Assessment and Treatment of Substance Use Disorders
Abstract
This is an overview of practices in addiction psychiatry. In particular, it refers to problems inherent in diagnosing and treating patients with alcohol and other substance use disorders. A proper assessment of substance use has the potential to decrease morbidity and mortality in those who are dependent on substances, and in those whose misuse leads to unsafe health and social practices. This article presents suggestions for working with patients who erect barriers to discussions about their substance use. There are tools clinicians can integrate into their practices that may simplify these discussions. New interventions have joined time-tested drug and alcohol treatments, in part as an outgrowth of the scientific understanding of addictions.
DEFINITION
The National Institute on Drug Abuse (NIDA) defines addiction as a chronic, often relapsing brain disease that causes compulsive drug seeking and use despite harmful consequences to the individuals who are addicted and to those around them (1). Among those who misuse substances, patients with chronic and relapsing use may be the easiest for physicians to identify. Frequently, there are negative consequences that have begun to spill into one or more life areas. Even so, it may be difficult to talk to these patients about their use and the trouble it creates in their lives. Not only is it important to identify patients with substance use disorders, but also there is a growing emphasis on identifying those with risky patterns of use. The aims are to prevent the development of an alcohol or drug use disorder and to thwart substance-related morbidity. NIDA defines addiction as follows:
Addiction is a chronic, often relapsing brain disease that causes compulsive drug seeking and use despite harmful consequences to the individuals who are addicted and to those around them.
This definition highlights the scientific underpinnings of addiction as well as behavioral, psychological, and social ramifications. Decades of crafting and refining definitions preceded this one. At center are people who continue to use substances even though it causes trouble for them. This definition recognizes that other people are affected, a fact that is not often highlighted in this way. The work to define alcohol dependence has helped clinicians think about other substance use disorders. Nearly 35 years ago, Edwards and Gross (2) introduced the term “alcohol dependence syndrome.” They made a significant contribution with an emphasis on criteria that pointed toward a spectrum of severity. Most of the criteria they set forth are stated or implied in the DSM-IV-TR.
In content, the NIDA definition and the DSM-IV-TR (Table 1) criteria are related. The physical manifestations of tolerance and withdrawal cited in DSM-IV-TR result from chronic, excessive use of a substance, as described in the NIDA definition. The next three DSM-IV-TR criteria refer to the NIDA-defined compulsive drug seeking: using more of the substance or for longer periods of time than intended, the desire to discontinue one's use or efforts to control one's use, and spending a great deal of time on activities related to one's use. The last two criteria, giving up important activities because of substance use and continued use despite consequences, are examples of compulsive drug seeking and of being unable to control using despite harmful consequences of the addiction. Addiction erodes the user's values and priorities. Another way one may think about substance dependence addresses patients' arguments that they can control their use. They recite instances of stopping for periods of time or limiting use for a particular occasion. Yet their statements fail to prove they are normal users because even though they are able to stop or reduce their intake, when they use their substance of choice, they cannot trust themselves. Their use is unpredictable in its timing, in the amount used, or in the consequences they experience. A salient point in understanding behaviors associated with addictions is the inability to predictably control one's use.
 |
Table 1. DSM-IV-TR Diagnostic Criteria for Substance Dependence and Abuse
SUBSTANCE USE AND AFFECTIVE STATES
In 1985, Khantzian (3) proposed the “self-medication hypothesis.” The idea that some patients use substances to deal with uncomfortable affective states was not new; however, publication of the hypothesis energized discussions about the impact that numbing negative affects could have on the development of addiction. Although substances can be a salve for unpleasant affective states, it is only one of the contributors to substance dependence. The following is an example of how medicating negative affects with alcohol or drugs might lead to substance dependence: an anxious patient begins to use alcohol before attending social functions to ease his or her tension; once alcohol is determined to be reliable in erasing negative affects, it is used in other situations to relieve emotionally painful states. Dawson et al. (4) showed that the successful navigation of transitional life events such as graduating from college, getting married, and gaining employment effect recovery, whereas failure to manage major life events has a negative impact on recovery.
The connection between affective states and substance use disorders is clear in patients with comorbidity. The term “dual diagnosis” frequently is applied to this concept; however, because the prefix “co” means “with” or “together,” the words “comorbidity” or “cooccurring disorders” more accurately reflect the conditions of those who have two or more interacting entities. A patient may have a myriad of psychiatric and medical disorders as well as a set of social problems. To assess such patients, clinicians try to untangle the interplay of one diagnosis on another.
Psychiatric disorders occur in patients with substance use disorders so frequently that every patient must be carefully assessed for comorbidity. Although some axis I and II psychiatric disorders are comorbid with substance use disorders more frequently than others, evaluations of all patients must be thorough.
Affective states contribute to some cases of substance use disorder and may have a role in perpetuating the course of addiction. However, those with substance use disorders are a heterogeneous group of people with complex etiologies. As scientific advances contribute to the field's growing knowledge, the multifaceted and intricate nature of addiction will probably become increasingly apparent.
EPIDEMIOLOGY
From 2001 to 2005, the National Institute on Alcohol and Alcohol Abuse (NIAAA) conducted the National Epidemiologic Survey on Alcohol and Related Conditions (NESARC) (5), the largest and one of the most comprehensive epidemiological studies on alcohol and other drug addiction. More than 43,000 people were surveyed for current and previous use of alcohol and other substances, comorbid conditions, and treatment services. Designers of the study included safeguards to help ensure that the sample adequately surveyed minorities. Among adults in the United States, 30% are estimated to be at risk for problems related to the use of alcohol; further, 25% of that group most likely have an alcohol use disorder. According to NESARC, 17.6 million people (8.46%) have an alcohol use disorder as defined in APA criteria (6).
NESARC provides a data set that researchers will glean for many years in an effort to understand how substance use, comorbidity, and treatment services impact patients. Not only did the study inquire about the ways respondents used alcohol and utilized treatment services, it made similar inquiries about the use of tobacco and illicit drugs. In addition to those with an alcohol abuse disorder, 2% of the population met the criteria for the abuse or dependence on an illicit drug, and 1.1% met the criteria for both a comorbid alcohol use disorder and an illicit drug use disorder (6, 7).
When NESARC was compared with its predecessor survey, the 1991–1992 National Longitudinal Alcohol Epidemiologic Survey (NLAES), NESCAR (2001–2002) showed that the number of people with an alcohol use disorder had increased by greater than 3 million individuals, more of whom abused alcohol than were dependent on it. This was a change from the previous decade. Of note, by 2002 higher rates of alcohol abuse were observed in young blacks and young Hispanics of both sexes. Rates of dependence rose among Asian men and young black women (8).
ASSESSMENT
The interview
Many patients who misuse substances present challenges in identification, diagnosis, or intervention. Some clinicians are uncomfortable dealing with these challenges. Motivation may come from the knowledge that a substance use disorder is a brain disease, similar to other familiar disorders. In addition, patients respond to treatment. In an analysis of NESARC data, Dawson et al. (4) found that among survey respondents who had met the alcohol dependence criteria for more than 1 year, nearly one-fifth were abstinent. Approximately one-half remained dependent or had only attained partial recovery. The remainder drank in either low-risk or at-risk amounts.
The psychiatrist's training in nonjudgmental support can be useful when working with addicted patients. The first evaluation is typically the best time to ask about substance use, as a part of the routine history. At some point the clinician must find out the patient's pattern of use and obtain an estimate of the amount he or she uses. Further inquiries include the age at which the patient began using each substance, information about the patient's peak use, whether the patient has stopped or has limited use, a description of withdrawal symptoms, and the episodes of detoxification or treatment. This line of questioning can raise defensiveness.
Starting with past use and associated events may ease the transition into questions about the present. Questions about spousal concern may allow the patient to divert attention to another. Furthermore, fact-based questions are easy to answer: the patient has either been involved in a treatment program or has not or has had a charge for driving under the influence or has not. Behaviorally oriented questions such as those in the DSM-IV-TR that refer to consequences of one's use may also be more palatable early in an interview (9, p. 47).
Collateral sources of information, such as family members and previous care providers, may be consulted if it seems prudent to gather additional information. Even though the patient does not often relate social or medical ills to substance use, clinicians can be aware of signs that point toward substance use. Table 2 shows a list of common signs. Domestic life is often the first area that is disrupted in the context of substance-related disorders. Social relationships, finances, work or school, and legal problems are other life areas that may be affected. In addition, the psychiatrist may witness a decline in the patient's condition or an inadequate response to psychiatric interventions. “Red flag” medical complaints include headaches, insomnia, erectile dysfunction, hypertension, and frequent accidents.
 |
Table 2. Common Signs That May Signal Substance Use Disorder
Drinking patterns in the United States
Information about U.S. drinking patterns can be an effective way for clinicians to show patients how their drinking patterns compare with those of others. Doing this at the first appointment indicates to the patient that he or she is not targeted for questions about drinking. To determine a patient's level of risk, NIAAA suggests asking the following three questions (10):
| 1. | How many days per week do you drink? | ||||
| 2. | How many drinks do you have on a typical drinking day? | ||||
| 3. | What is the maximum number of drinks you had on any given occasion during the last month? | ||||
Approximately 30% of patients are drinking “at risk.” Approximately 10% are among the heaviest drinkers (Table 3). The patient with an alcohol problem may believe that most people drink in a similar manner. Proof that this is a fallacy can be a powerful educational tool. If not initially, the clinician may later discuss health problems that result from heavy drinking. Table 4 shows the NIAAA-defined Maximum Drinking Limits For Healthy Adults (10). Both patients and clinicians can refer to the amount of alcohol consumed by referring to the “standard drink.” When patients tell a clinician the number of drinks consumed, the clinician should be certain both are using the same definition. NIAAA defines a standard drink as “12 oz of beer, or 5 oz of wine, or 1.5 oz of 80-proof spirits.”
 |
Table 3. Maximum Drinking Limits for Healthy Adults*
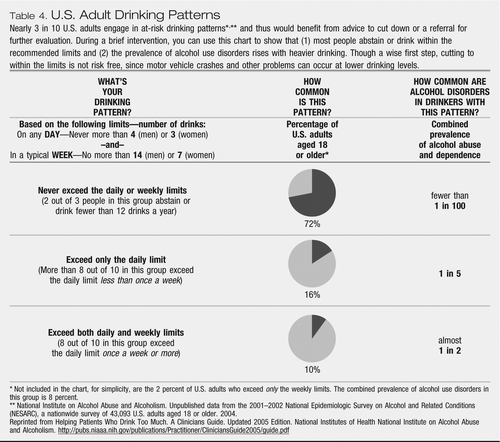 |
Table 4. U.S. Adult Drinking Patterns
Nearly 3 in 10 U.S. adults engage in at-risk drinking patterns*,** and thus would benefit from advice to cut down or a referral for further evaluation. During a brief intervention, you can use this chart to show that (1) most people abstain or drink within the recommended limits and (2) the prevalence of alcohol use disorders rises with heavier drinking. Though a wise first step, cutting to within the limits is not risk free, since motor vehicle crashes and other problems can occur at lower drinking levels.
Screening tools
Screening tools are not diagnostic; however, they are designed to identify individuals for whom further assessment is recommended. As such, sometimes they are used before the formal interview; at other times, they are woven into it. Screening instruments differ from the DSM-IV-TR criteria. The purpose is to establish a diagnosis for an individual suspected of having a substance use disorder.
The majority of patients have a positive attitude about screening (11). A positive screen indicates that more specific testing is needed to determine the presence or absence of disease. The ideal test should have high sensitivity, the ability to detect people who have the disorder; it should also have high specificity, the ability to exclude those without the disorder. A noninvasive test is ideal for screening populations. More than 100 screening tools are available to help determine whether or not a patient has a substance use disorder, although none has been proven to be successful as a diagnostic tool (12). Most of these were developed to screen for alcoholism; however, similar questions may be applied to drug use.
The CAGE is a four-question screening instrument that has gained widespread use because of its brevity and the ease with which it can be integrated into the patient history. Its acronym makes it simple to remember. It has been adapted for use in special populations, including use in screening for substances other than alcohol and in obstetrics. The questions ask the patient about having felt the need to cut down, feeling annoyed because of criticism about drinking, feeling guilty because of alcohol use, or ever having an eye-opener in the morning. Studies have borne out its utility, especially among moderately to severely impaired alcoholics (13). Two “yes” responses are considered a positive result, indicating the need for further inquiry. Studies have shown that the CAGE is relatively sensitive and specific in identifying those with a substance use disorder (14, 15). Although it may miss identifying patients who have a mild disorder (16).
CAGE-AID, adapted to include drugs, and DAST, the Drug Abuse Screening Test, are specifically for those with suspected illicit drug use (17). Another resource is the Alcohol, Smoking, and Substance Involvement Screening Test (ASSIST), available on the NIDA Web site (18). This questionnaire may be clinician-administered or computer-based. An affirmative response to prescreening questions about whether one has used drugs from any class gives way to questions about quantities and consequences, allowing the physician to determine level of use (19).
Other questionnaires that are frequently used and that can be easily scored are the Alcohol Use Disorders Identification Test (AUDIT) (20, 21) and the Michigan Alcohol Screening Test (MAST) (22). AUDIT is a 10-item questionnaire that has shown a high level of validity and reliability. It is more quantitative than the CAGE. It takes about 5 minutes to complete; thus, some clinicians like it because the patient can fill it out in the office beforehand. MAST is more qualitative, asks 25 questions, and is particularly useful for identifying alcohol dependence.
Other screening tests commonly used include the T-ACE (23) and the TWEAK (24), developed for use in pregnant women. The T-ACE, modeled on the CAGE, replaces the question that refers to guilt with one on tolerance, “How many drinks does it take to make you feel high?” An answer of more than two drinks is considered tolerance in the T-ACE. This is different from establishing the presence or absence of tolerance using DSM-IV-TR criteria, which is a qualitative rather than a quantitative assessment. Questions about tolerance are not thought to be as emotionally laden as those about guilt. Each question is 1 point, except the question on tolerance, which is 2 points. A score of 2 is required for a positive test. Researchers validated the T-ACE and the TWEAK, finding that each was more sensitive than the CAGE in pregnant women (24–26). The TWEAK borrows from the CAGE, T-ACE, and MAST.
Laboratory assessment
Most drugs are metabolized in the liver, with alcohol being notorious for causing hepatitis or cirrhosis. Even in the absence of disease, the liver may show signs of overwork, in which case abnormal liver function test results are found. An elevated γ-glutamyltransferase (GGT) level signals physicians that alcohol may be a problem. Another organ system susceptible to damage from excessive drinking is the bone marrow. Toxicity may result in an elevated mean corpuscular volume (MCV). Neither test alone is accurate enough to be used routinely to screen for alcohol dependence (12). However, the combination of elevations in GGT level and MCV is more suggestive of alcohol as a cause for the abnormal laboratory results than elevations in either result alone. Values for triglyceride and uric acid levels may also be elevated. A combination of abnormal laboratory findings that suggest organ toxicity related to alcohol should make the clinician suspicious of an alcohol use disorder (9, p. 51).
The presence of carbohydrate-deficient transferrin indicates recent heavy alcohol consumption. Normally, carbohydrates bind to transferrin in the liver; however, heavy drinking prevents the addition of carbohydrates. The test results normalize within 2–4 weeks of abstinence. Positive test results can be expected if the subject consumes at least 60 g (five drinks) of alcohol per day for 2 weeks, often before there are other signs of relapse (27). Its usefulness appears to be greatest as a tool to monitor relapse in heavily drinking men (28).
Drug tests are commonly used if the clinician suspects substance use. However, interpretation of the test depends on when the patient last used the drug and the length of time the drug is detectable. Some substances are rapidly cleared from the body; cocaine is a notorious example. On the other hand, a heavy user of cannabis may test positive for 3 or 4 weeks. Clinicians who order routine urine drug tests cannot assume that the test will pick up all illicit substances. A call to the testing laboratory can clarify which substances are included. If a situation demands a high level of accuracy, a gas chromatography-mass spectrometry analysis can be ordered. For instance, gas chromatography-mass spectrometry analysis is ordered in employee screening or when a treatment recommendation depends on the test (9, p. 176).
TREATMENT
General approaches
It may surprise some clinicians that substance-abusing patients are as compliant with treatment as most other patients with chronic illnesses such as asthma, diabetes, or hypertension (29). One can extend the analogy to include schizophrenia and affective disorders. When clinicians view substance abuse as a chronic disease, the logical approach becomes management.
A treatment program typically addresses a number of issues affected by one's addiction. Family therapy is often indicated. Psychotherapy is nearly always part of the recovery program, often in the form of group therapy. Many programs incorporate 12-step groups. Increasingly, treatment includes pharmacotherapy to target the addiction. The goal for most patients with substance dependence is abstinence. However, both short-term and long-term treatment goals depend on the severity of the substance use disorder, the presence of a psychiatric disorder, and the patient's medical condition. Initially, some patients with a substance use disorder cannot agree to long-term abstinence; an argument before detoxification or before they are engaged in the recovery process may lead to termination of the therapy. For them, a more effective approach is to focus on the first steps of the process rather than on what the patient must do in the coming months or years. Thinking long term is often overwhelming to the patient who cannot imagine being without the substance of choice.
Most patients are treated outside of the hospital. Outpatient programs range from the least restrictive level of care, rendered on an individual basis or in group counseling, to partial hospital programs. Outpatient treatment requires the patient to sustain abstinence long enough for the brain to recover so the patient can engage in treatment. Other considerations as to the appropriateness of outpatient care include withdrawal symptoms, support within the home and social environment, and stability of psychiatric and medical problems. Residential programs that occur outside of hospitals are designed for people who cannot achieve abstinence as outpatients but do not meet the criteria for hospitalization. Hospital-level care is most often indicated for those who are psychiatrically unstable or those at risk for severe or complicated withdrawal (30).
Psychotherapies
Self-help groups.
Twelve-step groups are a mainstay of contemporary addiction treatment. Alcoholics Anonymous (AA) chapters have proliferated throughout the United States and abroad, since William Wilson, Bill W., and Dr. Robert Smith, Dr. Bob, met in 1935. In 2010 TIME Magazine listed Mr. Wilson as one of the 100 most influential people of the century which was recognition not only of Bill Wilson but also of AA and other self-help groups that have become part of our culture (31).
Among those who receive a treatment service, 80% use AA as part of their recovery program (32). Although some patients' rejection of AA is part of their resistance, AA is not a fit for everyone. Chapters differ depending on the makeup of the groups, so it may be helpful to suggest that a patient try several groups before declining AA attendance. A common objection is the spiritual nature of AA and its emphasis on a “higher power.” Encouragement to broaden the concept beyond the traditional God may work for some; alternatively, a group such as Women for Sobriety, Smart Recovery, or Secular Organizations for Sobriety may be recommended. Others have objections that should be explored. Because AA is widespread, it has the advantage of easy access to meetings. Self-help groups for people with other drug addictions, such as Narcotics Anonymous, Cocaine Anonymous, and Marijuana Anonymous are modeled on AA. Little data on the efficacy of these groups exist; however, patients and clinicians continue to support self-help groups as a mainstay of treatment.
Behavioral therapy.
Behavioral therapies teach patients techniques to override the internal and external cues that move them toward their substances of choice. Cognitive behavior therapy (CBT) is one such therapy. Its aim is to teach patients to look at the rewards as well as the negative consequences of using. Patients study the sensibility of their choices. When they can recognize triggers to use, patients learn to anticipate and avoid situations that lead to using. Evidence supports its efficacy in the treatment of alcohol, cannabis, cocaine, methamphetamine, and nicotine abuse (33).
Contingency management interventions allow patients to earn inexpensive items in exchange for drug-free urine samples. This form of treatment has been effective for treatment retention and for helping patients remain drug-free after treatment. Studies have shown its usefulness in addictions to alcohol, stimulants, opioids, marijuana, and nicotine (34, 35). The community reinforcement approach is based on the principle that substance use is perpetuated because the patient's environment contains inadequate reinforcers. This approach encourages use of natural reinforcers that come from the family or social community (36, 37).
Motivational enhancement therapy.
The goal of motivational enhancement therapy (MET) is to move the patient closer to abstinence by exploring his or her ambivalence about change. The effectiveness of MET varies, depending on the type of substance the patient uses. Treatment for marijuana-dependent patients appears to be more successful when MET and CBT are combined. However, for other drugs (including heroin, cocaine, and nicotine) results are mixed (38). Evidence shows the effectiveness of MET in motivating alcohol-dependent patients to engage in treatment and to assist them in maintaining abstinence (39).
Motivational strategies share similarities with the tools psychiatrists use in supportive psychotherapy. Motivational interviewing includes the following techniques: giving advice, working on solutions to tangible problems that relate to one's sobriety, comparing the desirability of change with one's failure to act, practicing empathy, providing feedback, clarifying goals, and active helping, such as assisting a patient arrange for a medical appointment while he or she is still in the office (40).
The readiness for change model (precontemplation, contemplation, determination, action, and maintenance) helps to inform the clinician as to the motivational techniques that may be most beneficial to the patient (41). Patients in the precontemplation stage may be thought of as being in deep denial. These individuals may not realize there are problems or that those problems rest with them. For these patients the doctor might initially use an educational approach, such as comparing their use with that of others in the population by citing data on U.S. drinking patterns. In the contemplation stage, the individual struggles with the ideas of changing his or her behavior. The doctor may explore the patient's ambivalence by facilitating discussion about the gratification that using brings and about the patient's rationale for wanting to stop. In the determination stage the patient has made a decision. The clinician's role is to reinforce this resolve. Then the patient takes action and finally maintains the change. However, these stages do not necessarily flow smoothly from one to another, and the rate at which movement occurs is unpredictable.
Other therapies.
Couples therapies and family therapies have had a place in treatment programs for many years. Adolescents have unique needs, and most programs recognize the place for family therapy. An example of a successful form of family work is multisystemic therapy (MST), which addresses the adolescent's antisocial behaviors and substance abuse. MST occurs in a familiar environment, and treatment retention is high (42).
Brief interventions can be efficacious in reducing the misuse of alcohol in outpatients. The goal is to moderate a person's consumption and eliminate harmful drinking practices (43). There is hope that integration of brief interventions into primary care practices proves comfortable for patients, will reach large numbers of patients, and that the interventions will be easily administered to patients. The increased use of such interventions in emergency departments, in prenatal clinics, in psychiatric offices, and on college campuses holds the promise of reducing morbidity and mortality. Most studies have been conducted in outpatient settings. A recent study demonstrated that medical inpatients who misuse alcohol may benefit as well. Those receiving the most benefit tended to be younger adults, women, and individuals with alcohol abuse rather than dependence. The study also raised the possibility that patients who abuse drugs are candidates for brief intervention (44).
Brief therapy generally consists of fewer than five sessions that provide feedback about the individual's use of alcohol and his or her responsibility for change. More studies are needed about the populations that benefit from this form of therapy. There are questions about how to define a brief intervention (43). Unfortunately, the lack of a standard definition hinders research studies and their translation into practice.
An example of a brief intervention is a trial of low-risk drinking that adheres to the NIAAA guidelines for daily and weekly consumption in patients who are at-risk drinkers but who are less severely affected by alcohol. Along with a low-risk strategy, the patient would be warned about risky behaviors, such as drinking when driving and maintaining abstinence during pregnancy.
Pharmacotherapy
Medications used in the treatment of addictions are increasing in importance as more is learned about which ones to use and in whom to use them. Despite the growth in the number of effective medications, none will supplant the usefulness of psychotherapy or social attention in the foreseeable future.
The accelerated growth in pharmacotherapeutics may be attributed to scientific advancements, primarily in understanding the neurobiology of addiction. The length of time between the marketing of medications that target substance dependence has been shortening over the past 50 years. The best example is the treatment of alcohol dependence in the United States. Disulfiram (Antabuse) became available in 1951, followed by naltrexone (Revia) 40 years later and acamprosate (Campral) followed only 10 years after naltrexone. A similar pattern emerges for medications developed for both nicotine and for opioids.
Pharmacotherapy for opioid dependence.
Methadone. The medications available for treatment of opioid dependence are methadone, buprenorphine, and naltrexone. For four decades maintenance with methadone has been the mainstay of treatment. Methadone is a full μ-opioid agonist. This means it fully attaches to the receptor to produce effects similar to those of other opioids that fully attach to the receptor, such as heroin. The difference between methadone and heroin is pharmacodynamics. Methadone has a slower, steadier action compared with the quick burst of euphoria that heroin produces. When methadone is securely attached to the μ-opioid receptor, the “high” heroin generates is negligible. With compliance, methadone maintenance improves employment records, curtails criminal activity, decreases the risk of needle-sharing, and reduces morbidity from HIV infection (45, 46). The dose of methadone that is effective depends on the individual's habit.
Methadone has received a great deal of criticism despite the positive effect it has on life circumstances. It is dispensed through federally registered clinics; however, clinics are sometimes situated in locations with high crime rates. There may be a paucity of support resources, and addicts may divert methadone by selling their quotas for profit.
Buprenorphine. Buprenorphine, the other maintenance treatment for opioid dependence, became available for office-based prescription in 2002. Licensure to prescribe buprenorphine requires physicians to complete a brief course and have access to referral resources that are capable of providing psychosocial services. Buprenorphine is a μ-opioid partial agonist with enough agonist activity to prevent craving and enough antagonist activity to block the euphoria of opioids. Another benefit of a partial agonist is its ceiling effect. An increased dose does not correlate with an increased drug effect; thus, death from overdose is rare. Buprenorphine is typically administered in a sublingual tablet of a buprenorphine/naloxone combination in a 4:1 ratio. Adding the naloxone antagonist causes significant withdrawal symptoms when the combination tablet is used intravenously, which further decreases its potential for diversion to the street for profit (47).
Not all patients are good candidates for treatment with buprenorphine. High-dose heroin users may not be satisfied with partial agonist treatment. Sedatives and alcohol elevate the risk of death in buprenorphine overdose. The patient needs to follow through with an office-based treatment program and commit to the rules of the program (48).
Naltrexone. Naltrexone is an opioid antagonist. It blocks the receptor site, which prevents an agonist from attaching to produce drug effects. Because it does not activate the receptor site, it lacks opioid effects and does not prevent craving. Addicts seldom prefer antagonist treatment, and the retention rate is low. The incentive for abstinence must be greater than the desire to use opioids. Most heroin addicts are poor candidates for naltrexone. On the other hand, people whose livelihoods depend on remaining drug-free are good candidates for naltrexone. Medical professionals constitute one such group. Their licensing boards can mandate the requirements necessary to maintain licensure. These are likely to include documented administration of the medication and random urine samples. Naltrexone should not be administered until 7 days after one's last dose of an opiate (9, pp 162–169).
Pharmacotherapy for alcohol dependence.
Disulfiram. Disulfiram (Antabuse) was the first U.S. Food and Drug Administration (FDA)-approved treatment for alcohol dependence. To some degree, newer medications have supplanted its use. However, it remains a potent aversive medication for patients with relapsing alcohol dependence. Those with experience treating patients with disulfiram have a deep appreciation for the sobriety patients can achieve as part of a monitored, abstinence-based program. However, the liabilities of disulfiram are better known than its potential benefits. Patients who do best participate in a monitoring program administered through a clinic or other agency or perhaps a spouse. The added psychological benefit may be that each day the patient decides to take disulfiram, he or she decides not to drink. Some patients take it during high-risk situations such as social occasions where alcohol will be served. It may be administered on a 3- to 4-day cycle, which makes it more conveniently prescribed in an office-based practice. Cyclic administration works because enzyme inhibition can be adequately effective for 6 days, even though the product's insert carries the warning that a reaction can occur as long as 15 days after the last dose (49). The patient's blood alcohol content should be zero before the first dose of disulfiram. Some patients find they can drink while taking a 250-mg dose. In that case, an adjustment to 500 mg is indicated.
Disulfiram interferes with the liver's degradation of alcohol. Under normal circumstances ethyl alcohol is metabolized to acetaldehyde, which the enzyme aldehyde dehydrogenase further breaks down to acetate. However, when disulfiram inhibits the action of aldehyde dehydrogenase, then acetaldehyde increases to toxic levels. This leads to skin flushing, headache, and nausea. One safeguard for patients is that when they feel mild symptoms within minutes of drinking, they usually stop and avert advanced symptoms. The effect is dose-related, and a reaction typically lasts 30–60 minutes. In severe reactions there can be hypotension and respiratory distress. If an ethanol-disulfiram reaction occurs, the patient must know what to expect. The safest route is assessment in an emergency department where treatment is supportive; however, many episodes are self-limiting (50).
The risk of hepatitis is rare; however, it has the potential for significant morbidity and even mortality. Baseline liver function tests should be obtained, with testing again after 1 month and again after 3–4 months. Hepatitis is most likely to occur within the first several months of treatment (51). Patients must be carefully selected for their physical health and psychiatric health status, cognitive abilities, social stability, and treatment resources to make compliance realistic. The prescribing psychiatrist has a responsibility to become a miniexpert in the use of disulfiram before prescribing it.
Naltrexone. Alcohol has a complex mechanism of action, affecting several neurotransmitters including γ-aminobutyric acid (GABA), glutamate, dopamine, and opiate systems. Because of this, medications that act on different systems may be effective in treating alcohol dependence. In 1994, naltrexone, an opioid antagonist, became available for use in the treatment of alcohol dependence. Its ability to block the μ-opioid receptor leads to suppression of an alcohol-induced rise in β-endorphin levels. Because naltrexone attenuates the rewarding effects of alcohol, compliance may be a problem. In such a situation, the physician might consider the once-monthly depot preparation. A key to the effectiveness of naltrexone is enhancing adherence; greater efforts to counsel patients or the use of depot medications are ways to accomplish this.
The ideal patient for naltrexone therapy seems to be one who is motivated to stop drinking, has episodes of heavy intake, experiences strong craving, and has a family history of alcohol dependence (52–54). The individual should be past the risk of alcohol withdrawal. Drug testing should confirm the patient's report that neither illicit nor prescription opioids are in his or her system. Baseline liver enzyme levels should be obtained, and tests should be repeated every month during treatment. Hepatotoxicity may occur in some patients although it is most likely to occur in obese patients treated with doses of ≥100 mg daily (54). Any patient taking naltrexone should carry a medical card to alert health care providers that the patient takes the medication and that opiate analgesics are ineffective in patients taking naltrexone.
Acamprosate. Acamprosate acts on the GABA and glutamate systems. One theory is that it attenuates protracted withdrawal symptoms, theoretically making it ideal for patients who struggle with severe alcohol dependence. Another possible mechanism is to modulate the patient's response to cues that would typically trigger drinking episodes. Since its FDA approval, the medication has failed to gain a robust following. It is not known why results of European studies have been favorable but results in the United States have been less so. It may be that a subtype of alcoholism responds preferentially (53). Acamprosate is excreted by the kidneys and is not metabolized in the liver, which makes it advantageous in patients with liver disease. This medication is associated with few side effects.
Nonapproved medications. Of the nonapproved medications, topiramate holds the most promise at this stage. It potentiates GABAergic transmission, blocks certain glutamate receptors, and probably dampens the rewarding effects of alcohol. Side effects tend to be mild, with the exception of an increased risk of kidney stones. Baclofen, like topiramate, acts on the GABA system. Excreted by the kidneys, it too has potential for those with established liver disease. Its efficacy has been demonstrated in small trials (53). Until its utility is proven in larger studies it remains in the category of promising medications, especially in early-onset alcohol dependence. Odansetron, a serotonin-3 receptor antagonist, has garnered long-standing interest (55). At present, it is used to treat nausea from chemotherapy and other medical problems. It is not available in doses needed for patients with alcohol dependence, and its use is limited to research trials.
In a recent article, Johnson (56) presented cases in which treatment was based on clinical subtyping, informed by scientific knowledge of how medications exert their effects, patients' clinical presentations, and the medication side effects. Typology has long been considered key to the clinical understanding and ultimate treatment of alcohol-dependent patients (57–60). Subtyping alcoholism shows promise as a method of choosing the correct pharmacotherapeutic agents, especially as a broader range of medications become available. In the future, pharmacogenetics may provide additional guidance (56).
Pharmacotherapy for cocaine dependence and for cannabis dependence.
Researchers are working to find treatments for these commonly abused drugs. Thus far, there are hopeful areas of research on both drugs. Understanding the neural mechanisms they act on should help in finding medications to aid recovery. Marijuana is enjoying a surge in research largely because of the discovery of cannabinoid receptors and endocannabinoids, which are naturally occurring in humans (61).
Scientific advances have informed medication trials for cocaine addiction. Dopamine, simplistically termed the reward neurotransmitter, increases in the presence of cocaine, and chronic use dysregulates dopamine. Two medications, disulfiram and modafinil, blunt the dopamine-mediated euphoria that cocaine causes (62). Topiramate is another possibility for treatment of cocaine addiction because it may attenuate cue-induced craving (63). Most novel of the treatments under study are vaccines that stimulate production of cocaine-specific antibodies (64).
CONCLUSION
The rate of advancements in scientific discovery creates excitement in the field among those who wait for what Vocci et al. (65) terms “second-generation” medications for addictions. These would launch a series of events to target molecular sites, theoretically treating multiple addictions caused by similar neural pathways. The beneficiaries of advancements are patients and others who have substance-related hardships. Improved methods for assessing substance misuse and providing efficacious interventions in various populations are underway, with the promise of decreasing morbidity and mortality attributable to intoxication. Tools for assessment are available for clinicians who decide to incorporate them into their practices. Assessment begins the process of behavioral change for those with various degrees of substance misuse, abuse, and dependence. Both psychotherapeutic treatments and medication management of substance use disorders are a reality, and evidence points to more specific treatments being within reach.
1 National Institute of Drug Abuse: Understanding Drug Abuse and Addiction. InfoFacts, June 2008Google Scholar
2 Edwards G, Gross M: Alcohol dependence: provisional description of a clinical syndrome. Br Med J 1976; 1:1058–1061Crossref, Google Scholar
3 Khantzian EJ: The self-medication hypothesis of addictive disorders: focus on heroin and cocaine dependence. Am J Psychiatry 1985; 142:1259–1264Crossref, Google Scholar
4 Dawson DA, Grant BF, Stinson FS, Chou PS, Huang B, and Ruan WJ: Recovery from DSM-IV alcohol dependence: United States, 2001–2002. Addiction 2005; 100:281–292Crossref, Google Scholar
5 National Epidemiologic Survey on Alcohol and Related Conditions: Selected Findings, Vol. 29, No. 2, 2007. http://pubs.niaaa.nih.gov/publications/arh29–2/toc29-2.Google Scholar
6 National Institute of Alcohol Abuse and Alcoholism: National Epidemiologic Survey on Alcohol and Related Conditions. Alcohol Alert 2006; 70(Oct)Google Scholar
7 National Institute of Alcohol Abuse and Alcoholism: Alcohol and Other Drugs. Alcohol Alert 2008; 76(June)Google Scholar
8 Grant BF, Dawson DA, Stinson FS, Chou SP, Dufour MC, Pickering RP: The 12-month prevalence and trends in DSM-IV alcohol abuse and dependence: United States, 1991–1992 and 2001–2002. Drug Alcohol Depend 2004; 74:223–234Crossref, Google Scholar
9 Nace EP, Tinsley JA: Patients with Substance Abuse Problems: Effective Identification, Diagnosis, and Treatment. New York, Norton, 2007, p. 47Google Scholar
10 National Institute of Alcohol Abuse and Alcoholism: Helping Patients Who Drink Too Much: A Clinician's Guide: NIH Publication 07−3769. Bethesda, MD, DHEW, 2007Google Scholar
11 Miller PM, Thomas SE, Mallin R: Patient attitudes towards self-report and biomarker alcohol screening by primary care physicians. Alcohol Alcohol 2006; 41:306–310Crossref, Google Scholar
12 National Institute of Alcohol Abuse and Alcoholism:
13 Ewing JA: Detecting alcoholism: the CAGE questionnaire. JAMA 1984; 252:1905–1907Crossref, Google Scholar
14 Buchsbaum DG, Buchanan RG, Centor RM, Schnoll SH, Lawton MJ: Screening for alcohol abuse using CAGE scores and likelihood ratios. Ann Intern Med 1991; 115:774–777Crossref, Google Scholar
15 Soderstrom CA, Smith GS, Kufera JA, Dischinger PC, Hebel JR, McDuff DR, Gorelick DA, Ho SM, Kerns TJ, Read KM: The accuracy of the CAGE, the Brief Michigan Alcoholism Screening Test, and the Alcohol Use Disorders Identification Test in screening trauma center patients for alcoholism. J Trauma 1997; 43:962–969Crossref, Google Scholar
16 Adams WL, Barry KL, Fleming MF: Screening for problem drinking in older primary care patients JAMA 1996; 276:1964–1967Crossref, Google Scholar
17 Arnaout B, Petrakis I: Diagnosing co-morbid drug use in patients with alcohol use disorders. Alcohol Res Health 2008; 31:148–154Google Scholar
18 National Institute on Drug Abuse: Alcohol, Smoking, and Substance Involvement Screening Test. http://www.drugabuse.gov/nidamed/screening/nmassist.pdfGoogle Scholar
19 Henry-Edwards S, Humeniuk R, Ali R, Poznyak V, Monteiro M: The Alcohol, Smoking Substance Involvement Screening Test (ASSIST): Guidelines for Use in Primary Care. Geneva, World Health Organization, 2008Google Scholar
20 Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M: Development of the Alcohol Use Disorders Identification Test (AUDIT): World Health Organization Collaborative Project on Early Detection of Persons with Harmful Alcohol Consumption—II. Addiction 1993; 88:791–804Crossref, Google Scholar
21 Allen JP, Litten RZ, Fertig JB, Babor T: A review of research on the Alcohol Use Disorders Identification Test (AUDIT). Alcohol Clin Exp Res 1997; 21:613–617Crossref, Google Scholar
22 Selzer M: The Michigan Alcoholism Screening Test: the quest for a new diagnostic instrument. Am J Psychiatry 1971; 127:1653–1658Crossref, Google Scholar
23 Sokol RJ, Martier S, Ager JW: The T-ACE questions: practical prenatal detection of risk-drinking. Am J Obstet Gyenecol 1989; 160:863–870Crossref, Google Scholar
24 Chan AW, Pristach EA, Welte JW, Russell M: Use of the TWEAK test in screening for alcoholism/heavy drinking in three populations. Alcohol Clin Exp Res 1993; 17:1188–1192Crossref, Google Scholar
25 Chang G, Behr H, Goetz MA, Hiley A, Bigby J: Women and alcohol abuse in primary care: identification and intervention. Am J Addict 1997; 6:183–192Google Scholar
26 Russell M: New assessment tools for drinking in pregnancy. Alcohol Health Res World 1994; 18:55–61Google Scholar
27 Allen JO, Anthenelli RM: Problem drinking: the case for routine screening. Curr Psychiatry 2003; 2:27–44Google Scholar
28 Huseby NE, Nilssen O, Erfurth A, Wetterling T, Kanitz RD: Carbohydrate-deficient transferring and alcohol dependency: variation in response to alcohol intake in various groups of patients. Alcohol Clin Exp Res 1990; 21:201–205Google Scholar
29 McLellan AJ, Davis DC, O'Brien CP, Kleber HD: Drug dependence: a chronic medical illness. JAMA 2000; 284:1689–1694Crossref, Google Scholar
30 American Psychiatric Association Practice Guidelines. Treatment of patients with substance use disorders. Am J Psychiatry 2006; 163(suppl):14–15Google Scholar
31 TIME Magazine: The 2010 TIME 100: the most important people of the century. http://www.time.com/time/time100/index_2000_time100.htmlGoogle Scholar
32 Dawson DA, Grant BF, Stinson FS: Estimating the effect of help-seeking on achieving recovery from alcohol dependence. Addiction 2006; 10:824–834Crossref, Google Scholar
33 National Institute on Drug Abuse: Principles of Drug Addiction Treatment: A Research Based Guide. NIH Publication 09-4180. Bethesda, MD, DHEW, 2009Google Scholar
34 Prendergast M, Podus D, Finney J, Greenwell L, Roll J: Contingency management for treatment of substance use disorders: a meta-analysis. Addiction 2006; 101:1546–1560Crossref, Google Scholar
35 Budney AJ, Moore BA, Rocha HL, Higgins ST: Clinical trial of abstinence-based vouchers and cognitive behavioral therapy for cannabis dependence. J Consult Clin Psychol 2006; 74:307–316Crossref, Google Scholar
36 Smith JE, Meyers RJ, Delaney HD: The community reinforcement approach with homeless alcohol-dependent individuals. J Consult Clin Psychol 1998; 66:541–548Crossref, Google Scholar
37 Roozen HG, Boulogne JJ, van Tulder MW, van den Brink W, De Jong CAJ, Kerhof JFM: A systemic review of the effectiveness of the community reinforcement approach in alcohol, cocaine, and opioid addiction. Drug Alcohol Depend 2004; 74:1–13Crossref, Google Scholar
38 Miller WR, Yahne CE, Tonigan JS: Motivational interviewing in drug abuse services: a randomized trial. J Consult Clin Psychol 2003; 71:754–763Crossref, Google Scholar
39 Anton RF, O'Malley SS, Ciraulo DA, Cisler RA, Couper D, Donovan DM, Gastfriend DR, Hosking JD, Johnson BA, LoCastro JS, Longabaugh R, Mason BJ, Mattson ME, Miller WR, Pettinati HM, Randall CL, Swift R, Weiss RD, Williams LD, Zweben A: Combined pharmacotherapies and behavioral interventions for alcohol dependence: the COMBINE study: a randomized controlled trial. JAMA 2006; 295:2003–2017Crossref, Google Scholar
40 Miller WR, Rollnick S: Motivational approach, in Treating Substance Abuse: Theory and Techniques. Edited by Rogers F, Keller DS, Margenstern J. New York, Guilford, 1996, pp 266–285Google Scholar
41 DiClemente CC, Prochaska JO, Fairhurst S, Velicer WF, Velasquez MM, Rossi JS: The process of smoking cessation: an analysis of precontemplation, contemplation, and preparation stages of change. J Consult Clin Psychol 1991; 59:295–304Crossref, Google Scholar
42 Henggeler SW, Clingempeel WG, Brondino MJ and Pickrel SG: Four-year follow-up of multisystemic therapy with substance-abusing and substance-dependent juvenile offenders. J Am Acad Child Adolesc Psychiatry 2002; 41:868–874Crossref, Google Scholar
43 Moyer A, Finney JW, Swearingen CE, Vergun P: Brief interventions for alcohol problems: a meta-analytic review of controlled investigations in treatment-seeking and non-treatment-seeking populations. Addictions 2002; 97:279–292Crossref, Google Scholar
44 Saitz R, Palfai TP, Cheng DM, Horton NJ, Dukes K, Kreamer KL, Roberts MS, Guerriero R, Samet JH: Some medical inpatients with unhealthy alcohol use may benefit from brief intervention. J Stud Alcohol Drugs 2009; 70:426–435Crossref, Google Scholar
45 Gerada C: Drug misuse: a review of treatments. Clin Med 2005; 5:69–73Crossref, Google Scholar
46 Gerstein DR: The effectiveness of drug treatment, in Addictive States, vol 70. Edited by O'Brien CP, Jaffe JH. New York, Raven 1992, pp 253–282Google Scholar
47 O'Brien CP, Dackis CA: Treatment of substance-related disorders, in The American Psychiatric Publishing Textbook of Psychopharmacology. Edited by Schatzberg AF, Nemeroff CB. Arlington, VA, American Psychiatric Publishing, 2009, pp 1214Google Scholar
48 Center for Substance Abuse Treatment: Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction. Treatment Improvement Protocol (TIP) Series 40. US Department of Health and Human Services Publication (SMA) 04-3939. Rockville, MD, Substance Abuse and Mental Health Services Administration, 2004Google Scholar
49 Antabuse (disulfiram tablets) package insert. Florham Park, NJ, Odyssey Pharmaceuticals, 2005Google Scholar
50 O'Brien CP, Dackis CA: Treatment of substance-related disorders, in The American Psychiatric Publishing Textbook of Psychopharmacology. Edited by Schatzberg AF, Nemeroff CB. Arlington, VA, American Psychiatric Publishing, Inc, 2009, p 1214Google Scholar
51 Wright C, Moore RD: Disulfiram treatment of alcoholism. Am J Medicine 1990; 88:647–655Crossref, Google Scholar
52 Krishnan-Sarin S, Krystal JH, Shi J, Pittman B, O'Malley SS: Family History of alcoholism influences naltrexone-induced reduction in alcohol drinking. Biol Psychiatry 2007; 62:694–697Crossref, Google Scholar
53 Johnson BA: Update on neuropharmacological treatments for alcoholism: scientific basis and clinical findings. Biochem Pharmacol 2008; 75:34–56Crossref, Google Scholar
54 Anton RF: Naltrexone for the management of alcohol dependence. N Engl J Med 2008; 359:715–721Crossref, Google Scholar
55 Sellers EM, Toneatto T, Romach MK, Somer GR, Sobell LC, Sobell MD: Clinical efficacy of the 5-HT3 antagonist ondansetron in alcohol abuse and dependence. Alcohol Clin Exp Res 1994; 18:879–885Crossref, Google Scholar
56 Johnson BA: Medication treatment of different types of alcoholism. Am J Psychiatry 2010; 167:630–639Crossref, Google Scholar
57 Bogenschutz MP, Tonigan JS, Pettinati HM: Effects of alcoholism typology on response to naltrexone in the COMBINE study. Alcohol Clin Exp Res 2009; 33:10–18Crossref, Google Scholar
58 Babor TF, Dolinsky ZS: Alcoholic typologies: historical entities and empirical evaluation of some common classifications schemes, in Alcoholism: Origins and Outcomes. Edited by Rose RM, Barrett J. New York, Raven Press, 1998, pp 245–266Google Scholar
59 Project MATCH Research Group: Matching alcoholism treatment to clinical heterogeneity: Project MATCH posttreatment outcomes. J Stud Alcohol; 1997: 58: 7–29Crossref, Google Scholar
60 Cloninger CR: Neurogenetic adaptive mechanisms in alcoholism. Science 1987; 236:410–416Crossref, Google Scholar
61 Onaivi ES: Neuropsychobiological evidence for the functional presence and expression of cannabinoid CB2 receptors in the brain. Neuropsychobiology 2006; 54:231–246Crossref, Google Scholar
62 Dakis C, O'Brien CA: Glutamatergic agents for cocaine dependence. Ann NY Acad Sci 2003; 1003:328–345Crossref, Google Scholar
63 Kampman KM, Pettinati H, Lynch KG, et. A pilot trial of topiramate for the treatment of cocaine dependence. Drug and Alcohol Depend 2004; 75:233–240Crossref, Google Scholar
64 Sofuoglu M, Kosten TR: Novel approaches to the treatment of cocaine addiction. CNS Drugs 2005; 19:13–25Crossref, Google Scholar
65 Vocci FJ, Acri J, Elkashef A: Medication development for addictive disorders: the state of the science. Am J Psychiatry 2005; 162:1432–1440Crossref, Google Scholar



