Understanding and Managing Clinically Significant Pain in Patients with an Anxiety Disorder
Abstract
It is now well established that chronic pain and various anxiety disorders frequently co-occur. Co-occurrence of chronic pain is often unrecognized in anxiety disorder settings; yet, when unaddressed, it can complicate treatment, reduce treatment effectiveness, and exacerbate functional limitations and suffering for patients. The purpose of this clinical synthesis is to describe the core features of clinically significant pain experiences, summarize findings from epidemiological studies of co-occurrence, describe biopsychosocial models and associated mechanisms posited to account for co-occurrence, and provide anxiety disorder practitioners with time-efficient pain assessment strategies and promising options for treating clinically significant pain presentations in their patients. Future directions are also briefly highlighted.
There is considerable clinical and empirical evidence regarding the common co-occurrence of chronic pain and the anxiety disorders. The primary purpose of this clinical synthesis is to describe the core features of clinically significant pain experiences and to introduce anxiety disorder practitioners to efficient assessment and management methods. To achieve this purpose, it is necessary to define pain and its clinically significant chronic manifestation that frequently co-occur with the anxiety disorders, present data on the prevalence of co-occurrence, describe biopsychosocial models posited to account for the co-occurrence, and provide recommendations for improving assessment and treatment of patients who present with an anxiety disorder accompanied by significant pain. We also discuss directions for advancing this growing field of inquiry.
DEFINITIONS AND EPIDEMIOLOGY
Pain and chronic pain
Pain was once understood as a sensory experience resulting from stimulation of specific high-threshold sensory receptors (nociceptors) stemming from physical injury or progression of disease; as such, it was assumed that stimulation of nociceptors always produced pain. Contemporary models recognize that pain is a complex perceptual experience determined not only by stimulation of nociceptors but also by myriad psychological and social influences (1–3). Although typically perceived as a negative experience, pain has survival value, alerting us to potential or actual tissue damage and initiating behaviors that reduce chances of further injury and promote recovery (4). Pain associated with physical injury and disease typically abates with time and as healing occurs; however, some people continue to experience pain well beyond the time course of the healing process.
When pain persists for 3 months or longer, it is considered chronic (5). Chronic pain is currently one of the most common and costly chronic health conditions in North America, with approximately 10% of the general population reporting having experienced chronic idiopathic (i.e., noncancer-related) pain in the past 12 months (6). Chronic pain is often associated with physical decline, limited functional ability, and emotional distress (e.g., depression, anxiety, and anger), including an increased probability of comorbid psychopathology (for review, see 7). It is also associated with inappropriate use of medical services, reduced work performance or absenteeism, and high-cost insurance claims (8, 9). Translated into a dollar value, common chronic pain conditions cost the United States approximately $60 billion annually. Although chronic pain is often associated with negative outcomes, it is important to note that some people with chronic pain cope effectively and adapt in a manner that allows them to maintain their quality of life (10, 11).
Two terms common to the literature are used in the pages that follow to refer to clinically significant pain experiences. We use the term clinically significant chronic pain in reference to any noncancer-related pain condition that has persisted for a duration of 3 months or longer and is associated with maladaptive coping and functional limitations. We use the term pain disorder when referring to studies that have included this DSM-IV-TR diagnosis. In DSM-IV-TR, pain disorder is characterized by pain in one or more parts of the body that is severe enough to warrant clinical attention (criterion A) and for which there is significant distress or impairment in social or occupational functioning (criterion B), and evidence suggesting that psychological factors (e.g., other axis I or II conditions or symptoms that do not reach the threshold for a disorder) are involved in the etiology, severity, exacerbation, and maintenance of pain (criterion C). The pain must not be intentionally produced or feigned (criterion D) and must not be better accounted for by another disorder (criterion E). Subtypes of pain disorder are defined according to associated factors (i.e., pain disorder associated with psychological factors, pain disorder associated with a general medical condition, or pain disorder associated with both psychological factors and a general medical condition). It is noteworthy that some clinicians and researchers (12, 13) regard the diagnosis of pain disorder as problematic and of limited clinical utility (e.g., overinclusive, requiring a value judgment by clinicians while offering limited guidelines).
Epidemiology of co-occurrence with the anxiety disorders
The majority of research on co-occurrence of pain and the anxiety disorders has come from an examination of the prevalence of anxiety disorders among individuals reporting chronic pain. Epidemiological studies of community-dwelling adults have shown high rates of past year anxiety disorder diagnoses among individuals endorsing different pain syndromes, including fibromyalgia (14), arthritis/rheumatism (15–18), back/neck pain (15, 18, 19–21), ulcers (18), and migraines (15, 18). Back/neck pain has received substantial attention, as this is one of the most common pain conditions diagnosed in developed countries (19). Epidemiological studies spanning 17 countries indicate that anxiety disorders are at least twice as prevalent among persons with back/neck pain than in those without (19), with generalized anxiety disorder (GAD) and posttraumatic stress disorder (PTSD) being three times more likely. Similar results have been found using nationally representative samples from the United States (18, 21).
Among those seeking treatment for clinically significant chronic pain, some (20, 22), but not all (23–26), studies have reported elevated rates of current (i.e., at time of assessment) anxiety disorder diagnoses relative to the general population. In particular, rates of current social anxiety disorder (SAD), GAD, panic disorder (PD), and PTSD appear to be elevated among patients with clinically significant chronic pain. Lifetime prevalence rates appear similarly high among patients receiving treatment for clinically significant chronic pain (22, 27), although not all studies report an increased risk of lifetime anxiety disorder diagnoses (20, 26).
Researchers have recently turned attention to estimating the prevalence of pain conditions experienced by patients with anxiety disorders. High rates of comorbid pain disorder have been reported across the anxiety disorders; indeed, among patients with a current anxiety disorder, 61% reported lifetime unexplained pain symptoms and 28% reported a lifetime pain disorder, respectively, compared with 36% and 13% in the general population (29). There is emerging evidence that patients seeking treatment for various specific anxiety disorders have co-occurring clinically significant chronic pain experiences. For example, patients with PD experience significantly elevated rates of chronic pain (29–31). There is preliminary evidence that individuals with SAD are similar to individuals with PD with regard to the nature of (i.e., primarily musculoskeletal) and disability associated with comorbid chronic pain (32). Moreover, pain disorder rates among patients with GAD are three times greater than those found with a variety of other anxiety disorders (specific phobia, SAD, phobia not otherwise specified, agoraphobia without panic, PD without agoraphobia, and obsessive compulsive disorder), even after adjustment for age, sex, depressive and substance use disorders, and number of physical disorders. It is, however, the relationship between PTSD and chronic pain that has received the most empirical attention to date.
Individuals with PTSD report significantly elevated rates of chronic pain; specifically, more than 30% of patients seeking treatment for PTSD in outpatient clinics and 50%–80% of military veterans and volunteer firefighters seeking treatment for PTSD report chronic pain (for reviews, see 33, 34). Nationally representative data sets from both Canada and the United States have also been used to assess rates of chronic pain among persons with PTSD after adjustment for sociodemographic factors, mood disorders, substance use disorders, and commonly co-occurring anxiety disorders. The results suggest that rates of arthritis (43%), back problems (46%), fibromyalgia (7.7%), and migraine headaches (33.8%) reported by patients with PTSD are two to almost four times that found among individuals who do not meet the criteria for PTSD (35, 36).
Temporal relationship
The systematic investigation of the temporal relationship between co-occurring anxiety and pain is of critical importance in understanding the mechanisms linking these conditions. Although this line of research is in its early stages, there is reason to believe that, at least in some instances, anxiety disorders may predate the onset of pain. For example, research with injured workers experiencing chronic musculoskeletal pain indicates that current anxiety disorder diagnosis (including SAD, PD, PTSD, and specific phobia) preceded the onset of pain in all but one case wherein panic symptoms and pain had close temporal onset (23). Likewise, epidemiological research using a nationally representative sample of German community dwellers suggests that anxiety disorders precede the development of most arthritic conditions (73%) and migraine headaches (64%) (37). In contrast, there is evidence suggesting that the onset of a comorbid anxiety disorder may occur with equal frequency before (47%) and after (47%–53%) the onset of pain disorder (22, 28), with a minority reporting same year onset (6%).
Most studies documenting the temporal relationship between co-occurring anxiety and pain have conceptualized the anxiety disorders as a grouped variable (i.e., any anxiety disorder); however, anxiety disorders may not be comparable with respect to their temporal associations with pain experiences. For example, some self-report research (38) indicates that the anxiety disorder precedes the development of migraines for most people with specific phobia or SAD and follows migraine development for most with PD and GAD. Breslau et al. (39, 40) have likewise reported that specific phobia and SAD generally precede the onset of migraine attacks, whereas PD frequently develops after migraine onset. An epidemiological investigation of the correlates of PTSD suggests that onset of chronic pain tends to follow trauma exposure; however, retrospective recall bias may have influenced these results and further investigation is needed (41).
Differences may also exist in relation to specific symptoms of a given anxiety disorder. A recent prospective study (42) explored such relationships in persons experiencing PTSD symptoms and pain after undergoing posterolateral thoracotomy. Results indicated that pain intensity and trauma-related emotional numbing, but not trauma-related avoidance, contributed significantly to the prediction of pain-related disability at 6 months; however, at 12 months only emotional numbing predicted pain-related disability. These results suggest that trauma-related emotional numbing plays the most salient and important role in the development and/or maintenance of pain-related disability.
BIOPSYCHOSOCIAL MECHANISMS
Beyond studies on prevalence and temporal relationships of co-occurring anxiety disorders and chronic pain, theoretical and empirical work has been limited primarily to PTSD. Several biopsychosocial models have been proposed to account for the substantial co-occurrence of PTSD and chronic pain and identify associated mechanisms. These models, each representing slightly different iterations of the self-perpetuating interplay between PTSD and chronic pain that sustains distress and functional disability, include the mutual maintenance model (43), the shared vulnerability model (33), and several related iterations thereof (34, 44). It is beyond the scope of this clinical synthesis to comprehensively detail these models; instead, we provide a general overview of the key aspects of these models (Table 1).
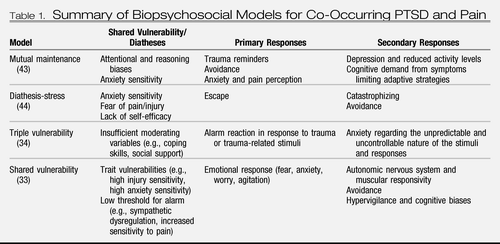 |
Table 1. Summary of Biopsychosocial Models for Co-Occurring PTSD and Pain
The mutual maintenance model (43) posits that the physiological, emotional, and behavioral components of PTSD maintain or exacerbate chronic pain and, likewise, that the cognitive, emotional, and behavioral components of chronic pain maintain or exacerbate symptoms of PTSD. Seven specific mechanisms of mutual maintenance (see the Shared Vulnerability/Diatheses, Primary Responses, and Secondary Responses columns of Table 1), each of which may have an impact along several bidirectional pathways, are posited in the model. The model predicts that pain sensations experienced by a person with chronic pain will be persistent and arousal-provoking reminders of the event that lead to the pain experience. Physiological arousal in response to recollection of the trauma will, in turn, promote avoidance of pain-related activities and (over time) physical deconditioning, which makes the experience of pain more likely.
The shared vulnerability model (33) and related iterations (34, 44) suggest that some of the maintenance factors proposed in the mutual maintenance model denote shared vulnerability, or diathesis, for developing both conditions (see Shared Vulnerability/Diathesis column of Table 1); accordingly, co-occurring PTSD and chronic pain are most likely to develop when a vulnerable person experiences an event that is both traumatic and painful, wherein subsequent trauma reminders and pain sensations trigger further negative emotion and associated functional disability.
Comprehensive reviews of related empirical work are provided elsewhere (7, 45). These reviews highlight a complex and emerging body of literature in which some mechanisms (i.e., anxiety sensitivity, depression, pain perception) have received more attention than others (i.e., trait variables beyond anxiety sensitivity, avoidance behavior, attentional biases, autonomic nervous system dysregulation). Asmundson and Katz (7) have recently extended the shared vulnerability model to the other anxiety disorders; however, its utility beyond PTSD awaits empirical scrutiny.
ASSESSMENT OF PAIN
Assessment of patients with anxiety disorders does not typically focus on clinically significant chronic pain or pain disorder. The lack of focus on pain experiences makes intuitive sense, given that the patient is often presenting to one specialist for help with their anxiety and to another specialist, if at all, for help with their pain; however, clinically significant chronic pain can be a critically complicating factor for both assessment and treatment of anxiety disorders. Results from randomized controlled trials comparing collaborative care with treatment as usual for PD or GAD indicate that pain severe enough to interfere with activities of daily living also interferes substantially with treatment success for anxiety (46). Accordingly, clinicians working with patients with anxiety disorders should consider incorporating pain assessments into their standard practice to facilitate case conceptualization and treatment planning for those patients who also experience clinically significant pain.
It is beyond the scope of this clinical synthesis to detail the complexities often associated with a comprehensive assessment of pain. Instead, we provide guidelines to facilitate a time-efficient yet reasonably comprehensive assessment (47–49). A time-efficient pain assessment focuses on seven key elements, including pain severity and stability, pain location(s), pain-related attitudes, pain-related beliefs, pain-specific emotional distress (i.e., fear, anxiety, and mood changes), pain-related coping styles, and pain-related functional capacity (Figure 1). The latter is particularly important given that, as noted above, some patients with chronic pain function well despite their pain whereas others do not.
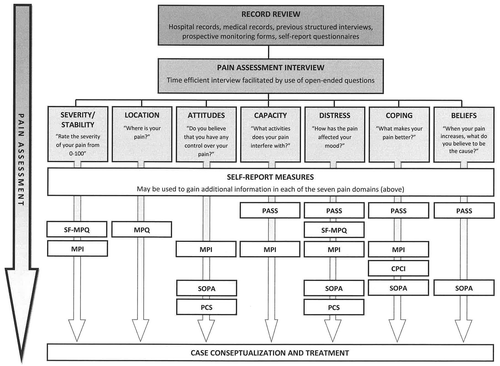
PASS, Pain Anxiety Symptoms Scale; MPQ, McGill Pain Questionnaire; SF-MPQ, Short Form McGill Pain Questionnaire; MPI, Multidimensional Pain Inventory; CPCI, Chronic Pain Coping Inventory; SOPA, Survey of Pain Attitudes; PCS, Pain Catastrophizing Scale.
Assessment begins with an evaluation of patient records when available. These records might include hospital medical records, records from other health care providers (e.g., psychologists and physiotherapists), previous clinician-administered structured clinical interviews, previous clinician observation techniques (e.g., pain behaviors and facial action coding), prospective monitoring forms, and self-report questionnaires. Although the information obtained from patient records may provide essential information, particularly for those who have received services from a number of clinicians, a brief clinical interview is essential for determining current biopsychosocial functioning.
Key open-ended questions can be used to facilitate identification of pain characteristics (i.e., location, severity, and stability), pain triggers, patterns of avoidance, and treatment goals. These include, for example, “Where is your pain?”, “On a scale from 0 (not at all) to 100 (the worst possible), how bad is your pain right now? In general?”, “Is your pain always present?”, “What makes your pain worse?”, “What makes your pain better?”, “What entertaining activities does your pain interfere with?”, “What work activities does your pain interfere with?”, “What would you do tomorrow if you knew you'd have no pain just for that day?” These open-ended questions can be supplemented with a selection of brief self-report measures that serve to facilitate assessment and gauge treatment progress. Although we recommend a set of specific supplemental measures that correspond to the seven key elements of an efficient pain assessment, other options are available (for example, see 50).
The psychometric properties for the self-report measures listed below are described in detail elsewhere (51, 52); as such, only the names and rationales for use are included here. The Short Form McGill Pain Questionnaire (53) is used to describe the quality and intensity of the pain experience, using descriptors such as throbbing and cramping, with intensity indicated using a 4-point Likert scale (0 = none to 3 = severe). A visual analog scale and present pain intensity index also provide indices of current overall pain intensity. We also recommend that a body diagram, such as that included in the original long form of the McGill Pain Questionnaire (54), be included as a means of assessing location of pain. The Multidimensional Pain Inventory (MPI) (55) assesses physical, psychological, and social factors related to the pain experience and permits identification of subtypes of pain characterized, for example, by adaptive versus dysfunctional coping (11). The Pain Anxiety Symptoms Scale (PASS) (56) and its short form (57) assess four theoretically distinct components of pain-related anxiety, providing useful information regarding pain-related cognition, emotion, and behavior. The Pain Catastrophizing Scale (PCS) (58) measures catastrophic beliefs regarding the consequences of anticipated or actual pain. The Survey of Pain Attitudes–Revised (SOPA) (59) measures attitudes about the appropriate treatment of pain as well as the personal ability to control pain. The Chronic Pain Coping Inventory (CPCI) (60) measures cognitive and behavioral strategies people might use in an attempt to prevent or relieve pain. Collectively, the PASS, PCS, SOPA, and CPCI provide a fairly comprehensive picture of cognitions and behaviors that may be contributing to the maintenance of pain and associated functional limitations. Finally, there is growing evidence that anxiety sensitivity—the fear of sensations related to anxiety (61)—may denote a key mechanism in the co-occurrence of the anxiety disorders and clinically significant chronic pain. Accordingly, the Anxiety Sensitivity Index-3 (ASI-3) (62), which assesses the dispositional tendency to fear anxiety-related sensations due to the belief such sensations signal harmful consequences, should also be considered for inclusion.
TREATMENT AND OUTCOMES
There are several evidence-based treatment options available for patients who present with an anxiety disorder accompanied by clinically significant chronic pain, including pharmacotherapy and several forms of psychotherapy. Combined pharmacotherapy and psychotherapy is also an option (63), although additional research is needed to establish whether the combined approach produces better outcomes for pain and anxiety symptoms relative to monotherapy within the context of the various anxiety disorders.
Pharmacotherapy has been shown to be effective in alleviating chronic pain (64, 65). The group of drugs known as the adjuvant analgesics—drugs that have primary indications other than pain but contain analgesic properties—may be particularly useful for patients with an anxiety disorder who present with clinically significant chronic pain (64). The adjuvant analgesics can be grouped according to the type of pain for which they are effective, including those for multiple pain types (e.g., tricyclic antidepressants, α2-adrenergic agonists, corticosteroids, selective serotonin reuptake inhibitors, and selective norepinephrine reuptake inhibitors), those for neuropathic pain (e.g., anticonvulsants, γ-aminobutyric acid agonists, and neuroleptics), those for musculoskeletal pain (e.g., muscle relaxants and some benzodiazepines), and those for cancer pain (e.g., osteoclast inhibitors and anticholinergic agents). In the absence of systematic evidence on efficacies of specific drugs for patients presenting with both an anxiety disorder and clinically significant chronic pain, drug selection must be based on the evidence available from applications to patients with either an anxiety disorder or chronic pain, careful assessment of the patient, and good clinical judgment. To illustrate, given its effectiveness in alleviating headache (66) and musculoskeletal pain (64) as well as PD, PTSD, SAD, and GAD, the anticonvulsant gabapentin may be useful in treating patients presenting with one of these anxiety disorders and clinically significant chronic pain (67–69). Likewise, the large body of literature indicating effectiveness of tricyclic antidepressants for various anxiety disorders as well as clinically significant chronic pain suggests that these drugs may be useful for patients presenting with both. However, the decision to prescribe a particular drug, if any, needs to be placed in the context of the nature of the pain the patient is experiencing as well as his or her medication preferences and tolerance.
Cognitive behavior therapy (CBT) for chronic pain does not focus on reducing pain but, instead, on improving mood, reducing pain-related catastrophic thinking, and increasing functional ability despite pain. The intensity of CBT (i.e., number of sessions) varies as a function of the severity of functional disability, with 10 to 20 hours typically delivered in once or twice weekly hourly sessions. Comprehensive reviews of CBT for chronic pain indicate that it has modest positive effects that, at least in the case of improved mood and reduced catastrophic thinking, persist at 6 and 9 months follow-up (70–72). Dropout rates vary from study to study but are generally around 20% (73). Because CBT has also been shown to be highly effective for the anxiety disorders (74), it seems reasonable to assume that treatment of clinically significant chronic pain in patients with an anxiety disorder may effectively integrate elements of CBT for both the anxiety disorder and chronic pain. There have to date been no published systematic evaluations of such an integrated treatment approach, although findings from case studies appear promising (75). Other promising options include provision of treatment as usual for a given anxiety disorder, or treatments that target putative shared mechanisms. As with the integrated treatment approach, there has been little systematic evaluation of these alternatives. Although results are preliminary, Shipherd et al. (76) have demonstrated that treatment as usual for veterans seeking treatment for PTSD (i.e., a 16-week group-based outpatient CBT program, combined with pharmacotherapy for most patients) resulted in reductions in pain over the course of PTSD treatment for those reporting chronic pain before treatment.
Strategies for targeting putative shared mechanisms remain in developmental stages and, to date, have focused primarily on the reduction of elevated anxiety sensitivity. This approach is predicated on the assumption that targeting the shared vulnerability will reduce severity of symptoms of both clinically significant chronic pain and the anxiety disorder. Interoceptive exposure (i.e., exposure to anxiety-provoking bodily sensations), a common component of treatment for various anxiety disorders (77), has recently been shown to be effective in reducing both PTSD symptoms (78) and fear of pain (79); consequently, it may have significant potential as treatment for patients who present with a co-occurring anxiety disorder and clinically significant chronic pain. Likewise, because exercise can serve the dual purpose of physical reconditioning in patients with chronic pain (80) and exposure-related amelioration of anxiety symptoms, particularly panic-related symptoms (81, 82), it is an attractive consideration for the patient with both clinically significant chronic pain and anxiety symptoms. It remains to be determined whether these and alternative methods for targeting putative shared mechanisms prove effective when systematically scrutinized.
FUTURE DIRECTIONS
Research on co-occurring chronic pain and anxiety disorders is increasing at an exponential rate. Although a comprehensive discussion of future research directions is beyond the scope of the current clinical synthesis, readers are directed to a recent review by Asmundson and Katz (7) for related discussion. Specific areas of interest include identifying mechanisms responsible for the co-occurrence of chronic pain and each of the anxiety disorders, as well as an investigation of the temporal primacy of co-occurring pain and anxiety. Given a relative dearth in the literature, studies targeting issues of treatment—options, sequencing, and patient preferences—and related factors that might improve treatment outcomes for patients presenting with clinically significant chronic pain and an anxiety disorder are of primary importance.
1 McWilliams LA, Cox BJ, Asmundson GJG: Symptom structure of posttraumatic stress disorder in a nationally representative sample. J Anxiety Disord 2005; 19:626–641Crossref, Google Scholar
2 Asmundson GJG, Wright KD, Stein MB: Pain and PTSD symptoms in female veterans. Eur J Pain 2004; 8:345–350Crossref, Google Scholar
3 Melzack R, Katz J: The Gate Control Theory: reaching for the brain, in Pain: Psychological Perspectives. Edited by Hadjistavropoulos T, Craig KD. Mahwah, NJ, Lawrence Erlbaum Associates, 2004, pp 13–34Google Scholar
4 Wall PW: On the relation of injury to pain. Pain 1978; 6:253–264Crossref, Google Scholar
5 International Association for the Study of Pain: Classification of chronic pain: descriptions of chronic pain syndromes and definitions of pain terms. Pain 1986; 1–222Google Scholar
6 Verhaak PF, Kerssens JJ, Dekker J, Sorbi MJ, Bensing JMM: Prevalence of chronic benign pain disorder among adults: a review of the literature. Pain 1998; 77:231–239Crossref, Google Scholar
7 Asmundson GJG, Katz J: Understanding the co-occurrence of anxiety disorders and chronic pain: state-of-the-art. Depress Anxiety 2009; 26:888–901Crossref, Google Scholar
8 Spengler DM, Bigos SJ, Martin NA: Back injuries in industry: a retrospective study. 1. Overview and cost analysis. Spine 1986; 11:241–245Crossref, Google Scholar
9 Stewart WF, Ricci JA, Chee E, Morganstein D, Lipton R: Lost productive time and cost due to common pain conditions in the US workforce. JAMA 2003; 12:2443–2454Crossref, Google Scholar
10 Asmundson GJG, Norton GR, Allerdings MD, Norton PJ, Larsen DK: Posttraumatic stress disorder and work-related injury. J Anxiety Disord 1998; 12:57–69Crossref, Google Scholar
11 Turk DC, Rudy TE: Towards a comprehensive assessment of chronic pain patients. Behav Res Ther 1987; 25:237–249Crossref, Google Scholar
12 Asmundson GJG, Carleton RN: Fear of pain, in Handbook of Anxiety and the Anxiety Disorders. Edited by Antony MM, Stein MB. New York, Oxford University Press, 2008, pp 551–561Google Scholar
13 Fishbain D: DSM-IV: implications and issues for the pain clinician. Am Pain Soc Bull 1995; 6:18Google Scholar
14 Raphael KG, Janal MN, Nayak S, Schwartx JE, Gallagher RM: Psychiatric comorbidities in a community sample of women with fibromyalgia. Pain 2006; 124:117–125Crossref, Google Scholar
15 Buist-Bouwman MA, de Graaf R, Vollebergh WAM, Ormel J: Comorbidity of physical and mental disorders and the effect on work loss days. Acta Psychiatr Scand 2005; 111:436–443Crossref, Google Scholar
16 Kessler RC, Ormel J, Demler O, Stang PE: Comorbid mental disorders account for the role impairment of commonly occurring chronic physical disorders: results from the National comorbidity Survey. J Occup Environ Med 2003; 45:1257–1266Crossref, Google Scholar
17 McWilliams LA, Cox BJ, Enns MW: Mood and anxiety disorders associated with chronic pain: an examination in a nationally representative sample. Pain 2003; 106:127–133Crossref, Google Scholar
18 McWilliams LA, Goodwin RD, Cox BJ: Depression and anxiety associated with three pain conditions: results from a nationally representative sample. Pain 2004; 111:77–83Crossref, Google Scholar
19 Demyttenaere K, Bruffaerts R, Lee S, Posada-Villa J, Kovess V, Angermeyer MC, Levinson D, de Girolamo G, Nakane H, Mneimneh Z, Lara C, de Graff R, Scott KM, Gureje O, Stein DJ, Haro JM, Bromet EJ, Kessler RC, Alonso J, Korff MV: Mental disorders among persons with chronic back or neck pain: results from the world mental health surveys. Pain 2007; 129:332–342Crossref, Google Scholar
20 Kinney RK, Gatchel RJ, Polatin PB, Fogarty WT, Mayer TG: Prevalence of psychopathology in acute and chronic low back pain patients. J Occup Rehabil 1993; 3:95–103Crossref, Google Scholar
21 Korff MV, Crane P, Lane M, Miglioretti DL, Simon G, Saunders K, Stang P, Brandenburg N, Kessler R: Chronic spinal pain and physical-mental comorbidity in the United States: results from the national comorbidity survey replication. Pain 2005; 113:331–339Crossref, Google Scholar
22 Atkinson JH, Slater MA, Patterson TL, Grant I, Garfin SR: Prevalence, onset, and risk of psychiatric disorders in men with chronic low back pain: a controlled study. Pain 1991; 45:111–121Crossref, Google Scholar
23 Asmundson GJG, Jacobson SJ, Allerdings MD, Norton GR: Social phobia in disabled workers with chronic musculoskeletal pain. Behav Res Ther 1996; 34:939–943Crossref, Google Scholar
24 Fishbain DA, Goldberg M, Meagher BR, Steele R, Rosomoff H: Male and female chronic pain patients categorized by DSM-III psychiatric diagnostic criteria. Pain 1986; 26:181–197Crossref, Google Scholar
25 Large RG: DSM-III diagnoses in chronic pain. Confusion or clarity? J Nerv Ment Dis 1986; 174:295–303Crossref, Google Scholar
26 Polatin PB, Kinney RK, Gatchel RJ, Lillo E, Mayer TG: Psychiatric illness and chronic low-back pain. The mind and the spine—which goes first? Spine 1993; 18:66–71Crossref, Google Scholar
27 Arnold LM, Hudson JI, Keck PE, Auchenbach MB, Javaras KN, Phil D, Hess EV: Comorbidity of fibromyalgia and psychotic disorders. J Clin Psychiatry 2006; 67:1219–1225Crossref, Google Scholar
28 Beesdo K, Jacobi F, Hoyer J, Low NCP, Hofler M, Wittchen HU: Pain associated with specific anxiety and depressive disorders in a nationally representative population sample. Soc Psychiat Epidemiol 2010; 45:89–104Crossref, Google Scholar
29 Kuch K, Cox BJ, Woszczyna CB, Swinson RP, Shulman I: Chronic pain in panic disorder. J Behav Ther Exp Psychiatry 1991; 22:255–259Crossref, Google Scholar
30 Schmidt NB, Telch MJ: Nonpsychiatric medical comorbidity, health perceptions, and treatment outcome in patients with panic disorder. Health Psychol 1997; 16:114–122Crossref, Google Scholar
31 Schmidt NB, Santiago HT, Trakowski JH, Kendren JM: Pain in patients with panic disorder: relation to symptoms, cognitive characteristics and treatment outcome. Pain Res Manag 2002; 7:134–141Crossref, Google Scholar
32 Asmundson GJ, Walker JR, Furer P, Kjemisted K: Beyond anxiety: a preliminary look at acute and chronic pain in individuals seeking treatment for anxiety disorders.
33 Asmundson GJG, Coons MJ, Taylor S, Katz J: PTSD and the experience of pain: research and clinical implications of shared vulnerability and mutual maintenance models. Can J Psychiatry 2002; 47:930–937Crossref, Google Scholar
34 Otis JD, Keane TM, Kerns RD: An examination of the relationship between chronic pain and post-traumatic stress disorder. J Rehabil Res Dev 2003; 40:397–405Crossref, Google Scholar
35 Sareen J, Cox BJ, Clara I, Asmundson GJG: The relationship between anxiety disorders and physical disorders in the U.S. National Comorbidity Survey. Depress Anxiety 2005; 21:193–202Crossref, Google Scholar
36 Sareen J, Cox BJ, Stein MB, Afifi TO, Fleet C, Asmundson GJG: Physical and mental comorbidity, disability, and suicidal behavior associated with posttraumatic stress disorder in a large community sample. Psychosom Med 2007; 69:242–248Crossref, Google Scholar
37 Sareen J, Jacobi F, Cox BJ, Belik S, Clara I, Stein MB: Disability and poor quality of life associated with comorbid anxiety disorders and physical conditions. Arch Intern Med 2006; 166:2109–2116Crossref, Google Scholar
38 Harter MC, Conway KP, Merlkangas. Associations between anxiety disorders and physical illness. Eur Arch Psychiatry Clin Neurosci 2003; 253:313–320Crossref, Google Scholar
39 Breslau N, Davis GC, Andreski P: Migraine, psychiatric disorders, and suicide attempts: an epidemiologic study of young adults. Psychiatry Res 1991; 37:11–23Crossref, Google Scholar
40 Breslau N, Davis G: Migraine, physical health and psychiatric disorder: a prospective epidemiologic study in young adults. J Psychiatr Res 1993; 27:211–221Crossref, Google Scholar
41 Sledjeski EM, Speisman B, Dierker LC: Does number of lifetime traumas explain the relationship between PTSD and chronic medical conditions? Answers from the National Comorbidity Survey-Replication (NCS-R). J Behav Med 2008; 31:341–349Crossref, Google Scholar
42 Katz J, Asmundson GJG, McRae K, Halket E: Emotional numbing and pain intensity predict the development of pain disability up to one year after lateral thoractomy. Eur J Pain 2009; 13:870–878Crossref, Google Scholar
43 Sharp TJ, Harvey AG: Chronic pain and posttraumatic stress disorder: mutual maintenance? Clin Psychol Rev 2001; 21:857–877Crossref, Google Scholar
44 Turk DC: A diathesis-stress model of chronic pain and disability following traumatic injury. Pain Res Manag 2002; 7:9–19Crossref, Google Scholar
45 Beck JG, Clapp JD. A different kind of comorbidity: understanding posttraumatic stress disorder and chronic pain. Psychol Trauma 2011; doi: https://doi.org/10.1037/a00211263Google Scholar
46 Teh CF, Morone NE, Karp JF, Belnap H, Zhu F, Weiner DK, Rollman B: Pain interference impacts response to treatment for anxiety disorders. Depress Anxiety 2009; 26:222–228Crossref, Google Scholar
47 Asmundson GJG: Pain assessment: state-of-the-art applications from the cognitive-behavioural perspective. Behav Res Ther 2002; 40:547–550Crossref, Google Scholar
48 Dworkin RH, Turk DC, Farrar JT, Haythomthwaited JA, Jensen MP, Katze NP, Kerns RD Stucki G, Allen RR, Bellamy N, Carr DB, Chandler J, Cowan P, Dionne R, Galer BS, Hertz S, Jadad AR, Kramer LD, Manning DC, Martin S, McCormick CG, McDermott MP, McGrath P, Quessy S, Rappaport BA, Robbins W, Robinson JP, Rothman M, Royal MA, Simon L, Stauffer JW, Stein W, Tollett J, Wernicke J, Witter J, IMMPACT: Core outcome measures for chronic pain in clinical trials: IMMPACT recommendations. Pain 2005; 113:9–19Crossref, Google Scholar
49 Tait R: Evaluation of treatment effectiveness in patients with intractable pain: measures and methods, in Psychosocial Factors in Pain: Critical Perspectives. Edited by Gatchel RJTD. New York, Guilford, 1999, pp 457–480Google Scholar
50 Thorn BE: Cognitive Therapy for Chronic Pain. New York, Guilford, 2004Google Scholar
51 Asmundson GJG, Abrams MP, Collimore KC: Pain and anxiety disorders, in Health Behaviors and Physical Illness in Anxiety and Its Disorders: Contemporary Theory and Research. Edited by Zvolensky MJ, Smits JAJ. New York, Springer, 2008, pp 207–235Google Scholar
52 Turk DC, Melzack R, Eds. Handbook of Pain Assessment, 3rd ed. New York, Guilford, 2011Google Scholar
53 Melzack R: The short-form McGill pain questionnaire. Pain 1987; 30:191–197Crossref, Google Scholar
54 Melzack R: The McGill Pain Questionnaire: major properties and scoring methods. Pain 1975; 1:277–299Crossref, Google Scholar
55 Kerns RD, Turk DC, Rudy TE: The West Haven-Yale Multidimensional Pain Inventory (WHYMPI). Pain 1985; 23:345–356Crossref, Google Scholar
56 McCracken LM, Zayfert C, Gross RT: The pain anxiety symptoms scale: development and validation of a scale to measure fear of pain. Pain 1992; 50:67–73Crossref, Google Scholar
57 McCracken LM, Dhingra L: A short version of the Pain Anxiety Symptom Scale (PASS-20): preliminary development and validity. Pain Res Manag 2002; 7:45–50Crossref, Google Scholar
58 Sullivan MJL, Bishop S, Pivik J: The Pain Catastrophizing Scale: development and validation. Psychol Assess 1995; 7:524–532Crossref, Google Scholar
59 Jensen MP, Turner JA, Romano JM: Pain belief assessment: a comparison of short and long versions of the Survey of Pain Attitudes. J Pain 2000; 1:138–150Crossref, Google Scholar
60 Romano JM, Jensen MP, Turner JA: The chronic pain coping inventory-42: reliability and validity. Pain 2003; 104:65–73Crossref, Google Scholar
61 Reiss S, McNally RJ: The expectancy model of fear, in Theoretical Issues in Behaviour Therapy. Edited by Reiss S, Bootzin RR. New York, Academic Press, 1985Google Scholar
62 Taylor S, Zvolensky MJ, Cox BJ, Deacon B, Heimberg RG, Ledley DR, Abramowitz JS, Holaway RM, Sandin B, Stewart SH, Coles M, Eng W, Daly ES, Arrindell WA, Bouvard M, Cardenas SJ: Robust dimensions of anxiety sensitivity: development and initial validation of the Anxiety Sensitivity index-3. Psychol Assess 2007; 19:176–188Crossref, Google Scholar
63 Foa EB, Davidson JRT, Frances A: The expert consensus guideline series: treatment of posttraumatic stress disorder. J Clin Psychiatry 1999; 60:4–76Google Scholar
64 Portenoy RK: Current pharmacotherapy of chronic pain. J Pain Symptom Manage 2000; 19(suppl 1):16–20Crossref, Google Scholar
65 Kroenke K, Krebs EE, Bair MJ: Pharmacotherapy of chronic pain: a synthesis of recommendations from systematic reviews. Gen Hosp Psychiatry 2009; 31:206–219Crossref, Google Scholar
66 Mathew N, Rapoport A, Saper J, Magnus L, Klapper J, Ramadan N, Stacey B, Tepper S: Efficacy of gabapentin in migraine prophylaxis. Headache 2001; 41:119–128Crossref, Google Scholar
67 Pande AC, Davidson JR, Jefferson JW, Janney CA, Katzelnick DJ, Weisler RH, Greist JH, Sutherland SM: Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol 1999; 19:341–348Crossref, Google Scholar
68 Pande AC, Pollack MH, Crockatt J, Greiner M, Chouinard G, Lydiard RB, Taylor CB, Dager SR, Shiovitz T: Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol 2000; 20:467–471Crossref, Google Scholar
69 Chouinard G, Beauclair L, Belanger MC: Gabapentin: long-term antianxiety and hypnotic effects in psychiatric patients with comorbid anxiety-related disorders. Can J Psychiatry 1998; 43:305Crossref, Google Scholar
70 Morley S, Eccleston C, Williams A: Systematic review and meta-analysis of randomized controlled trials of cognitive behavior therapy and behaviour therapy for chronic pain in adults, excluding headache. Pain 1990; 80:1–13Crossref, Google Scholar
71 Eccleston C, Williams AC, Morley S (2009). Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 2009; 2:CD007407Google Scholar
72 Morely S, Williams A, Hussain S. Estimating the clinical effectiveness of cognitive behavioural therapy in the clinic: evaluation of a CBT informed pain management programme. Pain 2008; 137:467–468Crossref, Google Scholar
73 Glombiewski JA, Hartwich-Tersek J, Rief W. Attrition in cognitive-behavioral treatment of chronic back pain. Clin J Pain 2010; 26:593–601Crossref, Google Scholar
74 Butler AC, Chapman JE, Forman EM, Beck AT: The empirical status of cognitive-behavioral therapy: a review of meta-analysis. Clin Psychol Rev 2006; 26:17–31Crossref, Google Scholar
75 Otis JD, Keane TM, Kerns RD, Monson C, Scioloi E: The development of an integrated treatment for veterans with comorbid chronic pain and posttraumatic stress disorder. Pain Med 2009; 10:1300–1311Crossref, Google Scholar
76 Shipherd JC, Keyes M, Jovanovic T, Ready DJ, Baltzell D, Worley V, Gordon-Brown V, Hayslett C, Duncan E: Veterans seeking treatment for posttraumatic stress disorder: what about comorbid chronic pain? J Rehabil Res Dev 2007; 44:153–166Crossref, Google Scholar
77 Taylor S, Ed. Anxiety Sensitivity: Theory, Research, and Treatment of the Fear of Anxiety. Mahwah, NJ: Lawrence Erlbaum Associates, 1999Google Scholar
78 Wald J, Taylor S: Efficacy of interoceptive exposure therapy combined with trauma-related exposure therapy for posttraumatic stress disorder: a pilot study. J Anxiety Disord 2007; 21:1050–1060Crossref, Google Scholar
79 Watt MC, Stewart SH, Lefaivre MJ, Uman LS: A brief cognitive-behavioral approach to reducing anxiety sensitivity decreases pain-related anxiety. Cogn Behav Ther 2006; 35:248–256Crossref, Google Scholar
80 Wright A, Sluka KA: Nonpharmacological treatments for musculoskeletal pain. Clin J Pain 2001; 17:33–46Crossref, Google Scholar
81 Smits JA, Berry AC, Rosenfield D, Powers MB, Behar E, Otto MW: Reducing anxiety sensitivity with exercise. Depress Anxiety 2008; 25:689–699Crossref, Google Scholar
82 Stathopoulou GPM, Berry AC, Smits JAJ, Otto MW: Exercise interventions for mental health: a quantitative and qualitative review. Clin Psychol Sci Pract 2006; 13:179–193Crossref, Google Scholar



