Morin et al. 1994; Murtagh and Greenwood 1995). Overall, a majority (70%–80%) of insomnia patients benefit from behavioral interventions, and improvements are maintained or enhanced at follow-up. Stimulus control, sleep restriction, and relaxation show the most robust effects among specific behavioral interventions. A third meta-analysis (Montgomery and Dennis 2003) investigated the efficacy of behavioral interventions in older adults. Overall, cognitive-behavioral therapy was effective at reducing sleep maintenance insomnia.
One additional meta-analysis (M.T. Smith et al. 2002b) compared the efficacy of cognitive-behavioral treatments and pharmacologic agents for insomnia. When compared with hypnotic medications, stimulus control and sleep restriction therapies were associated with similar improvements in wake time after sleep onset, number of awakenings, and sleep quality ratings. Behavioral interventions were associated with greater reductions in sleep onset latency, but hypnotics were associated with greater increases in total sleep time.
New developments in behavioral treatments of insomnia
New behavioral treatment modalities for insomnia are currently being investigated to disseminate these treatments and reduce patient burden and costs. These new developments include inhome visits (Espie et al. 2001), telephone interventions, Internet-based interventions (Strom et al. 2004), and self-help material (Mimeault and Morin 1999). Initial results suggest that behavioral interventions can be effectively delivered via these nontraditional methods. In elderly patients, however, one study suggest that in-person, group behavioral interventions may be more effective than home-based relaxation treatment to reduce insomnia complaints (Rybarczyk et al. 2002).
Psychological treatment of insomnia secondary to another condition
Cognitive-behavioral interventions for insomnia are also effective at improving sleep quality in patients with comorbid medical and psychiatric illnesses (Lichstein et al. 2000) and specific conditions such as cancer (D’Ambrosio et al. 1999; Simeit et al. 2004) and chronic pain (Currie et al. 2000; Espie et al. 2001; Morin et al. 1989). Multifaceted psychological insomnia interventions have been shown to improve sleep quality in caregivers of dementia patients (McCurry and Ancoli-Israel 2003). A growing number of studies show that cognitive-behavioral treatments delivered in the primary and psychiatric care settings are also efficacious (Edinger and Sampson 2003; Espie et al. 2001).
Clinical practice points
Most of the behavioral treatments described above have been evaluated in clinical trials using approximately six 1-hour sessions delivered by trained therapists. However, these treatments share a few basic principles that can be used in a variety of clinical settings as a basic behavioral treatment package. These specific interventions are reasonable straightforward and rely on principles of the “two-process” model of sleep regulation (see Chapter 1, “Introduction”) and simple conditioning. These recommendations are summarized in Table 4.
Pharmacologic treatment
The only drugs currently approved for the treatment of insomnia are benzodiazepine receptor agonists (BzRA). However, physicians frequently use drugs from other classes to treat insomnia. Between the years 1987 and 1996, the use of antidepressants to treat insomnia increased by 146% and the use of BzRA fell by over 50% (Walsh and Schweitzer 1999). Data from the Verispan Physician Drug and Diagnosis Audit (Verispan, Yardley, PA) in 2002 showed an apparent continuation of this trend, with antidepressants accounting for 5.3 million prescriptions, compared with approximately 4.2 million for BzRA (Walsh 2004). However, the use of other medications for insomnia, such as antihistamines, antipsychotics, and anticonvulsants, is based on sparse efficacy and safety data.
Benzodiazepine receptor agonists
Indications
BzRA are indicated for the treatment of primary insomnia and its various subtypes, as well as for acute situational insomnia. They are useful as adjunctive therapies for secondary insomnia related to certain medical conditions, psychiatric disorders, and other primary sleep disorders such as restless legs syndrome or circadian rhythm sleep disorders.
Pharmacodynamics
All BzRA bind at specific recognition sites on the γ-aminobutyric acid type A (GABAA) receptor complex. GABAA receptors are widely distributed in the CNS, including in the cortex, basal ganglia, and cerebellum (Bateson 2004). GABAA receptors contain several components, including the GABA receptor itself, a benzodiazepine recognition site, and a chloride ion channel (Bateson 2004). The GABA receptor complex is made up of five protein subunits. These subunits belong to different families (alpha, beta, gamma, theta, epsilon, delta, pi, and rho), most of which also have two subtypes (e.g., α1–6, β1–3). Most GABAA receptors that are sensitive to BzRA include two alpha, two beta, and one gamma subunit. BzRA bind to a specific site at the interface of alpha and gamma subunits (Figure 3). Some BzRA, such as zolpidem and zaleplon, have relatively selective binding at GABAA receptors containing α1 sub-units. The clinical significance of this selectivity is not clear, although such agents may be relatively more specific for hypnotic effects and may have lower abuse liability. GABAA receptors with α1 subunits are thought to mediate the sedative, amnestic, and anticonvulsant effects of BzRA, whereas those containing α2 and α3 subunits are more important for anxiolytic and myorelaxant effects (Mohler et al. 2002).
Pharmacokinetics
Although BzRA share a common mechanism of action, they have different clinical effects due to their different pharmacokinetic properties for rate of absorption, extent of distribution, and terminal elimination half-life. These pharmacokinetic properties, together with dose, determine a drug’s duration of action. Available agents range from those with half-lives of 1 hour (zaleplon) to those with half-lives of over 100 hours (flurazepam). Pharmacokinetic properties can be important in selecting a particular agent for a particular patient. For instance, a very short-acting drug such as zaleplon may be helpful for patients with predominantly sleep-onset problems, whereas longer-acting drugs such as temazepam may be more useful for nocturnal awakenings. Conversely, very short-acting drugs may wear off too soon, and longer-acting drugs may be associated with morning sedation. Table 5 shows the relevant clinical pharmacokinetic properties of BzRA commonly used to treat insomnia. Note that some of these drugs are not listed by the U.S. Food and Drug Administration as being indicated for insomnia but are still commonly used for this indication.
Effects on sleep
BzRA share a common set of actions, including hypnotic, anxiolytic, myorelaxant, and anticonvulsant effects. BzRA decrease sleep latency, decrease the number and duration of awakenings during the middle of the night, and (for agents with longer duration of action) increase sleep duration (Parrino and Terzano 1996). Most BzRA slightly decrease the amount of Stage 3/4 NREM sleep, delta EEG activity, and REM sleep. Other effects include an increase in duration and frequency of sleep spindles during Stage 2 NREM sleep (see Chapter 1, “Introduction”). BzRA have inconsistent effects on the number of periodic limb movements during sleep, but they do reduce associated wakefulness (Saletu et al. 2001).
Efficacy
Several meta-analyses have demonstrated that the BzRA are efficacious in the treatment of chronic insomnia (Holbrook et al. 2000; Nowell et al. 1997; Soldatos et al. 1999). On self-reported outcomes of sleep latency, sleep duration, number of awakenings, and sleep quality, BzRA are associated with effect sizes in the moderate to large range compared with placebo; this means that, on average, patients who are treated with BzRA in placebo-controlled trials show substantially more benefit than those treated with placebo. BzRA effects are comparable in magnitude to those of cognitive-behavioral therapies (M.T. Smith et al. 2002b).
A limitation of previous studies of BzRA has been their short overall duration. In the meta-analyses described above, the median duration of treatment was only 7 days. The brevity of these studies presents problems in terms of clinical utility, because the majority of patients treated with BzRA have chronic insomnia. One study demonstrated the efficacy of eszopiclone, compared with placebo, over 6 months of continued nightly use. The eszopiclone group had better outcomes in subjective sleep measures and in subjective daytime functioning (Krystal et al. 2003). This result suggests that BzRA may be efficacious over longer periods of time in at least some patients.
BzRA are often prescribed for intermittent use (e.g., to be used 3–5 times per week). Several studies have demonstrated the efficacy of zolpidem when used intermittently 3–5 times per week for up to 12 weeks (Perlis et al. 2004; Walsh et al. 2000a). Sleep outcomes for zolpidem pill nights were superior to outcomes for placebo pill nights and no-pill nights.
Side effects
BzRA have common side effects, which differ somewhat depending on their pharmacokinetic properties. The major side effects of BzRA include the following:
| • | Sedation. This is the most common side effect of BzRA and is a result of the desired effect continuing into the desired wake period (Vermeeren 2004). Daytime sedation is related to duration of action, with longer-acting drugs causing more sedation. | ||||
| • | Impaired psychomotor performance. BzRA consistently impair motor speed and coordination, as judged by both laboratory test and simulated driving tasks. This impairment is most severe during the time that blood levels are high and is proportional to the duration, action, and half-life of the drug (Roehrs et al. 1994; Vermeeren 2004). Some studies have demonstrated an association between BZRA use and cognitive decline in older adults, but these findings are not consistent (Allard et al. 2003; Curran et al. 2003). | ||||
| • | Falls and hip fractures. BzRA have been consistently associated with falls and hip fracture in elderly persons (Cumming and Le Couteur 2003). However, many studies have not consistently accounted for the separate effect of insomnia, which has been found to be independently associated with falls and hip fractures (Brassington et al. 2000; Koski et al. 1998). | ||||
| • | Motor vehicle accidents. The bulk of evidence suggests increased risk of motor vehicle accidents among BzRA users (Thomas 1998), but findings have been somewhat inconsistent. Some studies have found only long-half-life BzRA to be associated with motor vehicle accidents, and others have found no association at all. Different indications (e.g., insomnia vs. anxiety) and administration patterns (e.g., once-daily nighttime use vs. multiple daytime doses) may also influence these results. As noted above, insomnia itself may also increase the risk of accidents (Powell et al. 2002). | ||||
| • | Respiratory depression. BzRA suppress respiration to a small degree, but this can become clinically significant in patients with severely impaired pulmonary function. Studies in patients with mild to moderate sleep apnea have shown little effect of BzRA on oxyhemoglobin saturation or apnea-hypopnea index (Camacho and Morin 1995; Lofaso et al. 1997). (For a description of apnea-hypopnea index, see Chapter 3, “Sleep Apnea.”) | ||||
The other side effects of BzRA—tolerance, discontinuance effects, and abuse—warrant special consideration.
| • | Tolerance has long been a concern with BzRA, but recent evidence calls into question whether this is an inevitable finding. Specifically, the 6-month study cited above (Krystal et al. 2003) showed no loss of efficacy over 6 months of continued nightly treatment, which is consistent with other data using self-report outcomes (Oswald et al. 1982) and with data from 5-week polysomnographic studies of zolpidem and zaleplon (Scharf et al. 1994; Walsh et al. 2000b). Similar findings have been observed with intermittent use of BzRA (Perlis et al. 2004; Walsh et al. 2000b). Epidemiologic studies show that up to two-thirds of patients taking hypnotics chronically report substantial ongoing benefit (Ohayon and Guilleminault 1999). Finally, a meta-analysis of BzRA effects showed an apparent loss of efficacy for triazolam, but not for zolpidem or zopiclone, after 2 weeks of continued nightly use, suggesting that the tolerance phenomenon may be more likely with some drugs than with others (Soldatos et al. 1999). In many placebo-controlled BzRA trials, placebo-treated groups show gradual improvements over time, which may account for some of the apparent loss of efficacy with BzRA treatment. | ||||
| • | Discontinuance effects are another potentially important side effect of BzRA. A number of studies have clearly shown rebound insomnia, defined as an increase in sleep problems to greater than the baseline level on discontinuation of the drug, but other studies have not. In the meta-analysis described above, triazolam and zolpidem were associated with rebound insomnia on the first night of discontinuation but not over the first 3 nights of discontinuation, suggesting that rebound insomnia is a short-lived phenomenon (Soldatos et al. 1999). Nevertheless, patients should be cautioned that abrupt discontinuation of these medications could lead to a worsening of symptoms that lasts for a few days. | ||||
| • | The potential for abuse is perhaps the greatest concern regarding benzodiazepines. Unfortunately, adequate and accurate data are not available to address this potential concern. Benzodiazepines have a marginal tendency for self-administration in animals, and this tendency is often used as a model of abuse potential in humans (Woods and Winger 1995). BzRA are rarely the drug of choice or sole drug of abuse in humans who abuse drugs. Nevertheless, individuals with a past history of substance abuse, particularly substance abuse of sedative-hypnotics or alcohol, remain at increased risk and should be treated cautiously with these medications (Griffiths and Weerts 1997). | ||||
Use of BzRA as adjunctive medications
Limited evidence suggests that BzRA are efficacious as adjunctive treatment in patients with comorbid medical and psychiatric disorders. For instance, zolpidem improved sleep time and wakefulness after sleep onset in depressed patients treated with selective serotonin reuptake inhibitors (Asnis et al. 1999), and clonazepam helped to reduce sleep symptoms without worsening the overall response in patients treated with fluoxetine (W.T. Smith et al. 2002).
Trazodone
Trazodone is a sedating antidepressant drug that is a weak but a specific inhibitor of the serotonin reuptake transporter, with minimal affinity for nor-epinephrine or dopamine reuptake. Trazodone also inhibits serotonin 5-HT1A, 5-HT1C, and 5-HT2 receptors. Although it has no anticholinergic activity, it does have moderate antihistaminergic activity and is a weak antagonist of α2 receptors and a somewhat more potent agonist of α1 receptors (Golden et al. 2004). Trazodone is rapidly absorbed, with peak concentrations in 1–2 hours, and it has a half-life of approximately 5–9 hours. One of the metabolic products of trazodone is m-chlorophenylpiperazine (m-CPP), which itself possesses serotonergic activity and may be responsible for some of its side effects.
The sleep effects of trazodone have been described in small studies including control subjects and depressed patients, as well as one large study using only subjective outcomes in primary insomnia patients (reviewed in James and Mendelson 2004).
Sleep effects of trazodone and other nonbenzodiazepine medications used for insomnia are summarized in Table 6. Trazodone generally improves subjective sleep quality in healthy subjects and those with depression (Parrino et al. 1994; Saletu-Zyhlarz et al. 2002), but not consistently (van Bemmel et al. 1992). Trazodone has inconsistent effects in terms of improving PSG-defined sleep latency, wakefulness, total sleep time, and sleep efficiency (Saletu-Zyhlarz et al. 2002; van Bemmel et al. 1992). Unlike other sedating antidepressants, trazodone has little effect on REM sleep, with studies showing no significant change or a small decrease (Saletu-Zyhlarz et al. 2002; van Bemmel et al. 1992). Furthermore, trazodone is different from BzRA by virtue of increasing Stage 3/4 NREM sleep (Parrino et al. 1994; Saletu-Zyhlarz et al. 2002). In one large study comparing the effects of trazodone and zolpidem in patients with primary insomnia, trazodone improved subjective sleep latency, awakenings, wake time during sleep, and sleep time over 2 weeks of treatment, but these effects were significant only during the first week (Walsh et al. 1998).
Side effects of trazodone include sedation, orthostatic hypotension, lightheadedness, weakness, and the infrequent but serious risk of priapism.
Tricyclic antidepressants
Sedating tricyclic antidepressants (TCAs) such as doxepin, trimipramine, and amitriptyline have been used in low doses to treat insomnia. Doxepin and amitriptyline inhibit both serotonin and nor-epinephrine reuptake transporters, but trimipramine has few such effects. Sedating TCAs also antagonize peripheral α1- and α2-adrenergic receptors. Finally, they are potent cholinergic and histamine antagonists. TCAs are rapidly absorbed, with maximum concentrations in 2–6 hours. Their half-lives range from 15 to 30 hours (Nelson 2004).
Sedating tertiary TCAs have polysomnographic effects including reduced sleep latency, reduced wakefulness during sleep, and improved sleep efficiency (Feuillade et al. 1992; Roth et al. 1982; Shipley et al. 1985). Doxepin and amitriptyline also decrease REM sleep amount and increase eye movement activity during REM sleep (Roth et al. 1982; Shipley et al. 1985). Trimipramine, on the other hand, has very few effects on REM sleep. Sedating TCAs have little effect on Stage 3/4 sleep, although some reports show a small increase.
Small studies examining the effects of TCAs in primary insomnia have shown positive effects on both subjective measures and polysomnographic measures such as sleep deficiency (Hajak et al. 2001; Hohagen et al. 1994b).
Mirtazapine
Mirtazapine is a strong serotonin 5-HT2 receptor antagonist, with strong antihistamine and α1-antagonist properties as well. It also antagonizes α2 noradrenergic receptors (Flores and Schatzberg 2004). Mirtazapine is rapidly absorbed but has a half-life of approximately 20–40 hours (Flores and Schatzberg 2004). Few data are available regarding its effects on sleep. In one study of healthy adults, mirtazapine decreased sleep latency, awakening, and light NREM sleep, and it increased Stage 3/4 sleep (Ruigt et al. 1990). A small study of depressed patients showed similar effects but no change in REM or Stage 3/4 sleep (Winokur et al. 2000). Curiously, mirtazapine has stronger sedative effects at lower doses than at higher doses—presumably because higher doses are associated with more noradrenergic effect.
Antihistamines
Antihistamines are reversible antagonists of CNS histamine H1 receptors. Histamine, localized in the tuberomammillary nuclei, promotes wakefulness. Sedating antihistamines penetrate the blood-brain barrier readily, whereas nonsedating antihistamines do not. In addition, sedating antihistamines have anticholinergic and serotonergic effects. Diphenhydramine is well absorbed and has a half-life of approximately 4–8 hours. Despite the widespread use of antihistamines, their effects on sleep are not well documented. Clinical trials using doses of 12.5–50 mg have shown subjective improvements in sleep latency, nocturnal awakenings, sleep duration, and sleep quality (Kudo and Kurihara 1990; Meuleman et al. 1987; Rickels et al. 1983). Polysomnographic studies have focused on daytime sedation rather than nighttime sleep effects. In this context, antihistamines produce more rapid sleep onset than placebo, although tolerance to these effects is often seen within a few days (Richardson et al. 2002). Significant side effects of antihistamines include impaired psychomotor performance, cognitive impairment, decreased appetite, and constipation. In older adults, the anticholinergic effects of these medications can be associated with urinary retention.
Melatonin
Melatonin is a hormone normally produced by the pineal gland and secreted exclusively at night. Melatonin production is suppressed by bright light and regulated by the circadian timing system. The most likely effect of melatonin on sleep-wake regulation is its interaction with specific receptors in the suprachiasmatic nucleus of the hypothalamus, the site of the circadian pacemaker. Exogenous melatonin is rapidly absorbed, with peak levels occurring in 20–30 minutes and a very short elimination half-life of 40–60 minutes (DeMuro et al. 2000). Sustained-release preparations can extend the duration of melatonin’s effects to several hours.
Melatonin may affect sleep as both a chronobiotic and a hypnotic. As a chronobiotic, melatonin in appropriately timed administration can shift circadian rhythms to an earlier or later phase in a manner that is exactly opposite to that of bright light (see Chapter 7, “Circadian Rhythm Sleep Disorders”). As a hypnotic, melatonin has inconsistent effects. Studies of subjective effects in healthy young adults given melatonin during the daytime, when activity of the suprachiasmatic nucleus is greatest, support its hypnotic efficacy (Cajochen et al. 2003). When given at night, melatonin can decrease subjective sleep latency, although other effects are less consistent. The efficacy of melatonin demonstrated by polysomnographic measures is less well documented. Some studies have shown increased sleep efficiency, but there is no clear indication of an effect on sleep duration (Olde Rikkert and Rigaud 2001; Sack et al. 1997). Melatonin is generally well tolerated, sedation being its only substantial side effect.
Valerian
Valerian preparations are derived from roots of the plant genus Valeriana. The exact amount of various constituents in commercially available preparations is not well described. Likewise, the exact mechanism of valerian preparations is unknown. Some reports suggest GABA-like activity, as indicated by its sedative, anxiolytic, myorelaxant, and possible anticonvulsant effects. Some components of valerian extracts may inhibit GABA metabolism, and interactions with serotonin and adenosine receptors have also been discussed (Houghton 1999; Krystal and Ressler 2001). The pharmacokinetic properties of valerian are not well described because of its multiple constituents.
Sleep studies of valerian show subjective sedative effects, as well as decreased sleep latency and improved sleep quality. Polysomnographic studies have shown a reasonably consistent improvement in sleep latency, but other potential effects, including improved sleep efficiency, increased Stage 3/4 sleep, and reduced Stage 1 sleep, are inconsistent (Balderer and Borbély 1985; Donath et al. 2000). Side effects associated with valerian preparations are typically mild, including sedation, headache, and weakness.
Gabapentin
Gabapentin was initially developed and marketed as an anticonvulsant drug, although it is widely used for treatment of conditions such as chronic pain and insomnia. Its exact mechanism of action is not well understood, although it may promote formation of GABA in the CNS or antagonize N-methyl-d-aspartate receptors (Rose and Kam 2002; Stahl 2000). More recent evidence indicates that gabapentin and a related drug, pregabalin, are ligands for the α2δ subunit of voltage-sensitive calcium channels (VSCC). Binding of α2δ ligands to activated VSCC reduces calcium influx and reduces neurotransmiter release (Stahl 2004). Gabapentin has relatively low bioavailability and an elimination half-life of 5–9 hours. Although gabapentin is subjectively sedating, few studies have been conducted on its effects on sleep PSG. A study of epilepsy patients showed decreased awakenings and Stage 1 sleep, as well as increased REM sleep and slow-wave sleep (Foldvary-Schaefer et al. 2002). A study in restless legs syndrome showed similar findings, with increased sleep continuity and increased Stage 3/4 sleep (Garcia-Borreguero et al. 2002). No studies have formally evaluated gabapentin in insomnia.
Tiagabine
Tiagabine is an anticonvulsant drug with a well-defined mechanism of action. It inhibits the GABA transporter GAT-1, reducing GABA reuptake into presynaptic neurons. Tiagabine is rapidly absorbed and metabolized by the cytochrome P450 enzyme CYP3A4, with a half-life of approximately 8 hours. Efficacy studies have not been conducted in insomnia patients. One study in healthy adults demonstrated increased slow-wave sleep and improved sleep efficiency (Mathias et al. 2001). Tiagabine has been associated with new-onset seizures in a small number of patients. The exact frequency of this adverse effect is not known, but it can occur at low doses in patients with no seizure history.
Antipsychotics
Sedating antipsychotics have been used increasingly in the treatment of insomnia, particularly among patients with bipolar and psychotic disorders. The two most commonly used drugs are olanzapine and quetiapine. These drugs have a variety of receptor effects, including antagonist effects at 5-HT2 receptors, dopamine antagonism, and anti-histamine effects. Olanzapine is slowly absorbed and has a half-life of 20–54 hours. Quetiapine has a more rapid onset of action, with a peak concentration in 1.5 hours and a half-life of only 6 hours (Baldessarini and Tarazi 2001; Stahl 2000).
A limited number of polysomnographic studies suggest the hypnotic efficacy of olanzapine, which decreases wakefulness, Stage 1 sleep, and REM sleep but increases Stage 3/4 NREM sleep and subjective sleep quality (Lindberg et al. 2002; Sharpley et al. 2000). No published studies are yet available regarding quetiapine as a hypnotic. Consideration should be given to the potentially serious neurological side effects of these medications, as well as their potential effects of weight gain and impaired glucose metabolism.
Clinical practice points
Despite the wide array of agents used for the treatment of insomnia, there are no published guidelines regarding the selection of specific drugs, the optimal duration of treatment, the choice of drugs for specific types of insomnia, or strategies to use in cases of partial or complete nonresponsiveness. In most cases of primary insomnia, a short-acting benzodiazepine receptor agonist is the drug of first choice. Specific patients may require a benzodiazepine receptor agonist with a longer or shorter duration of action, depending on their pattern of symptoms, sensitivity to medication, and rate of metabolism. For older adults with primary insomnia, smaller doses should be used initially. In cases of partial or no response, substitution or addition of a drug from a different class, usually a sedating antidepressant, is a reasonable choice. Third-line agents would include tiagabine and gabapentin. For patients with concurrent mood or anxiety disorders, BzRA or sedating antidepressants are again appropriate, but only when a primary therapy for the underlying condition, such as psychotherapy or a selective serotonin reuptake inhibitor, is also being used. For patients with known substance use disorders and significant respiratory disease, non-BzRA such as sedating antidepressants should be considered as first-line agents.
In the absence of specific guidelines regarding duration of use, initial treatment should be aimed at short-term use of 2–4 weeks. Gradual taper and discontinuation should then be attempted, by decreasing both the dose per administration and the number of doses per week. If longer-term treatment is needed, intermittent use may be preferred to avoid potential tolerance and adverse effects. In some chronic insomnia patients, long-term use of BzRA or other hypnotic drugs is appropriate. Regular monitoring to establish continued efficacy and tolerability of side effects is essential.
Behavioral treatments should be considered and utilized whenever possible, even in patients receiving pharmacotherapy. Long-term efficacy is better established for behavioral treatments than for pharmacologic treatments, and it seems prudent to avoid medications and their potential side effects when such efficacious other treatments are available.
Summary
Insomnia is a prevalent health complaint that is strongly associated with psychiatric and medical disorders. Recent studies have suggested that increased CNS arousal may be a common final pathway for different subtypes of insomnia. In addition, cognitive and behavioral factors often play an important role. The assessment of insomnia rests on a detailed and accurate clinical history, supplemented by sleep diaries and other tools. Efficacious treatments for insomnia include behavioral and cognitive-behavioral therapy and pharmacotherapy with benzodiazepine receptor agonists and, potentially, antidepressant medications. Although a wide number of other medications have been used to treat insomnia, their efficacy remains to be demonstrated. Insomnia has a significant effect on quality of life and daytime function, and treatment can result in improved functioning.
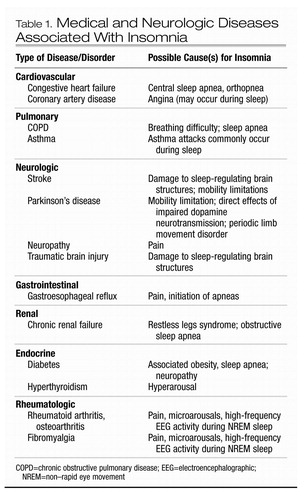 |
Table 1. Medical and Neurologic Diseases Associated With Insomnia
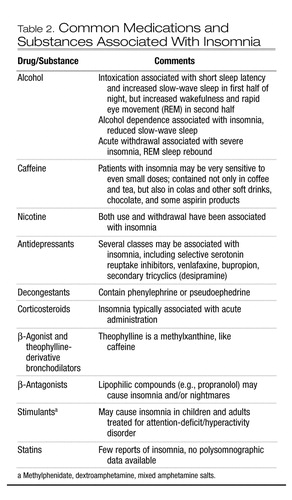 |
Table 2. Common Medications and Substances Associated With Insomnia
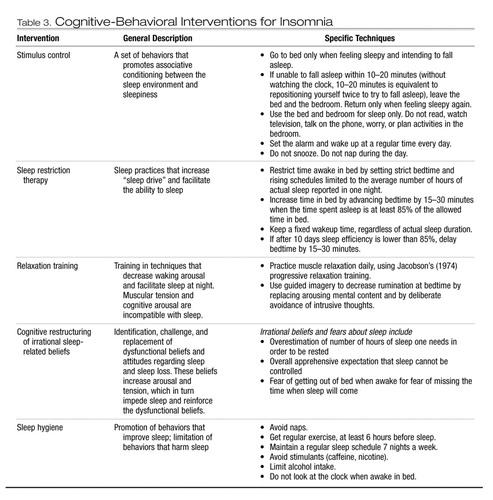 |
Table 3. Cognitive-Behavioral Interventions for Insomnia
 |
Table 4. Brief Behavioral Treatment for Insomnia
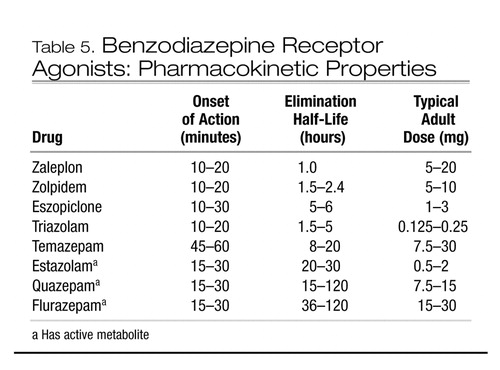 |
Table 5. Benzodiazepine Receptor Agonists: Pharmacokinetic Properties
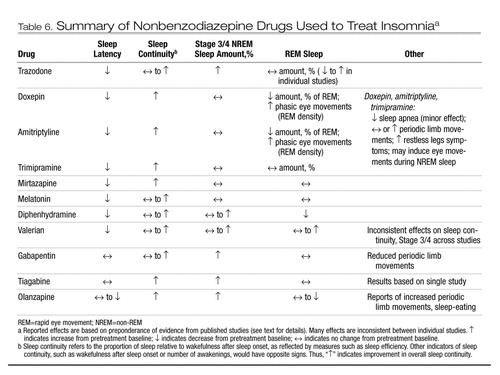 |
Table 6. Summary of Nonbenzodiazepine Drugs Used to Treat Insomniaa
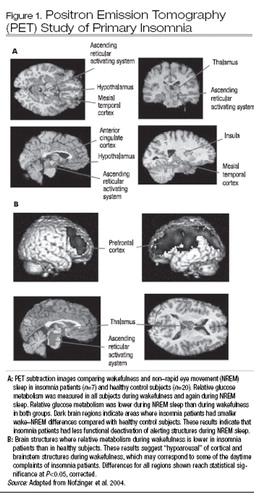
Figure 1. Positron Emission Tomography (PET) Study of Primary Insomnia
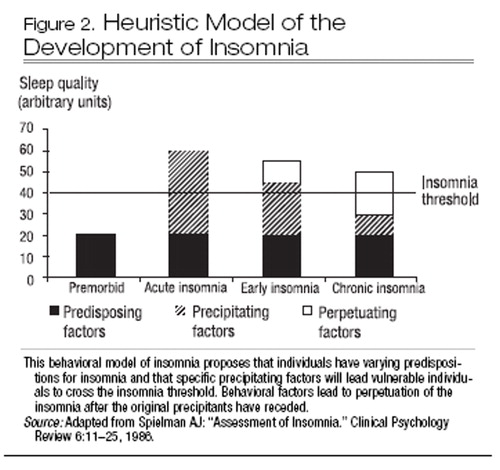
Figure 2. Heuristic Model of the Development of Insomnia
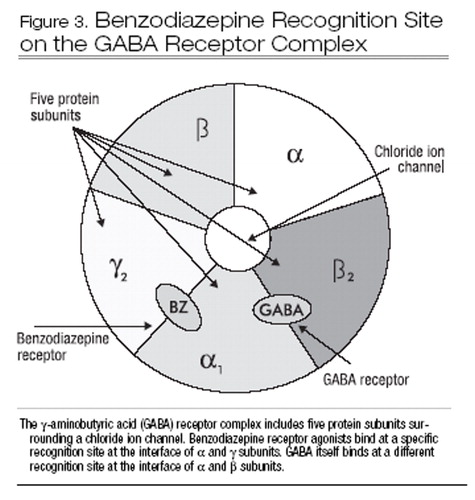
Figure 3. Benzodiazepine Recognition Site on the GABA Receptor Complex
Agargun MY, Kara H, Solmaz M: Subjective sleep quality and suicidality in patients with major depression. J Psychiatr Res 31:377–381, 1997Crossref, Google Scholar
Allard J, Artero S, Ritchie K: Consumption of psychotropic medication in the elderly: a re-evaluation of its effect on cognitive performance. Int J Geriatr Psychiatry 18:874–878, 2003Crossref, Google Scholar
American Academy of Sleep Medicine: International Classification of Sleep Disorders, 2nd Edition. Westchester, IL, American Academy of Sleep Medicine (in press)Google Scholar
American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision. Washington, DC, American Psychiatric Association, 2000Google Scholar
American Sleep Disorders Association: International Classification of Sleep Disorders, Revised: Diagnostic and Coding Manual. Rochester, MN, American Sleep Disorders Association, 1997Google Scholar
Asnis GM, Chakraburtty A, DuBoff EA, et al: Zolpidem for persistent insomnia in SSRI-treated depressed patients. J Clin Psychiatry 60:668–676, 1999Crossref, Google Scholar
Attenburrow ME, Dowling BA, Sharpley AL, et al: Case-control study of evening melatonin concentration in primary insomnia. BMJ 312:1263–1264, 1996Crossref, Google Scholar
Balderer G, Borbély AA: Effect of valerian on human sleep. Psychopharmacology (Berl) 87:406–409, 1985Crossref, Google Scholar
Baldessarini RJ, Tarazi FI: Drugs and the treatment of anxiety disorders: psychosis and mania, in Goodman and Gilman’s The Pharmacological Basis of Therapeutics. Edited by Hardman JG, Limbird LE. New York, McGraw-Hill, 2001, pp 485–520Google Scholar
Bateson AN: The benzodiazepine site of the GABAA receptor: an old target with new potential? Sleep Med 1 (5 suppl):S9–S15, 2004Google Scholar
Bonnet MH, Arand DL: 24-Hour metabolic rate in insomniacs and matched normal sleepers. Sleep 18:581–588, 1995Crossref, Google Scholar
Bonnet MH, Arand DL: Hyperarousal and insomnia. Sleep Med Rev 1:97–108, 1997aCrossref, Google Scholar
Bonnet MH, Arand DL: Physiological activation in patients with sleep state misperception. Psychosom Med 59:533–540, 1997bCrossref, Google Scholar
Bootzin RR, Nicassio PM: Behavioral treatments of insomnia, in Progress in Behavior Modification, Vol 6. Edited by Hersen M, Eisler RE, Miller PM. New York, Academic Press, 1978, pp 1–45Google Scholar
Brassington GS, King AC, Bliwise DL: Sleep problems as a risk factor for falls in a sample of community-dwelling adults aged 64–99 years. J Am Geriatr Soc 48:1234–1240, 2000Crossref, Google Scholar
Breslau N, Roth T, Rosenthal L, et al: Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry 39:411–418, 1996Crossref, Google Scholar
Brower KJ, Aldrich MS, Robinson EA, et al: Insomnia, self-medication, and relapse to alcoholism. Am J Psychiatry 158:399–404, 2001Crossref, Google Scholar
Buysse DJ, Reynolds CF 3rd, Hauri PJ, et al: Diagnostic concordance for DSM-IV sleep disorders: a report from the APA/NIMH DSM-IV field trial. Am J Psychiatry 151:1351–1360, 1994Crossref, Google Scholar
Buysse DJ, Tu XM, Cherry CR, et al: Pretreatment REM sleep and subjective sleep quality distinguish depressed psychotherapy remitters and nonremitters. Biol Psychiatry 45:205–213, 1999Crossref, Google Scholar
Cajochen C, Kräuchi K, Wirz-Justice A: Role of melatonin in the regulation of human circadian rhythms and sleep. J Neuroendocrinol 15:432–437, 2003Crossref, Google Scholar
Camacho ME, Morin CM: The effect of temazepam on respiration in elderly insomniacs with mild sleep apnea. Sleep 18:644–645, 1995Crossref, Google Scholar
Chang PP, Ford DE, Mead LA, et al: Insomnia in young men and subsequent depression: the Johns Hopkins Precursors Study. Am J Epidemiol 146:105–114, 1997Crossref, Google Scholar
Coull JT: Neural correlates of attention and arousal: insights from electrophysiology, functional neuroimaging and psychopharmacology. Prog Neurobiol 55:343–361, 1998Crossref, Google Scholar
Cumming RG, Le Couteur DG: Benzodiazepines and risk of hip fractures in older people: a review of the evidence. CNS Drugs 17:825–837, 2003Crossref, Google Scholar
Curran HV, Collins R, Fletcher S, et al: Older adults and withdrawal from benzodiazepine hypnotics in general practice: effects on cognitive function, sleep, mood and quality of life. Psychol Med 33:1223–1237, 2003Crossref, Google Scholar
Currie SR, Wilson KG, Pontefract AJ, et al: Cognitive-behavioral treatment of insomnia secondary to chronic pain. J Consult Clin Psychol 68:407–416, 2000Crossref, Google Scholar
D’Ambrosio C, Bowman T, Mohsenin V: Quality of life in patients with obstructive sleep apnea: effect of nasal continuous positive airway pressure—a prospective study. Chest 115:123–129, 1999Crossref, Google Scholar
DeMuro RL, Nafziger AN, Blask DE, et al: The absolute bioavailability of oral melatonin. J Clin Pharmacol 40:781–784, 2000Crossref, Google Scholar
Donath F, Quispe S, Diefenbach K, et al: Critical evaluation of the effect of valerian extract on sleep structure and sleep quality. Pharmacopsychiatry 33:47–53, 2000Crossref, Google Scholar
Edinger JD, Sampson WS: A primary care “friendly” cognitive behavior insomnia therapy. Sleep 26:177–182, 2003Crossref, Google Scholar
Espie CA, Inglis SJ, Harvey L: Predicting clinically significant response to cognitive behavior therapy for chronic insomnia in general medical practice: analyses of outcome data at 12 months posttreatment. J Consult Clin Psychol 69:58–66, 2001Crossref, Google Scholar
Feuillade P, Pringuey D, Belugou JL: Trimipramine: acute and lasting effects on sleep in healthy and major depressive subjects. J Affect Disord 24:135–145, 1992Crossref, Google Scholar
Flores BH, Schatzberg AF: Mirtazapine, in The American Psychiatric Publishing Textbook of Psychopharmacology, 3rd Edition. Edited by Schatzberg AF, Nemeroff CB. Washington, DC, American Psychiatric Publishing, 2004, pp 341–347Google Scholar
Foldvary-Schaefer N, De Leon Sanchez I, Karafa M, et al: Gabapentin increases slow-wave sleep in normal adults. Epilepsia 43:1493–1497, 2002Crossref, Google Scholar
Foley DJ, Monjan A, Simonsick EM, et al: Incidence and remission of insomnia among elderly adults: an epidemiologic study of 6,800 persons over three years. Sleep 22:S366–S372, 1999Google Scholar
Garcia-Borreguero D, Larrosa O, de la Llave Y, et al: Treatment of restless legs syndrome with gabapentin: a double-blind, cross-over study. Neurology 59:1573–1579, 2002Crossref, Google Scholar
Golden RN, Dawkins K, Nicholas L: Trazodone and nefazodone, in The American Psychiatric Textbook of Psychopharmacology, 3rd Edition. Edited by Schatzberg AF, Nemeroff CB. Washington, DC, American Psychiatric Publishing, 2004, pp 315–325Google Scholar
Griffiths RR, Weerts EM: Benzodiazepine self-administration in humans and laboratory animals: implications for problems of long-term use and abuse. Psychopharmacology (Berl) 134:1–37, 1997Crossref, Google Scholar
Gross RT, Borkovec TD: Effects of a cognitive intrusion manipulation on the sleep-onset latency of good sleepers. Behav Ther 13:112–116, 1982Crossref, Google Scholar
Hajak G, Rodenbeck A, Staedt J, et al: Nocturnal plasma melatonin levels in patients suffering from chronic primary insomnia. J Pineal Res 19:116–122, 1995Crossref, Google Scholar
Hajak G, Rodenbeck A, Voderholzer U, et al: Doxepin in the treatment of primary insomnia: a placebo-controlled, double-blind, polysomnographic study. J Clin Psychiatry 62:453–463, 2001Crossref, Google Scholar
Harvey AG: A cognitive model of insomnia. Behav Res Ther 40:869–893, 2002Crossref, Google Scholar
Hatoum HT, Kong SX, Kania CM, et al: Insomnia, health-related quality of life and healthcare resource consumption: a study of managed-care organisation enrollees. Pharmacoeconomics 14:629–637, 1998Crossref, Google Scholar
Hauri PJ: Case Studies in Insomnia. New York, Kluwer Academic, 1991Google Scholar
Hohagen F, Kappler C, Schramm E, et al: Sleep onset insomnia, sleep maintaining insomnia and insomnia with early morning awakening: temporal stability of subtypes in a longitudinal study on general practice attenders. Sleep 17:551–554, 1994aGoogle Scholar
Hohagen F, Montero RF, Weiss E, et al: Treatment of primary insomnia with trimipramine: an alternative to benzodiazepine hypnotics? Eur Arch Psychiatry Clin Neurosci 244:65–72, 1994bCrossref, Google Scholar
Holbrook AM, Crowther R, Lotter A, et al: Meta-analysis of benzodiazepine use in the treatment of insomnia. CMAJ 162:225–233, 2000Google Scholar
Houghton PJ: The scientific basis for the reputed activity of valerian. J Pharm Pharmacol 51:505–512, 1999Crossref, Google Scholar
Jacobson E: Progressive Relaxation: A Physiological and Clinical Investigation of Muscular States and Their Significance in Psychology and Medical Practice, 3rd Revised Edition. Chicago, IL, University of Chicago Press, 1974Google Scholar
James SP, Mendelson WB: The use of trazodone as a hypnotic: a critical review. J Clin Psychiatry 65:752–755, 2004Crossref, Google Scholar
Kappler C, Hohagen F: Psychosocial aspects of insomnia: results of a study in general practice. Eur Arch Psychiatry Clin Neurosci 253:49–52, 2003Crossref, Google Scholar
Karlsen KH, Larsen JP, Tandberg E, et al: Influence of clinical and demographic variables on quality of life in patients with Parkinson’s disease. J Neurol Neurosurg Psychiatry 66:431–435, 1999Crossref, Google Scholar
Katz DA, McHorney CA: Clinical correlates of insomnia in patients with chronic illness. Arch Intern Med 158:1099–1107, 1998Crossref, Google Scholar
Katz DA, McHorney CA: The relationship between insomnia and health-related quality of life in patients with chronic illness. J Fam Pract 51:229–235, 2002Google Scholar
Koski K, Luukinen H, Laippala P, et al: Risk factors for major injurious falls among the home-dwelling elderly by functional abilities: a prospective population-based study. Gerontology 44:232–238, 1998Crossref, Google Scholar
Krystal AD, Ressler I: The use of valerian in neuropsychiatry. CNS Spectr 6:841–847, 2001Crossref, Google Scholar
Krystal AD, Edinger JD, Wohlgemuth WK, et al: NREM sleep EEG frequency spectral correlates of sleep complaints in primary insomnia subtypes. Sleep 25:630–640, 2002Google Scholar
Krystal AD, Walsh JK, Laska E, et al: Sustained efficacy of eszopiclone over 6 months of nightly treatment: results of a randomized, double-blind, placebo-controlled study in adults with chronic insomnia. Sleep 26:793–799, 2003Crossref, Google Scholar
Kudo Y, Kurihara M: Clinical evaluation of diphenhydramine hydrochloride for the treatment of insomnia in psychiatric patients: a double-blind study. J Clin Pharmacol 30:1041–1048, 1990Crossref, Google Scholar
Lacks P, Morin CM: Recent advances in the assessment and treatment of insomnia. J Consult Clin Psychol 60:586–594, 1992Crossref, Google Scholar
Léger D, Scheuermaier K, Philip P, et al: SF-36: evaluation of quality of life in severe and mild insomniacs compared with good sleepers. Psychosom Med 63:49–55, 2001Crossref, Google Scholar
Léger D, Guilleminault C, Bader G, et al: Medical and socio-professional impact of insomnia. Sleep 25:625–629, 2002Google Scholar
Lichstein KL, Wilson NM, Johnson CT: Psychological treatment of secondary insomnia. Psychol Aging 15:232–240, 2000Crossref, Google Scholar
Lindberg N, Virkkunen M, Tani P, et al: Effect of a single-dose of olanzapine on sleep in healthy females and males. Int Clin Psychopharmacol 17:177–184, 2002Crossref, Google Scholar
Lofaso F, Goldenberg F, Thebault C, et al: Effect of zopiclone on sleep, nighttime ventilation, and daytime vigilance in upper airway resistance syndrome. Eur Respir J 10:2573–2577, 1997Crossref, Google Scholar
Mathias S, Wetter TC, Steiger A, et al: The GABA uptake inhibitor tiagabine promotes slow-wave sleep in normal elderly subjects. Neurobiol Aging 22:247–253, 2001Crossref, Google Scholar
McCall WV, Reboussin BA, Cohen W: Subjective measurement of insomnia and quality of life in depressed inpatients. J Sleep Res 9:43–48, 2000Crossref, Google Scholar
McCurry SM, Ancoli-Israel S: Sleep dysfunction in Alzheimer’s disease and other dementias. Curr Treat Options Neurol 5:261–272, 2003Crossref, Google Scholar
Mendelson WB: Long-term follow-up of chronic insomnia. Sleep 18:698–701, 1995Crossref, Google Scholar
Merica H, Gaillard JM: The EEG of the sleep onset period in insomnia: a discriminant analysis. Physiol Behav 52:199–204, 1992Crossref, Google Scholar
Merica H, Blois R, Gaillard JM: Spectral characteristics of sleep EEG in chronic insomnia. Eur J Neurosci 10:1826–1834, 1998Crossref, Google Scholar
Meuleman JR, Nelson RC, Clark RL: Evaluation of temazepam and diphenhydramine as hypnotics in a nursing-home population. Drug Intell Clin Pharm 21:716–720, 1987Crossref, Google Scholar
Mimeault V, Morin CM: Self-help treatment for insomnia: bibliotherapy with and without professional guidance. J Consult Clin Psychol 67:511–519, 1999Crossref, Google Scholar
Mohler H, Fritschy J, Rudolph U: A new benzodiazepine pharmacology. J Pharmacol Exp Ther 300:2–8, 2002Crossref, Google Scholar
Montgomery P, Dennis J: Cognitive behavioural interventions for sleep problems in adults aged 60+. Cochrane Database Syst Rev (1): CD003161, 2003Google Scholar
Moos RH, Cronkite RC: Symptom-based predictors of a 10-year chronic course of treated depression. J Nerv Ment Dis 187:360–368, 1999Crossref, Google Scholar
Morin CM: Insomnia: Psychological Assessment and Management. New York, Guilford, 1993Google Scholar
Morin CM, Kowatch RA, Wade JB: Behavioral management of sleep disturbances secondary to chronic pain. J Behav Ther Exp Psychiatry 20:295–302, 1989Crossref, Google Scholar
Morin CM, Culbert JP, Schwartz SM: Nonpharmacological interventions for insomnia: a meta-analysis of treatment efficacy. Am J Psychiatry 151:1172–1180, 1994Crossref, Google Scholar
Morin CM, Hauri PJ, Espie CA, et al: Nonpharmacologic treatment of chronic insomnia. Sleep 22:1135–1156, 1999Google Scholar
Moul DE, Nofzinger EA, Pilkonis PA, et al: Symptom reports in severe chronic insomnia. Sleep 25:553–563, 2002Google Scholar
Moul DE, Hall M, Pilkonis PA, et al: Self-report measures of insomnia adults: rationales, choices, and needs. Sleep Med Rev 8:177–198, 2004Crossref, Google Scholar
Murtagh DR, Greenwood KM: Identifying effective psychological treatments for insomnia: a meta-analysis. J Consult Clin Psychol 63:79–89, 1995Crossref, Google Scholar
Nelson JC: Tricyclic and tetracyclic drugs, in The American Psychiatric Publishing Textbook of Psychopharmacology, 3rd Edition. Edited by Schatzberg AF, Nemeroff CB. Washington, DC, American Psychiatric Publishing, 2004, pp 207–230Google Scholar
Nofzinger EA, Price JC, Meltzer CC, et al: Towards a neurobiology of dysfunctional arousal in depression: the relationship between beta EEG power and regional cerebral glucose metabolism during NREM sleep. Psychiatry Res 98:71–91, 2000Crossref, Google Scholar
Nofzinger EA, Buysse DJ, Germain A, et al: Functional neuroimaging evidence for hyperarousal in insomnia. Am J Psychiatry 161:2126–2128, 2004Crossref, Google Scholar
Nowell PD, Mazumdar S, Buysse DJ, et al: Benzodiazepines and zolpidem for chronic insomnia: a meta-analysis of treatment efficacy. JAMA 278:2170–2177, 1997Crossref, Google Scholar
Ohayon MM: Prevalence of DSM-IV diagnostic criteria of insomnia: distinguishing insomnia related to mental disorders from sleep disorders. J Psychiatr Res 31:333–346, 1997Crossref, Google Scholar
Ohayon MM: Epidemiology of insomnia: what we know and what we still need to learn. Sleep Med Rev 6:97–111, 2002Crossref, Google Scholar
Ohayon MM, Guilleminault C: Epidemiology of sleep disorders, in Sleep Disorders Medicine: Basic Science, Technical Considerations, and Clinical Aspects, 2nd Edition. Edited by Chokroverty S. Boston, MA, Butterworth-Heinemann, 1999, pp 301–316Google Scholar
Olde Rikkert MG, Rigaud AS: Melatonin in elderly patients with insomnia: a systematic review. Z Gerontol Geriatr 34:491–497, 2001Crossref, Google Scholar
Oswald I, French C, Adam K, et al: Benzodiazepine hypnotics remain effective for 24 weeks. Br Med J (Clin Res Ed) 284:860–863, 1982Crossref, Google Scholar
Parrino L, Terzano MG: Polysomnographic effects of hypnotic drugs: a review. Psychopharmacology (Berl) 126:1–16, 1996Crossref, Google Scholar
Parrino L, Spaggiari MC, Boselli M, et al: Clinical and polysomnographic effects of trazodone CR in chronic insomnia associated with dysthymia. Psychopharmacology (Berl) 116:389–395, 1994Crossref, Google Scholar
Perlis ML, Giles DE, Buysse DJ, et al: Self-reported sleep disturbance as a prodromal symptom in recurrent depression. J Affect Disord 42:209–212, 1997aCrossref, Google Scholar
Perlis ML, Giles DE, Mendelson WB, et al: Psychophysiological insomnia: the behavioural model and a neurocognitive perspective. J Sleep Res 6:179–188, 1997bCrossref, Google Scholar
Perlis ML, Smith MT, Andrews PJ, et al: Beta/gamma EEG activity in patients with primary and secondary insomnia and good sleeper controls. Sleep 24:110–117, 2001Crossref, Google Scholar
Perlis ML, McCall WV, Krystal AD, et al: Long-term, non-nightly administration of zolpidem in the treatment of patients with primary insomnia. J Clin Psychiatry 65:1128–1137, 2004Crossref, Google Scholar
Powell NB, Schechtman KB, Riley RW, et al: Sleepy driving: accidents and injury. Otolaryngol Head Neck Surg 126:217–227, 2002Crossref, Google Scholar
Reynolds CF 3rd, Frank E, Houck PR, et al: Which elderly patients with remitted depression remain well with continued interpersonal psychotherapy after discontinuation of antidepressant medication? Am J Psychiatry 154:958–962, 1997Crossref, Google Scholar
Richardson GS, Roehrs TA, Rosenthal L, et al: Tolerance to daytime sedative effects of H1 antihistamines. J Clin Psychopharmacol 22:511–515, 2002Crossref, Google Scholar
Rickels K, Morris RJ, Newman H, et al: Diphenhydramine in insomniac family practice patients: a double-blind study. J Clin Pharmacol 23:234–242, 1983Crossref, Google Scholar
Riemann D, Voderholzer U: Primary insomnia: a risk factor to develop depression? J Affect Disord 76:255–259, 2003Crossref, Google Scholar
Rodenbeck A, Huether G, Ruther E, et al: Interactions between evening and nocturnal cortisol secretion and sleep parameters in patients with severe chronic primary insomnia. Neurosci Lett 324:159–163, 2002Crossref, Google Scholar
Roehrs T, Merlotti L, Zorick F, et al: Sedative, memory, and performance effects of hypnotics. Psychopharmacology (Berl) 116:130–134, 1994Crossref, Google Scholar
Rose MA, Kam PC: Gabapentin: pharmacology and its use in pain management. Anaesthesia 57:451–462, 2002Crossref, Google Scholar
Roth T, Zorick F, Wittig R, et al: The effects of doxepin HCl on sleep and depression. J Clin Psychiatry 43:366–368, 1982Google Scholar
Ruigt GS, Kemp B, Groenhout CM, et al: Effect of the antidepressant Org 3770 on human sleep. Eur J Clin Pharmacol 38:551–554, 1990Crossref, Google Scholar
Rybarczyk B, Lopez M, Benson R, et al: Efficacy of two behavioral treatment programs for comorbid geriatric insomnia. Psychol Aging 17:288–298, 2002Crossref, Google Scholar
Sack RL, Hughes RJ, Edgar DM, et al: Sleep-promoting effects of melatonin: at what dose, in whom, under what conditions, and by what mechanisms? Sleep 20:908–915, 1997Crossref, Google Scholar
Sadeh A, Acebo C: The role of actigraphy in sleep medicine. Sleep Med Rev 6:113–124, 2002Crossref, Google Scholar
Saletu M, Anderer P, Saletu-Zyhlarz G, et al: Restless legs syndrome (RLS) and periodic limb movement disorder (PLMD): acute placebo-controlled sleep laboratory studies with clonazepam. Eur Neuropsychopharmacol 11:153–161, 2001Crossref, Google Scholar
Saletu-Zyhlarz G, Abu-Bakr M, Anderer P, et al: Insomnia in depression: differences in objective and subjective sleep awakening quality to normal controls and acute effects of trazodone. Prog Neuropsychopharmacol Biol Psychiatry 26:249–260, 2002Crossref, Google Scholar
Sateia MJ, Doghramji K, Hauri PJ, et al: Evaluation of chronic insomnia: an American Academy of Sleep Medicine review. Sleep 23:243–308, 2000Google Scholar
Scharf MB, Roth T, Vogel GW, et al: A multicenter, placebo-controlled study evaluating zolpidem in the treatment of chronic insomnia. J Clin Psychiatry 55:192–199, 1994Google Scholar
Sharpley AL, Vassallo CM, Cowen PJ: Olanzapine increases slow-wave sleep: evidence for blockade of central 5-HT(2C) receptors in vivo. Biol Psychiatry 47:468–470, 2000Crossref, Google Scholar
Shipley JE, Kupfer DJ, Griffin SJ, et al: Comparison of effects of desipramine and amitriptyline on EEG sleep of depressed patients. Psychopharmacology (Berl) 85:14–22, 1985Crossref, Google Scholar
Simeit R, Deck R, Conta-Marx B: Sleep management training for cancer patients with insomnia. Support Care Cancer 12:176–183, 2004Crossref, Google Scholar
Simon GE, Von Korff M: Prevalence, burden, and treatment of insomnia in primary care. Am J Psychiatry 154:1417–1423, 1997Crossref, Google Scholar
Smith MT, Perlis ML, Chengazi VU, et al: Neuroimaging of NREM sleep in primary insomnia: a Tc-99-HMPAO single photon emission computed tomography study. Sleep 25:325–335, 2002aGoogle Scholar
Smith MT, Perlis ML, Park A, et al: Comparative meta-analysis of pharmacotherapy and behavior therapy for persistent insomnia. Am J Psychiatry 159:5–11, 2002bCrossref, Google Scholar
Smith WT, Londborg PD, Glaudin V, et al: Is extended clonazepam cotherapy of fluoxetine effective for outpatients with major depression? J Affect Disord 70:251–259, 2002Crossref, Google Scholar
Sok SR, Erlen JA, Kim KB: Effects of acupuncture therapy on insomnia. J Adv Nurs 44:375–384, 2003Crossref, Google Scholar
Soldatos CR, Dikeos DG, Whitehead A: Tolerance and rebound insomnia with rapidly eliminated hypnotics: a meta-analysis of sleep laboratory studies. Int Clin Psychopharmacol 14:287–303, 1999Crossref, Google Scholar
Spielman AJ, Caruso LS, Glovinsky PB: A behavioral perspective on insomnia treatment. Psychiatr Clin North Am 10:541–553, 1987Crossref, Google Scholar
Stahl SM: Essential Pharmacology: Neuroscientific Basis and Practical Applications, 2nd Edition. New York, Cambridge University Press, 2000Google Scholar
Stahl SM: Mechanism of action of alpha2delta ligands: voltage sensitive calcium channel (VSCC) modulators. J Clin Psychiatry 65:1033–1034, 2004Crossref, Google Scholar
Strom L, Pettersson R, Andersson G: Internet-based treatment for insomnia: a controlled evaluation. J Consult Clin Psychol 72:113–120, 2004Crossref, Google Scholar
Thomas RE: Benzodiazepine use and motor vehicle accidents: systematic review of reported association. Can Fam Physician 44:799–808, 1998Google Scholar
van Bemmel AL, Havermans RG, van Diest R: Effects of trazodone on EEG sleep and clinical state in major depression. Psychopharmacology (Berl) 107:569–574, 1992Crossref, Google Scholar
Vermeeren A: Residual effects of hypnotics: epidemiology and clinical implications. CNS Drugs 18:297–328, 2004Crossref, Google Scholar
Vgontzas AN, Bixler EO, Lin HM, et al: Chronic insomnia is associated with nyctohemeral activation of the hypothalamic-pituitary-adrenal axis: clinical implications. J Clin Endocrinol Metab 86:3787–3794, 2001Crossref, Google Scholar
Vollrath M, Wicki W, Angst J: The Zurich study, VIII. Insomnia: association with depression, anxiety, somatic syndromes, and course of insomnia. Eur Arch Psychiatry Neurol Sci 239:113–124, 1989Crossref, Google Scholar
Walsh JK: Drugs used to treat insomnia in 2002: regulatory-based rather than evidence-based medicine. Sleep 1441–1442, 2004Google Scholar
Walsh JK, Engelhardt CL: The direct economic costs of insomnia in the United States for 1995. Sleep 22:S386–S393, 1999Google Scholar
Walsh JK, Erman M, Erwin CW, et al: Subjective hypnotic efficacy of trazodone and zolpidem in DSM-III-R primary insomnia. Hum Psychopharmacol 13:191–198, 1998Crossref, Google Scholar
Walsh JK, Schweitzer PK: Ten-year trends in the pharmacological treatment of insomnia. Sleep 22:371–375, 1999Crossref, Google Scholar
Walsh JK, Roth T, Randazzo A, et al: Eight weeks of non-nightly use of zolpidem for primary insomnia. Sleep 23:1087–1096, 2000aGoogle Scholar
Walsh JK, Vogel GW, Scharf M, et al: A five week, polysomnographic assessment of zaleplon 10 mg for the treatment of primary insomnia. Sleep Med 1:41–49, 2000bCrossref, Google Scholar
Winokur A, Sateia MJ, Hayes JB, et al: Acute effects of mirtazapine on sleep continuity and sleep architecture in depressed patients: a pilot study. Biol Psychiatry 48:75–78, 2000Crossref, Google Scholar
Woods JH, Winger G: Current benzodiazepine issues. Psychopharmacology (Berl) 118:107–115, 1995Crossref, Google Scholar
Woolfolk RL, McNulty TF: Relaxation treatment for insomnia: a component analysis. J Consult Clin Psychol 51:495–503, 1983Crossref, Google Scholar
World Health Organization: International Statistical Classification of Diseases and Related Health Problems, 10th Revision. Geneva, World Health Organization, 1992Google Scholar



