Perimenopause and First-Onset Mood Disorders: A Closer Look
Abstract
Perimenopause is often a time of social, emotional, and physical change. Various factors contribute to the development of mood disorders during this time. There is a known association among women with previous history of major depressive disorder or bipolar disorder and relapse during the menopausal transition. First-onset mood disorders during this time have been less studied. A literature review in PsycInfo Ovid of records pertaining to first-onset mood disorders during perimenopause showed that this multifactorial process involves hormonal fluctuations, with estrogen being a key player. In addition, vasomotor symptoms, previous negative life events, and socioeconomic status were found to contribute to first-onset mood disorders during perimenopause. Treatment options include established medication regimens for psychiatric conditions; however, hormone therapy also has proven beneficial for this patient population. Further research, particularly on bipolar disorder, is needed to develop a clear association between perimenopause and first-onset mood disorders.
Perimenopause represents a window of vulnerability in a woman's life where hormonal fluctuations can cause physical and psychiatric symptoms. Perimenopause, or the menopausal transition, is characterized as the time when women begin to display variability of menses, with cycle lengths differing by more than 7 days and late transition defined as greater than 60 days of amenorrhea (1). The existing literature suggests that perimenopause is a time of particular risk for mood disturbances, more so than pre- or postmenopause (2). Along with mood disorders, other symptoms of perimenopause consist of hot flashes, night sweats, insomnia, vaginal dryness, and weight gain (3).
Menopause results from declining reproductive hormone levels, with estrogen being the most implicated. It is understood that hormonal fluctuations pertaining to the reproductive period (including premenstrual and postpartum) are associated with increased risk of mood disorders (4). Similarly, the menopausal transition has been found to be associated with mood disorders, including major depressive disorder and bipolar disorder. Estrogen and progesterone are key players in the hormonal fluctuations of perimenopause, and research has shown their effect on serotonin and neuronal networks responsible for mood in human and animal studies.
An association has been established among women with a previous history of major depressive disorder or bipolar disorder and relapse during the menopausal transition. The odds of experiencing a major depressive episode (MDE) during the menopausal transition have been found to be three times more likely among women with a history of major depressive disorder (5). Along with previous major depression, other risk factors have been identified, such as later stage of menopausal transition, hot flashes, and prior life stressors (6). Sociodemographic variables also have an impact, as highlighted in a study (7) of midlife Portuguese women. Lower education has been significantly associated with more depressive symptoms in midlife, and more depressive symptoms have been seen in perimenopause compared with pre- or postmenopause, independent of the presence of any menopausal symptoms (7). However, there has been a paucity of studies conducted specifically on first- or new-onset mood disorders during this vulnerable time.
Bipolar disorder has been suggested to have bimodal distribution of onset during early adulthood and during later years (ages 45–54) (8). This later period coincides with the average age for perimenopause; however, the Kroon et al. study (8) included women and men and did not support a hormonal etiology. Other critical periods where hormonal changes are prominent include menarche and pregnancy. In a study (4) analyzing age of menarche and age of onset of psychiatric disorders, higher age of menarche was associated with early onset of bipolar disorder, illustrating a potential hormonal relationship between psychiatric illness onset and puberty.
Furthermore, differences during the course of bipolar disorder have been identified between men and women. Women experience more depressive episodes, rapid cycling, and mixed episodes (9). Like major depressive disorder, mood episodes in bipolar disorder have also been reported to be exacerbated by perimenopause and stressful life events. Psychosocial aspects, in addition to biological factors, need consideration when evaluating the interplay between the menopausal transition and mood disorders. In a study (10) following 13 women (who had been previously diagnosed as having bipolar disorder) throughout their respective pre-, peri- and postmenopausal stages, all 13 patients were found to have significantly greater syndromal depression during perimenopause than during their earlier reproductive stages. However, the evidence remains conflicting, with a case series (11) following five women previously diagnosed as having bipolar with postpartum psychosis that found dissimilar results. Four of the five women remained well both after subsequent postpartum episodes, as well as during perimenopause (11). A preliminary study (12) of 47 perimenopausal women with bipolar disorder showed that 68% of the women experienced at least one MDE during the study, and 27 of the women reported increased frequency of depression during perimenopause compared with the reproductive years. Both depressive and elevated mood symptoms among women with bipolar disorder have illustrated increased severity in late perimenopause and late menopause compared with early menopause (13).
The similar mechanisms which lead to relapse of bipolar and major depressive disorders among women during perimenopause can be speculated to cause new onset mood disorders. In this review article, we hypothesize that the menopausal transition has the propensity to precipitate mood disturbance among women with no history of mood disorders. We discuss literature surrounding perimenopausal hormone fluctuations, estrogen and other factors on mood, as well as studies and reviews evaluating first-onset mood disorders in relation to the menopausal transition. We also comment on treatment options and further areas where research is needed.
Methods
A narrative review of literature was performed using PsychInfo Ovid (Figure 1). The search included articles from 2005 to 2020 in the English language. The search terms were “menopause and mental disorders/or affective disorders/or anxiety disorders/or bipolar disorder.” The search produced 831 articles. Abstracts were screened, and duplicates were eliminated. Studies pertaining to first-onset or new-onset mood disorder were further analyzed. Literature was deemed eligible if it also provided background information on the hormonal implications of perimenopause and on other causes known to contribute to mood disturbances during the transition to menopause. Additional relevant articles were found by manual search of article reference lists from PubMed and Google Scholar.
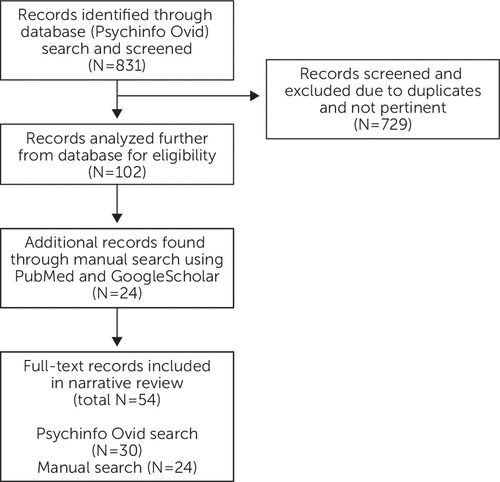
FIGURE 1. Selection of records for the narrative review
Hormonal Implications of Perimenopause
As recognized by the DSM-5, individuals with premenstrual dysphoric disorder may experience worsening symptoms when approaching menopause. Although the DSM-5 statement highlights the well-recognized influence of hormones on mood, the influence of the menopausal transition is likely more complex. Those experiencing perimenopausal symptoms across a longer period (e.g., 27 months) may have increased risk for depression, which suggests that independent of personal history of mood disorders, components of perimenopausal physiology contribute to a disturbance in mood (14). The specific hormones involved in perimenopausal mood changes are numerous. Recent investigations have suggested the influence of cortisol, hypothalamic-pituitary-adrenal (HPA) axis and inflammation (15), and testosterone (16). The hormonal influence of estrogen and progesterone, which have been extensively studied, will be the main focus of this section.
From premenopausal levels of 5 ng/dL to 35 ng/dL, estrogen declines to 1.3 ng/dL postmenopause, and the transition can be marked by mood disturbance, especially for those with a lengthy transition, personal or family history of mental illness, poor sleep, certain racial backgrounds, perimenstrual mood disturbance, vasomotor symptoms (VMS), social stressors, or impaired health (14, 15). Perhaps more important than perimenopausal estrogen decrease is the perimenopausal variance of anovulatory cycle frequency with increased variability of follicle-stimulating hormone (FSH), estrogen, and luteal progesterone, which can last up to 5 years (15). From a functional neuroanatomy perspective, the menopausal transition may alter the activity of ventral limbic regions, including the medial temporal lobe, which may influence consolidation of negative emotional information, possibly because of the presence of estrogen receptors in these regions (17).
Within the medial temporal lobe, estrogen may be linked to brain-derived neurotrophic factors (BDNF) expressed in the hippocampus. This process appears to be more sensitive to estrogen in acutely ovariectomized versus chronic ovariectomized mice (14). Furthermore, ovariectomized mice have been found to increase serotonin production after estradiol administration. Research (18) has illustrated that this effect could be related to a downregulation of monoamine oxidase A (MAO-A), and/or targeted expression of the serotonin transporter. MAO-A, an enzyme found in the brain, is responsible for apoptosis and oxidative stress. Elevated MAO-A levels occur in major depressive disorder in the prefrontal cortex, and greater MAO-A activity has been found in cortical and subcortical regions in animals with depression (19). MAO-A levels increase in the brain after a decrease in estrogen, which can be seen significantly in perimenopause. MAO-A VT has been shown to be increased by 34% in all regions of the brain among perimenopausal women compared with women of reproductive age, and by 16% compared with menopausal women (p<0.05) (19).
Preliminary findings suggest that estrogen receptor (ER) subtypes, ER alpha and ER beta, are expressed in serotonin (5-HT) neurons, including the dorsal raphe nucleus, which is a site of serotonin volume transmission. ER beta is thought to interact with serotonin through the serotonin transporter and to increase 5-HT2aR binding, with dysfunction thought to increase 5-HT1A in the amygdala (18). The effects of estrogen can thus affect the ventral neuroanatomical circuit of mood disturbance (15, 17).
Additionally, variability in progesterone and the downstream metabolite allopregnanolone (ALLO) from menstruation to perimenopause could imply variability in negative feedback on the HPA, as mediated by variance in ALLO action on gamma aminobutyric acid receptor subunit dynamics (15, 17). Exogenous estrogens may also play a role. A cross-sectional study (20) of women ages 45–55 has suggested that the concentration of urinary lignan phytoestrogen is inversely associated with depression as measured by the Patient Health Questionnaire–9. Each of these mechanisms in theory could contribute to a heightened sensitivity to stress during low or variable estrogen and/or progesterone states and to increased consolidation of fear-based stimuli. Additionally, links between estrogen and mood extend across the mood spectrum, potentially having implications for bipolar disorder and postpartum psychosis (21).
Menopause and Mood
The association between perimenopause and mood disorders has been established (22). The complexity of this association is far from understood, but a myriad of causes can contribute to this correlation. Hormonal changes, inflammatory markers, diet, and structural brain changes have been studied as potential contributors.
Hormonal changes occur during perimenopause. Biological changes include “programmed aging” of the HPA, with changes in sex hormones playing a role in perimenopausal depression. The “estrogen withdrawal hypothesis” suggests that estrogen insufficiency leads to depression (22). The above section on the hormonal implications of perimenopause provided details on the role of hormones, including estrogen, that are central to perimenopausal mood. Androgens also have some effect on mood. Testosterone may have anxiolytic and antidepressant effects, and lower levels have been found among women with depression (23). Mitogen-activated protein kinase 1 (MKP-1) has also been associated with depression, with a study (23) showing increased MKP-1 among perimenopausal women with depression. Higher cortisol levels are also found in severe depression, especially in the morning and evening. A study (24) investigated the association of depression and cortisol levels at awakening, 6 p.m., and 9 p.m. among midlife women. Women with more depressive symptoms had higher levels of cortisol at 6 p.m., but the finding was not significant after adjustment for smoking and education. A flatter diurnal slope of salivary cortisol levels has been associated with depressive symptoms among perimenopausal women (p<0.05).
Inflammatory processes also play a role in mood disorders among perimenopausal women. As previously mentioned, MAO-A activity has been found to be increased in certain areas of the brain in depression (19). Another study (25) noted a trend of increasing tumor necrosis factor alpha (TNFα) and decreasing interleukin 6 (IL-6) among perimenopausal women with depression, compared with those without depression and regardless of hot flashes, although the findings were not significant (p=0.53 for TNFα and p=0.90 for IL-6). However, in the same study, hot flashes were strongly associated with perimenopausal depression (p<0.05). The relationship between peripheral neurotrophic, oxidative, and inflammatory markers in new-onset depression among women during the menopause transition has also been researched (26). It has been suggested that 3-nitrotyrosine (p<0.05) and BDNF (p<0.05) are more likely to be related to first-onset depression among midlife women. Serum BDNF levels were decreased 12 months prior to development of depression and stayed low throughout the time leading to the episode (26). A different study (23) showed no difference in BDNF between perimenopausal women and nonperimenopausal women. Furthermore, heat shock protein (HSP) 70 is a member of the superfamily of HSPs. These proteins are upregulated in cells undergoing stressful stimuli, including inflammation and oxidative stress. HSP70 levels are higher at baseline among perimenopausal women who develop first-onset depression compared with those who do not (p<0.05). Therefore, it is speculated that HSP may be associated with protecting against free radicals because of an increase in 3-nitrotyrosine (26).
Diet plays an important role in mood disorders (27). High levels of lipids are present in the central nervous system, which change in response to dietary fat content (28). For example, saturated fatty acids have been shown to affect mood regulation by impairing the number of brain circuits (28). A study (28) of dietary saturated fatty acid intake of perimenopausal women at baseline and 4 years later showed a positive association between depression and saturated fatty acid intake. Similarly, trans fatty acids are linked to proinflammatory processes and gut dysbiosis, which is thought to negatively affect mental health (29). Trans fatty acid intake among women with depressive symptoms has been found to be higher than that of women without these symptoms (29). In contrast, omega-3 polyunsaturated fatty acid (n-3 PUFA) has been shown to be protective against mood changes (27). That study (27) showed that n-3 PUFA intake was inversely associated with depressive symptoms among perimenopausal women in a dose-response inverse relationship. N-3 PUFA supplementation is suggested to help alleviate some of the mood symptoms associated with depression (27).
Recent studies (30) have suggested that menopause may be associated with structural changes in the brain. Gray matter shrinking has been shown in various areas of the brain, including the left putamen, right pallidum, right inferior parietal gyrus, right superior frontal gyrus (orbital part), and right postcentral gyrus of perimenopausal women compared with premenopausal women (p<0.05) (30). These structures have different functions that are affected in perimenopause, including autonomic involvement, regulations of hormones such as FSH and luteinizing hormone (LH), higher cognitive functions, motor control, and sexual functions. Several studies (30) have illustrated that estrogen therapy may slow gray matter loss. White matter changes may also occur during perimenopause (22). Studies (22) have reported decreased fractional anisotropy, which assesses white matter integrity of the brain, in depression. Perimenopausal women with subsyndromal symptoms of depression have shown decreased fractional anisotropy values in the insula and increased values in the thalamus and midbrain, compared with nonperimenopausal age-matched controls (p<0.05) (22). The Wang et al. study (22) has suggested that subsyndromal depressive symptoms are a predictive factor of future depression and has shown that the altered microstructures in the above-mentioned areas make perimenopausal women more susceptible to depression.
First-Episode Depression in Menopause
With its changing hormonal milieu, the transition to menopause has been associated with new onset of depressive symptoms and actual diagnosed depression among women with no history of the disorder (31). The literature on first-episode depression among perimenopausal women has identified many contributing factors, in addition to hormonal changes. Among women with no history of depression, depressive symptoms were four times more likely when a woman was in the menopausal transition compared with the premenopausal state, and an actual diagnosis of major depressive disorder was twice as likely (31).
Many prior risk factors contribute to first-onset depression during perimenopause. When assessed by Bromberger et al. (32), 15.8% of perimenopausal and menopausal women with no past major depressive disorder met criteria for first onset of an MDE. Women more likely to develop first onset of a MDE were found to have low role functioning that was due to physical health limitation, low social functioning, or history of anxiety. Furthermore, women with negative life events had significantly increased risk of developing a first MDE as they transitioned to perimenopause compared with women remaining in premenopause (odds ratio [OR]:2.4, 95% confidence interval [CI]: 1.2–4.6) (33). Medical comorbidities, including cardiovascular disease (hazard ratio [HR]: 1.35, CI: 1.15–1.57), cerebrovascular disease (HR: 1.77, CI: 1.52–2.07), and liver disease (HR: 1.19, CI: 1.03–1.36), have also shown increased contribution to the development of depression during perimenopause (34). Mild increased risk for depression has been observed among women who report VMS (OR: 2.2, CI: 1.1–4.2) (33). Symptomatic menopausal transition has been identified as an independent risk factor for new depression (34). When symptomatic women during the menopausal transition were compared with those without symptoms, 11.4% of the symptomatic women were diagnosed with a psychiatric disorder, compared with 5.6% of those without symptoms (p<0.05) (35). Major depressive disorder (risk ratio [RR]: 2.15, CI : 1.91–2.42) was the most common psychiatric disorder identified; however bipolar disorder (RR : 1.71, CI: 1.01–2.96), anxiety disorder (RR : 2.07, CI: 1.81–2.37), and sleep disorder (RR : 2.01, CI : 1.73–2.34) were also associated with symptomatic perimenopause (35). VMS should be given more attention among those who develop first-onset depression during the menopausal transition to guide treatment options (36). VMS frequently lead to sleep disturbance, which can be severe enough to negatively affect functioning and quality of life (33).
Sleep disturbances, such as insomnia, are also common during the menopausal transition (37). Perimenopausal women are 40% to 56% more likely to report sleep issues, compared with 31% of premenopausal women (37). Nighttime awakening has been significantly associated with late menopausal transition stage and early postmenopause, as well as with a history of depression, with increased severity of depressed mood resulting in increased difficulty in falling asleep (38). In a cross-sectional study (39) controlled for specific menopausal stage, worsening depressive symptoms were associated with difficulties in sleeping more than five times a week (OR: 4.22, CI: 1.53–11.64) and with experiencing insomnia once a week or more (OR: 3.01, CI: 2.02–4.49). Specifically among women with newly diagnosed psychiatric conditions, new sleep disorders have been significantly associated with symptomatic menopausal transition (35). Conflicting research, however, has indicated that increased poor sleep is not significantly associated with newly diagnosed depression among perimenopausal women (OR: 3.68; CI: 1.22–11.15) (31) and that poor sleep is not a predictor of new onset major depressive disorder (HR: 1.23, CI: 0.64–2.35) (32). Although there is a lack of research in this area, awareness of this topic continues to be important.
Moreover, it has been identified that certain social determinants of health are protective against depression. These protective factors are high education level, high income, nonsmoker status, and social support, whereas psychosocial risk factors include low income and education level, nulliparity, and lack of social support (40). Depression is multifactorial, and many risks need to be considered.
A woman’s estradiol variability around her own mean level has been found to be significantly greater among women reporting depressive symptoms (31). Freeman et al. (31) found that women were 9.33 times more likely to have had higher FSH levels and 4.47 times more likely to have had higher LH levels than before the diagnosis of depression, offering support for a salient role of estradiol levels during perimenopause. That study also found that hot flashes, high body mass index, chronic smoking, and premenstrual syndrome were significant risk factors for depressive symptoms. In a study (40) assessing the relationship between menopause and depressive symptoms among midlife women, 18.8% of the women were classified as having depression, with both premature menopause (OR: 1.45, CI: 1.07–1.97) and use of hormone therapy (HT) (OR: 1.21, CI: 1.02–1.44) identified as risk factors for increased depression. Decreased exposure to endogenous estrogen, as occurs in premature menopause, can contribute to onset of depression among this population. Establishing this correlation may shed light on estrogen therapy as a possible treatment. It is interesting that use of HT was associated with increased depression as mentioned above. Furthermore, when comparing perimenopausal women with a hysterectomy to women without a hysterectomy, risk of new onset depressive symptoms was higher among women with hysterectomy (for hysterectomy only, RR: 1.20; CI: 1.06–1.36, and for hysterectomy–bilateral oophorectomy, RR: 1.44; CI: 1.22–1.68) (41). The role of exogenous hormones (HT) and association with depressive symptoms also was compared among women with hysterectomy and ovarian conservation versus those with hysterectomy and bilateral oophorectomy. Women with ovarian conservation who were using HT had a higher risk of developing depressive symptoms than women with hysterectomy and ovarian conservation who did not use HT (RR: 1.57; CI: 1.31–1.88). Interestingly, women with hysterectomy and bilateral oophorectomy who used menopausal HT did experience increased risk (41).
By recognizing menopausal transition as an independent risk factor for new onset depression, we can provide focused screening and early intervention (31). Psychiatric symptoms can present before physical symptoms of perimenopause. We know there is an impact on the neurotransmitters in the central nervous system years before peripheral serum assays detect hormonal changes in menopause (42).
Bipolar Disorder and Perimenopause
The association between perimenopause and first-onset of bipolar disorder has been demonstrated in a few studies; however, evidence remains low and mainly in the form of case reports. We mention these studies because the women in these studies had no previously diagnosed mood disorders before the onset of perimenopausal symptoms (43). It is understood that the transition to menopause can increase the risk of exacerbation of already existing psychiatric disorders, especially among those experiencing menopausal symptoms (35). A case report of a 46-year-old woman with a previous diagnosis of type 1 bipolar disorder showed an increase in frequency of hospitalizations and manic episodes following bilateral salpingo-oophorectomy for endometritis (44). Understanding how surgical or natural menopause plays a role in mood disorders is critical for proper psychiatric care. The authors of the case study (44) speculated that, like the postpartum period, surgically induced perimenopause may be a time of increased risk for onset or exacerbation of bipolar disorder, because of the rapid decline of estrogen and progesterone. Another case report (45) followed a 51-year-old woman with type 1 bipolar disorder posthysterectomy. Initially, the patient denied any mood concerns until 2 years postsurgery. Despite various psychopharmacological and lifestyle adjustments, complaints of mood lability, insomnia, and anhedonia remained. The patient subsequently experienced menopausal VMS, which were suggested as the underlying cause of her worsening moods. The patient was placed on HT by her general practitioner and the aforementioned symptoms subsided (45).
The actual effects of peri- and/or postmenopause on first-onset mood disorders are debatable. Although the correlation can seem distinct, it does not necessarily imply causation (11). A study (43) of midlife women diagnosed with major depressive disorder and no history of bipolar disorder divided the women into two subgroups on the basis of the presence or absence of symptomatic menopausal transition. In that study (43), symptomatic menopausal transition was associated with increased risk of developing bipolar disorder (HR: 1.14, CI: 1.07–1.23). The findings (43) highlighted that the effects of perimenopause can extend past already established depression and can contribute to a new diagnosis. However, the absence of appropriate hormonal follow-up has been a factor possibly negatively affecting the validity of studies (45). The risk of bipolar disorder in symptomatic transition to menopause was found to be increased when follow up was longer than 1 year. This finding suggests the importance of extended follow-up to assess the risk of bipolar disorder (35). Further research is needed on first-onset bipolar disorder during perimenopause and its relationship with other psychiatric disorders as well as with antidepressant and hormone use.
Treatment for Perimenopausal Mood Disorders
The relationship between first-onset depression and perimenopause highlights the importance of adequate treatment for women during this time. The Canadian Network for Mood and Anxiety Treatments (CANMAT) has established guidelines for the treatment of major depressive disorder among perimenopausal women, identifying desvenlafaxine and cognitive-behavioral therapy as first-line treatments. Second-line treatments include transdermal estradiol, citalopram, duloxetine, escitalopram, mirtazapine, quetiapine extended release, and venlafaxine extended release (46). Medication options need to be weighed with side effect profiles and other symptoms of perimenopause besides major depressive disorder, such as appetite, sleep, and VMS. The CANMAT guidelines (47) for bipolar disorder do not specifically comment on treatment for new-onset bipolar disorder during perimenopause, because of the paucity of research. Further research is needed for treatment of this specific population. Table 1 summarizes the effects of various interventions for depression (46, 48–53).
| Treatment | Treatment class | Effect |
|---|---|---|
| Desvenlafaxine | SNRI | Decreased depressive symptoms as monotherapy (46, 48) |
| Cognitive-behavioral therapy | Psychotherapy | Decreased depressive symptoms as monotherapy (46) |
| Escitalopram | SSRI | Decreased depressive symptoms as monotherapy (46, 49) |
| Transdermal estradiol (with and/or without progesterone, dependent on intact uterus) | Hormone therapy | Decreased depressive symptoms as monotherapy or adjunctive treatment (46, 49, 50) Prevention of depressive symptoms as monotherapy (51) |
| Tibolone | STEAR | Decreased depressive symptoms as adjunctive treatment (52) |
| Vortioxetine | Serotonin modulator | Decreased depressive symptoms as monotherapy (53) |
TABLE 1. Effects of treatment interventions on perimenopausal depressiona
In a randomized placebo-controlled study (48), desvenlafaxine was found to be effective and safe in treating depressive symptoms of perimenopausal women (p<0.05). It is important to note that the study (48) permitted HT treatment among the patient population, although this only affected 6% of the sample. The study authors speculated that serotonin-norepinephrine reuptake inhibitors (SNRIs) may alleviate 5-HT dysregulation during perimenopause and can potentially explain why selective serotonin reuptake inhibitors (SSRIs) are not always effective. To date it appears that desvenlafaxine is considered a first-line treatment, because it is the only SNRI that has been studied with randomized, placebo-controlled trials for antidepressant efficacy in perimenopause.
Despite CANMAT listing estradiol as a second-line agent, a meta-analysis (54) found that bioidentical estrogen (mainly estradiol) had no benefit on depression among menopausal women. However, the study did not specifically isolate for perimenopausal women and included postmenopausal women and thus concluded that for mild-to-moderate depression in perimenopause, a clinical benefit of estradiol could not be excluded.
Antidepressant effects versus those of HT have been studied. In an open-label study (49), both escitalopram and estrogen plus progesterone (EPT) significantly decreased depressive symptoms among perimenopausal women; however, the decrease was greater among the escitalopram group (p<0.05). The efficacy of escitalopram versus EPT for the improvement of depressive symptoms, menopause-related symptoms, sleep, and quality of life among peri- and postmenopausal women was studied. Interestingly, hot flashes were also significantly decreased in both groups, but only escitalopram decreased other menopause-related symptoms, including psychological, physical, and other VMS. The improvement in other menopause-related symptoms may have resulted from the improvement in depressive symptoms. Women treated with escitalopram or EPT demonstrated significant improvement in sleep characteristics (p<0.05), with no significant differences between treatment groups (49).
Sudden estradiol withdrawal, and its role in depressive symptom precipitation among women with and without a history of perimenopausal depression, has been studied (50). Participants were given 3 weeks of estradiol treatment and were then randomly assigned to receive placebo or continued estradiol (50). Interestingly, none of the women reported any depressive symptoms during the open-label phase of estradiol use; however, women with a history of perimenopausal depression who were subsequently assigned to the placebo group, experienced significantly increased depressive symptoms (P < 0.05). Women with past perimenopausal depression who continued with estradiol therapy, along with women in the control group who had no history of depression, had no change in their depressive symptoms. It is important to note that among the perimenopausal women with a history of depression, only 4 of 26 women had depression before perimenopause, and the remainder had first-onset depression at perimenopause. The study (50) highlighted the important relationship between perimenopausal depression and estrogen treatment and how caution regarding depression relapse should be exercised when discontinuing HT among women who have responded to estrogen therapy.
Prevention of depressive symptoms among perimenopausal and early postmenopausal women, rather than treatment of an established diagnosis, is also crucial. Treatment with transdermal estradiol plus intermittent micronized progesterone was found to prevent development of depressive symptoms among perimenopausal and early postmenopausal women who were euthymic at the start of the study, with 17.3% of the women taking treatment versus 32.3% of the women taking placebo developing significant depressive symptoms (OR: 2.5; CI: 1.1–5.7) (51). The study (51) included women with a history of depression, although this history was not associated specifically with benefits of the treatment on mood, suggesting that women with first-onset depression during perimenopause may benefit more. Transdermal estradiol and intermittent progesterone may be beneficial for perimenopausal women and for those with a history of stressful life events. By reducing estradiol variability, estradiol and progesterone can exert a prophylactic effect on depression among women with previous stressful life events (51).
In addition to HT acting as monotherapy or adjunctive therapy, research (52) has illustrated that nontraditional medications can be useful adjuncts. Tibilone is classified as a selective tissue estrogenic activity regulator and has estrogen-, androgen-, and progesterone-activating properties. The addition of tibilone to traditional SSRI or SNRI treatment resulted in less depressive symptoms compared with placebo among perimenopausal women with new-onset and recurrent cases of major depressive disorder, with no significant side effects (p<0.05). Vortioxetine, a newer antidepressant demonstrating multimodal serotonin activity, significantly decreased depressive symptoms among women with perimenopausal major depressive disorder (p<0.05) (53). Unfortunately, the study did not comment on first-episode versus recurrent major depressive disorder. Overall, treatment modalities vary for this population, and clear recommendations for specific first-onset mood disorders have not been defined, although these conditions appear to respond to established methods, with adjunctive treatments remaining as options.
Sleep difficulties during the menopausal transition can be multifactorial and, with specific guidelines lacking, treatment may vary on an individual basis. The reasons for sleep disturbances need to be investigated. Treatment options include HT, SSRI and/or SNRIs, and behavioral interventions such as cognitive-behavioral therapy, both as monotherapy and in combinations (37).
Conclusions
The research has indicated a relationship between perimenopause and first-onset mood disorders. In addition to variability of hormones, such as estrogen and progesterone, inflammatory markers, diet, and structural changes in the brain have been studied as potential contributors. Major depressive disorder can be attributed to the changing hormonal milieu during this vulnerable period among women with no history of previous psychiatric illness. However, other factors, such as VMS, prior negative life events, and socioeconomic status need to be considered. These associations were much stronger for first-onset depression; research on first-onset bipolar disorder among midlife women is lacking. Treatment options for this population need to consider psychosocial stressors and other perimenopausal symptoms, such as VMS and sleep disturbance, when establishing the best option for the patient in terms of antidepressant, HT, and psychotherapy use. Certain patients may merit adjunctive treatment and stronger focus on psychosocial factors and diet. Further research is needed in identifying a clear association between perimenopause and first-onset mood disorders, specifically bipolar disorder, because research in this area is limited. However, relationships have been established and remain multifactorial.
1. : In the clinic: perimenopause. Ann Intern Med 2015; 162:ITC1–ITC15Crossref, Google Scholar
2. : Menopausal symptoms and their management. Endocrinol Metab Clin North Am 2015; 44:497–515Crossref, Google Scholar
3. : Prevalence of menopausal symptoms among mid-life women: findings from electronic medical records. BMC Womens Health 2015; 15:58Crossref, Google Scholar
4. : Menarche, puberty and psychiatric disorders. Gynecol Endocrinol 2013; 29:1055–1058Crossref, Google Scholar
5. : Major depression during and after the menopausal transition: Study of Women’s Health Across the Nation (SWAN). Psychol Med 2011; 41:1879–1888Crossref, Google Scholar
6. : Depressed mood during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Menopause 2008; 15:223–232Crossref, Google Scholar
7. : A population-based assessment of the relationship between menopausal and depressive symptoms in Portuguese women. Health Care Women Int 2013; 34:86–100Crossref, Google Scholar
8. : Incidence rates and risk factors of bipolar disorder in the general population: a population-based cohort study. Bipolar Disord 2013; 15:306–313Crossref, Google Scholar
9. : Bipolar disorder in women: reproductive events and treatment considerations. Acta Psychiatr Scand 2005; 112:88–96Crossref, Google Scholar
10. : Progression of female reproductive stages associated with bipolar illness exacerbation. Bipolar Disord 2012; 14:515–526Crossref, Google Scholar
11. : Is the perimenopause a time of increased risk of recurrence in women with a history of bipolar affective postpartum psychosis? A case series. Arch Women Ment Health 2008; 11:75–78Crossref, Google Scholar
12. : Increased frequency of depressive episodes during the menopausal transition in women with bipolar disorder: preliminary report. J Psychiatr Res 2008; 42:247–251Crossref, Google Scholar
13. : Symptom severity of bipolar disorder during the menopausal transition. Int J Bipolar Disord 2015; 3:35Crossref, Google Scholar
14. :
15. : Ovarian hormone fluctuation, neurosteroids, and HPA axis dysregulation in perimenopausal depression: a novel heuristic model. Am J Psychiatry 2015; 172:227–236Crossref, Google Scholar
16. : Longitudinal change in reproductive hormones and depressive symptoms across the menopausal transition: results from the Study of Women’s Health Across the Nation (SWAN). Arch Gen Psychiatry 2010; 67:598–607Crossref, Google Scholar
17. : Estrogen, stress, and depression: a neurocognitive model. JAMA Psychiatry 2015; 72:727–729Crossref, Google Scholar
18. : Effect of estrogen-serotonin interactions on mood and cognition. Behav Cogn Neurosci Rev 2005; 4:43–58Crossref, Google Scholar
19. : Greater monoamine oxidase a binding in perimenopausal age as measured with carbon 11-labeled harmine positron emission tomography. JAMA Psychiatry 2014; 71:873–879Crossref, Google Scholar
20. : Urinary phytoestrogens and depression in perimenopausal US women: NHANES 2005–2008. J Affect Disord 2014; 156:200–205Crossref, Google Scholar
21. : The role of estrogen in bipolar disorder, a review. Nord J Psychiatry 2014; 68:81–87Crossref, Google Scholar
22. : Alterations in white matter fractional anisotropy in subsyndromal perimenopausal depression. BMC Psychiatry 2014; 14:367Crossref, Google Scholar
23. : Association between MKP-1, BDNF, and gonadal hormones with depression on perimenopausal women. J Womens Health (Larchmt) 2016; 25:71–77Crossref, Google Scholar
24. : Cortisol and depressive symptoms in a population-based cohort of midlife women. Psychosom Med 2010; 72:855–861Crossref, Google Scholar
25. : The role of cytokines and hot flashes in perimenopausal depression. Ann Gen Psychiatry 2012; 11:9Crossref, Google Scholar
26. : A longitudinal study of neurotrophic, oxidative, and inflammatory markers in first-onset depression in midlife women. Eur Arch Psychiatry Clin Neurosci 2018; 268:771–781Crossref, Google Scholar
27. : Association of dietary n-3 polyunsaturated fatty acids intake with depressive symptoms in midlife women. J Affect Disord 2020; 261:164–171Crossref, Google Scholar
28. : Association between saturated fatty acid intake and depressive symptoms in midlife women: a prospective study. J Affect Disord 2020; 267:17–22Crossref, Google Scholar
29. : Associations of dietary trans fatty acid intake with depressive symptoms in midlife women. J Affect Disord 2020; 260:194–199Crossref, Google Scholar
30. : Grey matter differences associated with age and sex hormone levels between premenopausal and perimenopausal women: a voxel-based morphometry study. J Neuroendocrinol 2018; 30:e12655Crossref, Google Scholar
31. : Associations of hormones and menopausal status with depressed mood in women with no history of depression. Arch Gen Psychiatry 2006; 63:375–382Crossref, Google Scholar
32. : Predictors of first lifetime episodes of major depression in midlife women. Psychol Med 2009; 39:55–64Crossref, Google Scholar
33. : Risk for new onset of depression during the menopausal transition: the Harvard study of moods and cycles. Arch Gen Psychiatry 2006; 63:385–390Crossref, Google Scholar
34. : Symptomatic menopausal transition increases the risk of new-onset depressive disorder in later life: a nationwide prospective cohort study in Taiwan. PLoS One 2013; 8:e59899Crossref, Google Scholar
35. : Risk of psychiatric disorders following symptomatic menopausal transition: a nationwide population-based retrospective cohort study. Medicine (Baltimore) 2016; 95:e2800Crossref, Google Scholar
36. : Risk factors for major depression during midlife among a community sample of women with and without prior major depression: are they the same or different? Psychol Med 2015; 45:1653–1664Crossref, Google Scholar
37. : Sleep problems during the menopausal transition: prevalence, impact, and management challenges. Nat Sci Sleep 2018; 10:73–95Crossref, Google Scholar
38. : Sleep symptoms during the menopausal transition and early postmenopause: observations from the Seattle Midlife Women’s Health Study. Sleep 2010; 33:539–549Crossref, Google Scholar
39. : Relations among menopausal symptoms, sleep disturbance and depressive symptoms in midlife. Maturitas 2009; 62:184–189Crossref, Google Scholar
40. : Depression, hormone therapy, and the menopausal transition among women aged 45 to 64 years using Canadian Longitudinal Study on Aging baseline data. Menopause 2020; 27:763–770Crossref, Google Scholar
41. : Hysterectomy and incidence of depressive symptoms in midlife women: the Australian Longitudinal Study on Women’s Health. Epidemiol Psychiatr Sci 2018; 27:381–392Crossref, Google Scholar
42. : A case of perimenopausal depression. Aust N Z J Psychiatry 2016; 50:1109Crossref, Google Scholar
43. : Symptomatic menopausal transition and subsequent bipolar disorder among midlife women with major depression: a nationwide longitudinal study. Arch Women Ment Health 2017; 20:463–468Crossref, Google Scholar
44. : Manic episode after total abdominal hysterectomy with bilateral salpingo-oophorectomy: case report. Menopause 2012; 19:476–478Crossref, Google Scholar
45. : Menopause manifesting as bipolar symptoms. J Psychiatr Pract 2007; 13:339–342Crossref, Google Scholar
46. : Canadian Network for Mood and Anxiety Treatments (CANMAT) 2016 clinical guidelines for the management of adults with major depressive disorder: section 6. Special populations: youth, women, and the elderly. Can J Psychiatry 2016; 61:588–603Crossref, Google Scholar
47. : Canadian Network for Mood and Anxiety Treatments (CANMAT) and International Society for Bipolar Disorders (ISBD) 2018 guidelines for the management of patients with bipolar disorder. Bipolar Disord 2018; 20:97–170Crossref, Google Scholar
48. : Efficacy and safety of desvenlafaxine 50 mg/d in a randomized, placebo-controlled study of perimenopausal and postmenopausal women with major depressive disorder. J Clin Psychiatry 2013; 74:1010–1017Crossref, Google Scholar
49. : Escitalopram versus ethinyl estradiol and norethindrone acetate for symptomatic peri- and postmenopausal women: impact on depression, vasomotor symptoms, sleep, and quality of life. Menopause 2006; 13:780–786Crossref, Google Scholar
50. : Effects of estradiol withdrawal on mood in women with past perimenopausal depression: a randomized clinical trial. JAMA Psychiatry 2015; 72:714–726Crossref, Google Scholar
51. : Efficacy of transdermal estradiol and micronized progesterone in the prevention of depressive symptoms in the menopause transition: a randomized clinical trial. JAMA Psychiatry 2018; 75:149–157Crossref, Google Scholar
52. : Tibolone improves depression in women through the menopause transition: a double-blind randomized controlled trial of adjunctive tibolone. J Affect Disord 2018; 236:88–92Crossref, Google Scholar
53. : Vortioxetine for major depressive disorder, vasomotor, and cognitive symptoms associated with the menopausal transition. Ann Clin Psychiatry 2017; 29:249–257Google Scholar
54. : Bioidentical estrogen for menopausal depressive symptoms: a systematic review and meta-analysis. J Womens Health (Larchmt) 2017; 26:18–28Crossref, Google Scholar



