The Genetics of Obsessive-Compulsive Disorder
Abstract
Obsessive-compulsive disorder (OCD) is a neuropsychiatric disease and a complex
Obsessive-compulsive disorder (OCD) is an anxiety disorder characterized by repetitive thoughts, images, impulses, or behaviors that cause significant time loss, distress, and/or impairment of functioning. This illness is highly morbid and disabling in its severe forms and affects children, adolescents, and adults. Genetic studies have demonstrated that both biological and environmental factors are important in the etiology of OCD (1). With respect to environmental factors, stressful events have been associated with OCD onset in childhood (2), perinatal, and adult forms of the illness. With respect to nongenetic biological factors, several illnesses have also been associated with OCD, including temporal lobe epilepsy (3), autoimmune diseases such as systemic lupus erythematosus (4, 5) and Crohn's disease (6, 7) and infectious processes (8–10). With respect to potential genetic factors, family studies of OCD report increased prevalence of this disorder among relatives, and twin studies have confirmed that a genetic component underlies the illness. Segregation analyses performed on multigenerational families suggest that this disorder is difficult to model using Mendelian approaches. In molecular genetic work, linkage studies have attempted to identify regions in which OCD vulnerability genes may lie and numerous candidate genes studies have been studied for potential association with the OCD phenotype. Most recently, a collaborative group of more than 20 OCD research sites has undertaken a genome-wide association study (GWAS) in search of common or rare susceptibility genetic variants.
Family studies of OCD-affected individuals published since the 1930s provide strong evidence for familiality of the disorder (Table 1). As OCD symptoms are frequently hidden, even from relatives, family studies in which relatives are not directly interviewed about their own diagnoses probably underestimate recurrence rates. To date there have been 15 OCD family studies in which relatives received structured interviews (11–26). Seven of these focused on childhood OCD (11–13, 19, 21, 22, 25), all of which reported significantly higher OCD rates among relatives than general population or control rates. Recurrence risks were also much higher than familial rates reported in adult OCD studies. Although reported rates of OCD among relatives of adults with OCD were approximately 2 times that among control subjects, the rate of OCD among relatives of children and adolescents with OCD was increased approximately 10-fold in those studies for which control rates were available. The most recent controlled family study (26) ascertained OCD probands from both European population and clinic samples, reporting increased OCD rates among relatives from both samples. This study's findings also parallel results of earlier studies completed in the United States (17, 18, 23) with families ascertained through treatment facilities. Finally, a meta-analysis of data from 1,209 first-degree relatives (27) studied before 2001 reported a significantly increased OCD recurrence risk among proband versus control relatives [Mantel-Haenszel summary odds ratio=4.0 (95% confidence interval=2.2–7.1)]. The unadjusted aggregate risk for relatives of OCD probands was 8.2%, compared with 2.0% for relatives of control subjects. These results demonstrate that at least some cases of OCD are familial, which may be attributed to combined genetic and/or environmental effects.
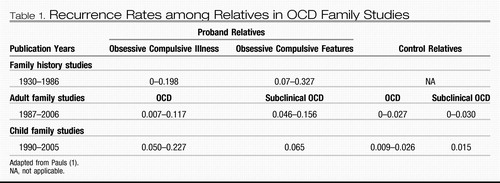 |
Table 1. Recurrence Rates among Relatives in OCD Family Studies
Twin studies help to determine whether genetic factors are important in the etiology of complex disorders, thus providing greater etiological specificity than family studies (Table 2). The concordance rate differences between monozygotic and dizygotic twins provide an estimate of heritability (defined as the percentage of phenotypic variance attributed to genetic factors). In a meta-analysis of twin studies published between 1929 and 2005, van Grootheest et al. (28) reported heritability estimates ranging between 45 and 65% in children and between 27 and 47% in adults. In the only additional twin study published since 2005, Bolton et al. (29) examined a community sample of 854 6-year-old twins and concluded that shared etiological factors exist for OCD and tics and for OCD and other anxiety disorders. Four complex segregation analyses of OCD have been conducted to estimate the “goodness of fit” for specific familial patterns of transmission (30–33). These suggested that some genes of major effect are probably involved in this disorder, although OCD transmission is difficult to model.
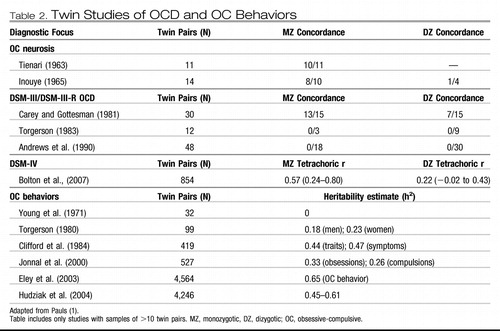 |
Table 2. Twin Studies of OCD and OC Behaviors
Genome-wide linkage studies are molecular genetic studies that attempt to identify regions in the genome that may contain vulnerability genes. None of the three of these studies completed to date in OCD (34–36) yielded genome-wide significant findings, although they did identify genomic regions of interest for future research. Hanna et al. (34) completed a genome scan on seven families identified through childhood OCD probands. They reported a lod score (logarithm of the odds ratio for linkage) of 2.25 (1.97 after fine mapping) for marker D9S288 on chromosome 9p. Willour et al. (37) genotyped 50 OCD pedigrees (37) in an attempt to replicate these findings. The largest lod scores observed in this study were for markers D9S1792 (heterogeneity lod=2.26) and D9S1813 (nonparametric linkage= 2.52, p=0.006), lying within 350 kb of the marker with the strongest finding reported by Hanna et al.
The second OCD genome-wide linkage study included sib-pairs and multigenerational members of 219 families. These authors reported the strongest linkage in examination of compulsive hoarding only on chromosome 3q27–28 [Kong and Cox lod(all) score [KAC(all)] score=2.67] for OCD (35) and on chromosome 14 [KAC(all)=2.9 for families with one affected relative and 3.7 for families with two or more affected relatives] (38). In the third OCD genome-wide linkage study 26 multigenerational families were genotyped. The maximum nonparametric lod (nlod) score was equal to 2.43 for markers located on chromosome 10p15. Unfortunately, when combined with data from the previous genome scan (34), the maximum nlod score was decreased to 1.79. In further follow-up via family-based association analysis of single nucleotide polymorphisms (SNPs) in this 10p15 region, however, association was detected with three adjacent SNPs. This included the amino acid variant rs2271275 in the 3′ region of adenosine deaminase acting on RNA 3 (ADAR3) (p<0.05).
Because sample sizes in all three linkage studies were quite small, these findings should be interpreted with caution. Nonetheless, the fact that Willour et al. (37) observed suggestive linkage in the same chromosome 9p region as reported by Hanna et al. (34, 36) is noteworthy. In addition, as discussed in the following paragraphs, four independent studies have reported an association between OCD and the glutamate transporter gene SLC1A1, which is located in this region of 9p. However, illustrating the inconsistency of OCD genetic findings (potentially related to OCD heterogeneity across samples), this region did not show any evidence for linkage in the study completed by Shugart et al. (35).
As noted above, family and twin studies have provided evidence that OCD is both familial and genetic. Segregation analyses have clarified that transmission does not occur in a simple Mendelian fashion and are in keeping with patterns seen for complex genetic disorders. In an effort to identify regions containing OCD vulnerability loci, whole genome linkage scans have reported some regions of suggestive linkage, and analyzing the subphenotype of hoarding led to an improved linkage signal. However, at best linkage scans are only able to identify broadly defined genomic regions, which contain numerous genes. An alternate approach, via candidate gene studies, searches for associations between OCD and specific genes, selected based upon their location or function.
More than 80 OCD candidate gene studies have been reported over the last decade (1). These have mainly been selected for study because of their proximal location to linkage peaks (positional candidates) or their putative etiological role in neurotransmitter pathways or anatomy related to OCD (functional candidates). Specifically, genes within serotonin, dopamine, and glutamate pathways and those involved in brain white matter have been the center of focus, outlined in the following paragraphs.
White matter volume abnormalities have been noted in both child (39) and adult (40) OCD samples. In addition, diffusion tensor imaging studies have reported white matter connectivity abnormalities in OCD (41). Following from this, white matter genes including OLIG2 (42) and MOG (43) have been studied with reported association in small family-based samples.
Until recently, serotonin remained the leading target for investigations of the neurochemical underpinnings of OCD, largely because of the efficacy of selective serotonin reuptake inhibitors (SSRIs) in its treatment (44). As such, numerous serotonin-related genes have been studied in OCD (Table 3). The most commonly studied of these is the serotonin transporter gene. In a meta-analysis stratifying OCD samples, a significant association was identified with the l-allele of the serotonin transporter polymorphism in family-based association studies with child and Caucasian samples (45).
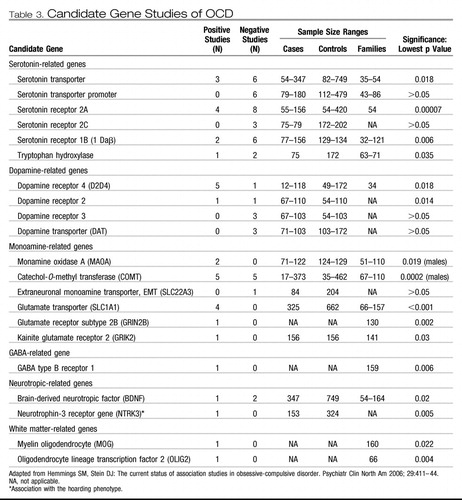 |
Table 3. Candidate Gene Studies of OCD
The dopamine neurotransmitter has also been implicated in OCD etiology. OCD-like behaviors in humans have been reported to emerge after administration of dopamine receptor 2 agonists and stimulants (46). Augmentation efficacy has also been reported for atypical antipsychotics, which act on serotonin and dopamine receptors (47). However, association studies of dopamine-related genes to date have reported only mixed results. There is also a complex interaction between glutamatergic, dopaminergic, and serotoninergic neurons (48, 49).
There has been a convergence of evidence across various lines of research supporting the putative role of glutamate in OCD (50, 51). Functional OCD neuroimaging studies have consistently reported metabolic hyperactivity in the cortico-striato-thalamo-cortical circuitry (52–55). Concentrations of glutamate have been found to be increased in the CSF of OCD-affected adults (56) and decreased in the anterior cingulate cortex of children with OCD (57). Rosenberg et al. (57, 58) conducted an elegant series of studies suggesting a notable role of glutamate in childhood OCD. Altered glutamate receptor levels have also been reported in OCD-implicated brain areas within subjects with OCD (59). Moreover, promising augmentation trials of glutamate-modulating agents riluzole (60) and memantine (61) have been completed. OCD animal model studies also support a role for glutamate in OCD. Knockout mice for the striatum-expressed SAPAP3 gene (coding for a protein at corticostriatal glutamatergic excitatory synapses) reportedly developed facial lesions, repetitive grooming behaviors, and anxiety that were reversed separately with SSRI administration and with gene replacement (62). In a recently published Nature paper (63), Slitrk5 deficient mice also demonstrated OCD-like behaviors diminishing with SSRI treatment and showed glutamate receptor composition changes. Moreover, glutamate receptor gene (GRIK2)-deficient mice have shown significantly less fear memory and fewer anxious behaviors than wild type mice (46, 64). Lastly, treatment with glutamate-related medications has reduced marble-burying behavior (another OCD animal model) in mice (65). Glutamate-related genes that have been associated with OCD in small studies to date include the glutamate transporter SLC1A1 (66–69) and glutamate receptors GRIN2B (70) and GRIK2 (71). In addition, an association between the previously mentioned SAPAP3 gene and pathological grooming behaviors (but not OCD) in humans has been found (72).
Unfortunately, there has been a failure to consistently replicate nearly all OCD candidate gene study findings. Further, none of the OCD candidate gene association studies reported to date have had sufficient power to demonstrate genome-wide significance. Among OCD candidate gene studies, the glutamate transporter gene SLCL1A1 (66–69) is the only one of these that has been repeatedly associated across OCD samples. Of note, this gene has also been associated with atypical antipsychotic-induced obsessive-compulsive symptoms (73) and with autism, a disorder frequently associated with obsessive-compulsive behaviors (Figure 1) (74).
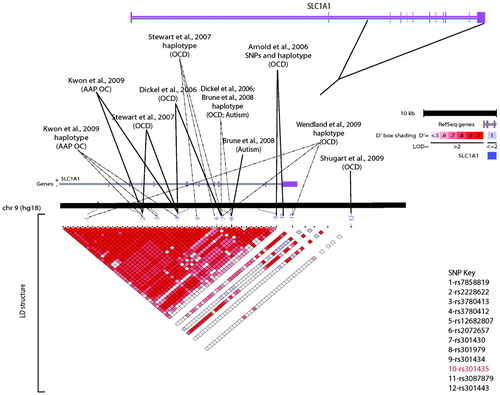
Shugart YY, Wang Y, Samuels JF, Grados MA, Greenberg BD, Knowles JA, McCracken JT, Rauch SL, Murphy DL, Rasmussen SA, Cullen B, Hoehn-Saric R, Pinto A, Fyer AJ, Piacentini J, Pauls DL, Bienvenu OJ, Riddle MA, Liang KY, Nestadt G: A family-based association study of the glutamate transporter gene SLC1A1 in obsessive-compulsive disorder in 378 families. Am J Med Genet B Neuropsychiatr Genet 2009; 150B(6):886–92. LD, linkage disequilibrium.
In summary, OCD is a complex genetic disorder that seems to also be strongly influenced by environmental and nongenetic biological processes. Moreover, it is possible that subtypes of this heterogeneous disorder exist, with independent or overlapping etiological factors. The twin and family studies summarized in this review suggest that combined influences from OCD vulnerability genes play a notable role in OCD etiology. However, given that the linkage studies and essentially all of the candidate genes studies to date provide only suggestive evidence for OCD risk genes of moderate to large effect, GWASs of OCD are warranted as the next step in understanding the genetic basis of OCD. GWASs are preferred over more traditional linkage studies or candidate gene studies as they provide enhanced ability to identify risk genes of relatively small effect. These GWASs have the capability to examine both common SNP markers as well as copy number variants and other rare genetic events. There is emerging evidence that complex disorders may result from both rare genes of major effect and a combination of common genes of lesser effect, requiring very large sample sizes. In an effort to meet this need, the International OCD Foundation Genetics Collaborative is currently conducting a GWAS of OCD on samples contributed from 21 different research sites from around the world. It is anticipated that individual or combined results from GWAS markers will eventually further illuminate knowledge about the impact of underlying genetic etiological factors on OCD, allowing for clinical translational applications of these findings.
1 Pauls DL: The genetics of obsessive compulsive disorder: a review of the evidence. Am J Med Genet C Semin Med Genet 2008; 148C: 133– 139Crossref, Google Scholar
2 Thomsen PH: Obsessive-compulsive disorder in children and adolescents: a study of parental psychopathology and precipitating events in 20 consecutive Danish cases. Psychopathology 1995; 28: 161– 167Crossref, Google Scholar
3 Isaacs KL, Philbeck JW, Barr WB, Devinsky O, Alper K: Obsessive-compulsive symptoms in patients with temporal lobe epilepsy. Epilepsy Behav 2004; 5: 569– 574Crossref, Google Scholar
4 Yu CH, Lee MB, Tseng MM, Liao SC: Obsessive-compulsive symptoms as a manifestation of neuropsychiatric systemic lupus erythematosus. J Formos Med Assoc 2008; 107: 68– 72Crossref, Google Scholar
5 Slattery MJ, Dubbert BK, Allen AJ, Leonard HL, Swedo SE, Gourley MF: Prevalence of obsessive-compulsive disorder in patients with systemic lupus erythematosus. J Clin Psychiatry 2004; 65: 301– 306Crossref, Google Scholar
6 Burke P, Meyer V, Kocoshis S, Orenstein D, Chandra R, Sauer J: Obsessive-compulsive symptoms in childhood inflammatory bowel disease and cystic fibrosis. J Am Acad Child Adolesc Psychiatry 1989; 28: 525– 527Crossref, Google Scholar
7 Derenne JL: Abrupt-onset obsessive-compulsive disorder (OCD) in a child with Crohn's disease. Psychosomatics 2009; 50: 425– 426Crossref, Google Scholar
8 Dale RC, Church AJ: Post-streptococcal neuropsychiatric disease: Sydenham's chorea and beyond, in Neuropsychiatric Disorders and Infection. Edited by Fatemi SH. London, Taylor & Francis, 2005, pp 154– 161Crossref, Google Scholar
9 Murphy TK, Sajid MW, Goodman WK: Immunology of obsessive-compulsive disorder. Psychiatr Clin N Am 2006; 29: 445– 469Crossref, Google Scholar
10 Swedo SE, Leonard HL, Garvey M, Mittleman B, Allen AJ, Perlmutter S, Lougee L, Dow S, Zamkoff J, Dubbert BK: Pediatric autoimmune neuropsychiatric disorders associated with streptococcal infections: clinical description of the first 50 cases. Am J Psychiatry 1998; 155: 264– 271Google Scholar
11 Lenane MC, Swedo SE, Leonard H, Pauls DL, Sceery W, Rapoport JL: Psychiatric disorders in first degree relatives of children and adolescents with obsessive compulsive disorder. J Am Acad Child Adolesc Psychiatry 1990; 29: 407– 412Crossref, Google Scholar
12 Riddle MA, Scahill L, King R, Hardin MT, Towbin KE, Ort SI, Leckman JF, Cohen DJ: Obsessive compulsive disorder in children and adolescents: phenomenology and family history. J Am Acad Child Adolesc Psychiatry 1990; 29: 766– 772Crossref, Google Scholar
13 Leonard HL, Lenane MC, Swedo SE, Rettew DC, Gershon ES, Rapoport JL: Tics and Tourette's disorder: a 2- to 7-year follow-up of 54 obsessive-compulsive children. Am J Psychiatry 1992; 149: 1244– 1251Crossref, Google Scholar
14 Bellodi L, Sciuto G, Diaferia G, Ronchi P, Smeraldi E: Psychiatric disorders in the families of patients with obsessive-compulsive disorder. Psychiatry Res 1992; 42: 111– 120Crossref, Google Scholar
15 Black DW, Noyes R Jr, Goldstein RB, Blum N: A family study of obsessive-compulsive disorder. Arch Gen Psychiatry 1992; 49: 362– 368Crossref, Google Scholar
16 Nicolini H, Weissbecker K, Mejia JM, Sanchez de Carmona M: Family study of obsessive-compulsive disorder in a Mexican population. Arch Med Res 1993; 24: 193– 198Google Scholar
17 Pauls DL, Alsobrook J II, Goodman WK, Rasmussen SA, Leckman JF: A family study of obsessive compulsive disorder. Am J Psychiatry 1995; 152: 76– 84Crossref, Google Scholar
18 Nestadt G, Samuels J, Riddle MA, Bienvenu OJ 3rd, Liang KY, LaBuda M, Walkup J, Grados M, Hoehn-Saric R: A family study of obsessive-compulsive disorder. Arch Gen Psychiatry 2000; 57: 358– 363Crossref, Google Scholar
19 Reddy PS, Reddy YC, Srinath S, Khanna S, Sheshadri SP, Girimaji SR: A family study of juvenile obsessive-compulsive disorder. Can J Psychiatry 2001; 46: 346– 351Crossref, Google Scholar
20 Albert U, Maina G, Ravizza L, Bogetto F: An exploratory study on obsessive-compulsive disorder with and without a familial component: are there any phenomenological differences? Psychopathology 2002; 35: 8– 16Crossref, Google Scholar
21 Chabane N, Delorme R, Millet B, Mouren MC, Leboyer M, Pauls D: Early-onset obsessive-compulsive disorder: a subgroup with a specific clinical and familial pattern? J Child Psychol Psychiatry 2005; 46: 881– 887Crossref, Google Scholar
22 Hanna GL, Himle JA, Curtis GC, Gillespie BW: A family study of obsessive-compulsive disorder with pediatric probands. Am J Med Genet B Neuropsychiatr Genet 2005; 134: 13– 19Crossref, Google Scholar
23 Fyer AJ, Lipsitz JD, Mannuzza S, Aronowitz B, Chapman TF: A direct interview family study of obsessive-compulsive disorder. I. Psychol Med 2005; 35: 1611– 1621Crossref, Google Scholar
24 Lipsitz JD, Mannuzza S, Chapman TF, Foa EB, Franklin ME, Goodwin RD, Fyer AJ: A direct interview family study of obsessive-compulsive disorder. II. Contribution of proband informant information. Psychol Med 2005; 35: 1623– 1631Crossref, Google Scholar
25 Rosario-Campos MC, Leckman JF, Curi M, Quatrano S, Katsovitch L, Miguel EC, Pauls DL: A family study of early-onset obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet 2005; 136: 92– 97Crossref, Google Scholar
26 Grabe HJ, Ruhrmann S, Ettelt S, Buhtz F, Hochrein A, Schulze-Rauschenbach S, Meyer K, Kraft S, Reck C, Pukrop R, Freyberger HJ, Klosterkötter J, Falkai P, John U, Maier W, Wagner M: Familiality of obsessive-compulsive disorder in nonclinical and clinical subjects. Am J Psychiatry 2006; 163: 1986– 1992Crossref, Google Scholar
27 Hettema JM, Neale MC, Kendler KS: A review and meta-analysis of the genetic epidemiology of anxiety disorders. Am J Psychiatry 2001; 158: 1568– 1578Crossref, Google Scholar
28 van Grootheest DS, Cath DC, Beekman AT, Boomsma DI: Twin studies on obsessive-compulsive disorder: a review. Twin Res Hum Genet 2005; 8: 450– 458Crossref, Google Scholar
29 Bolton D, Rijsdijk F, O'Connor TG, Perrin S, Eley TC: Obsessive-compulsive disorder, tics and anxiety in 6-year-old twins. Psychol Med 2007; 37: 39– 48Crossref, Google Scholar
30 Nicolini H, Hanna GL, Baxter L, Schwartz J, Weissbecker K, Spence MA: Segregation analysis of obsessive compulsive disorders. Preliminary results. Ursus Med 1991; 1: 25– 28Google Scholar
31 Alsobrook IJ, Leckman JF, Goodman WK, Rasmussen SA, Pauls DL: Segregation analysis of obsessive-compulsive disorder using symptom-based factor scores. Am J Med Genet 1999; 88: 669– 675Crossref, Google Scholar
32 Cavallini MC, Pasquale L, Bellodi L, Smeraldi E: Complex segregation analysis for obsessive compulsive disorder and related disorders. Am J Med Genet 1999; 88: 38– 43Crossref, Google Scholar
33 Nestadt G, Lan T, Samuels J, Riddle M, Bienvenu OJ, Liang K, Hoehn-Saric R, Cullen B, Grados M, Beaty TH, Shugart YY: Complex segregation analysis provides compelling evidence for a major gene underlying obsessive-compulsive disorder and for heterogeneity by sex. Am J Hum Genet 2000; 67: 1611– 1616Crossref, Google Scholar
34 Hanna GL, Veenstra-VanderWeele J, Cox NJ, Boehnke M, Himle JA, Curtis GC, Leventhal BL, Cook EH Jr: Genome-wide linkage analysis of families with obsessive-compulsive disorder ascertained through pediatric probands. Am J Med Genet B Neuropsychiatr Genet 2002; 114: 541– 552Crossref, Google Scholar
35 Shugart YY, Samuels J, Willour VL, Grados MA, Greenberg BD, Knowles JA, McCracken JT, Rauch SL, Murphy DL, Wang Y, Pinto A, Fyer AJ, Piacentini J, Pauls DL, Cullen B, Page J, Rasmussen SA, Bienvenu OJ, Hoehn-Saric R, Valle D, Liang KY, Riddle MA, Nestadt G: Genomewide linkage scan for obsessive-compulsive disorder: evidence for susceptibility loci on chromosomes 3q, 7p, 1q, 15q, and 6q. Mol Psychiatry 2006; 11: 763– 770Crossref, Google Scholar
36 Hanna GL, Veenstra-Vanderweele J, Cox NJ, Van Etten M, Fischer DJ, Himle JA, Bivens NC, Wu X, Roe CA, Hennessy KA, Dickel DE, Leventhal BL, Cook EH Jr: Evidence for a susceptibility locus on chromosome 10p15 in early-onset obsessive-compulsive disorder. Biol Psychiatry 2007; 62: 856– 862Crossref, Google Scholar
37 Willour VL, Yao Shugart Y, Samuels J, Grados M, Cullen B, Bienvenu OJ 3rd, Wang Y, Liang KY, Valle D, Hoehn-Saric R, Riddle M, Nestadt G: Replication study supports evidence for linkage to 9p24 in obsessive-compulsive disorder. Am J Hum Genet 2004; 75: 508– 513Crossref, Google Scholar
38 Samuels J, Shugart YY, Grados MA, Willour VL, Bienvenu OJ, Greenberg BD, Knowles JA, McCracken JT, Rauch SL, Murphy DL, Wang Y, Pinto A, Fyer AJ, Piacentini J, Pauls DL, Cullen B, Rasmussen SA, Hoehn-Saric R, Valle D, Liang KY, Riddle MA, Nestadt G: Significant linkage to compulsive hoarding on chromosome 14 in families with obsessive-compulsive disorder: results from the OCD Collaborative Genetics Study. Am J Psychiatry 2007; 164: 493– 499Crossref, Google Scholar
39 Macmaster F, Vora A, Easter P, Rix C, Rosenberg D: Orbital frontal cortex in treatment-naive pediatric obsessive-compulsive disorder. Psychiatry Res 2010; 181: 97– 100Crossref, Google Scholar
40 van den Heuvel OA, Remijnse PL, Mataix-Cols D, Vrenken H, Groenewegen HJ, Uylings HB, van Balkom AJ, Veltman DJ: The major symptom dimensions of obsessive-compulsive disorder are mediated by partially distinct neural systems. Brain 2009; 132: 853– 868Crossref, Google Scholar
41 Szeszko PR, Ardekani BA, Ashtari M, Malhotra AK, Robinson DG, Bilder RM, Lim KO: White matter abnormalities in obsessive-compulsive disorder: a diffusion tensor imaging study. Arch Gen Psychiatry 2005; 62: 782– 790Crossref, Google Scholar
42 Stewart SE, Platko J, Fagerness J, Birns J, Jenike E, Smoller JW, Perlis R, Leboyer M, Delorme R, Chabane N, Rauch SL, Jenike MA, Pauls DL: A genetic family-based association study of OLIG2 in obsessive-compulsive disorder. Arch Gen Psychiatry 2007; 64: 209– 214Crossref, Google Scholar
43 Zai G, Bezchlibnyk YB, Richter MA, Arnold P, Burroughs E, Barr CL, Kennedy JL: Myelin oligodendrocyte glycoprotein (MOG) gene is associated with obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet 2004; 129: 64– 68Crossref, Google Scholar
44 Soomro GM, Altman D, Rajagopal S, Oakley-Browne M: Selective serotonin re-uptake inhibitors (SSRIs) versus placebo for obsessive compulsive disorder (OCD). Cochrane Database Syst Rev 2008; 1: CD001765Google Scholar
45 Bloch MH, Landeros-Weisenberger A, Sen S, Dombrowski P, Kelmendi B, Coric V, Leckman JF: Association of the serotonin transporter polymorphism and obsessive-compulsive disorder: systematic review. Am J Med Genet B Neuropsychiatr Genet 2008; 147B: 850– 858Crossref, Google Scholar
46 Murphy TK, Voeller KK, Stewart SE. Neurobiology of obsessive-compulsive disorder, in Textbook of Child and Adolescent Psychopharmacology. Edited by Martin A, Scahill L, Kratochvil CJ. New York, Oxford University Press, in pressGoogle Scholar
47 Skapinakis P, Papatheodorou T, Mavreas V: Antipsychotic augmentation of serotonergic antidepressants in treatment-resistant obsessive-compulsive disorder: a meta-analysis of the randomized controlled trials. Eur Neuropsychopharmacol 2007; 17: 79– 93Crossref, Google Scholar
48 Schilman EA, Klavir O, Winter C, Sohr R, Joel D: The role of the striatum in compulsive behavior in intact and orbitofrontal-cortex-lesioned rats: possible involvement of the serotonergic system. Neuropsychopharmacology 2010; 35: 1026– 1039Crossref, Google Scholar
49 Carlsson ML: On the role of prefrontal cortex glutamate for the antithetical phenomenology of obsessive compulsive disorder and attention deficit hyperactivity disorder. Prog Neuropsychopharmacol Biol Psychiatry 2001; 25: 5– 26Crossref, Google Scholar
50 Ting JT, Feng G: Glutamatergic synaptic dysfunction and obsessive-compulsive disorder. Curr Chem Genomics 2008; 2: 62– 75Crossref, Google Scholar
51 Rotge JY, Aouizerate B, Tignol J, Bioulac B, Burbaud P, Guehl D: The glutamate-based genetic immune hypothesis in obsessive-compulsive disorder. An integrative approach from genes to symptoms. Neuroscience 2010; 165: 408– 417Crossref, Google Scholar
52 Baxter LR Jr: Positron emission tomography studies of cerebral glucose metabolism in obsessive compulsive disorder. J Clin Psychiatry 1994; 55( Suppl): 54– 59Google Scholar
53 Saxena S, Brody AL, Schwartz JM, Baxter LR: Neuroimaging and frontal-subcortical circuitry in obsessive-compulsive disorder. Br J Psychiatry Suppl 1998; 35: 26– 37Google Scholar
54 Swedo SE, Schapiro MB, Grady CL, Cheslow DL, Leonard HL, Kumar A, Friedland R, Rapoport SI, Rapoport JL: Cerebral glucose metabolism in childhood-onset obsessive-compulsive disorder. Arch Gen Psychiatry 1989; 46: 518– 523Crossref, Google Scholar
55 Horwitz B, Swedo SE, Grady CL, Pietrini P, Schapiro MB, Rapoport JL, Rapoport SI: Cerebral metabolic pattern in obsessive-compulsive disorder: altered intercorrelations between regional rates of glucose utilization. Psychiatry Res 1991; 40: 221– 237Crossref, Google Scholar
56 Chakrabarty K, Bhattacharyya S, Christopher R, Khanna S: Glutamatergic dysfunction in OCD. Neuropsychopharmacology 2005; 30: 1735– 1740Crossref, Google Scholar
57 Rosenberg DR, Mirza Y, Russell A, Tang J, Smith JM, Banerjee SP, R, Rose M, Ivey J, Boyd C, Moore GJ: Reduced anterior cingulate glutamatergic concentrations in childhood OCD and major depression versus healthy controls. J Am Acad Child Adolesc Psychiatry 2004; 43: 1146– 1153Crossref, Google Scholar
58 Rosenberg DR, MacMaster FP, Keshavan MS, Fitzgerald KD, Stewart CM, Moore GJ: Decrease in caudate glutamatergic concentrations in pediatric obsessive-compulsive disorder patients taking paroxetine. J Am Acad Child Adolesc Psychiatry 2000; 39: 1096– 1103Crossref, Google Scholar
59 Nissen JB, Thomsen PH: Clinicians' views on clinical examination and treatment of children and adolescents with obsessive-compulsive disorder (OCD). A Danish national survey study. Nord J Psychiatry 2008; 62: 309– 314Crossref, Google Scholar
60 Coric V, Taskiran S, Pittenger C, Wasylink S, Mathalon DH, Valentine G, Saksa J, Wu YT, Gueorguieva R, Sanacora G, Malison RT, Krystal JH: Riluzole augmentation in treatment-resistant obsessive-compulsive disorder: an open-label trial. Biol Psychiatry 2005; 58: 424– 428Crossref, Google Scholar
61 Stewart SE, Jenike EA, Hezel DM, Stack DE, Dodman NH, Shuster L, Jenike MA: A single-blinded case-control study of memantine in severe obsessive-compulsive disorder. J Clin Psychopharmacol 2010; 30: 34– 39Crossref, Google Scholar
62 Welch JM, Lu J, Rodriguiz RM, Trotta NC, Peca J, Ding JD, Feliciano C, Chen M, Adams JP, Luo J, Dudek SM, Weinberg RJ, Calakos N, Wetsel WC, Feng G: Cortico-striatal synaptic defects and OCD-like behaviors in Sapap3-mutant mice. Nature 2007; 448: 894– 900Crossref, Google Scholar
63 Shmelkov, S. V., A. Hormigo, et al. “Slitrk5 deficiency impairs corticostriatal circuitry and leads to obsessive-compulsive-like behaviors in mice.” Nat Med 16( 5): 598– 602, 591p following 602.Crossref, Google Scholar
64 Shaltiel G, Maeng S, Malkesman O, Pearson B, Schloesser RJ, Tragon T, Rogawski M, Gasior M, Luckenbaugh D, Chen G, Manji HK: Evidence for the involvement of the kainate receptor subunit GluR6 (GRIK2) in mediating behavioral displays related to behavioral symptoms of mania. Mol Psychiatry 2008; 13: 858– 872Crossref, Google Scholar
65 Egashira N, Okuno R, Harada S, Matsushita M, Mishima K, Iwasaki K, Nishimura R, Oishi R, Fujiwara M: Effects of glutamate-related drugs on marble-burying behavior in mice: implications for obsessive-compulsive disorder. Eur J Pharmacol 2008; 586: 164– 170Crossref, Google Scholar
66 Arnold PD, Sicard T, Burroughs E, Richter MA, Kennedy JL: Glutamate transporter gene SLC1A1 associated with obsessive-compulsive disorder. Arch Gen Psychiatry 2006; 63: 769– 776Crossref, Google Scholar
67 Dickel DE, Veenstra-VanderWeele J, Cox NJ, Wu X, Fischer DJ, Van Etten-Lee M, Himle JA, Leventhal BL, Cook EH Jr, Hanna GL: Association testing of the positional and functional candidate gene SLC1A1/EAAC1 in early-onset obsessive-compulsive disorder. Arch Gen Psychiatry 2006; 63: 778– 785Crossref, Google Scholar
68 Stewart SE, Fagerness JA, Platko J, Smoller JW, Scharf JM, Illmann C, Jenike E, Chabane N, Leboyer M, Delorme R, Jenike MA, Pauls DL: Association of the SLC1A1 glutamate transporter gene and obsessive-compulsive disorder. Am J Med Genet B Neuropsychiatr Genet 2007; 144: 1027– 1033Crossref, Google Scholar
69 Wendland JR, Moya PR, Kruse MR, Ren-Patterson RF, Jensen CL, Timpano KR, Murphy DL: A novel, putative gain-of-function haplotype at SLC6A4 associates with obsessive-compulsive disorder. Hum Mol Genet 2008; 17: 717– 723Crossref, Google Scholar
70 Arnold PD, Rosenberg DR, Mundo E, Tharmalingam S, Kennedy JL, Richter MA: Association of a glutamate (NMDA) subunit receptor gene (GRIN2B) with obsessive-compulsive disorder: a preliminary study. Psychopharmacology (Berl) 2004; 174: 530– 538Crossref, Google Scholar
71 Sampaio AS, Fagerness J, Crane J, Leboyer M, Delorme R, Pauls DL, Stewart SE: Association between polymorphisms in GRIK2 gene and obsessive-compulsive disorder: a family-based study. CNS Neurosci Ther ( in press)Google Scholar
72 Pinto A, Greenberg BD, Grados MA, Bienvenu OJ 3rd, Samuels JF, Murphy DL, Hasler G, Stout RL, Rauch SL, Shugart YY, Pauls DL, Knowles JA, Fyer AJ, McCracken JT, Piacentini J, Wang Y, Willour VL, Cullen B, Liang KY, Hoehn-Saric R, Riddle MA, Rasmussen SA, Nestadt G: Further development of YBOCS dimensions in the OCD Collaborative Genetics study: symptoms vs. categories. Psychiatry Res 2008; 160: 83– 93Crossref, Google Scholar
73 Kwon JS, Joo YH, Nam HJ, Lim M, Cho EY, Jung MH, Choi JS, Kim B, Kang DH, Oh S, Park T, Hong KS: Association of the glutamate transporter gene SLC1A1 with atypical antipsychotics-induced obsessive-compulsive symptoms. Arch Gen Psychiatry 2009; 66: 1233– 1241Crossref, Google Scholar
74 Brune C, Kim S, Hanna G, Courchesne E, Lord C, Leventhal B, Cook EH: Family-based association testing of OCD-associated SNPs of SLC1A1 in an autism sample. Autism Res 2008; 1: 108– 113Crossref, Google Scholar



