Motivational Interviewing and Psychiatry: Use in Addiction Treatment, Risky Drinking and Routine Practice
Abstract
This clinical synthesis focuses on the evidence-based practice of motivational interviewing (MI) and its use in the treatment of addictions, risky drinking, and general psychiatric practice. The authors review the definition and basic concepts of MI, present examples of some core MI clinical interventions, and provide a critical summary of the research supporting the usefulness of MI for the treatment of addictions. In addition, the article also discusses the problem of risky drinking and the ways that MI can help psychiatrists address problem drinking as well as more general behavioral issues and psychotherapy in routine practice. Pragmatic tools are provided to assist psychiatrists in this work.
WHAT IS MOTIVATIONAL INTERVIEWING?
First described by William Miller in 1983 (1) and further developed in collaboration with Stephen Rollnick over the next three decades (2–4), motivational interviewing (MI) represents a general theory of behavior change associated with a set of positive attitudes (“spirit of motivational interviewing”) and pragmatic, operational-defined intervention techniques.
MI is robustly evidence-based and widely disseminated, with broad applications in the fields of addictions, health behaviors, psychotherapies, criminal justice, and other domains. A recent review noted more than 200 current research projects, with more than 100 clinical trials, using or directly investigating the effectiveness of MI (5). In the United States, 47 states encourage the use of MI as a preferred treatment for substance abuse, and 8 states mandate the use of an empirically based treatment such as MI for addiction and/or mental health. “This widespread dissemination has led to a proliferation of MI adaptations as well as a burgeoning industry in training, continuing education, and certification of MI practitioners” (5).
There are 11 books on MI, more than 800 publications, and 1,200 trainers in 43 languages (William Miller, personal communication). Four meta-analyses demonstrate effectiveness across multiple areas of patient behavior including substance abuse, smoking, obesity, and medication nonadherence (6–9).
New data on life expectancy and general medical health of psychiatric patients confirm the relevance of MI for psychiatrists: the life expectancy of patients with severe mental illness is 32 years less than that of age- and sex-matched control subjects, and the risk of death from cardiovascular disease is two to three times higher than that of control subjects (10–17). Despite this evidence and the compelling relevance of MI for general psychiatric practice, many psychiatrists have little appreciation for the principles and practice of MI.
This article defines and describes MI, summarizes the evidence base supporting its use for the treatment of addictions, and discusses how MI can be integrated into the routine practice of psychiatry to improve efficiency and effectiveness for a broad range of other conditions and psychotherapies. For example, in addition to the formal treatment of addictions, MI approaches can be used in routine practice by helping psychiatrists address the much more common problem of risky drinking (discussed in a separate section below) as well as other applications to everyday practice issues, e.g., adherence to medications (18–20), lifestyle modifications (diet, exercise, and smoking) (21–25), and increasing the efficiency and effectiveness of various psychotherapies (26, 27).
Although the basic tenets of MI have remained intact over the last 25 years, Miller and Rollnick have revised the core definition several times. In 2002, they defined MI as a “directive, client-centered counseling style for eliciting behavior change by helping clients to explore and resolve ambivalence” (2). In the last few years, however, they have revised the concept of “directive counseling,” in favor of a style they name “guiding” which seems to better capture the essence of the approach they advocate. Thus, in 2008, MI was redefined as “a refined form of the familiar process of guiding” (4). More recently, explaining probable changes to the definition of MI, they described one possible new definition of MI as “a person-centered counseling method for addressing the common problem of ambivalence about behavior change” (unpublished keynote address, Second International Conference on Motivational Interviewing, Stockholm 2010). This possible new definition represents only one possible version that may be revised substantially before publication of Miller and Rollnick's third edition of “Motivational Interviewing,” expected in 2011. Despite these modest changes in concept and definition, MI throughout the years has retained the essence of its innovative approach to behavior change.
With respect to the application of MI in the general medical and psychiatric setting, four general principles for the application of MI can be summarized by the acronym RULE:
To resist the righting reflex, the clinician actively suppresses the reigning medical paradigm to tell patients what to do in an authoritarian manner, in contrast to helping patients express their own internal motivations for behavior change. Practitioners understand patients through the use of open questions about unhealthy behavior, e.g., asking “what is good and what is not so good” about a specific behavior. The clinician who can listen to the patient can more easily express accurate empathy and build trust and a working alliance for change. The practitioner helps empower a patient by building a sense of self-efficacy, through communicating support and confidence in the patient's inherent ability to change.In addition to the elements of RULE, MI also facilitates change by “rolling with resistance” (that is, not arguing directly with patients) and focusing dialogue on patient “change” talk. This focus on change talk leads to elicitation, clarification, and hopefully to resolution of a patient's inherent ambivalence about persisting unhealthy behaviors and about the desire, ability, reasons, needs, or commitment to change. At the most advanced levels, MI encourages positive change by eliciting “discrepancies” and then overt discussion about any differences that may exist between a patient's current behaviors and broader personal desires and values. Attention to this discrepancy (or inconsistency) functions as a powerful motivator for positive change. As discussed by Robert Cialdini in his authoritative review of the social psychology of persuasion and influence, “Psychologists have long understood the power of the consistency principle to direct human action … prominent theorists such as Leon Festinger, Fritz Hieder and Theodore Newcomb have viewed the desire for consistency as a central motivator of our behavior” (28, 29).
The underlying approach of MI practice must always communicate a sense of collaboration, evocation, and respect for autonomy, or what is called the “spirit of motivational interviewing.” Collaboration suggests that practitioners and patients should be equal in any discussion about change, evocation means that ideas for changing unhealthy behaviors should come from the patient and not be directed by the clinician, and respect for autonomy indicates that professionals must support and communicate acceptance of the right of patients to change or not to change. Measures of the spirit of MI, by itself, have been shown to predict subsequent adaptive behavior change in patients with persistent unhealthy behaviors (30).
MI challenges traditional intervention methods by suggesting that many patients already know what is best for them. MI recommends that professionals work collaboratively with their patients to make decisions about specific strategies for change, while acknowledging freedom of choice for patients. A menu of choices is one venue for offering recommendations while maintaining the patient's freedom of choice (31).
MI has been shown to have particular strengths for treating addictions and for addressing problems of persistent unhealthy behaviors, especially with patients who are angry or resistant to change (6). Although MI was originally developed as a treatment modality for alcohol and substance abuse, a growing evidence base supports its increasing use in specialized medical settings, such as hospital emergency departments, trauma centers, and obesity treatment programs as well as broad applications in primary care (32).
The following
The police brought a 40-year-old woman to the psychiatric emergency room because while intoxicated she threatened to kill her partner and herself. She had no history of violence or of legal or psychiatric problems. When she became sober the next day, she reported calmly that she was an alcoholic and was not violent and had no intention of hurting her partner or herself. She wanted to be discharged. The typical psychiatric approach to this problem would be a combination of education and confrontation; the psychiatrist would explain the dangers of alcoholism to the patient and encourage her to seek treatment, handing her a list of alcohol treatment centers.
In contrast, the actual MI conversation proceeded like this:
Patient: I am an alcoholic and don't want to change. I am not dangerous; just let me go home now.
Psychiatrist: OK, that's what we'll do. We can't force you to change. Can I just ask you a few questions and then we'll let you out of here.
[MI: Respect for autonomy—the psychiatrist respects the individual's right to change or not to make a change; collaboration—the psychiatrist is equal to the patient in power and asks permission for further inquiry.]
Patient: OK.
Psychiatrist: I'm interested in learning a little about your drinking. I understand you don't want to change. So I am assuming that the alcohol is mostly a good thing in your life. I am wondering if there is anything not so good about the alcohol in your life?
[MI: Elicit ambivalence.]
Patient: Well, they said my liver is not so good anymore. It's going to fail if I don't stop drinking.
Psychiatrist: OK, so that sounds like one part of the drinking that's not so good.
[MI: Explore ambivalence.]
Patient: Right.
Psychiatrist: But it doesn't sound important enough to make you want to change. I'm guessing that you don't care so much whether your liver fails or not.
[MI: Not at all sarcastic here; really respecting her autonomy.]
Patient: Well, I can't live without a liver.
Psychiatrist: OK. Then it sounds like you don't care so much whether you live or die.
[MI: Again, not at all sarcastic; simply reflecting content and respecting autonomy.]
Patient: No way! I love life!
Psychiatrist: Well, I'm not sure I understand then. On the one hand, you are very sure that you are not going to stop drinking, yet you also say you love life and don't want your liver to fail.
[MI: Develop discrepancy. Elicit change talk.]
Patient: Well, I know I'm going to have to cut down or stop sometime. This is just not the time.
Psychiatrist: OK. I hear what you are saying. You do want to stop drinking at some point, to save your liver and to save your life—it's just not the right time now.
[MI: Listen, understand, express empathy, and reflect feelings; respect autonomy.]
Patient: Right.
Psychiatrist: OK. Can I ask another question or two? … If you do think you're going to stop at some point, I wonder what thoughts you've had about when and how you might want to stop drinking? Would you want or need any help if and when you decided to cut down or stop drinking?
[MI: Open questions for understanding; encourage change talk.]
This conversation led quickly to discussion about where and when the patient might go for help. She developed a specific action plan for health. Of course, this patient may not have followed through with her action plan, but the interaction using an MI approach seemed much more likely to lead to change than the more traditional educational/confrontational one.
This review will now turn to the evidence supporting the use of MI for treatment of addictions, then discuss the use of MI in general psychiatry practice for addressing the more widespread and underrecognized problem of risky drinking, and, finally, discuss the integration of MI principles and techniques into the more routine general practice of psychiatry.
EFFICACY OF MI FOR TREATMENT OF ADDICTIONS
Overview
MI, with its brief adaptation motivational enhancement therapy (MET), is consistently ranked as one of the forms of addiction treatment with the strongest empirical support. A 2001 systematic review of 29 studies found a significant effect favoring MI in 18 of 26 studies (10 of 15 of those focusing on substance abuse), and effect size did not appear to diminish with length of follow-up (33). A 2003 meta-analysis reported effect sizes (relative to no treatment or placebo) of 0.56 for drug addition, 0.53 for diet and exercise, and 0.25–0.53 for alcohol abuse/dependence (34). These effects are larger than those of most empirically based addiction treatments. Another recent meta-analysis focusing exclusively on MI-based interventions for alcohol reported a mean effect size of 0.18 for MI versus no treatment and 0.43 for MI versus another treatment (35). For the studies in which MI was compared with no treatment, the effects were larger in studies with shorter periods of follow-up (≤3 months). Finally, a meta-analysis including 72 studies across a broad range of target behaviors (drinking, drug use, smoking, and other problem behaviors) reported an overall effect size of 0.77 in the month after treatment, but with a fairly rapid drop to 0.39 between 1 and 3 months, to 0.31 between 3 and 6 months, to 0.30 between 6 and 12 months, and to 0.11 (0.06–0.17) at follow-ups longer than 12 months (6).
These overall estimates of effect are useful, but they have limitations related to the heterogeneity of the studies they encompass. There are several basic designs that have been used in studies of MI. Some studies compare MI to no treatment or wait-list control. These studies tend to have larger effect sizes. Other studies compare MI plus another treatment to that other treatment alone. The other treatment could be “treatment as usual” or a specific treatment implemented as part of the study protocol. Another set of studies directly compares the effect of MI with a comparison treatment. These studies have yielded the smallest average effect sizes. There is no strong evidence that MI is more effective than other empirically supported treatments, most of which involve significantly greater duration of intervention than MI. In addition, there is significant variability in the MI intervention itself. Interventions have varied from single, unstructured sessions to several sessions of manualized MET, with a range from 15 minutes to 12 hours (6). The intensity of training and fidelity monitoring of the intervention also vary considerably. Finally, there is tremendous variability in the setting, population, and target behavior for which the interventions are tested. Some of these factors appear to have an impact on the effect of MI, as discussed below.
Moderators of effect size
Demographic factors.
Hettema et al. (6) examined the effects of age, ethnicity, gender, and problem severity and found that only ethnicity was significantly related to outcome. Effect sizes were significantly larger in minority samples (d = 0.79) than in white, nonminority samples (d = 0.26). However, not all recent studies have supported this finding (36). It is possible that the empathic, nonjudgmental, nonconfrontational approach of MI is more culturally acceptable than other approaches for some (but not necessarily all) minority groups or that it minimizes the power differential in the treatment relationship, which may be more troublesome to some minority patients.
Target behavior.
Although MI has been applied successfully to many problem behaviors, the evidence remains strongest for drug and alcohol abuse. Although effect sizes for alcohol versus those of other drugs (not including nicotine) appear to be similar, there are many more studies focusing on alcohol, so the weight of the evidence is much greater. However, for marijuana, a recent meta-analysis identified 17 studies focusing on marijuana dependence and reported an average effect size of d = 0.30 relative to no intervention (8). For nicotine dependence, MI has been less consistently successful in trials published to date. However, a recent meta-analysis reported a modest but significant effect (risk reduction 1.27) on quit rates relative to those with brief advice (7). Evidence for other problem behaviors such as HIV infection risk reduction and diet and exercise is largely positive, but more studies are needed.
Characteristics of the intervention.
There are several factors relating to the implementation of MI that seem to make a difference in its efficacy. “Dose” of treatment seems to make some difference, in that effect size is positively related to duration of treatment (6, 8). Hettema et al. (6) noted that treatment effects seem to persist longer in studies in which MI is used in addition to another treatment (often serving as a so-called “motivational induction” at the beginning of treatment). MI delivered in group format has not been shown to be effective and is less effective than treatment that included individual MI sessions (8).
One surprising finding of a recent meta-analysis was that effect sizes were smaller in studies that used manualized versions of MI (or MET) than those using less structured interventions. It has been proposed that pressure to adhere to the manual leads therapists in some cases to push too hard for commitment to a change plan toward the end of the intervention, increasing resistance and undoing the therapeutic effects of the session (37).
Health care settings
Recently there has been great interest in developing, implementing, and evaluating methods to identify and provide appropriate services to non-treatment-seeking individuals with substance use problems who are seen in health care settings such as primary care clinics, emergency departments, and trauma centers. “SBIRT” models have been developed to address this need. These models typically incorporate screening, brief intervention (which generally incorporates MI along with more structured elements such as feedback and change planning), and referral to treatment for patients for whom treatment is indicated.
A number of efficacy and effectiveness trials have demonstrated that screening, brief interventions, and/or referral to specialized treatment for those with alcohol use disorders or hazardous drinking contribute to subsequent reductions in both alcohol consumption and alcohol-related problems and to reduced medical service usage and costs (38–40). Considerably less is known about the effects of such methods for identifying and intervening with individuals using illicit drugs or abusing prescription medications. However, recent data suggest that that the effectiveness of such interventions may extend to drugs as well. In a primary care setting, Bernstein et al. (41) demonstrated significant decreases in heroin and cocaine use, documented by hair testing results, relative to those in individuals receiving usual care. The same group also recently reported significant effects of an SBIRT intervention targeting marijuana use in adolescent and young adult emergency department patients (42).
The large, multisite SBIRT demonstration program funded by the Center for Substance Abuse Treatment included multiple sites in six states, each state using somewhat different screening procedures and methods of intervening with alcohol and drug users across a variety of medical treatment settings including primary care, inpatient, and emergency department settings. Of 459,599 patients screened, 22.7% screened positive for a spectrum of risky/problematic use or abuse/addiction (43). The majority of those screening positive were recommended for a brief intervention (15.9%), with a smaller percentage recommended for brief treatment (3.2%) or referral to specialty treatment (3.7%). Six-month follow-up data were collected on a random 10% subsample of participants who screened positive at baseline. Among those reporting baseline illicit drug use, rates of drug use at the 6-month follow-up were 67.7% lower and heavy alcohol use was 38.6% lower, with comparable findings across sites, gender, race/ethnic, and age subgroups. Subset analyses from Washington State and Houston, Texas, confirmed large reductions in drug use across all levels of severity (44, 45). The findings suggest that implementation of SBIRT activities is feasible in a variety of health care settings and that they contribute to reduced substance use and improved psychosocial function and quality of life.
Psychiatric settings
Relatively little work has been done to determine the effects of MI on substance use in psychiatric patients with dual diagnoses or on treatment adherence in psychiatric patients without substance use disorders. MI has been incorporated into comprehensive outpatient behavioral treatments for patients with dual diagnoses, resulting in substantially improved outcomes (46). However, it is unclear to what degree MI is responsible for this improvement. Graeber et al. (47) reported a large improvement in rates of abstinence among patients with dual diagnoses of serious mental illness (mostly schizophrenia) and substance use disorders who received MI compared with those who received traditional recovery-based treatment (47). MI sessions with psychiatric inpatients seem to increase significantly their rates of follow-up with outpatient treatment, regardless of whether they had a dual diagnosis with an active substance use disorder (48). This latter finding suggests that another potential application of MI in psychiatry is the enhancement of psychiatric treatment adherence in patients with primary psychiatric disorders. Modifications to the usual format of MI have been suggested in working with patients with serious mental illnesses (49), but more work is needed in this area.
RISKY DRINKING: RECOGNITION AND MANAGEMENT IN ROUTINE PSYCHIATRIC PRACTICE
Although alcohol dependence is a familiar concept to psychiatrists, risky drinking may be less familiar. At the very least, established criteria for risky drinking are less well known by psychiatrists, other physicians, and the general public. A brief review of the concept of alcoholism will differentiate the concepts of alcoholism and dependence from the concept of risky drinking and help explain the reason that psychiatrists should routinely address risky drinking in clinical practice.
With the demise of prohibition in the 1930s, new policies were needed to deal with drunkenness (50). The search for a solution shifted away from a broad societal focus on alcohol as a toxic substance to anybody who consumed it toward an individual focus rooted in individual vulnerability, at that time labeled alcoholism. By the 1940s, well after prohibition ended, a confluence of factors highlighted the concept and catapulted it into the popular consciousness. Alcoholism became the focus of a national organization, a research institute, and medical and media attention. On the positive side, this concept increased medical research on the topic and led to more enlightened treatment of alcoholics. On the negative side, by creating a dichotomous entity, alcoholic or not alcoholic, the model had the potential to be misleading. Although people understood alcoholism as a severe and serious disease, they concluded that nonalcoholics do not need to concern themselves with how much they drank. Alcohol, the substance, was no longer the central problem and neither was the environment in which alcohol was served (restaurants and saloons) or the policies that influenced access; the focus shifted to a small number of individuals who were uniquely vulnerable to alcohol—alcoholics.
In this era, research with alcoholics documented that excessive drinking led to a variety of negative consequences, but only among patients who presented for specialized alcohol treatment. Studies of the association between alcohol and harm in the general population were not initiated until the 1960s. The most recent comprehensive, population-based survey shows that 71% of the U.S. civilian, noninstitutionalized population either does not drink or is drinking below the risky drinking threshold (51) (Figure 1). It also shows that a diagnosis of alcohol dependence applies to 3.8% of the population (52). That leaves about 25% of U.S. adults who are not alcohol dependent but drink too much. They have either already experienced alcohol-related harm or, compared with the 71%, are at elevated risk for future alcohol-related harm.

* National Institutes of Health, National Institute on Alcohol Abuse and Alcoholism: Helping Patients Who Drink Too Much: A Clinician's Guide. Updated 2005 Edition. NIH Publication No. 07-3769. Available for download at http://pubs.niaaa.nih.gov/publications/practitioner/cliniciansguide2005/guide.pdf. ** Patients who exceed either the daily or the weekly limits are drinking at risky levels and should be evaluated further and provided an intervention.
Those statistics mean that for each individual in the population who is alcohol dependent, six other individuals drink too much but are not dependent. That 6:1 ratio has enormous implications. First, it suggests that the bulk of alcohol-related harm in the United States is associated with individuals who are not alcoholic or alcohol dependent. Second, it broadens our understanding of the fundamental problematic nature regarding excessive alcohol consumption. If we focus exclusively on the alcohol-dependent portion of the population, we may be ignoring a majority of society's alcohol problems.
What kinds of risks accrue from drinking too much? From the point of view of mental health, the risk of interest is alcohol dependence. Clearly, individuals who pursue risky drinking with focus and intensity are more likely to become dependent. For those individuals, competent practitioners need to know diagnostic criteria. From the point of view of public health, however, the risk is not so singular. Although alcohol dependence can be a severe and disabling disease, it is only one chronic disease outcome associated with drinking too much. Even when risky drinking is not accompanied by dependence, it regularly results in a wide variety of diseases and injuries, ranging from increased accidents (e.g., motor vehicle accidents or accidental overdose) to violence and many chronic diseases (Figure 2).
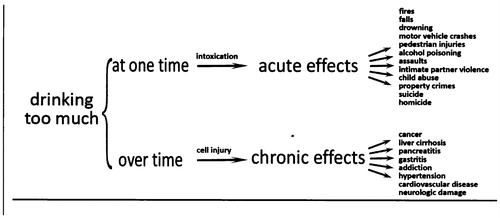
D. Hungerford, personal communication; 2010.
When patients drink too much at one time, i.e., on a single occasion (“binge drinking”), the increased blood alcohol concentration (BAC) affects the brain, leading to impaired judgment, increased risk taking, and decreased muscle control (53). The combination frequently leads to a variety of acute negative consequences. One example of a subacute problem of risky drinking would be fetal alcohol syndrome. From a chronic perspective, when people drink too much over time, cells in a variety of tissues throughout the body are damaged, leading to a variety of chronic diseases.
The National Institute on Alcohol Abuse and Alcoholism (NIAAA) has proposed a risky drinking threshold that accounts for both the acute and chronic effects of alcohol, making it a reasonable cut point for dividing populations into groups that do and do not need an alcohol intervention, brief or otherwise. However, consumption below that threshold should not be considered low-risk drinking. The term moderate drinking is avoided here because it has no uniform definition among health professionals (54) and is widely misunderstood by the public (54–56). The NIAAA based its daily consumption limit at a level that would produce BACs of 0.08 g/dl from a single drinking occasion, clearly a risky level. However, it is also clear that levels just below that threshold, say levels that produced a BAC of 0.06 or 0.07, are also risky. A recent emergency department study found that patients who drank at levels below the NIAAA daily thresholds accounted for 50%–85% of the acute injury visits for falls, transport-related events, and interpersonal violence (57). The U.S. Dietary Guidelines provide a more appropriate upper limit for low-risk drinking for the general population. They recommend up to two drinks a day for healthy men aged ≤65 years and up to one drink a day for healthy women regardless of age and healthy men aged >65 years (58).
There are several reasons for psychiatrists to screen all their patients for risky drinking. Risky drinking may
| 1. | frequently be occult, | ||||
| 2. | interact with medications to produce side effects or influence treatment efficacy, | ||||
| 3. | be a mode of self-medication, | ||||
| 4. | be a confounding factor for common psychiatric consultations, e.g., depression, | ||||
| 5. | be a factor in suicide attempts and suicide, | ||||
| 6. | be more prevalent in psychiatric practices than in the general population and other medical practices, and | ||||
| 7. | be the easiest way to identify patients early who are at the highest risk for future alcohol dependence. | ||||
The goal of screening is to quickly identify whether a patient's alcohol consumption is an issue that needs further evaluation. Although many alcohol screening instruments are available, most of them focus on identifying the DSM diagnoses of alcohol abuse and alcohol dependence and are, therefore, not useful in screening for risky drinking. The three self-report instruments described below are appropriate for identifying risky drinkers. Patients who receive positive screening results are either alcohol dependent or risky drinkers who are not dependent. Therefore, further evaluation will be necessary to identify which patients are dependent and so require more than a brief intervention. All three instruments are supported by research and easy to use.
The Alcohol Use Disorders Identification Test (AUDIT) (see Appendix 1) was developed as part of an international study of alcohol screening and brief intervention sponsored by the World Health Organization (62) and has been extensively studied (63–66). It has 10 self-report questions that measure the level of risk in three domains: alcohol consumption during the past year, alcohol-related harm, and alcohol dependence symptoms. It identifies four groups of people: abstainers and low-risk drinkers, those at risk because they drink excessively, those who have already experienced problems related to their drinking, and those who are likely to be alcohol dependent. The AUDIT is scored on a scale of 0–40 with a score of ≥8 as the most common threshold for a positive result. A manual for using the AUDIT is available online from World Health Organization. The AUDIT can be administered in 2–3 minutes through an interview, by the patient on paper, or by computer.
The AUDIT-C is a shorter instrument and uses the three AUDIT alcohol consumption questions to measure alcohol consumption during the past year (67, 68) (see Appendix 2). It is scored on a scale of 0–12, with scores of 0 indicating no alcohol use in the past year. For men a score ≥4 and for women a score ≥3 is typically considered positive for risky drinking. Higher scores indicate that drinking is more likely to be affecting the patient's health or safety. The instrument and a detailed, useful FAQ is available from the U.S. Department of Veterans Affairs.
The final screening option is a one-question instrument that focuses on binge drinking: “When was the last time you had more than x drinks in 1 day?” (x=5 for men; x=4 for women). Patients who report having had the requisite number of drinks in the last 3 months are considered to have positive results. Although the instrument is short, its validity has been evaluated (69, 70), and NIAAA recommends its use.
MI: RELEVANCE FOR ROUTINE PSYCHIATRIC PRACTICE
In addition to the relevance of MI for the treatment of addictions as well as for the recognition and treatment of risky drinking, considerable evidence is accumulating to support its relevance to routine psychiatric practice. MI can help psychiatrists address common problems of nonadherence in pharmacotherapy and follow-up care and can function as an adjunct to a variety of most other psychotherapies. With the growing awareness of physical problems and increased mortality in psychiatric patients, psychiatrists can also use the principles and practice of MI to help their patients make lifestyle adjustments to decrease general medical risk factors. Improved physical health through lifestyle change can contribute to improved quality of life, self-esteem, and better mental health outcomes.
HOW CAN PSYCHIATRISTS DEVELOP COMPETENCIES IN MI?
For psychiatrists interested in developing skills in MI for routine practice (training information is available at www.motivationalinterviewing.org), it is important to understand that learning to become proficient in MI is complex and time-consuming. Introductory 2- or 3-day workshops typically help practitioners develop the “spirit of MI”; however, these have not proven sufficient for building competencies in the formal techniques and skills of MI (30). Proficiency generally requires several intensive workshops as well as longitudinal individual feedback on cases and practice of skills under the supervision of an expert. The intensity and time commitment required to learn MI in this manner makes it unrealistic to expect most practicing psychiatrists to train to high levels of formal proficiency.
These practical limitations for training psychiatrists (and other health professionals) have led to considerable efforts to develop methods of “brief MI” or “adaptations of MI” (71). To give one example, Resnicow et al. (72) demonstrated that focused and limited training of pediatricians (e.g., 1 day of training plus individual supervision and feedback) was effective for developing motivational skills sufficient to improve outcomes in adolescent obesity. Brief interventions based on the principles of MI have also been shown to be effective in primary care physicians' offices for the treatment of risky drinking (73). With respect to psychiatric practice, however, (with the exception of the studies of the patients with dual diagnoses discussed above), the authors found only three published articles to date and only one with empirical data relating to questions about the use of MI in psychiatric practice or about training psychiatrists or psychiatric residents in MI (48, 74, 75).
BRIEF ACTION PLANNING (B.A.P.): A MOTIVATIONAL INTERVIEWING TOOL FOR ROUTINE PSYCHIATRIC PRACTICE
Brief action planning (B.A.P.) is one application of MI that has been developed and disseminated and may be useful for general medical as well as routine psychiatric practice. Originally developed by the lead author as a self-management support and motivational tool for the Health Resources Services Administration (HSRA)/Institute for Healthcare Improvement10 Health Disparities Collaboratives (http://www.healthcarecommunities.org/), B.A.P. was promoted by the American Medical Association in 2008 (Appendix A, “Physician Tip Sheet for Self-Management Support” in reference 76) and soon will be included by the Commonwealth Fund for its Tool-Kit on the Medical Home. It has been disseminated by programs of the Centers for Disease Control and Prevention, HRSA, the Robert Wood Johnson Foundation, and the Veteran's Administration and used in the Improvement in Patient Care Program of the Indian Health Service. Workshops or courses on B.A.P. have been presented at recent scientific sessions (77–79). Early evidence supporting its efficacy was presented at the First International Conference on Motivational Interviewing (Interlaken 2008) (80) and the Institute of Psychiatric Services (81).
The tool is organized around three core questions and four supporting skills (see Appendix 3), all of which are grounded in the scientific literature of self-management support, stage of change theory, MI, and atheoretical communication and psychotherapy research. B.A.P. has high face validity and is generally well accepted by most practitioners because of its simplicity and practicality.
The basic application of B.A.P. is relatively easy to teach and use. It serves as a useful motivational tool to help many patients, especially those who may be relatively ready, with support and focused encouragement, to make action plans for health. For effectiveness as a motivational tool, however, B.A.P. must be used in a patient-centered manner, aligned with the spirit of MI.
What about those patients who are more ambivalent, more angry, or more refractory to change, those patients with persistent unhealthy behavior? Strategic application of 13 other well-defined communication and MI skills enables practitioners to use B.A.P. effectively with these patients with more complex problems. For these patients, advanced skills can be integrated in a stepped-care manner into the basic B.A.P. template. This stepped-care application of MI is called comprehensive motivational interventions (CMI™). CMI skills are described in a textbook (82, 83) and web-based learning programs (http://www.comprehensiveMI.com).
SUMMARY
MI represents an important and powerful set of behavioral interventions shown to be useful for treating addictions, for treating risky drinking, and for helping psychiatric patients with many other health-related adverse behaviors (e.g., smoking, exercise, poor eating habits, and others). MI has also been shown to be useful for management of medication nonadherence and as an adjunct to other formal psychotherapies.
MI could be a useful addition to the general psychiatrist's skill set for routine practice settings. However, the dedication, cost, and time required to develop proficiency in formal MI make traditional MI training for psychiatrists somewhat impractical.
Psychiatrists interested in learning MI may choose to look for pragmatic adaptations or brief forms of MI to integrate into their routine psychiatric practice. In this paper, the authors present one such model, B.A.P., which has high face validity and is relatively easy to teach and learn.
Appendix 1: AUDIT
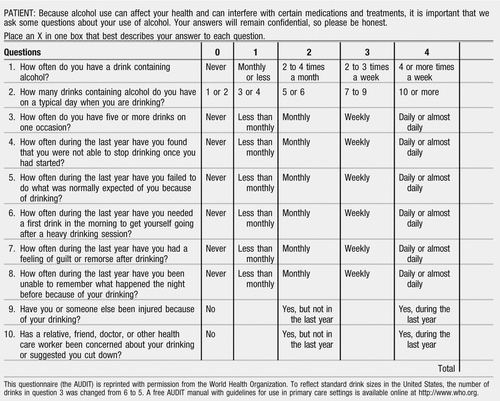 |
PATIENT: Because alcohol use can affect your health and can interfere with certain medications and treatments, it is important that we ask some questions about your use of alcohol. Your answers will remain confidential, so please be honest.
Place an X in one box that best describes your answer to each question.
Appendix 2: AUDIT-C
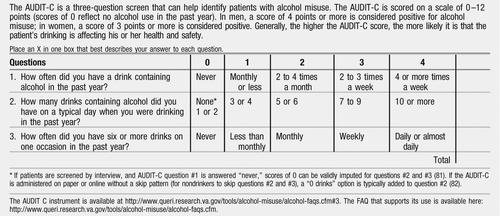 |
The AUDIT-C is a three-question screen that can help identify patients with alcohol misuse. The AUDIT-C is scored on a scale of 0–12 points (scores of 0 reflect no alcohol use in the past year). In men, a score of 4 points or more is considered positive for alcohol misuse; in women, a score of 3 points or more is considered positive. Generally, the higher the AUDIT-C score, the more likely it is that the patient's drinking is affecting his or her health and safety.
Place an X in one box that best describes your answer to each question.
Appendix 3: Brief action planning (B.A.P.)
B.A.P.™ is structured around three core questions:
| 1. | __ Elicit person's preferences/desires for behavior change “Is there anything you would like to do for your health in the next week or two?”* __ What? __ Where? __ When? __ How often? __ Elicit commitment statement “Just to make sure we understand each other, would you please tell me back what you've decided to do?” __ *Some persons need or request ideas for change. Clinicians can offer a behavioral menu: “If you would like, I can share some ideas that might help you feel better….” | ||||
| 2. | __ Evaluate confidence “I wonder how confident you feel about carrying out your plan. Considering a scale of 0 to 10, where ‘0' means you are not at all confident and ‘10' means you are very confident, about how confident do you feel?” __ If the confidence level is <7, problem solve overcoming barriers or adjusting plan: “5 is great. A lot higher than 0. I wonder if there is any way we might modify the plan to get you to a level of 7 or more? Maybe we could make the goal a little easier, or you could ask for help from a friend or family member, or even think of something else that might help you feel more confident?” | ||||
| 3. | __ Arrange follow-up (or accountability) “Sounds like a plan that's going to work for you. When would you like to check in with me to review how you're doing with your plan?” | ||||
BAPTM is a registered trademark of Steven Cole. © 2004, 2010. All rights reserved. May be used in clinical practice, education and research with permission. Stevencolemd@aol.
| 1. | Action planning is individual-centered, i.e., what the person wants, not what he or she is told to do. [This principle demonstrates alignment of B.A.P. with the “spirit” of motivational interviewing: evocation. See Miller and Rollnick S (1).] | ||||
| 2. | Action planning is collaborative. [Spirit of motivational interviewing: collaboration] | ||||
| 3. | Action planning respects the right of the individual to change or not to change. [Spirit of motivational interviewing: support autonomy] | ||||
| 4. | The most effective action plans are ‘SMART' (specific, measurable, achievable, relevant, and timed). | ||||
| 5. | After the plan has been formulated, the clinician/coach elicits a final “commitment statement.” | ||||
| 6. | Offer a behavioral menu when needed or requested. | ||||
| 7. | Confidence levels are elicited and problem-solving utilized for confidence levels less than 7. | ||||
| 8. | Action planning includes arranging follow-up or other accountability. | ||||
| 9. | Question 1 is routinely integrated into chronic care, preventive, coaching, and therapeutic visits. | ||||
1 Miller W: Motivational interviewing with problem drinkers. Behav Psychother 1983; 11:147–172Crossref, Google Scholar
2 Miller WR, Rollnick S: Motivational Interviewing : Preparing People for Change, 2nd ed. New York, Guilford, 2002Google Scholar
3 Miller WR, Rollnick S: Motivational Interviewing : Preparing People to Change Addictive Behavior. New York, Guilford, 1991Google Scholar
4 Rollnick SP, Miller WR, Butler CC: Motivational Interviewing in Health Care : Helping Patients Change Behavior. New York, Guilford, 2008Google Scholar
5 Glynn LH, Moyers TB: Chasing change talk: the clinician's role in evoking client language about change. J Subst Abuse Treat 2010; 39:65–70Crossref, Google Scholar
6 Hettema J, Steele J, Miller WR: Motivational interviewing. Annu Rev Clin Psychol 2005; 1:91–111Crossref, Google Scholar
7 Lai DT, Cahill K, Qin Y, Tang JL: Motivational interviewing for smoking cessation. Cochrane Database Syst Rev 2010; 1:CD006936Google Scholar
8 Lundahl B, Burke BL: The effectiveness and applicability of motivational interviewing: a practice-friendly review of four meta-analyses. J Clin Psychol 2009; 65:1232–1245Crossref, Google Scholar
9 Rubak S, Sandbaek A, Lauritzen T, Christensen B: Motivational interviewing: a systematic review and meta-analysis. Br J Gen Pract 2005; 55:305–312Google Scholar
10 Kopp M: Physical activity in persons with severe mental illness: research-based clinical recommendations. Neuropsychiatry 2009; 23:151–156Google Scholar
11 De Hert M, Dekker JM, Wood D, Kahl KG, Holt RI, Möller HJ: Cardiovascular disease and diabetes in people with severe mental illness position statement from the European Psychiatric Association (EPA), supported by the European Association for the Study of Diabetes (EASD) and the European Society of Cardiology (ESC). Eur Psychiatry 2009; 24:412–424Crossref, Google Scholar
12 Chwastiak LA, Rosenheck RA, McEvoy JP, Stroup TS, Swartz MS, Davis SM, Lieberman JA: The impact of obesity on health care costs among persons with schizophrenia. Gen Hosp Psychiatry 2009; 31:1–7Crossref, Google Scholar
13 von Hausswolff-Juhlin Y, Bjartveit M, Lindstrom E, Jones P: Schizophrenia and physical health problems. Acta Psychiatr Scand Suppl 2009; 15–21Google Scholar
14 Koponen H, Alaräisänen A, Saari K, Pelkonen O, Huikuri H, Raatikainen MJ, Savolainen M, Isohanni M: Schizophrenia and sudden cardiac death: a review. Nord J Psychiatry 2008; 62:342–345Crossref, Google Scholar
15 Miller BJ, Paschall CB 3rd, Svendsen DP: Mortality and medical comorbidity among patients with serious mental illness. Psychiatr Serv 2006; 57:1482–1487Crossref, Google Scholar
16 McDermott S, Moran R, Platt T, Dasari S: Variation in health conditions among groups of adults with disabilities in primary care. J Community Health 2006; 31:147–159Crossref, Google Scholar
17 Goff DC, Cather C, Evins AE, Henderson DC, Freudenreich O, Copeland PM, Bierer M, Duckworth K, Sacks FM: Medical morbidity and mortality in schizophrenia: guidelines for psychiatrists. J Clin Psychiatry 2005; 66:183–194; quiz 147, 273–274Crossref, Google Scholar
18 DiIorio C, McCarty F, Resnicow K, McDonnell Holstad M, Soet J, Yeager K, Sharma SM, Morisky DE, Lundberg B: Using motivational interviewing to promote adherence to antiretroviral medications: a randomized controlled study. AIDS Care 2008; 20:273–283Crossref, Google Scholar
19 Ogedegbe G, Chaplin W, Schoenthaler A, Statman D, Berger D, Richardson T, Phillips E, Spencer J, Allegrante JP: A practice-based trial of motivational interviewing and adherence in hypertensive African Americans. Am J Hypertens 2008; 21:1137–1143Crossref, Google Scholar
20 Solomon DH, Gleeson T, Iversen M, Avorn J, Brookhart MA, Lii J, Losina E, May F, Patrick A, Shrank WH, Katz JN: A blinded randomized controlled trial of motivational interviewing to improve adherence with osteoporosis medications: design of the OPTIMA trial. Osteoporos Int 2010; 21:137–144Crossref, Google Scholar
21 Hannöver W, Röske K, Thyrian JR, Grempler J, Rumpf HJ, Hapke U, John U: Interventions for smoking cessation in pregnancy and postpartum. Modalities, efficacy, introduction to motivational interviewing and social-cognitive models for behaviour changes. Z Geburtshilfe Neonatol 2008; 212:87–93Crossref, Google Scholar
22 Erol S, Erdogan S: Application of a stage based motivational interviewing approach to adolescent smoking cessation: the Transtheoretical Model-based study. Patient Educ Couns 2008; 72:42–48Crossref, Google Scholar
23 Reiner M, Carrard I, Golay A: Motivational interviewing integrated into cognitive behavioral therapy in obesity treatment. Rev Med Suisse 2010; 6:677–681Google Scholar
24 Schwartz RP: Motivational interviewing (patient-centered counseling) to address childhood obesity. Pediatr Ann 2010; 39:154–158Crossref, Google Scholar
25 Flattum C, Friend S, Neumark-Sztainer D, Story M: Motivational interviewing as a component of a school-based obesity prevention program for adolescent girls. J Am Diet Assoc 2009; 109:91–94Crossref, Google Scholar
26 Arkowitz H: Motivational Interviewing in the Treatment of Psychological Problems. New York, Guilford, 2008Google Scholar
27 Arkowitz H, Westra HA: Introduction to the special series on motivational interviewing and psychotherapy. J Clin Psychol 2009; 65:1149–1155Crossref, Google Scholar
28 Cialdini RB: Influence: Science and Practice, 5th ed. Boston, Pearson Education, 2009Google Scholar
29 Goldstein NJ, Martin SJ, Cialdini RB: Yes! 50 Scientifically Proven Ways to Be Persuasive, 1st hardcover ed. New York, Free Press, 2008Google Scholar
30 Miller WR, Rose GS: Toward a theory of motivational interviewing. Am Psychol 2009; 64:527–537Crossref, Google Scholar
31 Rollnick S, Butler CC, Kinnersley P, Gregory J, Mash B: Motivational interviewing. BMJ 2010; 340:c1900Crossref, Google Scholar
32 Anstiss T: Motivational interviewing in primary care. J Clin Psychol Med Settings 2009; 16:87–93Crossref, Google Scholar
33 Dunn C, Deroo L, Rivara FP: The use of brief interventions adapted from motivational interviewing across behavioral domains: a systematic review. Addiction 2001; 96:1725–1742Crossref, Google Scholar
34 Burke BL, Arkowitz H, Menchola M: The efficacy of motivational interviewing: a meta-analysis of controlled clinical trials. J Consult Clin Psychol 2003; 71:843–861Crossref, Google Scholar
35 Vasilaki EI, Hosier SG, Cox WM: The efficacy of motivational interviewing as a brief intervention for excessive drinking: a meta-analytic review. Alcohol Alcohol 2006; 41:328–335Crossref, Google Scholar
36 Arroyo JA, Miller WR, Tonigan JS: The influence of Hispanic ethnicity on long-term outcome in three alcohol-treatment modalities. J Stud Alcohol 2003; 64:98–104Crossref, Google Scholar
37 Amrhein PC, Miller WR, Yahne CE, Palmer M, Fulcher L: Client commitment language during motivational interviewing predicts drug use outcomes. J Consult Clin Psychol 2003; 71:862–878Crossref, Google Scholar
38 Gentilello LM, Rivara FP, Donovan DM, Jurkovich GJ, Daranciang E, Dunn CW, Villaveces A, Copass M, Ries RR: Alcohol interventions in a trauma center as a means of reducing the risk of injury recurrence. Ann Surg 1999; 230:473–480; discussion 80–83Crossref, Google Scholar
39 The impact of screening, brief intervention, and referral for treatment on emergency department patients' alcohol use. Ann Emerg Med 2007; 50:699–710; e1–6Crossref, Google Scholar
40 Saitz R: Screening and brief intervention enter their 5th decade. Subst Abus 2007; 28:3–6Crossref, Google Scholar
41 Bernstein J, Bernstein E, Tassiopoulos K, Heeren T, Levenson S, Hingson R: Brief motivational intervention at a clinic visit reduces cocaine and heroin use. Drug Alcohol Depend 2005; 77:49–59Crossref, Google Scholar
42 Bernstein E, Edwards E, Dorfman D, Heeren T, Bliss C, Bernstein J: Screening and brief intervention to reduce marijuana use among youth and young adults in a pediatric emergency department. Acad Emerg Med 2009; 16:1174–1185Crossref, Google Scholar
43 Madras BK, Compton WM, Avula D, Stegbauer T, Stein JB, Clark HW; Screening, brief interventions, referral to treatment (SBIRT) for illicit drug and alcohol use at multiple healthcare sites: comparison at intake and 6 months later. Drug Alcohol Depend 2009; 99:280–295Crossref, Google Scholar
44 Estee S, He L, Yang S, Doane J, Shah MF: Use of alcohol and other drugs declined among emergency department patients who received brief interventions for substance use disorders through WASBIRT. Olympia, Washington State Department of Social and Health Services, 2007Google Scholar
45 InSight Project Research Group: SBIRT outcomes in Houston: final report on InSight, a hospital district-based program for patients at risk for alcohol or drug use problems. Alcohol Clin Exp Res 2009; 33:1374–1381Crossref, Google Scholar
46 Bellack AS, Bennett ME, Gearon JS, Brown CH, Yang Y: A randomized clinical trial of a new behavioral treatment for drug abuse in people with severe and persistent mental illness. Arch Gen Psychiatry 2006; 63:426–432Crossref, Google Scholar
47 Graeber DA, Moyers TB, Griffith G, Guajardo E, Tonigan S: A pilot study comparing motivational interviewing and an educational intervention in patients with schizophrenia and alcohol use disorders. Community Ment Health J 2003; 39:189–202Crossref, Google Scholar
48 Swanson AJ, Pantalon MV, Cohen KR: Motivational interviewing and treatment adherence among psychiatric and dually diagnosed patients. J Nerv Ment Dis 1999; 187:630–635Crossref, Google Scholar
49 Martino S, Carroll K, Kostas D, Perkins J, Rounsaville B: Dual diagnosis motivational interviewing: a modification of motivational interviewing for substance-abusing patients with psychotic disorders. J Subst Abuse Treat 2002; 23:297–308Crossref, Google Scholar
50 Edwards G. Alcohol: The Ambiguous Molecule. New York, Penguin Books, 2000Google Scholar
51 Dawson DA, Grant BF, Stinson FS, Chou PS: Toward the attainment of low-risk drinking goals: a 10-year progress report. Alcohol Clin Exp Res 2004; 28:1371–1378Crossref, Google Scholar
52 Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP: The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend 2004; 74:223–234Crossref, Google Scholar
53 Babor T: Alcohol: No Ordinary Commodity: Research and Public Policy. Oxford, UK: Oxford University Press, 2003Google Scholar
54 Abel EL, Kruger ML, Friedl J: How do physicians define “light,” “moderate,” and “heavy” drinking? Alcohol Clin Exp Res 1998; 22:979–984Crossref, Google Scholar
55 Green CA, Polen MR, Janoff SL, Castleton DK, Perrin NA: “Not getting tanked”: definitions of moderate drinking and their health implications. Drug Alcohol Depend 2007; 86:265–273Crossref, Google Scholar
56 Mukamal KJ, Phillips RS, Mittleman MA: Beliefs, motivations, and opinions about moderate drinking: a cross-sectional survey. Fam Med 2008; 40:188–195Google Scholar
57 Kuendig H, Hasselberg M, Laflamme L, Daeppen JB, Gmel G: Acute alcohol consumption and injury: risk associations and attributable fractions for different injury mechanisms. J Stud Alcohol Drugs 2008; 69:218–226Crossref, Google Scholar
58 US Department of Health and Human Services, US Department of Agriculture, US Dietary Guidelines Advisory Committee: Dietary Guidelines for Americans, 2005. [6th Ed. Washington, D.C.: G.P.O.; 2005Google Scholar
59 Goldstein MG, Whitlock EP, DePue J: Multiple behavioral risk factor interventions in primary care. Summary of research evidence. Am J Prev Med 2004; 27(2 suppl):61–79Crossref, Google Scholar
60 Whitlock EP, Polen MR, Green CA, Orleans T, Klein J: Behavioral counseling interventions in primary care to reduce risky/harmful alcohol use by adults: a summary of the evidence for the U.S. Preventive Services Task Force. Ann Intern Med 2004; 140:557–568Crossref, Google Scholar
61 Screening and behavioral counseling interventions in primary care to reduce alcohol misuse: recommendation statement. Ann Intern Med 2004; 140:554–556Crossref, Google Scholar
62 Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M: Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction 1993; 88:791–804Crossref, Google Scholar
63 Reinert DF, Allen JP: The alcohol use disorders identification test: an update of research findings. Alcohol Clin Exp Res 2007; 31:185–199Crossref, Google Scholar
64 Aalto M, Alho H, Halme JT, Seppä K: AUDIT and its abbreviated versions in detecting heavy and binge drinking in a general population survey. Drug Alcohol Depend 2009; 103:25–29Crossref, Google Scholar
65 de Torres LA, Rebollo EM, Ruiz-Moral R, Fernández-García JA, Vega RA, Palomino MM: Diagnostic usefulness of the Alcohol Use Disorders Identification Test (AUDIT) questionnaire for the detection of hazardous drinking and dependence on alcohol among Spanish patients. Eur J Gen Pract 2009; 15:15–21Crossref, Google Scholar
66 Kokotailo PK, Egan J, Gangnon R, Brown D, Mundt M, Fleming M: Validity of the alcohol use disorders identification test in college students. Alcohol Clin Exp Res 2004; 28:914–920Crossref, Google Scholar
67 Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR: AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res 2007; 31:1208–1217Crossref, Google Scholar
68 Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA: The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 1998; 158:1789–1795Crossref, Google Scholar
69 Canagasaby A, Vinson DC: Screening for hazardous or harmful drinking using one or two quantity-frequency questions. Alcohol Alcohol 2005; 40:208–213Crossref, Google Scholar
70 Williams R, Vinson DC: Validation of a single screening question for problem drinking. J Fam Pract 2001; 50:307–312Google Scholar
71 Soderlund LL, Madson MB, Rubak S, Nilsen P: A systematic review of motivational interviewing training for general health care practitioners. Patient Educ Couns (in press)Google Scholar
72 Resnicow K, Davis R, Rollnick S: Motivational interviewing for pediatric obesity: conceptual issues and evidence review. J Am Diet Assoc 2006; 106:2024–2033Crossref, Google Scholar
73 Solberg LI, Maciosek MV, Edwards NM: Primary care intervention to reduce alcohol misuse ranking its health impact and cost effectiveness. Am J Prev Med 2008; 34:143–152Crossref, Google Scholar
74 Chanut F, Brown TG, Dongier M: Motivational interviewing and clinical psychiatry. Can J Psychiatry 2005; 50:548–554Crossref, Google Scholar
75 Renner JA Jr: How to train residents to identify and treat dual diagnosis patients. Biol Psychiatry 2004; 56:810–816Crossref, Google Scholar
76 American Medical Association: Physician Resource Guide to Patient Self-Management Support. Chicago, American Medical Association, 2008Google Scholar
77 Cole SV: Depression Management Using PHQ9, in
78 Cole SV: Motivational Interviewing for Routine Psychiatric Practice, in
79 Cole SV: in
80 Cole S, Waxenberg F, McCarthy D, McClure T, Lee R: Ultra-brief personal action planning (UB-PAP) and motivational interviewing: a prospective, controlled pilot efficacy study of stepped-care health coaching, in
81 Cole S, Blader J: UB-PAP (ultra-brief personal action planning): an innovative tool to support patient self-management, motivate healthy behavior change and improve adherence, in
82 Cole S, Bird J: The Medical Interview: The Three Function Approach, 3rd ed. St. Louis, MO, Elsevier (in press)Google Scholar
83 Cole S Bird J. The Medical Interview: The Three Function Approach, 2nd ed. St. Louis, MO, Elsevier, 2000Google Scholar
84 Bradley KA, DeBenedetti AF, Volk RJ, Williams EC, Frank D, Kivlahan DR: AUDIT-C as a brief screen for alcohol misuse in primary care. Alcohol Clin Exp Res 2007; 31:1208–1217Crossref, Google Scholar
85 Bush K, Kivlahan DR, McDonell MB, Fihn SD, Bradley KA: The AUDIT alcohol consumption questions (AUDIT-C): an effective brief screening test for problem drinking. Ambulatory Care Quality Improvement Project (ACQUIP). Alcohol Use Disorders Identification Test. Arch Intern Med 1998; 158:1789–1795Crossref, Google Scholar



