Substance-Related Disorders
Abstract
Substance-related disorders are common and cause significant medical, social, and psychological problems among users and those around them. This DSM category comprises a protean set of clinical syndromes, with the different substances exerting various effects. The authors review the diagnosis, natural history, biopsychosocial underpinnings, testing, and treatment of these disorders, focusing on the latest and most practical findings from the research and clinical literature. The future directions of the understanding and treatment of substance-related disorders are discussed.
In the United States, substance use disorders constitute a tremendous medical and social challenge: 13.8% of all Americans will have an alcohol-related substance use disorder in their lifetime, and an estimated 7.1% (15.1 million) of Americans 12 years old or older were current users of illicit drugs in 2001. The cost to the nation exceeds $275 billion annually. We provide a general review of the subject, focusing especially on advances in diagnosis, natural history, genetics, pathophysiology, psychopathology, and treatment.
Definition
In DSM-IV-TR (1), substance-related disorders are divided into two groups: substance use disorders, which include substance dependence and substance abuse, and substance-induced disorders, which encompass substance intoxication, substance withdrawal, and other psychiatric syndromes that are the direct pathophysiological consequence of use of a substance. DSM-IV-TR distinguishes substance-induced mental disorders from substance-induced disorders that are due to general medical conditions and those for which the causes are unknown.
DSM-IV-TR provides generic definitions for dependence, abuse, intoxication, and withdrawal and, with some exceptions, specific criteria sets for each of these states for almost all substances of abuse. For some substances, these diagnoses may be made in the absence of a specific criteria set. Table 1 indicates whether criteria sets have been established for certain states or substance combinations. Definitions of substance-induced syndromes other than intoxication and withdrawal are based on the DSM criteria for the syndromes they resemble and may be found in the relevant section of DSM-IV-TR—for example, cocaine-induced mood disorder is categorized as a mood disorder. This section addresses particular issues in the definition and diagnosis of the more complex conditions. An important caveat is that a majority of persons who use substances use more than one at a time, which complicates diagnosis.
“Addiction,” “cocainism,” and “alcoholism” are among many terms widely used to describe substance-related syndromes for which there are no DSM definitions. Nonetheless, the three terms generally refer to substance dependence, and in this review they are used to denote such conditions interchangeably with the types of substance dependence as defined in DSM-IV-TR.
Substance use disorders
Substance dependence
Dependence is a state comprising cognitive, behavioral, and physiological features that together signify continued substance use despite significant substance-related problems (Table 2). It is a pattern of repeated use lasting at least 12 months that can result in tolerance, withdrawal, and compulsive drug-taking behavior. Not all substances produce tolerance or withdrawal syndromes (see Table 1). Tolerance is the need for greatly increased amounts of a substance to produce a desired effect or a greatly diminished effect with constant use of the same amount of the substance. Withdrawal occurs when blood or tissue concentrations of a substance decline in an individual who had maintained prolonged heavy use of the substance. In this state the individual will likely take the substance to relieve or to avoid unpleasant withdrawal symptoms. Neither tolerance nor withdrawal is necessary or sufficient for a diagnosis of substance dependence, but a history of tolerance or withdrawal is usually associated with a poorer prognosis.
Substance abuse
Substance abuse as defined in DSM-IV-TR includes continued use of a substance, despite significant problems caused by its use, by an individual who does not meet criteria for substance dependence (Table 3). “Failure to fulfill major role obligations” is a criterion. To fulfill an abuse criterion, the substance-related problem must have occurred repeatedly or persistently during the same 12-month period. Substance abuse does not apply to caffeine and nicotine (see Table 1).
Substance-induced disorders
Substance intoxication and substance withdrawal are described in Tables 4 and 5, respectively. A diagnosis of a substance-induced mental disorder denotes the presence of a particular syndrome once it has been established that it is the direct result of the substance (via intoxication or withdrawal). Some disorders can persist after the substance has been eliminated from the body, but symptoms lasting more than 4 weeks after acute intoxication or withdrawal are considered manifestations either of a primary mental disorder or of a persisting substance-induced disorder. The differential diagnosis can be complicated: withdrawal from some substances (e.g., sedatives) may look like intoxication with others (e.g., amphetamines).
Alcohol
Alcohol use disorders: abuse and dependence
It is important to distinguish alcohol abuse from dependence; the prognostic implications of the two differ in terms of use, continued meeting of diagnostic criteria, and treatment. The alcohol abuser might not progress to dependence, and the alcohol-dependent person must become abstinent (2). Specifying whether physiological dependence is present has prognostic value, because it indicates a more severe clinical course (3), especially when withdrawal rather than tolerance is the basis for the diagnosis of physiological dependence. The term “primary alcoholism” is used in the context of psychiatric comorbidity. It refers to alcohol dependence that has led to psychopathology, most often an anxiety or mood disorder. “Secondary alcoholism” refers to dependence on alcohol that results from self-medication for an underlying psychiatric disorder. It commonly occurs with schizophrenia and bipolar disorder.
Alcohol-induced disorders
The alcohol-induced disorders include intoxication, withdrawal, intoxication delirium or withdrawal delirium, withdrawal seizures, alcohol-induced persisting dementia, alcohol-induced persisting amnestic disorder (Korsakoff’s syndrome), alcohol-induced psychotic disorder, and sexual dysfunction. Alcohol intoxication is time-limited and reversible, with onset depending on tolerance, amount ingested, and amount absorbed. It is affected by interactions with other substances, medical status, culture, and individual variation.
Delirium tremens can occur during either alcohol withdrawal or alcohol intoxication. Hallucinations may be auditory and of a persecutory nature, or they may be kinesthetic. Delirium tremens can appear suddenly, but usually it occurs gradually 2–3 days after cessation of drinking and reaches its peak intensity on day 4 or 5. It is usually benign and short-lived, subsiding after 3 days in the majority of cases, although subacute symptoms may last weeks. The fatality rate is estimated at less than 1%. Delirium tremens is associated with infections, subdural hematomas, trauma, liver disease, and metabolic disorders. If death occurs during delirium tremens, the cause is usually infectious fat embolism or cardiac arrhythmia (usually associated with hyperkalemia, hyperpyrexia, and poor hydration). Alcohol withdrawal seizures that are not a part of delirium tremens generally occur between 7 and 38 hours after the last alcohol use in chronic drinkers; the average is about 24 hours after the last drink. About 10% of all chronic drinkers experience a grand mal seizure at some time; of these, one-third go on to delirium tremens, and 2% go on to status epilepticus. Alcohol lowers the seizure threshold, which likely contributes to these seizures.
Thiamine deficiency associated with prolonged heavy alcohol use produces alcohol-induced persisting amnestic disorder, or Korsakoff’s syndrome, and its associated neurological deficits—peripheral neuropathy, cerebellar ataxia, and myopathy. Korsakoff’s syndrome often follows acute Wernicke’s encephalopathy—confusion, ataxia, nystagmus, ophthalmoplegia, and other neurological signs. As the encephalopathy subsides, severe impairment of anterograde and retrograde memory remains; confabulation is common. Early treatment of Wernicke’s encephalopathy with large doses of thiamine may prevent the development of Korsakoff’s syndrome.
Sedatives, hypnotics, and anxiolytics
These substances induce syndromes generally like those induced by alcohol, but their pharmacokinetics differ from alcohol’s in significant ways. Physical dependence on substances of this class is a predictable phenomenon that is closely related to dosage, half-life, and duration of use. At therapeutic doses, dependence may occur over the course of weeks, but at higher doses dependence develops more rapidly. The benzodiazepines vary in terms of half-life and predominant pharmacokinetics, leading to different abuse potentials. Salzman has provided a review (4).
Sedative, hypnotic, or anxiolytic withdrawal is a characteristic syndrome that develops in response to a relative decrease in intake after regular use. Grand mal seizures occur in 20%–30% of untreated individuals. The withdrawal syndrome produced by substances in this class may present as a life-threatening delirium.
Opioids
Opioid use disorders
Opioids refers to all drugs in this class, including heroin, morphine, synthetic drugs, and others. Most individuals with opioid dependence have significant tolerance and experience withdrawal. As noted in Table 1, there are no specific criteria for the diagnosis of opioid dependence or abuse other than the generic criteria. A diagnosis of dependence rather than of abuse is called for when use is accompanied by compulsive behavior related to opioids.
Opioid-induced disorders
Opioid intoxication and overdose are reversible by the administration of naloxone. Opioid withdrawal is a syndrome that follows a relative reduction in heavy and prolonged use. The syndrome begins within 6–8 hours after the last dose and peaks between 48 and 72 hours; symptoms disappear in 7–10 days. The signs and symptoms are the opposite of those of the acute agonist effects: lacrimation, rhinorrhea, pupillary dilation, piloerection, diaphoresis, diarrhea, yawning, mild hypertension, tachycardia, fever, and insomnia (Table 6). A flulike syndrome subsequently develops, with complaints, demands, and drug seeking. Uncomplicated withdrawal is usually not life-threatening unless a severe underlying disorder, such as cardiac disease, is present. Withdrawal occurs in 60%–94% of neonates of opioid-addicted mothers, beginning 12–24 hours after birth and sometimes persisting for months.
Cocaine
Cocaine can be chewed in the form of coca leaves, smoked in the form of coca paste, inhaled or injected as cocaine hydrochloride powder, or smoked in a cocaine alkaloid form (“freebase,” or “crack”). “Speedballing”—mixing cocaine and heroin—is particularly dangerous because of the potentiation of respiratory depressant effects.
Cocaine use disorders
Cocaine use can quickly produce dependence. With cocaine’s short half-life, frequent use is needed to maintain the high. Tolerance occurs with repeated use. The intensity and frequency of cocaine use are less in abuse than in dependence.
Cocaine-induced disorders
Cocaine-induced states must be clearly differentiated from schizophrenia, mood disorders, and anxiety disorders. Cocaine’s autonomic stimulant effects are more common than its depressant effects, which emerge only with chronic high-dose use. In overdose, cocaine’s effects on the noradrenergic system are significant and produce muscular twitching, rhabdomyolysis, seizures, cerebrovascular accident, myocardial infarction, arrhythmia, and respiratory failure. Metabolism is slowed when cocaine is combined with alcohol (producing cocaethylene), which increases the risk of death by 18 to 25 times. Tolerance to cocaine’s euphoric effects can develop during a binge. Often amphetamine or phencyclidine intoxication can be distinguished from cocaine intoxication only through toxicological studies. EEG abnormalities may be present during withdrawal from cocaine. Cocaine intoxication delirium may occur within 24 hours of use. Cocaine intoxication delirium and cocaine-induced psychotic disorder (usually with delusions) are not uncommon and should be kept in mind when evaluating patients with delirium or psychosis.
Amphetamines
In the DSM-IV-TR, amphetamines, which have a substituted-phenylethylamine structure (e.g., amphetamine, dextroamphetamine, and methamphetamine), are grouped with amphetamine-like substances that are structurally different, such as methylphenidate.
Amphetamine-induced disorders
In amphetamine intoxication, behavioral and psychological changes are accompanied by significant physiological and CNS syndromes. The state begins no more than 1 hour after use, depending on the drug and the method of delivery. “Perceptual disturbances” should be specified when hallucinations or illusions occur in intoxication without delirium if reality testing is intact. If hallucinations or illusions are accompanied by impaired reality testing, the diagnosis of amphetamine-induced psychotic disorder with hallucinations should be considered. Differentiating between amphetamine-induced psychosis and schizophrenia can be difficult (5).
Phencyclidine and ketamine
Variations of phencyclidine (PCP) include its thiophene analogue TCP (thienyl-cyclohexylpiperidine) and ketamine. These substances can be used orally or intravenously, and they can be smoked and inhaled. PCP is often mixed with other substances such as amphetamines, cannabis, cocaine, or hallucinogens.
Phencyclidine use disorders
Although the DSM-IV-TR diagnostic criteria for PCP dependence include the first seven items of the generic definition of substance dependence, criteria 2A and 2B may not apply, given that a clear-cut withdrawal pattern is difficult to establish. Adverse reactions to PCP, as with hallucinogens, may be more common among individuals with preexisting mental disorders. PCP dependence may have a rapid onset.
Phencyclidine-induced disorders
PCP intoxication produces both sympathetic and cholinergic effects. Intoxication begins within 5 minutes of use and peaks within 30 minutes. High-dose intoxication and overdose can produce hyperthermia and involuntary movements followed by amnesia, and coma, analgesia, seizures, and respiratory depression at the highest doses (i.e., greater than 20 mg). Because PCP is lipophilic, intoxication may persist for days. Mydriasis and nystagmus (vertical more than horizontal) are characteristic of PCP use. Perceptual disturbances should be specified if present. Psychosis is the most common PCP-induced mental disorder. Chronic psychosis may occur, along with long-term neuropsychological deficits.
Hallucinogens
This diverse group of substances includes indolamine derivatives (LSD, morning glory seeds), phenylalkylamine derivatives (mescaline, STP [2,5-dimethoxy-4-methylamphetamine], and ring-substituted amphetamines—MDA [methylenedioxyamphetamine; “old ecstasy”], MDMA [methylenedioxymethamphetamine; new “Ecstasy”], MDEA [methylenedioxyethamphetamine]), indole alkaloids (psilocybin, DMT [dimethyltryptamine]), and miscellaneous other compounds. Hallucinogens are usually taken orally, although dimethyltryptamine is smoked and sometimes injected.
Hallucinogen-induced disorders
In hallucinogen intoxication, perceptual changes occur alongside full alertness during or shortly after use. Changes include subjective intensification of perceptions, depersonalization, derealization, illusions, hallucinations, and synesthesias. DSM-IV-TR diagnosis requires the presence of at least two of seven physiological signs. Hallucinations are usually visual, often involving geometric forms but sometimes involving persons and objects. Auditory or tactile hallucinations are rare. Reality testing is usually preserved.
Hallucinogen persisting perception disorder (“flashbacks”) after cessation of hallucinogen use involves the reexperiencing of one or more of the same perceptual symptoms experienced while originally intoxicated. These are usually fleeting events, but in rare instances they may be lasting. Symptoms may be triggered by stress, drug use (including that of other drugs), or sensory deprivation, and they may be triggered intentionally. Psychotic disorder not otherwise specified is the proper diagnosis if the patient interprets the perceptions as reality or having origins that are unreal.
Cannabis
Cannabis-induced disorders
The cannabis class includes substances such as marijuana, hashish, and purified delta-9-tetrahydrocannabinol (THC). Usually smoked, these may also be mixed with food and eaten. Intoxication peaks 10–30 minutes after smoking cannabis and lasts about 3 hours. As lipophilic molecules, cannabinoids and their metabolites have a half-life of approximately 50 hours, and their effects may persist for 12–24 hours. At least two of the following signs develop within 2 hours of use: conjunctival injection, increased appetite, dry mouth, and tachycardia. Intoxication with CNS depressants (including alcohol, benzodiazepines, barbiturates, and other drugs), unlike with cannabis, usually decreases appetite, increases aggressive behavior, and produces nystagmus or ataxia. At low doses, hallucinogen intoxication may resemble cannabis intoxication. Acute adverse reactions to cannabis should be differentiated from panic, major depressive disorder, delusional disorder, bipolar disorder, and paranoid schizophrenia.
Cannabis-induced delusional disorder is a syndrome that may occur soon after use. It may be associated with persecutory delusions, marked anxiety, depersonalization, and emotional lability and is often misdiagnosed as schizophrenia. Subsequent amnesia of the episode can occur. Chronic use can cause a syndrome resembling dysthymic disorder.
Nicotine
Nicotine has euphoric effects and reinforcement properties similar to those of cocaine and opioids, and its use can lead to dependence. Nicotine withdrawal develops when use is stopped or reduced after a prolonged period (at least several weeks) of daily use. The withdrawal syndrome includes four or more of the following: dysphoric or depressed mood; insomnia; irritability, frustration, or anger; anxiety; difficulty concentrating; restlessness or impatience; decreased heart rate; and increased appetite or weight gain. After cessation, heart rate decreases 5–12 beats per minute within a few days, and weight increases on average 2–3 kg over the course of the first year.
Inhalants
This class of substances includes the aliphatic and aromatic hydrocarbons found in products such as gasoline, glue, paint thinners, and spray paints; the halogenated hydrocarbons found in cleaners, correction fluid, and spray-can propellants; and other volatile compounds containing esters, ketones, and glycols.
Inhalant use disorders
All of the substances in this class may produce dependence, abuse, or intoxication. There are no specific criteria sets for dependence or abuse of inhalants, partially because of uncertainty about whether tolerance or withdrawal syndromes develop with use of these substances.
Inhalant-induced disorders
Inhalant intoxication involves clinically significant behavioral or psychological changes accompanied by dizziness or visual disturbances, nystagmus, incoordination, slurred speech, unsteady gait, tremor, and euphoria. Higher doses of inhalants may lead to the development of lethargy and psychomotor retardation, generalized muscle weakness, depressed reflexes, stupor, or coma. Inhalant-induced mental disorders may be characterized by symptoms that resemble primary disorders. Mild to moderate intoxication with inhalants can be similar to intoxication caused by CNS depressants.
Caffeine
There is no DSM-IV-TR diagnosis for caffeine dependence or abuse. Although withdrawal headaches may occur, they are usually not severe enough to require treatment. Doses leading to caffeine intoxication can vary. At massive doses, grand mal seizures and respiratory failure can result. In persons who have developed tolerance, intoxication may not occur despite high caffeine intake. DSM-IV-TR recognizes other caffeine-induced disorders such as anxiety disorder and sleep disorder, and there is a category for caffeine-related disorder not otherwise specified.
Other (or unknown) substance-related disorders
The DSM-IV-TR category of other (or unknown) substance-related disorders encompasses symptoms that result either from use of a substance whose identity is unknown when the patient presents or from use of a substance that belongs to a class that does not have its own category. Examples of the latter include amyl nitrate, anticholinergic medications, gamma-hydroxybutyrate (GHB) (discussed below in Epidemiology and Natural History), corticosteroids, anabolic steroids (6), antihistamines, and a growing list of prescription drugs (e.g., angiotensin-converting enzyme [ACE] inhibitors, fluoroquinolones, and others) (7).
Polysubstance dependence
The DSM-IV-TR diagnosis of polysubstance dependence is used when an individual has, within the past year, repeatedly used at least three classes of substances (excluding nicotine and caffeine) without one predominating. Dependence is global and not for any one drug. There is no category of polysubstance abuse.
Psychiatric comorbidity
Dual diagnosis patients are those who have a substance abuse disorder and another major psychiatric diagnosis. Among the major psychopathologies, the reported odds ratios for comorbid substance use are 2 for major depressive disorder, 3 for panic disorder, 5 for schizophrenia, and 7 for bipolar illness (8).
Affective disorders
Prognosis is worsened by the presence of untreated major depression in a patient with primary alcoholism and by the presence of secondary alcoholism in a patient with primary depressive disorder. One study conducted with a large population of alcoholic patients found that primary major depressive episodes were more likely to occur in patients who were female, white, and married, who had experience with fewer drugs and less treatment for alcoholism, who had attempted suicide, and who had a close relative with a major mood disorder (9). Differentiating between primary and secondary alcoholism thus has clinical significance.
The prevalence of major depression ranges from 17% to 30% among persons who are addicted to heroin and is considerably higher among those being treated with methadone. Depressive episodes often are mild, may be related to treatment seeking, and frequently are stress related. In one study, depression was found to be a poor prognostic sign at 2.5-year follow-up in patients addicted to opioids, except in those who had coexistent antisocial personality; for this group, the presence of depression actually improved prognosis (10).
Affective disorders have been concurrently diagnosed in 30% of cocaine addicts, with a significant proportion of these patients having bipolar or cyclothymic disorder. One group has found that alcohol abuse may be more associated with depression, whereas cannabis abuse may be more commonly associated with mania (11).
Psychosis
Psychotic symptoms can result from the use of a wide range of psychoactive substances. Various studies have shown that 50%–60% of schizophrenia patients and 60%–80% of bipolar patients abuse substances (12). Persons with schizophrenia also are more likely than the general population to use tobacco and caffeine. Tobacco use has been associated with lowering of blood levels of neuroleptics. Among patients with cocaine dependence, craving is higher among those with schizophrenia than among those without schizophrenia (13).
Anxiety disorders
The relationship between anxiety and substance use has undergone a great deal of study, and the recent literature has focused particularly on substance use in posttraumatic stress disorder (PTSD). After the disasters of September 11, 2001, an increase was observed in relapse and in new use of substances (14). Alcohol and substance use can increase vulnerability to PTSD, and PTSD can worsen alcohol and drug problems. Pfefferbaum and Doughty (15) found that after the 1995 Oklahoma City bombing, alcohol use among victims increased. Alcohol use was not successful in alleviating symptoms in these individuals, and in fact it increased functional impairment. For a general review of anxiety and alcoholism, see Frances and Borg (16).
Neuropsychiatric disorders
Chronic abuse of alcohol, sedatives, or inhalants has been correlated with brain damage and neuropsychological impairment. Such impairment may be obvious (as in alcohol dementia and Korsakoff’s syndrome) or relatively mild and detectable only by neuropsychological testing. Cognitive impairment may be short-lived and recede after 3–4 weeks of abstinence, may improve gradually over several months or years of abstinence, or may be permanent.
Epidemiology and natural history
In 2001, some 7.1% (15.1 million) of Americans 12 years old or older were current users of illicit drugs, up from 6.3% in 2000 (17). In the most recent estimates of substance use disorder diagnoses (1999), 3.6 million Americans met diagnostic criteria for dependence on illicit drugs, and 1.1 million of these were 12 to 17 years old (18). Drug abuse deaths reported to the Drug Abuse Warning Network (DAWN) have increased in all metropolitan areas in recent years (19). In the Epidemiologic Catchment Area study, 30% of alcoholic individuals also met criteria for another substance use disorder. The actual monetary cost to the nation is estimated to exceed $275 billion annually.
The large-scale studies that have produced data on substance use disorders include DAWN (19), the Monitoring the Future study, which focuses on American adolescents and young adults (20); the National Household Survey (18); the Epidemiologic Catchment Area study (21); and the National Comorbidity Survey (22).
Alcohol
The Epidemiologic Catchment Area study found that 13.8% of Americans will have an alcohol-related substance use disorder in their lifetime. At least 9 million Americans are alcohol dependent, and another 6 million abuse alcohol. The annual costs attributed to alcohol use disorders exceed $166.5 billion annually (23).
Vaillant’s (24) studies found that some alcohol abusers drink until death, others stop drinking, and still others display a pattern of long abstinence followed by relapses. Risk-taking, sensation-seeking individuals are thought to be at particularly high risk of developing alcoholism. It has been established that alcoholism occurs in families, but the mode of transmission is unknown.
Biological markers may identify those at risk of developing alcohol use disorders. There is evidence that alcohol ingestion by not-yet-alcoholic children of alcoholics produces less subjective feelings of intoxication, less impairment of motor performance, less body sway or static ataxia, and less change in cortisol and prolactin levels than it does in others (25). There is also evidence of low-amplitude P300 event-related potential in alcoholic persons and in abstinent sons of alcoholic men.
Complications
Morbidity and mortality due to alcohol are high, especially given the effects of alcohol use on violence and on liver disease (26). About 25% of general hospital admissions are related to chronic alcohol use.
Violence. There is a strong association between alcohol and violence. The suicide rate among persons with alcoholism is 5%–6%. Nearly 50% of violent deaths in the United States, including traffic fatalities, are linked with alcohol (27, 28). In 2001, more than 1 in 10 Americans, or 25.1 million persons, reported having driven under the influence of alcohol at least once in the preceding 12 months (17).
Gastrointestinal effects. Alcoholism is the leading risk factor for cirrhosis, which occurs in 10% of alcoholic persons. Of patients who have chronic pancreatitis, 75% have an alcohol use disorder. Alcoholic hepatitis has a 5-year mortality rate of 50% (29).
Hematological effects. Alcoholism is part of the differential diagnosis for anemia, especially megaloblastic anemia. Alcohol impairs immune function and promotes oropharyngeal, esophageal, gastric, hepatic, breast, and pancreatic cancer.
Endocrinological effects. Alcohol affects male sexual function and fertility directly, through effects on testosterone levels, and indirectly, through testicular atrophy. Increased levels of estrogen in men lead to gynecomastia and body hair loss. In women there may also be severe gonadal failure. Hypoglycemia may result from excessive alcohol ingestion.
Neurological effects. Alcohol increases blood pressure and is associated with an increased risk of cerebrovascular accident. Alcoholic cerebellar degeneration is a slowly evolving condition in patients with a long-standing history of excessive alcohol use. Alcoholic peripheral neuropathy is characterized by stocking-and-glove paresthesia with decreased reflexes and autonomic nerve dysfunction. Other long-term neurological effects of alcoholism include central pontine myelinolysis, Marchiafava-Bignami disease, and muscular pathology.
Cardiovascular effects. Heavy drinkers have an elevated risk of cardiomyopathy and heart failure and lower post–myocardial infarction survival rates. On the other hand, moderate use of alcohol may have beneficial effects on both the risk of heart failure in the elderly (30) and on postinfarction mortality risk (31).
Sedatives, hypnotics, and anxiolytics
Abuse of the substances in this category is often iatrogenic. Chronic sedative abuse can produce “blackouts” and neuropsychological damage similar to those seen in alcoholic patients (32). This class of drugs may cause hypotension, sedation, cerebellar toxicity, psychomotor impairment (especially in the elderly), cognitive impairment, and, of course, dependence. The potential for morbidity and mortality is increased when these substances are used along with other CNS depressants. Flunitrazepam (trade name, Rohypnol) is a highly potent benzodiazepine sold outside the United States that is also used as a “date rape” drug because it produces anterograde amnesia (33).
Opioids
Lifetime heroin prevalence estimates were 2.3 million in 1979, 1.7 million in 1992, 2 million in 1997, and 2.4 million in 1998 (18). The Epidemiologic Catchment Area study showed that opioid use was greater among males, who were found to be three to four times as likely as females to use drugs of this type. Greater associated psychopathology is found in cultural groups in which heroin is less endemic. The course of heroin addiction generally involves an interval of 2–6 years between regular use and treatment seeking.
Complications
Impurities and contaminated needles may lead to endocarditis, septicemia, pulmonary embolism, pulmonary hypertension, skin infections, hepatitis B, and HIV infection. Because of infection, homicide, suicide, overdose, and AIDS, the death rate of young addicts is 20 times higher than that of nonaddicts.
Cocaine
In 1999 an estimated 1.5 million Americans over the age of 12 years were current cocaine users (18). Estimated current “crack” cocaine users numbered 413,000 in 1999 (15). More men than women use cocaine. The time from first intranasal use to cocaine addiction is 4 years in adults, but less in adolescents. The proportion of cocaine users in the United States underwent a statistically significant increase from 0.5% to 0.7% between 2000 and 2001 (17).
Complications
Violence. Cocaine was found to have been used immediately prior to death in 20% of suicides in New York City in the 1990s (34). Cocaine has also been detected in the urine of homicide victims, in persons arrested for murder, and in persons who have died from overdoses of other drugs.
Psychopathology. Chronic cocaine use depletes all neurotransmitters, leading to increased receptor sensitivity to dopamine and norepinephrine, which is associated with depression, fatigue, poor self-care, low self-esteem, low libido, and mild parkinsonism. Psychosis, an attention-deficit syndrome, and stereotypies may result from continued use. Levels of pathological gambling among cocaine-dependent patients have been found to be five times greater than in the general population (35).
Medical. Whether used intranasally, smoked, or injected, cocaine can lead to a variety of complications, including death, even in recreational, low-dose users. Acute complications include agitation, myocardial infarction, diaphoresis, tachycardia, QRS prolongation, subarachnoid hemorrhage, metabolic and respiratory acidosis, arrhythmia, grand mal seizure, and respiratory arrest. There is evidence of cerebrovascular disease secondary to chronic use (36). Chronic complications of intranasal abuse include nasoseptal defects and dental neglect as a result of cocaine’s anesthetic properties. Intravenous use may produce any of the sequelae of unsterile technique or contaminants.
Amphetamines
Some 4.7 million Americans have tried methamphetamine (18). Amphetamine abuse may start as attempts to lose weight or to increase energy. The long-term sequelae of amphetamine use mimic those of cocaine. Intravenous administration can produce the same medical complications as with other drugs.
Phencyclidine and ketamine
Chronic use of PCP may lead to neuropsychological deficits, psychotic episodes, and violence. Use of ketamine is growing, especially among youths and young adults. Ketamine and its active metabolite, norketamine, act at many CNS receptor sites, including the N-methyl-d-aspartate (NMDA) receptor. Ketamine is taken intranasally or orally. It quickly creates dissociation, and it may produce psychosis in normal individuals (37). The acute medical effects of both PCP and ketamine can be serious; they may produce neurological toxicity, rhabdomyolysis, and sympathetic stimulation. Respiratory arrest or apnea also can occur, with a risk of aspiration. In cases of death from overdose, pulmonary edema has been noted.
Hallucinogens
In 1999 there were an estimated 1.2 million new hallucinogen users in the United States, and the rate of current use nationwide was 0.4%. The number of persons reporting that they had ever tried Ecstasy (MDMA) increased from 6.5 million in 2000 to 8.1 million in 2001 (17).
Most hallucinogens can produce acute adverse effects (e.g., the “bad trip”). Hallucinogen use can also lead to chronic effects, including prolonged psychotic states that resemble psychosis or mania. Flashbacks, another potential chronic sequela of hallucinogen use, occur in 15%–30% of chronic users. Some of the drugs in this class, such as MPTP, have produced permanent parkinsonism by selective destruction of the substantia nigra.
MDMA, a serotonin releaser and serotonin reuptake inhibitor, may produce acute, fulminant states that variously include hepatotoxicity, disseminated intravascular coagulation, cardiotoxicity, hyperthermia, rhabdomyolysis, catatonia, and acute respiratory distress syndrome (38–42). There is growing evidence that MDMA causes neurodegeneration in users, affecting serotonin and possibly dopamine transmission (43). Animal models of CNS exposure to MDMA have complemented these findings (44).
Cannabis
Cannabis accounts for 75% of illicit drug use in the United States. Frequent users numbered 6.8 million in 1998, significantly more than in 1995, when there were 5.3 million (18). Marijuana use underwent a statistically significant increase from 4.8% to 5.4% between 2000 and 2001 (17).
Several acute neuropsychological changes and deficits have been identified with marijuana intoxication. Decreases in complex reaction-time tasks, digit-code memory tasks, fine motor function, time estimation, the ability to track information over time, tactual form discrimination, and concept formation have been found.
Chronic cannabis users have significant impairment of memory and attention that are persistent and worsen with ongoing use. CNS imaging in cannabis users has demonstrated altered function, blood flow, and metabolism in prefrontal and cerebellar regions (45). In cannabis users, emotional symptoms of anxiety, confusion, fear, and increased dependence can progress to panic or frank paranoid pathology. It can be a risk factor for schizophrenia or schizoid traits in vulnerable individuals. In a study that reassessed subjects from the 1980 Baltimore Epidemiologic Catchment Area study between 1994 and 1996, cannabis abusers were found to be four times as likely as nonusers to have depressive symptoms (46). Cannabis use increases the risks of endocrine, pulmonary, reproductive, vascular, and cancerous disease.
Nicotine
Tobacco use is the leading cause of preventable morbidity and mortality in the United States, causing approximately 430,000 deaths annually (47). Breslau et al. (48) provide epidemiological evidence of nicotine’s addictive potential by demonstrating that nearly half of those who ever smoked tobacco for a month or more before adulthood developed nicotine dependence. Patients with psychiatric problems and substance abuse disorders are likely to remain dependent on nicotine.
The prevalence of nicotine use is increasing: in 1999 an estimated 30.2% of Americans used cigarettes, up from 27.7% in 1998. An estimated 66.5 million Americans aged 12 years or older reported current use of a tobacco product in 2001; this number represents 29.5% of the population (17). Cigarette use is decreasing among men, but increasing among women. Of the 2.1 million Americans who began smoking cigarettes in 1997, most were under age 18 (18).
Inhalants
Inhalant use stabilized in 1996 after having increased for 4 years (20). Most users cease use quickly and move to other substances. Intoxication may produce aggressive, disruptive, antisocial behavior. Deaths have been reported from inhalant-induced CNS depression of the respiratory system, cardiac arrhythmia, and accidents. CNS studies using single photon emission computed tomography have documented the presence of abnormal perfusion in former users (49). Dermal or mucosal frostbite may occur after inhalation of fluorinated hydrocarbons from refrigerants or propellants (50).
Other substances
Gamma-hydroxybutyrate (GHB), a metabolite of gamma-aminobutyric acid (GABA), is a schedule I substance that is increasingly used worldwide as a recreational drug by party and nightclub attendees, as a growth hormone releaser by bodybuilders, and as a “date rape” drug. GHB increases CNS dopamine levels and has effects in the endogenous opioid system. GHB toxicity includes coma, seizures, respiratory depression, vomiting, anesthesia, and amnesia (51). The drug is missed in routine diagnostic urine screening. Although full recovery usually occurs, the toxic state is life threatening and may be complicated by severe muscular pathology leading to rhabdomyolysis and myoglobinuria (52). An growing number of reports have described clinically significant states of withdrawal from GHB (53). GHB occasionally leads to seizures, from which the user rapidly recovers. With the recent approval of the indication of GHB (under the name Xyrem) for the treatment of narcolepsy (54), GHB abuse may increase.
Biopsychosocial underpinnings
Addictive disorders are conceptualized as chronic medical disorders characterized by ongoing craving for and compulsion to use substances (55). A biopsychosocial approach to addiction accounts for social and psychological effects on use, including cultural sanctions (56), family dynamics, and both psychodynamic and behavioral contributions (57). The genetic contribution varies for each substance; evidence of a genetic component is likely greatest for alcohol use disorders (see above).
Neurobiology
Advances in cellular and molecular biology suggest that craving for addictive substances is related to a conditioned hyperactivity of CNS dopaminergic systems, most prominently the tract from the ventral tegmental area to the nucleus accumbens and areas to which the nucleus accumbens projects, the orbitofrontal cortex and the mesolimbic system, which ultimately affect the cingulate gyrus (58). Data suggest a network of limbic, paralimbic, and striatal brain regions, including structures involved in stimulus-reward association (amygdala), incentive motivation (subcallosal gyrus/ nucleus accumbens), and anticipation (anterior cingulate cortex) (59). This system is activated by “normal” pleasures but functions to a degree 10 times higher for users of substances of abuse. Using positron emission tomography studies, one group found low D2 dopamine receptors in the orbitofrontal cortices of methamphetamine abusers (60). Thus addiction is not only a degenerative disease or a lesion but also a learned process in which long-term memory occurs (inappropriately) at the molecular level (61–63).
Studies of particular drugs have built on these findings in alcohol (64) and opiates (65). Other neuroanatomic research focuses on the long-term changes in synaptic architecture and alterations in neurotransmitter physiology that may support the view that the brain of the addicted patient is different as a result of his or her excessive use—a difference that creates and maintains the compulsion to use (66).
A valuable line of investigation has used the newest techniques to study the neurochemistry and genetics of glutamate and dopamine in both abstinent alcoholics and in patients in alcohol withdrawal (67). Others have sought to use the methods of molecular biology to determine whether certain polymorphisms of the enzymes that produce these neurotransmitters or their receptors (e.g., the NMDA receptor) pose risks or are predictive of alcoholism or severe withdrawal states (e.g., delirium tremens) (68, 69).
Assessment and differential diagnosis
Diagnostic instruments and laboratory studies
Laboratory testing
Urinalysis remains the standard means of testing for alcohol and drugs. Most urinalyses begin with qualitative thin-layer chromatography and immunoassay techniques, including radioimmunoassay or enzyme multiplied immunoassay technique, fluorescence polarization immunoassay, and enzyme-linked immunoassay. More specific and sensitive quantitative testing with combined gas chromatography and mass spectrometry can be performed with certain drugs (e.g., marijuana); this technique constitutes the current gold standard for testing for these drugs, and it may reduce the rate of false positives (70). Advances in analysis of other tissues, including hair, sweat, and saliva, will likely be adopted in laboratory studies in the future.
Instruments and studies for alcohol
Assessment of alcohol use is more refined than for other substances, and new developments are expected. Serum levels of cellular enzymes, such as serum glutamic-oxaloacetic transaminase (SGOT), serum glutamic-pyruvic transaminase (SGPT), gamma-glutamyltransferase (GGT), and lactate dehydrogenase (LDH) as well as an elevated mean corpuscular volume (MCV) signal recent alcohol abuse. Detection of carbohydrate-deficient transferrin (CDT) will be increasingly important in detecting relapse as well as heavy alcohol use (71). Since the pathogenetic mechanisms of abnormal levels of CDT and SGGT are different, concurrent abnormal results may be a powerful indicator for alcohol use.
Several instruments are available for sensitive measurement of the attributes of alcohol abuse, including the Alcohol Use Inventory (AUI) (72, 73) and the Alcohol Dependence Scale (ADS) (74). Two alcoholism screening tests are the Michigan Alcoholism Screening Test (MAST) and the CAGE questionnaire (75). These tests have the advantage of being self-administered and brief. The short MAST (SMAST) is a 13-item scale that has a correlation of .90 with the 25-item MAST. The proportion of nonalcoholics correctly identified as such averages 74% for the MAST. The TWEAK has been found to be particularly helpful in screening female drinkers (76). The Addiction Severity Index (ASI) (77) has proved to be a useful instrument for use with the substance abuse population, particularly in treatment outcome research. An ASI adapted for teenagers is also available (78).
Treatment and outcomes
Standards contained in the American Psychiatric Association’s Practice Guideline for the Treatment of Patients With Substance Use Disorders (79), the American Society of Addiction Medicine’s Patient Placement Criteria for the Treatment of Substance-Related Disorders, now in a second, revised edition (80), the American Psychiatric Association’s Practice Guideline for the Treatment of Patients With Nicotine Dependence (81), and the National Institute on Drug Abuse’s Principles of Drug Addiction Treatment (82) may be of further help. In this section, we focus on the most important developments in the treatment of addictions.
Treatment outcomes
The primary goal of substance dependence treatment is abstinence from all substances (except for maintenance replacement and substitution therapies). For substance-dependent patients, recovery is a never-ending process; the term cure is avoided. Relapse is a process of attitudinal change that usually results in reuse of alcohol or drugs. In any one 12-month period after initiating abstinence, more than 90% of patients will likely use substances; of these, 45%–50% will return to pretreatment levels of use (83). As a result, a great deal of the field’s attention is focused on behavioral, psychological (e.g., reduction of guilt and shame), and pharmacological approaches to relapse prevention.
In general, treatment outcome is better for patients with high socioeconomic stability, antisocial personality, a lack of psychiatric and medical problems, and no family history of alcoholism. A history of participation in Alcoholics Anonymous (AA), a stable work and marriage history, and lack of a history of involvement with the legal system are correlated with better outcomes. Treatment outcome is related to duration of treatment; patients who complete day hospital substance abuse rehabilitation and then go on to participate in self-help groups have lower rates of alcohol and cocaine use at follow-up. Outcome in the treatment of cocaine dependence is related to severity of use at admission (84).
Differential therapeutics
Despite developments in treatment outcome research, the choice of the most appropriate treatments for addicted patients is still largely based on the conventional wisdom of clinical considerations. McLellan et al. (85) found that matching problems to treatment services improved treatment effectiveness and led to good overall effectiveness for alcohol treatment. Patients with severe psychiatric problems did poorly regardless of treatment modality or setting. Patients with legal problems did poorly in inpatient programs. Patients with the least severe psychiatric problems did well in both inpatient and outpatient settings. Patients whose psychiatric problems were in the middle range of severity usually also had employment or family problems, needed inpatient care, and improved when psychiatric services were also provided. It is often difficult to demonstrate differential treatment outcomes with different approaches.
Because of the modest long-term results obtained with use of medications in the treatment of addictive disorders (except in the case of opioid dependence), a number of major trials have been conducted to study and compare the psychotherapies. The National Institute on Drug Abuse Cocaine Psychotherapy Study was a four-site collaborative study of psychotherapy for cocaine dependence that compared the efficacy of group drug counseling only and group drug counseling plus either individual drug counseling, cognitive behavioral therapy, or supportive-expressive psychotherapy. The study unexpectedly found support for individual drug counseling over the other therapies when used with group counseling (86). Project MATCH (Matching Alcoholism Treatments to Client Heterogeneity), with 1,700 patients the largest study ever conducted of addictive disorders, examined the efficacy of cognitive behavioral therapy, motivational enhancement therapy, and 12-step facilitation for alcohol dependence. All of these modalities were found to be efficacious, and no significant differences were observed among them (87).
Treatment modalities
As in other areas of psychiatry, a combination of psychotherapy and pharmacotherapy is ultimately more effective in the treatment of addictive disorders than either modality alone. This has been demonstrated in opioid relapse prevention (88), in discontinuing methadone maintenance (82), and in treatment of alcohol dependence (89, 90). A detailed review of the principles of a psychotherapeutic approach to the substance-using patient can be found elsewhere (91).
Psychosocial treatments
Individual therapy. Individual therapy can be conducted alone or with other modalities such as pharmacotherapy, 12-step programs, and family and group treatments. The individual modalities include expressive psychotherapy, cognitive behavioral therapy, dialectical behavior therapy, and motivational enhancement therapy. Expressive psychotherapy may lead to deepening of the capacity to tolerate depression and anxiety without substance use (92). Cognitive behavioral therapy is very helpful for relapse prevention as well as for patients who are substance dependent or sociopathic or who suffer from primary psychiatric symptoms (93). Dialectical behavior therapy, a comprehensive, behaviorally oriented treatment designed for highly dysfunctional patients who meet criteria for borderline personality disorder, is being used in the treatment of addiction. This modality has been shown to be more effective than usual treatment for drug abuse in women with borderline personality disorder (94). Motivational enhancement therapy and motivational interviewing were specifically designed for patients with addictive disorders. These are directive, empathic, patient-centered counseling approaches that address the patient’s ambivalence and denial (95, 96).
Group therapy. Treatment programs frequently employ didactic groups, which may help keep patients in treatment, promote cohesiveness, and foster acceptance of long-term rehabilitation. Network therapy involves the creation of a support group—consisting of abstinent family and friends—and then applies cognitive behavioral therapy methods to environmental triggers, uses community reinforcement, and emphasizes the importance of the support of the patient’s social network (97).
Family evaluation and therapy. A family evaluation is warranted for all patients with a substance use disorder; information and aid from the family are crucial for both diagnosis and treatment. Techniques used by family therapists include conjoint family therapy, marital therapy groups, and conjoint hospitalization for married couples. The efficacy of these techniques has been documented (98). In the treatment of addiction in adolescents, family therapy is essential.
Self-help. Every clinician needs to be thoroughly familiar with the work of the 12-step self-help programs: AA, Al-Anon, Narcotics Anonymous (NA), Cocaine Anonymous, Gamblers Anonymous, and Overeaters Anonymous. There is a small but growing body of evidence documenting the efficacy of AA. Self-help also may be very important for cocaine addiction. Peer-led groups have been helpful as well (99).
Psychopharmacological modalities
In the treatment of substance use disorders, medications may be prescribed for detoxification, treatment of comorbid psychiatric disorders or of complicating neuropsychological and medical disorders, aversive treatment, and attenuation of craving or euphoria.
Naltrexone. Naltrexone, an opioid mu-receptor antagonist that is thought to block dopamine release in the nucleus accumbens, was originally indicated for opioid relapse prevention but is now also considered to have superior efficacy for alcohol relapse prevention (100). Because it causes withdrawal from opioid intoxication, naltrexone must be begun slowly over the course of days in the opioid user.
A recent multicenter, double-blind, placebo-controlled evaluation of naltrexone as an adjunct to standardized counseling for alcohol dependence in 627 veterans (almost all men) found no benefit from naltrexone in time to relapse, percentage of days on which drinking occurred, or number of drinks per drinking day (101). The authors concluded that their findings did not support the use of naltrexone for the treatment of chronic alcoholism. Nonetheless, 13 of 15 other studies have found naltrexone to have effectiveness in the treatment of alcohol dependence, which suggests that naltrexone likely is effective for this indication (101).
Methadone and levo-alpha acetyl methadol (LAAM). Methadone, an opioid agonist, has a well-established dose-dependent effect of diminishing the use of opioids and cocaine. Unfortunately, only a minority of opioid-dependent patients receive methadone. LAAM, another opioid agonist, has a longer half-life than methadone and offers the advantage of needing to be taken only three times a week. However, concerns that LAAM may be arrhythmogenic have resulted in a “black box” warning by the Food and Drug Administration. LAAM may not be used as a first-line treatment, and new guidelines for cardiac monitoring have been promoted. In addition, the use LAAM with other medications that inhibit cytochrome P-450 3A4 is contraindicated. Methadone may significantly increase serum levels of zidovudine (102).
Buprenorphine. Buprenorphine is a mixed opioid mu-receptor agonist-antagonist that has been proved superior to placebo for treatment of opioid dependence (103). Buprenorphine shows promise for opioid detoxification and opioid maintenance and for the transition to naltrexone. Suboxone, a 4:1 buprenorphine-naloxone combination in the form of a sublingual tablet, can be used for the same indications as buprenorphine and is not a likely candidate for abuse because if the tablet were crushed and injected, the naloxone would precipitate withdrawal. Buprenorphine is long acting, which means that it can be given three times a week instead of daily (104). At larger doses buprenorphine decreases respiratory drive. Clinicians may obtain formalized training in the use of buprenorphine before prescribing it in the office setting.
Disulfiram. Disulfiram is an aversive treatment for alcohol use. It acts by producing painful symptoms when alcohol is added to a hepatic system exposed to disulfiram and indirectly by blocking the “high” of intoxication unavailable, thus discouraging impulsive alcohol use. Although there is no convincing evidence that use of disulfiram affects long-term outcomes globally, disulfiram has been shown to be useful in certain subtypes of alcoholism, often when it can be administered by a patient’s significant other.
Lithium and mood stabilizers. Lithium and anticonvulsants such as divalproex are an integral component in the treatment of substance use disorders in the presence of an underlying primary bipolar or cyclothymic disorder.
Antidepressants. There is some evidence that sertraline may reduce drinking in alcohol-dependent patients who have no history of depression (105). Bupropion has been used successfully in nicotine cessation, which was recently demonstrated in African American populations (106). Otherwise, antidepressants do not have a direct effect on substance use disorders but are an important adjunct in the treatment of patients with primary mood disorders.
Acamprosate. Acamprosate is a synthetic compound intended to reduce craving for alcohol. It crosses the blood-brain barrier, is chemically similar to amino acid neurotransmitters, and acts at the NMDA receptor to reduce the glutamatergic hyperactivity of dependence. It is dose dependent and has no abuse potential, and giving acamprosate to a patient who is dependent on alcohol will not lead to an acute withdrawal syndrome. This medication may help change the nature of alcohol relapse prevention (107), although Food and Drug Administration approval is uncertain at this time.
Other somatic therapies
In addition to the medications discussed above, a number of other agents may be valuable adjuncts in the treatment of addictive disorders, including nonaddictive anxiolytics such as buspirone and selective serotonin reuptake inhibitors and antipsychotics for the treatment of aggression and psychosis in intoxicated states or delirium, especially alcohol hallucinosis (although they lower the seizure threshold). Among the atypical antipsychotics, olanzapine appears to be as effective as haloperidol in the treatment of cannabis-induced psychotic disorder and has a lower rate of extrapyramidal side effects (108). Opioid detoxification can be accomplished with clonidine, an α2-adrenergic agonist that has been shown to suppress signs and symptoms of autonomic sympathetic activation during withdrawal, although it has been less successful in decreasing the subjective discomfort of withdrawal (109). Benzodiazepines are the treatment of choice for alcohol or benzodiazepine detoxification. They are generally contraindicated in any substance use disorder except when used for detoxification, for manic patients acutely, or very selectively in compliant abstinent patients with anxiety disorders in whom other treatments have failed.
Rapid and ultrarapid detoxification methods employ opioid antagonists as well as adjuncts such as clonidine, sedatives, and even general anesthesia. However, ultrarapid detoxification has certain drawbacks: it involves the risks associated with anesthesia, it has led to deaths, it is expensive, and it is performed without rehabilitation.
Opioid maintenance treatment
Opioid agonist substitution is the key to maintenance treatment—interrupt the addict’s lifestyle, promote stability and employment, reduce intravenous drug use and the risk it carries of hepatitis B or HIV infection, and reduce criminal activity. Methadone is the standard treatment, and more than 170,000 Americans (only 20%–25% of addicted persons nationwide) are receiving methadone for this purpose. New agents such as buprenorphine (110) are also effective. Naltrexone, when used regularly, can lead to gradual extinction of drug-seeking behavior and can help in opioid relapse prevention. High rates of treatment refusal and treatment dropout limit its use to highly motivated individuals with a good prognosis who are likely to do well with a variety of treatment options and with whom the clinician has a good treatment alliance.
Rehabilitation
The rehabilitation model, pioneered in substance abuse treatment, has become an important organizing paradigm for a variety of categories of psychiatric illness. It combines self-help, counseling, education, relapse prevention, group treatment, a warm and supportive environment, and emphasis on a medical model geared toward reducing stigma and blame. Most treatment programs are highly structured, insist on the goal of abstinence, and offer lectures, films, and discussion groups as part of a complete cognitive and educational program. Patients frequently become active members of 12-step programs and are encouraged to continue in aftercare.
Medical comorbidity
An estimated 25%–50% of general hospital admissions are related to substance use (111), so vigilance among caregivers is important in detecting this relationship and its potential complications in every patient. Such vigilance includes providing treatment for intoxication, withdrawal, and any expected adverse medical effects of substance use; determining whether accidents (such as burns) are a result of substance use (substance use is associated with a worse prognosis for the burn patient, and alcohol is an independent predictor of death in such patients [112]); and accounting for any substance-induced alterations in medication metabolism (which includes tolerance for pain medications in opioid addicts). There is evidence that organ and patient survival among selected recovering alcoholics who receive organ transplants are as good as those for patients who receive a liver for non-alcohol-related diseases (113).
Prevention
Educational programs targeting students, parents, and employees have significantly increased knowledge about the risks of substance abuse. In “life skills” training, a model that focuses on value-clarification abilities, decision making, and drug refusal (114), participants rehearse how to say “no” successfully. The legal drinking age has been raised to 21 years throughout the nation, and studies indicate that this has decreased motor vehicle accidents and deaths among teenagers. Limiting tobacco advertising and its use in public places and imposing taxes on tobacco sales have very likely helped move the nation toward the goal expressed by former Surgeon General David Satcher as a “smokeless” society. Finally, to avoid loss of employee productivity resulting from the safety hazards, illness, absenteeism, poor work quality, and low morale that are associated with intoxication and chronic drug abuse, prevention of substance abuse in the workplace is a significant endeavor (115).
Special populations
Women
Women with substance use disorders suffer greater secondary medical morbidity from substance use (116) and suffer a higher mortality rate by all causes (including suicide). Female alcohol abusers start later than men, progress to abuse faster, abuse less total alcohol, and have higher rates of comorbid psychopathology. Comorbid psychopathology in women with substance use disorders more often involves anxiety disorders (particularly PTSD), mood disorders, and eating disorders (117). Women are more likely to have a significant other who is also a substance abuser. They also tend to have a history of sexual or physical abuse and to date the onset of their substance use disorder to a stressful event.
Alcohol’s neurotoxic effects may be greater in women than in men (118). Sexual function is affected by almost all drugs of abuse, and sexual function improves with sobriety (119). For unknown reasons, women alcohol abusers have a higher risk of developing breast cancer than nonabusers (120).
Prenatal exposure to alcohol remains one of the leading preventable causes of birth defects, mental retardation, and neurodevelopmental disorders (possibly including attention deficit hyperactivity disorder [(121]) in the United States (122). The treatment of neonates for methadone withdrawal is a common procedure; thus, methadone use is not an absolute contraindication to becoming pregnant (123). Finally, despite intense debate, it is becoming increasingly apparent that that babies of cocaine-using mothers are at risk of neurodevelopmental deficits (124).
Treatment services
Large-scale studies have suggested that women with substance-related disorders are undertreated (125). In one group of pregnant women with identified psychiatric or substance use disorders, a very poor rate of treatment for substance use (23%) was observed (126).
Children and adolescents
Substance use disorders among adolescents constitute a significant public health problem. The prevalence of substance use disorders is rising, the age at first use is dropping, and morbidity and mortality among youths with substance use disorders are increasing (127). In 2001, 10.8% of youths 12 to 17 years of age were current drug users, compared with 9.7% in 2000. Similarly, among adults 18 to 25 years of age, current drug use increased from 15.9% to 18.8% between 2000 and 2001 (17).
Diagnosis of substance dependence in adolescents is difficult, because adolescents are less likely to report withdrawal symptoms, have shorter periods of addiction, and may recover more rapidly from withdrawal. Among children and adolescents, the family is a very important unit for conceptualizing influences that promote substance use and for conceptualizing treatment (128–131).
The American Academy of Child and Adolescent Psychiatry’s practice parameters for substance use disorders provide the basis of the care of adolescents (132). In addition, the American Academy of Pediatrics has established guidelines for the assessment, treatment, and referral of such problems (133), and the Substance Abuse and Mental Health Services Administration has published a guide (134). For details about general aspects of adolescent substance use disorders, particularly epidemiology, risk factors, genetics, prevention, and neurobiology, the reader is referred to other sources (135, 136).
Epidemiology
Until late 2002, epidemiological studies on adolescent substance abuse had found few positive findings. Among the findings from the best available studies from recent years are that 7%–17% of adolescents 13 to 18 years old meet DSM criteria for substance abuse or dependence (137) and that alcohol use (17) and substance use in general (138) are widespread among adolescents. However, preliminary data from 2001 suggest that there may be significant declines in use of some substances in many age groups (17).
Psychiatric comorbidity
Most adolescents in substance treatment programs have comorbid psychopathology, including conduct disorder, depression, attention deficit hyperactivity disorder (ADHD), and others. The Oregon Adolescent Depression Project (139) found that 66.2% of adolescents with a substance use disorder also had a psychiatric disorder but that only 31.3% of those with a psychiatric disorder had a substance use disorder. Substance use disorders are a major risk factor for suicide and suicide attempts among adolescents. The use of methylphenidate in the treatment of ADHD in adolescents has been found to significantly reduce their risk of developing substance abuse in later life (140).
Bipolar disorder in adolescence may be a major risk factor for adult substance use disorders. Biederman et al. found that adolescent-onset bipolar disorder was associated with a greater risk of later having a substance use disorder than child-onset bipolar disorder, regardless of whether conduct disorder was present (141). Geller conducted a double-blind, placebo-controlled, random-assignment, parallel-group, pharmacokinetically dosed study of lithium treatment for adolescents with bipolar disorders and temporally secondary substance dependence disorders (142). Those who received lithium had better outcomes in terms of relapse than those who received placebo, indicating the utility of lithium in bipolar disorder in adolescents and perhaps also in adolescents with primary substance use disorders (143).
Treatment issues
Adolescent programs rely heavily on peer support groups, family therapy, organized education, education on drug abuse, 12-step programs such as AA and Alateen, vocational programs, patient-staff meetings, and activity therapy (136). Predictors of treatment completion include greater severity of alcohol abuse, worse abuse of drugs other than alcohol, nicotine, or cannabis; a higher degree of internalizing problems, and lower self-esteem (143).
Interpersonal therapy and cognitive behavioral therapy (144) approaches have been shown to be generally valuable and efficacious for adolescents. Motivational enhancement therapy, originally developed for adults, has been applied to adolescents (145). Contingency management, in which the patient is rewarded for proof of abstinence, is also effective (146). Few investigations of group therapy among adolescent alcohol abusers have been conducted, although this modality’s efficacy has been documented (147, 148). Among the structured group psychotherapies, cognitive behavioral therapy groups have been shown to be superior to interactional groups in reducing use (149). Twelve-step programs geared to younger persons may be of benefit for the few adolescents who have sufficient insight.
The elderly
The incidence of alcoholism is lower among the elderly (perhaps because persons with chronic alcoholism die prematurely), but the abuse of prescription drugs is common. In addition, age-related changes in the pharmacokinetics of prescribed medications compound the consequences of substance abuse in the elderly.
Abuse of benzodiazepines and other hypnotics is common. Cognitive decline may lead to unintentional misuse. Nursing home patients are sometimes overmedicated to reduce disruptiveness. Abusers of prescription medications may require hospitalization to discontinue use.
The chronically mentally ill
Chronic mental illness frequently contributes to the chronicity and severity of substance abuse problems. Facilities that combine psychiatric treatment with substance abuse rehabilitation techniques are often unavailable to these patients. Severely disturbed abusers may be shunned by drug rehabilitation units. Confrontational methods and overemphasis on abstinence, which may lead to underuse of appropriate psychotropic medications, can be detrimental to mentally ill substance abusers.
Mildly retarded patients (those with an IQ in the range of 60–85) are concrete in their thinking, are easily manipulated, and may have problems learning from experience. Most of these patients live in the community, and they may attempt to socialize in neighborhood bars, which are viewed as warm and nonjudgmental social environments. An emphasis on providing acceptance, warmth, and emotional support is especially important in working with this population.
The homeless population is no longer principally composed of “skid row” alcoholics or single, older, chronically alcoholic men (150). The population is now younger and includes a growing proportion of women. In addition, as many as 90% of homeless persons have a primary psychiatric diagnosis. In various urban samples in the United States, 20%–60% of homeless patients reported alcohol dependence (150).
Minorities
Substance use disorders have a major impact on overall life expectancy among blacks, Hispanics, and Native Americans (151). Black teenagers report less alcohol abuse than do their white peers; in their twenties and thirties, however, rates of abuse among blacks are comparable to those of whites, and blacks suffer a disproportionate degree of medical (notably cirrhosis, esophageal cancer, and AIDS), psychological, and social sequelae. Excessive alcohol consumption is reported among rural and urban Native Americans (152).
Future directions
Over the past two decades, American medicine has seen tremendous developments in the understanding and treatment of substance-related disorders, and further developments are promising. The neurobiology of addiction will likely be further refined in terms of specific neuroanatomical effects of specific substances. Other areas currently being investigated include variation in pharmacokinetics, especially in hepatic metabolism, and genetic factors in addiction. The effects of the newest drugs of abuse—MDMA, GHB, and others—will need a great deal of attention in the coming years.
Investigation of optimal treatment planning will be informed by ongoing studies comparing existing treatment modalities. Ideally, particular modalities can be used for specific diagnoses, psychiatric comorbid states, and socioeconomic groups. Expansion of treatment options with opiate antagonists or buprenorphine may become a reality soon (153). Interesting developments in novel treatment strategies include vaccines against cocaine (154), corticotropin-releasing hormone antagonists to decrease cocaine and heroin addiction, (155), and antagonists to cannabinoid receptors to block the effects of cannabis. Thus the future of addiction psychiatry is bright and promises greater understanding, refinements of established treatments, and the creative development of novel therapies for this costly group of disorders.
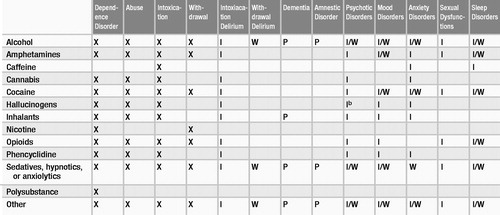 |
Table 1. Diagnoses Associated With Class of Substancesa
| A. Maladaptive pattern of substance use, leading to clinically significant impairment or distress, as manifested by three (or more) of the following, occurring at any time in the same 12-month period: |
| (1) Tolerance, as defined by either of the following: |
| (a) A need for markedly increased amounts of the substance to achieve intoxication or desired effect |
| (b) Markedly diminished effect with continued use of the same amount of the substance |
| (2) Withdrawal, as manifested by either of the following: |
| (a) The characteristic withdrawal syndrome for the substance (refer to Criteria A and B of the criteria sets for withdrawal from the specific substances) |
| (b) The same (or a closely related) substance is taken to relieve or avoid withdrawal symptoms |
| (3) The substance is often taken in larger amounts or over a longer period than was intended. |
| (4) There is a persistent desire or unsuccessful efforts to cut down or control substance use. |
| (5) A great deal of time is spent in activities necessary to obtain the substance (e.g., visiting multiple doctors or driving long distances), use the substance (e.g., chain-smoking), or recover from its effects. |
| (6) Important social, occupational, or recreational activities are given up or reduced because of substance use. |
| (7) The substance use is continued despite knowledge of having a persistent or recurrent physical or psychological problem that is likely to have been caused or exacerbated by the substance (e.g., current cocaine use despite recognition of cocaine-induced depression, or continued drinking despite recognition that an ulcer was made worse by alcohol consumption). |
| Specify if: |
| With physiological dependence: evidence of tolerance or withdrawal (i.e., either item 1 or 2 is present) |
| Without physiological dependence: no evidence of tolerance or withdrawal (i.e., neither item 1 nor 2 is present) |
| Course specifiers (see DSM-IV-TR text for definitions): |
| Early full remission |
| Early partial remission |
| Sustained full remission |
| Sustained partial remission |
| On agonist therapy |
| In a controlled environment |
| A. A maladaptive pattern of substance use leading to clinically significant impairment or distress, as manifested by one (or more) of the following, occurring within a 12-month period: |
| (1) Recurrent substance use resulting in a failure to fulfill major role obligations at work, school, or home (e.g., repeated absences or poor work performance related to substance use; substance-related absences, suspensions, or expulsions from school; neglect of children or household) |
| (2) Recurrent substance use in situations in which it is physically hazardous (e.g., driving an automobile or operating a machine when impaired by substance use) |
| (3) Recurrent substance-related legal problems (e.g., arrests for substance-related disorderly conduct) |
| (4) Continued substance use despite having persistent or recurrent social or interpersonal problems caused or exacerbated by the effects of the substance (e.g., arguments with spouse about consequences of intoxication, physical fights) |
| B. The symptoms have never met the criteria for substance dependence for this class of substance. |
| A. The development of a reversible substance-specific syndrome due to recent ingestion of (or exposure to) a substance. Note: Different substances may produce similar or identical syndromes. |
| B. Clinically significant maladaptive behavioral or psychological changes that are due to the effect of the substance on the central nervous system (e.g., belligerence, mood lability, cognitive impairment, impaired judgment, impaired social or occupational functioning) and develop during or shortly after use of the substance |
| C. The symptoms are not due to a general medical condition and are not better accounted for by another mental disorder. |
| A. The development of a substance-specific syndrome due to the cessation of (or reduction in) substance use that has been heavy and prolonged |
| B. The substance-specific syndrome causes clinically significant distress or impairment in social, occupational, or other important areas of functioning. |
| C. The symptoms are not due to a general medical condition and are not better accounted for by another mental disorder. |
| A. Either of the following: |
| (1) Cessation of (or reduction in) opioid use that has been heavy and prolonged (several weeks or longer) |
| (2) Administration of an opioid antagonist after a period of opioid use |
| B. Three (or more) of the following, developing within minutes to several days after Criterion A: |
| (1) Dysphoric mood |
| (2) Nausea or vomiting |
| (3) Muscle aches |
| (4) Lacrimation or rhinorrhea |
| (5) Pupillary dilation, piloerection, or sweating |
| (6) Diarrhea |
| (7) Yawning |
| (8) Fever |
| (9) Insomnia |
| C. The symptoms in Criterion B cause clinically significant distress or impairment in social, occupational, or other important areas of functioning. |
| D. The symptoms are not due to a general medical condition and are not better accounted for by another mental disorder. |
1 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, 4th Edition, Text Revision. Washington, DC, American Psychiatric Association, 2000Google Scholar
2 Schuckit MA, Smith TL, Danko GP: Five-year clinical course associated with DSM-IV alcohol abuse or dependence in a large group of men and women. American Journal of Psychiatry 2001; 158:1084–1090Crossref, Google Scholar
3 Schuckit MA, Smith TL, Daeppen JB, Eng M, Li TK, Hesselbrock VM, Nurnberger JI Jr, Bucholz KK: Clinical relevance of the distinction between alcohol dependence with and without a physiological component. American Journal of Psychiatry 1998; 155:733–740Google Scholar
4 Salzman C: Addiction to benzodiazepines. Psychiatric Quarterly 1998; 69:251–261Crossref, Google Scholar
5 Flaum M, Schultz SK: When does amphetamine-induced psychosis become schizophrenia? American Journal of Psychiatry 1996; 153:812–815Crossref, Google Scholar
6 Brower KJ, Blow FC, Young JP, Hill EM: Symptoms and correlates of anabolic-androgenic steroid dependence. British Journal of Addiction 1991; 86:759–768Crossref, Google Scholar
7 Drugs that cause psychiatric symptoms. Medical Letter on Drugs and Therapeutics 2002; 44:59–62Google Scholar
8 Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK: Co-morbidity of mental disorders with alcohol and other drug abuse: results from the Epidemiologic Catchment Area study. JAMA 1990; 264:2511–2518Crossref, Google Scholar
9 Schuckit MA, Tipp JE, Bergman M, Reich W, Hesselbrock VM, Smith TL: Comparison of induced and independent major depressive disorders in 2,945 alcoholics. American Journal of Psychiatry 1997; 154:948–957Crossref, Google Scholar
10 Brooner RK, King VL, Kidorf M, Schmidt CW Jr, Bigelow GE: Psychiatric and substance use comorbidity among treatment seeking opioid abusers. Archives of General Psychiatry 1997; 54:71–80Crossref, Google Scholar
11 Strakowski SM, DelBello MP, Fleck DE, Arndt S: The impact of substance abuse on the course of bipolar disorder. Biological Psychiatry 2000; 48:477–485Crossref, Google Scholar
12 Schneier FR, Siris SG: A review of psychoactive substance use and abuse in schizophrenia: patterns of drug choice. Journal of Nervous and Mental Disorders 1987; 175:641–652Crossref, Google Scholar
13 Carol G, Smelson DA, Losonczy MF, Ziedonis D: A preliminary investigation of cocaine craving among persons with and without schizophrenia. Psychiatric Services 2001; 52:1029–1031Crossref, Google Scholar
14 Vlahov D, Galea S, Resnick H, Ahern J, Boscarino JA, Bucuvalas M, Gold J, Kilpatrick D: Increased use of cigarettes, alcohol, and marijuana among Manhattan, New York, residents after the September 11th terrorist attacks. American Journal of Epidemiology 2002; 155:988–996Crossref, Google Scholar
15 Pfefferbaum B, Doughty DE: Increased alcohol use in a treatment sample of Oklahoma City bombing victims. Psychiatry 2001; 64:296–303Crossref, Google Scholar
16 Frances RJ, Borg L: The treatment of anxiety in patients with alcoholism. Journal of Clinical Psychiatry 1993; 54(suppl):37–43Google Scholar
17 Office of Applied Studies: Results from the 2001 National Household Survey on Drug Abuse, vol 1. Summary of National Findings. NHSDA Series H-17, DHHS Pub No SMA 02-3758. Rockville, MD, Substance Abuse and Mental Health Services Administration, 2002Google Scholar
18 Office of Applied Studies: National Household Survey on Drug Abuse. Rockville, MD, Substance Abuse and Mental Health Services Administration, 2000Google Scholar
19 Office of Applied Studies: Mortality data from the Drug Abuse Warning Network, 2000. DAWN, Series D-19, DHHS Series No SMA 02-3633. Rockville, MD, Substance Abuse and Mental Health Services Administration, 2002Google Scholar
20 Johnston LD, O’Malley PM, Bachman JG: Monitoring the Future National Survey Results on Adolescent Drug Use: Overview of Key Findings, 2000. NIH Pub No 01-4923. Bethesda, MD, National Institute on Drug Abuse, 2001Google Scholar
21 Regier DA, Boyd JH, Burke JD Jr, Rae DS, Myers JK, Kramer M, Robins LN, George LK, Karno M, Locke BZ: One-month prevalence of mental disorders in the United States, based on five Epidemiologic Catchment Area sites. Archives of General Psychiatry 1988; 45:977–986Crossref, Google Scholar
22 Kessler RC, Crum RM, Warner LA, Nelson CB, Schulenberg J, Anthony JC: Lifetime co-occurrence of DSM-III-R alcohol abuse and dependence with other psychiatric disorders in the National Comorbidity Survey. Archives of General Psychiatry 1997; 54:313–321Crossref, Google Scholar
23 Harwood H, Fountain D, Livermore G: The Economic Costs of Alcohol and Drug Abuse in the United States, 1992. Washington, DC, National Institute on Drug Abuse and National Institute on Alcohol Abuse and Alcoholism, 1998Google Scholar
24 Vaillant GE: A long-term follow-up of male alcohol abuse. Archives of General Psychiatry 1996; 53:243–249Crossref, Google Scholar
25 Schuckit MA: Biological vulnerability to alcoholism. Journal of Consulting and Clinical Psychology 1987; 55:301–309Crossref, Google Scholar
26 Murphy SL: Deaths: Final Data for 1998: National Vital Statistics Reports, vol 48, no 11. Hyattsville, MD, National Center for Vital Statistics, 2000Google Scholar
27 Institute of Medicine: Workshop Summary: The Role of Co-occurring Substance Abuse and Mental Illness in Violence. Division of Neuroscience and Behavioral Health, Institute of Medicine. Washington, DC, National Academy Press, 1999Google Scholar
28 Centers for Disease Control and Prevention: Alcohol involvement in fatal motor vehicle crashes—United States, 1997–1998. Morbidity and Mortality Weekly Report 1999; 48:1086–1087Google Scholar
29 Lieber CS: Medical disorders of alcoholism. New England Journal of Medicine 1995; 333:1058–1065Crossref, Google Scholar
30 Abramson JL, Williams SA, Krumholz HM, Vaccarino V: Moderate alcohol consumption and risk of heart failure among older persons. JAMA 2001; 285:1971–1977Crossref, Google Scholar
31 Mukamal KJ, Maclure M, Muller JE, Sherwood JB, MIttelman MA: Prior alcohol consumption and mortality following acute myocardial infarction. JAMA 2001; 285:1965–1970Crossref, Google Scholar
32 Tonne U, Hiltunen AJ, Vikander B, Engelbrektsson K, Bergman H, Bergman I, Leifman H, Borg S: Neuropsychological changes during steady-state drug use: withdrawal and abstinence in primary benzodiazepine-dependent patients. Acta Psychiatrica Scandinavica 1995; 91:299–304Crossref, Google Scholar
33 Druid H, Holmgren P, Ahlner J: Flunitrazepam: an evaluation of use, abuse, and toxicity. Forensic Science International 2001; 122:136–141Crossref, Google Scholar
34 Marzuk PM, Tardiff KL, Leon AC, Stajic M, Morgan EB, Mann JJ: Prevalence of cocaine use among residents of New York City who committed suicide during a one-year period. American Journal of Psychiatry 1992; 149:371–375Crossref, Google Scholar
35 Hall GW, Carriero NJ, Takushi RY: Pathological gambling among cocaine-dependent outpatients. American Journal of Psychiatry 2000; 157:1127–1133Crossref, Google Scholar
36 Bartzokis G, Beckson M, Hance DB, Lu PH, Foster JA, Mintz J, Ling W, Bridge P: Magnetic resonance imaging evidence of “silent” cerebrovascular toxicity in cocaine dependence. Biological Psychiatry 1999; 45:1203–1211Crossref, Google Scholar
37 Krystal JH, Karper LP, Seibyl JP, Freeman GK, Delaney R, Bremner JD, Heninger GR, Bowers MB Jr, Charney DS: Subanesthetic effects of the noncompetitive NMDA antagonist, ketamine, in humans: psychotomimetic, perceptual, cognitive, and neuroendocrine responses. Archives of General Psychiatry 1994; 51:199–214Crossref, Google Scholar
38 Jonas MM, Graeme-Cook FM: A 17-year-old girl with marked jaundice and weight loss. New England Journal of Medicine 2001; 344:591–599Crossref, Google Scholar
39 Van Kampen J, Katz M: Persistent psychosis after a single ingestion of “ecstasy.” Psychosomatics 2001; 42:525–527Crossref, Google Scholar
40 Vaiva G, Bailly D, Boss V, Thomas P, Lestavel P, Goudemand M: A case of acute psychotic episode after a single dose of ecstasy. Encephale 2001; 27:198–202Google Scholar
41 Masi G, Mucci M, Floriani C: Acute catatonia after a single dose of ecstasy. Journal of the American Academy of Child and Adolescent Psychiatry 2002; 41:892Crossref, Google Scholar
42 Reneman L, Lavalaye J, Schmand B: Cortical serotonin transporter density and verbal memory in individuals who stopped using 3,4-methylenedioxymethamphetamine (MDMA or “Ecstasy”): preliminary findings. Archives of General Psychiatry 2001; 58:901–906Crossref, Google Scholar
43 McCann UD, Szabo Z, Scheffel U, Dannals RF, Ricaurte GA: Positron emission tomographic evidence of toxic effect of MDMA (“Ecstasy”) on brain serotonin neurons in human beings. Lancet 1998; 352(9138):1433–1437Crossref, Google Scholar
44 Meechan AO, Moran PM, Elliot JM: A study of the effect of a single neurotoxic dose of 3,4-methylenedioxymethamphetamine (MDMA; “ecstasy”) on the subsequent long-term behaviour of rats in the plus maze and open field. Psychopharmacology 2002; 159:167–175Crossref, Google Scholar
45 Solowij N, Stephens RS, Roffman RA, Babor T, Kadden R, Miller M, Christiansen K, McRee B, Vendetti J: Cognitive functioning of long-term heavy cannabis users seeking treatment. JAMA 2002; 287:1123–1131Crossref, Google Scholar
46 Bovasso GB: Cannabis abuse as a risk factor for depressive symptoms. American Journal of Psychiatry 2001; 158:2033–2037Crossref, Google Scholar
47 Centers for Disease Control and Prevention: Cigarette smoking among adults: United States, 1997. Morbidity and Mortality Weekly Report 1999; 48:993–996Google Scholar
48 Breslau N, Johnson EO, Hiripi E, Kessler R: Nicotine dependence in the United States: prevalence, trends, and smoking persistence. Archives of General Psychiatry 2001; 58:810–816Crossref, Google Scholar
49 Kucuk NO, Kilic EO, Ibis E, Aysev A, Gencoglu EA, Aras G, Soylu A, Erbay G: Brain SPECT findings in long-term inhalant abuse. Nuclear Medicine Communications 2000; 21:769–773Crossref, Google Scholar
50 Kuspis DA, Krenzelok EP: Oral frostbite injury from intentional abuse of a fluorinated hydrocarbon. Journal of Toxicology: Clinical Toxicology 1999; 37:873–875Crossref, Google Scholar
51 Centers for Disease Control and Prevention: Gamma hydroxybutyrate use: New York and Texas, 1995–1996. JAMA 1997; 277:1511Crossref, Google Scholar
52 Graeme KA: New drugs of abuse. Emergency Medicine Clinics of North America 2000; 18:625–636Crossref, Google Scholar
53 Craig K, Gomez HF, McManus JL, Bania TC: Severe gamma-hydroxybutyrate withdrawal: a case report and literature review. Journal of Emergency Medicine 2000; 18:65–70Crossref, Google Scholar
54 US Xyrem Multicenter Study Group: A randomized, double-blind, placebo-controlled multicenter trial comparing the effects of three doses of orally administered sodium oxybate with placebo for the treatment of narcolepsy. Sleep 2002; 25:42–49Crossref, Google Scholar
55 McLellan AT, Lewis DC, O’Brien CP, Kleber H: Drug dependence, a chronic medical illness: implications for treatment, insurance, and outcomes evaluation. JAMA 2000; 284:1689–1695Crossref, Google Scholar
56 Westermeyer J: Historical and social context of psychoactive substance disorders, in Textbook of Addictive Disorders. Edited by Frances RJ, Miller S. New York, Guilford Press, 1998Google Scholar
57 Frances RJ, Frances J, Borg L, Franklin JE: Psychodynamics, in Textbook of Substance Abuse Treatment, 2nd ed. Edited by Galanter M, Kleber H. Washington, DC, American Psychiatric Press, 1999Google Scholar
58 Leshner AI, Koob GF: Drugs of abuse and the brain. Proceedings of the Association of American Physicians 1999; 111:99–108Crossref, Google Scholar
59 Kilts CD, Schweitzer JB, Quinn CK: Neural activity related to drug craving in cocaine addiction. Archives of General Psychiatry 2001; 58:334–341Crossref, Google Scholar
60 Volkow ND, Chang L, Wang GJ, Fowler JS, Ding YS, Sedler M, Logan J, Franceschi D, Gatley J, Hitzemann R, Gifford A, Wong C, Pappas N: Low level of brain dopamine D2 receptors in methamphetamine abusers: association with metabolism in the orbitofrontal cortex. American Journal of Psychiatry 2001; 158:2015–2021Crossref, Google Scholar
61 Kreek MJ: Methadone-related opioid agonist pharmacotherapy for heroin addiction: history, recent molecular and neurochemical research, and future in mainstream medicine. Annals of the New York Academy of Sciences 2000; 909:186–216Crossref, Google Scholar
62 Volkow ND, Fowler JS: Addiction, a disease of compulsion and drive: involvement of the orbitofrontal cortex. 2000Cerebral Cortex 10:318–325Crossref, Google Scholar
63 Volkow ND, Chang L, Wang G-J, Fowler JS, Leonido-Yee M, Franceschi D, Sedler MJ, Gatley SJ, Hitzemann R, Ding YS, Logan J, Wong C, Miller EN: Association of dopamine transporter reduction with psychomotor impairment in methamphetamine abusers. American Journal of Psychiatry 2001; 158:377–382Crossref, Google Scholar
64 George MS, Anton RF, Bloomer C, Teneback C, Drobes DJ, Lorberbaum JP, Nahas Z, Vincent DJ: Activation of prefrontal cortex and anterior thalamus in alcoholic subjects on exposure to alcohol-specific cues. Archives of General Psychiatry 2001; 58:345–352Crossref, Google Scholar
65 Daglish MR, Weinstein A, Malizia AL, Wilson S, Melichar JK, Britten S, Brewer C, Lingford-Hughes A, Myles JS, Grasby P, Nutt DJ: Changes in regional cerebral blood flow elicited by craving memories in abstinent opiate-dependent subjects. American Journal of Psychiatry 2001; 158:1680–1686Crossref, Google Scholar
66 Leshner AI: Addiction is a brain disease and it matters. Science 1997; 278:45–47Crossref, Google Scholar
67 Tsai GE, Ragan P, Chang R, Chen S, Linnoila VMI, Coyle JT: Increased glutaminergic neurotransmission and oxidative stress after alcohol withdrawal. American Journal of Psychiatry 1998; 155:726–732Google Scholar
68 Kohnke MD, Zabetian CP, Anderson GM, Kolb W, Gaertner I, Buchkremer G, Vonthein R, Schick S, Lutz U, Kohnke AM, Cubells JF: A genotype-controlled analysis of plasma dopamine beta-hydroxylase in healthy and alcoholic subjects: evidence for alcohol-related differences in noradrenergic function. Biological Psychiatry 2002; 52:1151–1158Crossref, Google Scholar
69 Fiellin DA, O’Connor PG, Holmboe ES, Horwitz RI: Risk for delirium tremens in patients with alcohol withdrawal syndrome. Substance Abuse 2002; 23:83–94Google Scholar
70 Tests for Drugs of Abuse. Medical Letter on Drugs and Therapeutics 2002; 44:71–73Google Scholar
71 Schmidt LG, Schmidt K, Dufeu P, Ohse A, Rommelspacher H, Muller C: Superiority of carbohydrate-deficient transferrin to gamma-glutamyltransferase in detecting relapse in alcoholism. American Journal of Psychiatry 1997; 154:75–80Crossref, Google Scholar
72 Horn JL, Warburg KW, Foster FM: Alcohol Use Inventory. Minneapolis, MN, National Computer Systems, 1986Google Scholar
73 Warburg K, Horn J: The Alcohol Use Inventory. Port Logan, CO, Multivariate Measurement Consultants, 1985Google Scholar
74 Skinner HA, Horn JL: Alcohol Dependence Scale: Users’ Guide. Toronto, Ontario, Canada, Addiction Research Foundation, 1984Google Scholar
75 Mack A, Franklin JE, Frances RJ: Substance use disorders, in Textbook of Psychiatry, 4th ed. Edited by Hales RE, Yudofsky S. Washington, DC, American Psychiatric Press, 2002Google Scholar
76 Russell M, Martier SS, Sokol RJ, Mudar P, Bottoms S, Jacobson S, Jacobson J: Screening for pregnancy risk-drinking. Alcoholism: Clinical and Experimental Research 1994; 18:1156–1161Crossref, Google Scholar
77 McLellan AT, Luborsky L, Woody GE, O’Brien CP, Kron R: Are the “addiction-related” problems of substance abusers really related? Journal of Nervous and Mental Disorders 1981; 169:232–239Crossref, Google Scholar
78 Kaminer Y, Bukstein O, Tarter RE: The Teen-Addiction Severity Index: rationale and reliability. International Journal of the Addictions 1991; 26:219–226Crossref, Google Scholar
79 American Psychiatric Association: Practice Guideline for the Treatment of Patients With Substance Use Disorders: Alcohol, Cocaine, Opioids. American Journal of Psychiatry 1995; 152(Nov suppl):1–59Crossref, Google Scholar
80 American Society of Addiction Medicine: Patient Placement Criteria for the Treatment of Substance-Related Disorders, 2nd ed. Chevy Chase, MD, American Society of Addiction Medicine, 2001Google Scholar
81 American Psychiatric Association: Practice Guideline for the Treatment of Patients With Nicotine Dependence. American Journal of Psychiatry 1996; 153:(Oct suppl):1–31Google Scholar
82 National Institute on Drug Abuse: Principles of Drug Addiction Treatment: A Research-Based Guide. NIH Pub 00-4180. Rockville, MD, National Institute on Drug Abuse, 1999Google Scholar
83 Armor DJ, Polish SM, Stambul HB: Alcoholics and Treatment. New York, Wiley, 1978Google Scholar
84 Simpson D, Joe GW, Broome KM: A national 5-year follow-up of treatment outcomes for cocaine dependence. Archives of General Psychiatry 2002; 59:538–544Crossref, Google Scholar
85 McLellan AT, Grissom GR, Zanis D, Randall M, Brill P, O’Brien CP: Problem-service matching in addiction treatment: a prospective study in 4 programs. Archives of General Psychiatry 1997; 54:730–735Crossref, Google Scholar
86 Crits-Christoph P, Lynne Siqueland L, Blaine J, Frank A, Luborsky L, Onken LS, Muenz LR, Thase ME, Weiss RD, Gastfriend DR, Woody GE, Barber JP, Butler SF, Daley D, Salloum I, Bishop S, Najavits LM, Lis J, Mercer D, Griffin ML, Moras K, Beck AT: Psychosocial treatments for cocaine dependence: results of the NIDA Cocaine Collaborative Study. Archives of General Psychiatry 1999; 57:493–502Google Scholar
87 Project MATCH research group: Matching alcohol treatments to client heterogeneity: Project MATCH post-treatment drinking outcomes. Journal of Studies on Alcohol 1997; 58:7–29Crossref, Google Scholar
88 O’Malley SS, Jaffe AJ, Chang G, Rode S, Schottenfeld R, Meyer RE, Rounsaville B: Six-month follow-up of naltrexone and psychotherapy for alcohol dependence. Archives of General Psychiatry 1996; 53:217–224Crossref, Google Scholar
89 Sees KL, Delucci KL, Masson C, Rosen A, Clark HW, Robillard H, Banys P, Hall SM: Methadone maintenance versus 180-day psychosocially enriched detoxification for treatment of opioid dependence. JAMA 2000; 283:1303–1310Crossref, Google Scholar
90 Anton RF, Moak DH, Waid LR, Malcolm RJ, Dias JK, Roberts JS: Naltrexone and cognitive behavioral therapy for the treatment of outpatient alcoholics: results of a placebo-controlled trial. American Journal of Psychiatry 156:1758–1764, 1999Google Scholar
91 Mack A, Franklin JE, Frances RJ: Concise Guide to the Treatment of Alcoholism and Addictions. Washington, DC, American Psychiatric Press, 2001Google Scholar
92 Frances RJ, Mack AH, Borg L, Franklin JE: Individual treatment: psychodynamics and the treatment of substance-related disorders, in Treatment of Psychiatric Disorders, 3rd ed. Edited by Gabbard GO. Washington, DC, American Psychiatric Press, 2001Google Scholar
93 Wright FD, Beck AT, Newman CF: Cognitive therapy of substance abuse: theoretical rationale, in Behavioral Treatments for Drug Abuse and Dependence. NIDA Research Monograph 137. Edited by Onken LS, Blaine JD, Boren JJ. Rockville, MD, National Institute on Drug Abuse, 1993Google Scholar
94 Linehan MM, Schmidt H III, Dimeff LA, Craft JC, Kanter J, Comtois KA: Dialectical behavior therapy for patients with borderline personality disorder and drug dependence. American Journal of Addiction 1999; 8:279–292Crossref, Google Scholar
95 Prochaska JO, DiClemente CC, Norcross JC: In search of how people change: applications to addictive behavior. American Psychologist 1992; 47:1102–1114Crossref, Google Scholar
96 Samet JH, Rollnick S, Barnes H: Beyond CAGE: a brief clinical approach after detection of substance abuse. Archives of Internal Medicine 1996; 156:2287–2293Crossref, Google Scholar
97 Galanter M: Healing through social and spiritual affiliation. Psychiatric Services 2002; 53:1072–1074Crossref, Google Scholar
98 Edwards ME, Steinglass P: Family therapy treatment outcomes for alcoholism. Journal of Marital and Family Therapy 1995; 21:475–509Crossref, Google Scholar
99 Galanter M: Self-help treatment for combined addiction and mental illness. Psychiatric Services 2000; 51:977–979Crossref, Google Scholar
100 Volpicelli JR, Alterman AI, Hayashida M, O’Brien CP: Naltrexone in the treatment of alcohol dependence. Archives of General Psychiatry 1992; 49:876–887Crossref, Google Scholar
101 Krystal JH, Cramer JA, Krol WF, Kirk GF, Rosenheck RA: Naltrexone in the treatment of alcohol dependence. New England Journal of Medicine 2001; 345:1734–1739Crossref, Google Scholar
102 McCance-Katz EF, Rainey PM, Jatlow P, Friedland G: Methadone effects on zidovudine disposition. AIDS Clinical Trials Group 262. Journal of Acquired Immune Deficiency Syndromes and Human Retrovirology 1998; 18:435–443Crossref, Google Scholar
103 Johnson RE, Eissenberg T, Stitzer ML, Strain EC, Liebson IA, Bigelow GE: A placebo-controlled clinical trial of buprenorphine as a treatment for opioid dependence. Drug and Alcohol Dependence 1995; 40:17–25Crossref, Google Scholar
104 Bickel WK, Amass L, Higgins ST, Badger GJ, Esch RA: Effects of adding behavioral treatment to opioid detoxification with buprenorphine. Journal of Consulting and Clinical Psychology 1997; 65:803–810Crossref, Google Scholar
105 Pettinati HM, Volpicelli JR, Luck G, Kranzler HR, Rukstalis MR, Cnaan A: Double-blind clinical trial of sertraline treatment of alcohol dependence. Journal of Clinical Psychopharmacology 2001; 21:143–153Crossref, Google Scholar
106 Ahluwalia JS, Harris KJ, Catley D: Sustained-release bupropion for smoking cessation in African Americans: a randomized controlled trial. JAMA 2002; 288:468–474Crossref, Google Scholar
107 Mason BJ: Treatment of alcohol-dependent outpatients with acamprosate: a clinical review. Journal of Clinical Psychiatry 2001; 62(suppl):42–48Google Scholar
108 Berk M, Brook S, Trandafir AI: A comparison of olanzapine with haloperidol in cannabis-induced psychotic disorder: a double-blind randomized controlled trial. International Clinical Psychopharmacology 1999; 14:177–180Google Scholar
109 O’Connor PG, Carroll KM, Shi JM, Schottenfeld RS, Kosten TR, Rounsaville BJ: Three methods of opioid detoxification in a primary care setting: a randomized trial. Annals of Internal Medicine 1997; 127:526–530Crossref, Google Scholar
110 Ling W, Charuvastra C, Collins JF, Batki S, Brown LS Jr, Kintaudi P, Wesson DR, McNicholas L, Tusel DJ, Malkerneker U, Renner JA Jr, Santos E, Casadonte P, Fye C, Stine S, Wang RI, Segal D: Buprenorphine maintenance treatment of opiate dependence: a multicenter, randomized clinical trial. Addiction 1998; 93:475–486Crossref, Google Scholar
111 Moore RD, Bone LR, Geller G, Mamon JA, Stokes EJ, Levine DM: Prevalence, detection, and treatment of alcoholism in hospitalized patients. JAMA 1989; 261:403–407Crossref, Google Scholar
112 McGill V, Kowal-Vern A, Fisher SG: The impact of substance use on mortality and morbidity from thermal injury. Journal of Trauma 1995; 38:931–934Crossref, Google Scholar
113 Berlakovich GA, Steininger R, Herbst F, Barlan M, Mittlbock M, Muhlbacher F: Efficacy of liver transplantation for alcoholic cirrhosis with respect to recidivism and compliance. Transplantation 58:560–565, 1994Crossref, Google Scholar
114 Botvin GJ, Baker E, Dusenbury L, Botvin EM, Diaz T: Long-term follow-up results of a randomized drug abuse prevention trial in a white middle-class population. JAMA 1995; 273:1106–1112Crossref, Google Scholar
115 Mack A, Rosecan J, Frances RJ: Drugs: abuse and dependence, in Mental Health and Productivity in the Workplace. Edited by Kahn J, Langlieb A. Baltimore, Jossey-Bass, 2002Google Scholar
116 Ashley MJ, Olin JS, LeRiche WH, Kornaczewski A, Schmidt W, Rankin JG: Morbidity in alcoholics: evidence for accelerated development of physical disease in women. Archives of Internal Medicine 1977; 137:883–887Crossref, Google Scholar
117 Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB: Posttraumatic stress disorder in the National Comorbidity Survey. Archives of General Psychiatry 1995; 52:1048–1060Crossref, Google Scholar
118 Hommer DW, Momenan R, Kaiser E, Rawlings R: Evidence for a gender-related effect of alcoholism on brain volumes. American Journal of Psychiatry 2001; 158:198–204Crossref, Google Scholar
119 Gavaler JS, Rizzo A, Rossaro L, Van Thiel DH, Brezza E, Deal SR: Sexuality of alcoholic women with menstrual cycle function: effects of duration of alcohol abstinence. Alcoholism: Clinical and Experimental Research 1995; 17:778–781Google Scholar
120 Singletary KW, Gapstur SM: Alcohol and breast cancer: review of epidemiologic and experimental evidence and potential mechanisms. JAMA 2001; 286:2143–2151Crossref, Google Scholar
121 Mick E, Biederman J, Faraone SV, Sayer J, Kleinman S: Case-control study of attention-deficit hyperactivity disorder and maternal smoking: alcohol use and drug use during pregnancy. Journal of the American Academy of Child and Adolescent Psychiatry 2002; 41:378–385Crossref, Google Scholar
122 Jacobs EA, Copperman SM, Jeffe A, Kulig J: Fetal alcohol syndrome and alcohol-related neurodevelopmental disorders. Pediatrics 2000; 106:358–361Crossref, Google Scholar
123 Brown HL, Britton KA, Mahaffey D, Brizendine E, Hiett AK, Turnquest MA: Methadone maintenance in pregnancy: a reappraisal. American Journal of Obstetrics and Gynecology 1998; 179:459–463Crossref, Google Scholar
124 Singer LT, Arendt R, Minnes S, Farkas K, Salvator A, Kirchner HL, Kliegman R: Cognitive and motor outcomes of cocaine-exposed infants. JAMA 2002; 287:1952–1960Crossref, Google Scholar
125 Roeloffs CA, Fink A, Unützer J: Problematic substance use, depressive symptoms, and gender in primary care. Psychiatric Services 2001; 52:1251–1253Crossref, Google Scholar
126 Kelly RH, Zatzick DF, Anders TF: The detection and treatment of psychiatric disorders and substance use among pregnant women cared for in obstetrics. American Journal of Psychiatry 2001; 158:213–219Crossref, Google Scholar
127 Weinberg NZ, Rahdert E, Colliver JD, Glantz MD: Adolescent substance abuse: a review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry 1998; 37:252–261Crossref, Google Scholar
128 Ozechowski TJ, Liddle HA: Family-based therapy for adolescent drug abuse: knowns and unknowns. Clinical Child and Family Psychology Review 2000; 3:269–299Crossref, Google Scholar
129 Stanton MD, Shadish WR: Outcome, attrition, and family/couples treatment for drug abuse: a meta-analysis and review of the controlled, comparative studies. Psychological Bulletin 1997; 122:170–191Crossref, Google Scholar
130 Liddle HA, Dakof GA: Family-based treatment for adolescent drug use: state of the science, in Adolescent Drug Abuse: Clinical Assessment and Therapeutic Interventions. NIDA Research Monograph 156, NIH Pub No 95-3908. Edited by Rahdert E, Czechowicz D. Rockville, MD, National Institute on Drug Abuse, 1995Google Scholar
131 Schmidt SE, Liddle HA, Dakof GA: Changes in parenting practices and adolescent drug abuse during multidimensional family therapy. Journal of Family Psychology 1996; 10:12–27Crossref, Google Scholar
132 American Academy of Child and Adolescent Psychiatry: Practice parameters for the assessment and treatment of children and adolescents with substance use disorders. Journal of the American Academy of Child and Adolescent Psychiatry 1997; 36(10 suppl):140S–156SCrossref, Google Scholar
133 American Academy of Pediatrics: Tobacco, alcohol, and other drugs: the role of the pediatrician in prevention and management of substance abuse. Pediatrics 1998; 101:125–128Crossref, Google Scholar
134 Treatment of Adolescents With Substance Use Disorders. TIPS 32. Rockville, MD, Center for Substance Abuse Treatment, 1999Google Scholar
135 Weinberg NZ, Rahdert E, Colliver JD, Glantz MD: Adolescent substance abuse: a review of the past 10 years. Journal of the American Academy of Child and Adolescent Psychiatry 1998; 37:252–261Crossref, Google Scholar
136 Jainchill N: Substance dependency treatment for adolescents: practice and research. Substance Use and Misuse 2000; 35:2031–2060Crossref, Google Scholar
137 Harrison PA, Fulkerson JA, Beebe TJ: DSM-IV substance use disorder criteria for adolescents: a critical examination based on a statewide school survey. American Journal of Psychiatry 1998; 155:486–492Crossref, Google Scholar
138 Hser Y-I, Grella CE, Hubbard RL, Hsieh SC, Fletcher BW, Brown BS, Anglin MD: An evaluation of drug treatments for adolescents in 4 US cities. Archives of General Psychiatry 2001; 58:689–695Crossref, Google Scholar
139 Lewisohn PM, Hopps H, Roberts RE: Adolescent psychopathology: I. prevalence and incidence of depression and other DSM-III-R disorders in high school students. Journal of Abnormal Psychology 1993; 102:133–144Crossref, Google Scholar
140 Biederman J, Wilens T, Mick E, Faraone SV, Weber W, Curtis S, Thornell A, Pfister K, Jetton JG, Soriano J: Is ADHD a risk factor for psychoactive substance use disorders? Findings from a four-year prospective follow-up study. Journal of the American Academy of Child and Adolescent Psychiatry 1997; 36:21–29Crossref, Google Scholar
141 Wilens TE, Biederman J, Millstein RB, Wozniak J, Hahesy AL, Spencer TJ: Risk for substance use disorder in youths with child- and adolescent-onset bipolar disorder. Journal of the American Academy of Child and Adolescent Psychiatry 1999; 38:680–685Crossref, Google Scholar
142 Geller B, Cooper T, Sun K, Zimerman B, Frazier J, Williams M, Heath J: Double-blind and placebo-controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. Journal of the American Academy of Child and Adolescent Psychiatry 1998; 37:171–178Crossref, Google Scholar
143 Blood L, Cornwall A: Pretreatment variables that predict completion of an adolescent substance abuse treatment program. Journal of Nervous and Mental Disorders 1994; 182:14–19Crossref, Google Scholar
144 Kaminer Y: Adolescent substance abuse: a comprehensive guide to theory and practice. New York, Plenum, 1994Google Scholar
145 Azrin NH, Donohue B, Besalel VA, McMahon P: Youth drug abuse treatment: a controlled outcome study. Journal of Child and Adolescent Substance Abuse 1994; 3:1–16Crossref, Google Scholar
146 Kaminer Y: Contingency management reinforcement procedures for adolescent substance abuse. Journal of the American Academy of Child and Adolescent Psychiatry 2000; 39:1324–1326Crossref, Google Scholar
147 Fisher MS, KJ Bentley: Two group therapy models for clients with a dual diagnosis of substance abuse and personality disorder. Psychiatric Services 1996; 47:1244–1250Crossref, Google Scholar
148 Kelly JF, Myers MG, Brown SA: A multivariate process model of adolescent 12-step attendance and substance use outcome following inpatient treatment. Psychology of Addictive Behaviors 2000; 14:376–389Crossref, Google Scholar
149 Kaminer Y, Burleson J, Blitz C, Sussman J, Rounsaville BJ: Psychotherapies for adolescent substance abusers: a pilot study. Journal of Nervous and Mental Disorders 1998; 186:684–690Crossref, Google Scholar
150 Koegel P, Burnam A: Alcoholism among homeless adults in the inner city of Los Angeles. Archives of General Psychiatry 1988; 45:1011–1018Crossref, Google Scholar
151 Franklin J: Minority populations, in Clinical Textbook of Addictive Disorders, 2nd ed. Edited by Frances RJ, Miller SI. New York, Guilford, 1998Google Scholar
152 Shore J, Manson SM, Buchwald D: Screening for alcohol abuse among urban Native Americans in a primary care setting. Psychiatric Services 2002; 53:757–760Crossref, Google Scholar
153 Fiellin DA, O’Connon PG: Office-based treatment of opioid-dependent patients. New England Journal of Medicine 2002; 347:817–823Crossref, Google Scholar
154 Carrera M, Ashley JA, Wirsching P, Koob GF, Janda KD: A second-generation vaccine protects against the psychoactive effects of cocaine. Proceedings of the National Academy of Sciences of the United States of America 2001; 98:1988–1992Crossref, Google Scholar
155 Shaham Y, Erb S, Leung S, Buczek Y, Stewart J: CP-154,526, a selective, non-peptide antagonist of the corticotropin-releasing factor 1 receptor attenuates stress-induced relapse to drug seeking in cocaine- and heroin-trained rats. Psychopharmacology (Berlin) 1998; 137:184–190Crossref, Google Scholar



