Practice Guideline for the Treatment of Patients With Bipolar Disorder (Revision)
Part A: Treatment recommendations for patients with bipolar disorder
I. Executive summary of recommendations
Each recommendation is identified as falling into one of three categories of endorsement, indicated by a bracketed Roman numeral following the statement. The three categories represent varying levels of clinical confidence regarding the recommendation:
[I] Recommended with substantial clinical confidence.
[II] Recommended with moderate clinical confidence.
[III] May be recommended on the basis of individual circumstances.
A. Psychiatric management
At this time, there is no cure for bipolar disorder; however, treatment can decrease the associated morbidity and mortality [I]. Initially, the psychiatrist should perform a diagnostic evaluation and assess the patient’s safety and level of functioning to arrive at a decision about the optimum treatment setting [I]. Subsequently, specific goals of psychiatric management include establishing and maintaining a therapeutic alliance, monitoring the patient’s psychiatric status, providing education regarding bipolar disorder, enhancing treatment compliance, promoting regular patterns of activity and of sleep, anticipating stressors, identifying new episodes early, and minimizing functional impairments [I].
B. Acute treatment
1. Manic or mixed episodes
The first-line pharmacological treatment for more severe manic or mixed episodes is the initiation of either lithium plus an antipsychotic or valproate plus an antipsychotic [I]. For less ill patients, monotherapy with lithium, valproate, or an antipsychotic such as olanzapine may be sufficient [I]. Short-term adjunctive treatment with a benzodiazepine may also be helpful [II]. For mixed episodes, valproate may be preferred over lithium [II]. Atypical antipsychotics are preferred over typical antipsychotics because of their more benign side effect profile [I], with most of the evidence supporting the use of olanzapine or risperidone [II]. Alternatives include carbamazepine or oxcarbazepine in lieu of lithium or valproate [II]. Antidepressants should be tapered and discontinued if possible [I]. If psychosocial therapy approaches are used, they should be combined with pharmacotherapy [I].
For patients who, despite receiving maintenance medication treatment, experience a manic or mixed episode (i.e., a “breakthrough” episode), the first-line intervention should be to optimize the medication dose [I]. Introduction or resumption of an antipsychotic is sometimes necessary [II]. Severely ill or agitated patients may also require short-term adjunctive treatment with a benzodiazepine [I].
When first-line medication treatment at optimal doses fails to control symptoms, recommended treatment options include addition of another first-line medication [I]. Alternative treatment options include adding carbamazepine or oxcarbazepine in lieu of an additional first-line medication [II], adding an antipsychotic if not already prescribed [I], or changing from one antipsychotic to another [III]. Clozapine may be particularly effective in the treatment of refractory illness [II]. ECT may also be considered for patients with severe or treatment-resistant mania or if preferred by the patient in consultation with the psychiatrist [I]. In addition, ECT is a potential treatment for patients experiencing mixed episodes or for patients experiencing severe mania during pregnancy [II].
Manic or mixed episodes with psychotic features usually require treatment with an antipsychotic medication [II].
2. Depressive episodes
The first-line pharmacological treatment for bipolar depression is the initiation of either lithium [I] or lamotrigine [II]. Antidepressant monotherapy is not recommended [I]. As an alternative, especially for more severely ill patients, some clinicians will initiate simultaneous treatment with lithium and an antidepressant [III]. In patients with life-threatening inanition, suicidality, or psychosis, ECT also represents a reasonable alternative [I]. ECT is also a potential treatment for severe depression during pregnancy [II].
A large body of evidence supports the efficacy of psychotherapy in the treatment of unipolar depression [I]. In bipolar depression, interpersonal therapy and cognitive behavior therapy may be useful when added to pharmacotherapy [II]. While psychodynamic psychotherapy has not been empirically studied in patients with bipolar depression, it is widely used in addition to medication [III].
For patients who, despite receiving maintenance medication treatment, suffer a breakthrough depressive episode, the first-line intervention should be to optimize the dose of maintenance medication [II].
When an acute depressive episode of bipolar disorder does not respond to first-line medication treatment at optimal doses, next steps include adding lamotrigine [I], bupropion [II], or paroxetine [II]. Alternative next steps include adding other newer antidepressants (e.g., a selective serotonin reuptake inhibitor [SSRI] or venlafaxine) [II] or a monoamine oxidase inhibitor (MAOI) [II]. For patients with severe or treatment-resistant depression or depression with psychotic or catatonic features, ECT should be considered [I].
The likelihood of antidepressant treatment precipitating a switch into a hypomanic episode is probably lower in patients with bipolar II depression than in patients with bipolar I depression. Therefore, clinicians may elect to recommend antidepressant treatment earlier in patients with bipolar II disorder [II].
Depressive episodes with psychotic features usually require adjunctive treatment with an antipsychotic medication [I]. ECT represents a reasonable alternative [I].
3. Rapid cycling
As defined in DSM-IV-TR (1) and applied in this guideline, rapid cycling refers to the occurrence of four or more mood disturbances within a single year that meet criteria for a major depressive, mixed, manic, or hypomanic episode. These episodes are demarcated either by partial or full remission for at least 2 months or a switch to an episode of opposite polarity (e.g., from a major depressive to a manic episode). The initial intervention in patients who experience rapid cycling is to identify and treat medical conditions, such as hypothyroidism or drug or alcohol use, that may contribute to cycling [I]. Certain medications, particularly antidepressants, may also contribute to cycling and should be tapered if possible [II]. The initial treatment for patients who experience rapid cycling should include lithium or valproate [I]; an alternative treatment is lamotrigine [I]. For many patients, combinations of medications are required [II].
C. Maintenance treatment
Following remission of an acute episode, patients may remain at particularly high risk of relapse for a period of up to 6 months; this phase of treatment, sometimes referred to as continuation treatment, is considered in this guideline to be part of the maintenance phase. Maintenance regimens of medication are recommended following a manic episode [I]. Although few studies involving patients with bipolar II disorder have been conducted, consideration of maintenance treatment for this form of the illness is also strongly warranted [II]. The medications with the best empirical evidence to support their use in maintenance treatment include lithium [I] and valproate [I]; possible alternatives include lamotrigine [II] or carbamazepine or oxcarbazepine [II]. If one of these medications was used to achieve remission from the most recent depressive or manic episode, it generally should be continued [I]. Maintenance sessions of ECT may also be considered for patients whose acute episode responded to ECT [II].
For patients treated with an antipsychotic medication during the preceding acute episode, the need for ongoing antipsychotic treatment should be reassessed upon entering maintenance treatment [I]; antipsychotics should be discontinued unless they are required for control of persistent psychosis [I] or prophylaxis against recurrence [III]. While maintenance therapy with atypical antipsychotics may be considered [III], there is as yet no definitive evidence that their efficacy in maintenance treatment is comparable to that of agents such as lithium or valproate.
During maintenance treatment, patients with bipolar disorder are likely to benefit from a concomitant psychosocial intervention—including psychotherapy—that addresses illness management (i.e., adherence, lifestyle changes, and early detection of prodromal symptoms) and interpersonal difficulties [II].
Group psychotherapy may also help patients address such issues as adherence to a treatment plan, adaptation to a chronic illness, regulation of self-esteem, and management of marital and other psychosocial issues [II]. Support groups provide useful information about bipolar disorder and its treatment [I].
Patients who continue to experience subthreshold symptoms or breakthrough mood episodes may require the addition of another maintenance medication [II], an atypical antipsychotic [III], or an antidepressant [III]. There are currently insufficient data to support one combination over another. Maintenance sessions of ECT may also be considered for patients whose acute episode responded to ECT [II].
II. Formulation and implementation of a treatment plan
The following discussion regarding the formulation and implementation of a treatment plan refers specifically to patients with bipolar disorder. Every effort has been made to identify and highlight distinctions between bipolar I and bipolar II disorder in terms of patient response to treatment. However, with few exceptions, data from large trials have been presented in such a way that making such distinctions is difficult. For the treatment of patients with major depressive disorder, readers should refer to the APA Practice Guideline for the Treatment of Patients With Major Depressive Disorder (2).
Initial treatment of bipolar disorder requires a thorough assessment of the patient, with particular attention to the safety of the patient and those around him or her as well as attention to possible comorbid psychiatric or medical illnesses. In addition to the current mood state, the clinician needs to consider the longitudinal history of the patient’s illness. Patients frequently seek treatment during an acute episode, which may be characterized by depression, mania, hypomania, or a mixture of depressive and manic features. Treatment is aimed at stabilization of the episode with the goal of achieving remission, defined as a complete return to baseline level of functioning and a virtual lack of symptoms. (Following remission of a depressive episode, patients may remain at particularly high risk of relapse for a period up to 6 months; this phase of treatment, sometimes referred to as continuation treatment [4], is considered in this guideline to be part of maintenance treatment.) After successfully completing the acute phase of treatment, patients enter the maintenance phase. At this point, the primary goal of treatment is to optimize protection against recurrence of depressive, mixed, manic, or hypomanic episodes. Concurrently, attention needs to be devoted to maximizing patient functioning and minimizing subthreshold symptoms and adverse effects of treatment.
Of note, in the treatment recommendations outlined in this guideline, several references are made to adding medications or offering combinations of medications. Patients with bipolar disorder often require such combinations in order to achieve adequate symptom control and prophylaxis against future episodes. However, each additional medication generally increases the side effect burden and the likelihood of drug-drug interactions or other toxicity and therefore must be assessed in terms of the risk-benefit ratio to the individual patient. This guideline has attempted to highlight medication interactions used in common clinical practice that are of particular concern (e.g., interactions between lamotrigine and valproate or between carbamazepine and oral contraceptives). In addition, for several of the medications addressed in this guideline, different preparations or forms are available (e.g., valproic acid and divalproex). Although the guideline refers to these medications in general terms, the form of medication with the best tolerability and fewest drug interactions should be preferred.
At other times in treatment, it may be necessary to discontinue a medication (e.g., because of intolerable side effects) or substitute one medication for another. It is preferable to slowly taper the medication to be discontinued rather than discontinuing it abruptly.
In this revision of the previously published Practice Guideline for the Treatment of Patients With Bipolar Disorder (5), the term “mood stabilizer” has been omitted. Several definitions of what constitutes a mood stabilizer have been proposed and generally include such criteria as proven efficacy for the treatment of mania or depression, absence of exacerbation of manic or mixed symptoms, or prophylactic efficacy. Because of the absence of a consensus definition, this guideline will instead generally refer to specific medications or to the phase of illness in which they may be used.
A. Psychiatric management
The cross-sectional (i.e., current clinical status) and longitudinal (i.e., frequency, severity, and consequences of past episodes) context of the treatment decision should guide the psychiatrist and bipolar disorder patient in choosing from among various possible treatments and treatment settings. Such treatment decisions must be based on knowledge of the potential beneficial and adverse effects of available options along with information about patient preferences. In addition, treatment decisions should be continually reassessed as new information becomes available, the patient’s clinical status changes, or both. Lack of insight or minimization is often a prominent part of bipolar disorder and may at times interfere with the patient’s ability to make reasoned treatment decisions, necessitating the involvement of family members or significant others in treatment whenever possible.
At this time, there is no cure for bipolar disorder; however, treatment can significantly decrease the associated morbidity and mortality. The general goals of bipolar disorder treatment are to assess and treat acute exacerbations, prevent recurrences, improve interepisode functioning, and provide assistance, insight, and support to the patient and family. Initially, the psychiatrist will perform a diagnostic evaluation and assess the patient’s safety, level of functioning, and clinical needs in order to arrive at a decision about the optimum treatment setting. Subsequently, specific goals of psychiatric management include establishing and maintaining a therapeutic alliance, monitoring the patient’s psychiatric status, providing education regarding bipolar disorder, enhancing treatment compliance, promoting regular patterns of activity and of sleep, anticipating stressors, identifying new episodes early, and minimizing functional impairments.
1. Perform a diagnostic evaluation
The evaluation for bipolar disorder requires careful and thorough attention to the clinical history. Patients with bipolar disorder most often exhibit symptoms of depression but may also exhibit substance use, impulsivity, irritability, agitation, insomnia, problems with relationships, or other concerns. Patients rarely volunteer information about manic or hypomanic episodes, so clinicians must probe about time periods with mood dysregulation, lability, or both that are accompanied by associated manic symptoms (e.g., decreased need for sleep, increased energy).
One way to improve efficiency and increase sensitivity in detecting bipolar disorder is to screen for it, particularly in patients with depression, irritability, or impulsivity. The Mood Disorder Questionnaire is a 13-item, self-report screening instrument for bipolar disorder that has been used successfully in psychiatric clinics (6) and in the general population (unpublished 2001 study of R.M.A. Hirschfeld). The general principles and components of a complete psychiatric evaluation have been outlined in the APA Practice Guideline for Psychiatric Evaluation of Adults (7).
2. Evaluate the safety of the patient and others and determine a treatment setting
Suicide completion rates in patients with bipolar I disorder may be as high as 10%–15% (8–13); thus, a careful assessment of the patient’s risk for suicide is critical. The overwhelming majority of suicide attempts are associated with depressive episodes or depressive features during mixed episodes. The elements of an evaluation for suicide risk are summarized in Table 1. All patients should be asked about suicidal ideation, intention to act on these ideas, and extent of plans or preparation for suicide. Collateral information from family members or others is critical in assessing suicide risk. Access to means of committing suicide (e.g., medications, firearms) and the lethality of these means should also be determined. Other clinical factors that may increase the risk of a patient acting on suicidal ideation should be assessed; these may include substance abuse or other psychiatric comorbidity, such as psychosis. The nature of any prior suicide attempts, including their potential for lethality, should be considered.
The ability to predict suicide or violence risk from clinical data is somewhat limited. Consequently, patients who exhibit suicidal or violent ideas or intent require close monitoring. Whenever suicidal or violent ideas are expressed or suspected, careful documentation of the decision-making process is essential. Hospitalization is usually indicated for patients who are considered to pose a serious threat of harm to themselves or others. If patients refuse, they can be hospitalized involuntarily if their condition meets criteria of the local jurisdiction for involuntary admission. Severely ill patients who lack adequate social support outside of a hospital setting or demonstrate significantly impaired judgment should also be considered for admission to a hospital. Additionally, those patients who have psychiatric or general medical complications or who have not responded adequately to outpatient treatment may need to be hospitalized. The optimal treatment setting and the patient’s ability to benefit from a different level of care should be reevaluated on an ongoing basis throughout the course of treatment.
During the manic phase of bipolar disorder, a calm and highly structured environment is optimal. Such stimuli as television, videos, music, and even animated conversations can heighten manic thought processes and activities. Patients and their families should be advised that during manic episodes, patients may engage in reckless behavior and that, at times, steps should be taken to limit access to cars, credit cards, bank accounts, and telephones or cellular phones.
3. Establish and maintain a therapeutic alliance
Bipolar disorder is a long-term illness that manifests in different ways in different patients and at different points during its course. Establishing and maintaining a supportive and therapeutic relationship is critical to the proper understanding and management of an individual patient. A crucial element of this alliance is the knowledge gained about the course of the patient’s illness that allows new episodes to be identified as early as possible.
4. Monitor treatment response
The psychiatrist should remain vigilant for changes in psychiatric status. While this is true for all psychiatric disorders, it is especially important in bipolar disorder because limited insight on the part of the patient is so frequent, especially during manic episodes. In addition, small changes in mood or behavior may herald the onset of an episode, with potentially devastating consequences. Such monitoring may be enhanced by knowledge gained over time about particular characteristics of a patient’s illness, including typical sequence (e.g., whether episodes of mania are usually followed by episodes of depression) and typical duration and severity of episodes.
5. Provide education to the patient and to the family
Patients with bipolar disorder benefit from education and feedback regarding their illness, prognosis, and treatment. Frequently, their ability to understand and retain this information will vary over time. Patients will also vary in their ability to accept and adapt to the idea that they have an illness that requires long-term treatment. Education should therefore be an ongoing process in which the psychiatrist gradually but persistently introduces facts about the illness. Over an extended period of time, such an approach to patient education will assist in reinforcing the patient’s collaborative role in treating this persistent illness. In this capacity, the patient will know when to report subsyndromal symptoms. Printed material on cross-sectional and longitudinal aspects of bipolar illness and its treatment can be helpful, including information available on the Internet (such as that found in the Medical Library at www.medem.com). Similar educational approaches are also important for family members and significant others. They too may have difficulty accepting that the patient has an illness and may minimize the consequences of the illness and the patient’s need for continuing treatment (14–17).
6. Enhance treatment compliance
Bipolar disorder is a long-term illness in which adherence to carefully designed treatment plans can improve the patient’s health status. However, patients with this disorder are frequently ambivalent about treatment (18). This ambivalence often takes the form of noncompliance with medication and other treatments (19, 20), which is a major cause of relapse (21, 22).
Ambivalence about treatment stems from many factors, one of which is lack of insight. Patients who do not believe that they have a serious illness are not likely to be willing to adhere to long-term treatment regimens. Patients with bipolar disorder may minimize or deny the reality of a prior episode or their own behavior and its consequences. Lack of insight may be especially pronounced during a manic episode.
Another important factor for some patients is their reluctance to give up the experience of hypomania or mania (19). The increased energy, euphoria, heightened self-esteem, and ability to focus may be very desirable and enjoyable. Patients often recall this aspect of the experience and minimize or deny entirely the subsequent devastating features of full-blown mania or the extended demoralization of a depressive episode. As a result, they are often reluctant to take medications that prevent elevations in mood.
Medication side effects, cost, and other demands of long-term treatment may be burdensome and need to be discussed realistically with the patient and family members. Many side effects can be corrected with careful attention to dosing, scheduling, and preparation. Troublesome side effects that remain must be discussed in the context of an informed assessment of the risks and benefits of the current treatment and its potential alternatives.
7. Promote awareness of stressors and regular patterns of activity and sleep
Patients and families can also benefit from an understanding of the role of psychosocial stressors and other disruptions in precipitating or exacerbating mood episodes. Psychosocial stressors are consistently found to be increased before both manic and depressive episodes (23). Although this relationship was previously thought to hold true only for the first few episodes of bipolar disorder, more recent studies have found that stressors commonly precede episodes in all phases of the illness (24). Social rhythm disruption with disrupted sleep/wake cycles may specifically trigger manic (but not depressive) episodes (25). Of course, some episodes may not be associated with any discernible life events or stressors. Clinically, the pharmacological management of manic or depressive episodes does not depend on whether stressors preceded the episode. However, patients and families should be informed about the potential consequences of sleep disruption on the course of bipolar disorder (26). To target vulnerable times and to generate coping strategies for these stressors, the unique association between specific types of life stressors and precipitating episodes for each patient should also be addressed (27). It is similarly important to recognize distress or dysfunction in the family of a patient with bipolar disorder, since such ongoing stress may exacerbate the patient’s illness or interfere with treatment (14, 15, 28, 29).
Patients with bipolar disorder may benefit from regular patterns of daily activities, including sleeping, eating, physical activity, and social and emotional stimulation. The psychiatrist should help the patient determine the degree to which these factors affect mood states and develop methods to monitor and modulate daily activities. Many patients find that if they establish regular patterns of sleeping, other important aspects of life will fall into regular patterns as well.
8. Work with the patient to anticipate and address early signs of relapse
The psychiatrist should help the patient, family members, and significant others recognize early signs and symptoms of manic or depressive episodes. Such identification can help the patient enhance mastery over his or her illness and can help ensure that adequate treatment is instituted as early as possible in the course of an episode. Early markers of episode onset vary from patient to patient but are often usefully predictable across episodes for an individual patient. Many patients experience changes in sleep patterns early in the development of an episode. Other symptoms may be quite subtle and specific to the individual (e.g., participating in religious activities more or less often than usual). The identification of these early prodromal signs or symptoms is facilitated by the presence of a consistent relationship between the psychiatrist and the patient as well as a consistent relationship with the patient’s family (27). The use of a graphic display or timeline of life events and mood symptoms can be very helpful in this process (30). First conceived by Kraepelin (31) and Meyer (32) and refined and advanced by Post et al. (30), a life chart provides a valuable display of illness course and episode sequence, polarity, severity, frequency, response to treatment, and relationship (if any) to environmental stressors. A graphic display of sleep patterns may be sufficient for some patients to identify early signs of episodes.
9. Evaluate and manage functional impairments
Episodes of mania or depression often leave patients with emotional, social, family, academic, occupational, and financial problems. During manic episodes, for example, patients may spend money unwisely, damage important relationships, lose jobs, or commit sexual indiscretions. Following mood episodes, they may require assistance in addressing the psychosocial consequences of their actions.
Bipolar disorder is associated with functional impairments even during periods of euthymia, and the presence, type, and severity of dysfunction should be evaluated (33–35). Impairments can include deficits in cognition, interpersonal relationships, work, living conditions, and other medical or health-related needs (36, 37). Identified impairments in functioning should be addressed. For example, some patients may require assistance in scheduling absences from work or other responsibilities, whereas others may require encouragement to avoid major life changes while in a depressive or manic state. Patients should also be encouraged to set realistic, attainable goals for themselves in terms of desirable levels of functioning. Occupational therapists may be helpful with addressing functional impairments caused by bipolar disorder.
Patients who have children may need help assessing and addressing their children’s needs. In particular, children of individuals with bipolar disorder have genetic as well as psychosocial risk factors for developing a psychiatric disorder; parents may need help in obtaining a psychiatric evaluation for children who show early signs of mood instability.
B. Acute treatment
1. Manic or mixed episodes
For patients experiencing a manic or mixed episode, the primary goal of treatment is the control of symptoms to allow a return to normal levels of psychosocial functioning. The rapid control of agitation, aggression, and impulsivity is particularly important to ensure the safety of patients and those around them.
Lithium, valproate, and antipsychotic medications have shown efficacy in the treatment of acute mania, although the time to onset of action for lithium may be somewhat slower than that for valproate or antipsychotics. The combination of an antipsychotic with either lithium or valproate may be more effective than any of these agents alone. Thus, the first-line pharmacological treatment for patients with severe mania is the initiation of either lithium plus an antipsychotic or valproate plus an antipsychotic. For less ill patients, monotherapy with lithium, valproate, or an antipsychotic such as olanzapine may be sufficient. Alternatives with less supporting evidence for treatment of manic and mixed states include ziprasidone or quetiapine in lieu of another antipsychotic and carbamazepine or oxcarbazepine in lieu of lithium or valproate. (Although efficacy data for oxcarbazepine remain limited, this medication may have equivalent efficacy and better tolerability than carbamazepine.) Short-term adjunctive treatment with a benzodiazepine may also be helpful. In contrast, antidepressants may precipitate or exacerbate manic or mixed episodes and generally should be tapered and discontinued if possible.
Selection of the initial treatment should be guided by clinical factors such as illness severity, by associated features (e.g., rapid cycling, psychosis), and by patient preference where possible, with particular attention to side effect profiles. A number of factors may lead the clinician to choose one particular agent over another. For example, some evidence suggests a greater efficacy of valproate compared with lithium in the treatment of mixed states. Also, severely ill and agitated patients who are unable to take medications by mouth may require antipsychotic medications that can be administered intramuscularly. Because of the more benign side effect profile of atypical antipsychotics, they are preferred over typical antipsychotics such as haloperidol and chlorpromazine. Of the atypical antipsychotics, there is presently more placebo-controlled evidence in support of olanzapine and risperidone.
If psychosocial therapies are used, they should be combined with pharmacotherapy. Perhaps the only indications for psychotherapy alone for patients experiencing acute manic or mixed episodes are when all established treatments have been refused, involuntary treatment is not appropriate, and the primary goals of therapy are focused and crisis-oriented (e.g., resolving ambivalence about taking medication).
For patients who, despite receiving the aforementioned medications, experience a manic or mixed episode (i.e., a “breakthrough” episode), the first-line intervention should be to optimize the medication dose. Optimization of dosage entails ensuring that the blood level is in the therapeutic range and in some cases achieving a higher serum level (although one still within the therapeutic range). Introduction or resumption of an antipsychotic is often necessary. Severely ill or agitated patients may require short-term adjunctive treatment with an antipsychotic agent or benzodiazepine.
With adequate dosing and serum levels, medications for the treatment of mania generally exert some appreciable clinical effect by the 10th to the 14th day of treatment. When first-line medications at optimal doses fail to control symptoms, recommended treatment options include addition of another first-line medication. Alternative treatment options include adding carbamazepine or oxcarbazepine in lieu of an additional first-line medication, adding an antipsychotic if not already prescribed, or changing from one antipsychotic to another. Of the antipsychotic agents, clozapine may be particularly effective for treatment of refractory illness. As always, caution should be exercised when combining medications, since side effects may be additive and metabolism of other agents may be affected.
ECT may also be considered for patients with severe or treatment-resistant illness or when preferred by the patient in consultation with the psychiatrist. In addition, ECT is a potential treatment for patients with mixed episodes or for severe mania experienced during pregnancy.
Patients displaying psychotic features during a manic episode usually require treatment with an antipsychotic medication. Atypical antipsychotics are favored because of their more benign side effect profile.
2. Depressive episodes
The primary goal of treatment in bipolar depression, as with nonbipolar depression, is remission of the symptoms of major depression with return to normal levels of psychosocial functioning. An additional focus of treatment is to avoid precipitation of a manic or hypomanic episode.
The first-line pharmacological treatment for bipolar depression is the initiation of either lithium or lamotrigine. The better supported of these is lithium. While standard antidepressants such as SSRIs have shown good efficacy in the treatment of unipolar depression, for bipolar disorder they generally have been studied as add-ons to medications such as lithium or valproate; antidepressant monotherapy is not recommended, given the risk of precipitating a switch into mania. For severely ill patients, some clinicians will initiate treatment with lithium and an antidepressant simultaneously, although there are limited data to support this approach. In patients with life-threatening inanition, suicidality, or psychosis, ECT also represents a reasonable alternative. In addition, ECT is a potential treatment for severe depression during pregnancy. Selection of the initial treatment should be guided by clinical factors such as illness severity, by associated features (e.g., rapid cycling, psychosis), and by patient preference, with particular attention to side effect profiles.
Small studies have suggested that interpersonal therapy and cognitive behavior therapy may also be useful when added to pharmacotherapy during depressive episodes in patients with bipolar disorder. There have been no definitive studies to date of psychotherapy in lieu of antidepressant treatment for bipolar depression. However, a larger body of evidence supports the efficacy of psychotherapy in the treatment of unipolar depression (2).
For patients who, despite receiving maintenance medication treatment, suffer a breakthrough depressive episode, the first-line intervention should be to optimize the dose of the maintenance medication. Optimization of dosage entails ensuring that the serum drug level is in the therapeutic range and in some cases achieving a higher serum level (although one still within the therapeutic range).
For patients who do not respond to optimal maintenance treatment, next steps include adding lamotrigine, bupropion, or paroxetine. Alternative next steps include adding other newer antidepressants (e.g., another SSRI or venlafaxine) or an MAOI. Although there are few empirical data that directly compare risk of switch or efficacy among antidepressants in the treatment of bipolar disorder, tricyclic antidepressants may carry a greater risk of precipitating a switch into hypomania or mania. Also, while MAOIs have generally demonstrated good efficacy, their side effect profile may make other agents preferable as initial interventions (2). ECT should be considered for patients with severe or treatment-resistant depressive episodes or for those episodes with catatonic features.
Patients with psychotic features during a depressive episode usually require adjunctive treatment with an antipsychotic medication. ECT represents a reasonable alternative.
Studies of bipolar depression rarely separate results for patients with bipolar I disorder from those of patients with bipolar II disorder. It is not known whether specific pharmacotherapy regimens differ in efficacy for treatment of bipolar I versus bipolar II depression. However, existing data suggest that for patients with bipolar II disorder, antidepressant treatment—either alone or in combination with a maintenance medication—is less likely to result in a switch into a hypomanic episode relative to those with bipolar I disorder (38).
3. Rapid cycling
The initial intervention for patients who experience rapid-cycling episodes of illness is to identify and treat medical conditions that may contribute to cycling, such as hypothyroidism or drug or alcohol use. Since antidepressants may also contribute to cycling, the need for continued antidepressant treatment should be reassessed; antidepressants should be tapered if possible. The initial treatment for patients who experience rapid-cycling episodes of illness should include lithium or valproate; an alternative treatment is lamotrigine. In many instances, combinations of medications are required (39, 40); possibilities include combining two of these agents or combining one of them with an antipsychotic. Because of their more benign side effect profile, atypical antipsychotics are preferred over typical antipsychotics.
C. Maintenance treatment
Maintenance medication treatment is generally recommended following a single manic episode. Although few studies have been conducted involving patients with bipolar II disorder, consideration of maintenance treatment for this form of the illness is also warranted. Primary goals of treatment include relapse prevention, reduction of subthreshold symptoms, and reduction of suicide risk. Goals also need to include reduction of cycling frequency and mood instability as well as improvement in overall functioning. Pharmacotherapy must be employed in ways that yield good tolerability and do not predispose the patient to nonadherence.
Options with the best empirical evidence to support their use as maintenance treatments include lithium or valproate; possible alternatives include lamotrigine, carbamazepine, or oxcarbazepine. Despite limited data, oxcarbazepine is included—as it was for acute treatment of mania—because its efficacy may be similar to that of carbamazepine but with better tolerability. In general, if one of these medications was used to achieve remission from the most recent depressive or manic episode, it should be continued. Maintenance ECT may also be considered for patients whose acute episode responded to ECT. Selection of the initial treatment should be guided by clinical factors such as illness severity, by associated features (e.g., rapid cycling, psychosis), and by patient preference, with particular attention to side effect profiles.
For patients treated with an antipsychotic medication during the preceding acute episode, the need for ongoing antipsychotic treatment should be reassessed upon entering the maintenance phase. Since antipsychotic agents, particularly typical antipsychotics, may cause tardive dyskinesia with long-term use, antipsychotics should be slowly tapered and discontinued unless they are required to control persistent psychosis or provide prophylaxis against recurrence. While maintenance therapy with atypical antipsychotics may be considered, there is as yet no definitive evidence that their efficacy in maintenance is comparable to that of agents such as lithium or valproate.
Patients with bipolar disorder are likely to gain some additional benefit during the maintenance phase from a concomitant psychosocial intervention that addresses illness management (i.e., adherence, lifestyle changes, and early detection of prodromal symptoms) and interpersonal difficulties. Although not adequately studied to provide evidence-based documentation, supportive and psychodynamic psychotherapy are widely used in addition to medication.
Group psychotherapy, in conjunction with appropriate medication, may also help patients address such issues as adherence to a treatment plan, adaptation to a chronic illness, regulation of self-esteem, and management of marital as well as other psychosocial issues.
Support groups provide useful information about bipolar disorder and its treatment. Patients in these groups often benefit from hearing the experiences of others who are struggling with such issues as denial versus acceptance of the need for medication, problems with side effects, and how to shoulder other burdens associated with the illness and its treatment. Advocacy groups such as the National Depressive and Manic-Depressive Association and the National Alliance for the Mentally Ill have many local chapters that provide both support and educational material to patients and their families.
Although maintenance medication combinations are often associated with increases in side effects, use of such regimens should be considered for patients who have not responded adequately to simpler regimens. The addition of another maintenance medication, an atypical antipsychotic, or an antidepressant may be necessary for patients who experience either continuing high levels of subthreshold symptoms or a breakthrough episode of illness. There are currently insufficient data to support one combination over another. Maintenance ECT may also be considered for patients whose acute episode responded to ECT.
III. Special clinical features influencing the treatment plan
A. Psychiatric features
1. Psychosis
Psychotic symptoms (e.g., delusions, hallucinations) are commonly seen during episodes of either mania or depression but are more common in the former, appearing in over one-half of manic episodes (41). Mood-congruent features during a manic episode probably are not predictive of a poorer outcome, although early onset (before age 21) of psychotic mania may predict a more severe disorder (42). Mood-incongruent features have been identified in some (43) but not all (44) studies to be a predictor of a shorter time in remission. The presence of psychotic features during a manic episode may not require an antipsychotic medication, although most clinicians prescribe them in addition to a maintenance agent (45).
2. Catatonia
Catatonic features may develop in up to one-third of patients during a manic episode (46). The most commonly observed symptoms of catatonia in mania are motor excitement, mutism, and stereotypic movements. Because catatonic symptoms are seen in other psychiatric and neurological disorders, a careful assessment is indicated for an accurate diagnosis. In addition, patients who exhibit catatonic stupor may go on to show more typical signs and symptoms of mania during the same episode of illness (47). The presence of catatonic features during the course of a manic episode is associated with greater episode severity, mixed states, and somewhat poorer short-term outcomes (46). In treating catatonia, neuroleptics have generally exhibited poor efficacy (48). In contrast, prospective studies have demonstrated the efficacy of lorazepam in the treatment of catatonic syndromes, including those associated with mania (49–52). Since ECT is probably the most effective treatment for catatonic syndromes regardless of etiology, ECT should be considered if benzodiazepines do not result in symptom resolution (48).
3. Risk of suicide, homicide, and violence
Like those suffering from major depression, patients with bipolar disorder are at high risk for suicide (53, 54). The frequency of suicide attempts appears similar for the bipolar I and bipolar II subtypes (55, 56). Individuals with bipolar disorder repeatedly have been shown to have greater overall mortality than the general population (41). Although much of this risk reflects the higher rate of suicide, cardiovascular and pulmonary mortality among patients with untreated bipolar disorder is also high (41, 57).
Known general risk factors for suicide also apply to patients with bipolar disorder. These include a history of suicide attempts, suicidal ideation, comorbid substance abuse, comorbid personality disorders (58), agitation, pervasive insomnia, impulsiveness (59), and family history of suicide. Among the phases of bipolar disorder, depression is associated with the highest suicide risk, followed by mixed states and presence of psychotic symptoms, with episodes of mania being least associated with suicide (8, 56). Suicidal ideation during mixed states has been correlated with the severity of depressive symptoms (10). In general, a detailed evaluation of the individual patient is necessary to assess suicidal risk (Table 1). Judgment of suicidal risk is inherently imperfect; therefore, risks and benefits of intervention should be carefully weighed and documented.
Long-term treatment with lithium has been associated with reduction of suicide risk (56, 60). Whether this reflects an anti-impulsivity factor beyond lithium’s mood-stabilizing effect is not yet clear. Lithium may also diminish the greater mortality risk observed among bipolar disorder patients from causes other than suicide (61). It is unknown whether prolonged survival is also seen with the anticonvulsant maintenance agents.
Clinical experience attests to the presence of violent behavior in some patients with bipolar disorder, and violence may be an indication for hospitalization (41). Comorbid substance abuse and psychosis may contribute to the threat of criminal violence or aggression (62–64).
4. Substance use disorders
Bipolar disorder with a comorbid substance use disorder is a very common presentation, with bipolar disorder patients of both sexes showing much higher rates of substance use than the general population (65). For example, the Epidemiologic Catchment Area study found rates of alcohol abuse or dependence in 46% of patients with bipolar disorder compared with 13% for the general population. Comparable drug abuse and dependence figures are 41% and 6%, respectively (66, 67). Substance abuse may obscure or exacerbate endogenous mood swings. Conversely, comorbid substance use disorder may be overlooked in patients with bipolar disorder (68, 69). Substance abuse may also precipitate mood episodes or be used by patients to ameliorate the symptoms of such episodes. Comorbid substance use is typically associated with fewer and slower remissions, greater rates of suicide and suicide attempts, and poorer outcome (70–73).
Treatment for substance abuse and bipolar disorder should proceed concurrently when possible. It is also helpful to obtain consultation from an addiction expert, such as an addiction psychiatrist, or to arrange for concomitant treatment of the bipolar disorder and the substance use disorder in a dual-diagnosis program.
Alcohol abuse and its effects may affect bipolar disorder pharmacotherapy. For instance, alcohol-related dehydration may raise lithium levels to toxicity. Hepatic dysfunction from chronic alcohol abuse or from hepatitis associated with intravenous substance use may alter plasma levels of valproate and carbamazepine (74). If the hepatic dysfunction is severe, the use of these hepatically metabolized medications may be problematic. In these cases, coordination with the patient’s primary care physician or gastroenterologist is recommended (75).
5. Comorbid psychiatric conditions
Patients with comorbid personality disorders pose complicated diagnostic pictures. They are clearly at greater risk for experiencing intrapsychic and psychosocial stress that can precipitate or exacerbate mood episodes. Patients with comorbid personality disorders generally have greater symptom burden, lower recovery rates from episodes, and greater functional impairment (76). In addition, these patients may have particular difficulty adhering to long-term treatment regimens (77).
Relative to the general population, individuals with bipolar disorder are at greater risk for comorbid anxiety disorders, especially panic disorder and obsessive-compulsive disorder. Comorbid anxiety disorders may predict a longer time to recovery of mood episodes (78). Treatment for the bipolar disorder and the comorbid anxiety disorder should proceed concurrently.
The presence of comorbid attention deficit hyperactivity disorder (ADHD) in adults and children with bipolar disorder may make it difficult to monitor changes in mood states. Of note, adults with bipolar disorder and comorbid ADHD are likely to have experienced a much earlier age at onset of their mood disorder relative to those without comorbid ADHD (79).
B. Demographic and psychosocial factors
1. Gender
A number of issues related to gender must be considered when treating patients with bipolar disorder. Hypothyroidism is more common in women, and women may be more susceptible to the antithyroid effects of lithium (80). Additionally, rapid cycling is more common in women (81, 82). Treatment with antipsychotics and, to a lesser extent, SSRIs may elevate serum levels of prolactin and result in galactorrhea, sexual dysfunction, menstrual disorders, and impaired fertility (83, 84).
2. Pregnancy
Because many medications used to treat bipolar disorder are associated with a higher risk of birth defects, the psychiatrist should encourage effective contraceptive practices for all female patients of childbearing age who are receiving pharmacological treatment (85, 86). Since carbamazepine, oxcarbazepine, and topiramate increase the metabolism of oral contraceptives, women taking these medications should not rely on oral contraceptives for birth control (87–89). This effect does not occur with other medications used to treat bipolar disorder.
Multiple clinical issues arise in relationship to pregnancy in bipolar disorder patients. In order to permit discussion of the risks and benefits of therapeutic options, a pregnancy should be planned in consultation with the psychiatrist whenever possible. Because of the higher genetic risk for bipolar disorder (90–92), patients with bipolar disorder who are considering having children may also benefit from genetic counseling (22).
a) Continuing/discontinuing medications. Around the time of pregnancy, the risks and benefits of continuing versus discontinuing treatment require the most thoughtful judgment and discussion among the patient, the psychiatrist, the obstetrician, and the father. Specific options include continuing medication throughout pregnancy, discontinuing medications at the beginning of pregnancy or before conception, and discontinuing the medication only for the first trimester.
In clinical decision making, the potential teratogenic risks of psychotropic medications must be balanced against the risk of no prophylactic treatment, with the attendant risks of illness (93). Although the course of bipolar disorder during pregnancy is still unclear, some evidence suggests that pregnancy does not alter the rate of mood episodes compared with other times (94). However, in patients who have been stable on a regimen of lithium, the rate of recurrent mood episodes is clearly increased by lithium discontinuation, particularly when discontinuation is abrupt (94). Should the decision be made to discontinue medication, the woman should be advised about the potentially greater risk of mood episode recurrence with rapid discontinuation of lithium (and possibly other maintenance agents) compared with a slower taper over many weeks (95).
Although direct evidence of a negative effect of untreated psychiatric disorders on fetal development is lacking, antenatal stress, depression, and anxiety are linked with a variety of abnormalities in newborns (96–101). Additionally, during a manic episode, women are at risk of increasing their consumption of alcohol and other drugs, thus conferring additional dangers to the fetus.
b) Prenatal exposure to medications. First-trimester exposure to lithium, valproate, or carbamazepine is associated with a greater risk of birth defects. With lithium exposure the absolute risk for Ebstein’s anomaly, a cardiovascular defect, is 1–2 per 1,000. This is approximately 10–20 times greater than the risk in the general population (102). Exposure to carbamazepine and valproate during the first trimester is associated with neural tube defects at rates of up to 1% and 3%–5%, respectively (85). Both carbamazepine and valproate exposure have also been associated with craniofacial abnormalities (103, 104). Other congenital defects that have been observed with valproate include limb malformations and cardiac defects (104). Little is known about the potential teratogenicity of lamotrigine, gabapentin, or other newer anticonvulsants.
No teratogenic effects have been demonstrated with tricyclic antidepressants. Near term, however, their use has been associated with side effects in the neonate (105). The SSRIs seem to be relatively benign in their risks to exposed fetuses (106), with safety data being strongest for fluoxetine and citalopram. Although data with bupropion, mirtazapine, nefazodone, trazodone, and venlafaxine are limited (105), none of the newer antidepressants has been shown to be teratogenic (106, 107). Nonetheless, caution must be exercised if they are prescribed to treat bipolar depression in pregnant women (93).
Antipsychotic agents may be needed to treat psychotic features of bipolar disorder during pregnancy, but they may also represent an alternative to lithium for treating symptoms of mania (105). High-potency antipsychotic medications are preferred during pregnancy, since they are less likely to have associated anticholinergic, antihistaminergic, or hypotensive effects. In addition, there is no evidence of teratogenicity with exposure to haloperidol, perphenazine, thiothixene, or trifluoperazine (105). When high-potency antipsychotic medications are used near term, neonates may show extrapyramidal side effects, but these are generally short-lived (108). To limit the duration of such effects, however, long-acting depot preparations of antipsychotic medications are not recommended during pregnancy (105). For newer antipsychotic agents such as risperidone, olanzapine, clozapine, quetiapine, and ziprasidone, little is known about the potential risks of teratogenicity or the potential effects in the neonate.
The risk of teratogenicity with benzodiazepines is not clear (108). Early studies, primarily with diazepam and chlordiazepoxide, suggested that first-trimester exposure may have led to malformations, including facial clefts, in some infants. Later studies showed no significant increases in specific defects or in the overall incidence of malformations (108). A recent meta-analysis of the risk of oral cleft or major malformations showed no association with fetal exposure to benzodiazepines in pooled data from cohort studies, but a greater risk was reported on the basis of pooled data from case-control studies (109). In general, however, teratogenic risks are thought likely to be small with benzodiazepines (105). Near term, use of benzodiazepines may be associated with sedation in the neonate. Withdrawal symptoms resulting from dependence may also be seen in the neonate (108). As a result, if benzodiazepines are used during pregnancy, lorazepam is generally preferred (105).
ECT is also a potential treatment for severe mania or depression during pregnancy (110). In terms of teratogenicity, the short-term administration of anesthetic agents with ECT may present less risk to the fetus than pharmacological treatment options (111). The APA Task Force Report on ECT contains additional details on the use of ECT during pregnancy (110).
c) Prenatal monitoring. Women who choose to remain on regimens of lithium, valproate, or carbamazepine during pregnancy should have maternal serum α-fetoprotein screening for neural tube defects before the 20th week of gestation, with amniocentesis as well as targeted sonography performed for any elevated α-fetoprotein values (105). Women should also be encouraged to undergo high-resolution ultrasound examination at 16–18 weeks gestation to detect cardiac abnormalities in the fetus. Since hepatic metabolism, renal excretion, and fluid volume are altered during pregnancy and the perinatal period, serum levels of medications should be monitored and doses adjusted if indicated. At delivery, the rapid fluid shifts in the mother will markedly increase lithium levels unless care is taken to either lower the lithium dose, ensure hydration, or both (112). Discontinuing lithium on the day of delivery is probably not necessary and may be unwise given the high risk for postpartum mood episodes and the greater risk of recurrence if lithium is discontinued in women with bipolar disorder (94, 112).
d) Postpartum issues. The postpartum period is consistently associated with a markedly greater risk for relapse into mania, depression, or psychosis. For women with bipolar disorder, the rate of postpartum relapse is as high as 50% (86, 94). Women who have had severe postpartum affective episodes in the past are at highest risk to have another episode of illness after subsequent pregnancies. Despite a paucity of studies, it is generally considered that prophylactic medications such as lithium or valproate may prevent postpartum mood episodes in women with bipolar disorder (113). Also, since changes in sleep are common in the postpartum period, women should be educated about the need to maintain normal sleep patterns to avoid precipitating episodes of mania.
e) Infant medication exposure through breast-feeding. All medications used in the treatment of bipolar disorder are secreted in breast milk in varying degrees, thereby exposing the neonate to maternally ingested medication (114). However, as with the risks of medications during pregnancy, risks of breast-feeding with psychotropic medications must be weighed against the benefits of breast-feeding (115, 116). Because lithium is secreted in breast milk at 40% of maternal serum concentration, most experts have recommended against its use in mothers who choose to breast-feed (105). Fewer data on breast-feeding are available for carbamazepine and valproate. Although it is generally considered safe, potential risks should always be considered. Little is known about lamotrigine exposure in breast-fed neonates; however, levels in the infant may reach 25% of maternal serum levels (117). Consequently, the potential for pharmacological effects, including a risk for life-threatening rash, should be taken into consideration (118). With other psychotropic medications (including antipsychotics, antidepressants, and benzodiazepines), there are few reports of specific adverse effects in breast-feeding infants. Nonetheless, these drugs are found in measurable quantities in breast milk and could conceivably affect central nervous system functioning in the infant (118).
3. Cross-cultural issues
Culture can influence the experience and communication of symptoms of depression and mania. Underdiagnosis or misdiagnosis, as well as delayed detection of early signs of recurrence, can be reduced by being alert to specific ethnic and cultural differences in reporting complaints of a major mood episode. Specifically, minority patients (particularly African and Hispanic Americans) with bipolar disorder are at greater risk for being misdiagnosed with schizophrenia (119, 120). This greater risk appears to result from clinicians failing to elicit affective symptoms in minority patients with affective psychoses (121).
Ethnicity and race must also be taken into consideration when prescribing medications, since ethnic and racial groups may differ in their metabolism of some medications (122, 123). For example, relative to Caucasian patients, Chinese patients have a lower average activity of the cytochrome P-450 isoenzyme 2D6 (123). As a result, they typically require lower doses of antidepressants and antipsychotics that are metabolized by this enzyme (122). Similar deficits in average activity of the cytochrome P-450 isoenzyme 2C19 have been found in Chinese, Japanese, and Korean patients compared with Caucasians (123).
4. Children and adolescents
The prevalence of bipolar disorder in a community sample of children and adolescents was 1%; an additional 5.7% had mood symptoms that met criteria for bipolar disorder not otherwise specified (124). Although DSM-IV-TR criteria are used to diagnose bipolar disorder in childhood and adolescence, the clinical features of childhood bipolar disorder differ from bipolar disorder in adults. Children with bipolar disorder often have mixed mania, rapid cycling, and psychosis (125). Child and adolescent bipolar disorder is often comorbid with attention deficit and conduct disorders (126–128). For children and adolescents in a current manic episode, 1-year recovery rates of 37.1% and relapse rates of 38.3% have been reported (1, 129). In a 5-year prospective follow-up of adolescents experiencing bipolar disorder, relapse rates of 44% were found (130). Despite the severity and chronicity of this disorder in children and adolescents and its devastating impact on social, emotional, and academic development, treatment research has lagged far behind that of adult bipolar disorder.
Although there is more information available about the use of lithium and divalproex in children and adolescents with bipolar disorder, other medication treatment options include atypical antipsychotics, carbamazepine, and combinations of these medications.
Treatment with a maintenance agent should continue for a minimum of 18 months after stabilization of a manic episode. There is evidence that ultimate stabilization takes a number of years (131). In addition, lithium discontinuation has been shown to increase relapse rates in adolescents with bipolar disorder: relapse occurred within 18 months in 92% of those who discontinued lithium versus 37% of those who continued lithium (132). Consequently, medication discontinuation should be done gradually at a time when there are no major anticipated stressors.
Psychiatric comorbidity may complicate the diagnosis and treatment of bipolar disorder in children and adolescents. The presence of ADHD, especially in children and adolescents, confounds the assessment of mood changes in patients with bipolar disorder. Early manifestations of mania and hypomania can be particularly difficult to distinguish from the ongoing symptoms of ADHD. Careful tracking of symptoms and behaviors is helpful. In addition, the presence of ADHD is associated with higher rates of learning disabilities, which should be addressed in treatment planning.
Youths with bipolar disorder are at greater risk for substance use disorders (133, 134). Comorbid substance use has been shown to complicate the course of bipolar disorder and its treatment (135). Short-term treatment with lithium (136) and divalproex (137) may be useful in these conditions. However, in a 2-year follow-up of hospitalized manic adolescents, the bipolar disorder patients who continued to abuse substances had more manic episodes and poorer functioning than early-onset bipolar disorder patients who did not exhibit comorbid substance abuse. In contrast, cessation of substance use was associated with fewer episodes and greater functional improvement at the 4-year follow-up point (135).
5. Geriatric patients
In patients over 65 years of age, prevalence rates of bipolar disorder range from 0.1% to 0.4% (138). In addition, 5%–12% of geriatric psychiatry admissions are for bipolar disorder (138). Relative to patients with onset of mania at a younger age, those with onset at an older age tend to have less of a family history of bipolar disorder. They may also have longer episode durations or more frequent episodes of illness (139). Of individuals with onset of mania at older ages, one-half have had previous depressive episodes, often with a long latency period before the first manic episode (140).
Manic syndromes in geriatric patients may also be associated with general medical conditions, medications used to treat those conditions, or substance use (138–140). The new onset of mania in later life is particularly associated with high rates of medical and neurological diseases (139–141). Right hemispheric cortical or subcortical lesions are especially common. Relative to elderly patients with multiple episodes of mania, geriatric patients with a first episode of mania have a higher risk of mortality (141). Therefore, any patient with a late onset of manic symptoms should be evaluated carefully for general medical and neurological causes (138–140).
General principles for treating geriatric mania are similar to those for younger adults. Older patients will usually require lower doses of medications, since aging is associated with reductions in renal clearance and volume of distribution (142). Concomitant medications and medical conditions may also alter the metabolism or excretion of psychotropic medications (139). Older patients may also be more sensitive to side effects because of greater end-organ sensitivity. Many elderly patients tolerate only low serum levels of lithium (e.g., 0.4–0.6 meq/liter) (138) and can respond to these levels. Those who tolerate low serum lithium levels but who are not showing benefit should have slow dose increases to yield serum levels in the usual therapeutic range.
Older patients may be more likely to develop cognitive impairment with medications such as lithium or benzodiazepines (138). They may also have difficulty tolerating antipsychotic medications and are more likely to develop extrapyramidal side effects and tardive dyskinesia than younger individuals (143). With some antipsychotics and antidepressants, orthostatic hypotension may be particularly problematic and increases the risk of falls. Use of benzodiazepines and of neuroleptics also has been associated with greater risks of falls and hip fractures in geriatric patients (144).
C. Concurrent general medical conditions
In the presence of a severe medical disorder, the disorder itself or the medications used to treat it should always be considered as possible causes of a manic episode. Neurological conditions commonly associated with secondary mania are multiple sclerosis, lesions involving right-sided subcortical structures, and lesions of cortical areas with close links to the limbic system (145). l-Dopa and corticosteroids are the most common medications associated with secondary mania (146).
The presence of a general medical condition may also exacerbate the course or severity of bipolar disorder or complicate its treatment (147). For example, the course of bipolar disorder may be exacerbated by any condition that requires intermittent or regular use of steroids (e.g., asthma, inflammatory bowel disease) or that leads to abnormal thyroid functioning. In addition, treatment of patients with bipolar disorder may be complicated by conditions requiring the use of diuretics, angiotensin-converting enzyme inhibitors, nonsteroidal anti-inflammatory drugs, cyclooxygenase-2 inhibitors, or salt-restricted diets, all of which affect lithium excretion. Conditions or their treatments that are associated with abnormal cardiac conduction or rhythm or that affect renal or hepatic function may further restrict the choice or dosage of medications. In HIV-infected patients, lower doses of medications are often indicated because of patients’ greater sensitivity to side effects and because of the potential for drug-drug interactions. Special considerations in the treatment of HIV-infected patients are presented in the APA Practice Guideline for the Treatment of Patients With HIV/AIDS (148).
Whenever patients are taking more than one medication, the possibility of adverse drug-drug interactions should always be considered. Patients should be educated about the importance of informing their psychiatrist and other physicians about their current medications whenever new medications are prescribed. Clinicians should also inquire about patient use of herbal preparations and over-the-counter medications.
Part B: Background information and review of available evidence
IV. Disease definition, natural history and course, and epidemiology
A. Definition of bipolar disorder
According to DSM-IV-TR (1), patients with bipolar I disorder have had at least one episode of mania (criteria for a manic episode are presented in Table 2). Some patients have had previous depressive episodes (Table 3), and most patients will have subsequent episodes that can be either manic or depressive. Hypomanic and mixed episodes (Table 4 and Table 5, respectively) can occur, as well as significant subthreshold mood lability between episodes. Patients meeting criteria for bipolar II disorder have a history of major depressive episodes and hypomanic episodes only. Patients may also exhibit significant evidence of mood lability, hypomania, and depressive symptoms but fail to meet duration criteria for bipolar II disorder, thereby leading to a diagnosis of bipolar disorder not otherwise specified. Finally, cyclothymic disorder may be diagnosed in those patients who have never experienced a manic, mixed, or major depressive episode but who experience numerous periods of depressive symptoms and numerous periods of hypomanic symptoms for at least 2 years (1 year in children), with no symptom-free period greater than 2 months. The subtypes of bipolar disorder, as well as selected other affective illnesses, are summarized and compared in Table 6.
In addition to providing definitions of bipolar disorder, DSM-IV-TR also includes specifiers describing the course of recurrent episodes, such as seasonal pattern, longitudinal course (with or without full interepisode recovery), and rapid cycling.
Some investigators have advocated moving from a categorical to a more dimensional perspective in characterizing bipolar disorder. In particular, this perspective includes the concept of a bipolar spectrum that would encompass a range of presentations not currently considered bipolar (149). For example, a patient with antidepressant-induced hypomanic symptoms would be considered to have a form of bipolar disorder under the spectrum conceptualization.
B. Natural history and course
Bipolar disorder is generally an episodic, lifelong illness with a variable course. The first episode of bipolar disorder may be manic, hypomanic, mixed, or depressive. Men are more likely than women to be initially manic, but both are more likely to have a first episode of depression. Patients with untreated bipolar disorder may have more than 10 total episodes of mania and depression during their lifetime, with the duration of episodes and interepisode periods stabilizing after the fourth or fifth episode (150). Often, 4 years or more may elapse between the first and second episodes, but the intervals between subsequent episodes usually narrow. However, it must be emphasized that variability is the hallmark of this illness. Thus, when taking a history, a number of longitudinal issues must be considered, including the number of prior episodes, the average length and severity of episodes, average interepisode duration, and the interval since the last episode of mania or depression.
Frequently, a patient will experience several episodes of depression before a manic episode occurs (34, 151). Consequently, bipolar disorder should always be considered in the differential diagnosis of depression. Patients very often do not report prior episodes of mania and hypomania and instead seek treatment for complaints of depression, delaying correct diagnosis (5, 152–157). For a patient who is not educated about bipolar disorder, symptoms of dysphoric hypomania may not be recognized or reported. Therefore, the psychiatrist needs to ask explicitly about prior manic or hypomanic episodes, since knowledge of their presence can influence treatment decisions. The psychiatrist should also ask about a family history of mood disorders, including mania and hypomania. Consultation with family members and significant others may be extremely useful in establishing family history and identifying prior affective episodes.
In addition to substance abuse and risk-taking behavior, other cross-sectional features that can have an impact on diagnosis and treatment planning include the presence of psychotic symptoms or cognitive impairment and the risk of suicide or violence to persons or property (41).
Suicide rates are high among bipolar disorder patients. Completed suicide occurs in an estimated 10%–15% of individuals with bipolar I disorder. Suicide is more likely to occur during a depressive or a mixed episode (8–13). Pharmacotherapy may substantially reduce the risk of suicide (56, 60, 153). For example, in an 11-year follow-up study of 103 patients with bipolar disorder who were receiving lithium, death rates were well below those expected for this group on the basis of age and sex (154).
Bipolar disorder causes substantial psychosocial morbidity, frequently affecting patients’ relationships with spouses or partners, children, and other family members as well as their occupation and other aspects of their lives. Even during periods of euthymia, patients may experience impairments in psychosocial functioning or residual symptoms of depression or mania/hypomania. It is estimated that as many as 60% of people diagnosed with bipolar I disorder experience chronic interpersonal or occupational difficulties and subclinical symptoms between acute episodes (13, 33, 34, 158–164). Divorce rates are substantially higher in patients with bipolar disorder, approaching two to three times the rate of comparison subjects (152). The occupational status of patients with bipolar disorder is twice as likely to deteriorate as that of comparison subjects (152). Patients’ ability to care for themselves, degree of disability or distress, childbearing status or plans, availability of supports such as family or friends, and resources such as housing and finances also bear on treatment plans.
C. Epidemiology
Bipolar I disorder affects approximately 0.8% of the adult population, with estimates from community samples ranging between 0.4% and 1.6%. These rates are consistent across diverse cultures and ethnic groups (165). Bipolar II disorder affects approximately 0.5% of the population (156). While bipolar II disorder is apparently more common in women (81), bipolar I disorder affects men and women fairly equally. These estimates of prevalence are considered conservative. Reasons for this underestimate may include differences in diagnostic definitions and inclusion of persons who fall within the bipolar spectrum but who do not meet DSM-IV-TR criteria for bipolar I or bipolar II disorder (166).
The Epidemiologic Catchment Area study reported a mean age at onset of 21 years for bipolar disorder (6). When studies examining age at onset are stratified into 5-year intervals, the peak age at onset of first symptoms falls between ages 15 and 19, followed closely by ages 20–24. There is often a 5- to 10-year interval, however, between age at onset of illness and age at first treatment or first hospitalization (34, 151). Onset of mania before age 15 has been less well studied (167). Bipolar disorder may be difficult to diagnose in this age group because of its atypical presentation with ADHD (13, 157–163). Thus, the true age at onset of bipolar disorder is still unclear and may be younger than reported for the full syndrome, since there is uncertainty about the symptom presentation in children. Research that follows cohorts of offspring of patients with bipolar disorder may help to clarify early signs in children.
Onset of mania after age 60 is less likely to be associated with a family history of bipolar disorder and is more likely to be associated with identifiable general medical factors, including stroke or other central nervous system lesion (34, 155, 168).
Evidence from epidemiological and twin studies strongly suggests that bipolar disorder is a heritable illness (164, 169). First-degree relatives of patients with bipolar disorder have significantly higher rates of mood disorder than do relatives of nonpsychiatrically ill comparison groups. However, the mode of inheritance remains unknown. In clinical practice, a family history of mood disorder, especially of bipolar disorder, provides strong corroborative evidence of the potential for a primary mood disorder in a patient with otherwise predominantly psychotic features.
Likewise, the magnitude of the role played by environmental stressors, particularly early in the course of the illness, remains uncertain. However, there is growing evidence that environmental and lifestyle features can have an impact on severity and course of illness (170–172). Stressful life events, changes in sleep-wake schedule, and current alcohol or substance abuse may affect the course of illness and lengthen the time to recovery (26, 71, 73, 173–175).
V. Review and synthesis of available evidence
A. Somatic treatments of acute manic and mixed episodes
In general, the primary goal of treatment for patients experiencing a manic or mixed episode is symptom control to allow a return to normal levels of psychosocial functioning. The rapid control of symptoms such as agitation and aggression may be particularly important for the safety of the patient and others.
1. Lithium
Lithium has been used for the treatment of acute bipolar mania for over 50 years. Five studies have demonstrated that lithium is superior to placebo (176–180). Pooled data from these studies reveal that 87 (70%) of 124 patients displayed at least partial reduction of mania with lithium. However, the use of a crossover design in four of these trials (176–179), nonrandom assignment in two studies (177, 178), and variations in diagnostic criteria and trial duration limit interpretation of the results of all but one trial (180). Nevertheless, in the only placebo-controlled, parallel-design trial in which lithium served as an active comparator to divalproex, lithium and divalproex exerted comparable efficacy (180). In active comparator trials, lithium displayed efficacy comparable to that of carbamazepine (181, 182), risperidone (183), olanzapine (184), and chlorpromazine and other typical antipsychotics (185–190). Among active comparator trials, however, only three (185, 186, 189) were likely to be of sufficient size to detect possible differences in efficacy between treatments. Open studies (191–194) and randomized, active comparator-controlled studies (195–197) indicate that lithium is likely to be effective for treatment of pure or elated mania but is less often effective in the treatment of mixed states.
a) Side effects. Up to 75% of patients treated with lithium experience some side effects (41, 198). These side effects vary in clinical significance; most are either minor or can be reduced or eliminated by lowering the lithium dose or changing the dosage schedule. For example, Schou (199) reported a 30% reduction in side effects among patients treated with an average lithium level of 0.68 meq/liter compared with those treated with an average level of 0.85 meq/liter. Side effects that appear to be related to peak serum levels (e.g., tremor that peaks within 1 to 2 hours of a dose) may be reduced or eliminated by using a slow-release preparation or changing to a single bedtime dose.
Dose-related side effects of lithium include polyuria, polydipsia, weight gain, cognitive problems (e.g., dulling, impaired memory, poor concentration, confusion, mental slowness), tremor, sedation or lethargy, impaired coordination, gastrointestinal distress (e.g., nausea, vomiting, dyspepsia, diarrhea), hair loss, benign leukocytosis, acne, and edema (200). Side effects that persist despite dosage adjustment may be managed with other medications (e.g., β blockers for tremor; diuretics for polyuria, polydipsia, or edema; topical antibiotics or retinoic acid for acne). Gastrointestinal disturbances can be managed by administering lithium with meals or changing lithium preparations (especially to lithium citrate).
Lithium may cause benign ECG changes associated with repolarization. Less commonly, cardiac conduction abnormalities have been associated with lithium treatment. Anecdotal reports have linked lithium with other ECG changes, including the exacerbation of existing arrhythmias and, less commonly, the development of new arrhythmias (201).
The most common renal effect of lithium is impaired concentrating capacity caused by reduced renal response to ADH, manifested as polyuria, polydipsia, or both (202, 203). Although the polyuria associated with early lithium treatment may resolve, persistent polyuria (ranging from mild and well tolerated to severe nephrogenic diabetes insipidus) may occur. Polyuria can frequently be managed by changing to a once-daily bedtime dose. If the polyuria persists, management includes ensuring that fluid intake is adequate and that the lithium dose is as low as possible. If these measures do not ameliorate the problem, then concurrent administration of a thiazide diuretic (e.g., hydrochlorothiazide at a dose of 50 mg/day) may be helpful. The lithium dose will usually need to be decreased (typically by 50%) to account for the increased reabsorption induced by thiazides (198). In addition, potassium levels will need to be monitored, and potassium replacement may be necessary. Amiloride, a potassium-sparing diuretic, is reported to be effective in treating lithium-induced polyuria and polydipsia (203). Its advantages are that it does not alter lithium levels and does not cause potassium depletion. Amiloride may be started at 5 mg b.i.d. and may be increased to 10 mg b.i.d. as needed (204).
Hypothyroidism occurs in 5%–35% of patients treated with lithium. It occurs more frequently in women, tends to appear after 6–18 months of lithium treatment, and may be associated with rapid cycling (41, 80, 198, 205). Lithium-induced hypothyroidism is not a contraindication to continuing lithium and is easily treated by the administration of levothyroxine (198, 205). In addition to the other signs and symptoms of hypothyroidism, patients with bipolar disorder are at risk of developing depression or rapid cycling. If these symptoms occur in the presence of laboratory evidence of suboptimal thyroid functioning, then thyroid supplementation, discontinuation of lithium, or both should be considered (206–208). Hyperparathyroidism has also been noted with lithium treatment (209–211).
A small number of case reports have described exacerbation or first occurrences of psoriasis associated with lithium treatment (212). Some of these patients improved with appropriate dermatologic treatment or when the lithium dose was lowered. In some cases, however, lithium seemed to block the effects of dermatologic treatment, with psoriasis clearing only after lithium was discontinued. In addition, patients occasionally experience severe pustular acne that does not respond well to standard dermatologic treatments and only resolves once the lithium treatment is discontinued (212). This is in contrast to the more common mild to moderate acne that can occur with lithium treatment, which is usually responsive to standard treatments (198).
Approximately 10%–20% of patients receiving long-term lithium treatment (i.e., for more than 10 years) display morphological kidney changes—usually interstitial fibrosis, tubular atrophy, and sometimes glomerular sclerosis. These changes may be associated with impairment of water reabsorption but not with reduction in glomerular filtration rate or development of renal insufficiency (41, 198, 213–216). Although irreversible renal failure caused by lithium has not been unequivocally established, there are a number of case reports of probable lithium-induced renal insufficiency (215, 217, 218). Additionally, several studies have shown that a small percentage of patients treated with lithium may develop rising serum creatinine concentrations after 10 years or more of treatment (215, 218).
b) Toxicity/overdose. Toxic effects of lithium become more likely as the serum level rises (219). Most patients will experience some toxic effects with levels above 1.5 meq/liter; levels above 2.0 meq/liter are commonly associated with life-threatening side effects. For many patients, the therapeutic range within which beneficial effects outweigh toxic effects is quite narrow, so that small changes in serum level may lead to clinically significant alterations in the beneficial and harmful effects of lithium. Elderly patients may experience toxic effects at lower levels and have a correspondingly narrower therapeutic window (138).
Signs and symptoms of early intoxication (with levels above 1.5 meq/liter) include marked tremor, nausea and diarrhea, blurred vision, vertigo, confusion, and increased deep tendon reflexes. With levels above 2.5 meq/liter, patients may experience more severe neurological complications and eventually experience seizures, coma, cardiac dysrhythmia, and permanent neurological impairment. The magnitude of the serum level and the duration of exposure to a high level of lithium are both correlated with risk of adverse effects (219). Therefore, rapid steps to reduce the serum level are essential. In addition, during treatment for severe intoxication, patients may experience “secondary peaks” during which the serum level rises after a period of relative decline; the clinician must therefore continue to monitor serum levels during treatment for severe intoxication. The patient with lithium intoxication should be treated with supportive care (e.g., maintenance of fluid and electrolyte balance), and steps should be taken to prevent further absorption of the medication (e.g., gastric lavage or, in the alert patient, induction of emesis).
Hemodialysis is the only reliable method of rapidly removing excess lithium from the body and is more effective than peritoneal dialysis for this purpose (220). Criteria for the use of hemodialysis in lithium intoxication are not firmly established, and the decision to dialyze must take into account both the patient’s clinical status and the serum lithium level (219, 221). When serum lithium levels are below 2.5 meq/liter, hemodialysis usually is unnecessary. The need for hemodialysis differs in patients who have developed toxicity after an acute overdose compared with those who have developed gradual toxicity or have an acute overdose superimposed on long-term lithium treatment. In acute poisoning, hemodialysis is generally required with serum lithium levels over 6–8 meq/liter, whereas hemodialysis may be needed with serum levels over 4 meq/liter in those who have been on long-term regimens of lithium treatment. Hemodialysis may also be necessary at lower serum levels in patients who are more susceptible to complications because of underlying illnesses (e.g., cardiac disease, renal impairment). Regardless of serum lithium level, hemodialysis is generally indicated in patients with progressive clinical deterioration or severe clinical signs of intoxication such as coma, convulsions, cardiovascular symptoms, or respiratory failure (219, 221). Because serum levels of lithium may rebound after initial hemodialysis, repeat dialysis may be needed (219, 222).
In cases of overdose with sustained-release preparations of lithium, development of toxicity is likely to be delayed, and the duration of toxicity is likely to be prolonged (223, 224). This should be taken into consideration in decisions about the need for initial or repeat hemodialysis (219).
c) Implementation and dosing. Before beginning lithium treatment, the patient’s general medical history should be reviewed, with special reference to those systems that might affect or be affected by lithium therapy (e.g., renal, thyroid, and cardiac functioning). In addition, pregnancy or the presence of a dermatologic disorder must be ascertained. Patient education should address potential side effects of lithium treatment as well as the need to avoid salt-restricted diets or concomitant medications that could elevate serum lithium levels (e.g., diuretics, angiotensin-converting enzyme inhibitors, nonsteroidal anti-inflammatory drugs, cyclooxygenase-2 inhibitors). Patients should be cautioned, particularly if nephrogenic diabetes insipidus is present, that lithium toxicity might occur with dehydration from environmental heat, gastrointestinal disturbance, or inadequate fluid intake.
Laboratory measures and other diagnostic tests are generally recommended on the basis of pathophysiological knowledge and anticipated clinical decisions rather than on empirical evidence of their clinical utility. The decision to recommend a test is based on the probability of detecting a finding that would alter treatment as well as the expected benefit of such alterations in treatment. Recommended tests fall into three categories: 1) baseline measures to facilitate subsequent interpretation of laboratory tests (e.g., ECG, CBC); 2) tests to determine conditions requiring different or additional treatments (e.g., pregnancy, thyroid-stimulating hormone level); and 3) tests to determine conditions requiring alteration of the standard dosage regimen of lithium (e.g., creatinine level).
On the basis of these considerations, the following procedures are generally recommended before beginning lithium therapy: a general medical history, a physical examination, BUN and creatinine level measurement, a pregnancy test, thyroid function evaluation, and, for patients over age 40, ECG monitoring with rhythm strip. Some authorities also suggest a CBC.
Lithium is usually started in low, divided doses to minimize side effects (e.g., 300 mg t.i.d. or less, depending on the patient’s weight and age), with the dose titrated upward (generally to serum concentrations of 0.5–1.2 meq/liter) according to response and side effects (225). Lithium levels should be checked after each dose increase and before the next. Steady-state levels are likely to be reached approximately 5 days after dose adjustment, but levels may need to be checked sooner if a rapid increase is necessary (e.g., in the treatment of acute mania) or if toxicity is suspected. As levels approach the upper limits of the therapeutic range (i.e., ≥1.0 meq/liter), they should be checked at shorter intervals after each dose increase to minimize the risk of toxicity.
Serum concentrations required for prophylaxis may be, in some cases, as high as those required for treatment of the acute episode. A controlled study by Gelenberg et al. (225) found that patients randomly assigned to a “low” lithium level (0.4–0.6 meq/liter) had fewer side effects but more illness episodes than patients in the “standard” lithium group (0.8–1.0 meq/liter). However, the lithium levels of some of the patients in the low-lithium group decreased relatively rapidly from their previous treatment levels, a decrease that could have increased their risk of relapse. Although the prophylactic efficacy of lithium levels between 0.6 and 0.8 meq/liter has not been formally studied, this range is commonly chosen by patients and their psychiatrists (226). Despite the lack of formal study, it is likely that for many patients, increases in maintenance lithium levels will result in a trade-off between greater protection from illness episodes at the cost of an increase in side effects. The “optimal” maintenance level may therefore vary somewhat from patient to patient. Some patients find that a single, daily dose facilitates treatment compliance and reduces or does not change side effects.
The clinical status of patients receiving lithium needs to be monitored especially closely. The frequency of monitoring depends on the individual patient’s clinical situation but generally should be no less than every 6 months for stable patients. The optimal frequency of serum level monitoring in an individual patient depends on the stability of lithium levels over time for that patient and the degree to which the patient can be relied upon to notice and report symptoms.
In general, renal function should be tested every 2–3 months during the first 6 months of treatment, and thyroid function should be evaluated once or twice during the first 6 months of lithium treatment. Subsequently, renal and thyroid function may be checked every 6 months to 1 year in stable patients or whenever clinically indicated (e.g., in the presence of breakthrough affective symptoms, changes in side effects, or new medical or psychiatric signs or symptoms) (198, 214).
2. Divalproex/valproate/valproic acid
Divalproex and its sodium valproate and valproic acid formulations have been studied in four randomized, placebo-controlled trials: two small crossover trials (227, 228) and two parallel-group trials (180, 229). All four studies found significantly greater efficacy for valproate compared with placebo, with response rates ranging from 48% to 53%. Secondary analyses (150, 197) of data from the largest parallel-group trial (180) suggested that patients with prominent depressive symptoms during mania and with multiple prior mood episodes were more likely to respond to acute treatment with divalproex than with lithium. An additional randomized comparison also reported valproate to be more efficacious than lithium among manic patients with mixed symptoms (195). In patients with acute mania, divalproex was comparable in efficacy to haloperidol in an open trial (230) and to olanzapine in a randomized, controlled trial (231) in the reduction of symptoms of mania and psychosis. In contrast, in a second head-to-head comparison trial (232), olanzapine was superior to divalproex in the mean reduction of manic symptoms and in the proportion of patients in remission at the end of the study.
a) Side effects. Minor side effects of valproate, such as sedation or gastrointestinal distress, are common initially and typically resolve with continued treatment or dose adjustment. In addition, valproate has a wide therapeutic window. Inadvertent overdose is uncommon, and purposeful overdose is less likely to be lethal than it is with lithium. However, in rare instances, valproate can cause life-threatening side effects, and patients must be relied upon to report the often subtle symptoms of these reactions promptly.
Common dose-related side effects of valproate include gastrointestinal distress (e.g., anorexia, nausea, dyspepsia, vomiting, diarrhea), benign hepatic transaminase elevations, osteoporosis (233, 234), tremor, and sedation. Patients with past or current hepatic disease may be at greater risk for hepatotoxicity (235). Mild, asymptomatic leukopenia and thrombocytopenia occur less frequently and are reversible upon drug discontinuation. Other side effects that are often bothersome to the patient include hair loss (236, 237), increased appetite, and weight gain. Persistent gastrointestinal distress associated with valproate can be alleviated by dose reduction, change of preparation (use of the divalproex sodium formulation rather than valproic acid), or by administration of a histamine-2 antagonist (e.g., famotidine or cimetidine) (238–242). Tremor can be managed with dose reduction or coadministration of β blockers. Cases of mild, asymptomatic leukopenia (total WBC count >3000/mm3 and polymorphonuclear leukocyte count <1500/mm3) are usually reversible upon dose reduction or discontinuation. Similarly, if mild, asymptomatic thrombocytopenia occurs, a decrease in valproate dose will usually restore the platelet count to normal. However, more severe cases of thrombocytopenia have been reported (243).
The relationship between polycystic ovarian syndrome and valproate treatment is unclear (244–246). One uncontrolled report indicated that 80% of women receiving long-term valproate treatment for epilepsy before the age of 20 had polycystic ovaries or hyperandrogenism (247). Other cross-sectional studies have demonstrated higher rates of polycystic ovaries and polycystic ovarian syndrome in women with epilepsy (244–246). However, none of the studies examined whether the polycystic ovarian syndrome began before or after the development of epilepsy or the initiation of valproate therapy (246). Furthermore, women with bipolar disorder may differ from women with epilepsy in their rates of polycystic ovarian syndrome independent of treatment. An accurate assessment of risk will require a longitudinal study of women with bipolar disorder before and after initiation of valproate treatment (246). Consequently, although the risks are unclear, psychiatrists should be aware that polycystic ovarian syndrome may be possible with valproate treatment, and thus patients should be monitored accordingly (244).
Rare, idiosyncratic, but potentially fatal adverse events with valproate include irreversible hepatic failure, hemorrhagic pancreatitis, and agranulocytosis. Thus, patients taking valproate need to be instructed to contact their psychiatrist or primary care physician immediately if they develop symptoms of these conditions.
b) Toxicity/overdose. Valproate has a wide therapeutic window, so unintentional overdose is uncommon (248). Signs of overdose include somnolence, heart block, and eventually coma. Deaths have been reported. Overdose can be treated with hemodialysis (249, 250).
c) Implementation and dosing. Before initiating valproate treatment, a general medical history should be taken, with special attention to hepatic, hematologic, and bleeding abnormalities. Results of liver function tests and hematologic measures should be obtained at baseline to evaluate general medical health.
Data from a number of open trials (230, 251–253) and one randomized controlled trial (254) indicate that divalproex can be administered at a therapeutic initial starting dose of 20–30 mg/kg per day in inpatients. This strategy appears to be well tolerated and may be more rapidly efficacious than more gradual titration from a lower starting dose (254). After a serum valproate level is obtained, the dose is then adjusted downward to achieve a target level between 50 and 125 μg/ml.
Among outpatients, elderly patients, or patients who are hypomanic or euthymic, valproate may be initiated in low, divided doses to minimize gastrointestinal and neurological toxicity. Valproate should generally be started at 250 mg t.i.d., with the dose increased every few days as side effects allow (204). Depending upon clinical response and side effects, the dose is then titrated upward by 250–500 mg/day every few days, generally to a serum concentration of 50–125 μg/ml, with a maximum adult daily dose of 60 mg/kg per day (250). Once the patient is stable, valproate regimens can be simplified to enhance convenience and compliance, since many patients do well with once- or twice-a-day dosing.
Extended-release divalproex, a new formulation that allows for once-a-day dosing, has become available. Bioavailability is approximately 15% lower than the immediate-release formulation (hence usually requiring slightly higher doses), and side effect profiles appear to be better than that of the immediate-release formulation (255). Demonstration of efficacy in patients with bipolar disorder is limited to open studies (255–257).
Asymptomatic hepatic enzyme elevations, leukopenia, and thrombocytopenia do not reliably predict life-threatening hepatic or bone marrow failure. In conjunction with careful monitoring of clinical status, educating patients about the signs and symptoms of hepatic and hematologic dysfunction and instructing them to report these symptoms if they occur are essential. Some investigators believe that in otherwise healthy patients with epilepsy receiving long-term valproate treatment, routine monitoring of hematologic and hepatic function is not necessary (258). Nevertheless, most psychiatrists perform clinical assessments, including tests of hematologic and hepatic function, at a minimum of every 6 months for stable patients who are taking valproate (252, 259, 260). Patients who cannot reliably report signs or symptoms of toxicity need to be monitored more frequently.
Psychiatrists should be alert to the potential for interactions between valproate and other medications (261). For example, valproate displaces highly protein-bound drugs from their protein binding sites. In addition, valproate inhibits lamotrigine metabolism and more than doubles its elimination half-life by competing for glucuronidation enzyme sites in the liver (262, 263). Consequently, in patients treated with valproate, lamotrigine must be initiated at a dose that is less than half that used in patients who are not receiving concomitant valproate.
3. Carbamazepine
Many controlled trials of carbamazepine have been conducted in the treatment of acute bipolar mania, but interpretation of the results of a number of these studies is difficult because of the confounding effects of other medications administered as part of study protocols (264). Carbamazepine was superior to placebo in one randomized, crossover trial (265). Carbamazepine was less effective and associated with more need for adjunctive “rescue medication” than valproate in a randomized, blind, parallel-group trial of 30 hospitalized manic patients (266). Carbamazepine was comparable to lithium in two randomized comparison trials (181, 182) and comparable to chlorpromazine in two other randomized trials (267, 268).
a) Side effects. Up to 50% of patients receiving carbamazepine experience side effects, and the drug is associated with potentially serious adverse reactions (258, 269, 270).
The most common dose-related side effects of carbamazepine include neurological symptoms, such as diplopia, blurred vision, fatigue, nausea, and ataxia. These effects are usually transient and often reversible with dose reduction. Elderly patients, however, may be more sensitive to side effects. Less frequent side effects include skin rashes (271), mild leukopenia, mild thrombocytopenia, hyponatremia, and (less commonly) hypo-osmolality. Mild liver enzyme elevations occur in 5%–15% of patients. Mild asymptomatic leukopenia is not related to serious idiopathic blood dyscrasias and usually resolves spontaneously with continuation of carbamazepine treatment or with dose reduction. In the event of asymptomatic leukopenia, thrombocytopenia, or elevated liver enzymes, the carbamazepine dose can be reduced or, in the case of severe changes, discontinued. Hyponatremia may be related to water retention caused by carbamazepine’s antidiuretic effect (272). Hyponatremia occurs in 6%–31% of patients, is rare in children but probably more common in the elderly, occasionally develops many months after the initiation of carbamazepine treatment, and sometimes necessitates carbamazepine discontinuation. In addition, carbamazepine may decrease total and free thyroxine levels and increase free cortisol levels, but these effects are rarely clinically significant. Weight gain is also a common side effect of carbamazepine.
Rare, idiosyncratic, but serious and potentially fatal side effects of carbamazepine include agranulocytosis, aplastic anemia, thrombocytopenia, hepatic failure, exfoliative dermatitis (e.g., Stevens-Johnson syndrome), and pancreatitis (243, 258, 273–275). Although these side effects usually occur within 3–6 months of carbamazepine initiation, they have also occurred after more extended periods of treatment. Routine blood monitoring does not reliably predict blood dyscrasias, hepatic failure, or exfoliative dermatitis. Thus, in addition to careful monitoring of clinical status, it is essential to educate patients about the signs and symptoms of hepatic, hematologic, or dermatologic reactions and instruct them to report symptoms if they occur. Other rare side effects include systemic hypersensitivity reactions, cardiac conduction disturbances, psychiatric symptoms (including sporadic cases of psychosis), and, very rarely, renal effects (including renal failure, oliguria, hematuria, and proteinuria).
b) Toxicity/overdose. Carbamazepine may be fatal in overdose; deaths have been reported with ingestions of more than 6 g. Signs of impending carbamazepine toxicity include dizziness, ataxia, sedation, and diplopia. Acute intoxication can result in hyperirritability, stupor, or coma. The most common symptoms of carbamazepine overdose are nystagmus, ophthalmoplegia, cerebellar and extrapyramidal signs, impaired consciousness, convulsions, and respiratory dysfunction. Cardiac symptoms may include tachycardia, arrhythmia, conduction disturbances, and hypotension. Gastrointestinal and anticholinergic symptoms may also occur. Management of carbamazepine intoxication includes symptomatic treatment, gastric lavage, and hemoperfusion.
c) Implementation and dosing. A pretreatment evaluation for carbamazepine should include a general medical history and physical examination, with special emphasis on prior history of blood dyscrasias or liver disease. Most authorities recommend that the minimum baseline evaluation include a CBC with differential and platelet count, a liver profile (evaluation of LDH, SGOT, SGPT, bilirubin, and alkaline phosphatase), and renal function tests (204). Serum electrolyte levels may also be obtained, especially in the elderly, who may be at higher risk for hyponatremia.
Although doses can range from 200 to 1800 mg/day, the relationships among dose, serum concentration, response, and side effects are variable. Therefore, the dose should be titrated upward according to response and side effects. In patients over the age of 12, carbamazepine is usually begun at a total daily dose of 200–600 mg, given in three to four divided doses. In hospitalized patients with acute mania, the dose may be increased in increments of 200 mg/day up to 800–1000 mg/day (unless side effects develop), with slower increases thereafter as indicated. In less acutely ill outpatients, dose adjustments should be slower, since rapid increases may cause patients to develop nausea and vomiting or mild neurological symptoms such as drowsiness, dizziness, ataxia, clumsiness, or diplopia. Should such side effects occur, the dose can be decreased temporarily and then increased again more slowly once these side effects have passed.
While therapeutic serum levels of carbamazepine have not been established for patients with bipolar disorder, serum concentrations established for treatment of seizure disorders (4–12 μg/ml) are generally applied. Trough levels are most meaningful for establishing an effective level for a given patient and are conveniently drawn before the first morning dose. Serum levels should be determined 5 days after a dose change or sooner if toxicity or noncompliance is suspected. Maintenance doses average about 1000 mg/day but may range from 200–1600 mg/day in routine clinical practice (204). Doses higher than 1600 mg/day are not recommended.
CBCs, platelet measurements, and liver function tests should be performed every 2 weeks during the first 2 months of carbamazepine treatment. Thereafter, if results of laboratory tests remain normal and no symptoms of bone marrow suppression or hepatitis appear, blood counts and liver function tests should be performed at least every 3 months (204). More frequent monitoring is necessary in patients with laboratory findings, signs, or symptoms consistent with hematologic or hepatic abnormalities. Life-threatening reactions, however, are not always detected by routine monitoring. The psychiatrist should educate patients about signs and symptoms of hepatic, hematologic, or dermatologic reactions and instruct patients to report these symptoms if they occur. More frequent clinical and laboratory assessments are needed for those patients who cannot reliably report symptoms.
Psychiatrists should be aware that carbamazepine is able to induce drug metabolism, including its own, through cytochrome P-450 oxidation and conjugation (261, 263, 276). This enzymatic induction may decrease levels of concomitantly administered medications such as valproate, lamotrigine, oral contraceptives, protease inhibitors, benzodiazepines, and many antipsychotic and antidepressant medications. In addition, carbamazepine has an active epoxide metabolite and is metabolized primarily through a single enzyme, cytochrome P-450 isoenzyme 3A3/4, making drug-drug interactions even more likely. Consequently, carbamazepine levels may be increased by medications that inhibit the cytochrome P-450 isoenzyme 3A3/4, such as fluoxetine, fluvoxamine, cimetidine, and some antibiotics and calcium channel blockers. Thus, in patients treated with carbamazepine, more frequent clinical and laboratory assessments may be needed with addition or dose adjustments of other medications.
4. Other anticonvulsants
Oxcarbazepine, the 10-keto analog of carbamazepine, was comparable in efficacy to lithium and haloperidol in two small trials (277, 278). However, these studies lacked sufficient power to detect possible drug-drug differences. While direct comparisons with carbamazepine in studies of bipolar disorder are lacking, studies of epilepsy suggest that oxcarbazepine may have a lower rate of severe side effects (279) and be well tolerated overall (280), although it has been associated with clinically significant hyponatremia (281). Moreover, unlike carbamazepine, oxcarbazepine does not induce its own metabolism (282). However, it may still decrease plasma concentrations of oral contraceptives and dihydropyridine calcium channel blockers, requiring medication change or dose adjustment. (For a more complete review, see the bipolar disorder treatment algorithm of the Texas Medication Algorithm Project [283].)
Three controlled studies, all with methodological limitations, have evaluated lamotrigine in the treatment of bipolar mania. In the first trial, 28 patients with bipolar I or bipolar II disorder were assessed in a double-blind, randomized, crossover series of three 6-week monotherapy trials of lamotrigine, gabapentin, or placebo (284). The response rate for manic symptom improvement, as measured by the Clinical Global Impression Scale for Bipolar Illness, did not differ significantly among the three treatment groups. However, the low mean Young Mania Rating Scale scores at baseline, the crossover design, and the small number of subjects may have limited the findings. In the second study, 16 outpatients with mania, hypomania, or mixed episodes who were inadequately responsive to or unable to tolerate lithium were randomly assigned to lamotrigine or placebo as mono- or adjunctive therapy (285). There were no significant differences between lamotrigine and placebo groups on changes in Young Mania Rating Scale scores or response rates. Limitations of this study included the small study group size and high (50%) placebo response rate. In the third study, 30 inpatients were randomly assigned to lamotrigine or lithium for 4 weeks (286). Both treatment groups displayed significant and comparable reductions in manic symptoms from baseline to endpoint. Limitations of this study included lack of a placebo group, small patient group size, and use of relatively low lithium levels (mean plasma concentration of 0.7 meq/liter at study endpoint). Adverse events and implementation and dosing issues associated with lamotrigine treatment are described in detail in section V.B.2.c.
Two controlled studies have evaluated the efficacy of gabapentin in the treatment of bipolar manic symptoms. In the first study (284), there were no significant differences in efficacy between gabapentin monotherapy and placebo in improvement in manic symptoms. The second controlled trial (287) compared gabapentin with placebo added to lithium, valproate, or both in 114 outpatients with manic, hypomanic, or mixed symptoms. Both treatment groups displayed a decrease in Young Mania Rating Scale scores from baseline to endpoint, but this decrease was significantly greater in the placebo group.
Finally, one small placebo-controlled trial also suggested efficacy for the anticonvulsant phenytoin in the treatment of mania when added to haloperidol treatment (288).
5. Olanzapine
Olanzapine was superior to placebo in the treatment of acute bipolar mania in two large, multicenter randomized controlled trials. In the first trial (289), olanzapine versus placebo differences did not reach statistical significance until the third week of treatment. In the second study (290), significant reductions in manic symptoms were apparent in olanzapine-treated patients compared with those receiving placebo at the first assessment point (after 1 week). These differences were probably due to differences in initial starting dose, since the initial olanzapine dose was 10 mg/day in the first study and 15 mg/day in the second trial. In a secondary analysis of data from the second trial, in which sufficient proportions of patients with mixed episodes or rapid cycling were included for comparison, olanzapine response was comparable in patients with or without these features (291). In other randomized, controlled trials, olanzapine exerted comparable efficacy to lithium (184), divalproex (231), and haloperidol (292) in the reduction of manic symptoms. Olanzapine was superior to divalproex in a randomized comparison trial (232). Last, olanzapine was superior to placebo as adjunctive therapy to lithium or divalproex in a randomized, controlled acute treatment trial (292).
a) Side effects. In short-term, placebo-controlled clinical trials, somnolence was the most common side effect associated with olanzapine. Other common side effects included constipation, dry mouth, increased appetite, and weight gain (291). Especially during initial dose titration, olanzapine may induce orthostatic hypotension associated with dizziness, tachycardia, and, in some patients, syncope. Syncope was reported in 0.6% of olanzapine-treated patients in phase II and III trials.
In clinical trials, seizures occurred in 0.9% of olanzapine-treated patients. Although confounding factors may have contributed to seizures in many instances, olanzapine should be used cautiously in patients with a history of seizure disorder or in clinical conditions associated with lowered seizure threshold. Transient elevations in plasma prolactin concentrations were also observed in short-term trials (293). These elevations typically remained within the normal physiological range and decreased with continued treatment. Clinically significant hepatic transaminase elevations (≥3 times the upper limit of the normal range) were observed in 2% of olanzapine-treated patients.
In long-term studies, 56% of olanzapine-treated patients gained >7% of their baseline weight. In retrospective analyses of patients followed for a median of 2.54 years, the mean and median weight gains were 6.26 kg and 5.9 kg, respectively (294). Weight gain did not appear to be dose related, occurred most rapidly within the first 39 weeks of treatment, was greatest in patients with the lowest baseline body mass index, and was not correlated with increases in serum glucose. Increases in serum glucose in olanzapine-treated patients did not differ significantly from those in patients treated with haloperidol (294). Weight gain and hyperglycemia in patients treated with atypical antipsychotics have been reviewed in detail elsewhere (295, 296).
In short-term trials, there were no significant differences in the incidence of dystonic reactions, parkinsonism, akathisia, or dyskinetic events among patients receiving placebo or olanzapine (291). Also, extrapyramidal side effects with olanzapine were substantially less than those seen with conventional antipsychotic medications such as haloperidol (297). In a 1-year haloperidol-controlled trial, the incidence of dyskinetic movements among olanzapine-treated patients with schizophrenia was 0.6% compared with 7.5% in patients receiving haloperidol (298). This incidence rate is confounded by prior treatment with typical antipsychotics and the rate of spontaneous dyskinesia in patients with schizophrenia. In 98 patients with bipolar disorder who received olanzapine for 1 year, some in combination with lithium or fluoxetine, no patients developed dyskinetic movements (291).
b) Implementation and dosing. In the two placebo-controlled studies of olanzapine in patients with bipolar mania, the mean final dose was approximately 15 mg/day. In the first study in which olanzapine was initiated at 10 mg/day and then titrated according to response and side effects, olanzapine did not differentiate from placebo until the third week of the trial (289). The second trial used a starting dose of 15 mg/day and found a significant difference in efficacy in favor of olanzapine at 1 week (the time of the first rating) (290). Taken together, the results of these trials suggest that for inpatients with acute mania, a starting dose of 15 mg/day may be more rapidly efficacious. For outpatients, lower starting doses of 5–10 mg/day may be indicated (299).
6. Other antipsychotics
Only one randomized, placebo-controlled study of typical antipsychotic medications has been reported in the treatment of acute bipolar mania (300). In this study, chlorpromazine was superior to placebo in global improvement of manic symptoms. Typical antipsychotics were comparable to lithium in reducing manic and psychotic symptoms in acute treatment comparison trials (185–190).
Among the atypical antipsychotic agents, risperidone and ziprasidone have also been studied in the treatment of acute bipolar mania with randomized, placebo-controlled trials. As an adjunct to treatment with lithium or divalproex, risperidone was comparable to haloperidol and superior to placebo (301). Ziprasidone was also superior to placebo in a large, multicenter monotherapy trial, with significant differences in favor of ziprasidone apparent at the time of the first rating, day 2 of treatment (302). While no placebo-controlled trials exist for the use of clozapine in the treatment of bipolar disorder, one randomized 1-year trial in patients with refractory bipolar or schizoaffective disorder showed greater clinical improvement with the addition of clozapine than with treatment as usual (303). An open trial of clozapine in the treatment of refractory mania was also associated with improvement in manic symptoms (304, 305). In general, these trials have used dose ranges similar to those used in schizophrenia trials, with similar rates of adverse events.
7. Combination therapy
Controlled trials of lithium plus an antipsychotic and of valproate plus an antipsychotic suggest greater efficacy or more rapid onset of action with these combinations than with any of these agents alone. All of these studies involved patients who were currently being treated but who experienced breakthrough episodes of mania or incomplete response to monotherapy. The studies compared combination therapies: an antipsychotic combined with either valproate or placebo (306); lithium or valproate combined with either olanzapine or placebo (290); lithium or valproate combined with either risperidone or placebo (301); or lithium, valproate, or carbamazepine combined with either risperidone or placebo (307). This last trial supported combination therapy only when the carbamazepine-treated group was excluded.
8. ECT
Three prospective studies have assessed clinical outcomes of treatment of acute mania with ECT. In a prospective, randomized controlled trial (308), patients who received ECT followed by lithium maintenance treatment exhibited greater improvement after 8 weeks than did patients who received lithium as both acute and maintenance treatment. Clinical outcomes with ECT were also found to be superior to outcomes with a combination of lithium and haloperidol (309). In a third study (310), 30 manic patients were all treated with chlorpromazine but were randomly assigned to receive a course of either six ECT sessions or six sham ECT sessions. Patients treated with sham ECT did significantly worse than those treated with real ECT. Although all of these studies had small study group sizes, the results were consistent with other earlier retrospective comparisons of outcome in mania (311, 312) and with earlier naturalistic case series (see Mukherjee et al. [309] and the APA Task Force Report on ECT [110] for reviews).
Although there are no prospective, randomized controlled studies of the use of ECT in the treatment of mixed states, in the aforementioned trial of ECT for treatment of mania (308), the strongest predictor of clinical response was the baseline rating of depressive symptoms. Case reports also suggest that ECT may be efficacious in treatment of mixed states (313–315).
Information on side effects and implementation of ECT can be found in the APA Task Force Report on ECT (110).
9. Novel treatments
A number of new agents are under active investigation as potential treatments for patients with acute bipolar mania, but data regarding their efficacy from randomized controlled trials are not yet available. These agents include the atypical antipsychotics quetiapine and aripiprazole; the antiepileptics zonisamide, acamprosate, and levetiracetam; and omega-3 fatty acids (316).
Two other medication classes, benzodiazepines and calcium channel blockers, have been studied in randomized controlled trials for treatment of acute bipolar mania. Among the benzodiazepines, clonazepam and lorazepam have been studied alone and in combination with lithium (317–322). Interpretation of many of these studies is confounded by small study group sizes, short treatment durations, concomitant antipsychotic use, and difficulties in distinguishing putative antimanic effects from nonspecific sedative effects. Taken together, however, these studies suggest that the sedative effects of benzodiazepines may make them effective treatment adjuncts while awaiting the effects of a primary antimanic agent to become evident. The fact that lorazepam, unlike other benzodiazepines, is well absorbed after intramuscular injection has made it particularly useful for the management of agitation. However, intramuscular olanzapine was superior to intramuscular lorazepam in ameliorating agitation in patients with bipolar mania (322).
Two randomized, controlled trials found little support for the efficacy of the calcium channel antagonist verapamil in the treatment of acute mania. In the first study, verapamil was compared with lithium in 40 patients hospitalized for an acute manic episode (323). The mean reduction in manic symptoms was significantly greater in the group of patients receiving lithium compared with the verapamil-treated group. The second trial, a 3-week double-blind study involving 32 patients with acute mania (324), showed no significant differences in efficacy between verapamil and placebo. These studies indicate that lithium was superior to verapamil and that verapamil, in turn, was not superior to placebo as an antimanic agent. In contrast, in a crossover trial involving 12 patients with refractory ultrarapid-cycling bipolar disorder (325), the calcium channel antagonist nimodipine was superior to placebo in ameliorating mood cycling.
B. Somatic treatments of acute depressive episodes
Somatic treatments that have been studied in bipolar depression include lithium, anticonvulsants, antidepressants, and ECT. Open studies and case reports comprise most of the literature on the treatment of bipolar depression, with the best-controlled data relating to treatment with lithium, lamotrigine, and paroxetine.
In general, the goals for treatment of acute depression in a patient with bipolar disorder are identical to those for patients with nonbipolar depression. The primary goal is remission of the symptoms of major depression and a return to normal levels of psychosocial functioning. Concerns about precipitation of a manic or hypomanic episode introduce management issues in the treatment of bipolar depression that do not exist for unipolar depression. This section will present efficacy data on lithium, anticonvulsants, antidepressants, ECT, and novel treatments. Information on side effects and implementation and dosing issues for lithium and the anticonvulsants are presented in this guideline in their respective sections under “Somatic Treatments of Acute Manic and Mixed Episodes” (section V.A.). Information on side effects and implementation and dosing issues for the antidepressants is provided in the APA Practice Guideline for the Treatment of Patients With Major Depressive Disorder (2).
1. Lithium
There have been eight placebo-controlled studies of lithium in the treatment of bipolar depression that had five or more subjects. All of these studies employed crossover designs, and all were completed before 1980 (for a review, see Zornberg and Pope [326]). Among a total of 160 patients, the overall rate of response to lithium, regardless of the degree of improvement or relapse with placebo, was 79%. However, the “unequivocal” lithium response rate, defined as a good or moderate response to lithium with a subsequent relapse when given placebo, was much lower (36%). An additional consideration in the use of lithium as an antidepressant is its time to onset (6–8 weeks), which is later than its antimanic effect (326).
2. Anticonvulsants
a) Divalproex and sodium valproate. There have been no published controlled studies of valproate in the treatment of bipolar depression. In an unpublished study, 43 subjects with bipolar I or bipolar II depression were entered into an 8-week, double-blind, placebo-controlled trial of divalproex. Forty-three percent of divalproex-treated patients and 27% of placebo-treated patients achieved recovery, defined as an improvement of ≥50% in score on the 16-item Hamilton Depression Rating Scale in the absence of hypomania (Young Mania Rating Scale score <10). This difference was not statistically significant (Gary Sachs and Michelle Collins, personal communication). While these results suggest that divalproex may be useful in the treatment of bipolar depression, a more definitive study is needed.
b) Carbamazepine. In a double-blind, placebo-controlled crossover study (327), four of nine patients with bipolar depression showed significant improvement from baseline in depressive symptoms with carbamazepine treatment.
In an open study of carbamazepine (328), there were significant reductions from baseline in 17-item Hamilton depression scale scores among 27 patients with bipolar depression and nine patients with mixed episodes. Patients with mixed episodes were significantly less likely to have a remission than those with bipolar depression.
c) Lamotrigine. Lamotrigine at doses of 50 mg/day and 200 mg/day was compared with placebo in a 7-week double-blind trial in 195 patients with bipolar I disorder with major depression (329). Both lamotrigine groups reported significantly better response rates on the Montgomery-Åsberg Depression Rating Scale but not on the Hamilton depression scale. The first significant lamotrigine versus placebo difference in Hamilton depression scale scores occurred at week 5 in the patients receiving 200 mg/day, whereas it occurred at week 7 in those given 50 mg/day. Switches into manic or hypomanic episodes occurred at equivalent rates (3%–8%) among the three groups.
In a flexible-dose, placebo-controlled study of lamotrigine in 206 patients with bipolar I or bipolar II major depression (330), both treatment groups improved significantly (response rate to lamotrigine was 50%, response rate to placebo was 49%), but lamotrigine did not distinguish itself from placebo. Lamotrigine was started at 25 mg/day and titrated over 5–6 weeks to the target dose of 400 mg/day. In a subgroup analysis, the patients with bipolar I disorder given lamotrigine did respond significantly better than those given placebo in terms of Montgomery-Åsberg Depression Rating Scale score (mean change of 13.5 versus 10.1, respectively).
In a double-blind, crossover study of patients with refractory, rapid-cycling bipolar I or bipolar II disorder who were treated with lamotrigine, gabapentin, or placebo, 45% of the depressed patients responded to lamotrigine, compared with response rates of 26% for gabapentin and 19% for placebo (284).
Finally, in an open study of patients with refractory bipolar disorder, 48% of 40 depressed patients treated with lamotrigine showed a marked response, and 20% showed a moderate response (331).
The most common side effects of lamotrigine in the treatment of depression are headache, nausea, infection, and xerostomia (39, 329). However, none of these occurred at significantly higher percentage than with placebo (332).
The risk of serious rash, including Stevens-Johnson syndrome and toxic epidermal necrolysis, was found to be higher in patients treated for epilepsy in the first year after the introduction of lamotrigine in Europe (333). In clinical trials for epilepsy, the incidence of serious rash was approximately 0.3% in adults and approximately 1% in children (334, 335). However, with a slow titration schedule, the risk of serious rash was reduced to 0.01% in adults (329), which is comparable to that of other anticonvulsant medications. Rash can occur at any time during treatment but is more likely to occur early in treatment. It may also be more likely if lamotrigine and valproate are administered concomitantly (334, 335). Whenever lamotrigine is prescribed, patients should be apprised of the risk of rash and urged to contact the psychiatrist or primary care physician immediately if a rash occurs. At rash onset, it is difficult to distinguish between a serious and a more benign rash. Particularly worrisome are rashes accompanied by fever or sore throat, those that are diffuse and widespread, and those with prominent facial or mucosal involvement. In such circumstances lamotrigine (and concurrent valproate) should be discontinued.
Lamotrigine should be administered at 25 mg/day for the first 2 weeks, then 50 mg/day for weeks 3 and 4. After that, 50 mg can be added per week as clinically indicated. With concurrent valproate treatment, pharmacokinetic interactions lead to lamotrigine levels that are approximately twice normal. To minimize the risk of potentially serious rash in patients who are receiving valproate, the dose schedule should be cut in half (i.e., 12.5 mg/day or 25 mg every other day for 2 weeks, then 25 mg/day for weeks 3 and 4). Similarly, concurrent carbamazepine treatment leads to an increase in lamotrigine metabolism and requires dosing to be doubled. Further details of lamotrigine dosing and adverse effects can be found in several reviews (262, 334–337).
d) Topiramate. There are no placebo-controlled trials of topiramate in the treatment of bipolar depression, but several trials have suggested its efficacy as an add-on therapy. McIntyre et al. (338) conducted a single-blind, add-on study of topiramate and sustained-release bupropion in depressed patients with bipolar I or bipolar II disorder. Both groups had significant baseline-to-endpoint reduction in 17-item Hamilton depression scale and Clinical Global Impression (CGI) improvement scores, with no difference between the two groups. Thirty-three percent of patients receiving cotreatment with topiramate discontinued treatment because of adverse events compared with 22% of the patients receiving bupropion alone. The most common adverse events were sweating, blurred vision, difficulty sleeping, tremors, and paresthesia.
Hussain (339) conducted an open-label, add-on, 6-month study with topiramate in depressed patients with bipolar I or bipolar II disorder. Of 45 patients, 19 fully responded (Hamilton depression scale score=3–7), and 12 partially responded (Hamilton depression scale score=8–12). Five patients discontinued treatment because of lack of efficacy, and nine discontinued because of adverse events.
Conversely, in an open study of patients with bipolar I or bipolar II disorder, the 11 patients who were initially depressed and received add-on topiramate treatment had no significant improvements in either CGI or Inventory of Depressive Symptomatology scores (316).
3. MAOI antidepressants
a) Tranylcypromine. The efficacy of tranylcypromine was compared with that of imipramine in 56 outpatients with bipolar I or bipolar II depression (340). Compared with imipramine (at doses of at least 150 mg/day), tranylcypromine (at doses of at least 30 mg/day) produced significantly superior outcomes in terms of lower attrition, greater symptomatic improvement, and higher global response without a greater risk of treatment-emergent hypomania or mania.
In a second study (341), tranylcypromine was compared with imipramine in a double-blind crossover fashion for the 16 nonresponsive patients with bipolar I or bipolar II disorder from the previous trial. Tranylcypromine had comparatively better results, including lower attrition, greater symptomatic improvement, higher global response, and no greater risk of precipitating a switch into hypomania or mania.
b) Moclobemide. Moclobemide was compared to imipramine in a 4-week, multicenter, randomized study of 381 patients (342). No significant differences in efficacy were observed between the groups (both had response rates of 58%). The number of patients with adverse events and the total number of adverse events were greater in the imipramine group.
4. SSRIs and other newer antidepressant agents
a) Fluoxetine. Fluoxetine was compared with imipramine and placebo in 89 patients with bipolar depression. Twenty-two of the 89 patients were also taking lithium during the study. Eighty-six percent of the patients receiving fluoxetine over 6 weeks improved compared with 57% receiving imipramine and 38% given placebo. The response rate with fluoxetine was significantly better than that of both imipramine (p<0.05) and placebo (p=0.005). There were significantly fewer fluoxetine patients who discontinued treatment because of adverse events (343).
b) Paroxetine. Paroxetine was studied as an add-on treatment in three double-blind studies of patients with bipolar depression. In one study (344), depressed patients with bipolar I or bipolar II disorder maintained on regimens of lithium or divalproex were randomly assigned either to addition of paroxetine or a combination of lithium and divalproex in a 6-week outpatient trial. In terms of improvement from baseline in 17-item Hamilton depression scale scores, both treatments were equally effective at week 6: the mean scores of 6 and 9 in the subjects given lithium plus divalproex and those treated with adjunctive paroxetine, respectively, represented a decrease of 50%–70% (p<0.001). There were more dropouts among those treated with the combination of lithium and divalproex.
In a placebo-controlled multicenter trial of paroxetine and imipramine in the treatment of patients with bipolar I depression maintained on a regimen of lithium (345), imipramine and paroxetine were found to be superior to placebo in patients whose serum lithium level was ≤0.8 meq/liter. In those patients with serum lithium levels >0.8 meq/liter, there were no differences among the groups. Of the patients receiving imipramine, treatment-induced switches into manic or hypomanic episodes occurred in 6% of those with lithium levels >0.8 meq/liter and 11% of those with lithium levels ≤0.8 meq/liter. Switches occurred in none of the paroxetine-treated patients and in 2% of the placebo group (all of whom had lithium levels ≤0.8 meq/liter).
Paroxetine and venlafaxine were studied in the treatment of patients with bipolar depression on a maintenance medication regimen (346). Forty-three percent of the paroxetine group and 48% of the venlafaxine group were rated as having responded (difference not significant). Whereas switches to episodes of mania or hypomania occurred in 3% of those treated with paroxetine, the rate of switching in the venlafaxine group was 13%.
c) Citalopram. In a 24-week, open-label trial, the use of citalopram as an add-on treatment was studied in 45 patients with bipolar depression (30 [67%] with bipolar I disorder) who were receiving lithium, valproate, or carbamazepine (347). Of the 33 patients who completed the 8-week acute phase, 64% responded, and most of these patients continued to improve through the 16-week continuation phase.
d) Bupropion. There have been two controlled studies of bupropion in the treatment of bipolar depression. In a double-blind, 8-week study (348), patients who had been maintained on regimens of lithium, valproate, or carbamazepine were randomly assigned to bupropion or desipramine treatment. The response rate was 55% for bupropion and 50% for desipramine, a nonsignificant difference. In the first 8 weeks, 30% of the patients receiving desipramine switched into a manic episode, whereas 11% of those receiving bupropion did. Over the entire study, with follow-up to 1 year, the observed rate of switching into manic or hypomanic episodes in patients receiving desipramine was 50%, whereas the rate was 11% with bupropion.
In a 6-week, double-blind study of bupropion versus idazoxan (a selective α-2 antagonist) in 16 patients with bipolar I disorder—some of whom were also on a maintenance regimen of lithium—no significant differences were seen between the groups (349).
e) Venlafaxine. In addition to the aforementioned double-blind study that compared venlafaxine with paroxetine (346), another study reported on 15 depressed women with bipolar II disorder who were treated with venlafaxine (350). Sixty-three percent of the patients experienced a Δ50% reduction from baseline in scores on the 21-item Hamilton depression scale. Two patients (13%) discontinued treatment because of adverse events.
5. Tricyclic antidepressants
Imipramine and desipramine have been used as active control treatments in studies of tranylcypromine, fluoxetine, paroxetine, and bupropion. In general, the tricyclic antidepressants had response rates that were equivalent to or poorer than that of the active comparator (yet superior to placebo). In addition, treatment with tricyclic antidepressants was associated with higher rates of switching into manic or hypomanic episodes.
6. Antipsychotics
In an 8-week, double-blind study of olanzapine monotherapy, olanzapine and fluoxetine combination therapy, and placebo in the treatment of 833 patients with acute bipolar I depression, olanzapine monotherapy and combination therapy were both significantly better than placebo at endpoint (M. Tohen, personal communication, 2001). Furthermore, both of these treatment regimens showed significant separation from placebo at week 1.
7. ECT
Several controlled studies of ECT in patients with bipolar depression were conducted several decades ago (326). All found ECT to be as or more effective than MAOIs, tricyclic antidepressants, or placebo. ECT is a viable option for patients with severe bipolar depression, especially if psychotic features are present (110). For information on side effects and implementation of ECT, see the APA Task Force Report on ECT (110).
8. Novel treatments
Several studies have suggested that sleep deprivation has an antidepressant effect in patients with bipolar depression, although its effect is usually short-lived (351). It has been studied in conjunction with pindolol in a placebo-controlled protocol (352). Forty patients with bipolar depression were randomly assigned to receive either pindolol or placebo in combination with total sleep deprivation. Fourteen of 20 patients who underwent total sleep deprivation while receiving pindolol were rated as having responded (Hamilton depression scale score <8), whereas only one patient receiving placebo and pindolol responded. No switches into manic episodes were observed. Another study examined the value of phototherapy or lithium in conjunction with total sleep deprivation among 115 patients with bipolar depression (353). The authors reported that each adjunctive treatment improved total sleep-deprivation response rates, but the combination of all three added nothing.
Thyroid hormones, particularly thyroxine (T4), have been reported to be useful in the treatment of bipolar disorder, particularly rapid cycling (205). In patients with nonbipolar depression, triiodothyronine (T3) augmentation is associated with an antidepressant effect. The use of thyroid hormones in patients with bipolar depression remains to be studied.
The use of other agents, such as risperidone, olanzapine, ziprasidone, omega-3 fatty acids (354), pramipexole (355), or interventions such as phototherapy (353), vagus nerve stimulation (356), or repetitive transcranial magnetic stimulation (357) requires further study.
C. Rapid Cycling
Rapid cycling is generally difficult to treat (358, 359). An important first step is to assess for and treat medical conditions that may contribute to cycling, such as hypothyroidism or drug or alcohol use. Medications, particularly antidepressants, may also contribute to cycling. Such medications should be discontinued if possible. Increases in cycling frequency or precipitation of hypomanic or manic episodes have been reported in association with essentially all currently approved antidepressants (340, 343, 360). Use of some form of mood chart can aid in identifying a link between a medication and cycling frequency.
Rapid cycling is relatively unresponsive to lithium or carbamazepine (358, 361–363). Among 41 lithium-treated patients with rapid-cycling bipolar disorder followed for 5 years, all patients experienced at least one recurrence. Twenty-six percent derived limited or no prophylactic benefit (364). The limited benefit of lithium in rapid cycling may be a function of its lack of efficacy for depressive symptoms, despite its efficacy for manic symptoms (365, 366). In the open-stabilization phase of a study of lithium and divalproex in patients with rapid-cycling bipolar disorder, those who failed to meet criteria for random assignment were more likely to have refractory depression (76%) than manic or mixed states (24%) (40). These results suggest that 1) the major benefit of treatment with lithium or lithium combined with divalproex is on the manic aspects of rapid-cycling bipolar disorder and 2) rapid cycling is principally characterized by recurrent depression.
In a randomized, blind, placebo-controlled study of 182 patients with rapid-cycling bipolar I or bipolar II disorder who were receiving maintenance treatment (39), lamotrigine was superior to placebo on overall study survival (p<0.04) but not on the primary measure, which was the time elapsed until the onset of a mood episode that required additional pharmacotherapy. The lamotrigine over placebo advantage was greatest (p=0.01) among the 52 patients with bipolar II disorder: the median time to discontinuation for any reason among patients with bipolar II disorder was 17 weeks for the patients receiving lamotrigine and 7 weeks for those given placebo (the discontinuation times among the entire group were 18 weeks and 12 weeks for the lamotrigine-treated and placebo-treated patients, respectively). Similarly, the rate of study completion without relapse in patients receiving medication monotherapy was significantly greater among the lamotrigine-treated than among the placebo-treated patients with bipolar II disorder (46% versus 18%, p=0.04); this difference was not seen among those with bipolar I disorder (39). An open study comparing response to lamotrigine in patients with rapid-cycling versus non-rapid-cycling bipolar disorder also indicated efficacy, with some evidence that rapid-cycling patients with more severe manic symptoms at the start of treatment respond less well (367).
Divalproex was effective as monotherapy or as an add-on therapy in an open study of 107 rapid-cycling patients followed for a mean of 17 months. Marked benefit occurred among 77% of the patients who entered the study when manic or hypomanic. However, only 38% of those who entered the study depressed reached the maintenance stage (368, 369).
These limited data provide support for the use of lamotrigine in rapid-cycling bipolar disorder—especially for depressive features, which appear to dominate the bipolar II form of rapid cycling—and suggest that combination drug therapy is often superior to use of a single drug.
D. Maintenance treatment
Maintenance treatment of patients with bipolar disorder has multiple goals. In addition to relapse prevention, reduction of subthreshold symptoms, and reduction of suicide risk, aims need to include reduction of cycling frequency and mood instability as well as improvement of functioning. Maintenance medication is generally recommended following a manic episode (370, 371). Although few studies involving patients with bipolar II disorder have been conducted in this area, consideration of maintenance treatment for this form of the illness is also strongly warranted.
Maintenance studies pose two difficulties not central to acute episode studies. The multiple treatment goals make it impractical to select a single goal as an adequate index of efficacy. Also, because of risks associated with full relapse and of suicidal behavior, few placebo-controlled studies have been conducted, and many of those have enrolled somewhat less severely ill patients than seen in the spectrum of clinical practice with bipolar disorder (372).
This section will present efficacy data on lithium, anticonvulsants, antipsychotics, and ECT as maintenance treatment agents. Information on side effects and implementation and dosing issues for lithium and the anticonvulsants are presented in this guideline in their respective sections under “Somatic Treatments of Acute Manic and Mixed Episodes” (section V.A.), with the exception of lamotrigine, the data for which are presented under “Somatic Treatments of Acute Depressive Episodes” (section V.B.2.c.).
1. Lithium
Studies conducted over 25 years ago consistently reported lithium to be more effective than placebo with regard to the proportion of patients who did not relapse (373–377). Most of these studies used discontinuation study designs, in which patients taking stable doses of lithium were abruptly discontinued from lithium if randomly assigned to placebo. It has subsequently become clear that such discontinuation of lithium increases early relapse into mania or depression (378). These studies had additional design limitations, including enrollment of both unipolar and bipolar depressed patients, lack of specification of diagnostic criteria, reporting of results only for patients who completed the study, and failure to report reasons for premature discontinuation. These studies raised expectations for lithium therapy unrealistically.
In large, open, naturalistic studies on the effectiveness of lithium as a maintenance treatment agent in patients with bipolar disorder, good outcomes (e.g., no relapse and only mild symptoms) were seen in approximately one-third of the subjects (226, 364, 379–382). At a 2-year follow-up evaluation, Markar and Mander (379) reported no difference in the rate of hospital readmissions between patients who received lithium and those who did not. Harrow et al. (380) reported equivalent 1-year outcomes for patients receiving lithium and those not taking medication, with 40% of patients taking lithium for the year developing manic episodes. Coryell et al. (381) reported a lower risk of relapse during the first 32 weeks of treatment for patients taking lithium than for those receiving no prophylactic medication, but no difference in relapse risk was seen for weeks 33–96. Other large, open studies that have employed varying methods have reported similar results (226, 364, 383, 384). In general, these studies have also reported high dropout rates.
However, two recent randomized, double-blind, parallel-group studies have indicated evidence of efficacy for lithium compared with placebo in extending time until a new manic episode (385, 386). Each study enrolled patients who were currently experiencing or recently had experienced a manic episode. Symptoms were initially controlled through open treatment with medications (including those to which the subjects would be randomly assigned). Subjects were then randomly assigned either to treatment with lithium, placebo, or divalproex (385) or treatment with lithium, placebo, or lamotrigine (386). The first study measured the time until 25% of subjects undergoing 1 year of maintenance lithium treatment suffered recurrent mania. In this study, lithium extended the time until recurrence by 55% compared with placebo (385). In the second study, an 18-month trial that enrolled patients during or shortly after a manic episode, lithium significantly extended time until intervention for a recurrent manic episode relative to placebo (p=0.006). The relapse rate into mania was 17% for lithium-treated patients, compared with 41% for placebo-treated patients (386). However, lithium did not significantly extend time until a new depressive episode in either study and tended to worsen subthreshold depressive symptoms in the first study (385). These two studies were the first maintenance studies to use modern methods, enroll patients during an index manic episode, and taper lithium taken during the open phase for those patients entering the randomized, placebo-controlled maintenance phase. Earlier randomized, placebo-controlled studies and a crossover study also have reported efficacy for lithium with regard to manic, but not depressive, symptoms (362, 365, 366).
A randomized, open 2.5-year study compared lithium maintenance treatment with that of carbamazepine (387). The primary efficacy measure, time until hospitalization, did not indicate a significant difference between the treatments. However, broader secondary analyses, such as time until relapse or need for concomitant medication, favored lithium (44% versus 67%, p=0.04). Rapid cycling is associated with relatively poor response to lithium (358); however, in a small prospective study, both rapid-cycling and non-rapid-cycling patients had fewer manic episodes with lithium therapy than did those receiving placebo (365). In addition, one small study has suggested that combining lithium and carbamazepine improves the proportion of response among rapid-cycling patients to a rate equivalent to that of non-rapid-cycling patients (362).
Serum-level guidelines are not well established for maintenance treatment with lithium. In clinical settings, doses and serum levels somewhat lower than those employed for treatment of acute mania are generally used (316). One randomized study of high- and low-dose lithium ranges indicated better efficacy for lithium at 0.8–1.0 meq/liter than at 0.4–0.6 meq/liter in the prevention of manic, but not depressed, episodes (225). However, tolerability was much worse at the higher range. An open study similarly reported rates of rehospitalization lower than those before treatment for the subset of patients whose serum levels were consistently above 0.5 meq/liter (364).
2. Divalproex or valproate
Valproate has been studied in one placebo-controlled, double-blind, randomized trial (385) and two randomized comparisons with lithium (254, 388). In the placebo-controlled study, there was no significant difference in the primary efficacy measure (time until development of any mood episode) among patients treated with divalproex, lithium, and placebo, although there was a nonsignificant difference favoring divalproex over lithium (p=0.06). Divalproex was superior to placebo on rate of early termination for any mood episode (24% versus 38%, respectively; p<0.02), early termination for depression (6% versus 16%; p<0.02), and termination due to failure to adhere to protocol, intercurrent illness, or administrative reasons (16% versus 25%; p<0.02). Early termination for intolerance or noncompliance favored divalproex over lithium (22% versus 35%, respectively; p<0.03). The divalproex advantage over placebo was greater in the subset of 149 patients who had received divalproex treatment for their manic episode during the open period, with rates of early termination for any mood episode of 29% and 50%, respectively (p<0.04). One randomized, 18-month open study of valproate (formulated as valpromide) versus lithium reported a 20% lower rate of new episodes among valpromide-treated patients than among lithium-treated patients (388). Relative to patients given lithium, a lower proportion of patients given valpromide had their treatment discontinued because of intolerance or lack of efficacy. Divalproex and lithium were comparably effective in a 1-year, open, naturalistic, longitudinal study that allowed addition of any needed medication (254). Finally, divalproex was effective both as monotherapy and when added to lithium therapy in a large, open maintenance trial of patients with rapid-cycling presentations (368). These findings indicate efficacy and generally good tolerability of divalproex in maintenance treatment, with effectiveness at least comparable to lithium.
As with lithium, dosing guidelines for maintenance treatment are less evidence-based than for acute treatment of mania, and lower levels are sometimes used for maintenance treatment. A 1-year study of divalproex found an association between higher serum levels and increased appetite, reduced platelet counts, and reduced WBC counts (371).
3. Lamotrigine
Lamotrigine has been studied in one large, 18-month, randomized, double-blind, placebo-controlled study of patients who had experienced a manic or hypomanic episode within 60 days of entry into an open treatment phase (386). Patients who improved during the open-treatment phase were randomly assigned to maintenance treatment with lamotrigine, lithium, or placebo. For the primary outcome measure (time until additional pharmacotherapy required for treatment of a mood episode), both lamotrigine and lithium were superior to placebo (p<0.02 and p=0.003, respectively). The median time until one-quarter of the patients in each treatment group developed a mood episode was 72 weeks for those given lamotrigine, 58 weeks for those receiving lithium, and 35 weeks for those given placebo. On a secondary outcome measure (time until discontinuation for any reason), lamotrigine was superior to placebo, but lithium was not (p=0.03 and p=0.07, respectively). Lamotrigine did not significantly prolong the time until a manic episode but was superior to placebo in prolonging the time until a depressive episode (p<0.02), whereas lithium was not (p<0.17). Lamotrigine was also superior to placebo in a 26-week study of rapid-cycling patients with bipolar I or bipolar II disorder (39). The primary efficacy measure, time until additional medication required for treatment of a mood episode, did not differ significantly (p=0.07). However, among patients with bipolar II disorder, the median time until additional pharmacotherapy was required was significantly greater for those receiving lamotrigine than for those given placebo (17 weeks versus 7 weeks, p=0.01). Time until additional pharmacotherapy was required did not differ significantly among patients with bipolar I disorder. Also, the proportion of patients who completed the study without requiring additional medication was greater among those treated with lamotrigine than for those given placebo (41% versus 26%, p=0.03). Among patients requiring additional pharmacotherapy, 80% required medication for depressive symptoms; 20% required medication for manic, hypomanic, or mixed symptoms (39). These results are consistent with those of an open study of patients with bipolar disorder treated with lamotrigine for up to 48 weeks either as monotherapy or as part of combination therapy (329).
4. Carbamazepine
The effectiveness of carbamazepine for maintenance treatment of bipolar disorder is unclear (362). Carbamazepine was inferior to lithium on most outcome measures in one randomized, open, 2.5-year study (387). Carbamazepine was nonsignificantly better than lithium among patients with mood-incongruent illnesses, comorbidity, mixed states, and bipolar II disorder (389). Crossover studies have reported carbamazepine somewhat less effective than lithium in maintenance treatment of bipolar disorder (362, 390). The proportion of time spent in a manic episode dropped from 25% before treatment to 19% in patients treated with carbamazepine and 9% in patients treated with lithium (p<0.01). The proportion of time spent in a depressive episode did not change after initiation of either drug (before treatment: 32%, in patients treated with carbamazepine: 26%, in patients treated with lithium: 31%) (362).
5. Antipsychotic medications
The one placebo-controlled study of prophylactic treatment with an antipsychotic drug did not show an advantage of flupentixol plus lithium compared with lithium alone (391). Open case reports and one randomized, open study of clozapine plus usual care compared with usual care alone have indicated benefits of maintenance clozapine treatment over 1 year (303).
6. ECT
The use of ECT on a maintenance basis to prevent mood episodes in patients with bipolar disorder was initially described over 50 years ago (392, 393). While efficacy of maintenance ECT for bipolar disorder patients has never been assessed in a randomized, controlled trial, multiple case reports and case series have suggested its utility (51, 356, 394–403). A more extensive naturalistic review (404) identified 56 patients, including nine with bipolar disorder, who received maintenance ECT following successful index treatment. Of the patients with bipolar disorder, 78% showed at least some improvement, and 33% were much improved.
Vanelle et al. (405) prospectively followed 22 medication-resistant or medication-intolerant patients for more than 18 months of maintenance ECT treatment. Seven of these individuals were diagnosed with bipolar disorder, and four had shown a rapid-cycling course. When the study period was compared with the 1-year period before ECT initiation, the maintenance ECT group as a whole showed a significant decrease in time spent in the hospital and in the number of episodes of illness that necessitated hospitalization. For the patients with bipolar disorder, as well as in those with major depressive disorder, the mean number of mood episodes significantly decreased during the maintenance ECT course. None of the bipolar disorder patients failed to show a response to maintenance ECT.
Schwarz et al. (406), using a case-control approach, compared depressed patients who responded to an acute course of ECT and then received maintenance ECT to patients who responded to acute ECT but received no maintenance ECT. A third comparison group received only pharmacotherapy. In each group, four of the 21 patients had a diagnosis of bipolar disorder. Although this number was too small to permit subgroup analysis, the rate of rehospitalization decreased by 67% for the study patients as a whole with implementation of maintenance ECT. In depressed patients who had responded to an acute course of ECT, Gagné et al. (407) also used a case-control approach to compare patients who received maintenance pharmacotherapy alone with those who received maintenance ECT in combination with maintenance pharmacotherapy. The two groups differed only in the number of “adequate” pharmacotherapy trials before ECT, with patients receiving maintenance ECT showing greater resistance to pharmacotherapy. Of the 58 depressed patients in the study, 12 had a diagnosis of bipolar disorder. For the group as a whole, patients receiving maintenance ECT had a greater cumulative probability of surviving without relapse or recurrence at 2 years than patients receiving only pharmacotherapy after the index ECT course (93% versus 52%, respectively). At 5 years, the difference in survival between the two groups was even more striking (73% versus 18%, respectively). Proportional hazards regression did not demonstrate statistically significant rate differences between patients with bipolar disorder and those with major depressive disorder.
Thus, in studies of maintenance ECT, study group sizes have been small, and patients with bipolar disorder have made up a small proportion of those groups, making subgroup analyses impossible. Nonetheless, the findings suggest that maintenance ECT may be helpful for individual patients with severe bipolar illness who are unable to tolerate or do not respond to maintenance pharmacotherapy.
E. Psychosocial interventions
Although psychiatric management and pharmacotherapy are essential components of bipolar disorder treatment, specific forms of psychotherapy also are critical components of the treatment plan for many patients. Patients with bipolar disorder suffer from the psychosocial consequences of past episodes, the ongoing vulnerability to future episodes, and the burdens of adhering to a long-term treatment plan that may involve unpleasant side effects. In addition, many patients have clinically significant residual symptoms or mood instability between major episodes. The primary goals of psychotherapeutic treatments are to reduce distress and improve the patient’s functioning between episodes as well as decrease the likelihood and severity of future episodes (408).
Most patients with bipolar disorder struggle with some of the following issues: 1) emotional consequences of episodes of mania and depression; 2) coming to terms with having a potentially chronic mental illness; 3) problems associated with stigmatization; 4) delays or major deviations in development; 5) fears of recurrence and consequent inhibition of more autonomous functioning; 6) interpersonal difficulties, including issues pertaining to marriage, family, childbearing, and parenting; 7) academic and occupational problems; and 8) other legal, social, and emotional problems that arise from reckless, inappropriate, withdrawn, or violent behavior that may occur during episodes. Although a specific psychotherapeutic approach (in addition to psychiatric management) may be needed to address these issues, the form, intensity, and focus of psychotherapy will vary over time for each patient.
There are now a range of specific psychotherapeutic interventions that have been shown to be helpful when used in combination with pharmacotherapy and psychiatric management for treatment of bipolar disorder. The best-studied treatment approaches have been developed around psychoeducational, interpersonal, family, and cognitive behavior therapies. Formal studies have been conducted for these treatments, and additional investigations are underway. Further, psychodynamic and other forms of therapy may be indicated for some patients. The available psychotherapeutic treatments are discussed as separate entities, even though psychiatrists commonly use a combination or synthesis of different approaches depending on both training and the patient’s needs and preferences.
1. Efficacy
Evidence concerning the utility of specific psychosocial interventions for patients with bipolar disorder is slowly building. The research summarized here involves the specific forms of psychotherapy that have been studied in randomized, controlled clinical trials.
Perry et al. (27) evaluated a relatively brief (average: seven sessions) individual psychoeducational intervention that focused on illness management, recognition of risk factors, and prevention of relapses. When compared with a group randomly assigned to a treatment-as-usual condition, patients receiving psychoeducation (in addition to pharmacotherapy) experienced a significant reduction in risk of manic relapses as well as improved social and vocational functioning.
A brief (approximately six sessions) inpatient family intervention (409) has been developed for patients with schizophrenia or bipolar disorder. Goals include accepting the reality of the illness, identifying precipitating stressors and likely future stressors inside and outside the family, elucidating family interactions that produce stress on the patient, planning strategies for managing or minimizing future stressors, and bringing about the patient’s family’s acceptance of the need for continued treatment after hospital discharge. In the initial study (410), the family intervention resulted in improved outcomes for female patients with affective disorders but not for male patients. In a subsequent study by this group (410), ongoing couples therapy (extending for up to 11 months after hospitalization) was found to significantly enhance treatment adherence and improve global functioning. Unfortunately, this study was too small (intent-to-treat N=42) to reliably detect more modest effects, such as a reduction of relapse risk.
When the functional impairments of bipolar disorder are severe and persistent, other services may be necessary, such as case management, assertive community treatment, psychosocial rehabilitation, and supported employment. These approaches, which have traditionally been studied in patients with schizophrenia, also show effectiveness for certain individuals with bipolar disorder.
Family-focused treatment was developed for patients who have recently had an episode of mania or depression (411). Family-focused therapy is behaviorally based and includes psychoeducation, communication skills training, and problem-solving skills training. One adequately sized trial of behavioral family treatment has been completed; the investigators found that behavioral family management (in concert with adequate pharmacotherapy) resulted in a substantial decrease in depressive relapse rates when compared with a treatment-as-usual control condition (412).
A cognitive behavior therapy program for patients with bipolar disorder has been developed by Basco and Rush (413). The goals of the program are to educate the patient regarding bipolar disorder and its treatment, teach cognitive behavior skills for coping with psychosocial stressors and attendant problems, facilitate compliance with treatment, and monitor the occurrence and severity of symptoms. A large study of the impact of cognitive behavior therapy for prophylaxis against bipolar recurrences is underway. Preliminary studies suggest that this approach may help reduce depressive symptoms (414), improve longer-term outcomes (415), and improve treatment adherence (416).
The observation that many patients with bipolar disorder experience less mood lability when they maintain a regular pattern of daily activities (including sleeping, eating, physical activity, and emotional stimulation) has led to the development of a formalized psychotherapy called interpersonal and social rhythm therapy (417). This form of psychotherapy builds upon the traditional focus of interpersonal psychotherapy by incorporating a behavioral self-monitoring program intended to help patients with bipolar disorder initiate and maintain a lifestyle characterized by more regular sleep-wake cycles, meal times, and other so-called social zeitgebers. The ultimate goal is to help regulate circadian disturbances that may provoke or exaggerate episodes of mood disorder.
Frank and colleagues have reported several findings from their ongoing study of interpersonal and social rhythm therapy. First, interpersonal and social rhythm therapy (in combination with pharmacotherapy) was associated with significant increases in targeted lifestyle regularities when compared with a clinical management plus pharmacotherapy control group (418). However, interpersonal and social rhythm therapy was not associated with a faster time to recovery from manic (419) or depressive (420) episodes. The withdrawal of interpersonal and social rhythm therapy after stabilization was associated with a significant increase in relapse rates (421). Across 2 years of maintenance treatment, interpersonal and social rhythm therapy led to a reduction of both depressive symptoms and manic/hypomanic symptoms and an increase in days of euthymia when compared with treatment as usual (unpublished 2001 study of E. Frank and D.J. Kupfer).
Finally, preliminary results of a trial comparing group psychoeducation to standard medical care alone among a group of patients with bipolar disorder suggest that patients receiving psychoeducation had significantly fewer manic episodes, depressive episodes, and hospitalizations (422).
2. Psychotherapeutic treatment of mania
Psychosocial therapies alone are generally not useful treatments for acute mania. Perhaps the only indications for psychotherapy alone are when all established treatments have been refused, involuntary treatment is not appropriate, and the primary focus of therapy is focused and crisis-oriented (e.g., resolving ambivalence about taking medication). In one study of bipolar I disorder patients with acute mania or hypomania, treatment with the combination of interpersonal and social rhythm therapy and pharmacotherapy did not produce an additive effect on manic symptoms or reduce time to remission when compared with an intensive clinical paradigm plus medication (419). Moreover, patients withdrawn from this psychotherapy after completion of acute treatment had a poorer prognosis when compared with those who either received monthly maintenance psychotherapy sessions or recovered with intensive clinical management and pharmacotherapy (421).
3. Psychotherapeutic treatment of depression
Several psychotherapeutic approaches, including cognitive behavior therapy (423) and interpersonal therapy (424–426), have demonstrated efficacy in patients with unipolar depression, either in lieu of or in addition to pharmacotherapy. Efficacy data are discussed in the APA Practice Guideline for the Treatment of Patients With Major Depressive Disorder (2).
For unipolar depression, the application of a specific, effective psychotherapy in lieu of pharmacotherapy may be considered for patients with mild to moderate symptoms. For bipolar depression, the use of focused psychotherapy instead of antidepressant pharmacotherapy has potential appeal, particularly with respect to avoiding antidepressant side effects and minimizing the risk of treatment-emergent mania or induction of rapid cycling. However, only a handful of reports have described such an approach, and there have been no definitive studies to date.
Cole et al. (420) evaluated the impact of a modified form of interpersonal psychotherapy as part of a larger study relating thyroid function to clinical course in 65 patients with bipolar I depression. Patients were randomly assigned to receive weekly interpersonal and social rhythm therapy sessions or treatment as usual. All patients received pharmacotherapy (principally lithium salts); about two-thirds of the patients also received antidepressants. Cole et al. found that the addition of weekly psychotherapy did not enhance depressive symptom reduction or accelerate time to remission in comparison with treatment as usual across up to 6 months of treatment.
Zaretsky et al. (414) treated 11 patients with bipolar depression with individual cognitive behavior therapy (20 weekly sessions) in addition to ongoing pharmacotherapy. They compared their patients’ outcomes to a contemporaneous group of age and sex-matched patients with unipolar depression. Among the eight completers in the bipolar depression group (seven with bipolar I disorder, one with bipolar II disorder), improvements were comparable to those in the unipolar depression group. Further, no depressed patient receiving cognitive behavior therapy developed treatment-emergent mania or hypomania.
4. Maintenance treatment
Since the 1994 publication of the first APA practice guideline for bipolar disorder (5), a number of reports on the value of concomitant psychosocial treatment during the maintenance phase of treatment for bipolar disorder have been published. All studies used “add-on” designs, with patients continuing pharmacotherapies such as lithium and divalproex. Many of these reports described preliminary or pilot studies; nevertheless, results of three larger, more definitive studies have been published for psychoeducation (27), interpersonal and social rhythm therapy (427), and family-focused (412) interventions.
Overall, these studies demonstrated that the addition of a time-limited individual psychosocial intervention appropriately modified for bipolar disorder is likely to improve outcomes across 1–2 years of follow-up. When feasible, group psychoeducational interventions also appear useful (428), which may improve the cost efficiency of treatment. Despite these promising results, however, improvements have not been consistently documented across studies on the full range of syndromal, functional, adherence, and interpersonal domains. On the basis of a methodological review of the more numerous studies of unipolar depression (429), such inconsistencies in findings are more likely to be attributable to differences in patient populations and statistical power than true therapeutic specificity.
Nevertheless, the weight of the evidence suggests that patients with bipolar disorder are likely to gain some additional benefit during the maintenance phase from a concomitant psychosocial intervention, including psychotherapy, that addresses illness management (i.e., adherence, lifestyle changes, and early detection of prodromal symptoms) and interpersonal difficulties. The more commonly practiced supportive and dynamic-eclectic therapies have not been studied in randomized, controlled trials as maintenance treatments for patients with bipolar disorder.
5. Addressing comorbid disorders and psychosocial consequences
Patients in remission from bipolar disorder suffer from the psychosocial consequences of past episodes and ongoing vulnerability to future episodes. In addition, patients with this disorder remain vulnerable to other psychiatric disorders, including, most commonly, substance use disorders (66) and personality disorders (430, 431). Each of these comorbid disorders has particular consequences and increases the overall psychosocial vulnerability of the patient with bipolar disorder. Psychosocial treatments, including psychotherapy, should address issues of comorbidity and complications that are present.
F. Somatic therapies for children and adolescents
To date, there has been only one double-blind, placebo-controlled, randomized study of pharmacotherapy in the treatment of adolescents with bipolar disorder (432). The majority of information available about pharmacological treatments for bipolar disorder in youth relies upon open studies, case series, and case reports.
1. Lithium
There are more data available for lithium than for any other medication in the treatment of bipolar disorder in children and adolescents. Geller et al. (432) conducted the only double-blind, placebo-controlled, parallel-group study of lithium treatment in 25 adolescent outpatients with comorbid bipolar disorder and substance dependence. Subjects were randomly assigned to lithium or placebo for a 6-week trial. There was significantly greater improvement in global functioning with lithium treatment than with placebo. Significantly more patients in the lithium-treatment group experienced thirst, polyuria, nausea, vomiting, and dizziness.
In four double-blind, placebo-controlled, crossover studies of children with bipolar disorder, significant improvement in mood lability, explosive outbursts, aggressive behavior, and psychosis was found with lithium compared with placebo (433–436). However, small study group sizes, diagnostic issues, and short treatment durations limit the interpretation of these findings. There have also been open studies, case series, and case reports with clinical responses ranging from 50% to 100% (437–455).
2. Valproate/divalproex
There have been no placebo-controlled studies of divalproex in the treatment of bipolar disorder in children and adolescents, but divalproex response rates in four open studies ranged from 60% to 83% (127, 456–458).
In the only multisite open study of divalproex treatment for children and adolescents with bipolar disorder (458), 40 subjects ages 7–17 years received divalproex for 2–8 weeks. Sixty-one percent of the subjects showed a Δ≥50% improvement from baseline scores on the Young Mania Rating Scale. Twenty-three patients (58%) discontinued the study, of whom 16 had a comorbid psychiatric diagnosis such as ADHD, conduct disorder, or oppositional defiant disorder. The most commonly occurring side effects (>10% incidence) were headache, nausea, vomiting, diarrhea, and somnolence. No significant laboratory abnormalities were noted.
There have also been four case reports or series of divalproex sodium treatment of bipolar disorder in youth. Response rates have ranged from 66% to 100% in these reports (459–462).
Divalproex also showed efficacy in an active-comparator study in which 42 children and adolescents (ages 8–18 years) with bipolar disorder were randomly assigned to 6 weeks of open treatment with lithium, divalproex, or carbamazepine (463). No significant differences in response rates (>50% change from baseline to last Young Mania Rating Scale score) were found among the patients receiving divalproex (53%), lithium (38%), or carbamazepine (38%). There were no serious adverse events reported with any of these medications.
In the continuation phase of this study, 35 patients received open treatment for an additional 16–18 weeks (463). Response during the continuation phase was defined as a score of 1 or 2 on the Bipolar Clinical Global Improvement Scale. Thirty patients (85%) were classified as having responded at the end of the continuation phase. Only 13 patients (37%) were receiving a single study drug (lithium, divalproex, or carbamazepine) and no other psychotropic medication at the end of the continuation phase. For the 22 patients who required additional psychotropic medication, 11 received a second study drug (lithium, divalproex, or carbamazepine), and 11 received a stimulant.
3. Carbamazepine
Information about the use of carbamazepine in the treatment of adolescent bipolar disorder is limited to case reports. Woolston (464) described three cases of carbamazepine monotherapy for adolescents with bipolar disorder in whom clinical improvement of manic symptoms was demonstrated. A positive response was reported with the combination of carbamazepine and lithium in seven adolescents with bipolar disorder (192, 449).
4. Atypical antipsychotics
There are two case series and one open trial of olanzapine as primary or adjunctive treatment for children and adolescents with bipolar disorder. In an open study, 23 children ages 5–14 years with bipolar disorder received olanzapine 2.5–20 mg/day for 8 weeks (465). Response was defined as Δ≥30% improvement in score on the Young Mania Rating Scale, and the response rate was 61%. There were no significant side effects reported except weight gain (mean=5 kg). In case reports of three youths (ages 9–19 years) with bipolar disorder, olanzapine was used as an adjunctive treatment in addition to existing medication regimens (466). Within a week, CGI scores were rated markedly improved. Sedation and weight gain were the common side effects. Finally, in a report of seven cases of adolescents with bipolar disorder (467), olanzapine was used as adjunctive treatment to existing psychotropic medication regimens. Seventy-one percent of adolescents showed marked to moderate response on CGI scores with adjunctive olanzapine treatment.
A retrospective chart review of 28 outpatient children and adolescents ages 4–17 years with bipolar disorder assessed adjunctive risperidone treatment (468). These subjects received risperidone over an average of 6 months. Improvement (CGI score ≤2) in manic and aggressive symptoms was seen in 82% of the patients, and 69% exhibited improvement in psychotic symptoms. No serious adverse effects were reported, although common side effects were weight gain and sedation.
5. Newer antiepileptics
There are few reports of the use of the newer antiepileptic agents in the treatment of children and adolescents with bipolar disorder. In a retrospective study of 18 adolescents for whom prior medication trials had failed (469), subjects with bipolar disorder not otherwise specified (N= 15), bipolar II disorder (N=1), or schizoaffective disorder (N=2) received gabapentin at doses between 900 and 2400 mg/day. Sixteen of the adolescents who continued gabapentin treatment had cessation of cycling. Of these patients, six reported improved mood. Gabapentin was also reported to be effective in the treatment of an adolescent patient with mania (470).
6. ECT
ECT has been used to treat refractory mania in two prepubertal children (471). A review of literature on ECT use in young people (472) reported its efficacy for mania in adolescents.
The following coding system is used to indicate the nature of the supporting evidence in the references:
[A] Randomized clinical trial. A study of an intervention in which subjects are prospectively followed over time; there are treatment and control groups; subjects are randomly assigned to the two groups; both the subjects and the investigators are blind to the assignments.
[B] Clinical trial. A prospective study in which an intervention is made and the results of that intervention are tracked longitudinally; study does not meet standards for a randomized clinical trial.
[C] Cohort or longitudinal study. A study in which subjects are prospectively followed over time without any specific intervention.
[D] Control study. A study in which a group of patients and a group of control subjects are identified in the present and information about them is pursued retrospectively or backward in time.
[E] Review with secondary data analysis. A structured analytic review of existing data, e.g., a meta-analysis or a decision analysis.
[F] Review. A qualitative review and discussion of previously published literature without a quantitative synthesis of the data.
[G] Other. Textbooks, expert opinion, case reports, and other reports not included above.
 |
Table 1. Characteristics to Evaluate in an Assessment of Suicide Risk in Patients With Bipolar Disordera
 |
Table 2. Diagnostic Criteria for a Manic Episodea
 |
Table 3. Diagnostic Criteria for a Major Depressive Episodea
 |
Table 4. Diagnostic Criteria for a Hypomanic Episodea
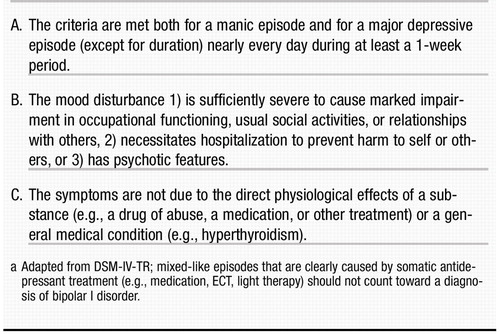 |
Table 5. Diagnostic Criteria for a Mixed Episodea
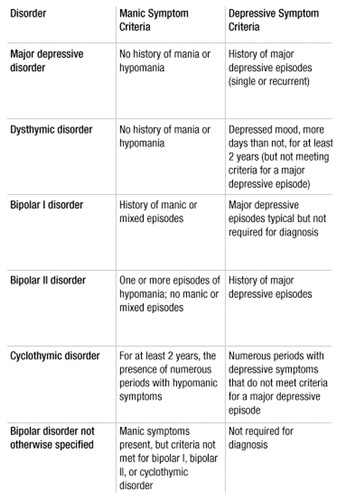 |
Table 6. Summary of Manic and Depressive Symptom Criteria in DSM-IV-TR Mood Disorders
1 American Psychiatric Association: Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (text revision). Washington, DC, APA, 2000 [G]Google Scholar
2 American Psychiatric Association: Practice Guideline for the Treatment of Patients With Major Depressive Disorder (Revision). Am J Psychiatry 2000; 157 (April suppl) [G]Google Scholar
3 American Academy of Child and Adolescent Psychiatry: AACAP official action: practice parameters for the assessment and treatment of children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 1997; 36:138–157 [G]Crossref, Google Scholar
4 Frank E, Prien RF, Jarrett RB, Keller MB, Kupfer DJ, Lavori PW, Rush AJ, Weissman MM: Conceptualization and rationale for consensus definitions of terms in major depressive disorder: remission, recovery, relapse, and recurrence. Arch Gen Psychiatry 1991; 48:851–855 [F]Crossref, Google Scholar
5 American Psychiatric Association: Practice Guideline for the Treatment of Patients With Bipolar Disorder. Am J Psychiatry 1994; 151(Dec suppl) [G]Google Scholar
6 Hirschfeld RMA, Williams JBW, Spitzer RL, Calabrese JR, Flynn L, Keck PE Jr, Lewis L, McElroy SL, Post RM, Rapport DJ, Russell JM, Sachs GS, Zajecka J: Development and validation of a screening instrument for bipolar spectrum disorder: the Mood Disorder Questionnaire. Am J Psychiatry 2000; 157:1873–1875 [G]Crossref, Google Scholar
7 American Psychiatric Association: Practice Guideline for Psychiatric Evaluation of Adults. Am J Psychiatry 1995; 152(Nov suppl) [G]Google Scholar
8 Isometsa ET, Henriksson MM, Aro HM, Lonnqvist JK: Suicide in bipolar disorder in Finland. Am J Psychiatry 1994; 151:1020–1024 [D]Crossref, Google Scholar
9 Dilsaver SC, Chen YW, Swann AC, Shoaib AM, Krajewski KJ: Suicidality in patients with pure and depressive mania. Am J Psychiatry 1994; 151:1312–1315 [D]Crossref, Google Scholar
10 Strakowski SM, McElroy SL, Keck PE Jr, West SA: Suicidality among patients with mixed and manic bipolar disorder. Am J Psychiatry 1996; 153:674–676 [C]Crossref, Google Scholar
11 Muller-Oerlinghausen B, Wolf T, Ahrens B, Glaenz T, Schou M, Grof E, Grof P, Lenz G, Simhandl C, Thau K, Vestergaard P, Wolf R: Mortality of patients who dropped out from regular lithium prophylaxis: a collaborative study by the International Group for the Study of Lithium-Treated Patients (IGSLI). Acta Psychiatr Scand 1996; 94:344–347 [C]Crossref, Google Scholar
12 Baldessarini RJ, Tondo L, Hennen J: Effects of lithium treatment and its discontinuation on suicidal behavior in bipolar manic-depressive disorders. J Clin Psychiatry 1999; 60(suppl 2):77–84 [E]Crossref, Google Scholar
13 Angst J, Preisig M: Outcome of a clinical cohort of unipolar, bipolar and schizoaffective patients: results of a prospective study from 1959 to 1985. Schweiz Arch Neurol Psychiatr 1995; 146:17–23 [C]Google Scholar
14 Rosenfarb IS, Miklowitz DJ, Goldstein MJ, Harmon L, Nuechterlein KH, Rea MM: Family transactions and relapse in bipolar disorder. Fam Process 2001; 40:5–14 [C]Crossref, Google Scholar
15 Mino Y, Shimodera S, Inoue S, Fujita H, Tanaka S, Kanazawa S: Expressed emotion of families and the course of mood disorders: a cohort study in Japan. J Affect Disord 2001; 63:43–49 [C]Crossref, Google Scholar
16 Tompson MC, Rea MM, Goldstein MJ, Miklowitz DJ, Weisman AG: Difficulty in implementing a family intervention for bipolar disorder: the predictive role of patient and family attributes. Fam Process 2000; 39:105–120 [G]Crossref, Google Scholar
17 Simoneau TL, Miklowitz DJ, Saleem R: Expressed emotion and interactional patterns in the families of bipolar patients. J Abnorm Psychol 1998; 107:497–507 [G]Crossref, Google Scholar
18 Jamison KR, Gerner RH, Goodwin FK: Patient and physician attitudes toward lithium: relationship to compliance. Arch Gen Psychiatry 1979; 36:866–869 [G]Crossref, Google Scholar
19 Gutheil TG: The psychology of psychopharmacology. Bull Menninger Clin 1982; 46:321–330 [G]Google Scholar
20 Jamison KR: Manic-depressive illness: the overlooked need for psychotherapy, in Integrating Pharmacotherapy and Psychotherapy. Washington, DC, American Psychiatric Press, 1991 [F]Google Scholar
21 Jamison KR, Akiskal HS: Medication compliance in patients with bipolar disorder. Psychiatr Clin North Am 1983; 6:175–192 [F]Crossref, Google Scholar
22 Pardes H, Kaufmann CA, Pincus HA, West A: Genetics and psychiatry: past discoveries, current dilemmas, and future directions. Am J Psychiatry 1989; 146:435–443 [G]Crossref, Google Scholar
23 Johnson SL, Roberts JE: Life events and bipolar disorder: implications from biological theories. Psychol Bull 1995; 117:434–449 [F]Crossref, Google Scholar
24 Hammen C, Gitlin M: Stress reactivity in bipolar patients and its relation to prior history of disorder. Am J Psychiatry 1997; 154:856–857 [D]Crossref, Google Scholar
25 Malkoff-Schwartz S, Frank E, Anderson B, Sherrill JT, Siegel L, Patterson D, Kupfer DJ: Stressful life events and social rhythm disruption in the onset of manic and depressive bipolar episodes: a preliminary investigation. Arch Gen Psychiatry 1998; 55:702–707 [D]Crossref, Google Scholar
26 Leibenluft E, Suppes T: Treating bipolar illness: focus on treatment algorithms and management of the sleep-wake cycle (case conf). Am J Psychiatry 1999; 156:1976–1986 [G]Google Scholar
27 Perry A, Tarrier N, Morriss R, McCarthy E, Limb K: Randomised controlled trial of efficacy of teaching patients with bipolar disorder to identify early symptoms of relapse and obtain treatment. Br Med J 1999; 318:149–153 [A]Crossref, Google Scholar
28 Butzlaff RL, Hooley JM: Expressed emotion and psychiatric relapse: a meta-analysis. Arch Gen Psychiatry 1998; 55:547–552 [E]Crossref, Google Scholar
29 Miklowitz DJ, Goldstein MJ, Nuechterlein KH, Snyder KS, Mintz J: Family factors and the course of bipolar affective disorder. Arch Gen Psychiatry 1988; 45:225–231 [C]Crossref, Google Scholar
30 Post RM, Roy-Byrne PP, Uhde TW: Graphic representation of the life course of illness in patients with affective disorder. Am J Psychiatry 1988; 145:844–848 [G]Crossref, Google Scholar
31 Kraepelin E: Manic-Depressive Insanity and Paranoia (1921). Translated by Barclay RM. Salem, NH, Ayer, 1976 [G]Google Scholar
32 Meyer A: The Collected Papers of Adolph Meyer. Baltimore, Johns Hopkins University Press, 1950 [G]Google Scholar
33 Tohen M, Hennen J, Zarate CM Jr, Baldessarini RJ, Strakowski SM, Stoll AL, Faedda GL, Suppes T, Gebre-Medhin P, Cohen BM: Two-year syndromal and functional recovery in 219 cases of first-episode major affective disorder with psychotic features. Am J Psychiatry 2000; 157:220–228 [D]Crossref, Google Scholar
34 Suppes T, Leverich GS, Keck PE Jr: The Stanley Foundation Bipolar Network: demographics and illness characteristics of the first 261 patients with bipolar disorder. J Affect Disord (in press) [C]Google Scholar
35 Keck PE Jr, McElroy SL, Strakowski SM, West SA, Sax KW, Hawkins JM, Bourne ML, Haggard P: 12-month outcome of patients with bipolar disorder following hospitalization for a manic or mixed episode. Am J Psychiatry 1998; 155:646–652 [C]Crossref, Google Scholar
36 Rossi A, Arduini L, Daneluzzo E, Bustini M, Prosperini P, Stratta P: Cognitive function in euthymic bipolar patients, stabilized schizophrenic patients, and healthy controls. J Psychiatr Res 2000; 34:333–339 [G]Crossref, Google Scholar
37 Bearden CE, Hoffman KM, Cannon TD: The neuropsychology and neuroanatomy of bipolar affective disorder: a critical review. Bipolar Disord 2001; 3:106–150 [F]Crossref, Google Scholar
38 Howland RH: Induction of mania with serotonin reuptake inhibitors. J Clin Psychopharmacol 1996; 16:425–427 [B]Crossref, Google Scholar
39 Calabrese JR, Suppes T, Bowden CL, Sachs GS, Swann AC, McElroy SL, Kusumakar V, Ascher JA, Earl NL, Greene PL, Monaghan ET (Lamictal 614 Study Group): A double-blind, placebo-controlled, prophylaxis study of lamotrigine in rapid-cycling bipolar disorder. J Clin Psychiatry 2000; 61:841–850 [A]Crossref, Google Scholar
40 Calabrese JR, Shelton MD, Bowden CL, Rapport DJ, Suppes T, Shirley ER, Kimmel SE, Caban SJ: Bipolar rapid cycling: focus on depression as its hallmark. J Clin Psychiatry 2001; 62(suppl 14): 34–41 [F]Crossref, Google Scholar
41 Goodwin FK, Jamison KR: Manic-Depressive Illness. New York, Oxford University Press, 1990 [G]Google Scholar
42 Carlson GA, Bromet EJ, Sievers S: Phenomenology and outcome of subjects with early- and adult-onset psychotic mania. Am J Psychiatry 2000; 157:213–219 [C]Crossref, Google Scholar
43 Tohen M, Tsuang MT, Goodwin DC: Prediction of outcome in mania by mood-congruent or mood-incongruent psychotic features. Am J Psychiatry 1992; 149:1580–1584 [C]Crossref, Google Scholar
44 Fennig S, Bromet EJ, Karant MT, Ram R, Jandorf L: Mood-congruent versus mood-incongruent psychotic symptoms in first-admission patients with affective disorder. J Affect Disord 1996; 37:23–29 [D]Crossref, Google Scholar
45 McElroy SL, Keck PE Jr, Strakowski SM: Mania, psychosis, and antipsychotics. J Clin Psychiatry 1996; 57(suppl 3):14–26 [F]Google Scholar
46 Braunig P, Kruger S, Shugar G: Prevalence and clinical significance of catatonic symptoms in mania. Compr Psychiatry 1998; 39:35–46 [D]Crossref, Google Scholar
47 Taylor MA, Abrams R: Catatonia: prevalence and importance in the manic phase of manic-depressive illness. Arch Gen Psychiatry 1977; 34:1223–1225 [C]Crossref, Google Scholar
48 Hawkins JM, Archer KJ, Strakowski SM, Keck PE: Somatic treatment of catatonia. Int J Psychiatry Med 1995; 25:345–369 [F]Crossref, Google Scholar
49 Rosebush PI, Hildebrand AM, Furlong BG, Mazurek MF: Catatonic syndrome in a general psychiatric inpatient population: frequency, clinical presentation, and response to lorazepam. J Clin Psychiatry 1990; 51:357–362 [B]Google Scholar
50 Northoff G, Wenke J, Demisch L, Eckert J, Gille B, Pflug B: Catatonia: short-term response to lorazepam and dopaminergic metabolism. Psychopharmacology (Berl) 1995; 122:182–186 [B]Crossref, Google Scholar
51 Bush G, Fink M, Petrides G, Dowling F, Francis A: Catatonia, II: treatment with lorazepam and electroconvulsive therapy. Acta Psychiatr Scand 1996; 93:137–143 [B]Crossref, Google Scholar
52 Lee JW: Serum iron in catatonia and neuroleptic malignant syndrome. Biol Psychiatry 1998; 44:499–507 [B]Crossref, Google Scholar
53 Cooper TB, Bergner PE, Simpson GM: The 24-hour serum lithium level as a prognosticator of dosage requirements. Am J Psychiatry 1973; 130:601–603 [C]Crossref, Google Scholar
54 Simpson SG, Jamison KR: The risk of suicide in patients with bipolar disorders. J Clin Psychiatry 1999; 60(suppl 2):53–56 [F]Crossref, Google Scholar
55 Lester D: Suicidal behavior in bipolar and unipolar affective disorders: a meta-analysis. J Affect Disord 1993; 27:117–121 [E]Crossref, Google Scholar
56 Tondo L, Baldessarini RJ: Reduced suicide risk during lithium maintenance treatment. J Clin Psychiatry 2000; 61(suppl 9):97–104 [F]Google Scholar
57 Norton B, Whalley LJ: Mortality of a lithium-treated population. Br J Psychiatry 1984; 145:277–282 [F]Crossref, Google Scholar
58 Vieta E, Colom F, Martinez-Aran A, Benabarre A, Gasto C: Personality disorders in bipolar II patients. J Nerv Ment Dis 1999; 187:245–248 [G]Crossref, Google Scholar
59 Fawcett J: Treating impulsivity and anxiety in the suicidal patient. Ann NY Acad Sci 2001; 932:94–102 [F]Crossref, Google Scholar
60 Tondo L, Jamison KR, Baldessarini RJ: Effect of lithium maintenance on suicidal behavior in major mood disorders. Ann NY Acad Sci 1997; 836:339–351 [E]Crossref, Google Scholar
61 Ahrens B, Muller-Oerlinghausen B, Schou M, Wolf T, Alda M, Grof E, Grof P, Lenz G, Simhandl C, Thau K: Excess cardiovascular and suicide mortality of affective disorders may be reduced by lithium prophylaxis. J Affect Disord 1995; 33:67–75 [D]Crossref, Google Scholar
62 Brennan PA, Mednick SA, Hodgins S: Major mental disorders and criminal violence in a Danish birth cohort. Arch Gen Psychiatry 2000; 57:494–500 [D]Crossref, Google Scholar
63 Barlow K, Grenyer B, Ilkiw-Lavalle O: Prevalence and precipitants of aggression in psychiatric inpatient units. Aust NZ J Psychiatry 2000; 34:967–974 [C]Crossref, Google Scholar
64 Asnis GM, Kaplan ML, Hundorfean G, Saeed W: Violence and homicidal behaviors in psychiatric disorders. Psychiatr Clin North Am 1997; 20:405–425 [F]Crossref, Google Scholar
65 Hendrick V, Altshuler LL, Gitlin MJ, Delrahim S, Hammen C: Gender and bipolar illness. J Clin Psychiatry 2000; 61:393–396 [D]Crossref, Google Scholar
66 Regier DA, Farmer ME, Rae DS, Locke BZ, Keith SJ, Judd LL, Goodwin FK: Comorbidity of mental disorders with alcohol and other drug abuse: results from the Epidemiologic Catchment Area (ECA) study. JAMA 1990; 264:2511–2518 [D]Crossref, Google Scholar
67 Regier DA, Boyd JH, Burke JD Jr, Rae DS, Myers JK, Kramer M, Robins LN, George LK, Karno M, Locke BZ: One-month prevalence of mental disorders in the United States: based on five Epidemiologic Catchment Area sites. Arch Gen Psychiatry 1988; 45:977–986 [D]Crossref, Google Scholar
68 Tohen M, Waternaux CM, Tsuang MT, Hunt AT: Four-year follow-up of twenty-four first-episode manic patients. J Affect Disord 1990; 19:79–86 [C]Crossref, Google Scholar
69 Tohen M, Waternaux CM, Tsuang MT: Outcome in mania: a 4-year prospective follow-up of 75 patients utilizing survival analysis. Arch Gen Psychiatry 1990; 47:1106–1111 [C]Crossref, Google Scholar
70 Potash JB, Kane HS, Chiu Y-F, Simpson SG, MacKinnon DF, McInnis MG, McMahon FJ, DePaulo JR Jr: Attempted suicide and alcoholism in bipolar disorder: clinical and familial relationships. Am J Psychiatry 2000; 157:2048–2050 [D]Crossref, Google Scholar
71 Goldberg JF, Garno JL, Leon AC, Kocsis JH, Portera L: A history of substance abuse complicates remission from acute mania in bipolar disorder. J Clin Psychiatry 1999; 60:733–740 [D]Crossref, Google Scholar
72 Sonne SC, Brady KT: Substance abuse and bipolar comorbidity. Psychiatr Clin North Am 1999; 22:609–627 [F]Crossref, Google Scholar
73 Tondo L, Baldessarini RJ, Hennen J, Minnai GP, Salis P, Scamonatti L, Masia M, Ghiani C, Mannu P: Suicide attempts in major affective disorder patients with comorbid substance use disorders. J Clin Psychiatry 1999; 60(suppl 2):63–69 [C]Google Scholar
74 Hagan H, Des J: HIV and HCV infection among injecting drug users. Mt Sinai J Med 2000; 67:423–428 [F]Google Scholar
75 American Psychiatric Association: Practice Guideline for the Treatment of Patients With Substance Use Disorders: Alcohol, Cocaine, Opioids. Am J Psychiatry 1995; 152(Nov suppl) [G]Google Scholar
76 Dunayevich E, Sax KW, Keck PE Jr, McElroy SL, Sorter MT, McConville BJ, Strakowski SM: Twelve-month outcome in bipolar patients with and without personality disorders. J Clin Psychiatry 2000; 61:134–139 [C]Crossref, Google Scholar
77 Colom F, Vieta E, Martinez-Aran A, Reinares M, Benabarre A, Gasto C: Clinical factors associated with treatment noncompliance in euthymic bipolar patients. J Clin Psychiatry 2000; 61:549–555 [D]Crossref, Google Scholar
78 Feske U, Frank E, Mallinger AG, Houck PR, Fagiolini A, Shear MK, Grochocinski VJ, Kupfer DJ: Anxiety as a correlate of response to the acute treatment of bipolar I disorder. Am J Psychiatry 2000; 157:956–962 [D]Crossref, Google Scholar
79 Sachs GS, Baldassano CF, Truman CJ, Guille C: Comorbidity of attention deficit hyperactivity disorder with early- and late-onset bipolar disorder. Am J Psychiatry 2000; 157:466–468 [G]Crossref, Google Scholar
80 Johnston AM, Eagles JM: Lithium-associated clinical hypothyroidism: prevalence and risk factors. Br J Psychiatry 1999; 175:336–339 [C]Crossref, Google Scholar
81 Leibenluft E: Women with bipolar illness: clinical and research issues. Am J Psychiatry 1996; 153:163–173 [F]Crossref, Google Scholar
82 Tondo L, Baldessarini RJ, Hennen J, Floris G, Silvetti F, Tohen M: Lithium treatment and risk of suicidal behavior in bipolar disorder patients. J Clin Psychiatry 1998; 59:405–414 [B]Crossref, Google Scholar
83 Dickson RA, Seeman MV, Corenblum B: Hormonal side effects in women: typical versus atypical antipsychotic treatment. J Clin Psychiatry 2000; 61(suppl 3):10–15 [F]Google Scholar
84 Goodnick PJ, Chaudry T, Artadi J, Arcey S: Women’s issues in mood disorders. Expert Opin Pharmacother 2000; 1:903–916 [F]Crossref, Google Scholar
85 Viguera AC, Cohen LS: The course and management of bipolar disorder during pregnancy. Psychopharmacol Bull 1998; 34:339–346 [F]Google Scholar
86 Altshuler LL, Hendrick V, Cohen LS: Course of mood and anxiety disorders during pregnancy and the postpartum period. J Clin Psychiatry 1998; 59(suppl 2):29–33 [F]Google Scholar
87 Rosenfeld WE, Doose DR, Walker SA, Nayak RK: Effect of topiramate on the pharmacokinetics of an oral contraceptive containing norethindrone and ethinyl estradiol in patients with epilepsy. Epilepsia 1997; 38:317–323 [B]Crossref, Google Scholar
88 Wilbur K, Ensom MH: Pharmacokinetic drug interactions between oral contraceptives and second-generation anticonvulsants. Clin Pharmacokinet 2000; 38:355–365 [F]Crossref, Google Scholar
89 Spina E, Pisani F, Perucca E: Clinically significant pharmacokinetic drug interactions with carbamazepine: an update. Clin Pharmacokinet 1996; 31:198–214 [F]Crossref, Google Scholar
90 Potash JB, DePaulo JR Jr: Searching high and low: a review of the genetics of bipolar disorder. Bipolar Disord 2000; 2:8–26 [F]Crossref, Google Scholar
91 Berrettini WH: Genetics of psychiatric disease. Annu Rev Med 2000; 51:465–479 [F]Crossref, Google Scholar
92 Duffy A, Grof P, Robertson C, Alda M: The implications of genetics studies of major mood disorders for clinical practice. J Clin Psychiatry 2000; 61:630–637 [F]Crossref, Google Scholar
93 Wisner KL, Zarin D, Holmboe E, Appelbaum P, Gelenberg AJ, Leonard HL, Frank E: Risk-benefit decision making for treatment of depression during pregnancy. Am J Psychiatry 2000; 157:1933–1940 [F]Crossref, Google Scholar
94 Viguera AC, Nonacs R, Cohen LS, Tondo L, Murray A, Baldessarini RJ: Risk of recurrence of bipolar disorder in pregnant and nonpregnant women after discontinuing lithium maintenance. Am J Psychiatry 2000; 157:179–184 [D]Crossref, Google Scholar
95 Viguera AC, Tondo L, Baldessarini RJ: Sex differences in response to lithium treatment. Am J Psychiatry 2000; 157:1509–1511 [D]Crossref, Google Scholar
96 Hoffman S, Hatch MC: Depressive symptomatology during pregnancy: evidence for an association with decreased fetal growth in pregnancies of lower social class women. Health Psychol 2000; 19:535–543 [C]Crossref, Google Scholar
97 Paarlberg KM, Vingerhoets AJ, Passchier J, Dekker GA, Heinen AG, van Geijn HP: Psychosocial predictors of low birthweight: a prospective study. Br J Obstet Gynaecol 1999; 106:834–841 [C]Crossref, Google Scholar
98 Spielvogel A, Wile J: Treatment and outcomes of psychotic patients during pregnancy and childbirth. Birth 1992; 19:131–137 [D]Crossref, Google Scholar
99 Coverdale JH, Chervenak FA, McCullough LB, Bayer T: Ethically justified clinically comprehensive guidelines for the management of the depressed pregnant patient. Am J Obstet Gynecol 1996; 174:169–173 [F]Crossref, Google Scholar
100 Stocky A, Lynch J: Acute psychiatric disturbance in pregnancy and the puerperium. Baillieres Best Pract Res Clin Obstet Gynaecol 2000; 14:73–87 [F]Crossref, Google Scholar
101 Cohen LS, Rosenbaum JF: Psychotropic drug use during pregnancy: weighing the risks. J Clin Psychiatry 1998; 59(suppl 2):18–28 [F]Google Scholar
102 Cohen LS, Friedman JM, Jefferson JW, Johnson EM, Weiner ML: A reevaluation of risk of in utero exposure to lithium. JAMA 1994; 271:146–150 [F]Crossref, Google Scholar
103 Holmes LB, Harvey EA, Coull BA, Huntington KB, Khoshbin S, Hayes AM, Ryan LM: The teratogenicity of anticonvulsant drugs. N Engl J Med 2001; 344:1132–1138 [C]Crossref, Google Scholar
104 Arpino C, Brescianini S, Robert E, Castilla EE, Cocchi G, Cornel MC, de Vigan C, Lancaster PA, Merlob P, Sumiyoshi Y, Zampino G, Renzi C, Rosano A, Mastroiacovo P: Teratogenic effects of antiepileptic drugs: use of an International Database on Malformations and Drug Exposure (MADRE). Epilepsia 2000; 41:1436–1443 [C]Crossref, Google Scholar
105 American Academy of Pediatrics Committee on Drugs: Use of psychoactive medication during pregnancy and possible effects on the fetus and newborn. Pediatrics 2000; 105:880–887 [F]Crossref, Google Scholar
106 Wisner KL, Gelenberg AJ, Leonard H, Zarin D, Frank E: Pharmacologic treatment of depression during pregnancy. JAMA 1999; 282:1264–1269 [F]Crossref, Google Scholar
107 Ericson A, Kallen B, Wiholm B: Delivery outcome after the use of antidepressants in early pregnancy. Eur J Clin Pharmacol 1999; 55:503–508 [C]Crossref, Google Scholar
108 McElhatton PR: The effects of benzodiazepine use during pregnancy and lactation. Reprod Toxicol 1994; 8:461–475 [F]Crossref, Google Scholar
109 Dolovich LR, Addis A, Vaillancourt JM, Power JD, Koren G, Einarson TR: Benzodiazepine use in pregnancy and major malformations or oral cleft: meta-analysis of cohort and casecontrol studies. Br Med J 1998; 317:839–843 [E]Crossref, Google Scholar
110 American Psychiatric Association: The Practice of Electroconvulsive Therapy: Recommendations for Treatment, Training, and Privileging: A Task Force Report of the American Psychiatric Association, 2nd ed. Washington, DC, American Psychiatric Press, 2001 [G]Google Scholar
111 Shnider SM, Levinson G: Anesthesia for Obstetrics, 3rd ed. Baltimore, Williams & Wilkins, 1993 [G]Google Scholar
112 Llewellyn A, Stowe ZN, Strader JR Jr: The use of lithium and management of women with bipolar disorder during pregnancy and lactation. J Clin Psychiatry 1998; 59(suppl 6):57–64 [F]Google Scholar
113 Cohen LS, Sichel DA, Robertson LM, Heckscher E, Rosenbaum JF: Postpartum prophylaxis for women with bipolar disorder. Am J Psychiatry 1995; 152:1641–1645 [D]Crossref, Google Scholar
114 Yoshida K, Smith B, Kumar R: Psychotropic drugs in mothers’ milk: a comprehensive review of assay methods, pharmacokinetics and of safety of breast-feeding. J Psychopharmacol 1999; 13:64–80 [F]Crossref, Google Scholar
115 Chaudron LH, Jefferson JW: Mood stabilizers during breastfeeding: a review. J Clin Psychiatry 2000; 61:79–90 [F]Crossref, Google Scholar
116 Burt VK, Suri R, Altshuler L, Stowe Z, Hendrick VC, Muntean E: The use of psychotropic medications during breast-feeding. Am J Psychiatry 2001; 158:1001–1009 [F]Crossref, Google Scholar
117 Tomson T, Ohman I, Vitols S: Lamotrigine in pregnancy and lactation: a case report. Epilepsia 1997; 38:1039–1041 [G]Crossref, Google Scholar
118 American Academy of Pediatrics Committee on Drugs: Transfer of drugs and other chemicals into human milk. Pediatrics 2001; 108:776–789 [F]Crossref, Google Scholar
119 Strakowski SM, McElroy SL, Keck PE Jr, West SA: Racial influence on diagnosis in psychotic mania. J Affect Disord 1996; 39:157–162 [D]Crossref, Google Scholar
120 Strakowski SM, Flaum M, Amador X, Bracha HS, Pandurangi AK, Robinson D, Tohen M: Racial differences in the diagnosis of psychosis. Schizophr Res 1996; 21:117–124 [D]Crossref, Google Scholar
121 Strakowski SM, Hawkins JM, Keck PE Jr, McElroy SL, West SA, Bourne ML, Sax KW, Tugrul KC: The effects of race and information variance on disagreement between psychiatric emergency service and research diagnoses in first-episode psychosis. J Clin Psychiatry 1997; 58:457–463 [D]Crossref, Google Scholar
122 Lin KM, Anderson D, Poland RE: Ethnicity and psychopharmacology: bridging the gap. Psychiatr Clin North Am 1995; 18:635–647 [F]Crossref, Google Scholar
123 Bertilsson L: Geographical/interracial differences in polymorphic drug oxidation: current state of knowledge of cytochromes P450 (CYP) 2D6 and 2C19. Clin Pharmacokinet 1995; 29:192–209 [F]Crossref, Google Scholar
124 Lewinsohn PM, Klein DN, Seeley JR: Bipolar disorders in a community sample of older adolescents: prevalence, phenomenology, comorbidity, and course. J Am Acad Child Adolesc Psychiatry 1995; 34:454–463 [C]Crossref, Google Scholar
125 Geller B, Zimerman B, Williams M, Bolhofner K, Craney JL, DelBello MP, Soutullo CA: Diagnostic characteristics of 93 cases of a prepubertal and early adolescent bipolar disorder phenotype by gender, puberty and comorbid attention deficit hyperactivity disorder. J Child Adolesc Psychopharmacol 2000; 10:157–164 [C]Crossref, Google Scholar
126 Kovacs M, Pollock M: Bipolar disorder and comorbid conduct disorder in childhood and adolescence. J Am Acad Child Adolesc Psychiatry 1995; 34:715–723 [C]Crossref, Google Scholar
127 West SA, McElroy SL, Strakowski SM, Keck PE Jr, McConville BJ: Attention deficit hyperactivity disorder in adolescent mania. Am J Psychiatry 1995; 152:271–273 [D]Crossref, Google Scholar
128 Faraone SV, Biederman J, Wozniak J, Mundy E, Mennin D, O’Donnell D: Is comorbidity with ADHD a marker for juvenile-onset mania? J Am Acad Child Adolesc Psychiatry 1997; 36:1046–1055 [D]Crossref, Google Scholar
129 Altshuler LL, Cohen LS, Moline ML, Kahn DA, Carpenter D, Docherty JP: The Expert Consensus Guideline Series: Treatment of Depression in Women. New York, McGraw-Hill Companies, 2001 [F]Google Scholar
130 Strober M, Schmidt-Lackner S, Freeman R, Bower S, Lampert C, DeAntonio M: Recovery and relapse in adolescents with bipolar affective illness: a five-year naturalistic, prospective followup. J Am Acad Child Adolesc Psychiatry 1995; 34:724–731 [C]Crossref, Google Scholar
131 Biederman J, Mick E, Bostic JQ, Prince J, Daly J, Wilens TE, Spencer T, Garcia-Jetton J, Russell R, Wozniak J, Faraone SV: The naturalistic course of pharmacologic treatment of children with manic-like symptoms: a systematic chart review. J Clin Psychiatry 1998; 59:628–637 [B]Crossref, Google Scholar
132 Strober M, Morrell W, Lampert C, Burroughs J: Relapse following discontinuation of lithium maintenance therapy in adolescents with bipolar I illness: a naturalistic study. Am J Psychiatry 1990; 147:457–461 [C]Crossref, Google Scholar
133 Biederman J, Faraone SV, Wozniak J, Monuteaux MC: Parsing the association between bipolar, conduct, and substance use disorders: a familial risk analysis. Biol Psychiatry 2000; 48:1037–1044 [C]Crossref, Google Scholar
134 Wilens TE, Biederman J, Millstein RB, Wozniak J, Hahesy AL, Spencer TJ: Risk for substance use disorders in youths with child- and adolescent-onset bipolar disorder. J Am Acad Child Adolesc Psychiatry 1999; 38:680–685 [C]Crossref, Google Scholar
135 Carlson GA, Lavelle J, Bromet EJ: Medication treatment in adolescents vs adults with psychotic mania. J Child Adolesc Psychopharmacol 1999; 9:221–231 [C]Crossref, Google Scholar
136 Geller B, Luby J: Child and adolescent bipolar disorder: a review of the past 10 years. J Am Acad Child Adolesc Psychiatry 1997; 36:1168–1176; correction, 36:1642 [F]Crossref, Google Scholar
137 Donovan SJ, Nunes EV: Treatment of comorbid affective and substance use disorders: therapeutic potential of anticonvulsants. Am J Addict 1998; 7:210–220 [F]Google Scholar
138 Van Gerpen MW, Johnson JE, Winstead DK: Mania in the geriatric patient population: a review of the literature. Am J Geriatr Psychiatry 1999; 7:188–202 [F]Crossref, Google Scholar
139 Young RC, Klerman GL: Mania in late life: focus on age at onset. Am J Psychiatry 1992; 149:867–876 [F]Crossref, Google Scholar
140 Shulman KI, Herrmann N: The nature and management of mania in old age. Psychiatr Clin North Am 1999; 22:649–665 [F]Crossref, Google Scholar
141 Tohen M, Shulman KI, Satlin A: First-episode mania in late life. Am J Psychiatry 1994; 151:130–132 [C]Crossref, Google Scholar
142 Sproule BA, Hardy BG, Shulman KI: Differential pharmacokinetics of lithium in elderly patients. Drugs Aging 2000; 16:165–177 [F]Crossref, Google Scholar
143 Caligiuri MR, Jeste DV, Lacro JP: Antipsychotic-induced movement disorders in the elderly: epidemiology and treatment recommendations. Drugs Aging 2000; 17:363–384 [F]Crossref, Google Scholar
144 Leipzig RM, Cumming RG, Tinetti ME: Drugs and falls in older people: a systematic review and meta-analysis, I: psychotropic drugs. J Am Geriatr Soc 1999; 47:30–39 [E]Crossref, Google Scholar
145 Strakowski SM, McElroy SL, Keck PW Jr, West SA: The co-occurrence of mania with medical and other psychiatric disorders. Int J Psychiatry Med 1994; 24:305–328 [F]Crossref, Google Scholar
146 Peet M, Peters S: Drug-induced mania. Drug Saf 1995; 12:146–153 [F]Crossref, Google Scholar
147 Cozza KL, Armstrong SC (eds): Concise Guide to the Cytochrome P450 System: Drug Interaction Principles for Medical Practice. Washington, DC, American Psychiatric Press, 2001, pp 103–200 [G]Google Scholar
148 American Psychiatric Association: Practice Guideline for the Treatment of Patients With HIV/AIDS. Am J Psychiatry 2000; 157(Nov suppl) [G]Google Scholar
149 Akiskal HS, Pinto O: The evolving bipolar spectrum: prototypes I, II, III, and IV. Psychiatr Clin North Am 1999; 22:517–534 [F]Crossref, Google Scholar
150 Swann AC, Bowden CL, Calabrese JR, Dilsaver SC, Morris DD: Differential effect of number of previous episodes of affective disorder on response to lithium or divalproex in acute mania. Am J Psychiatry 1999; 156:1264–1266 [E]Google Scholar
151 Lish JD, Dime-Meenan S, Whybrow PC, Price RA, Hirschfeld RM: The National Depressive and Manic-Depressive Association (DMDA) survey of bipolar members. J Affect Disord 1994; 31:281–294 [G]Crossref, Google Scholar
152 Manning JS, Haykal RF, Connor PD, Akiskal HS: On the nature of depressive and anxious states in a family practice setting: the high prevalence of bipolar II and related disorders in a cohort followed longitudinally. Compr Psychiatry 1997; 38:102–108 [D]Crossref, Google Scholar
153 Perugi G, Akiskal HS, Lattanzi L, Cecconi D, Mastrocinque C, Patronelli A, Vignoli S, Bemi E: The high prevalence of “soft” bipolar (II) features in atypical depression. Compr Psychiatry 1998; 39:63–71 [G]Crossref, Google Scholar
154 Benazzi F: Prevalence of bipolar II disorder in outpatient depression: a 203-case study in private practice. J Affect Disord 1997; 43:163–166 [G]Crossref, Google Scholar
155 Akiskal HS, Maser JD, Zeller PJ, Endicott J, Coryell W, Keller M, Warshaw M, Clayton P, Goodwin F: Switching from “unipolar” to bipolar II: an 11-year prospective study of clinical and temperamental predictors in 559 patients. Arch Gen Psychiatry 1995; 52:114–123 [C]Crossref, Google Scholar
156 Mitchell PB, Wilhelm K, Parker G, Austin MP, Rutgers P, Malhi GS: The clinical features of bipolar depression: a comparison with matched major depressive disorder patients. J Clin Psychiatry 2001; 62:212–216 [D]Crossref, Google Scholar
157 Ghaemi SN, Sachs GS, Chiou AM, Pandurangi AK, Goodwin K: Is bipolar disorder still underdiagnosed? are antidepressants overutilized? J Affect Disord 1999; 52:135–144 [G]Crossref, Google Scholar
158 Fogarty F, Russell JM, Newman SC, Bland RC: Epidemiology of psychiatric disorders in Edmonton: mania. Acta Psychiatr Scand Suppl 1994; 376:16–23 [G]Crossref, Google Scholar
159 Faedda GL, Baldessarini RJ, Suppes T, Tondo L, Becker I, Lipschitz DS: Pediatric-onset bipolar disorder: a neglected clinical and public health problem. Harv Rev Psychiatry 1995; 3:171–195 [F]Crossref, Google Scholar
160 Winokur G, Coryell W, Akiskal HS, Endicott J, Keller M, Mueller T: Manic-depressive (bipolar) disorder: the course in light of a prospective ten-year follow-up of 131 patients. Acta Psychiatr Scand 1994; 89:102–110 [C]Crossref, Google Scholar
161 Dion GL, Tohen M, Anthony WA, Waternaux CS: Symptoms and functioning of patients with bipolar disorder six months after hospitalization. Hosp Community Psychiatry 1988; 39:652–657 [C]Google Scholar
162 Goodnick PJ, Fieve RR, Schlegel A, Baxter N: Predictors of interepisode symptoms and relapse in affective disorder patients treated with lithium carbonate. Am J Psychiatry 1987; 144:367–369 [B]Crossref, Google Scholar
163 Goldberg JF, Harrow M, Grossman LS: Course and outcome in bipolar affective disorder: a longitudinal follow-up study. Am J Psychiatry 1995; 152:379–384 [C]Crossref, Google Scholar
164 Coryell W, Scheftner W, Keller M, Endicott J, Maser J, Klerman GL: The enduring psychosocial consequences of mania and depression. Am J Psychiatry 1993; 150:720–727 [C]Crossref, Google Scholar
165 Weissman MM, Bland RC, Canino GJ, Faravelli C, Greenwald S, Hwu HG, Joyce PR, Karam EG, Lee CK, Lellouch J, Lepine JP, Newman SC, Rubio-Stipec M, Wells JE, Wickramaratne PJ, Wittchen H, Yeh EK: Cross-national epidemiology of major depression and bipolar disorder. JAMA 1996; 276:293–299 [E]Crossref, Google Scholar
166 Angst J: The emerging epidemiology of hypomania and bipolar II disorder. J Affect Disord 1998; 50:143–151 [C]Crossref, Google Scholar
167 Geller B, Craney JL, Bolhofner K, DelBello MP, Williams M, Zimerman B: One-year recovery and relapse rates of children with a prepubertal and early adolescent bipolar disorder phenotype. Am J Psychiatry 2001; 158:303–305 [C]Crossref, Google Scholar
168 McDonald WM, Nemeroff CB: The diagnosis and treatment of mania in the elderly. Bull Menninger Clin 1996; 60:174–196 [F]Google Scholar
169 Tohen M, Bromet E, Murphy JM, Tsuang MT: Psychiatric epidemiology. Harvard Rev Psychiatry 2000; 8:111–125 [G]Crossref, Google Scholar
170 Leverich GS, McElroy SL, Suppes T: Early physical or sexual abuse and the course of bipolar illness. Biological Psychiatry (in press) [G]Google Scholar
171 Johnson SL, Miller I: Negative life events and time to recovery from episodes of bipolar disorder. J Abnorm Psychol 1997; 106:449–457 [C]Crossref, Google Scholar
172 Frank E, Thase ME: Natural history and preventative treatment of recurrent mood disorders. Annu Rev Med 1999; 50:453–468 [F]Crossref, Google Scholar
173 Colombo C, Benedetti F, Barbini B, Campori E, Smeraldi E: Rate of switch from depression into mania after therapeutic sleep deprivation in bipolar depression. Psychiatry Res 1999; 86:267–270 [B]Crossref, Google Scholar
174 Ashman SB, Monk TH, Kupfer DJ, Clark CH, Myers FS, Frank E, Leibenluft E: Relationship between social rhythms and mood in patients with rapid cycling bipolar disorder. Psychiatry Res 1999; 86:1–8 [C]Crossref, Google Scholar
175 Strakowski SM, DelBello MP: The co-occurrence of bipolar and substance use disorders. Clin Psychol Rev 2000; 20:191–206 [F]Crossref, Google Scholar
176 Schou M, Juel-Nielson, Stroomgren E, Voldby H: The treatment of manic psychoses by administration of lithium salts. J Neurol Neurosurg Psychiatry 1954; 17:250–260 [B]Crossref, Google Scholar
177 Goodwin FK, Murphy DL, Bunney WE Jr: Lithium-carbonate treatment in depression and mania: a longitudinal doubleblind study. Arch Gen Psychiatry 1969; 21:486–496 [B]Crossref, Google Scholar
178 Stokes PE, Shamoian CA, Stoll PM, Patton MJ: Efficacy of lithium as acute treatment of manic-depressive illness. Lancet 1971; 1:1319–1325 [B]Crossref, Google Scholar
179 Maggs R: Treatment of manic illness with lithium carbonate. Br J Psychiatry 1963; 109:56–65 [B]Crossref, Google Scholar
180 Bowden CL, Brugger AM, Swann AC, Calabrese JR, Janicak PG, Petty F, Dilsaver SC, Davis JM, Rush AJ, Small JG (Depakote Mania Study Group): Efficacy of divalproex vs lithium and placebo in the treatment of mania. JAMA 1994; 271:918–924 [A]Crossref, Google Scholar
181 Lerer B, Moore N, Meyendorff E, Cho SR, Gershon S: Carbamazepine versus lithium in mania: a double-blind study. J Clin Psychiatry 1987; 48:89–93 [A]Google Scholar
182 Small JG, Klapper MH, Milstein V, Kellams JJ, Miller MJ, Marhenke JD, Small IF: Carbamazepine compared with lithium in the treatment of mania. Arch Gen Psychiatry 1991; 48:915–921 [A]Crossref, Google Scholar
183 Segal J, Berk M, Brook S: Risperidone compared with both lithium and haloperidol in mania: a double-blind randomized controlled trial. Clin Neuropharmacol 1998; 21:176–180 [A]Google Scholar
184 Berk M, Ichim L, Brook S: Olanzapine compared to lithium in mania: a double-blind randomized controlled trial. Int Clin Psychopharmacol 1999; 14:339–343 [A]Crossref, Google Scholar
185 Takahashi R, Sakuma A, Itoh K, Itoh H, Kurihara M: Comparison of efficacy of lithium carbonate and chlorpromazine in mania: report of collaborative study group on treatment of mania in Japan. Arch Gen Psychiatry 1975; 32:1310–1318 [A]Crossref, Google Scholar
186 Platman SR: A comparison of lithium carbonate and chlorpromazine in mania. Am J Psychiatry 1970; 127:351–353 [A]Crossref, Google Scholar
187 Spring G, Schweid D, Gray C, Steinberg J, Horwitz M: A double-blind comparison of lithium and chlorpromazine in the treatment of manic states. Am J Psychiatry 1970; 126:1306–1310 [A]Crossref, Google Scholar
188 Johnson G, Gershon S, Burdock EI, Floyd A, Hekimian L: Comparative effects of lithium and chlorpromazine in the treatment of acute manic states. Br J Psychiatry 1971; 119:267–276 [A]Crossref, Google Scholar
189 Prien RF, Caffey EM Jr, Klett CJ: Comparison of lithium carbonate and chlorpromazine in the treatment of mania: report of the Veterans Administration and National Institute of Mental Health Collaborative Study Group. Arch Gen Psychiatry 1972; 26:146–153 [A]Crossref, Google Scholar
190 Shopsin B, Gershon S, Thompson H, Collins P: Psychoactive drugs in mania: a controlled comparison of lithium carbonate, chlorpromazine, and haloperidol. Arch Gen Psychiatry 1975; 32:34–42 [A]Crossref, Google Scholar
191 Secunda SK, Katz MM, Swann A, Koslow SH, Maas JW, Chuang S, Croughan J: Mania: diagnosis, state measurement and prediction of treatment response. J Affect Disord 1985; 8:113–121 [E]Crossref, Google Scholar
192 Himmelhoch JM, Garfinkel ME: Sources of lithium resistance in mixed mania. Psychopharmacol Bull 1986; 22:613–620 [C]Google Scholar
193 Prien RF, Himmelhoch JM, Kupfer DJ: Treatment of mixed mania. J Affect Disord 1988; 15:9–15 [B]Crossref, Google Scholar
194 Kramlinger KG, Post RM: Adding lithium carbonate to carbamazepine: antimanic efficacy in treatment-resistant mania. Acta Psychiatr Scand 1989; 79:378–385 [B]Crossref, Google Scholar
195 Freeman TW, Clothier JL, Pazzaglia P, Lesem MD, Swann AC: A double-blind comparison of valproate and lithium in the treatment of acute mania. Am J Psychiatry 1992; 149:108–111 [A]Crossref, Google Scholar
196 Bowden CL: Predictors of response to divalproex and lithium. J Clin Psychiatry 1995; 56(suppl 3):25–30 [E]Google Scholar
197 Swann AC, Bowden CL, Morris D, Calabrese JR, Petty F, Small J, Dilsaver SC, Davis JM: Depression during mania: treatment response to lithium or divalproex. Arch Gen Psychiatry 1997; 54:37–42 [E]Crossref, Google Scholar
198 Jefferson JW, Greist JH, Acherman DL, Carroll JA: Lithium Encyclopedia for Clinical Practice, 2nd ed. Washington, DC, American Psychiatric Press, 1987 [F]Google Scholar
199 Schou M: Lithium prophylaxis: myths and realities. Am J Psychiatry 1989; 146:573–576 [F]Crossref, Google Scholar
200 Peet M, Pratt JP: Lithium: current status in psychiatric disorders. Drugs 1993; 46:7–17 [F]Crossref, Google Scholar
201 Burggraf GW: Are psychotropic drugs at therapeutic levels a concern for cardiologists? Can J Cardiol 1997; 13:75–80 [F]Google Scholar
202 Gitlin M: Lithium and the kidney: an updated review. Drug Saf 1999; 20:231–243 [F]Crossref, Google Scholar
203 Bendz H, Aurell M: Drug-induced diabetes insipidus: incidence, prevention and management. Drug Saf 1999; 21:449–456 [F]Crossref, Google Scholar
204 Arana GW, Hyman SE: Handbook of Psychiatric Drug Therapy, 2nd ed. Boston, Little, Brown, 1991 [F]Google Scholar
205 Bauer MS, Whybrow PC: Rapid cycling bipolar affective disorder, II: treatment of refractory rapid cycling with high-dose levothyroxine: a preliminary study. Arch Gen Psychiatry 1990; 47:435–440 [A]Crossref, Google Scholar
206 Kleiner J, Altshuler L, Hendrick V, Hershman JM: Lithium-induced subclinical hypothyroidism: review of the literature and guidelines for treatment. J Clin Psychiatry 1999; 60:249–255 [F]Crossref, Google Scholar
207 Smigan L, Wahlin A, Jacobsson L, von Knorring L: Lithium therapy and thyroid function tests: a prospective study. Neuropsychobiology 1984; 11:39–43 [B]Crossref, Google Scholar
208 Bocchetta A, Bernardi F, Burrai C, Pedditzi M, Loviselli A, Velluzzi F, Martino E, Del Zompo M: The course of thyroid abnormalities during lithium treatment: a two-year follow-up study. Acta Psychiatr Scand 1992; 86:38–41 [C]Crossref, Google Scholar
209 Haden ST, Stoll AL, McCormick S, Scott J, Fuleihan GE-H: Alterations in parathyroid dynamics in lithium-treated subjects. J Clin Endocrinol Metab 1997; 82:2844–2848 [B]Google Scholar
210 Kallner G, Petterson U: Renal, thyroid and parathyroid function during lithium treatment: laboratory tests in 207 people treated for 1–30 years. Acta Psychiatr Scand 1995; 91:48–51 [C]Crossref, Google Scholar
211 Mak TW, Shek CC, Chow CC, Wing YK, Lee S: Effects of lithium therapy on bone mineral metabolism: a two-year prospective longitudinal study. J Clin Endocrinol Metab 1998; 83:3857–3859 [B]Google Scholar
212 Chan HH, Wing Y, Su R, Van Krevel C, Lee S: A control study of the cutaneous side effects of chronic lithium therapy. J Affect Disord 2000; 57:107–113 [D]Crossref, Google Scholar
213 Vestergaard P, Schou M, Thomsen K: Monitoring of patients in prophylactic lithium treatment: an assessment based on recent kidney studies. Br J Psychiatry 1982; 140:185–187 [C]Crossref, Google Scholar
214 Schou M: Effects of long-term lithium treatment on kidney function: an overview. J Psychiatr Res 1988; 22:287–296 [F]Crossref, Google Scholar
215 Gitlin MJ: Lithium-induced renal insufficiency. J Clin Psychopharmacol 1993; 13:276–279 [C]Google Scholar
216 Bendz H, Sjodin I, Aurell M: Renal function on and off lithium in patients treated with lithium for 15 years or more: a controlled, prospective lithium-withdrawal study. Nephrol Dial Transplant 1996; 11:457–460 [B]Crossref, Google Scholar
217 von Knorring L, Walton SA, Okuma T, Bohman SO: Uraemia induced by long-term lithium treatment. Lithium 1990; 1:251–253 [G]Google Scholar
218 Markowitz GS, Radhakrishnan J, Kambham N, Valeri AM, Hines WH, D’Agati VD: Lithium nephrotoxicity: a progressive combined glomerular and tubulointerstitial nephropathy. J Am Soc Nephrol 2000; 11:1439–1448 [G]Google Scholar
219 Ellenhorn MJ: Lithium, in Ellenhorn’s Medical Toxicology: Diagnosis and Treatment of Human Poisoning. Baltimore, Williams & Wilkins, 1997, pp 1579–1585 [G]Google Scholar
220 Scharman EJ: Methods used to decrease lithium absorption or enhance elimination. J Toxicol Clin Toxicol 1997; 35:601–608 [F]Crossref, Google Scholar
221 Jaeger A, Sauder P, Kopferschmitt J, Tritsch L, Flesch F: When should dialysis be performed in lithium poisoning? a kinetic study in 14 cases of lithium poisoning. J Toxicol Clin Toxicol 1993; 31:429–447 [B]Crossref, Google Scholar
222 van Bommel EF, Kalmeijer MD, Ponssen HH: Treatment of life-threatening lithium toxicity with high-volume continuous venovenous hemofiltration. Am J Nephrol 2000; 20:408–411 [G]Crossref, Google Scholar
223 Friedberg RC, Spyker DA, Herold DA: Massive overdoses with sustained-release lithium carbonate preparations: pharmacokinetic model based on two case studies. Clin Chem 1991; 37:1205–1209 [G]Google Scholar
224 Bosse GM, Arnold TC: Overdose with sustained-release lithium preparations. J Emerg Med 1992; 10:719–721 [G]Crossref, Google Scholar
225 Gelenberg AJ, Kane JM, Keller MB, Lavori P, Rosenbaum JF, Cole K, Lavelle J: Comparison of standard and low serum levels of lithium for maintenance treatment of bipolar disorder. N Engl J Med 1989; 321:1489–1493 [A]Crossref, Google Scholar
226 Vestergaard P, Licht RW, Brodersen A, Rasmussen NA, Christensen H, Arngrim T, Gronvall B, Kristensen E, Poulstrup I, Wentzer LR: Outcome of lithium prophylaxis: a prospective follow-up of affective disorder patients assigned to high and low serum lithium levels. Acta Psychiatr Scand 1998; 98:310–315 [B]Crossref, Google Scholar
227 Emrich HM, von Zerssen D, Kissling W: On a possible role of GABA in mania: therapeutic efficacy of sodium valproate, in GABA and Benzodiazepine Receptors. Edited by Costa E, Dicharia G, Gessa GL. New York, Raven Press, 1981, pp 287–296 [B]Google Scholar
228 Brennan MJW, Sandyk R, Borsook D: Use of sodium valproate in the management of affective disorders: basic and clinical aspects, in Anticonvulsants in Affective Disorders. Edited by Emrich HM, Okuma T, Muller AA. Amsterdam, Excerpta Medica, 1984, pp 56–65 [A]Google Scholar
229 Pope HG Jr, McElroy SL, Keck PE Jr, Hudson JI: Valproate in the treatment of acute mania: a placebo-controlled study. Arch Gen Psychiatry 1991; 48:62–68 [A]Crossref, Google Scholar
230 McElroy SL, Keck PE, Stanton SP, Tugrul KC, Bennett JA, Strakowski SM: A randomized comparison of divalproex oral loading versus haloperidol in the initial treatment of acute psychotic mania. J Clin Psychiatry 1996; 57:142–146 [B]Google Scholar
231 Zajecka JM, Weisler R, Swann AC: Divalproex sodium versus olanzapine for the treatment of mania in bipolar disorder, in American College of Neuropsychopharmacology Annual Meeting Poster Abstracts. Nashville, Tenn, ACNP, 2000 [A]Google Scholar
232 Tohen MF, Milton DR, Davis AR: Olanzapine versus divalproex for the treatment of acute mania, in Congress Poster Abstracts. Munich, European College of Neuropsychopharmacology, 2000 [A]Google Scholar
233 Sheth RD, Wesolowski CA, Jacob JC, Penney S, Hobbs GR, Riggs JE, Bodensteiner JB: Effect of carbamazepine and valproate on bone mineral density. J Pediatr 1995; 127:256–262 [D]Crossref, Google Scholar
234 Tannirandorn P, Epstein S: Drug-induced bone loss. Osteoporos Int 2000; 11:637–659 [F]Crossref, Google Scholar
235 Davis R, Peters DH, McTavish D: Valproic acid: a reappraisal of its pharmacological properties and clinical efficacy in epilepsy. Drugs 1994; 47:332–372 [F]Crossref, Google Scholar
236 Mercke Y, Sheng H, Khan T, Lippmann S: Hair loss in psychopharmacology. Ann Clin Psychiatry 2000; 12:35–42 [F]Crossref, Google Scholar
237 Gautam M: Alopecia due to psychotropic medications. Ann Pharmacother 1999; 33:631–637 [F]Crossref, Google Scholar
238 Stoll AL, Walton SA, McElroy SL: Histamine-2-receptor antagonists for the treatment of valproate-induced gastrointestinal distress. Ann Clin Psychiatry 1991; 3:301–304 [G]Crossref, Google Scholar
239 Spiller HA, Krenzelok EP, Klein-Schwartz W, Winter ML, Weber JA, Sollee DR, Bangh SA: Multicenter case series of valproic acid ingestion: serum concentrations and toxicity. J Toxicol Clin Toxicol 2000; 38:755–760 [G]Crossref, Google Scholar
240 Loscher W: Valproate: a reappraisal of its pharmacodynamic properties and mechanisms of action. Prog Neurobiol 1999; 58:31–59 [F]Crossref, Google Scholar
241 Bryant AE III, Dreifuss FE: Valproic acid hepatic fatalities, III: US experience since 1986. Neurology 1996; 46:465–469 [B]Crossref, Google Scholar
242 Gidal B, Spencer N, Maly M, Pitterle M, Williams E, Collins M, Jones J: Valproate-mediated disturbances of hemostasis: relationship to dose and plasma concentration. Neurology 1994; 44:1418–1422 [C]Crossref, Google Scholar
243 Finsterer J, Pelzl G, Hess B: Severe, isolated thrombocytopenia under polytherapy with carbamazepine and valproate. Psychiatry Clin Neurosci 2001; 55:423–426 [G]Crossref, Google Scholar
244 Chappell KA, Markowitz JS, Jackson CW: Is valproate pharmacotherapy associated with polycystic ovaries? Ann Pharmacother 1999; 33:1211–1216 [F]Crossref, Google Scholar
245 Genton P, Bauer J, Duncan S, Taylor AE, Balen AH, Eberle A, Pedersen B, Salas-Puig X, Sauer MV: On the association between valproate and polycystic ovary syndrome. Epilepsia 2001; 42:295–304 [F]Crossref, Google Scholar
246 Joffe H, Taylor AE, Hall JE: Polycystic ovarian syndrome—relationship to epilepsy and antiepileptic drug therapy. J Clin Endocrinol Metab 2001; 86:2946–2949 [F]Google Scholar
247 Isojarvi JI, Laatikainen TJ, Pakarinen AJ, Juntunen KT, Myllyla VV: Polycystic ovaries and hyperandrogenism in women taking valproate for epilepsy. N Engl J Med 1993; 329:1383–1388 [G]Crossref, Google Scholar
248 Ellenhorn MJ: Valproate, in Ellenhorn’s Medical Toxicology: Diagnosis and Treatment of Human Poisoning. Baltimore, Williams & Wilkins, 1997, pp 610–612 [G]Google Scholar
249 Janicak P, Davis JM, Preskorn SH, Ayd FJ: Principles and Practice of Psychopharmacotherapy. Baltimore, Williams & Wilkins, 1993 [F]Google Scholar
250 Gilman AG, Rall TW, Nies AS, Taylor P (eds): Goodman and Gilman’s The Pharmacological Basis of Therapeutics, 8th ed. New York, Pergamon Press, 1990 [G]Google Scholar
251 Keck PE Jr, McElroy SL, Tugrul KC, Bennett JA: Valproate oral loading in the treatment of acute mania. J Clin Psychiatry 1993; 54:305–308 [B]Google Scholar
252 McElroy SL, Keck PE Jr, Tugrul KC, Bennett JA: Valproate as a loading treatment in acute mania. Neuropsychobiology 1993; 27:146–149 [G]Crossref, Google Scholar
253 Martinez JM, Russell JM, Hirschfeld RM: Tolerability of oral loading of divalproex sodium in the treatment of acute mania. Depress Anxiety 1998; 7:83–86 [D]Crossref, Google Scholar
254 Hirschfeld RM, Allen MH, McEvoy JP, Keck PE Jr, Russell JM: Safety and tolerability of oral loading divalproex sodium in acutely manic bipolar patients. J Clin Psychiatry 1999; 60:815–818 [A]Crossref, Google Scholar
255 Longo LP: Divalproex sodium for alcohol withdrawal and relapse prevention: a case report. J Clin Psychiatry 2000; 61:947–948 [G]Crossref, Google Scholar
256 Horne M, Lindley SE: Divalproex sodium in the treatment of aggressive behavior and dysphoria in patients with organic brain syndromes (letter). J Clin Psychiatry 1995; 56:430–431 [G]Google Scholar
257 Miller PR: Clozapine therapy for a patient with a history of Hodgkin’s disease (letter). Psychiatr Serv 2001; 52:110–111 [G]Crossref, Google Scholar
258 Pellock JM, Willmore LJ: A rational guide to routine blood monitoring in patients receiving antiepileptic drugs. Neurology 1991; 41:961–964 [G]Crossref, Google Scholar
259 McElroy SL, Keck PE Jr, Pope HG Jr, Hudson JI: Valproate in the treatment of bipolar disorder: literature review and clinical guidelines. J Clin Psychopharmacol 1992; 12:42S–52S [F]Crossref, Google Scholar
260 McElroy SL, Keck PE Jr, Pope HG Jr, Hudson JI, Faedda GL, Swann AC: Clinical and research implications of the diagnosis of dysphoric or mixed mania or hypomania. Am J Psychiatry 1992; 149:1633–1644 [F]Crossref, Google Scholar
261 Riva R, Albani F, Contin M, Baruzzi A: Pharmacokinetic interactions between antiepileptic drugs: clinical considerations. Clin Pharmacokinet 1996; 31:470–493 [F]Crossref, Google Scholar
262 Matsuo F: Lamotrigine. Epilepsia 1999; 40(suppl 5):S30–S36 [F]Crossref, Google Scholar
263 Tanaka E: Clinically significant pharmacokinetic drug interactions between antiepileptic drugs. J Clin Pharmacol Ther 1999; 24:87–92 [F]Crossref, Google Scholar
264 Keck PE Jr, McElroy SL, Nemeroff CB: Anticonvulsants in the treatment of bipolar disorder. J Neuropsychiatry Clin Neurosci 1992; 4:395–405 [F]Crossref, Google Scholar
265 Ballenger JC, Post RM: Therapeutic effects of carbamazepine in affective illness: a preliminary report. Commun Psychopharmacol 1978; 2:159–175 [A]Google Scholar
266 Vasudev K, Goswami U, Kohli K: Carbamazepine and valproate monotherapy: feasibility, relative safety and efficacy, and therapeutic drug monitoring in manic disorder. Psychopharmacology (Berl) 2000; 150:15–23 [A]Crossref, Google Scholar
267 Grossi E, Sacchetti E, Vita A: Anticonvulsants in affective disorders, in Carbamazepine Versus Chlorpromazine in Mania: A Double-Blind Trial. Edited by Emirch HM, Okuma T, Muller AA. Amsterdam, Excerpta Medica, 1984, pp 177–187 [B]Google Scholar
268 Okuma T, Inanaga K, Otsuki S, Sarai K, Takahashi R, Hazama H, Mori A, Watanabe M: Comparison of the antimanic efficacy of carbamazepine and chlorpromazine: a double-blind controlled study. Psychopharmacology (Berl) 1979; 66:211–217 [A]Crossref, Google Scholar
269 Rimmer EM, Richens A: An update on sodium valproate. Pharmacotherapy 1985; 5:171–184 [F]Crossref, Google Scholar
270 Smith MC, Bleck TP: Convulsive disorders: toxicity of anticonvulsants. Clin Neuropharmacol 1991; 14:97–115 [F]Crossref, Google Scholar
271 Kramlinger KG, Phillips KA, Post RM: Rash complicating carbamazepine treatment. J Clin Psychopharmacol 1994; 14:408–413 [C]Crossref, Google Scholar
272 Van Amelsvoort T, Bakshi R, Devaux CB, Schwabe S: Hyponatremia associated with carbamazepine and oxcarbazepine therapy: a review. Epilepsia 1994; 35:181–188 [F]Crossref, Google Scholar
273 Seetharam MN, Pellock JM: Risk-benefit assessment of carbamazepine in children. Drug Saf 1991; 6:148–158 [F]Crossref, Google Scholar
274 Knowles SR, Shapiro LE, Shear NH: Anticonvulsant hypersensitivity syndrome: incidence, prevention and management. Drug Saf 1999; 21:489–501 [F]Crossref, Google Scholar
275 Schweiger FJ, Kelton JG, Messner H, Klein M, Berger S, McIlroy WJ, Falk J, Keating A: Anticonvulsant-induced marrow suppression and immune thrombocytopenia. Acta Haematol 1988; 80:54–58 [G]Crossref, Google Scholar
276 Ketter TA, Frye MA, Cora-Locatelli G, Kimbrell TA, Post RM: Metabolism and excretion of mood stabilizers and new anticonvulsants. Cell Mol Neurobiol 1999; 19:511–532 [F]Crossref, Google Scholar
277 Muller AA, Stoll KD: Anticonvulsants in affective disorders, in Carbamazepine and Oxcarbazepine in the Treatment of Manic Syndromes: Studies in Germany. Edited by Emrich HM, Okuma T, Muller AA. Amsterdam, Exerpta Medica, 1984, pp 134–147 [B]Google Scholar
278 Emrich HM, Zihl J, Raptis C, Wendl A: Reduced dark-adaptation: an indication of lithium’s neuronal action in humans. Am J Psychiatry 1990; 147:629–631 [G]Crossref, Google Scholar
279 Dam M, Ekberg R, Loyning Y, Waltimo O, Jakobsen K: A doubleblind study comparing oxcarbazepine and carbamazepine in patients with newly diagnosed, previously untreated epilepsy. Epilepsy Res 1989; 3:70–76 [A]Crossref, Google Scholar
280 Glauser TA, Nigro M, Sachdeo R, Pasteris LA, Weinstein S, Abou-Khalil B, Frank LM, Grinspan A, Guarino T, Bettis D, Kerrigan J, Geoffroy G, Mandelbaum D, Jacobs T, Mesenbrink P, Kramer L, D’Souza J (Oxcarbazepine Pediatric Study Group): Adjunctive therapy with oxcarbazepine in children with partial seizures. Neurology 2000; 54:2237–2244 [A]Crossref, Google Scholar
281 Smith PE: Clinical recommendations for oxcarbazepine. Seizure 2001; 10:87–91 [F]Crossref, Google Scholar
282 Wellington K, Goa KL: Oxcarbazepine: an update of its efficacy in the management of epilepsy. CNS Drugs 2001; 15:137–163 [F]Crossref, Google Scholar
283 Suppes T, Swann AC, Dennehy EB, Habermacher ED, Mason M, Crismon ML, Toprac MG, Rush AJ, Shon SP, Altshuler KZ: Texas Medication Algorithm Project: development and feasibility testing of a treatment algorithm for patients with bipolar disorder. J Clin Psychiatry 2001; 62:439–447 [B]Crossref, Google Scholar
284 Frye MA, Ketter TA, Kimbrell TA, Dunn RT, Speer AM, Osuch EA, Luckenbaugh DA, Cora-Locatelli G, Leverich GS, Post RM: A placebo-controlled study of lamotrigine and gabapentin monotherapy in refractory mood disorders. J Clin Psychopharmacol 2000; 20:607–614 [A]Crossref, Google Scholar
285 Anand A, Oren DA, Berman RM: Lamotrigine treatment of lithium failure in outpatient mania: a double-blind, placebo-controlled trial, in Abstract Book, Third International Bipolar Conference. Edited by Soares JC, Gershon S. Pittsburgh, Munksgaard, 1999, p 23 [A]Google Scholar
286 Ichim L, Berk M, Brook S: Lamotrigine compared with lithium in mania: a double-blind randomized controlled trial. Ann Clin Psychiatry 2000; 12:5–10 [A]Crossref, Google Scholar
287 Pande AC, Crockatt JG, Janney CA, Werth JL, Tsaroucha G (Gabapentin Bipolar Disorder Study Group): Gabapentin in bipolar disorder: a placebo-controlled trial of adjunctive therapy. Bipolar Disord 2000; 2:249–255 [A]Crossref, Google Scholar
288 Mishory A, Yaroslavsky Y, Bersudsky Y, Belmaker RH: Phenytoin as an antimanic anticonvulsant: a controlled study. Am J Psychiatry 2000; 157:463–465 [B]Crossref, Google Scholar
289 Tohen M, Sanger TM, McElroy SL, Tollefson GD, Chengappa KNR, Daniel DG, Petty F, Centorrino F, Wang R, Grundy SL, Greaney MG, Jacobs TG, David SR, Toma V (Olanzapine HGEH Study Group): Olanzapine versus placebo in the treatment of acute mania. Am J Psychiatry 1999; 156:702–709 [A]Google Scholar
290 Tohen M, Jacobs TG, Grundy SL, McElroy SL, Banov MC, Janicak PG, Sanger T, Risser R, Zhang F, Toma V, Francis J, Tollefson GD, Breier A (Olanzapine HGGW Study Group): Efficacy of olanzapine in acute bipolar mania: a double-blind, placebo-controlled study. Arch Gen Psychiatry 2000; 57:841–849 [A]Crossref, Google Scholar
291 Sanger TM, Grundy SL, Gibson PJ, Namjoshi MA, Greaney MG, Tohen MF: Long-term olanzapine therapy in the treatment of bipolar I disorder: an open-label continuation phase study. J Clin Psychiatry 2001; 62:273–281 [E]Crossref, Google Scholar
292 Tohen M, Zhang F, Feldman PD, Evans AR, Brier A: Olanzapine versus haloperidol in the treatment of acute mania, in 1998 Annual Meeting Syllabus and Proceedings Summary. Washington, DC, American Psychiatric Association, 1998 [A]Google Scholar
293 Crawford AM, Beasley CM Jr, Tollefson GD: The acute and longterm effect of olanzapine compared with placebo and haloperidol on serum prolactin concentrations. Schizophr Res 1997; 26:41–54 [A]Crossref, Google Scholar
294 Kinon BJ, Basson BR, Gilmore JA, Tollefson GD: Long-term olanzapine treatment: weight change and weight-related health factors in schizophrenia. J Clin Psychiatry 2001; 62:92–100 [A]Crossref, Google Scholar
295 Allison DB, Casey DE: Antipsychotic-induced weight gain: a review of the literature. J Clin Psychiatry 2001; 62(suppl 7):22–31 [F]Crossref, Google Scholar
296 Lindenmayer JP, Nathan AM, Smith RC: Hyperglycemia associated with the use of atypical antipsychotics. J Clin Psychiatry 2001; 62(suppl 23):30–38 [F]Crossref, Google Scholar
297 Leucht S, Pitschel-Walz G, Abraham D, Kissling W: Efficacy and extrapyramidal side-effects of the new antipsychotics olanzapine, quetiapine, risperidone, and sertindole compared to conventional antipsychotics and placebo: a meta-analysis of randomized controlled trials. Schizophr Res 1999; 35:51–68 [E]Crossref, Google Scholar
298 Beasley CM, Dellva MA, Tamura RN, Morgenstern H, Glazer WM, Ferguson K, Tollefson GD: Randomised double-blind comparison of the incidence of tardive dyskinesia in patients with schizophrenia during long-term treatment with olanzapine or haloperidol. Br J Psychiatry 1999; 174:23–30 [A]Crossref, Google Scholar
299 Chou JC, Czobor P, Charles O, Tuma I, Winsberg B, Allen MH, Trujillo M, Volavka J: Acute mania: haloperidol dose and augmentation with lithium or lorazepam. J Clin Psychopharmacol 1999; 19:500–505 [A]Crossref, Google Scholar
300 Klein DF: Importance of psychiatric diagnosis in prediction of clinical drug effects. Arch Gen Psychiatry 1967; 16:118–126 [A]Crossref, Google Scholar
301 Sachs GS: Emerging data: atypical antipsychotics in bipolar disorder, in Program and Abstracts of the 52nd Institute on Psychiatric Services. Washington, DC, APA, 2001 [A]Google Scholar
302 Keck PE Jr: Atypical antipsychotics in the treatment of aggressive behaviors, in 2001 Annual Meeting Syllabus and Proceedings Summary. Washington, DC, American Psychiatric Association, 2001 [A]Google Scholar
303 Suppes T, Webb A, Paul B, Carmody T, Kraemer H, Rush AJ: Clinical outcome in a randomized 1-year trial of clozapine versus treatment as usual for patients with treatment-resistant illness and a history of mania. Am J Psychiatry 1999; 156:1164–1169 [B]Google Scholar
304 Calabrese JR, Kimmel SE, Woyshville MJ, Rapport DJ, Faust CJ, Thompson PA, Meltzer HY: Clozapine for treatment-refractory mania. Am J Psychiatry 1996; 153:759–764 [B]Crossref, Google Scholar
305 Green AI, Tohen M, Patel JK, Banov M, DuRand C, Berman I, Chang H, Zarate C Jr, Posener J, Lee H, Dawson R, Richards C, Cole JO, Schatzberg AF: Clozapine in the treatment of refractory psychotic mania. Am J Psychiatry 2000; 157:982–986 [B]Crossref, Google Scholar
306 Muller-Oerlinghausen B, Retzow A, Henn FA, Giedke H, Walden J (European Valproate Mania Study Group): Valproate as an adjunct to neuroleptic medication for the treatment of acute episodes of mania: a prospective, randomized, double-blind, placebo-controlled, multicenter study. J Clin Psychopharmacol 2000; 20:195–203 [A]Crossref, Google Scholar
307 Yatham LN: Safety and efficacy of risperidone as combination therapy for the manic phase of bipolar disorder: preliminary findings of a randomized double blind study (RIS-INT-46). Int J Neuropsychopharmacol 2000; 3(suppl 1):S142 [A]Google Scholar
308 Small JG, Klapper MH, Kellams JJ, Miller MJ, Milstein V, Sharpley PH, Small IF: Electroconvulsive treatment compared with lithium in the management of manic states. Arch Gen Psychiatry 1988; 45:727–732 [A]Crossref, Google Scholar
309 Mukherjee S, Sackeim HA, Schnur DB: Electroconvulsive therapy of acute manic episodes: a review of 50 years’ experience. Am J Psychiatry 1994; 151:169–176 [F]Crossref, Google Scholar
310 Sikdar S, Kulhara P, Avasthi A, Singh H: Combined chlorpromazine and electroconvulsive therapy in mania. Br J Psychiatry 1994; 164:806–810 [A]Crossref, Google Scholar
311 Black DW, Winokur G, Nasrallah A: Treatment of mania: a naturalistic study of electroconvulsive therapy versus lithium in 438 patients. J Clin Psychiatry 1987; 48:132–139 [C]Google Scholar
312 Thomas J, Reddy B: The treatment of mania: a retrospective evaluation of the effects of ECT, chlorpromazine, and lithium. J Affect Disord 1982; 4:85–92 [D]Crossref, Google Scholar
313 Ciapparelli A, Dell’Osso L, Tundo A, Pini S, Chiavacci MC, Di Sacco I, Cassano GB: Electroconvulsive therapy in medication-nonresponsive patients with mixed mania and bipolar depression. J Clin Psychiatry 2001; 62:552–555 [B]Crossref, Google Scholar
314 Devanand DP, Polanco P, Cruz R, Shah S, Paykina N, Singh K, Majors L: The efficacy of ECT in mixed affective states. J ECT 2000; 16:32–37 [B]Crossref, Google Scholar
315 Gruber NP, Dilsaver SC, Shoaib AM, Swann AC: ECT in mixed affective states: a case series. J ECT 2000; 16:183–188 [G]Crossref, Google Scholar
316 McElroy SL, Keck PE Jr: Pharmacologic agents for the treatment of acute bipolar mania. Biol Psychiatry 2000; 48:539–557 [F]Crossref, Google Scholar
317 Lenox RH, Newhouse PA, Creelman WL, Whitaker TM: Adjunctive treatment of manic agitation with lorazepam versus haloperidol: a double-blind study. J Clin Psychiatry 1992; 53:47–52 [A]Google Scholar
318 Edwards R, Stephenson U, Flewett T: Clonazepam in acute mania: a double blind trial. Aust NZ J Psychiatry 1991; 25:238–242 [A]Crossref, Google Scholar
319 Chouinard G, Young SN, Annable L: Antimanic effect of clonazepam. Biol Psychiatry 1983; 18:451–466 [B]Google Scholar
320 Chouinard G: Clonazepam in acute and maintenance treatment of bipolar affective disorder. J Clin Psychiatry 1987; 48(Oct suppl):29–37 [B]Google Scholar
321 Chouinard G, Annable L, Turnier L, Holobow N, Szkrumelak N: A double-blind randomized clinical trial of rapid tranquilization with IM clonazepam and IM haloperidol in agitated psychotic patients with manic symptoms. Can J Psychiatry 1993; 38(suppl 4):S114–S121 [A]Google Scholar
322 Meehan K, Zhang F, David S, Tohen M, Janicak P, Small J, Koch M, Rizk R, Walker D, Tran P, Breier A: A double-blind, randomized comparison of the efficacy and safety of intramuscular injections of olanzapine, lorazepam, or placebo in treating acutely agitated patients diagnosed with bipolar mania. J Clin Psychopharmacol 2001; 21:389–397 [A]Crossref, Google Scholar
323 Walton SA, Berk M, Brook S: Superiority of lithium over verapamil in mania: a randomized, controlled, single-blind trial. J Clin Psychiatry 1996; 57:543–546 [A]Crossref, Google Scholar
324 Janicak PG, Sharma RP, Pandey G, Davis JM: Verapamil for the treatment of acute mania: a double-blind, placebo-controlled trial. Am J Psychiatry 1998; 155:972–973 [A]Crossref, Google Scholar
325 Pazzaglia PJ, Post RM, Ketter TA, George MS, Marangell LB: Preliminary controlled trial of nimodipine in ultra-rapid cycling affective dysregulation. Psychiatry Res 1993; 49:257–272 [A]Crossref, Google Scholar
326 Zornberg GL, Pope HG Jr: Treatment of depression in bipolar disorder: new directions for research. J Clin Psychopharmacol 1993; 13:397–408 [F]Crossref, Google Scholar
327 Ballenger JC, Post RM: Carbamazepine in manic-depressive illness: a new treatment. Am J Psychiatry 1980; 137:782–790 [B]Crossref, Google Scholar
328 Dilsaver SC, Swann SC, Chen YW, Shoaib A, Joe B, Krajewski KJ, Gruber N, Tsai Y: Treatment of bipolar depression with carbamazepine: results of an open study. Biol Psychiatry 1996; 40:935–937 [G]Crossref, Google Scholar
329 Calabrese JR, Bowden CL, Sachs GS, Ascher JA, Monaghan E, Rudd GD (Lamictal 602 Study Group): A double-blind placebo-controlled study of lamotrigine monotherapy in outpatients with bipolar I depression. J Clin Psychiatry 1999; 60:79–88 [A]Crossref, Google Scholar
330 Bowden CL: Novel treatments for bipolar disorder. Expert Opin Investig Drugs 2001; 10:661–671 [F]Crossref, Google Scholar
331 Calabrese JR, Bowden CL, McElroy SL, Cookson J, Andersen J, Keck PE Jr, Rhodes L, Bolden-Watson C, Zhou J, Ascher JA: Spectrum of activity of lamotrigine in treatment-refractory bipolar disorder. Am J Psychiatry 1999; 156:1019–1023 [B]Google Scholar
332 Deveaugh-Geiss J, Ascher J, Brrok S, Cedrone J, Earl N, Emsley R, Frangou S, Huffman R: Safety and tolerability of lamotrigine in controlled monotherapy, in American College of Neuropsychopharmacology Annual Meeting Poster Abstracts. Nashville, Tenn, ACNP, 2000 [G]Google Scholar
333 Sullivan JR, Shear NH: The drug hypersensitivity syndrome: what is the pathogenesis? Arch Dermatol 2001; 137:357–364 [G]Google Scholar
334 Guberman AH, Besag FM, Brodie MJ, Dooley JM, Duchowny MS, Pellock JM, Richens A, Stern RS, Trevathan E: Lamotrigine-associated rash: risk/benefit considerations in adults and children. Epilepsia 1999; 40:985–991 [G]Crossref, Google Scholar
335 Messenheimer J, Mullens EL, Giorgi L, Young F: Safety review of adult clinical trial experience with lamotrigine. Drug Saf 1998; 18:281–296 [F]Crossref, Google Scholar
336 Fitton A, Goa KL: Lamotrigine: an update of its pharmacology and therapeutic use in epilepsy. Drugs 1995; 50:691–713 [F]Crossref, Google Scholar
337 Rambeck B, Wolf P: Lamotrigine clinical pharmacokinetics. Clin Pharmacokinet 1993; 25:433–443 [F]Crossref, Google Scholar
338 McIntyre RS, Mancini D, McCann JM: Randomized, single-blind comparison of topiramate and bupropion SR as add-on therapy in bipolar depression (abstract). Acta Neuropsychiatrica 2000; 12:163 [B]Google Scholar
339 Hussein MZ: Treatment of bipolar depression with topiramate (abstract). Eur Neuropsychopharmacol 1999; 9(suppl 5):S222 [B]Google Scholar
340 Himmelhoch JM, Thase ME, Mallinger AG, Houck P: Tranylcypromine versus imipramine in anergic bipolar depression. Am J Psychiatry 1991; 148:910–916 [A]Crossref, Google Scholar
341 Thase ME, Mallinger AG, McKnight D, Himmelhoch JM: Treatment of imipramine-resistant recurrent depression, IV: a double-blind crossover study of tranylcypromine for anergic bipolar depression. Am J Psychiatry 1992; 149:195–198 [A]Crossref, Google Scholar
342 Baumhackl U, Biziere K, Fischbach R, Geretsegger C, Hebenstreit G, Radmayr E, Stabl M: Efficacy and tolerability of moclobemide compared with imipramine in depressive disorder (DSM-III): an Austrian double-blind, multicentre study. Br J Psychiatry Suppl 1989; 6:78–83 [A]Google Scholar
343 Cohn JB, Collins G, Ashbrook E, Wernicke JF: A comparison of fluoxetine, imipramine, and placebo in patients with bipolar depressive disorder. Int Clin Psychopharmacol 1989; 4:313–322 [A]Crossref, Google Scholar
344 Young LT, Joffe RT, Robb JC, MacQueen GM, Marriott M, Patelis-Siotis I: Double-blind comparison of addition of a second mood stabilizer versus an antidepressant to an initial mood stabilizer for treatment of patients with bipolar depression. Am J Psychiatry 2000; 157:124–126 [A]Crossref, Google Scholar
345 Nemeroff CB, Evans DL, Gyulai L, Sachs GS, Bowden CL, Gergel IP, Oakes R, Pitts CD: Double-blind, placebo-controlled comparison of imipramine and paroxetine in the treatment of bipolar depression. Am J Psychiatry 2001; 158:906–912 [A]Crossref, Google Scholar
346 Vieta E: Martinez-Arán A, Goikolea JM: A randomized trial comparing paroxetine and venlafaxine in the treatment of bipolar depressed patients taking mood stabilizers. J Clin Psychiatry (in press) [A]Google Scholar
347 Kupfer DJ, Chengappa KN, Gelenberg AJ, Hirschfeld RM, Goldberg JF, Sachs GS, Grochochinski VJ, Houck PR, Kolar KB: Citalopram as adjunctive therapy in bipolar depression. J Clin Psychiatry 2001; 62:985–990 [B]Crossref, Google Scholar
348 Sachs GS, Lafer B, Stoll AL, Banov M, Thibault AB, Tohen M, Rosenbaum JF: A double-blind trial of bupropion versus desipramine for bipolar depression. J Clin Psychiatry 1994; 55:391–393 [A]Google Scholar
349 Grossman F, Potter WZ, Brown EA, Maislin G: A double-blind study comparing idazoxan and bupropion in bipolar depressed patients. J Affect Disord 1999; 56:237–243 [A]Crossref, Google Scholar
350 Amsterdam JD, Garcia-Espana F: Venlafaxine monotherapy in women with bipolar II and unipolar major depression. J Affect Disord 2000; 59:225–229 [A]Crossref, Google Scholar
351 Wehr TA, Sack DA, Rosenthal NE: Sleep reduction as a final common pathway in the genesis of mania. Am J Psychiatry 1987; 144:201–204; correction, 144:542 [F]Crossref, Google Scholar
352 Smeraldi E, Benedetti F, Barbini B, Campori E, Colombo C: Sustained antidepressant effect of sleep deprivation combined with pindolol in bipolar depression: a placebo-controlled trial. Neuropsychopharmacology 1999; 20:380–385 [A]Crossref, Google Scholar
353 Colombo C, Lucca A, Benedetti F, Barbini B, Campori E, Smeraldi E: Total sleep deprivation combined with lithium and light therapy in the treatment of bipolar depression: replication of main effects and interaction. Psychiatry Res 2000; 95:43–53 [A]Crossref, Google Scholar
354 Stoll AL, Severus WE, Freeman MP, Rueter S, Zboyan HA, Diamond E, Cress KK, Marangell LB: Omega 3 fatty acids in bipolar disorder: a preliminary double-blind, placebo-controlled trial. Arch Gen Psychiatry 1999; 56:407–412 [A]Crossref, Google Scholar
355 Sporn J, Ghaemi SN, Sambur MR, Rankin MA, Recht J, Sachs GS, Rosenbaum JF, Fava M: Pramipexole augmentation in the treatment of unipolar and bipolar depression: a retrospective chart review. Ann Clin Psychiatry 2000; 12:137–140 [D]Crossref, Google Scholar
356 George MS, Sackeim HA, Rush AJ, Marangell LB, Nahas Z, Husain MM, Lisanby S, Burt T, Goldman J, Ballenger JC: Vagus nerve stimulation: a new tool for brain research and therapy. Biol Psychiatry 2000; 47:287–295 [F]Crossref, Google Scholar
357 Nahas Z, Molloy MA, Hughes PL, Oliver NC, Arana GW, Risch SC, George MS: Repetitive transcranial magnetic stimulation: perspectives for application in the treatment of bipolar and unipolar disorders. Bipolar Disord 1999; 1:73–80 [F]Crossref, Google Scholar
358 Cole AJ, Scott J, Ferrier IN, Eccleston D: Patterns of treatment resistance in bipolar affective disorder. Acta Psychiatr Scand 1993; 88:121–123 [C]Crossref, Google Scholar
359 Dunner DL, Fieve RR: Clinical factors in lithium carbonate prophylaxis failure. Arch Gen Psychiatry 1974; 30:229–233 [F]Crossref, Google Scholar
360 Peet M: Induction of mania with selective serotonin re-uptake inhibitors and tricyclic antidepressants. Br J Psychiatry 1994; 164:549–550 [E]Crossref, Google Scholar
361 Okuma T, Inanaga K, Otsuki S, Sarai K, Takahashi R, Hazama H, Mori A, Watanabe S: A preliminary double-blind study on the efficacy of carbamazepine in prophylaxis of manic-depressive illness. Psychopharmacology (Berl) 1981; 73:95–96 [A]Crossref, Google Scholar
362 Denicoff KD, Smith-Jackson EE, Disney ER, Ali SO, Leverich GS, Post RM: Comparative prophylactic efficacy of lithium, carbamazepine, and the combination in bipolar disorder. J Clin Psychiatry 1997; 58:470–478 [B]Crossref, Google Scholar
363 Bauer MS, Whybrow PC, Gyulai L, Gonnel J, Yeh HS: Testing definitions of dysphoric mania and hypomania: prevalence, clinical characteristics and inter-episode stability. J Affect Disord 1994; 32:201–211 [C]Crossref, Google Scholar
364 Maj M, Pirozzi R, Magliano L, Bartoli L: Long-term outcome of lithium prophylaxis in bipolar disorder: a 5-year prospective study of 402 patients at a lithium clinic. Am J Psychiatry 1998; 155:30–35 [C]Crossref, Google Scholar
365 Dunner DL, Stallone F, Fieve RR: Lithium carbonate and affective disorders, V: a double-blind study of prophylaxis of depression in bipolar illness. Arch Gen Psychiatry 1976; 33:117–120 [A]Crossref, Google Scholar
366 Shapiro DR, Quitkin FM, Fleiss JL: Response to maintenance therapy in bipolar illness: effect of index episode. Arch Gen Psychiatry 1989; 46:401–405 [E]Crossref, Google Scholar
367 Bowden CL, Calabrese JR, McElroy SL, Rhodes LJ, Keck PE Jr, Cookson J, Anderson J, Bolden-Watson C, Ascher J, Monaghan E, Zhou J: The efficacy of lamotrigine in rapid cycling and nonrapid cycling patients with bipolar disorder. Biol Psychiatry 1999; 45:953–958 [G]Crossref, Google Scholar
368 Calabrese JR, Delucchi GA: Spectrum of efficacy of valproate in 55 patients with rapid-cycling bipolar disorder. Am J Psychiatry 1990; 147:431–434 [G]Crossref, Google Scholar
369 Calabrese JR, Woyshville MJ, Kimmel SE, Rapport DJ: Predictors of valproate response in bipolar rapid cycling. J Clin Psychopharmacol 1993; 13:280–283 [E]Crossref, Google Scholar
370 Sachs GS, Printz DJ, Kahn DA, Carpenter D, Docherty JP: The Expert Consensus Guideline Series: Medication Treatment of Bipolar Disorder. Postgrad Med Special Issue 2000; 1:1–104 [G]Google Scholar
371 Bowden CL, Lecrubier Y, Bauer M, Goodwin G, Greil W, Sachs G, von Knorring L: Maintenance therapies for classic and other forms of bipolar disorder. J Affect Disord 2000; 59(suppl 1): S57–S67 [F]Crossref, Google Scholar
372 Baldessarini RJ, Tohen M, Tondo L: Maintenance treatment in bipolar disorder. Arch Gen Psychiatry 2000; 57:490–492 [G]Crossref, Google Scholar
373 Baastrup PC, Poulsen JC, Schou M, Thomsen K, Amdisen A: Prophylactic lithium: double blind discontinuation in manic-depressive and recurrent-depressive disorders. Lancet 1970; 2:326–330 [A]Crossref, Google Scholar
374 Melia PI: Prophylactic lithium: a double-blind trial in recurrent affective disorders. Br J Psychiatry 1970; 116:621–624 [A]Crossref, Google Scholar
375 Coppen A, Peet M, Bailey J, Noguera R, Burns BH, Swani MS, Maggs R, Gardner R: Double-blind and open prospective studies on lithium prophylaxis in affective disorders. Psychiatr Neurol Neurochir 1973; 76:501–510 [F]Google Scholar
376 Cundall RL, Brooks PW, Murray LG: A controlled evaluation of lithium prophylaxis in affective disorders. Psychol Med 1972; 2:308–311 [B]Crossref, Google Scholar
377 Prien RF, Caffey EM Jr, Klett CJ: Prophylactic efficacy of lithium carbonate in manic-depressive illness: report of the Veterans Administration and National Institute of Mental Health collaborative study group. Arch Gen Psychiatry 1973; 28:337–341 [A]Crossref, Google Scholar
378 Suppes T, Baldessarini RJ, Faedda GL, Tohen M: Risk of recurrence following discontinuation of lithium treatment in bipolar disorder. Arch Gen Psychiatry 1991; 48:1082–1088 [E]Crossref, Google Scholar
379 Markar HR, Mander AJ: Efficacy of lithium prophylaxis in clinical practice. Br J Psychiatry 1989; 155:496–500 [F]Crossref, Google Scholar
380 Harrow M, Goldberg JF, Grossman LS, Meltzer HY: Outcome in manic disorders: a naturalistic follow-up study. Arch Gen Psychiatry 1990; 47:665–671 [C]Crossref, Google Scholar
381 Coryell W, Winokur G, Solomon D, Shea T, Leon A, Keller M: Lithium and recurrence in a long-term follow-up of bipolar affective disorder. Psychol Med 1997; 27:281–289 [C]Crossref, Google Scholar
382 Gitlin MJ, Swendsen J, Heller TL, Hammen C: Relapse and impairment in bipolar disorder. Am J Psychiatry 1995; 152:1635–1640 [C]Crossref, Google Scholar
383 Licht RW, Vestergaard P, Rasmussen NA, Jepsen K, Brodersen A, Hansen PE: A lithium clinic for bipolar patients: 2-year outcome of the first 148 patients. Acta Psychiatr Scand 2001; 104:387–390 [B]Crossref, Google Scholar
384 Peselow ED, Fieve RR, Difiglia C, Sanfilipo MP: Lithium prophylaxis of bipolar illness: the value of combination treatment. Br J Psychiatry 1994; 164:208–214 [D]Crossref, Google Scholar
385 Bowden CL, Calabrese JR, McElroy SL, Gyulai L, Wassef A, Petty F, Pope HG Jr, Chou JC, Keck PE Jr, Rhodes LJ, Swann AC, Hirschfeld RM, Wozniak PJ (Divalproex Maintenance Study Group): A randomized, placebo-controlled 12-month trial of divalproex and lithium in treatment of outpatients with bipolar I disorder. Arch Gen Psychiatry 2000; 57:481–489 [A]Crossref, Google Scholar
386 Calabrese JR, Bowden CL, DeVeaugh-Geiss J, Earl NL, Gyulai L, Sachs GS, Montgomery P: Lamotrigine demonstrates longterm mood stabilization in manic patients, in 2001 Annual Meeting New Research Program and Abstracts. Washington, DC, American Psychiatric Association, 2001, p 110 [A]Google Scholar
387 Greil W, Ludwig-Mayerhofer W, Erazo N, Schochlin C, Schmidt S, Engel RR, Czernik A, Giedke H, Muller-Oerlinghausen B, Osterheider M, Rudolf GA, Sauer H, Tegeler J, Wetterling T: Lithium versus carbamazepine in the maintenance treatment of bipolar disorders—a randomised study. J Affect Disord 1997; 43:151–161 [B]Crossref, Google Scholar
388 Lambert PA, Venaud G: [Comparative study of valpromide versus lithium as prophylactic treatment in affective disorders.] Nervure 1992; 5:57–65 (French) [B]Google Scholar
389 Greil W, Kleindienst N, Erazo N, Muller-Oerlinghausen B: Differential response to lithium and carbamazepine in the prophylaxis of bipolar disorder. J Clin Psychopharmacol 1998; 18:455–460 [E]Crossref, Google Scholar
390 Stromgren LS: The combination of lithium and carbamazepine in treatment and prevention of manic-depressive disorder: a review and a case report. Compr Psychiatry 1990; 31:261–265 [E]Crossref, Google Scholar
391 Esparon J, Kolloori J, Naylor GJ, McHarg AM, Smith AH, Hopwood SE: Comparison of the prophylactic action of flupenthixol with placebo in lithium treated manic-depressive patients. Br J Psychiatry 1986; 148:723–725 [A]Crossref, Google Scholar
392 Stevenson GH, Geoghegan JJ: Prophylactic electroshock; a five-year study. Am J Psychiatry 1951; 107:743–748 [B]Crossref, Google Scholar
393 Geoghegan JJ, Stevenson GH: Prophylactic electroshock. Am J Psychiatry 1949; 105:494–496 [B]Crossref, Google Scholar
394 Godemann F, Hellweg R: [20 years unsuccessful prevention of bipolar affective psychosis recurrence.] Nervenarzt 1997; 68:582–585 (German) [F]Crossref, Google Scholar
395 Chanpattana W: Combined ECT and clozapine in treatment-resistant mania. J ECT 2000; 16:204–207 [G]Crossref, Google Scholar
396 Decina P, Schlegel AM, Fieve RR: Lithium poisoning. NY State J Med 1987; 87:230–231 [G]Google Scholar
397 Kramer BA: A naturalistic review of maintenance ECT at a university setting. J ECT 1999; 15:262–269 [G]Google Scholar
398 Rhodes LJ: Maintenance ECT replaced with lamotrigine (letter). Am J Psychiatry 2000; 157:2058 [G]Crossref, Google Scholar
399 Gupta S, Austin R, Devanand DP: Lithium and maintenance electroconvulsive therapy. J ECT 1998; 14:241–244 [G]Crossref, Google Scholar
400 Barnes RC, Hussein A, Anderson DN, Powell D: Maintenance electroconvulsive therapy and cognitive function. Br J Psychiatry 1997; 170:285–287 [G]Crossref, Google Scholar
401 Jaffe R, Dubin WR: Oral versus intravenous caffeine augmentation of ECT (letter). Am J Psychiatry 1992; 149:1610 [G]Crossref, Google Scholar
402 Karliner W: Accidental convulsion induced by atropine. Am J Psychiatry 1965; 122:578–579 [G]Crossref, Google Scholar
403 Clarke L: Psychiatric nursing and electroconvulsive therapy. Nurs Ethics 1995; 2:321–331 [F]Crossref, Google Scholar
404 Kramer BA: A seasonal schedule for maintenance ECT. J ECT 1999; 15:226–231 [G]Google Scholar
405 Vanelle JM, Loo H, Galinowski A, de Carvalho W, Bourdel MC, Brochier P, Bouvet O, Brochier T, Olie JP: Maintenance ECT in intractable manic-depressive disorders. Convuls Ther 1994; 10:195–205 [B]Google Scholar
406 Schwarz T, Loewenstein J, Isenberg KE: Maintenance ECT: indications and outcome. Convuls Ther 1995; 11:14–23 [B]Google Scholar
407 Gagné GG Jr, Furman MJ, Carpenter LL, Price LH: Efficacy of continuation ECT and antidepressant drugs compared to long-term antidepressants alone in depressed patients. Am J Psychiatry 2000; 157:1960–1965 [B]Crossref, Google Scholar
408 Kahn DA: The use of psychodynamic psychotherapy in manic-depressive illness. J Am Acad Psychoanal 1993; 21:441–455 [A]Crossref, Google Scholar
409 Haas GL, Glick ID, Clarkin JF, Spencer JH, Lewis AB, Peyser J, DeMane N, Good-Ellis M, Harris E, Lestelle V: Inpatient family intervention: a randomized clinical trial, II: results at hospital discharge. Arch Gen Psychiatry 1988; 45:217–224 [A]Crossref, Google Scholar
410 Clarkin JF, Carpenter D, Hull J, Wilner P, Glick I: Effects of psychoeducational intervention for married patients with bipolar disorder and their spouses. Psychiatr Serv 1998; 49:531–533 [A]Crossref, Google Scholar
411 Miklowitz DJ, Goldstein MJ: Bipolar Disorder: A Family-Focused Treatment Approach. New York, Guilford Press, 1997 [G]Google Scholar
412 Miklowitz DJ, Simoneau TL, George EL, Richards JA, Kalbag A, Sachs-Ericsson N, Suddath R: Family-focused treatment of bipolar disorder: 1-year effects of a psychoeducational program in conjunction with pharmacotherapy. Biol Psychiatry 2000; 48:582–592 [A]Crossref, Google Scholar
413 Basco MR, Rush AJ: Cognitive-Behavioral Therapy for Bipolar Disorder. New York, Guilford Press, 1996 [G]Google Scholar
414 Zaretsky AE, Segal ZV, Gemar M: Cognitive therapy for bipolar depression: a pilot study. Can J Psychiatry 1999; 44:491–494 [B]Crossref, Google Scholar
415 Fava GA, Bartolucci G, Rafanelli C, Mangelli L: Cognitive-behavioral management of patients with bipolar disorder who relapsed while on lithium prophylaxis. J Clin Psychiatry 2001; 62:556–559 [B]Crossref, Google Scholar
416 Cochran SD: Preventing medical noncompliance in the outpatient treatment of bipolar affective disorders. J Consult Clin Psychol 1984; 52:873–878 [A]Crossref, Google Scholar
417 Ehlers CL, Frank E, Kupfer DJ: Social zeitgebers and biological rhythms: a unified approach to understanding the etiology of depression. Arch Gen Psychiatry 1988; 45:948–952 [F]Crossref, Google Scholar
418 Frank E, Hlastala S, Ritenour A, Houck P, Tu XM, Monk TH, Mallinger AG, Kupfer DJ: Inducing lifestyle regularity in recovering bipolar disorder patients: results from the maintenance therapies in bipolar disorder protocol. Biol Psychiatry 1997; 41:1165–1173 [A]Crossref, Google Scholar
419 Frank E, Swartz HA, Kupfer DJ: Interpersonal and social rhythm therapy: managing the chaos of bipolar disorder. Biol Psychiatry 2000; 48:593–604 [F]Crossref, Google Scholar
420 Cole DP, Thase ME, Mallinger AG, Soares JC, Luther JF, Kupfer DJ, Frank E: Slower treatment response in bipolar depression predicted by lower pretreatment thyroid function. Am J Psychiatry 2002; 159:116–121 [A]Crossref, Google Scholar
421 Frank E, Swartz HA, Mallinger AG, Thase ME, Weaver EV, Kupfer DJ: Adjunctive psychotherapy for bipolar disorder: effects of changing treatment modality. J Abnorm Psychol 1999; 108:579–587 [A]Crossref, Google Scholar
422 Colom F, Vieta E, Benabarre A, Martinez-Aran A, Reinares M, Corbella B, Gasto C: Topiramate abuse in a bipolar patient with an eating disorder. J Clin Psychiatry 2001; 62:475–476 [G]Crossref, Google Scholar
423 Beck AT, Rush AJ, Shaw BF, Emery G: Cognitive Therapy of Depression. New York, Guilford, 1979 [G]Google Scholar
424 Klerman GL, Weissman MM, Rounsaville BJ, Chevron ES: Interpersonal Psychotherapy of Depression. New York, Basic Books, 1984 [G]Google Scholar
425 Frank E, Kupfer DJ, Perel JM, Cornes C, Jarrett DB, Mallinger AG, Thase ME, McEachran AB, Grochocinski VJ: Three-year outcomes for maintenance therapies in recurrent depression. Arch Gen Psychiatry 1990; 47:1093–1099 [A]Crossref, Google Scholar
426 Weissman MM, Markowitz JC, Klerman GL: Comprehensive Guide to Interpersonal Psychotherapy. New York, Basic Books, 2000 [G]Google Scholar
427 Frank E, Kupfer DJ, Gibbons R, Houck P, Kostelnik B, Mallinger AG, Swartz HA, Thase ME: Interpersonal and social rhythm therapy prevents depressive symptomatology in patients with bipolar I disorder. Arch Gen Psychiatry (in press) [A]Google Scholar
428 Bauer MS, McBride L, Chase C, Sachs G, Shea N: Manual-based group psychotherapy for bipolar disorder: a feasibility study. J Clin Psychiatry 1998; 59:449–455 [B]Crossref, Google Scholar
429 Rush AJ, Thase ME: Psychotherapies for depressive disorders: a review, in Evidence and Experience in Psychiatry, vol 1: Depressive Disorders. Edited by Maj M, Sartorious N. Chichester, UK, John Wiley & Sons, 1999, pp 161–206 [G]Google Scholar
430 Blacker D, Tsuang MT: Contested boundaries of bipolar disorder and the limits of categorical diagnosis in psychiatry. Am J Psychiatry 1992; 149:1473–1483 [F]Crossref, Google Scholar
431 Akiskal HS, Hirschfeld RM, Yerevanian BI: The relationship of personality to affective disorders. Arch Gen Psychiatry 1983; 40:801–810 [F]Crossref, Google Scholar
432 Geller B, Cooper TB, Sun K, Zimerman B, Frazier J, Williams M, Heath J: Double-blind and placebo-controlled study of lithium for adolescent bipolar disorders with secondary substance dependency. J Am Acad Child Adolesc Psychiatry 1998; 37:171–178 [A]Crossref, Google Scholar
433 Gram LF, Rafaelsen OJ: Lithium treatment of psychotic children and adolescents: a controlled clinical trial. Acta Psychiatr Scand 1972; 48:253–260 [A]Crossref, Google Scholar
434 Lena B: Lithium in child and adolescent psychiatry. Arch Gen Psychiatry 1979; 36:854–855 [A]Crossref, Google Scholar
435 McKnew DH, Cytryn L, Buchsbaum MS, Hamovit J, Lamour M, Rapoport JL, Gershon ES: Lithium in children of lithium-responding parents. Psychiatry Res 1981; 4:171–180 [A]Crossref, Google Scholar
436 DeLong GR, Nieman GW: Lithium-induced behavior changes in children with symptoms suggesting manic-depressive illness. Psychopharmacol Bull 1983; 19:258–265 [A]Google Scholar
437 Annell AL: Manic-depressive illness in children and effect of treatment with lithium carbonate. Acta Paedopsychiatr 1969; 36:292–301 [G]Google Scholar
438 Dyson WL, Barcai A: Treatment of children of lithium-responding parents. Curr Ther Res Clin Exp 1970; 12:286–290 [G]Google Scholar
439 Dugas M, Gueriot C, Frohwirth C: [Has lithium a value in child psychiatry]. Rev Neuropsychiatr Infant 1975; 23:365–372 (French) [B]Google Scholar
440 Watanabe S, Ishino H, Otsuki S: Double-blind comparison of lithium carbonate and imipramine in treatment of depression. Arch Gen Psychiatry 1975; 32:659–668 [A]Crossref, Google Scholar
441 Brumback RA, Weinberg WA: Mania in childhood, II: therapeutic trial of lithium carbonate and further description of manic-depressive illness in children. Am J Dis Child 1977; 131:1122–1126 [B]Crossref, Google Scholar
442 Horowitz HA: Lithium and the treatment of adolescent manic depressive illness. Dis Nerv Syst 1977; 38:480–483 [G]Google Scholar
443 Carlson GA, Strober M: Manic-depressive illness in early adolescence: a study of clinical and diagnostic characteristics in six cases. J Am Acad Child Psychiatry 1978; 17:138–153 [C]Crossref, Google Scholar
444 Davis RE: Manic-depressive variant syndrome of childhood: a preliminary report. Am J Psychiatry 1979; 136:702–706 [G]Crossref, Google Scholar
445 Hassanyeh F, Davison K: Bipolar affective psychosis with onset before age 16 years: report of 10 cases. Br J Psychiatry 1980; 137:530–539 [G]Crossref, Google Scholar
446 Rogeness GA, Riester AE, Wicoff JS: Unusual presentation of manic depressive disorder in adolescence. J Clin Psychiatry 1982; 43:37–39 [G]Google Scholar
447 Sylvester CE, Burke PM, McCauley EA, Clark CJ: Manic psychosis in childhood: report of two cases. J Nerv Ment Dis 1984; 172:12–15 [G]Crossref, Google Scholar
448 DeLong GR, Aldershof AL: Long-term experience with lithium treatment in childhood: correlation with clinical diagnosis. J Am Acad Child Adolesc Psychiatry 1987; 26:389–394 [B]Crossref, Google Scholar
449 Hsu LK, Starzynski JM: Mania in adolescence. J Clin Psychiatry 1986; 47:596–599 [C]Google Scholar
450 Hsu LK: Lithium-resistant adolescent mania. J Am Acad Child Psychiatry 1986; 25:280–283 [G]Crossref, Google Scholar
451 Varanka TM, Weller RA, Weller EB, Fristad MA: Lithium treatment of manic episodes with psychotic features in prepubertal children. Am J Psychiatry 1988; 145:1557–1559 [B]Crossref, Google Scholar
452 Tomasson K, Kuperman S: Bipolar disorder in a prepubescent child. J Am Acad Child Adolesc Psychiatry 1990; 29:308–310 [F]Crossref, Google Scholar
453 Carlson GA, Rapport MD, Pataki CS, Kelly KL: Lithium in hospitalized children at 4 and 8 weeks: mood, behavior and cognitive effects. J Child Psychol Psychiatry 1992; 33:411–425 [A]Crossref, Google Scholar
454 Kafantaris V, Coletti DJ, Dicker R, Padula G, Pollack S: Are childhood psychiatric histories of bipolar adolescents associated with family history, psychosis, and response to lithium treatment? J Affect Disord 1998; 51:153–164 [A]Crossref, Google Scholar
455 Strober M, DeAntonio M, Schmidt-Lackner S, Freeman R, Lampert C, Diamond J: Early childhood attention deficit hyperactivity disorder predicts poorer response to acute lithium therapy in adolescent mania. J Affect Disord 1998; 51:145–151 [B]Crossref, Google Scholar
456 Papatheodorou G, Kutcher SP: Divalproex sodium treatment in late adolescent and young adult acute mania. Psychopharmacol Bull 1993; 29:213–219 [B]Google Scholar
457 Papatheodorou G, Kutcher SP, Katic M, Szalai JP: The efficacy and safety of divalproex sodium in the treatment of acute mania in adolescents and young adults: an open clinical trial. J Clin Psychopharmacol 1995; 15:110–116 [B]Crossref, Google Scholar
458 Wagner KD: Safety and efficacy of divalproex in childhood bipolar disorder, in Abstracts of Posters Presented at the 2000 Annual Meeting of the American Academy of Child and Adolescent Psychiatry. Washington, DC, AACAP, 2000 [G]Google Scholar
459 Kastner T, Friedman DL: Verapamil and valproic acid treatment of prolonged mania. J Am Acad Child Adolesc Psychiatry 1992; 31:271–275 [D]Crossref, Google Scholar
460 Kastner T, Friedman DL, Plummer AT, Ruiz MQ, Henning D: Valproic acid for the treatment of children with mental retardation and mood symptomatology. Pediatrics 1990; 86:467–472 [G]Google Scholar
461 Whittier MC, West SA, Galli VB, Raute NJ: Valproic acid for dysphoric mania in a mentally retarded adolescent. J Clin Psychiatry 1995; 56:590–591 [G]Google Scholar
462 Deltito JA, Levitan J, Damore J, Hajal F, Zambenedetti M: Naturalistic experience with the use of divalproex sodium on an in-patient unit for adolescent psychiatric patients. Acta Psychiatr Scand 1998; 97:236–240 [E]Crossref, Google Scholar
463 Kowatch RA, Suppes T, Carmody TJ, Bucci JP, Hume JH, Kromelis M, Emslie GJ, Weinberg WA, Rush AJ: Effect size of lithium, divalproex sodium, and carbamazepine in children and adolescents with bipolar disorder. J Am Acad Child Adolesc Psychiatry 2000; 39:713–720 [A]Crossref, Google Scholar
464 Woolston JL: Case study: carbamazepine treatment of juvenile-onset bipolar disorder. J Am Acad Child Adolesc Psychiatry 1999; 38:335–338 [G]Crossref, Google Scholar
465 Frazier JA, Biederman J, Jacobs TG, Tohen MF, Toma V, Feldman PD, Rater MA, Tarazi RA, Kim GA, Garfield SB, Gonzalez-Heydrich J, Nowlin ZM: Olanzapine in the treatment of bipolar disorder in juveniles, in New Clinical Drug Evaluation Unit 2000 Program Abstracts. Washington, DC, NCDEU, 2000, poster 46 [G]Google Scholar
466 Chang KD, Ketter TA: Mood stabilizer augmentation with olanzapine in acutely manic children. J Child Adolesc Psychopharmacol 2000; 10:45–49 [G]Crossref, Google Scholar
467 Soutullo CA, Sorter MT, Foster KD, McElroy SL, Keck PE: Olanzapine in the treatment of adolescent acute mania: a report of seven cases. J Affect Disord 1999; 53:279–283 [E]Crossref, Google Scholar
468 Frazier JA, Meyer MC, Biederman J, Wozniak J, Wilens TE, Spencer TJ, Kim GS, Shapiro S: Risperidone treatment for juvenile bipolar disorder: a retrospective chart review. J Am Acad Child Adolesc Psychiatry 1999; 38:960–965 [F]Crossref, Google Scholar
469 Ryback RS, Brodsky L, Munasifi F: Gabapentin in bipolar disorder (letter). J Neuropsychiatry Clin Neurosci 1997; 9:301 [G]Crossref, Google Scholar
470 Soutullo CA, Casuto LS, Keck PE Jr: Gabapentin in the treatment of adolescent mania: a case report. J Child Adolesc Psychopharmacol 1998; 8:81–85 [G]Crossref, Google Scholar
471 Hill MA, Courvoisie H, Dawkins K, Nofal P, Thomas B: ECT for the treatment of intractable mania in two prepubertal male children. Convuls Ther 1997; 13:74–82 [G]Google Scholar
472 Rey JM, Walter G: Half a century of ECT use in young people. Am J Psychiatry 1997; 154:595–602 [F]Crossref, Google Scholar



