Clinical Importance of Sleep Disturbance as a Treatment Target in PTSD
Abstract
In this review, evidence for the importance of sleep-related disturbances in PTSD as a target for treatment is presented. Examination of efficacy studies of the first-line treatments for posttraumatic stress disorder (PTSD)—selective serotonin reuptake inhibitors and cognitive behavior therapy—indicates that neither adequately treats PTSD nightmares and insomnia. The published guidelines that recommend these treatments do not provide advice on treating posttraumatic sleep disturbance, suggesting a possible lack of awareness of the problem. Based on clinical reports, imaging, and polysomnographic studies, a theoretical model in which REM dysregulation in PTSD plays a key role in the development and persistence of PTSD will be presented. Finally, evidence regarding the beneficial effects of prazosin and other agents in the treatment of PTSD-related sleep disturbance will be highlighted.
CLINICAL CONTEXT: SLEEP DISTURBANCE AS A CORE SYMPTOM IN PTSD
Persistent and severe posttraumatic nightmares and sleep disturbance are reported by more than 70% of combat veterans and civilians with posttraumatic stress disorder (PTSD) (1). It is surprising that there has been relatively little research in this area. In 1989 Ross et al. (2) speculated that sleep disturbance and nightmares in particular were relatively specific for the disorder, a “hallmark” symptom of PTSD. Individuals with severe PTSD-related nightmares often develop conditioned fear of going back to sleep or going to sleep (3). Germain et al. (4) studied 367 individuals with PTSD of 6 months' to more than 30 years' duration and found that PTSD severity was most closely associated with the severity of sleep disturbances.
Other sleep-related problems that are common in PTSD have been described. Periodic limb movement disorder, a condition characterized by repetitive movements of the arms and legs during sleep (5), occurs in 30%–70% of individuals with PTSD. Sleep-disordered breathing (SDB) disorders such as obstructive sleep apnea occur in 40%–91% of individuals with PTSD, far exceeding the rates in demographically similar individuals without PTSD or in the general population (6). Both of these disorders cause miniarousals during sleep and contribute to sleep disturbance (Figure 1). Finally, the cumulative sleep deprivation resulting from the above problems acts as an additional stressor that contributes to allostatic load by increasing inflammatory cytokine levels and promoting sympathetic activation (7). Even brief experimental sleep deprivation in normal subjects has clear-cut adverse effects on cognition, mood, and frontal lobe function (8, 9).
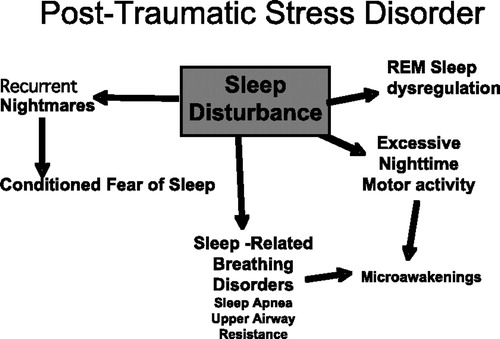
Figure 1. PTSD Sleep-related Disturbances.
PTSD-related sleep disturbance has been viewed by many as an accompanying or secondary symptom that should improve with successful treatment of PTSD (10, 11). The prevalence of this perspective is apparent in published expert consensus statements and treatment guidelines intended to advise clinical practice but do not mention sleep (12). In the DSM-IV-TR diagnostic criteria for PTSD (Table 1), nightmares and insomnia appear in different symptom clusters and neither is required for a diagnosis of PTSD. This fact may also reflect the complexity of the disorder or insufficient information about which symptoms are most fundamental. It is not widely recognized that in the aftermath of a traumatic event, sleep disturbance appears before the onset of PTSD (13) as does polysomnographic evidence of sleep disruption; both are predictors for PTSD (14, 15).
 |
Table 1. DSM-IV-TR Criteria for PTSD
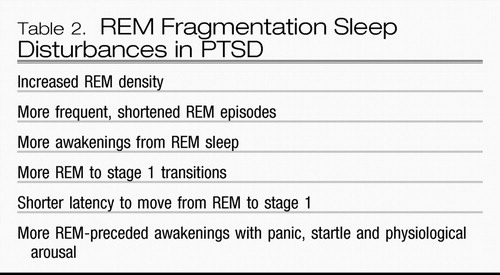 |
Table 2.
TREATMENT STRATEGIES AND EVIDENCE
DO FIRST-LINE TREATMENTS FOR PTSD EFFECTIVELY TREAT SLEEP DISTURBANCE?
Maher et al. (16) recently reviewed the empirical evidence for efficacy of pharmacotherapy and certain cognitive behavior treatments for PTSD and concluded that the recommended first-line treatments do not effectively resolve PTSD-related sleep disruption. Recommendations made for PTSD pharmacotherapy by several expert consensus groups (17) were in unanimous agreement that the selective serotonin reuptake inhibitors (SSRIs) should be considered as first-line pharmacotherapy for PTSD. Improvements in all three symptom clusters (B, C, and D) (Table 1) were reported in the pivotal studies of the three Food and Drug Administration-approved SSRIs (fluoxetine, paroxetine, and sertraline). However, there were minimal effects on insomnia and no evidence for clinically meaningful reduction of nightmares (16) in these studies. In summary, the evidence to date suggests that the SSRIs have some beneficial effects on PTSD, minimal effects on insomnia, and no effect on nightmares in PTSD.
Cognitive behavior therapy (CBT) with exposure has been shown to benefit PTSD and is recommended as the first-line non-drug treatment (18, 19) but only a few studies provided sleep outcome data. In one report, 48% of 24 of individuals completing treatment with CBT for civilian PTSD and no longer meeting the DSM-IV-TR criteria still had clinically significant sleep disturbance (primarily insomnia) (3); some of these patients derived a benefit from additional insomnia-targeted CBT (20). In one open label (21) and one controlled trial (22) of imagery rehearsal therapy (IRT) focused on PTSD-related nightmares and insomnia, there were clinically significant improvements in sleep measures and PTSD, but most participants continued to have at least moderately severe insomnia, nightmares, and PTSD at the end of the study.
These data indicate that neither of the first-line PTSD treatments—SSRIs or CBT with exposure—is particularly effective for normalizing sleep. SSRIs are ineffective for nightmares and have only minimal benefit on other sleep disturbances. The few data available suggest that there is residual sleep disturbance after standard CBT. Importantly, the IRT studies that targeted sleep disruption provide evidence suggesting that targeting sleep disturbance appears to reduce PTSD severity.
NEUROBIOLOGICAL SUBSTRATES OF PTSD RELEVANT TO SLEEP DISTURBANCE
One reason for reconsideration of the importance of sleep disruption in PTSD is that sleep is regulated in part by brain areas in which PTSD-related changes occur, suggesting that the stress response, arousal, fear conditioning/extinction, emotional memory, and, importantly, sleep may be biologically linked. Exaggerated activity of the amygdala in conjunction with reduced activation in the medial prefrontal cortex (mPFC) and hippocampal dysfunction have been most consistently reported (23). These changes are thought to interfere with extinction of the fear response (24, 25). Imaging studies show that exposure to trauma-related stimuli results in excessive activation in the amygdala and decreased activation in the mPFC/anterior cingulate and hippocampus (25). The magnitude of these changes correlates with the clinical severity of PTSD symptoms (26) and appears to increase with repeated reexposure (27). Persistently increased release of norepinephrine (NE), a monoamine that plays a critical role in fear conditioning/extinction and in part mediates stress-related deficits in hippocampal function, may not only interfere with extinction but also could actually promote fear conditioning (23, 28). Excessive NE activity is also thought to mediate in part symptoms of reexperiencing, hyperarousal, vigilance, and, importantly, sleep disturbance in PTSD (15, 29–33).
POLYSOMNOGRAPHY IN PTSD
Normal human sleep is characterized by cycles of REM sleep and non-REM (NREM) sleep, each of which takes 70–120 minutes (1). NREM sleep includes four stages (1 through 4) in which stages 3 and 4 represent deep sleep. In healthy young adults, stages 3 and 4 are most prevalent during the first one third of the 8- to 9-hour night. REM episodes occur mostly during deeper stages 3 and 4 and are characterized by rapid ocular movements and motor paralysis. REM episodes typically increase in length as the night progresses. During REM and NREM sleep in normal subjects, there are changes in activation and deactivation of different brain areas as measured by regional cerebral blood flow (rCBF) (4). During REM sleep there is a relative increase in rCBF in the amygdala, paralimbic areas, and wake-promoting areas (medial pons and thalamus) and reduced rCBF in lateral prefrontal, parietal, and sensory cortices compared with wakefulness. During NREM sleep, rCBF is decreased in the pons, midbrain reticular and thalamic areas, and associated cortices and increased in areas that promote sleep (dorsal pontine tegmentum and basal forebrain) (34). On the basis of their respective patterns of brain activation and deactivation, NREM sleep is hypothesized to be a restorative, low-activation state and REM sleep may be related to learning, memory consolidation, and/or emotional processing (1, 11).
Despite such consistent and high rates of subjective reports of sleep problems (35), results of polysomnographic studies in PTSD were surprisingly inconsistent and inconclusive (36) and failed to reflect the severe sleep disturbances reported. In 2007, Kobayashi et al. (37) completed a metaanalysis of 20 polysomnography studies in PTSD that used control groups for a total of 772 patients. This analysis revealed that there were, in fact, characteristic polysomnographic abnormalities in individuals with PTSD than in those without PTSD: more light (stage 1) sleep, less deep (stages 3 and 4), slow-wave sleep, and greater REM density. Sleep abnormalities in PTSD were modulated by age, comorbid depression, sex, and substance abuse.
Nightmares in PTSD.
A number of researchers have speculated that nightmares are a core feature of PTSD (2, 10, 11) that predict chronicity of sleep disturbance (38). Compared with those without PTSD, PTSD nightmares are more frequent, occur earlier in the sleep cycle, are consistent in content or theme, and may replicate real events (35). Fear nightmares may generate further sleep-disruptive behaviors such as fear of going to sleep or going back to sleep after checking or other safety behaviors, nighttime eating, sleeping in lounge chairs, and others (3, 10), thus contributing to overall sleep deprivation.
REM sleep disruption in PTSD.
As more is learned about REM disruption in PTSD, several investigators have suggested that disordered REM sleep may be important in the genesis and maintenance of PTSD (2, 10, 11, 15). In addition to increased REM density, others have reported more REM-to-stage-1 transitions (39), more awakenings from REM sleep (6), and more frequent but shorter REM episodes (14) in subjects with PTSD than in control subjects. This REM fragmentation (14) may theoretically be caused by excessive NE activity (15), prematurely terminating REM episodes or perturbing the normal rhythm of cholinergic REM-on and aminergic REM-off signals (10, 11, 31, 32, 40). REM fragmentation may effectively mimic REM deprivation with consequences resembling the behavioral aspects of REM sleep behavior syndrome (41) and possibly the metabolic effects of REM deprivation (7).
PERIODIC LIMB MOVEMENTS IN PTSD
Excessive motor movements during sleep in PTSD have been described by a number of investigators (4–6, 42, 43). Because nightmares in PTSD tend to emerge just after REM episodes, during which there is normally motor paralysis, Ross et al. (42) investigated motor behavior during REM sleep in war veterans via electromyographic recording of anterior tibialis muscle movements. Prolonged anterior tibialis twitches were more frequent in subjects with PTSD than in control subjects during REM sleep. Periodic limb movements also occurred more often during NREM sleep in subjects with PTSD (6); these were similar to symptoms of REM sleep behavior disorder (41), leading to speculation that disturbed REM-NREM phasic mechanisms may play a role in sleep-motor behavior (6, 10, 11, 42).
SDB in PTSD.
There are numerous reports of SDB, especially sleep apnea in individuals with PTSD (37, 43–49). A survey of 256 sexual assault survivors showed that 51% exhibited SDB and 61% exhibited sleep-related movement disorders. The literature suggests a complicated interaction between trauma and disordered sleep. Whether sleep-related airway obstruction contributes to the etiology of PTSD, is a result of PTSD, or is related more generally to sleep deprivation remains unclear.
QUESTIONS AND CONTROVERSY: IS REM DYSREGULATION A MODEL FOR SLEEP DISTURBANCE IN PTSD?
During normal sleep, REM sleep is initiated in the ventral part of the oral pontine reticular nucleus (40) by firing of cholinergic neurons (31). This can occur only during requisite “silence of the locus ceruleus” (32) (i.e., inhibition of locus ceruleus and raphe activity). REM sleep is terminated by increased aminergic input from sleep-off nuclei including the raphe and locus ceruleus, thereby creating a dynamic, alternating cycle of cholinergic-aminergic activity. In PTSD, the persistently overactive noradrenergic system has been hypothesized to escape normal inhibitory processes, resulting in early termination or disruption of REM episodes as evidenced by increased REM density and more frequent REM awakenings (10, 11) (Figure 2). With perturbation of the normal cadence of REM-on and REM-off cycles, periodic limb movements during REM episodes could theoretically result from disrupted timing or incomplete termination of noradrenergic signaling in or near REM episodes. In addition, the occurrence of nightmares during REM episodes may be related to amygdala hyperactivity with incompletely inhibited, intrusive α-adrenergic signals during REM episodes. This hypothesis is consistent with pharmacologic induction of nightmares with α-agonists (50, 51) and indirectly supported by recent preclinical evidence that membrane potential changes related to REM sleep deprivation are mediated via an α1-adrenergic mechanism (52). This model suggests that agents which block central postsynaptic norepinephrine receptors or decrease central NE release (33, 53) and/or otherwise normalize REM sleep might have benefits in PTSD-related sleep disturbances.
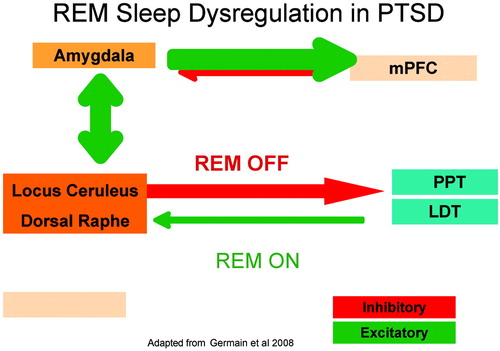
Figure 2. Schematic Model of REM Sleep Dysregulation in PTSD.
RECOMMENDATIONS: IMPLICATIONS FOR TREATMENT SLEEP DISTURBANCE IN PTSD
The focus of this brief review was to examine the importance of sleep disturbance in PTSD and discuss the importance of treating disrupted sleep. There is accruing evidence that some agents may be specifically useful for treating patients with PTSD who have residual sleep disturbance.
PRAZOSIN
Prazosin, a centrally and peripherally acting α1-receptor antagonist, was shown to meaningfully reduce or eliminate PTSD-related nightmares in veterans (54, 55) and civilians (56) in three placebo-controlled studies. Target doses of 10–15 mg at bedtime in the first two studies in veterans with combat PTSD were well tolerated with only a few terminations due to orthostatic hypotension (54, 55). The third study was a placebo-controlled crossover study in 13 civilians with PTSD. In addition, this study included sleep measures. Treatment periods were 3 weeks (random assignment to prazosin or placebo) separated by a 1-week washout; the average dose was approximately 3 mg nightly. Prazosin treatment was superior to placebo in improving sleep disturbance and nightmares and was associated with increased total sleep time, total REM time, and increased duration of REM episodes (56). In one small study of subjects for whom nightmares were controlled well with prazosin, adding small daytime doses reduced reactivity to trauma cues during the day (57). These findings lend support to the model linking noradrenergic overdrive and REM dysregulation for PTSD-related nightmares, sleep disturbance, and perhaps other PTSD symptoms.
Prazosin was originally marketed as an antihypertensive agent (Minipress) in doses up to 20 daily. Dosage forms are 1-, 2-, or 5-mg capsules. For nightmares, bedtime dosing is used, with an initial dose of 1–2 mg with monitoring for postural hypotension and with advancement every few weeks to 10–15 mg unless improvement occurs earlier. Clinical experience indicates that suppression of nightmare symptoms can occur at doses as low as 1 mg nightly within 1–2 weeks. The most common side effects are postural dizziness in about 10% of patients, to which most develop tolerance. Sedation occurs in about 10% of patients, but for sleep disturbance this is desirable for most patients. Prazosin is also used off-label to treat benign prostatic hypertrophy (2 mg b.i.d.) and Raynaud's disease (0.5–3 mg b.i.d.).
OTHER ANTIADRENERGIC AGENTS
The evidence for efficacy of other antiadrenergic agents is much less well-developed than that for prazosin. Both clonidine, an α2-adrenergic agonist, and guanfacine have been reported to improve symptoms of PTSD in children and in adults (33, 53). In one controlled study guanfacine, an α2-adrenergic agonist that is less selective for the α2-receptor than clonidine, was not found to be efficacious for PTSD-related nightmares (58). Propranolol, a β-adrenergic antagonist, modestly reduced the percentage of traumatized individuals who develop PTSD (59) and is anecdotally helpful in children with PTSD, but there are no reports on sleep effects.
GABAPENTIN
Gabapentin is an anticonvulsant that also exerts anxiolytic, antinociceptive, and sleep-promoting effects in humans (60). The broad actions of gabapentin in the central and peripheral nervous systems, as well as those of its derivative pregabalin, are thought to be effected via its high affinity for and binding to the α2δ subunit of voltage-gated neuronal Ca2+ channels. This results in reduced release of norepinephrine, serotonin (5-HT), glutamate, and substance P from neurons with pathologically rapid firing rates (60). In normal subjects and sample patients with epilepsy, gabapentin enhances slow-wave sleep, increases total sleep time, reduces arousals and awakenings, and reduces periodic motor movements during sleep (61, 62). Hamner et al. (63) reported the off label effects of adjunctive gabapentin (300–2100 mg daily) added to the existing drug treatment regimen of 30 veterans with PTSD to reduce nightmares and sleep disturbance. Twenty of these had been receiving SSRIs shown to have efficacy in PTSD. Of 30 patients, 24 (77%) showed moderate to marked improvement in sleep disturbance and global PTSD severity after the adjunctive gabapentin treatment. It is unclear whether these therapeutic effects are mediated via antiadrenergic or other mechanisms such as effects on γ-aminobutyric acid (GABA) neurotransmission. In controlled studies, gabapentin showed clinically and (nearly statistically) significant benefits in the treatment of panic disorder and social anxiety disorder (64, 65). Both gabapentin and its derivative pregabalin promote nonsynaptosomal glial uptake and release of GABA (66). There is presumed dysregulation of GABA functioning in both panic disorder and PTSD as evidenced by reduced GABAA-benzodiazepine receptor binding (67, 68). In addition, preclinical evidence suggests that gabapentin normalizes GABA function in the central nucleus of the amygdala and in laboratory models using human neocortex synaptosomal preparations, which may be relevant to its therapeutic effects in humans (69, 70). In summary, the existing human data and interesting preclinical evidence support the hypothesis that gabapentin and probably pregabalin show promise as potential alternative or adjunctive treatments for SSRI-treated patients with PTSD who have residual sleep disturbance.
SUMMARY
The evidence presented supports the concept that disturbed sleep is an important issue in PTSD treatment. More research is needed to confirm whether normalizing REM abnormalities in PTSD is consistently associated with improvement. The findings that generated this hypothesis and the recently reported effects of prazosin on sleep architecture are certainly encouraging, and the convincing controlled data on the effects of prazosin on sleep disturbance are of immediate clinical utility. Gabapentin and it derivative pregabalin should be studied in controlled trials as monotherapy and as adjunctive treatments in prospective clinical trials. The benefit of CBT treatment, which targets nightmares (IRT), also supports the theory that targeting sleep in PTSD is clinically relevant. It is hoped that further studies will clarify this understudied area.
1 Harvey AG, Jones C, Schmidt A: Sleep and posttraumatic stress disorder: a review. Clin Psychol Rev 2003; 23: 377– 407Crossref, Google Scholar
2 Ross RJ, Ball WA, Sullivan KA, Caroff SN: Sleep disturbance as the hallmark of posttraumatic stress disorder. Am J Psychiatry 1989; 146: 697– 707Crossref, Google Scholar
3 Zayfert C, DeViva JC: Residual insomnia following cognitive behavioral therapy for PTSD. J Trauma Stress 2004; 17: 69– 73Crossref, Google Scholar
4 Germain A, Buysse DJ, Shear MK, Fayyad R, Austin C: Clinical correlates of poor sleep quality in posttraumatic stress disorder. J Trauma Stress 2004; 17: 477– 484Crossref, Google Scholar
5 Brown TM, Boudewyns PA: Periodic limb movements of sleep in combat veterans with posttraumatic stress disorder. J Trauma Stress 1996; 9: 129– 136Crossref, Google Scholar
6 Mellman TA, Kulick-Bell R, Ashlock LE, Nolan B: Sleep events among veterans with combat-related posttraumatic stress disorder. Am J Psychiatry 1995; 152: 110– 115Crossref, Google Scholar
7 McEwen BS: Sleep deprivation as a neurobiologic and physiologic stressor: allostasis and allostatic load. Metabolism 2006; 55 ( 10 Suppl 2): S20– S23Google Scholar
8 Wu JC, Gillin JC, Buchsbaum MS, Chen P, Keator DB, Khosla Wu N, Darnall LA, Fallon JH, Bunney WE: Frontal lobe metabolic decreases with sleep deprivation not totally reversed by recovery sleep. Neuropsychopharmacology 2006; 31: 2783– 2792Crossref, Google Scholar
9 Killgore WD, Kahn-Greene ET, Lipizzi EL, Newman RA, Kamimori GH, Balkin TJ: Sleep deprivation reduces perceived emotional intelligence and constructive thinking skills. Sleep Med 2008; 9: 517– 526Crossref, Google Scholar
10 Spoormaker1. VI, Montgomery P: Disturbed sleep in post-traumatic stress disorder: Secondary symptom or core feature? Sleep Med Rev 2008; 12: 169– 184Google Scholar
11 Germain A, Buysse DJ, Nofzinger E: Sleep-specific mechanisms underlying posttraumatic stress disorder: integrative review and neurobiological hypotheses. Sleep Med Rev 2008; 12: 185– 195Crossref, Google Scholar
12 Ballenger JC, Davidson JR, Lecrubier Y, Nutt DJ, Marshall RD, Nemeroff CB, Shalev AY, Yehuda R: Consensus statement update on posttraumatic stress disorder from the international consensus group on depression and anxiety. J Clin Psychiatry 2004; 65 ( Suppl 1): 55– 62Google Scholar
13 Koren D, Arnon I, Lavie P, Klein E: Sleep complaints as early predictors of posttraumatic stress disorder: a 1-year prospective study of injured survivors of motor vehicle accidents. Am J Psychiatry 2002; 159: 855– 857Crossref, Google Scholar
14 Mellman TA, Bustamante V, Fins AI, Pigeon WR, Nolan B: REM sleep and the early development of posttraumatic stress disorder. Am J Psychiatry 2002; 159: 1696– 1701Crossref, Google Scholar
15 Mellman TA, Knorr BR, Pigeon WR, Leiter JC, Akay M: Heart rate variability during sleep and the early development of posttraumatic stress disorder. Biol Psychiatry 2004; 55: 953– 956Crossref, Google Scholar
16 Maher MJ, Rego SA, Asnis GA: Sleep disturbances in patients with post-traumatic stress disorder: epidemiology, impact and approaches to management. CNS Drugs 2006; 20: 567– 591Crossref, Google Scholar
17 Stein DJ, Ipser JC, Seedat S: Pharmacotherapy for post traumatic stress disorder (PTSD). Cochrane Database Syst Rev. 2006; 1: CD00279Google Scholar
18 Foa EB, Davidson J, Frances A: The expert consensus guideline series: treatment of post-traumatic stress disorder: the expert consensus panels for PTSD. J Clin Psychiatry 1999; 60( Suppl 16): 13– 76Google Scholar
19 Foa EB: Psychosocial therapy for posttraumatic stress disorder. J Clin Psychiatry 2006; 67( Suppl 2): 40– 45Google Scholar
20 DeViva JC, Zayfert C, Pigeon WR, Mellman TA: Treatment of residual insomnia after CBT for PTSD: case studies. J Trauma Stress 2005; 18: 155– 159Crossref, Google Scholar
21 Krakow B, Johnston L, Melendrez D, Hollifield M, Warner TD, Chavez-Kennedy D, Herlan MJ: An open-label trial of evidence-based cognitive behavior therapy for nightmares and insomnia in crime victims with PTSD. Am J Psychiatry 2001; 158: 2043– 2047Crossref, Google Scholar
22 Krakow B, Hollifield M, Johnston L, Koss M, Schrader R, Warner TD, Tandberg D, Lauriello J, McBride L, Cutchen L, Cheng D, Emmons S, Germain A, Melendrez D, Sandoval D, Prince H: Imagery rehearsal therapy for chronic nightmares in sexual assault survivors with posttraumatic stress disorder: a randomized controlled trial. JAMA 2001; 286: 537– 545Crossref, Google Scholar
23 Bremner JD, Vermetten E, Schmahl C, Vaccarino V, Vythilingam M, Afzal N, Grillon C, Charney DS: Positron emission tomographic imaging of neural correlates of a fear acquisition and extinction paradigm in women with childhood sexual-abuse-related post-traumatic stress disorder. Psychol Med 2005; 35: 791– 806Crossref, Google Scholar
24 Etkin A, Wager TD: Functional neuroimaging of anxiety: a meta-analysis of emotional processing in PTSD, social anxiety disorder, and specific phobia. Am J Psychiatry 2007; 164: 1476– 1488Crossref, Google Scholar
25 Bremner JD, Elzinga B, Schmahl C, Vermetten E: Structural and functional plasticity of the human brain in posttraumatic stress disorder. Prog Brain Res 2008; 167: 171– 186Crossref, Google Scholar
26 Moreya RA, Petty CM, Cooper DA, LaBara KS, McCarthy G: Neural systems for executive and emotional processing are modulated by symptoms of posttraumatic stress disorder in Iraq War veterans. Psychiatry Res Neuroimaging 2008; 162: 59– 72Crossref, Google Scholar
27 Gilboa A, Shalev AY, Laor L, Lester H, Louzoun Y, Chisin R, Bonne O: Functional connectivity of the prefrontal cortex and the amygdala in posttraumatic stress disorder. Biol Psychiatry 2004; 55: 263– 272Crossref, Google Scholar
28 Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK: De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. J Abnorm Psychol 2000; 109: 290– 298Crossref, Google Scholar
29 Debiec J, LeDoux JE: Noradrenergic signaling in the amygdala contributes to the reconsolidation of fear memory: treatment implications for PTSD. Ann NY Acad Sci 2006; 1071: 521– 524Crossref, Google Scholar
30 Mellman TA, Kumar A, Kulick-Bell R, Kumar M, Nolan B: Nocturnal/daytime urine noradrenergic measures and sleep in combat-related PTSD. Biol Psychiatry 1995; 38: 174– 179Crossref, Google Scholar
31 Hobson JA, Stickgold R, Pace-Schott EF: The neuropsychology of REM sleep dreaming. Neuroreport 1998; 9: R1– R14Crossref, Google Scholar
32 Gottesmann C: Noradrenaline involvement in basic and higher integrated REM sleep processes. Prog Neurobiol 2008; 85: 237– 272Crossref, Google Scholar
33 Strawn JR, Geracioti TD Jr: Noradrenergic dysfunction and the psychopharmacology of posttraumatic stress disorder. Depress Anxiety 2008; 25: 260– 271Crossref, Google Scholar
34 Nofzinger E: Human regional cerebral glucose metabolism during non-rapid eye movement sleep in relation to waking. Brain 2002; 125: 1105– 1115Crossref, Google Scholar
35 Inman DJ, Silver SM, Doghramji K: Sleep disturbance in post-traumatic stress disorder: a comparison with non-PTSD insomnia. J. Trauma Stress 1990; 3: 429– 437Crossref, Google Scholar
36 Pillar G, Malhotra A, Lavie P: Post-traumatic stress disorder and sleep: what a nightmare! Sleep Med Rev 2000; 4: 183– 200Crossref, Google Scholar
37 Kobayashi I, Boarts JM, Delahanty DL: Polysomnographically measured sleep abnormalities in PTSD: a meta-analytic review. Psychophysiology 2007; 44: 660– 669Crossref, Google Scholar
38 Kobayashi I, Sledjeski EM, Spoonster E, Fallon WF Jr, Delahanty DL: Effects of early nightmares on the development of sleep disturbances in motor vehicle accident victims. J Trauma Stress 2008; 21: 548– 555Crossref, Google Scholar
39 Breslau N, Roth T, Burduvali E, Kapke A, Schultz L, Roehrs T: Sleep in lifetime posttraumatic stress disorder: a community-based polysomnographic study. Arch Gen Psychiatry 2004; 61: 508– 516Crossref, Google Scholar
40 Reinoso-Suárez F, de Andrés I, Rodrigo-Angulo ML, Garzón M: Brain structures and mechanisms involved in the generation of REM sleep. Sleep Med Rev 2001; 5: 63– 77Crossref, Google Scholar
41 Chiu HF, Wing YK: REM sleep behaviour disorder: an overview. Int J Clin Pract 1997; 51: 451– 454Google Scholar
42 Ross RJ, Ball WA, Dinges DF, Kribbs NB, Morrison AR, Silver SM, Mulvaney FD: Motor dysfunction during sleep in posttraumatic stress disorder. Sleep 1994; 17: 723– 732Crossref, Google Scholar
43 Krakow B, Germain A, Tandberg D, Koss M, Schrader R, Hollifield M, Cheng D, Edmond T: Sleep breathing and sleep movement disorders masquerading as insomnia in sexual-assault survivors. Compr Psychiatry 2000; 41: 49– 56Crossref, Google Scholar
44 Krakow B, Germain A, Warner TD, Schrader R, Koss M, Hollifield M, Tandberg D, Melendrez D, Johnston L: The relationship of sleep quality and posttraumatic stress to potential sleep disorders in sexual assault survivors with nightmares, insomnia, and PTSD. J Trauma Stress 2001; 14: 647– 665Crossref, Google Scholar
45 Krakow B, Haynes PL, Warner TD, Santana E, Melendrez D, Johnston L, Hollifield M, Sisley BN, Koss M, Shafer L: Nightmares, insomnia, and sleep-disordered breathing in fire evacuees seeking treatment for posttraumatic sleep disturbance. J Trauma Stress 2004; 17: 257– 268Crossref, Google Scholar
46 Krakow B, Lowry C, Germain A, Gaddy L, Hollifield M, Koss M, Tandberg D, Johnston L, Melendrez D: A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res 2000; 49: 291– 298Crossref, Google Scholar
47 Krakow B, Melendrez D, Johnston L, Warner TD, Clark JO, Pacheco M, Pedersen B, Koss M, Hollifield M, Schrader R: Sleep-disordered breathing, psychiatric distress, and quality of life impairment in sexual assault survivors. J Nerv Ment Dis 2002; 190: 442– 452Crossref, Google Scholar
48 Lamarche LJ, De Koninck J: Sleep disturbance in adults with posttraumatic stress disorder: a review. J Clin Psychiatry 2007; 68: 1257– 1270Crossref, Google Scholar
49 Ocasio-Tascon ME, Alicea-Colon E, Torres-Palacios A, Rodriguez-Cintron W: The veteran population: one at high risk for sleep-disordered breathing. Sleep Breath 2006; 10: 70– 75Crossref, Google Scholar
50 Thompson DF, Pierce DR: Drug-induced nightmares. Ann Pharmacother 1999; 33: 93– 98Crossref, Google Scholar
51 Pagel JF, Helfter P: Drug induced nightmares—an etiology based review. Hum Psychopharmacol 2003; 18: 59– 67Crossref, Google Scholar
52 Das G, Mallick BN: Noradrenaline acting on α1-adrenoceptor mediates REM sleep deprivation-induced increased membrane potential in rat brain synaptosomes. Neurochem Int 2008; 52: 734– 740Crossref, Google Scholar
53 Boehnlein JK, Kinzie JD: Pharmacologic reduction of CNS noradrenergic activity in PTSD: the case for clonidine and prazosin. J Psychiatr Pract 2007; 13: 72– 78Crossref, Google Scholar
54 Raskind MA, Peskind ER, Hoff DJ, Hart KL, Holmes HA, Warren D, Shofer J, O'Connell J, Taylor F, Gross C, Rohde K, McFall ME: A parallel group placebo controlled study of prazosin for trauma nightmares and sleep disturbance in combat veterans with post-traumatic stress disorder. Biol Psychiatry 2007; 61: 928– 934Crossref, Google Scholar
55 Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, Dobie DJ, Hoff D, Rein RJ, Straits-Troster K, Thomas RG, McFall MM: Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry 2003; 160: 371– 373Crossref, Google Scholar
56 Taylor FB, Martin P, Thompson C, Williams J, Mellman TA, Gross C, Peskind ER, Raskind MA: Prazosin effects on objective sleep measures and clinical symptoms in civilian trauma posttraumatic stress disorder: a placebo-controlled study. Biol Psychiatry 2008; 63: 629– 632Crossref, Google Scholar
57 Taylor FB, Lowe K, Thompson C, McFall MM, Peskind ER, Kanter ED, Allison N, Williams J, Martin P, Raskind MA: Daytime prazosin reduces psychological distress to trauma specific cues in civilian trauma posttraumatic stress disorder. Biol Psychiatry 2006; 59: 577– 581Crossref, Google Scholar
58 Neylan TC, Lenoci M, Samuelson KW, Metzler TJ, Henn-Haase C, Hierholzer RW, Lindley SE, Otte C, Schoenfeld FB, Yesavage JA, Marmar CR: No improvement of posttraumatic stress disorder symptoms with guanfacine treatment. Am J Psychiatry 2006; 163: 2186– 2188Crossref, Google Scholar
59 Pitman RK, Sanders KM, Zusman RM, Healy AR, Cheema F, Lasko NB, Cahill L, Orr SP: Pilot study of secondary prevention of posttraumatic stress disorder with propranolol. Biol Psychiatry 2002; 51: 189– 192Crossref, Google Scholar
60 Taylor CP, Gee NS, Su TZ, Kocsis JD, Welty DF, Brown JP, Dooley DJ, Boden P, Singh L: A summary of mechanistic hypotheses of gabapentin pharmacology. Epilepsy Res 1998; 29: 233– 249Crossref, Google Scholar
61 Happe S, Sauter C, Klosch G, Saletu B, Zeitlhofer J: Gabapentin versus ropinirole in the treatment of idiopathic restless legs syndrome. Neuropsychobiology 2003; 48: 82– 86Crossref, Google Scholar
62 Garcia-Borreguero D, Larrosa O, de la Llave Y, Verger K, Masramon X, Hernandez G: Treatment of restless legs syndrome with gabapentin: a double-blind, cross-over study. Neurology 2002; 59: 1573– 1579Crossref, Google Scholar
63 Hamner MB, Brodrick PS, Labbate LA: Gabapentin in PTSD: a retrospective, clinical series of adjunctive therapy. Ann Clin Psychiatry 2001; 13: 141– 146Crossref, Google Scholar
64 Pande AC, Pollack MH, Crockatt J, Greiner M, Chouinard G, Lydiard RB, Taylor CB, Dager SR, Shiovitz T: Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol 2000; 20: 467– 471Crossref, Google Scholar
65 Pande AC, Davidson JR, Jefferson JW, Janney CA, Katzelnick DJ, Weisler RH, Greist JH, Sutherland SM: Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol 1999; 19: 341– 348Crossref, Google Scholar
66 Lydiard RB: The role of GABA in anxiety disorders. J Clin Psychiatry 2003; 64( Suppl 3): 21– 27Google Scholar
67 Hasler G, Nugent AC, Carlson PJ, Carson RE, Geraci M, Drevets WC: Altered cerebral γ-aminobutyric acid type A-benzodiazepine receptor binding in panic disorder determined by [11C]flumazenil positron emission tomography. Arch Gen Psychiatry 2008; 65: 1166– 1175Crossref, Google Scholar
68 Geuze E, van Berckel BN, Lammertsma AA, Boellaard R, de Kloet CS, Vermetten E, Westenberg HG: Reduced GABAA benzodiazepine receptor binding in veterans with post-traumatic stress disorder. Mol Psychiatry 2008; 13: 74– 83Crossref, Google Scholar
69 Clemens KJ, Vendruscolo LF: Anxious to drink: gabapentin normalizes GABAergic transmission in the central amygdala and reduces symptoms of ethanol dependence. J Neurosci 2008; 28: 9087– 9089Crossref, Google Scholar
70 Brawek B, Loffler M, Weyerbrock A, Feuerstein TJ: Effects of gabapentin and pregabalin on K+-evoked 3H-GABA and 3H-glutamate release from human neocortical synaptosomes. Naunyn Schmiedebergs Arch Pharmacol 2009; 379: 361– 369Crossref, Google Scholar



