Pharmacological and Somatic Treatment Effects on Suicide in Adults: A Systematic Review and Meta-Analysis
Abstract
Background:
Suicide is a public health crisis. We conducted a systematic review and meta-analysis of the effects of psychopharmacologic and somatic therapies on suicide risk.
Methods:
A systematic search of MEDLINE for studies evaluating the effects of pharmacologic (excluding antidepressants) or somatic interventions on suicide risk was conducted. Studies were included if they used a comparison group, reported on suicide death, assessed a psychopharmacological or somatic intervention, and included adults. Study quality was assessed using the Newcastle–Ottawa scale. Fifty-seven studies were included from 2940 reviewed citations.
Results:
In bipolar disorder, lithium was associated with a reduction in the odds of suicide compared to active controls (odds ratio [OR] = .58, p = .005; k = 12) and compared to placebo/no lithium (OR = .46, p = .009; k = 9). In mixed diagnostic samples, lithium was associated with a reduction in the odds of suicide compared to placebo/no lithium (OR = .27, p < .001; k = 12), but not compared to active controls (OR = .89, p = .468; k = 7). In psychotic disorders, clozapine was associated with a reduction in the odds of suicide (OR = .46, p = .007; k = 7). Associations between suicide death and electroconvulsive therapy (OR = .77, p = .053; k = 11), non-clozapine antipsychotics in bipolar disorder (OR = .73, p = .090; k = 6) and antipsychotics in psychotic disorders (OR = .39, p = .069; k = 6) were not significant. There was no consistent relationship between antiepileptic mood stabilizers and suicide. There were insufficient studies to meta-analyze associations of suicide risk with vagus nerve stimulation, transcranial magnetic stimulation, magnetic seizure therapy, or transcranial direct current stimulation.
Conclusion:
Lithium and clozapine have consistent data supporting protective effects against suicide in certain clinical contexts.
Reprinted from Depress Anxiety 2022; 39:100–112, with permission from John Wiley and Sons. Copyright © 2022
INTRODUCTION
Suicide is a growing public health crisis. In response to this threat to public health, several national organizations have developed initiatives aimed at preventing and reducing suicide in the United States. Some examples include the National Action Alliance for Suicide Prevention promulgating a national strategy to improve public recognition of suicide warning signs (National Action Alliance) and the Suicide Prevention Resource Center developing Zero Suicide, a quality improvement framework for transforming suicide prevention in health care systems (Zero Suicide). In addition, the National Institute of Mental Health has given high priority to suicide prevention research (Collins & Gordon, 2018).
Yet despite these efforts, the number of deaths by suicide in the US has unfortunately increased by over 60% from 1999 to 2018 (Underlying Cause of Death 1999-2018, CDC Database). Furthermore, suicide is now one of the 10 leading causes of death in the United States. There have been particularly sharp increases in suicide deaths among teenagers and young adults, middle-aged adults, and those living in rural areas (Stein et al., 2017). Furthermore, the increase in suicide deaths has contributed to a recent decline in overall US life expectancy (Murphy et al., 2017).
Prevention of suicide and suicidal behavior can involve public health interventions that aim at suicide reduction within defined populations or clinical interventions that focus on reducing the risk for individual patients. Promising public health approaches include limiting access to lethal methods (Yip et al., 2012), school-based psychoeducation and mental health screening programs (Aseltine & DeMartino, 2004), integrated policies and educational efforts focused on reducing suicide risk in defined populations (Hampton, 2010; Knox et al., 2010), and implementing recommended mental health care policies in defined catchment areas (While et al., 2012).
At the patient level, several clinical interventions have evi- dence of lowering the risk of suicidal behavior in patients with high-risk mental health conditions. Several recent meta-analyses have showed the benefits of targeted psychotherapies (i.e., cognitive behavioral therapy, dialectical behavior therapy) to reduce suicide behaviors (DeCou et al., 2019; Leavey & Hawkins, 2017; Riblet et al., 2017). In light of collective efforts to reduce the rising trend of suicide, we conducted a systematic review and meta-analysis of the evidence of standard clinical interventions with evidence of reducing the risk of suicide. Given issues with ascertainment of nonfatal suicide attempts, we focused our analysis on fatal suicide attempts as an outcome. Given recent meta-analyses of psychotherapy approaches, we focus on pharmacotherapies and somatic therapies, including electroconvulsive therapy (ECT), lithium and other mood stabilizers, clozapine and other antipsychotics, transcranial magnetic stimulation (TMS), vagus nerve stimulation (VMS), and direct current stimulation (DCS). We sought to examine the effects of these treatment approaches on suicide death in patients with mental health disorders via systematic review and meta-analysis.
METHODS
Search Strategy
A systematic search was performed on MEDLINE using the following search criteria without language restriction before December 15, 2019: ([Mood stabilizers OR Depakote OR valproate OR valproic acid OR lamotrigine OR lamictal OR carbamazepine OR tegretol] OR [Antipsychotics or neuroleptics or quetiapine or Seroquel or olanzapine or Zyprexa or zydis or Latuda or lurasidone or aripiprazole or abilify or rexulta or brexpiprazole or ziprasidone or Geodon or risperidone or Risperdal or asenapine or saphris] OR [Lithium] OR [clozapine] OR [ect OR electroconvulsive therapy] OR [TMS or transcranial magnetic stimulation] OR [vagus nerve stimulation OR VNS]) AND suicide. Two authors (Anubhav Kidambi and Victor J. Avila-Quintero) independently identified relevant studies among the results of the systematic search. References of included studies were also screened for relevant studies. Resources from Yale University were used to translate studies published in languages other than English. This study was con- ducted according to the MOOSE guidelines (Stroup et al., 2000; see Supporting Information).
Inclusion and Exclusion Criteria
We only included studies that
Used suicide as an outcome (we did not include studies where fatal and nonfatal suicide attempts could not be separated)
Used a comparison group (such as placebo or another intervention); within-group comparisons (i.e., when participants were receiving the intervention compared to when they were not) were permitted. Studies using historical comparisons were excluded.
The comparison group had similar diagnostic relevance as the treatment group (i.e., we excluded studies that compared suicide rates in a clinical population to rates in the general population)
Assessed a psychopharmacological or somatic intervention. For the purpose of this study, somatic interventions include ECT, TMS, VNS, and DCS
Were not comprised exclusively of pediatric patients (younger than 18 years of age)
Randomized trials, observational cohort studies, and case-control studies were included. Given the broad scope of this meta-analysis, we did not include psychotherapeutic treatments in our review (D'Anci et al., 2019; Fox et al., 2020). Furthermore, given the extensive literature on the topic, we did not include standard oral antidepressants (such as selective serotonin reuptake inhibitors) in this review (Carpenter et al., 2011; Gibbons et al., 2012; Jick et al., 2004; Perlis et al., 2007).
Data Extraction
Two authors (Samuel T. Wilkinson and Daniel T. Diaz) independently extracted the data using a standardized form: study design, intervention of interest, comparison group, odds ratio (OR)/hazard ratio (HR)/relative risk, number of suicide deaths, and denominator in each comparison group. Where studies had some but not all necessary data for meta-analysis, efforts were made to contact corresponding au- thors. In extracting data, we prioritized adjusted ORs or HRs calculated by the authors of the original papers. Where this information was not available, we calculated unadjusted ORs using raw data. For observational studies where cohort groups often differed with respect to person-time of exposure, we favored (where available) the use of person-years in the denominator. For events with rare occurrences such as suicide, ORs and relative risk yield approximately equal results (Rothman et al., 2012). In reporting total number of subjects exposed to a given intervention, total numbers of subjects were used; in instances where the published literature only reported person-years, these were used but are separated from counts as noted below. Quality of studies was assessed using the Newcastle–Ottawa scale. In specific, the Newcastle–Ottawa scale scores studies on quality based on selection of cohorts, comparability of cohorts, and outcome. Four possible points are given for selection of cohorts (maximum points awarded for a cohort that is truly representative of patients in the community with mental illness, if the nonexposed cohort is drawn from the same community as the exposed cohort, if the exposure is ascertained through a secure record, and there is clear demonstration that the outcome was not present at the start of the study. Two possible points are given for comparability if the study controls for basic potential confounders (socioeconomic status, age, race, and gender) as well as content-specific confounders. Three possible points are given for outcome if the assessment of outcome is done independently or by reference to secured records (i.e., medical records), if the follow-up was long enough for the outcome to occur, and if there is adequacy of follow-up cohorts (Wells et al., 2000).
Analysis
Statistical analyses were performed using STATA 15.1 and Comprehensive Meta-Analysis 3.0 (CMA; Biostat). The primary outcome for each comparison was suicide death, which was assessed as described in each individual study (either through prospective observation or through records database matching [i.e., the National Death Index or similar death registries]). The time over which suicide was assessed varied by study; as a rule, we used the follow-up time given in each individual study.
Our primary outcome measure for this meta-analysis was effect sizes of pharmacological and somatic interventions with respect to suicide death. We report the random-effects model as our primary analysis because it is likely to be more conservative and more appropriate for the data as we expected significant heterogeneity between studies given differences in design, approach, doses of drug used, and other features.
The Q-statistic was used to evaluate heterogeneity between studies. This metric provides a statistical test indicating whether different effect sizes between studies are attributable to subject level sampling error alone or other sources of variability. We estimated the degree of heterogeneity using the I-squared statistic, which approximates the proportion of total variance attributable to between-study variance. We also assessed publication bias graphically (by use of funnel plot, which plots effect size vs. standard error for each study) and statistically by use of the Egger's test (Egger et al., 1997).
Where possible, we stratified meta-analyses by clinical diagnosis and comparison group. This involved comparison of the intervention of interest to another active control (almost always, a psychotropic medication) or to placebo/no intervention. The placebo/no intervention groups were comprised of randomized trials and reports of cohort studies that did not specify what treatments patients were receiving who were not receiving the intervention of interest.
RESULTS
We identified 57 studies eligible for inclusion in this review from 2903 reviewed citations. A PRISMA diagram outlining our selection of studies is presented in Figure S1. Tables S1–S5 depict the characteristics of the included studies.
Lithium
Meta-analysis included 36 studies involving 58,244 subjects treated with Lithium and 87,965 controls (in addition to 103,487 person-years of lithium and 160,729.2 person-years of control follow-up). Studies included 20 cohort studies, 3 case-control studies, and 13 randomized trials. Seventeen studies included subjects exclusively with bipolar disorder and 19 studies included subjects with other diagnoses.
Figure 1 displays the effects of pharmacological treatment with lithium on suicide risk. Among studies conducted with individuals with bipolar disorder, lithium was associated with a significant reduction in the odds of suicide compared to an active control (OR = .58, 95% confidence interval [CI] = 0.40–0.85, z = −2.78, p = .005, k = 12; heterogeneity: Q = 14.5, I2 = 24.1%; Figure 1) and when compared to placebo or no specific intervention (OR = .46, 95% CI = 0.25–0.82, z = −2.61, p = .009, k = 9; heterogeneity: Q = 18.9, I2 = 57.1%). Among studies conducted with individuals with mixed psychiatric disorders, lithium was similarly associated with a reduction in odds of suicide compared to placebo or no intervention (OR = .27, 95% CI = 0.17–0.45, z = −5.21, p < .001, k = 12; heterogeneity: Q = 9.67, I2 = 0.0%) but not compared to an active control (OR = .89, 95% CI = 0.64–1.23, z = −0.73, p = .468, k = 7; heterogeneity: Q = 6.77, I2 = 11.4%).
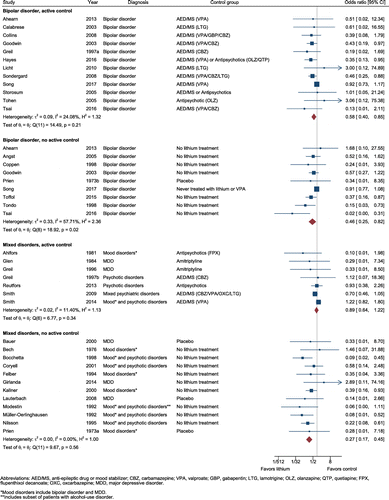
FIGURE 1. Protective effects of lithium against suicidea
a A color version of the figure, as originally published, appears in the online version of this article (focus.psychiatryonline.org).
There was evidence of publication bias when lithium was compared against no active control among patients with bipolar disorder using the Egger's test (z = −3.30, p = .013) and was also suggested by funnel plot asymmetry. A reduction in suicide odds was no longer significant when the trim and fill method was used to adjust for funnel plot asymmetry (adjusted OR = 0.63, 95% CI = 0.35–1.15, p = .12). There was no evidence of publication bias for the other lithium analyses. Funnel plots examining all lithium outcomes are provided in Figure S2.
In sensitivity analyses where only randomized trials were included, estimates were generally similar to the overall analysis though without statistical significance (bipolar disorder, vs. active control OR = 0.74, 95% CI = 0.21–2.69, z = −0.45, p = .65, k = 5; insufficient data for bipolar disorder without active control; mixed disorders, vs. active control, OR = 0.34, 95% CI = 0.07 to 1.55, z = −1.40, p = .16, k = 4; mixed disorders, vs. placebo, OR = 0.65, 95% CI = 0.10–4.23, z = −0.45, p = .65).
Clozapine
Meta-analysis included seven studies involving 5424 subjects treated with clozapine and 56,548 controls (in addition to 100,204 person-years of clozapine and 36,473 person-years of control follow-up). Included studies were comprised of four cohorts, two case-control studies, and one randomized trial. All studies were conducted in patients with psychotic disorders. Most studies (four out of seven) reported that the comparison group was taking a non-clozapine antipsychotic; one study specified that the comparison group was not taking an antipsychotic. Figure 2 displays the effects of pharmacological treatment with clozapine on the risk of suicide. Clozapine was associated with a reduction in the odds of suicide (OR = 0.46, 95% CI = 0.27–0.81, z = −2.70, p = .007, k = 7; Figure 2). There was a large amount of heterogeneity among studies for this comparison (Q = 18.3, I2 = 67.3%). There was no evidence of publication bias according to a funnel plot (Figure S3) or the Egger's test (z = 1.36, p = .233). There were insufficient randomized trials to conduct sensitivity analyses.
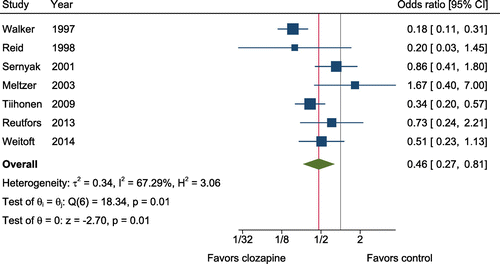
FIGURE 2. Protective effects of clozapine against suicidea
aAll studies were done in patients with psychotic disorders. [Publisher's Note: A color version of the figure, as originally published, appears in the online version of this article at focus.psychiatryonline.org.]
Electroconvulsive Therapy
Meta-analysis included 11 studies involving 1100 subjects treated with ECT and 6077 controls (in addition to 8508 person-years of ECT and 29,221 person-years of control follow-up). Included studies were comprised of seven cohorts, three case-control studies, and one randomized trial. No ECT was the comparison group in all studies and in most cases involved some form of psychotropic medicine therapy. Figure 3 depicts the association of ECT with risk of suicide death. The association between ECT and lower odds of suicide did not achieve statistical significance (OR = 0.77, 95% CI = 0.58–1.00, z = −1.93, p = .053, k = 11; Figure 3). There was a small amount of heterogeneity between studies (Q = 11.8, I2 = 15.6%). Neither the funnel plot (Figure S4) nor Egger's test (z = −1.24, p = .245) suggested evidence of publication bias. Notably, many of these studies were older and of lower quality (Table S3). There were insufficient randomized trials to conduct sensitivity analyses.
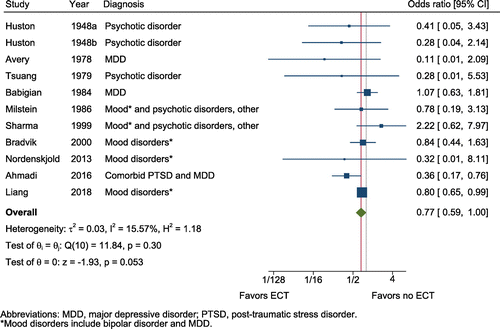
FIGURE 3. Protective effects of electroconvulsive therapy (ECT) against suicidea
a A color version of the figure, as originally published, appears in the online version of this article (focus.psychiatryonline.org).
Antipsychotics (Excluding Clozapine)
Meta-analysis included 12 studies involving 46,569 subjects treated with antipsychotics and 10,454 controls (in addition to 16,907 person-years of antipsychotics and 11,349 person-years of control follow-up). Included studies were comprised of five cohorts, two case-control studies, and five randomized trials. Given protective effects against suicide previously demonstrated with lithium, we stratified the analysis based on comparison group (lithium vs. non-lithium comparator). Additionally, studies were stratified by the psychiatric diagnosis of the study sample.
Among patients with bipolar disorder, the association between antipsychotic use and a reduction in odds of suicide when compared to non-lithium therapies or no therapies did not achieve statistical significance (OR = .73, 95% CI = 0.51–1.05, z = −1.70, p = .090, k = 6; het-erogeneity: Q = 4.2, I2 = 0.0%; Figure 4). Among patients with psychotic disorders, the association between antipsychotic use and a reduction in risk of suicide when compared to non-lithium therapies or no therapies did not achieve statistical significance (OR = 0.39, 95% CI = 0.14–1.07, z = −1.82, p = .069, k = 6; heterogeneity: Q = 8.91, I2 = 43.9%). Neither the funnel plots nor Egger's tests (z = 1.80, p = .15 for bipolar disorder; z = 0.36, p = .74 for psychotic disorder) suggested publication bias for these comparisons. When compared to lithium, no statistically significant association was seen (OR = 2.68, 95% CI = 0.75–9.55, z = 1.52, p = .128; heterogeneity: Q = 2.44, I2 = 18.0%).
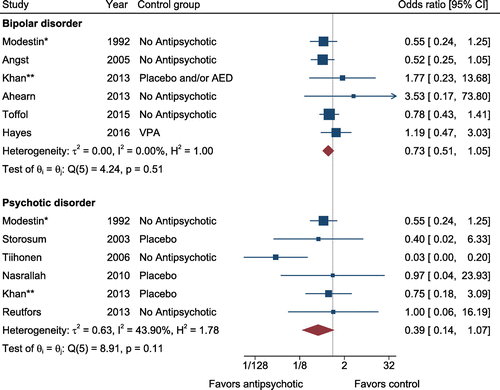
FIGURE 4. Protective effects of non-clozapine antipsychotics against suicide.
AED, antiepileptic drug; VPA, valproic acid; *Same study did not distinguish between bipolar and psychotic disorder populations; **Same study that did distinguish between bipolar and psychotic disorder populations. [Publisher's Note: A color version of the figure, as originally published, appears in the online version of this article at focus.psychiatryonline.org.]
There were insufficient randomized trials to conduct sensitivity analyses of antipsychotics in patients with bipolar disorder. Sensitivity analyses using only randomized trials in patients with psychotic disorder, antipsychotic versus non-lithium therapies, or no therapies showed (OR = 0.69, 95% CI = 0.21–2.24, z = −0.62, p = .54, k = 3).
Antiepileptic Mood Stabilizers
Meta-analysis included 16 studies involving 54,135 subjects treated with antiepileptic mood stabilizers and 61,875 controls (in addition to 93,629 person-years of antiepileptic mood stabilizers and 87,104 person-years of control follow-up). Included studies were comprised of 11 cohorts, 1 case-control study, and 4 randomized trials. Given the protective effects against suicide previously demonstrated with lithium (Cipriani et al., 2013), we stratified the analysis based on comparison group (lithium vs. non-lithium comparator). When compared to non-lithium therapies or no therapies, there was no significant association with risk of suicide (OR = 0.91, 95% CI = 0.36–2.27, z = −0.21, p = .84, k = 8; heterogeneity: Q = 31.2, I2 = 77.6%; Figure 5). Neither the funnel plot nor Egger's test suggested publication bias (z = 1.24, p = .261). When compared to lithium, antiepileptic mood stabilizers were associated with a higher risk of suicide death (OR = 1.35, 95% CI = 1.01–1.81, z = 2.04, p = .042, k = 13; heterogeneity: Q = 16.8, I2 = 28.6%). Both inspection of the funnel plot and Egger's test (z = 2.28, p = .043) suggested publication bias for this comparison. When the Trim and Fill method was used to adjust for potential funnel plot asymmetry, the adjusted odds ratio was 1.23 (adjusted OR = 1.23, 95% CI = 0.91–1.68, p = .18, Q = 23.0) for antiepileptic mood stabilizers compared to lithium.
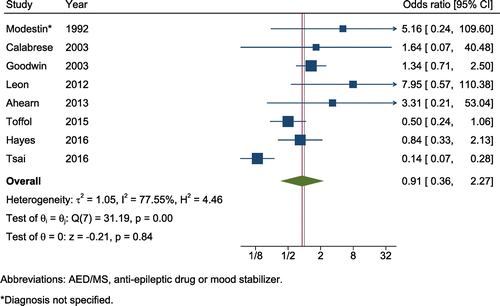
FIGURE 5. Protective effects of antiepileptic mood stabilizers against suicidea
aFor the scope of this analysis, we focused on valproic acid, lamotrigine, and carbamazepine as antiepileptic mood stabilizers. [Publisher's Note: A color version of the figure, as originally published, appears in the online version of this article at focus.psychiatryonline.org.]
There were insufficient data to conduct sensitivity analyses in randomized trials.
Other Somatic Therapies
There was an insufficient number of studies (k = 2) to meta-analyze the effects of VNS on suicide-related outcomes (Aaronson et al., 2017; Olin et al., 2012). Our search revealed no studies assessing the effect of TMS, Magnetic Seizure Therapy, or transcranial direct current stimulation on risk of suicide.
DISCUSSION
The current meta-analysis summarizes research for pharmacologic (excluding antidepressants) and somatic therapies that have demonstrated protective effects against risk of suicide. Our systematic review included 57 studies drawn from 2903 citations. Our meta-analysis shows lithium to have significant protective effects against placebo or no lithium regardless of diagnosis (OR = .46, p = .009 in bipolar disorder; OR = .27, p < .001 in mixed disorders). Our data also show that lithium has protective effects against suicide versus an active comparator (mostly antiepileptic mood stabilizers or antipsychotics) in bipolar disorder (OR = .58, p = .005) but not in mixed disorders (major depressive disorder or psychotic disorders). Clozapine also has evidence that it can protect against suicide death in psychotic disorders (OR = .46, p = .007). Associations with reduced risk of suicide death for ECT (OR = .77, p = .053), non-clozapine antipsychotics in bipolar disorder (OR = .73, p = .069) and antipsychotics in psychotic disorder (OR = .39, p = .069) did not achieve statistical significance. There was no consistent relationship between antiepileptic mood stabilizers and suicide death. Given the rising rates of suicide in the United States, these data can help guide prescribers in the treatment of patients at highest risk for suicide.
Lithium
Lithium is indicated for use in bipolar disorder (acute treatment for mixed, manic, or depressive phases as well as for maintenance therapy) and as adjunctive therapy for unipolar depression. Findings suggest that lithium is effective in protecting against suicide in bipolar disorder. In some clinical contexts (compared to no intervention), lithium may also be protective in unipolar depression and psychotic disorders. The finding that lithium is not better than an active comparator in unipolar depression and psychotic disorders suggests that interventions specifically indicated for these disorders (i.e., antipsychotics for psychotic disorders) may more effectively treat the underlying disorder and consequently reduce the risk of suicide.
Despite evidence of its protective effects, the use of lithium has waned in recent decades (Kessing et al., 2016; Rhee, Olfson, Nierenberg, et al., 2020; Rhee, Olfson, Sint, et al., 2020). A number of factors have likely contributed to this trend, including the availability of newer generation antipsychotics that are also indicated for bipolar disorder and have active marketing support from pharmaceutical companies. Also, lithium has tolerability concerns, a need for frequent plasma monitoring, and risks of long-term renal impairment that may limit its use in select populations. However, the decline in lithium use in concert with the rapid increase in the use of antipsychotics for bipolar disorder highlights the importance of investigation into the comparative protective effects of these treatments.
Clozapine
Antipsychotic clozapine was associated with a reduction in risk of suicide death. Because of the risk of agranulocytosis associated with clozapine, this medicine is subject to a strict Risk Evaluation and Mitigation Strategy (REMS), that requires regular complete blood count monitoring. Along with an unfavorable metabolic safety profile, strict REMS requirements have limited its use in practice. Clozapine is generally not used until other antipsychotics have failed to produce adequate symptom relief. Notably, clozapine is approved by the Food and Drug Administration for reducing the risk of recurrent suicidal behavior in patients with schizophrenia or schizoaffective disorder.
Molecular Mechanisms
Understanding treatment approaches that reduce the risk of suicide may shed light on the mechanistic basis of suicide risk. Advances in elucidating the mechanism of lithium suggest that it may serve to upregulate neuroprotective factors (i.e., brain-derived neurotrophic factor and BCL-2) and downregulate apoptotic factors (Malhi et al., 2013). Furthermore, lithium may also work through the modulation of dopaminergic, glutamatergic, and GABAergic pathways (Malhi et al., 2013). Lithium's beneficial effects on impulsivity and aggression may mediate this reduction in suicide risk from a psychological perspective (Cipriani et al., 2013). The mechanisms by which clozapine reduces suicide risk is less clear, but given its wide array of effects may include simultaneous modulation of multiple neurotransmitters (Meltzer et al., 2000) (monoamine-based) and intracellular (Leveque et al., 2000) (i.e., modulation of N-methyl-d-aspartate receptor expression) systems.
Electroconvulsive Therapy
While practice standards suggest ECT is to be used for patients with mood disorders at risk of suicide, the evidence of the effects of ECT on suicide death have been inconsistent. Given the nature of ECT, blinded studies are essentially no longer possible due to ethical and practical concerns. Furthermore, because ECT is usually reserved for the most severely ill patients, observational studies that adequately account for confounding by indication are inherently difficult to conduct. While ECT has consistently been associated with lower risks of all-cause mortality (Jorgensen et al., 2020; Rhee et al., 2021), further carefully conducted research is needed to examine the extent to which ECT may provide protection against suicide risk.
Need for Improvement in the Implementation of Protective Therapies
One critical area of future investigation involves improving access to therapies that have been shown to reduce the risk of suicidal behaviors. For instance, lithium, clozapine, and ECT are all underused in community settings (Baldessarini et al., 2007; Kelly et al., 2018; Rhee, Olfson, Nierenberg, et al., 2020; Rhee, Olfson, Sint, et al., 2020; Wilkinson et al., 2018), limiting their potential public health impact on suicide prevention.
Implications for Clinical Practice
While several treatments have demonstrated evidence of reducing the risk of suicide or suicidal behaviors, improved therapies are needed given the side effect profiles of many of these medicines. A key challenge involves the development of treatments that are targeted to treat patients with suicidal ideation. Over the last several years, clinical trials of antidepressants and antipsychotics have had more restrictive eligibility criteria (Zimmerman et al., 2015), to the point that a large majority of clinical trials excluded patients with even low risk of suicide. As a result, several of the most commonly used treatments have little evidence of the efficacy of protective effects against suicide in patients who are at the highest risk. Reasons for excluding patients at some risk of suicide from clinical trials involve liability, the ethical quandary of allocating someone at risk of suicide to a placebo condition, higher study costs due to more safeguards, and the theoretical concern that treatments may worsen suicidal ideation (Pearson et al., 2001). In 2018, the FDA issued im- portant guidance on including patients with suicidal ideation in clinical trials (Food and Drug Administration, 2018); this guidance advises that patients at risk of suicide can be included in clinical trials for the development of interventions that target those most at risk. One key principle from this guidance is that a standard of care option should be included in lieu of standalone placebo in the comparator arm in trials where patients at significant risk of suicide are included.
Limitations
Several limitations of this meta-analysis require comment. First, the inclusion of non-randomized studies can bias results if comparator groups are different in ways that are not measured or controlled for. For instance, the severity of illness (i.e., in mood disorders) is difficult to account for in non-randomized studies and may introduce confounding. However, all studies included passed peer review and the majority were of high quality with moderate to good comparability between groups (Tables S1–S5). While the Newcastle–Ottawa Scale can have fair to high interrater reliability (Oremus et al., 2012), its use for reducing bias in meta-analyses is recommended by the Cochrane Collaboration (Higgins et al., 2019). Second, while not a perfect assessment, there was significant heterogeneity in three of the meta-analyses performed: lithium in bipolar disorder versus no active control; clozapine; and antiepileptic mood stabilizers versus no antiepileptic mood stabilizers. (For the other seven analyses, heterogeneity was not significant.) Third, among some studies included, the comparator groups were not consistently defined. However, significant findings were also found where comparator groups were consistently defined (i.e., lithium vs. antiepileptic mood stabilizers). Fourth, we did not conduct subgroup analyses when significant heterogeneity was found because there were too few studies for each outcome. Finally, a lack of inclusion of studies involving children and adolescents limits the generalizability of these findings to broader populations.
Conclusions
Lithium and clozapine have consistent data supporting protective effects against suicide in certain psychiatric disorders.
(2017). A 5‐year observational study of patients with treatment-resistant depression treated with vagus nerve stimulation or treatment as usual: Comparison of response, remission, and suicidality. American Journal of Psychiatry, 174(7), 640–648. https://doi.org/10.1176/appi.ajp.2017.16010034 Crossref, Google Scholar
(2013). Suicide attempts in veterans with bipolar disorder during treatment with lithium, divalproex, and atypical antipsychotics. Journal of Affective Disorders, 145(1), 77–82. https://doi.org/10.1016/j.jad.2012.07.015 Crossref, Google Scholar
(1981). Flupenthixol decanoate in recurrent manic‐depressive illness. A comparison with lithium. Acta Psychiatrica Scandinavica, 64(3), 226–237. https://doi.org/10.1111/j.1600-0447.1981.tb00778.x Crossref, Google Scholar
(2016). Efficacy and long‐term clinical outcome of comorbid posttraumatic stress disorder and major depressive disorder after electroconvulsive therapy. Depression and Anxiety, 33(7), 640–647. https://doi.org/10.1002/da.22451 Crossref, Google Scholar
(2005). Suicide in 406 mood‐disorder patients with and without long‐term medication: A 40 to 44 years' follow‐up. Archives of Suicide Research: Official Journal of the International Academy for Suicide Research, 9(3), 279–300. https://doi.org/10.1080/13811110590929488 Crossref, Google Scholar
(2004). An outcome evaluation of the SOS Suicide Prevention Program. American Journal of Public Health, 94(3), 446–451. https://doi.org/10.2105/ajph.94.3.446 Crossref, Google Scholar
(1978). Suicide, attempted suicide, and relapse rates in depression. Archives of General Psychiatry, 35(6), 749–753. Crossref, Google Scholar
(1984). Epidemiologic considerations in electroconvulsive therapy. Archives of General Psychiatry, 41(3), 246–253. https://doi.org/10.1001/archpsyc.1984.01790140036005 Crossref, Google Scholar
(2007). Patterns of psychotropic drug prescription for U.S. patients with diagnoses of bipolar disorders. Psychiatric Services, 58(1), 85–91. https://doi.org/10.1176/ps.2007.58.1.85 Crossref, Google Scholar
(2000). Double‐blind, placebo‐controlled trial of the use of lithium to augment antidepressant medication in continuation treatment of unipolar major depression. American Journal of Psychiatry, 157(9), 1429–1435. https://doi.org/10.1176/appi.ajp.157.9.1429 Crossref, Google Scholar
(1976). Lithium maintenance treatment of manic‐melancholic patients: Its role in the daily routine. Acta Psychiatrica Scandinavica, 53(1), 70–81. https://doi.org/10.1111/j.1600-0447.1976.tb00060.x Crossref, Google Scholar
(1998). Suicidal behavior on and off lithium prophylaxis in a group of patients with prior suicide attempts. Journal of Clinical Psychopharmacology, 18(5), 384–389. https://doi.org/10.1097/00004714-199810000-00006 Crossref, Google Scholar
(2000). Treatment and suicide in severe depression: A case‐control study of antidepressant therapy at last contact before suicide. The Journal of ECT, 16(4), 399–408. https://doi.org/10.1097/00124509-200012000-00010Crossref, Google Scholar
(2003). A placebo‐ controlled 18‐month trial of lamotrigine and lithium maintenance treatment in recently depressed patients with bipolar I disorder. Journal of Clinical Psychiatry, 64(9), 1013–1024. https://doi.org/10.4088/jcp.v64n0906 Crossref, Google Scholar
(2011). Meta‐analysis of efficacy and treatment‐emergent suicidality in adults by psychiatric indication and age subgroup following initiation of paroxetine therapy: A complete set of randomized placebo‐controlled trials. Journal of Clinical Psychiatry, 72(11), 1503–1514. https://doi.org/10.4088/JCP.08m04927blu Crossref, Google Scholar
(2013). Lithium in the prevention of suicide in mood disorders: Updated systematic review and meta‐analysis. British Medical Journal, 346, f3646. https://doi.org/10.1136/bmj.f3646 Crossref, Google Scholar
. (2018, November 28). We're deeply committed to reducing suicide. USA Today. https://www.usatoday.com/story/opinion/2018/11/28/nih-nimh-deeply-committed-reducing-suicide-editorials-debates/2128221002/ Google Scholar
(2008). Divalproex, lithium and suicide among Medicaid patients with bipolar disorder. Journal of Affective Disorders, 107(1–3), 23–28. https://doi.org/10.1016/j.jad.2007.07.014 Crossref, Google Scholar
(1998). Suicide mortality in patients on lithium maintenance therapy. Journal of Affective Disorders, 50(2–3), 261–267. https://doi.org/10.1016/s0165-0327(98)00067-6Crossref, Google Scholar
(2001). Lithium and suicidal behavior in major affective disorder: A case‐control study. Acta Psychiatrica Scandinavica, 104(3), 193–197. https://doi.org/10.1034/j.1600- 0447.2001.00338.x Crossref, Google Scholar
(2019). Treatments for the prevention and management of suicide: A systematic review. Annals of Internal Medicine, 171(5), 334–342. https://doi.org/10.7326/ m19-0869 Crossref, Google Scholar
(2019). Dialectical behavior therapy is effective for the treatment of suicidal behavior: A meta‐analysis. Behavior Therapy, 50(1), 60–72. https://doi.org/10.1016/j. beth.2018.03.009 Crossref, Google Scholar
(1997). Bias in meta‐analysis detected by a simple, graphical test. British Medical Journal, 315(7109), 629–634. Crossref, Google Scholar
(1994). Suizide und Parasuizide whrend und auerhalb einer Lithium‐Prophylaxe, Ziele und Ergebnisse der medikamentsen Prophylaxe affektiver Psychosen (p. 5359). Thieme. Google Scholar
(2020). Interventions for suicide and self‐injury: A meta‐analysis of randomized controlled trials across nearly 50 years of research. Psychological Bulletin, 146(12), 1117–1145. https://doi.org/10.1037/bul0000305 Crossref, Google Scholar
(2012). Suicidal thoughts and behavior with antidepressant treatment: Reanalysis of the randomized placebo‐controlled studies of fluoxetine and venlafaxine. Archives of General Psychiatry, 69(6), 580–587. https://doi.org/10.1001/archgenpsychiatry.2011.2048 Crossref, Google Scholar
(1984). Continuation therapy with lithium and amitriptyline in unipolar depressive illness: A randomized, double‐blind, controlled trial. Psychological Medicine, 14(1), 37–50. https://doi.org/10.1017/s0033291700003068 Crossref, Google Scholar
(2003). Suicide risk in bipolar disorder during treatment with lithium and divalproex. Journal of the American Medical Association, 290(11), 1467–1473. https://doi.org/10.1001/jama.290.11.1467 Crossref, Google Scholar
(1996). Comparative efficacy of lithium and amitriptyline in the maintenance treatment of recurrent unipolar depression: A randomised study. Journal of Affective Disorders, 40(3), 179–190. https://doi.org/10.1016/0165-5 Crossref, Google Scholar
(1997). Lithium vs carbamazepine in the maintenance treatment of schizoaffective disorder: A randomised study. European Archives of Psychiatry and Clinical Neuroscience, 247(1), 42–50. https://doi.org/10.1007/bf02916252 Crossref, Google Scholar
(1997). Lithium versus carbamazepine in the maintenance treatment of bipolar disorders—a randomised study. Journal of Affective Disorders, 43(2), 151–161. https://doi.org/10.1016/s0165-0327(96)01427-9 Crossref, Google Scholar
(2010). Depression care effort brings dramatic drop in large HMO population's suicide rate. Journal of the American Medical Association, 303(19), 1903–1905. https://doi.org/10.1001/jama.2010.595 Crossref, Google Scholar
(2016). Self‐harm, unintentional injury, and suicide in bipolar disorder during maintenance mood stabilizer treatment: A UK population‐based electronic health records study. JAMA Psychiatry, 73(6), 630–637. https://doi.org/10.1001/jamapsychiatry.2016.0432 Crossref, Google Scholar
(2019). Cochrane handbook for systematic reviews of interventions. John Wiley & Sons. Crossref, Google Scholar
(1948a). Involutional psychosis: Course when untreated and when treated with electric shock. Archives of Neurology & Psychiatry, 59(3), 385–394. Crossref, Google Scholar
(1948b). Manic‐depressive psychosis; Course when treated and untreated with electric shock. Archives of Neurology and Psychiatry, 60(1), 37–48. Crossref, Google Scholar
(2004). Antidepressants and the risk of suicidal behaviors. Journal of the American Medical Association, 292(3), 338–343. https://doi.org/10.1001/jama.292.3.338 Crossref, Google Scholar
(2020). Electroconvulsive therapy, depression severity and mortality: Data from the Danish National Patient Registry. Journal of Psychopharmacology, 34(3), 273–279. Crossref, Google Scholar
(2000). Mortality in 497 patients with affective disorders attending a lithium clinic or after having left it. Pharmacopsychiatry, 33(1), 8–13. https://doi.org/10.1055/s-2000-7965 Crossref, Google Scholar
(2018). Addressing barriers to clozapine underutilization: A national effort. Psychiatric Services, 69(2), 224–227. https://doi.org/10.1176/appi. ps.201700162 Crossref, Google Scholar
(2016). Nationwide and population‐based prescription patterns in bipolar disorder. Bipolar Disorders, 18(2), 174–182. https://doi.org/10.1111/bdi.12371 Crossref, Google Scholar
(2013). Comparative mortality risk in adult patients with schizophrenia, depression, bipolar disorder, anxiety disorders, and attention‐deficit/ hyperactivity disorder participating in psychopharmacology clinical trials. JAMA Psychiatry, 70(10), 1091–1099. https://doi.org/10.1001/jamapsychiatry.2013.149 Crossref, Google Scholar
(2010). The US Air Force suicide prevention program: Implications for public health policy. American Journal of Public Health, 100(12), 2457–2463. https://doi.org/10.2105/ajph.2009.159871 Crossref, Google Scholar
(2008). Adjunctive lithium treatment in the prevention of suicidal behaviour in depressive disorders: A randomised, placebo‐controlled, 1‐year trial. Acta Psychiatrica Scandinavica, 118(6), 469–479. https://doi.org/10.1111/j.1600-0447.2008.01266.x Crossref, Google Scholar
(2017). Is cognitive behavioural therapy effective in reducing suicidal ideation and behaviour when delivered face‐to‐face or via e‐health? A systematic review and meta‐analysis. Cognitive Behaviour Therapy, 46(5), 353–374. https://doi.org/10.1080/16506073.2017.1332095 Crossref, Google Scholar
(2012). Antiepileptic drugs for bipolar disorder and the risk of suicidal behavior: A 30‐year observational study. American Journal of Psychiatry, 169(3), 285–291. https://doi.org/10.1176/appi.ajp.2011.11060948 Crossref, Google Scholar
(2000). Intracellular modulation of NMDA receptor function by antipsychotic drugs. Journal of Neuroscience, 20(11), 4011–4020. https://doi.org/10.1523/jneurosci.20-11-04011.2000 Crossref, Google Scholar
(2018). Superior anti‐suicidal effects of electroconvulsive therapy in unipolar disorder and bipolar depression. Bipolar Disorders, 20(6), 539–546. https://doi.org/10.1111/bdi.12589 Crossref, Google Scholar
(2010). Lamotrigine versus lithium as maintenance treatment in bipolar I disorder: An open, randomized effectiveness study mimicking clinical practice. The 6th trial of the Danish University Antidepressant Group (DUAG‐6). Bipolar Disorders, 12(5), 483–493. https://doi.org/10.1111/j.1399-5618.2010.00836.x Crossref, Google Scholar
. (2018). Major depressive disorder: Developing drugs for treatment – guidance for industry. Google Scholar
(2013). Potential mechanisms of action of lithium in bipolar disorder. Current understanding. CNS Drugs, 27(2), 135–153. https://doi.org/10.1007/s40263-013-0039-0 Crossref, Google Scholar
(2003). Clozapine treatment for suicidality in schizophrenia: International Suicide Prevention Trial (InterSePT). Archives of General Psychiatry, 60(1), 82–91. Crossref, Google Scholar
(2000). Reducing suicide risk in Schizophrenia. CNS Drugs, 14(5), 355–365. Crossref, Google Scholar
(1986). Does electroconvulsive therapy prevent suicide? Convulsive Therapy, 2(1), 3–6. Google Scholar
(1992). Effect of psychopharmacotherapy on suicide risk in discharged psychiatric inpatients. Acta Psychiatrica Scandinavica, 85(2), 173–175. https://doi.org/10.1111/j.1600-0447.1992.tb01464.x Crossref, Google Scholar
(1992). The effect of long‐term lithium treatment on the mortality of patients with manic‐depressive and schizoaffective illness. Acta Psychiatrica Scandinavica, 86(3), 218–222. https://doi.org/10.1111/j.1600-0447.1992.tb03255.x Crossref, Google Scholar
(2017). Mortality in the United States. NCHS Data Brief, 2018(328), 1–8. Google Scholar
(2010). A controlled, evidence‐based trial of paliperidone palmitate, a long‐acting injectable antipsychotic, in schizophrenia. Neuropsychopharmacology, 35(10), 2072–2082. https://doi.org/10.1038/npp.2010.79 Crossref, Google Scholar
. Retrieved August 26, 2020, from https://theactionalliance.org/ Google Scholar
(1995). Mortality in recurrent mood disorders during periods on and off lithium. A complete population study in 362 patients. Pharmacopsychiatry, 28(1), 8–13. https://doi.org/10.1055/s-2007-979581 Crossref, Google Scholar
(2013). Continuation electroconvulsive therapy with pharmacotherapy versus pharmacotherapy alone for prevention of relapse of depression: A randomized controlled trial. The Journal of ECT, 29(2), 86–92. https://doi.org/10.1097/YCT.0b013e318276591f Crossref, Google Scholar
(2012). Mortality and suicide risk in treatment‐resistant depression: An observational study of the long‐term impact of intervention. PLOS One, 7(10): e48002. https://doi.org/10.1371/journal.pone.0048002 Crossref, Google Scholar
(2012). Inter‐rater and test‐retest reliability of quality assessments by novice student raters using the Jadad and Newcastle‐Ottawa Scales. BMJ Open, 2(4), https://doi.org/10.1136/bmjopen-2012-001368 Crossref, Google Scholar
(2001). Intervention research with persons at high risk for suicidality: Safety and ethical considerations. Journal of Clinical Psychiatry, 62(Suppl 25), 17–26. Google Scholar
(2007). Treatment‐associated suicidal ideation and adverse effects in an open, multicenter trial of fluoxetine for major depressive episodes. Psychotherapy and Psychosomatics, 76(1), 40–46. Crossref, Google Scholar
(1973). Prophylactic efficacy of lithium carbonate in manic‐depressive illness. Report of the Veterans Administration and National Institute of Mental Health collaborative study group. Archives of General Psychiatry, 28(3), 337–341. https://doi.org/10.1001/archpsyc.1973.01750330035006 Crossref, Google Scholar
(1973). Lithium carbonate and imipramine in prevention of affective episodes. A comparison in recurrent affective illness. Archives of General Psychiatry, 29(3), 420–425. https://doi.org/10.1001/archpsyc.1973.04200030104017 Crossref, Google Scholar
(1998). Suicide prevention effects associated with clozapine therapy in schizophrenia and schizoaffective disorder. Psychiatric Services, 49(8), 1029–1033. https://doi.org/10.1176/ps.49.8.1029 Crossref, Google Scholar
(2013). Medication and suicide risk in schizophrenia: A nested case‐control study. Schizophrenia Research, 150(2–3), 416–420. https://doi.org/10.1016/j.schres.2013.09.001 Crossref, Google Scholar
(2020). 20‐year trends in the pharmacologic treatment of bipolar disorder by psychiatrists in outpatient care settings. American Journal of Psychiatry, 177(8), 706–715. Crossref, Google Scholar
(2020). Characterization of the quality of electroconvulsive therapy among older medicare beneficiaries. Journal of Clinical Psychiatry, 81(4), https://doi.org/10.4088/JCP.19m13186 Google Scholar
(2021). Association of ECT with risks of all‐cause mortality and suicide in older Medicare patients. American Journal of Psychiatry,2021; 210:40351. https://doi.org/10.1176/appi.ajp.2021.21040351 Google Scholar
(2017). Strategies to prevent death by suicide: Meta‐analysis of randomised controlled trials. British Journal of Psychiatry, 210(6), 396–402. https://doi.org/10.1192/bjp.bp.116.187799 Crossref, Google Scholar
(2014). Mortality, attempted suicide, re‐hospitalisation and prescription refill for clozapine and other antipsychotics in Sweden‐a register‐based study. Pharmacoepidemiology and Drug Safety, 23(3), 290–298. https://doi.org/10.1002/pds.3567 Crossref, Google Scholar
(2012). Modern epidemiology. Wolters Kluwer Health/Lippincott Williams & Wilkins. Google Scholar
(2001). Impact of clozapine on completed suicide. American Journal of Psychiatry, 158(6), 931–937. https://doi.org/10.1176/appi.ajp.158.6.931 Crossref, Google Scholar
(1999). Retrospective controlled study of inpatient ECT: Does it prevent suicide? Journal of Affective Disorders, 56(2–3), 183–187. https://doi.org/10.1016/s0165-0327(98)00207-9 Crossref, Google Scholar
(2014). Suicide risk in Veterans Health Administration patients with mental health diagnoses initiating lithium or valproate: A historical prospective cohort study. BMC Psychiatry, 14, 357. https://doi.org/10.1186/s12888-014-0357-x Crossref, Google Scholar
(2009). Association between consistent purchase of anticonvulsants or lithium and suicide risk: A longitudinal cohort study from Denmark, 1995‐2001. Journal of Affective Disorders, 117(3), 162–167. https://doi.org/10.1016/j.jad.2009.01.013 Crossref, Google Scholar
(2017). Suicidal behavior during lithium and valproate treatment: A within‐individual 8‐year prospective study of 50,000 patients with bipolar disorder. American Journal of Psychiatry, 174(8), 795–802. https://doi.org/10.1176/appi.ajp.2017.16050542 Crossref, Google Scholar
(2017). The epidemic of despair among white Americans: Trends in the leading causes of premature death, 1999‐2015. American Journal of Public Health, 107(10), 1541–1547. https://doi.org/10.2105/ajph.2017.303941 Crossref, Google Scholar
(2005). Suicide risk in placebo‐controlled trials of treatment for acute manic episode and prevention of manic‐depressive episode. American Journal of Psychiatry, 162(4), 799–802. https://doi.org/10.1176/appi.ajp.162.4.799 Crossref, Google Scholar
(2003). Suicide risk in placebo vs active treatment in placebo‐controlled trials for schizophrenia. Archives of General Psychiatry, 60(4), 365–368. https://doi.org/10.1001/archpsyc.60.4.365 Crossref, Google Scholar
(2000). Meta‐analysis of observational studies in epidemiology: a proposal for reporting. Meta‐analysis Of Observational Studies in Epidemiology (MOOSE) group. Journal of the American Medical Association, 283(15), 2008–2012. https://doi.org/10.1001/jama.283.15.2008 Google Scholar
(2008). Mood‐stabilizing pharmacological treatment in bipolar disorders and risk of suicide. Bipolar Disorders, 10(1), 87–94. https://doi.org/10.1111/j.1399-5618.2008.00464.x Crossref, Google Scholar
(2009). 11‐year follow‐up of mortality in patients with schizophrenia: A population‐based cohort study (FIN11 study). Lancet, 374(9690), 620–627. https://doi.org/10.1016/s0140-6736(09)60742-x Crossref, Google Scholar
(2006). Effectiveness of antipsychotic treatments in a nationwide cohort of patients in community care after first hospitalisation due to schizophrenia and schizoaffective disorder: Observational follow‐up study. British Medical Journal, 333(7561), 224. https://doi.org/10.1136/bmj.38881.382755.2F Crossref, Google Scholar
(2015). Lithium is associated with decrease in all‐cause and suicide mortality in high‐risk bipolar patients: A nationwide registry‐based prospective cohort study. Journal of Affective Disorders, 183, 159–165. https://doi.org/10.1016/j.jad.2015.04.055 Crossref, Google Scholar
(2005). Olanzapine versus lithium in the maintenance treatment of bipolar disorder: A 12‐month, randomized, double‐blind, controlled clinical trial. American Journal of Psychiatry, 162(7), 1281–1290. https://doi.org/10.1176/appi.ajp.162.7.1281 Crossref, Google Scholar
(1998). Lithium treatment and risk of suicidal behavior in bipolar disorder patients. Journal of Clinical Psychiatry, 59(8), 405–414. https://doi.org/10.4088/jcp.v59n0802 Crossref, Google Scholar
(2016). The rapid suicide protection of mood stabilizers on patients with bipolar disorder: A nationwide observational cohort study in Taiwan. Journal of Affective Disorders, 196, 71–77. https://doi.org/10.1016/j.jad.2016.02.014 Crossref, Google Scholar
(1979). Can ECT prevent premature death and suicide in 'schizoaffective' patients? Journal of Affective Disorders, 1(3), 167–171. https://doi.org/10.1016/0165-0327(79)90001-6 Crossref, Google Scholar
Underlying Cause of Death 1999‐2018 on CDC WONDER Online Data- base, released in 2020. Data are from the Multiple Cause of Death Files, 1999‐2018, as compiled from data provided by the 57 vital statistics jurisdictions through the Vital Statistics Cooperative Program. http://wonder.cdc.gov/ucd-icd10.html Google Scholar
(1997). Mortality in current and former users of clozapine. Epidemiology, 8(6), 671–677. Crossref, Google Scholar
(2000). The Newcastle‐Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta‐analyses. Google Scholar
(2012). Implementation of mental health service recommendations in England and Wales and suicide rates, 1997‐2006: A cross‐sectional and before‐and‐after observational study. The Lancet, 379(9820), 1005–1012. https://doi.org/10.1016/s0140-6736(11)61712-1 Crossref, Google Scholar
(2018). Identifying recipients of electroconvulsive therapy: Data from privately insured Americans. Psychiatric Services, 69, 542–548. https://doi.org/10.1176/appi.ps.201700364 Crossref, Google Scholar
(2012). Means restriction for suicide prevention. The Lancet, 379(9834), 2393–2399. https://doi.org/10.1016/s0140-6736(12)60521-2 Crossref, Google Scholar
Zero Suicide from https://zerosuicide.edc.org/ Google Scholar
(2015). Have treatment studies of depression become even less generalizable? A review of the inclusion and exclusion criteria used in placebo‐controlled antidepressant efficacy trials published during the past 20 years. Mayo Clinic Proceedings, 90(9), 1180–1186. https://doi.org/10.1016/j.mayocp.2015.06.016 Crossref, Google Scholar



