Pharmacotherapy for Anxiety Disorders: From First-Line Options to Treatment Resistance
Abstract
In this review, the author examines the evidence for psychopharmacologic treatments among adults for generalized anxiety disorder, panic disorder, and social anxiety disorder derived from clinical trials. For each disorder, major categories of drugs are reviewed, and then the evidence-based medications in each category are discussed. The author reviews key safety and tolerability considerations for each of the medications or classes. Evidence-based dosing for most specific agents is displayed in a comprehensive reference table. Subsequently, the author synthesizes the available information to suggest a pragmatic stepwise approach to treatment that accounts for patient-specific factors. To inform the guidance, the author incorporates and refines perspectives from treatment guidelines already written by clinical professional organizations. The author also briefly reviews the relatively new quantitative systematic review methodology of network meta-analysis (NMA) and discusses how NMA may help guide pharmacologic treatment sequencing decisions in the future by way of ranking treatments according to effect size and the relative amount of study to which treatments have been subject. Caveats of NMA studies are briefly discussed, as are results of recent NMAs regarding the pharmacologic treatment of anxiety disorders.
Anxiety disorders are frequently encountered conditions that historically have been underrecognized and undertreated; moreover, they can be associated with considerable morbidity and suicide risk (1). In this review, I examine the wide array of evidence for pharmacologic treatments for the primary anxiety disorders, including generalized anxiety disorder, panic disorder, and social anxiety disorder. Subsequently, I synthesize the available evidence to create a structured and pragmatic approach to medication trials for these disabling disorders when first-line treatments alone are insufficient. Table 1 summarizes all the drugs discussed with recommended dosing schedules.
| Medication (brand name) | Starting dosage (mg/day) | Target dose range (mg) | Anxiety disorder FDA indications | Specific notes |
|---|---|---|---|---|
| SSRIs | ||||
| Escitalopram (Lexapro) | 5–10 | 10–30 | Generalized anxiety disorder | May prolong QT interval similarly to citalopram |
| Sertraline (Zoloft) | 25 | 50–200 | Panic disorder, social anxiety disorder | |
| Citalopram (Celexa) | 10 | 20–40 | None | FDA warning for QT prolongation at doses>40 mg/day; FDA recommended dose limit 20 mg/day in older adults |
| Fluoxetine (Prozac) | 10 | 20–80 | Panic disorder | Very long elimination half life; CYP2D6 strong inhibitor |
| Paroxetine (Paxil) | 10 | 20–60 | Panic disorder, generalized anxiety disorder, social anxiety disorder | Anticholinergic and antihistaminergic side effects; strong CYP2D6 inhibitor; difficult discontinuation, taper slowly |
| Paroxetine CR (Paxil CR) | 12.5 | 25–75 | Panic disorder, generalized anxiety disorder, social anxiety disorder | Same as paroxetine |
| Fluvoxamine (Luvox) | 25 | 100–300 | CYP1A2 strong inhibitor; inhibits own metabolism; increases serum levels of clozapine, caffeine | |
| SNRIs | ||||
| Venlafaxine XR (Effexor XR) | 37.5 | 75–300 | Panic disorder, generalized anxiety disorder, social anxiety disorder | Monitor BP for patients at doses≥225mg/day; difficult discontinuation, taper slowly |
| Duloxetine (Cymbalta) | 20 | 60–120 | Generalized anxiety disorder | FDA-approved for fibromyalgia and chronic pain |
| Milnacipran (Savella) | 25 BID | 50 BID | FDA-approved for fibromyalgia; may be useful for panic disorder | |
| Novel serotonin-modulating antidepressants | ||||
| Vilazodone (Viibryd) | 10 | 20–40 | Mechanism is SSRI+5-HT1A partial agonist (like SSRI+buspirone) | |
| Vortioxetine (Trintellix) | 5 | 5–20 | Improves cognitive processing speed | |
| TCAs | ||||
| Imipramine (Tofranil) | 10 | 100–300 | Target serum levels 110–140 ng/ml | |
| Clomipramine (Anafranil) | 10 | 50–250 | Strongest evidence for panic disorder; consider in comorbid refractory obsessive-compulsive disorder | |
| Despiramine (Norpramin) | 10 | 100–300 | Consider in comorbid attention-deficit hyperactivity disorder | |
| MAOIs | ||||
| Phenelzine (Nardil) | 15 | 60–90 divided BID–TID | Low-tyramine diet; wash out prior antidepressant 5 half lives | |
| Tranylcypromine (Parnate) | 10 | 30–60 divided BID | See above | |
| Other antidepressants | ||||
| Bupropion (Wellbutrin) | 100 SR; 150 XL | 150 SR BID; 300 XL daily | Lowers seizure threshold; no sexual side effects | |
| Mirtazapine (Remeron) | 7.5–15 QHS | 15–30 QHS | May cause significant weight gain | |
| Nefazodone (Serzone) | 50 BID | 200–600 divided BID | Rare liver toxicity, can be fatal | |
| Trazodone (Desyrel) | 50 QHS | 200–400 divided BID | Metabolite MCPP can cause agitation | |
| Beta blockers (for performance-only social anxiety disorder) | ||||
| Propranolol (Inderal) | 10 PRN | 10–40 PRN | Test dose before performance situation; monitor BP, HR | |
| Atenolol (Tenormin) | 25 PRN | 25 | Test dose before performance situation; monitor BP, HR | |
| Azapirones | ||||
| Buspirone (Buspar) | 5 BID–TID | 10–20 BID–TID | Generalized anxiety disorder | No efficacy as monotherapy except in generalized anxiety disorder |
| Antihistamines | ||||
| Hydroxyzine (Vistaril, Atarax) | 25 QHS | 12.5–50 BID–TID | Evidence for efficacy in generalized anxiety disorder at total daily dose of 50 mg | |
| Benzodiazepines | ||||
| Lorazepam (Ativan) | 0.5 QD–TID | 0.5–2 QD–TID | Anxiety disorders | Not reliant on CYP450-mediated hepatic metabolism |
| Clonazepam (Klonopin) | 0.25–0.5 BID | 0.5–1 BID (maximum 4 daily) | Panic disorder | No proven increased efficacy advantage but increased side effects at doses>2mg/day |
| Alprazolam (Xanax) | 0.25 TID–QID | 2–6 mg divided TID–QID | Anxiety disorders | Prone to rebound anxiety; most misuse liability |
| Alprazolam XR | 0.5–1 | 2–6 daily or divided BID | Panic disorder | Less risk of rebound anxiety; slow onset |
| Diazepam (Valium) | 2–5 | 40 divided BID–QID | Anxiety | Accumulates active metabolites with very long half life |
| Anticonvulsants, including gabapentinoids | ||||
| Gabapentin (Neurontin) | 100–300 QHS | 900–3,600 divided TID | Best evidence for social anxiety disorder; likely requires near-maximal doses | |
| Pregabalin (Lyrica) | 75–150 QHS | 300–600 divided BID | Social anxiety disorder requires higher doses (450–600) | |
| Valproic acid (Depakote) | 250 QHS | 500–2,500 daily or divided BID | No target therapeutic serum level; requires periodic monitoring of CBC, LFTs, and drug levels | |
| Second-generation antipsychotics | ||||
| Quetiapine (Seroquel) | 25 QHS | 50–150 QHS | Mostly studied in XR formulation | |
| Risperidone (Risperdal) | 0.25 daily | 0.25–1 daily | Best evidence for panic disorder | |
| Olanzapine (Zyprexa) | 2.5 QHS | 5–10 QHS | Very high risk of metabolic adverse effect | |
| Aripiprazole (Abilify) | 2–2.5 daily | 2–15 mg daily | Less likely to cause metabolic effects |
TABLE 1 Clinically studied medications for anxiety disordersa
Generalized Anxiety Disorder
Generalized anxiety disorder is a syndrome of prominent anxiety and worries about several components of one’s life, accompanied by symptoms of hyperarousal that may include disturbed sleep, irritability, muscle tension, restlessness, and associated fatigue and lack of concentration. This condition carries a high burden of morbidity via impairment of social and occupational functioning and is often associated with comorbid depression. It has been estimated that more than 100 million disability days per year can be attributed to generalized anxiety disorder. Thus, effective treatment of these individuals is of paramount importance (1).
Review of Specific Agents and Their Efficacy and Tolerability in Treatment of Generalized Anxiety Disorder
Selective serotonin reuptake inhibitors (SSRIs) and serotonin-norepinephrine reuptake inhibitors (SNRIs).
SSRIs and SNRIs (referred to collectively as SRIs hereafter) are the treatment of choice for pharmacologic management of generalized anxiety disorder. SRIs increase overall serotonergic transmission via selective blockade of presynaptic serotonin transporters (SERTs) that normally transport serotonin (5-HT) back into the neuron for recycling, causing a generalized increase in 5-HT neurotransmission. This process leads to a variety of downstream changes hypothesized to play a role in treating anxiety and depression, including desensitization of postsynaptic 5-HT1A receptors in the raphe nucleus of the brainstem (2); central nervous system (CNS) anti-inflammatory effects (3); and induction of increased neuroplasticity and subsequent neurogenesis in key brain circuitry that is mediated by serotonin-induced, brain-derived neurotrophic factor signaling (4). SNRIs similarly inhibit the SERT and increase synaptic concentrations of 5-HT; additionally, SNRIs increase norepinephrine (NE) levels by interfering with the activity of the NE transporter (NET). Of note, the SNRIs vary significantly in their binding affinity for SERT compared with NET; these variations range from functioning predominantly as SSRIs (venlafaxine, desvenlafaxine, and duloxetine at low doses) to having a 2:1 ratio of noradrenergic to serotonergic activity (levomilnacipran) (5). A subset of SRIs have U.S. Food and Drug Administration (FDA) indications for generalized anxiety disorder, including escitalopram and paroxetine among the SSRIs and venlafaxine and duloxetine among the SNRIs. Nevertheless, randomized controlled trial (RCT) evidence supports the use of other medications from these classes in generalized anxiety disorder, including sertraline and fluoxetine (6).
Recommended starting doses and target therapeutic ranges for each medication as per the FDA are notated in Table 1. An adjustment period on the order of 2 months is often needed to see the full clinical response; however, compiled data from multiple trials suggest that partial response is common in the first 2–4 weeks and is a good prognostic sign (7, 8). Expert consensus supports starting at lower doses than in depression and titrating slowly. Despite lower initial doses, ultimately patients tend to require doses at the higher end of the therapeutic range for a significant response, although dose-response studies have not yielded consistent results. The forgoing recommendations also apply to other patients with anxiety disorders, particularly patients with panic disorder. These higher doses will subject patients to increased risk of dose-dependent side effects, especially sexual dysfunction (9) and the poorly quantified, but clinically described, apathy syndrome (10). There is also a risk of dose-dependent prolongation of the cardiac QT interval, especially in citalopram (which carries a related FDA warning) and escitalopram, although the clinical significance of this effect remains controversial (11). Thus, careful monitoring of side effects is warranted; moreover, treatment goals may favor doses that do not achieve full remission but sufficiently reduce symptoms to improve quality of life and functioning. In this case, concomitant psychotherapy may help the patient approach full remission.
Buspirone.
Buspirone, an azapirone class 5-HT1A receptor partial agonist, is FDA approved for use in generalized anxiety disorder and may prove useful among patients who do not respond to or who are unable to tolerate SRIs. RCT evidence also exists for its usefulness as an adjunct to first-line treatment with antidepressants in the case of partial response (12). Buspirone is generally well-tolerated medication, is not habit forming, and notably is not associated with sexual side effects. Like the SRIs, buspirone has a delayed onset of anxiolytic effects on the order of weeks. The pharmacokinetics of buspirone require dividing the dose at least twice daily, which may present adherence challenges. For patients with mild to moderate generalized anxiety disorder with hesitance about taking SRIs, buspirone may be tried as a first-line agent.
Anticonvulsants.
The gamma-aminobutyric acid (GABA)–analog pregabalin (trade name Lyrica) has approval from the European Medicines Agency for use in generalized anxiety disorder; it also has FDA indications for fibromyalgia, neuropathic pain, and epilepsy. Several trials have demonstrated good efficacy and tolerability of pregabalin for generalized anxiety disorder (13). Until recently, cost barriers have prevented widespread prescribing of pregabalin in the United States, but the drug has become more affordable after the recent introduction of multiple generic versions. Although structurally related to GABA, pregabalin does not work by modulating GABA neurotransmission. Instead, the drug binds the alpha-2-delta subunit of voltage-gated calcium channels in a state-dependent fashion in hyperexcited neurons, resulting in CNS inhibition (14). On the basis of the evidence supporting pregabalin, it has been suggested that gabapentin, an older GABA analog with similar structure and mechanism of action, may also effectively treat generalized anxiety disorder. However, only case report evidence exists to suggest efficacy of gabapentin in generalized anxiety disorder (15). Noting its other clinical uses, pregabalin occasionally may be considered as a first-line agent among patients whose anxiety is comorbid with the other FDA-approved indications. The most common side effects of pregabalin are sedation and dizziness, although weight gain, peripheral edema, and visual changes may occur. Both pregabalin and gabapentin require dose adjustment for renal impairment.
Benzodiazepines (BZDs).
BZDs may play a role in treatment of generalized anxiety disorder for appropriate patients who fail to respond to or tolerate multiple trials of SRIs and buspirone. They potentiate the CNS inhibitory effects of endogenous GABA by allosteric modulation of the GABA receptor. For the most part, since the establishment of SRIs as first line, most experts have recommended using BZDs in the short term only, citing concerns about tolerance and dependence and the lack of established efficacy for comorbid depression. Chemical dependence after long-term use of BZDs is well-established; however, the common assertion that they lead to tolerance and dose escalation has not been observed in studies following patients taking them for up to 2 years (16, 17). BZDs should be avoided among patients with active substance use disorders and history of misuse of BZDs. However, clinicians should carefully weigh the benefits and risks in prescribing BZDs to patients with more remote history of disordered use of alcohol or other substances such as cannabis or stimulants. The majority of patients misusing BZDs tend to have disordered use of other substances as well (18). Increased risk of death from coadministration of BZDs and opioids, even at typical prescribed doses, may occur because of synergistic respiratory depressive effects (19). BZDs also have a strong relative contraindication in elderly patients owing to increased fall risk and potential for cognitive impairment.
Among the BZDs, four are most-commonly used for anxiety: low-potency diazepam, mid-potency lorazepam, and the high-potency alprazolam and clonazepam. All of these BZDs are used in the treatment of generalized anxiety disorder, although only alprazolam has an FDA indication for generalized anxiety disorder. Alprazolam remains one of the most widely prescribed drugs in the United States. Nevertheless, the literature suggests that alprazolam has the highest misuse liability among the BZDs because of its reinforcing pharmacokinetic and pharmacodynamic properties as well the way it has been shown to enhance dopamine (DA) release in the striatum similarly to stimulants (18). Other disadvantages of alprazolam include very fast onset and offset of therapeutic effect, indicating a need for 3–4 times daily dosing, potential for interdose rebound anxiety, and risk of life-threatening withdrawal in abrupt cessation (20).
Lorazepam lends itself well to use as a short-term, as-needed agent for anxiety because of its medium potency and moderately short clearance half life. Another unique property of lorazepam is that its metabolism bypasses hepatic CYP450 enzymes and only involves direct glucuronidation. Thus, it can be used among patients with impaired liver and renal function. Meanwhile, clonazepam tends to be favored as a standing agent among patients with anxiety because of longer duration of action and half life, typically allowing for twice daily dosing without significant interdose anxiety (20). Diazepam is the oldest of the aforementioned BZDs and is less commonly used at present. It has a fast time to onset and duration of therapeutic effect similar to that or lorazepam or clonazepam; however, it is notable for very slow clearance, with elimination half life of diazepam and its active metabolites varying from 30 to 100 hours. This trait raises concern for accumulation-related side effects, especially fall risk among older adults. Diazepam is relatively unique in having a rectal suppository gel formulation, which has very fast onset and obviates the need for enteral or parenteral access.
Tricyclic antidepressants (TCAs).
A few TCAs have demonstrated efficacy in generalized anxiety disorder. They work by binding and inhibiting both the SERT and NET as well as directly modulating specific 5-HT receptors. Unfortunately, they are particularly nonselective in their receptor affinities, leading to a broad range of adverse effects. Antagonist effects of TCAs at muscarinic acetylcholine receptors can lead to prominent dry mouth, constipation, urinary retention, blurred vision, tachycardia, and impaired cognition. Patients may have significant weight gain or sedation because of strong histamine-1 receptor blockade by TCAs. Alpha-1 adrenergic blockade often leads to significant orthostatic hypotension and dizziness. Finally, the SSRI-like inhibition of the SERT may lead to long-term sexual side effects. TCAs also have a narrow therapeutic index and may be lethal in overdose via hypotension, cardiac arrhythmias, anticholinergic poisoning, and neurologic compromise ranging from seizures to coma. Thus, TCAs should be used with care among patients with a history of suicide attempts by medication overdose (21, 22).
Imipramine, the prototypical TCA, has undergone multiple RCTs against placebo and other established treatments supportive of efficacy for symptoms of generalized anxiety disorder (23, 24). Clomipramine has preliminary evidence for use in generalized anxiety disorder from a small open-label trial (25), and nortriptyline showed evidence of efficacy in a small, merged analysis of three placebo-controlled trials among patients with generalized anxiety disorder after a stroke (26). Whether TCAs have equivalent or superior efficacy to SRIs is an important question in the context of considering next steps for patients with conditions that are treatment resistant. Unfortunately, head-to-head data comparing drugs from the two classes are limited. One such study showed similar efficacy between imipramine and paroxetine, with a higher proportion of patients dropping out in the imipramine group (27). Although evidence of superior efficacy to SRIs is lacking, TCAs are a heterogeneous group of antidepressants with very divergent pharmacodynamic profiles from both each other and the SRIs. This feature allows these medications to play niche roles in treating other conditions besides anxiety (e.g., obsessive-compulsive disorder for clomipramine, attention-deficit hyperactivity disorder for desipramine, headache for amitriptyline, and chronic pain for both amitriptyline and nortriptyline). Thus, both by being sufficiently different from the first-line agents and by having more potential to address comorbid conditions, TCAs may be useful among patients who can titrate to therapeutic doses without intolerable side effects.
Miscellaneous antidepressants.
A variety of other antidepressants with unique mechanisms of action have shown promise for off-label treatment of generalized anxiety disorder, although they have been subject to limited study. A pilot study (N=24) found bupropion extra long (XL), which has the advantage of not being associated with sexual side effects, to be noninferior to escitalopram among patients with generalized anxiety disorder (28). An open-label trial of mirtazapine 30 mg daily for generalized anxiety disorder showed a high response rate of almost 80% (29). A meta-analysis of four RCTs supports the efficacy of the novel antidepressant vortioxetine, which acts as an SRI in addition to modulating a few specific 5-HT receptors; furthermore, effect size was more robust among patients with more severe anxiety at baseline (Hamilton Anxiety Rating Scale score>25) (30). Notable limitations of the studies include lower dosages (2.5–10 mg vs. usual maximum for depression of 20 mg, and short duration of 8 weeks). Vortioxetine has demonstrated cognitive-enhancing effects among patients with major depressive disorder, which may also be advantageous in the population with generalized anxiety disorder because concentration difficulties are part of the core symptom cluster (31).
A meta-analysis of three RCTs using 20–40 mg daily of vilazodone, a combined SRI and 5-HT1A partial agonist, achieved statistical significance for efficacy but with a small effect size and increased dropout for adverse events (32). Nefazodone, which acts as a 5-HT2A and 5-HT2C receptor antagonist, has preliminary support from a small open trial among adults with generalized anxiety disorder (33). This drug was temporarily withdrawn from market in the United States because of association with rare cases of fulminant and sometimes fatal hepatic failure, but it was later reinstated (34). Close monitoring for hepatotoxicity by liver function tests and education about hepatitis symptoms should be used if nefazodone is prescribed. In a placebo-controlled study mentioned earlier in the section on TCAs, trazodone demonstrated similar efficacy to diazepam and imipramine for generalized anxiety disorder. It should be noted that patients taking trazodone titrated to a mean dose of 255 mg daily for anxiolytic effect (24). Trazodone is rarely used in such high doses in contemporary practice because of often prohibitive sedation.
Antihistamines.
The histamine blocking medication hydroxyzine has support from double-blind RCTs with placebo and active comparators, such as BZDs or buspirone, and has demonstrated comparable efficacy with these established generalized anxiety disorder treatments (35, 36). A Cochrane review in 2010 (37) characterized hydroxyzine as having support for use in generalized anxiety disorder but could not recommend it as a first-line treatment because of limited studies and sample size along with high risk of bias in the available trials. Usual daily dosing per the trials is 50 mg, divided twice a day to three times a day, and the most prominent adverse effect is sedation (37).
Neuroleptics.
Finally, RCT evidence has been found for the use of second-generation antipsychotics in generalized anxiety disorder, particularly quetiapine. However, citing significant cardiovascular and metabolic risks, an FDA panel denied approval for this indication. A meta-analysis in 2016 found quetiapine to be efficacious as a monotherapy for generalized anxiety disorder at doses between 50 and 150 mg daily, with discontinuation and drop-out rates similar to those in clinical trials of antidepressants (38). Specifically, two studies found quetiapine to have essentially equivalent effects on generalized anxiety disorder to standard doses of 10 mg of escitalopram and 20 mg of paroxetine, respectively. Although the studies were performed with quetiapine extended release (XR), pharmacokinetic studies showed no significant difference in side-effect profile between the immediate release and XR formulations; however, the XR version did show decreased sedation in the initial few hours after dosing (39), which is easily circumvented with dosing all at bedtime. A few small trials have provided support for adjunctive use of risperidone, ziprasidone, and aripiprazole (40–44). With all antipsychotics, major risks include weight gain, metabolic syndrome and associated sequelae, corrected QT prolongation, drug-induced extrapyramidal symptoms, tardive dyskinesia, and akathisia. Given these risks, trials of antipsychotics should be reserved after failure of several prior adequate trials of safer agents.
Rational Pharmacologic Approach to Generalized Anxiety Disorder
Comprehensive, evidence-based guides to direct treatment of generalized anxiety disorder are limited, with one noteworthy exception being the “Canadian Clinical Practice Guidelines for the Management of Anxiety, Posttraumatic Stress and Obsessive-Compulsive Disorders” (45). In this sizeable review, the authors stratified treatment interventions according to established efficacy, quality of evidence, and tolerability and safety considerations; furthermore, they proposed a hierarchy of first-, second-, and third-line monotherapy options as well as ranked adjunctive treatment options. The authors concluded that SSRIs and SNRIs along with pregabalin should be considered first-line treatments. They characterized TCAs, BZDs, vortioxetine, bupropion XL, buspirone, quetiapine, and hydroxyzine as second-line treatments. They qualified miscellaneous antidepressants and valproic acid (VPA) in rare formulations as third-line treatments. Finally, for adjunctive treatment, they favored pregabalin over second-generation antipsychotics (45).
Investigators have devised evidence-based algorithms to guide sequencing of medication trials in generalized anxiety disorder on the basis of estimates of treatment effect size from meta-analyses (46). Traditional pairwise systematic review and meta-analysis have the limitation of only being able to compare two interventions with each other at once and fail to account for the relative amount of study that different interventions have received. Network meta-analysis (NMA) is a novel approach to ranking the efficacy of various interventions by using data from a systematic review of the literature and drawing on both direct and indirect comparisons. An important part of a NMA is the network graph (Figure 1), which displays a network of circular nodes of varying sizes with lines called edges of varying thickness between the nodes. The nodes represent interventions, and the size of the nodes indicates the number of participants in each intervention. The edges between nodes represent studies directly comparing two interventions, with the thickness proportional to the number of such trials. This visuospatial mapping of the literature gives context to the quantitative analysis of effect size, which uses complex statistical methods to identify the effect sizes of various interventions relative to each other and to placebo (47).
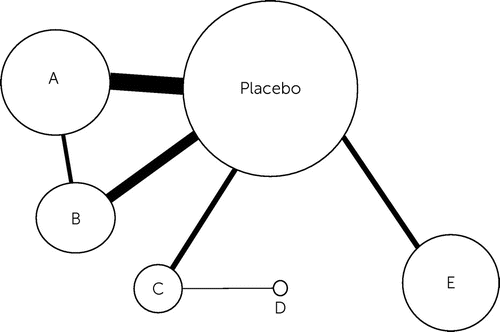
FIGURE 1. Generic example of a network grapha
For instance, one NMA comparing various psychopharmacologic and psychological interventions for generalized anxiety disorder reported bupropion and mirtazapine as more efficacious than all other treatments, even though they have a paucity of data backing them. The network plot clearly displays this contradiction by showing the nodes representing bupropion and mirtazapine as small dots in comparison with the much more studied first-line treatments (6). Using traditional pairwise meta-analysis, clinicians interpreting the research may have (probably falsely) concluded that they should resort to bupropion and mirtazapine as first-line agents. Another network analysis in 2019 showed significant positive effects combined with good tolerability relative to placebo for escitalopram, duloxetine, venlafaxine, and pregabalin. This same study also measured quetiapine as having the strongest effect size, although poorly tolerated (48). As the methodology of NMA continues to advance, the results of such studies will have more clinical utility. Of course, even with the additional dimensions added by NMAs, no method has been established to computationally incorporate additional factors such as side-effect profile and cost. Eventually choice of agents comes down to a human integrating available quantitative evidence for efficacy with other qualitative considerations.
On the basis of the data presented earlier, the following is one of multiple possible models for rational stepwise pharmacologic treatment of patients with generalized anxiety disorder. At all stages, proper informed consent and shared decision making should be incorporated into the process. In this model, and at points throughout this review, the concept of an “incomplete” or “partial” response is referenced; for the reader, these terms may be defined as a roughly 50% improvement but with residual symptoms continuing to distress the patient or impair functioning.
First line: Treatment should typically begin with an SSRI; one might choose the initial antidepressant on the basis of characteristics of the agent rather than presence or absence of FDA approval. Sertraline and escitalopram are often strong choices on the basis of demonstrated efficacy in generalized anxiety disorder, along with lower potential than the FDA-approved agents for drug-drug interactions or idiosyncratic adverse effects. Among patients with certain comorbid conditions such as fibromyalgia or chronic pain, an SNRI such as duloxetine would be a logical first choice. Among patients with significant hesitation about sexual side effects with SSRIs, consider buspirone monotherapy as first line.
Second line: In most cases, clinicians may resort to SSRIs and SNRIs for the first several medication trials because the SSRIs each exhibit idiosyncratic effects in different patients; moreover, the SNRIs are clinically a heterogenous group of medications in terms of their differential effects on 5-HT and NE signaling. Because of its benign side-effect profile, buspirone may be the rational next step, either as monotherapy or as augmentation to an antidepressant that achieved an incomplete response. Pregabalin as monotherapy or in combination with another drug should also be considered at this stage. Finally, for patients without contraindications, BZDs warrant a trial.
Third line: TCAs are relegated to this stage because of tolerability and safety concerns, although available evidence shows that they may have equal efficacy to the first-line treatments. Despite efficacy, quetiapine as monotherapy or augmentation similarly should be reserved for patients with conditions that are truly treatment refractory because of the previously mentioned significant risk of long-term adverse effects from antipsychotics.
Fourth line: Augmentation with other second-generation antipsychotics and use of the miscellaneous antidepressants fall into this category because of limited supportive data and significant adverse effects.
Panic Disorder
The hallmark of panic disorder is anxiety and pathological avoidance surrounding recurrent panic attacks, which are brief episodes of intense fear associated with unpleasant physical symptoms. Treatment emphasizes reducing frequency and severity of panic attacks, with the goal of reversing learned patterns of avoidance that interfere with functioning and quality of life, often through psychotherapy. Panic disorder has been characterized as a relapsing-remitting chronic disorder for which the severity of symptoms varies over time. Psychotherapy, particularly cognitive-behavioral therapy (CBT), can promote lifelong remission in some. However, indefinite pharmacologic treatment is indicated among many patients because of the nature of the disorder (1).
Review of Specific Agents and Their Efficacy and Tolerability in Treatment of Panic Disorder
SRIs.
As in generalized anxiety disorder, first-line (and second- and sometimes third-line) pharmacotherapy for panic disorder uses SRIs. The specific SSRIs that have FDA approval for panic disorder include fluoxetine, sertraline, and paroxetine (regular and controlled release). Venlafaxine XR is the sole SNRI with an FDA indication for panic disorder. Nevertheless, placebo-controlled and more preliminary clinical trial evidence supports off-label use of the other SSRIs (escitalopram, citalopram, fluvoxamine) (49–51) and SNRIs for panic disorder (duloxetine and milnacipran) (52, 53). The evidence base supports dosing of SSRIs and SNRIs within the usual FDA-approved dosing ranges.
BZDs.
Before the establishment of SRIs as first-line treatment for panic disorder, the high-potency BZDs (HPBs) clonazepam and alprazolam were preferred treatments because of their rapid onset of effect, tolerability, and sustained antipanic efficacy over time. For reasons already discussed, these medications have fallen out of favor. However, HPBs remain a valuable second-line option among appropriate patients who are resistant to or cannot tolerate the SRIs. Clonazepam is the preferred HPB for panic disorder, and clear efficacy has been demonstrated for doses ranging from 1 to 4 mg daily, usually divided twice daily (54, 55). A dose-finding trial indicated that dose-dependent sedation and ataxia became more problematic when exceeding 2 mg total daily; in addition, 1–2 mg daily of clonazepam provided the best tradeoff of therapeutic efficacy and tolerability (56). Alprazolam XR, although rarely used in practice, may also be used to address the frequent dosing and interdose anxiety associated with immediate-release alprazolam (57). At high doses, the XR form does not eliminate the misuse liability of the drug (18). The midpotency BZD lorazepam was shown in a head-to-head, double-blind trial with alprazolam to have equivalent efficacy for panic disorder (58). A study comparing the low-potency benzo diazepam with alprazolam found similar antipanic efficacy (59). Interestingly, a 2019 Cochrane meta-analysis comparing BZDs with placebo for treatment of panic disorder slightly favored BZDs; however, the authors took care to point out the low quality of evidence and high risk of bias across most reviewed studies (55).
TCAs.
TCAs have well-demonstrated efficacy for panic disorder. Imipramine has the most supportive clinical trial evidence (24, 60–62). It was found to have equivalent antipanic efficacy in a head-to-head RCT with sertraline among patients with comorbid panic disorder and major depressive disorder (63). This medication may cause high levels of initial jitteriness and anxiety among patients with panic disorder, so a low starting dose of 10 mg daily is recommended, with slow titration in 25-mg increments to the ultimate target dose (64). Serum levels may be useful for guiding treatment, with one trial showing that levels of 110–140 ng/ml yielded optimal results (61). The other two TCAs that have been validated for panic disorder are clomipramine and desipramine, with multiple RCTs supporting the antipanic efficacy of each (65–68).
Monoamine oxidase inhibitors (MAOIs).
MAOIs block the activity of enzymes involved in the breakdown of serotonin (5-HT) and the catecholamine neurotransmitters DA and NE by oxidative deamination, thereby leading to increased levels of these neurotransmitters in the synaptic cleft. The monoamine oxidase (MAO) enzymes include MAO-A and MAO-B. The catecholamines are metabolized by both MAO-A and MAO-B, whereas 5-HT is metabolized only by MAO-A. The four irreversible MAOIs marketed in the United States include the nonselective MAOIs phenelzine and tranylcypromine and the MAO-B selective drugs selegiline and isocarboxazid ((2)). Presumably because they increase 5-HT transmission in addition to DA and NE, only the nonselective MAOIs phenelzine and tranylcypromine have demonstrated significant efficacy for anxiety disorders, specifically panic disorder and social anxiety disorder (69–72).
Despite sufficient evidence for their efficacy, MAOIs should be reserved for patients with anxiety disorders that are severely treatment resistant because of their heavy side-effect burden and risk of dangerous complications. Roughly half of patients taking MAOIs will experience significant orthostatic dizziness. Other very common side effects include sexual dysfunction, insomnia or sedation, headache, constipation, and dry mouth (73). Patients taking irreversible MAOIs must adhere to dietary restrictions to avoid the dreaded tyramine-induced hypertensive crisis, sometimes called the “cheese effect” because it can be triggered by eating foods such as certain aged cheeses that have high tyramine content (74). Furthermore, coadministration of MAOIs with other medications that increase serotonergic or catecholaminergic neurotransmission may lead to life-threatening drug interactions such as serotonin syndrome or dangerous hypertension. Thus, before starting an MAOI, patients must allow at least five half-lives time for their previous antidepressant to wash out. This gap may vary from 1 to 2 weeks in the case of most antidepressants and from 4 to 5 weeks in the case of fluoxetine. Similarly, because the effects of irreversible MAO inhibition can persist for 2–3 weeks, a washout period of the same length is recommended before switching to another class of antidepressant to prevent problematic drug interactions (75).
Although the decision to start an MAOI should not be taken lightly for all the reasons discussed earlier, risk-benefit calculation may yet favor prescribing these medications in subpopulations of patients with panic or social anxiety disorder with severe, disabling symptoms and failure to respond to or tolerate several adequate trials of lower-risk medications. Dietary restrictions are the most common reason for hesitancy; however, it is worth noting that the tyramine content of foods has decreased over time and that after reexamination, earlier low-tyramine diets have been deemed unnecessarily expansive. With advances in food science and the usual tyramine content of different foods, researchers have developed less-restrictive, more user-friendly diets for patients taking MAOIs (75).
The reversible inhibitors of MAO-A (RIMAs) have been targets of interest for the treatment of panic disorder because of the toxicity and drug-interaction concerns raised by phenelzine and tranylcypromine. In the presence of substrates such as tyramine or monoamine neurotransmitters, RIMAs are easily displaced from the MAO enzyme, making major adverse events such as tyramine-associated hypertensive crisis or serotonin syndrome very unlikely. Thus, limited to no dietary adjustments are required to take these medications (74). The most widely used RIMA is moclobemide, which is not marketed in the United States but is commercially available in Canada and many European countries. RCT evidence for using moclobemide to treat panic disorder has been supportive of efficacy and improved safety and tolerability in comparison with other MAOIs or TCAs (74, 76–80). Similarly, the MAO-B selective inhibitor selegiline has drawn growing interest because of the availability of a transdermal patch for treatment of major depressive disorder, which at the lowest dose form of 6 mg does not require dietary modifications. Unfortunately, no studies have supported its use for panic disorder.
Other antidepressants.
A handful of the unclassified antidepressants are supported by multiple small RCTs. A small, open-label, flexibly-dosed trial of vortioxetine was suggestive of benefit for panic disorder (81). Mirtazapine has demonstrated efficacy for panic disorder in multiple open-label trials and in one double-blind, head-to-head study with fluoxetine, with effective dosing ranging from 15 to 30 mg daily (82–84). Bupropion has been studied with mixed results (85). Nefazodone has multiple open trials supportive of efficacy for panic disorder at doses between 200 and 600 mg daily (86, 87). The evidence for trazodone is weak, with positive results in a small open-label trial; however, high attrition and low response rate were found in an 8-week, double-blind trial comparing it with the established agents imipramine and alprazolam (60).
Anticonvulsants.
VPA has preliminary evidence for efficacy in treating panic disorder from small open trials in doses from 500 to 2,250 mg daily. Generally, VPA is well tolerated, but it may cause weight gain, tremor, and hair loss. Because of the potential for cytopenias, hepatic injury, and buildup of toxic levels, patients taking VPA require periodic monitoring of complete blood counts, liver function tests, and serum VPA levels (88, 89). Trials with carbamazepine and gabapentin have been negative and equivocal, respectively (90, 91).
Second-generation antipsychotics.
An 8-week, single-blind RCT (N=56) of moderately-dosed paroxetine against low-dose risperidone among patients with panic disorder either alone or comorbid with major depressive disorder showed significant antipanic efficacy that was not different between treatment groups. Moreover, presumably because of the low doses used, risperidone was generally well tolerated (92). However, a double-blind, placebo-controlled trial (N=111) of risperidone 0.5–4.0 mg daily monotherapy for patients with comorbid bipolar disorder and generalized anxiety disorder or panic disorder failed to show significant improvements in anxiety symptoms (93). In a 12-week, open-label study (N=31) of SSRI augmentation with olanzapine 5 mg daily, 57.7% of patients achieved remission (94).
Rational Pharmacologic Approach to Panic Disorder
As with generalized anxiety disorder, studies informing rational sequencing of medication trials for panic disorder are limited. The American Psychiatric Association has developed treatment guidelines that are relatively nonspecific (95). Katzman and colleagues (45) sorted the available interventions into first line (SSRIs and SNRIs), second line (BZDs, TCAs, and the miscellaneous antidepressants reboxetine and mirtazapine), and third line (MAOIs and RIMAs, second-generation antipsychotics, assorted antidepressants, and a few anticonvulsants). For adjunctive therapy, they supported BZDs, the beta blocker pindolol, and second-generation antipsychotics. They also discussed the strength of the various medications for maintenance treatment of panic disorder (45). In 2009, a three-phase, 24-week RCT did provide an interesting comparison of various commonly pursued pathways all starting at the same first-line SSRI therapy. The trial began with an open-label, 6-week SSRI (sertraline or escitalopram) targeted to a usual therapeutic dose (i.e., 100 mg sertraline) (96). Of the patients, 20% achieved remission status after phase 1 and terminated the study at this point. Next phases, in succession, included increased dose of SSRI or same dose as phase 1 plus placebo; subsequent phases included “medication optimization” with flexibly dosed clonazepam or psychotherapy with CBT. The end results tended to favor allowing adequate time for standard therapeutic doses of SSRI to take effect and using combination with CBT (96).
NMA studies were discussed in the section on generalized anxiety disorder as a novel method of ranking treatment interventions. Unfortunately, although NMAs have been published comparing various psychological treatments for panic disorder, review of the literature yielded no published NMAs of psychopharmacologic therapies. The addition of high-quality NMAs would add significantly to the current evidence base for stratifying treatments.
The following hierarchy of treatments on the basis of the evidence presented earlier is proposed:
First line: Begin with SRIs with particular consideration of specific drug characteristics and comorbid conditions.
Second line: For panic disorder, a standing high-potency benzo (usually clonazepam) should be considered sooner in appropriate patients because of its well-demonstrated track record of efficacy and tolerability. If SRIs were not tolerated and the patient has only panic disorder, monotherapy with HPBs would be reasonable. Otherwise, especially with comorbid conditions treated by SRIs, HPBs should be used in combination with the SRI.
Third line: As in generalized anxiety disorder, TCAs are arguably third line despite strong data for efficacy in treating panic disorder because they have a formidable side-effect burden and potential for toxicity. Another disadvantage is lack of efficacy in treating comorbid social anxiety disorder. At this stage, other antidepressants such as mirtazapine and nefazodone should also be considered, given their moderately strong clinical evidence coupled with low-moderate adverse effect risks.
Fourth line: At this point, the remaining studied interventions can all be considered, including adjunctive second-generation antipsychotics, MAOIs, and VPA. Although any of these medications may prove efficacious among patients with conditions that are treatment resistant, these medications should be end-of-the-line options because of either paucity of data or mixed results of studies, dangerous or highly intolerable adverse effect potential, or some combination of the two.
Social Anxiety Disorder
Social anxiety disorder involves pathological levels of anxiety in social or performance situations in which intense scrutiny by others is perceived or anticipated. Patients tend to altogether avoid the feared social situations; only approach them with a companion; or endure them with distress and physical symptoms that can approach the intensity of full panic attacks, such as flushing, sweating, palpitations, and tremor. Social anxiety disorder includes both a “performance-only” variant and generalized social anxiety disorder, which extends to a variety of social interactions (1).
Review of Specific Agents and Their Efficacy and Tolerability in Treatment of Social Anxiety Disorder
SRIs.
Pharmacologic treatment aims to decrease anticipatory anxiety and avoidance behaviors before, and distress during, essential social activities to improve social and occupational functioning. As with the other anxiety disorders, SRIs are the pharmacologic treatment of choice for generalized social anxiety disorder. Performance-only social anxiety disorder is generally not treated with maintenance medications because most people infrequently encounter the feared performance situations. The drugs in these classes that carry the FDA indication for social anxiety disorder include the SSRIs sertraline, paroxetine, and paroxetine CR as well as the SNRI venlafaxine XR. Data from RCTs also support the use of the SSRIs fluvoxamine, citalopram, and escitalopram (97–100). Some initial data support SNRI duloxetine for the treatment of social anxiety disorder (101). Fluoxetine has less consistent evidence (98, 102, 103). Escitalopram, paroxetine, sertraline, and venlafaxine were shown in a meta-analysis to have roughly equivalent effect size and superiority to placebo (104). Principles guiding titration and dosing are similar to those applied to generalized anxiety disorder and panic disorder.
Beta-adrenergic blockers.
Beta blockers have been validated for the treatment of performance-only social anxiety, which generally arises in the context of public speaking or other performative situations. Their hypothesized mechanism of action is suppression of the physiological hyperarousal that occurs in the fear response to such situations. This process interrupts an escalating feedback loop in which patients become distressed and self-conscious about physical manifestations such as flushing, palpitations, and sweating, which subsequently causes them to further amplify these exhibitions; this amplification, in turn, begets more psychic anxiety about these manifestations being scrutinized by others and so on (105, 106). For reasons that are not fully understood, beta blockers have not demonstrated efficacy in treating generalized social anxiety disorder. For instance, a head-to-head trial of beta blocker atenolol compared with the validated treatment phenelzine and placebo found atenolol ineffective for generalized social anxiety (107).
The nonselective beta blocker propranolol and the cardio-selective agent atenolol are the two primary agents that have been studied for use in performance-only social anxiety disorder. Recommended use is as needed 1–2 hours before a performance situation at total daily doses of 10–80 mg propranolol and 50–150 mg atenolol. One should take a “test” dose on a day with no performance obligations to get accustomed to the feeling of the medication. Typical adverse effects include orthostatic hypotension and lightheadedness, bradycardia, sedation, and nausea; in the case of nonselective propranolol, effects include worsening of underlying airway obstruction among patients with asthma or chronic obstructive pulmonary disease (105, 106, 108, 109).
BZDs.
BZDs have moderate support in clinical studies for use in treating social anxiety disorder and are associated with a faster response than the first-line antidepressant agents (110). Used as needed before performance situations or occasional feared social situations, BZDs may be quite helpful given their rapid onset of effect; however, they may also be associated with cognitive and psychomotor impairment and ataxia, which, in turn, could impair performance. A high incidence of patients with social anxiety disorder use alcohol to self-medicate symptoms. Thus, thorough substance use evaluation should be undertaken with all patients, and practitioners should carefully discuss risks and benefits of BZD prescriptions with patients who have histories of problematic alcohol use and document these conversations thoroughly. Concomitant use of alcohol with BZDs risks synergistic CNS depression, which could result in death (111). However, if BZDs lead to significant improvement of social anxiety disorder symptoms, patients who historically have used alcohol to self-medicate for social anxiety may no longer feel compelled to do so.
The available evidence suggests minimizing use of as-needed BZDs for generalized social anxiety disorder because rapid relief of anxiety with medication eliminates exposure to anxiety symptoms required for fear-extinction learning, interfering with the efficacy of CBT interventions (112). For patients with refractory generalized social anxiety symptoms, standing BZD regimens may be appropriate, preferably with a long-acting agent initiated at low dose and titrated to the minimum dose needed for efficacy. In one study, adding 1–2 mg per day of clonazepam to flexibly-dosed paroxetine yielded superior results compared with paroxetine monotherapy (110). For as-needed use, lorazepam given 1–2 hours before the occasional feared situation is a reasonable choice. As with beta blockers, patients naïve to BZDs should first take a test dose in a safe setting to ensure toleration of the medication.
Anticonvulsants.
Gabapentin and pregabalin both have RCTs showing efficacy over placebo for social anxiety disorder; however, it should be noted that improvement was associated with higher doses than are often tolerated (e.g., >2,100 mg daily for gabapentin and 600 mg total daily for pregabalin) (113–115). However, one study supported slightly lower maintenance dosing for pregabalin (114). Small open-label trials have raised the possibility of treating social anxiety disorder effectively with VPA, levetiracetiram, topiramate, and tiagabine, but these trials either were not successfully replicated with, or not followed by, subsequent RCTs.
MAOIs.
The MAOIs phenelzine and tranylcypromine formerly were regarded as the standard of care pharmacologic treatment for social anxiety disorder before the emergence of the safer, less complicated SSRIs and SNRIs. This practice was based initially on the observation that MAOIs seemed to uniquely treat patients with “atypical” depression and prominent rejection sensitivity; this practice was subsequently supported by multiple positive RCTs (69, 73). The RIMAs represent a potentially helpful and easier to administer MAOI category for the treatment of social anxiety disorder, but limitations include lack of availability in the United States and mixed evidence (79, 104, 116). Selegiline transdermal patch also lacks evidence for use in treating social anxiety disorder.
Miscellaneous medications: other antidepressants, buspirone, and neuroleptics.
Contrary to their well-supported use in panic disorder and generalized anxiety disorder, TCAs have no established role in treating social anxiety disorder because of negative results in the few available open-label and double-blind, placebo-controlled trials. Vilazodone was studied in one double-blind, placebo-controlled RCT (N=39) of patients who, on average, had severe social anxiety disorder at baseline; their symptoms significantly improved compared with placebo, with a moderate effect size (117). An RCT (N=40) of vortioxetine against placebo in a patient sample with comorbid major depressive disorder and social anxiety disorder showed marked reductions in measures of social anxiety (118). Mirtazapine has positive evidence from open-label studies as well as a more rigorous RCT with a sample of only women, but a larger placebo-controlled RCT of both men and women failed to replicate those results (119–121). At least one small open trial suggests possible efficacy of bupropion (122). As an adjunctive agent to SSRI therapy, buspirone has limited evidence suggesting efficacy (123). Small trials and case series provide preliminary evidence of efficacy for the second-generation antipsychotics quetiapine and olanzapine in treating social anxiety disorder (124–126). Overall, the body of evidence for each of these treatments is small. The favorable outcomes in studies of vilazodone and vortioxetine are not surprising given their pharmacodynamic similarity to SSRIs.
Rational Pharmacologic Approach to Social Anxiety Disorder
The Anxiety and Depression Association of America has published a clinical practice review for social anxiety disorder that separates psychopharmacologic treatments into a first-line category (essentially the SSRIs and SNRIs), followed by a second-line category, which contains the broad range of treatments discussed earlier; however, notable option rankings are not included except to suggest first a trial of a different SSRI or SNRI (127). Other important conclusions from this report included a recommendation against standing beta blockers for generalized social anxiety disorder and as-needed quetiapine for performance-only social anxiety disorder; however, cautious optimism was given for both quetiapine and olanzapine as adjunct treatment (127). The Canadian Clinical Practice Guidelines similarly recommend SSRIs and SNRIs and high-dose pregabalin as first line. BZDs, MAOIs, and gabapentin were recommended as second line. Finally, a variety of antidepressants, anticonvulsants, and second-generation antipsychotics were recommended as third line (45). In 2014, Pollack and colleagues (128) conducted a double-blind RCT examining next-step treatment options for patients with social anxiety disorder who did not respond to sertraline after 10 weeks. The patients who did not respond to sertraline were randomly assigned to either receive continued sertraline plus placebo, sertraline augmentation with up to 3.0 mg per day of clonazepam, or switch from sertraline to optimally titrated venlafaxine. Augmentation with clonazepam as a strategy resulted in significant reductions in measures of social anxiety compared with maintenance sertraline or switch to venlafaxine, although no significant difference was found in the number of patients achieving remission (128).
In 2020, an NMA of pharmacologic treatments for social anxiety disorder was published and yielded interesting results (129). The authors identified paroxetine as superior to other treatments for reducing the symptom severity of social anxiety disorder; therefore, they recommended paroxetine as first-line treatment. However, paroxetine is associated with several practical problems, including an association with weight gain, anticholinergic effects, and drug-drug interactions via strong inhibition of the important cytochrome P450 enzyme 2D6. On the basis of a single included study, the authors of this analysis found olanzapine to be considered very efficacious, which suggests a need for higher-quality studies, especially in light of the medication’s long-term safety and tolerability profile. The authors also identified a broad group of agents as superior to placebo for social anxiety disorder, including the SSRIs paroxetine, escitalopram, fluvoxamine, and sertraline; the BZDs bromazepam and clonazepam; and the MAOI phenelzine (129).
The following proposed hierarchy of pharmacologic treatments for social anxiety disorder integrates all the evidence previously discussed:
First line: As with the other major anxiety disorders, start with SSRIs or SNRIs, noting idiosyncratic drug characteristics and comorbid conditions. If the patient exhibits performance-only social anxiety, then trial an as-needed beta blocker, typically propranolol first.
Second line: Pursue one or more additional trials of supported SRIs, including venlafaxine. Despite the paucity of evidence, on the basis of the quality and effect size from available studies, one may subsequently consider a trial of vortioxetine or vilazodone. For performance-only social anxiety disorder that is not responsive to beta blockers, consider as-needed, short-acting BZDs such as lorazepam.
Third line: Consider adequacy of engagement with psychotherapy before additional medication trials. For generalized social anxiety disorder, in the event of incomplete response of an antidepressant, one may pursue adjunctive treatment with an HPB such as clonazepam 1–2 mg daily in an appropriate patient for BZDs. If numerous SRIs were not tolerated or failed to achieve even partial (e.g., >50%) response and the patient does not have comorbid conditions requiring an antidepressant, one should consider monotherapy with a standing HPB.
Fourth line: Depending on patient preference and other considerations, next steps would include the anticonvulsants gabapentin and pregabalin or the MAOIs phenelzine and tranylcypromine.
Fifth line: Other treatments with inconsistent trials (mirtazapine) or only open-label trial evidence should be considered as last resort. Even though a small trial and a recent NMA study provide support for strong efficacy of olanzapine, because of long-term safety and tolerability issues, I would reserve monotherapy or adjunct second-generation antipsychotics only for the most severe patients with conditions that are the most treatment refractory.
Conclusions and Future Directions
Psychiatry has had a lack of novel pharmacologic treatments developed specifically to target anxiety disorders for decades. Nevertheless, the field continues to make incremental progress via rigorous study of repurposing medications primarily approved for other conditions to good effect for anxiety. In this review, I examined the wide evidence base for medication treatment of the primary anxiety disorders, with updates to account for the latest studies supporting off-label use of classes of medications such as novel antidepressants, anticonvulsants, and second-generation antipsychotics. Additionally, I reviewed the novel systematic review methodology of NMA, which is unique and promising for its capacity to develop relative ranking of treatments even if they were never studied head to head. I also critically assessed available NMAs of pharmacologic treatments for incorporation into clinical decision making. Finally, for generalized anxiety disorder, panic disorder, and social anxiety disorder, I constructed pragmatic and flexible hierarchies for evidence-based sequencing of medication trials.
A few points covered in this review bear reemphasizing. The first is the potential for wider use of pregabalin. Because of lack of FDA approval, pregabalin is underutilized in the United States for anxiety disorders. The drug has recently come off patent, and new generic formulations have become available, decreasing costs. Thus, clinicians treating patients with anxiety should consider it earlier in treatment algorithms, especially for generalized anxiety disorder, for which it has strong evidence of efficacy at moderate, well-tolerated doses. A second point of emphasis is the continued role of BZDs for pharmacologic treatment of moderate to severe anxiety, particularly panic disorder and more refractory generalized anxiety disorder. Effective clinicians should be comfortable prescribing these medications among patients for whom they are appropriate and in situations that necessitate more rapid stabilization of symptoms than first-line SRIs can provide. If possible, they should be used in a time-limited manner to avoid the development of physical dependence, but long-term use is indicated in a subset of patients.
Another key finding from the literature is that second-generation antipsychotics are evidence-based therapies for anxiety disorders, especially quetiapine as monotherapy or adjunct for generalized anxiety disorder. These drugs remain nonpreferred treatments because of significant long-term risks, especially weight gain and metabolic abnormalities. However, if well informed about risks and benefits, patients with anxiety disorders that are highly refractory warrant trials of these agents. I would assert that clinicians’ hesitance to prescribe them for anxiety disorders in the absence of additional novel low-risk medications may reflect a tendency to underpathologize severely disordered anxiety. Disordered anxiety imposes substantial morbidity on society via impairment of patients’ occupational and social functioning; moreover, it is associated with suicide, as evidenced by the epidemiological data showing an increased risk of suicide among patients with panic disorder (130).
In this review, I did not address novel drug targets for anxiety disorders. I also did not discuss use of psychopharmacologic agents to facilitate psychotherapy, as in psychedelic-assisted psychotherapy, which is currently the subject of rigorous RCTs for posttraumatic stress disorder and depression; if approved, it warrants further study in the primary anxiety disorders. Other issues not covered include best practices for integrating psychopharmacologic treatments with evidence-based psychotherapy, such as CBT, and the incorporation of agents from complementary and alternative medicine. All of the aforementioned issues warrant additional research. With reference to optimizing the use of the currently available drug armamentarium as discussed in this review, next steps include development of highly sophisticated sequenced treatment algorithms that factor in specific patient characteristics and cost-effectiveness, among other considerations. With ongoing refinement of methodology, additional NMAs of pharmacologic treatments will contribute significantly to the development of decision-support tools for busy general psychiatrists. Given all these considerations, a great deal of work remains to advance medication treatment of anxiety disorders.
1. Diagnostic and Statistical Manual of Mental Disorders, 5th ed. Arlington, VA, American Psychiatric Publishing, 2013Google Scholar
2. : Mechanism of action of serotonin selective reuptake inhibitors. Serotonin receptors and pathways mediate therapeutic effects and side effects. J Affect Disord 1998; 51:215–235Crossref, Google Scholar
3. : The anti-inflammatory mechanism of antidepressants—SSRIs, SNRIs. Prog Neuropsychopharmacol Biol Psychiatry 2018; 80:291–294Crossref, Google Scholar
4. : iPlasticity: induced juvenile-like plasticity in the adult brain as a mechanism of antidepressants. Psychiatry Clin Neurosci 2018; 72:633–653Crossref, Google Scholar
5. : Serotonin norepinephrine reuptake inhibitors: a pharmacological comparison. Innov Clin Neurosci 2014; 11:37–42Google Scholar
6. : Comparative efficacy and acceptability of first-line drugs for the acute treatment of generalized anxiety disorder in adults: a network meta-analysis. J Psychiatr Res 2019; 118:21–30Crossref, Google Scholar
7. : Chronological milestones to guide drug change. When should clinicians switch antidepressants? Arch Gen Psychiatry 1996; 53:785–792Crossref, Google Scholar
8. : How long should a trial of escitalopram treatment be in patients with major depressive disorder, generalised anxiety disorder or social anxiety disorder? An exploration of the randomised controlled trial database. Hum Psychopharmacol 2009; 24:269–275Crossref, Google Scholar
9. : Incidence of sexual dysfunction associated with antidepressant agents: a prospective multicenter study of 1022 outpatients. J Clin Psychiatry 2001; 62(Suppl 3):10–21Google Scholar
10. : SSRI-induced apathy syndrome: a clinical review. J Psychiatr Pract 2004; 10:196–199Crossref, Google Scholar
11. : QT prolongation, Torsades de Pointes, and psychotropic medications: a 5-year update. Psychosomatics 2018; 59:105–122Crossref, Google Scholar
12. : Azapirones for generalized anxiety disorder. Cochrane Database Syst Rev 2006; 3:CD006115Google Scholar
13. : Pregabalin for generalized anxiety disorder: an updated systematic review and meta-analysis. Int Clin Psychopharmacol 2017; 32:49–55Crossref, Google Scholar
14. : Pregabalin for the treatment of generalized anxiety disorder: an update. Neuropsychiatr Dis Treat 2013; 9:883–892Crossref, Google Scholar
15. : Treatment of generalized anxiety disorder with gabapentin. Case Rep Psychiatry 2017; 2017:6045017Google Scholar
16. : Long-term experience with clonazepam in patients with a primary diagnosis of panic disorder. Psychopharmacol Bull 1998; 34:199–205Google Scholar
17. : Lack of relationship between long-term use of benzodiazepines and escalation to high dosages. Psychiatr Serv 2003; 54:1006–1011Crossref, Google Scholar
18. : A review of alprazolam use, misuse, and withdrawal. J Addict Med 2018; 12:4–10Crossref, Google Scholar
19. : Alcohol or benzodiazepine co-involvement with opioid overdose deaths in the United States, 1999–2017. JAMA Netw Open 2020; 3:e202361Crossref, Google Scholar
20. : The alprazolam to clonazepam switch for the treatment of panic disorder. J Clin Psychopharmacol 1987; 7:175–178Crossref, Google Scholar
21. : Adverse effects associated with selective serotonin reuptake inhibitors and tricyclic antidepressants: a meta-analysis. CMAJ 1998; 159:1245–1252Google Scholar
22. : Tricyclic Antidepressant Toxicity. Treasure Island, FL, StatPearls Publishing, 2020Google Scholar
23. : Imipramine and chlordiazepoxide in depressive and anxiety disorders. II. Efficacy in anxious outpatients. Arch Gen Psychiatry 1986; 43:79–85Crossref, Google Scholar
24. : Antidepressants for the treatment of generalized anxiety disorder. A placebo-controlled comparison of imipramine, trazodone, and diazepam. Arch Gen Psychiatry 1993; 50:884–895Crossref, Google Scholar
25. : Clomipramine treatment for generalized anxiety disorder. J Clin Psychopharmacol 1992; 12:214–215Crossref, Google Scholar
26. : Treatment of poststroke generalized anxiety disorder comorbid with poststroke depression: merged analysis of nortriptyline trials. Am J Geriatr Psychiatry 2003; 11:320–327Crossref, Google Scholar
27. : Paroxetine efficacy in the treatment of generalized anxiety disorder. Acta Psychiatr Scand 1997; 95:444–450Crossref, Google Scholar
28. : A pilot controlled trial of bupropion XL versus escitalopram in generalized anxiety disorder. Psychopharmacol Bull 2008; 41:46–51Google Scholar
29. : Mirtazapine treatment of generalized anxiety disorder: a fixed dose, open label study. J Psychopharmacol 2005; 19:483–487Crossref, Google Scholar
30. : Vortioxetine, a multimodal antidepressant for generalized anxiety disorder: a systematic review and meta-analysis. J Psychiatr Res 2015; 64:88–98Crossref, Google Scholar
31. : Treatment effects on residual cognitive symptoms among partially or fully remitted patients with major depressive disorder: a randomized, double-blinded, exploratory study with vortioxetine. J Affect Disord 2019; 250:35–42Crossref, Google Scholar
32. : Efficacy and tolerability of vilazodone for the acute treatment of generalized anxiety disorder: a meta-analysis. Asian J Psychiatr 2017; 26:115–122Crossref, Google Scholar
33. : An open trial of nefazodone in adult patients with generalized anxiety disorder. Psychopharmacol Bull 1996; 32:671–676Google Scholar
34. : Involvement of mitochondrial dysfunction in nefazodone-induced hepatotoxicity. Food Chem Toxicol 2016; 94:148–158Crossref, Google Scholar
35. : Efficacy and safety of hydroxyzine in the treatment of generalized anxiety disorder: a 3-month double-blind study. J Clin Psychiatry 2002; 63:1020–1027Crossref, Google Scholar
36. : A multicentre double-blind comparison of hydroxyzine, buspirone and placebo in patients with generalized anxiety disorder. Psychopharmacology (Berl) 1998; 139:402–406Crossref, Google Scholar
37. : Hydroxyzine for generalised anxiety disorder. Cochrane Database Syst Rev 2010; 12:CD006815Google Scholar
38. : Quetiapine monotherapy in acute treatment of generalized anxiety disorder: a systematic review and meta-analysis of randomized controlled trials. Drug Des Devel Ther 2016; 10:259–276Crossref, Google Scholar
39. : Self-reported sedation profile of quetiapine extended-release and quetiapine immediate-release during 6-day initial dose escalation in bipolar depression: a multicenter, randomized, double-blind, phase IV study. Clin Ther 2012; 34:2202–2211Crossref, Google Scholar
40. : Adjunctive risperidone in generalized anxiety disorder: a double-blind, placebo-controlled study. J Clin Psychiatry 2005; 66:1321–1325Crossref, Google Scholar
41. : Atypical antipsychotics in primary generalized anxiety disorder or comorbid with mood disorders. Expert Rev Neurother 2009; 9:1147–1158Crossref, Google Scholar
42. : Adjunctive risperidone in the treatment of generalized anxiety disorder: a double-blind, prospective, placebo-controlled, randomized trial. Psychopharmacol Bull 2007; 40:41–57Google Scholar
43. : Ziprasidone treatment of refractory generalized anxiety disorder: a placebo-controlled, double-blind study. J Clin Psychopharmacol 2010; 30:185–189Crossref, Google Scholar
44. : An open-label trial of aripiprazole augmentation for treatment-resistant generalized anxiety disorder. J Clin Psychopharmacol 2007; 27:207–210Crossref, Google Scholar
45. : Canadian clinical practice guidelines for the management of anxiety, posttraumatic stress and obsessive-compulsive disorders. BMC Psychiatry 2014; 14(Suppl 1):S1Crossref, Google Scholar
46. : The Psychopharmacology Algorithm Project at the Harvard South Shore Program: an algorithm for generalized anxiety disorder. Harv Rev Psychiatry 2016; 24:243–256Crossref, Google Scholar
47. : Network meta-analysis: an introduction for clinicians. Intern Emerg Med 2017; 12:103–111Crossref, Google Scholar
48. : Pharmacological and psychological interventions for generalized anxiety disorder in adults: a network meta-analysis. J Psychiatr Res 2019; 118:73–83Crossref, Google Scholar
49. : Escitalopram in the treatment of panic disorder: a randomized, double-blind, placebo-controlled trial. J Clin Psychiatry 2003; 64:1322–1327Crossref, Google Scholar
50. : Fluvoxamine in the treatment of panic disorder: a multi-center, double-blind, placebo-controlled study in outpatients. Psychiatry Res 2001; 103:1–14Crossref, Google Scholar
51. : A double-blind placebo-controlled trial comparing fluvoxamine and imipramine in the treatment of panic disorder with or without agoraphobia. Psychopharmacol Bull 1996; 32:135–141Google Scholar
52. : Open-label support for duloxetine for the treatment of panic disorder. CNS Neurosci Ther 2009; 15:19–23Crossref, Google Scholar
53. : The efficacy of milnacipran in panic disorder: an open trial. Int Clin Psychopharmacol 2007; 22:153–158Crossref, Google Scholar
54. : Efficacy, safety, and gradual discontinuation of clonazepam in panic disorder: a placebo-controlled, multicenter study using optimized dosages. J Clin Psychiatry 1999; 60:604–612Crossref, Google Scholar
55. : Benzodiazepines versus placebo for panic disorder in adults. Cochrane Database Syst Rev 2019; 3:CD010677Google Scholar
56. : Clonazepam in the treatment of panic disorder with or without agoraphobia: a dose-response study of efficacy, safety, and discontinuance. J Clin Psychopharmacol 1997; 17:390–400Crossref, Google Scholar
57. : A double-blind, placebo-controlled, multicenter study with alprazolam and extended-release alprazolam in the treatment of panic disorder. J Clin Psychopharmacol 1994; 14:314–321Crossref, Google Scholar
58. : Lorazepam vs. alprazolam in the treatment of panic disorder. Pharmacopsychiatry 1990; 23:90–93Crossref, Google Scholar
59. : Diazepam versus alprazolam for the treatment of panic disorder. J Clin Psychiatry 1996; 57:349–355Google Scholar
60. : Drug treatment of panic disorder: the comparative efficacy of imipramine, alprazolam, and trazodone. J Clin Psychiatry 1986; 47:580–586Google Scholar
61. : Imipramine treatment of panic disorder with agoraphobia: dose ranging and plasma level-response relationships. Am J Psychiatry 1995; 152:673–682Crossref, Google Scholar
62. : Maintenance drug treatment of panic disorder. I. Results of a prospective, placebo-controlled comparison of alprazolam and imipramine. Arch Gen Psychiatry 1993; 50:51–60Crossref, Google Scholar
63. : Sertraline versus imipramine treatment of comorbid panic disorder and major depressive disorder. J Clin Psychiatry 2003; 64:654–662Crossref, Google Scholar
64. : Antidepressant-induced jitteriness/anxiety syndrome: systematic review. Br J Psychiatry 2009; 194:483–490Crossref, Google Scholar
65. : A double-blind crossover comparison of clomipramine and desipramine in the treatment of panic disorder. Eur Neuropsychopharmacol 1999; 9:191–196Crossref, Google Scholar
66. : The Galway Study of Panic Disorder. I: clomipramine and lofepramine in DSM-III-R panic disorder: a placebo controlled trial. J Affect Disord 1992; 25:63–75Crossref, Google Scholar
67. : Paroxetine, clomipramine, and cognitive therapy in the treatment of panic disorder. J Clin Psychiatry 1999; 60:831–838Crossref, Google Scholar
68. : Superiority of clomipramine over imipramine in the treatment of panic disorder: a placebo-controlled trial. J Clin Psychopharmacol 1992; 12:251–261Crossref, Google Scholar
69. : Psychopharmacologic validation of atypical depression. J Clin Psychiatry 1984; 45:22–25Google Scholar
70. : Therapeutic response to phenelzine in patients with panic disorder and agoraphobia with panic attacks. J Clin Psychiatry 1987; 48:55–59Google Scholar
71. : Treatment of endogenous anxiety with phobic, hysterical, and hypochondriacal symptoms. Arch Gen Psychiatry 1980; 37:51–59Crossref, Google Scholar
72. : Double-blind comparison of 30 and 60 mg tranylcypromine daily in patients with panic disorder comorbid with social anxiety disorder. Psychiatry Res 2010; 175:260–265Crossref, Google Scholar
73. : MAO Inhibitors. Treasure Island, FL, StatPearls Publishing, 2020Google Scholar
74. : On tyramine, food, beverages and the reversible MAO inhibitor moclobemide. J Neural Transm Suppl 1988; 26:31–56Google Scholar
75. : Switching antidepressants for treatment-resistant major depression. J Clin Psychiatry 2001; 62(Suppl 18):12–17Google Scholar
76. : The efficacy and safety of moclobemide compared to clomipramine in the treatment of panic disorder. Eur Arch Psychiatry Clin Neurosci 1999; 249(Suppl 1):S19–S24Crossref, Google Scholar
77. : Moclobemide and fluoxetine for panic disorder. Eur Arch Psychiatry Clin Neurosci 1999; 249(Suppl 1):S7–S10Crossref, Google Scholar
78. : Moclobemide for anxiety disorders: a focus on moclobemide for panic disorder. Int Clin Psychopharmacol 1997; 12(Suppl 6):S27–S30Crossref, Google Scholar
79. : Pharmacotherapy of social phobia. A controlled study with moclobemide and phenelzine. Br J Psychiatry 1992; 161:353–360Crossref, Google Scholar
80. : Reversible monoamine oxidase-A inhibitors in panic disorder. Clin Neuropharmacol 1993; 16(Suppl 2):S77–S82Google Scholar
81. : An open-label, flexible dose adaptive study evaluating the efficacy of vortioxetine in subjects with panic disorder. Ann Gen Psychiatry 2018; 17:19Crossref, Google Scholar
82. : The effect of mirtazapine in panic disorder: an open label pilot study with a single-blind placebo run-in period. Int Clin Psychopharmacol 2001; 16:363–368Crossref, Google Scholar
83. : Mirtazapine in the treatment of panic disorder: an open-label trial. Int Clin Psychopharmacol 2003; 18:35–38Crossref, Google Scholar
84. : Mirtazapine versus fluoxetine in the treatment of panic disorder. Braz J Med Biol Res 2001; 34:1303–1307Crossref, Google Scholar
85. : Bupropion sustained release for panic disorder. Psychopharmacol Bull 2003; 37:66–72Google Scholar
86. : Efficacy of open-label nefazodone treatment in patients with panic disorder. J Clin Psychopharmacol 2000; 20:544–546Crossref, Google Scholar
87. : Pilot open-label study of nefazodone in panic disorder. Depress Anxiety 1999; 10:137–139Crossref, Google Scholar
88. : Panic disorder: treatment with valproate. J Clin Psychiatry 1994; 55:134–136Google Scholar
89. : Valproic acid and panic disorder. Can J Psychiatry 1990; 35:248–250Crossref, Google Scholar
90. : Lack of efficacy of carbamazepine in the treatment of panic disorder. Am J Psychiatry 1988; 145:1104–1109Crossref, Google Scholar
91. : Placebo-controlled study of gabapentin treatment of panic disorder. J Clin Psychopharmacol 2000; 20:467–471Crossref, Google Scholar
92. : A comparison of low-dose risperidone to paroxetine in the treatment of panic attacks: a randomized, single-blind study. BMC Psychiatry 2009; 9:25Crossref, Google Scholar
93. : Randomized, placebo-controlled trial of risperidone for acute treatment of bipolar anxiety. J Affect Disord 2009; 115:376–385Crossref, Google Scholar
94. : Olanzapine augmentation in treatment-resistant panic disorder: a 12-week, fixed-dose, open-label trial. J Clin Psychopharmacol 2006; 26:45–49Crossref, Google Scholar
95. : Practice Guideline for the Treatment of Patients With Panic Disorder, 2nd ed. Arlington, VA, American Psychiatric Association, 2010. https://psychiatryonline.org/pb/assets/raw/sitewide/practice_guidelines/guidelines/panicdisorder.pdf. Accessed Feb 18, 2021Google Scholar
96. : Next-step strategies for panic disorder refractory to initial pharmacotherapy: a 3-phase randomized clinical trial. J Clin Psychiatry 2009; 70:1563–1570Crossref, Google Scholar
97. : Controlled-release fluvoxamine in obsessive-compulsive disorder and social phobia. Drugs Today (Barc) 2008; 44:887–893Crossref, Google Scholar
98. : Fluoxetine in social phobia: a double-blind, placebo-controlled pilot study. J Clin Psychopharmacol 2002; 22:257–262Crossref, Google Scholar
99. : Fluvoxamine treatment of social phobia (social anxiety disorder): a double-blind, placebo-controlled study. Am J Psychiatry 1999; 156:756–760Google Scholar
100. : Psychopharmacological treatment of social phobia; a double blind placebo controlled study with fluvoxamine. Psychopharmacology (Berl) 1994; 115:128–134Crossref, Google Scholar
101. : Duloxetine for the treatment of generalized social anxiety disorder: a preliminary randomized trial of increased dose to optimize response. CNS Spectr 2010; 15:367–373Crossref, Google Scholar
102. : Cognitive therapy versus fluoxetine in generalized social phobia: a randomized placebo-controlled trial. J Consult Clin Psychol 2003; 71:1058–1067Crossref, Google Scholar
103. : Fluoxetine, comprehensive cognitive behavioral therapy, and placebo in generalized social phobia. Arch Gen Psychiatry 2004; 61:1005–1013Crossref, Google Scholar
104. : Pharmacotherapy for social anxiety disorder (SAnD). Cochrane Database Syst Rev 2017; 10:CD001206Google Scholar
105. : Beta-adrenergic blocking drugs in anxiety and stress. Psychiatr Clin North Am 1985; 8:119–132Crossref, Google Scholar
106. : Effect of beta blockade and beta stimulation on stage fright. Am J Med 1982; 72:88–94Crossref, Google Scholar
107. : Phenelzine vs atenolol in social phobia. A placebo-controlled comparison. Arch Gen Psychiatry 1992; 49:290–300Crossref, Google Scholar
108. : The effect of propranolol versus placebo on resident surgical performance. Trans Am Ophthalmol Soc 1998; 96:283–294Google Scholar
109. : Use of beta-blocking agents to reduce the stress of presentation at an international cardiology meeting: results of a survey. Am J Cardiol 1984; 54:240–241Crossref, Google Scholar
110. : Double-blind, placebo-controlled assessment of combined clonazepam with paroxetine compared with paroxetine monotherapy for generalized social anxiety disorder. J Clin Psychiatry 2004; 65:244–248Google Scholar
111. : Benzodiazepine misuse in adults with alcohol use disorder: prevalence, motives and patterns of use. J Subst Abuse Treat 2020; 117:108061Crossref, Google Scholar
112. : Benzodiazepine treatment can impair or spare extinction, depending on when it is given. Behav Res Ther 2014; 56:22–29Crossref, Google Scholar
113. : Efficacy of pregabalin in generalized social anxiety disorder: results of a double-blind, placebo-controlled, fixed-dose study. Int Clin Psychopharmacol 2011; 26:213–220Crossref, Google Scholar
114. : Efficacy of pregabalin in preventing relapse in patients with generalized social anxiety disorder: results of a double-blind, placebo-controlled 26-week study. Int Clin Psychopharmacol 2011; 26:243–251Crossref, Google Scholar
115. : Treatment of social phobia with gabapentin: a placebo-controlled study. J Clin Psychopharmacol 1999; 19:341–348Crossref, Google Scholar
116. : Moclobemide in social phobia: a controlled dose-response trial. J Clin Psychopharmacol 1997; 17:247–254Crossref, Google Scholar
117. : A 12-week double-blind, placebo-controlled, flexible-dose trial of vilazodone in generalized social anxiety disorder. Prim Care Companion CNS Disord 2015; 17. doi: 10.4088/PCC.15m01831Google Scholar
118. : Vortioxetine versus placebo in major depressive disorder comorbid with social anxiety disorder. Depress Anxiety 2017; 34:1164–1172Crossref, Google Scholar
119. : Mirtazapine in generalized social anxiety disorder: a randomized, double-blind, placebo-controlled study. Int Clin Psychopharmacol 2010; 25:302–304Crossref, Google Scholar
120. : Mirtazapine treatment of social phobia in women: a randomized, double-blind, placebo-controlled study. J Clin Psychopharmacol 2005; 25:580–583Crossref, Google Scholar
121. : Mirtazapine in social anxiety disorder: a pilot study. Int Clin Psychopharmacol 2002; 17:315–317Crossref, Google Scholar
122. : Bupropion-SR in treatment of social phobia. Depress Anxiety 2000; 12:111–113Crossref, Google Scholar
123. : Buspirone augmentation of selective serotonin reuptake inhibitors (SSRIs) in social phobia. J Affect Disord 1996; 39:115–121Crossref, Google Scholar
124. : Efficacy of quetiapine in generalized social anxiety disorder: results from an open-label study. J Clin Psychiatry 2005; 66:540–542Crossref, Google Scholar
125. : Quetiapine as monotherapy for social anxiety disorder: a placebo-controlled study. Prog Neuropsychopharmacol Biol Psychiatry 2007; 31:1464–1469Crossref, Google Scholar
126. : Efficacy of olanzapine in social anxiety disorder: a pilot study. J Psychopharmacol 2002; 16:365–368Crossref, Google Scholar
127. : Clinical Practice Review for Social Anxiety Disorder. Silver Spring, MD, Anxiety and Depression Association of America, 2015. https://adaa.org/resources-professionals/clinical-practice-review-social-anxiety. Accessed Feb 18, 2021Google Scholar
128. : A double-blind randomized controlled trial of augmentation and switch strategies for refractory social anxiety disorder. Am J Psychiatry 2014; 171:44–53Crossref, Google Scholar
129. : Pharmacological treatments for social anxiety disorder in adults: a systematic review and network meta-analysis. Acta Neuropsychiatr 2020; 32:169–176Crossref, Google Scholar
130. : A comprehensive model of predictors of suicide attempt in individuals with panic disorder: results from a national 3-year prospective study. Gen Hosp Psychiatry 2020; 67:127–135Crossref, Google Scholar



